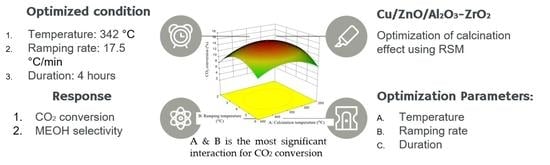
Optimization of Calcination Conditions for Cu/ZnO/Al2O3-ZrO2 Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Model Fitting
2.2. Regression Model Equation and Analysis of Variance (ANOVA)
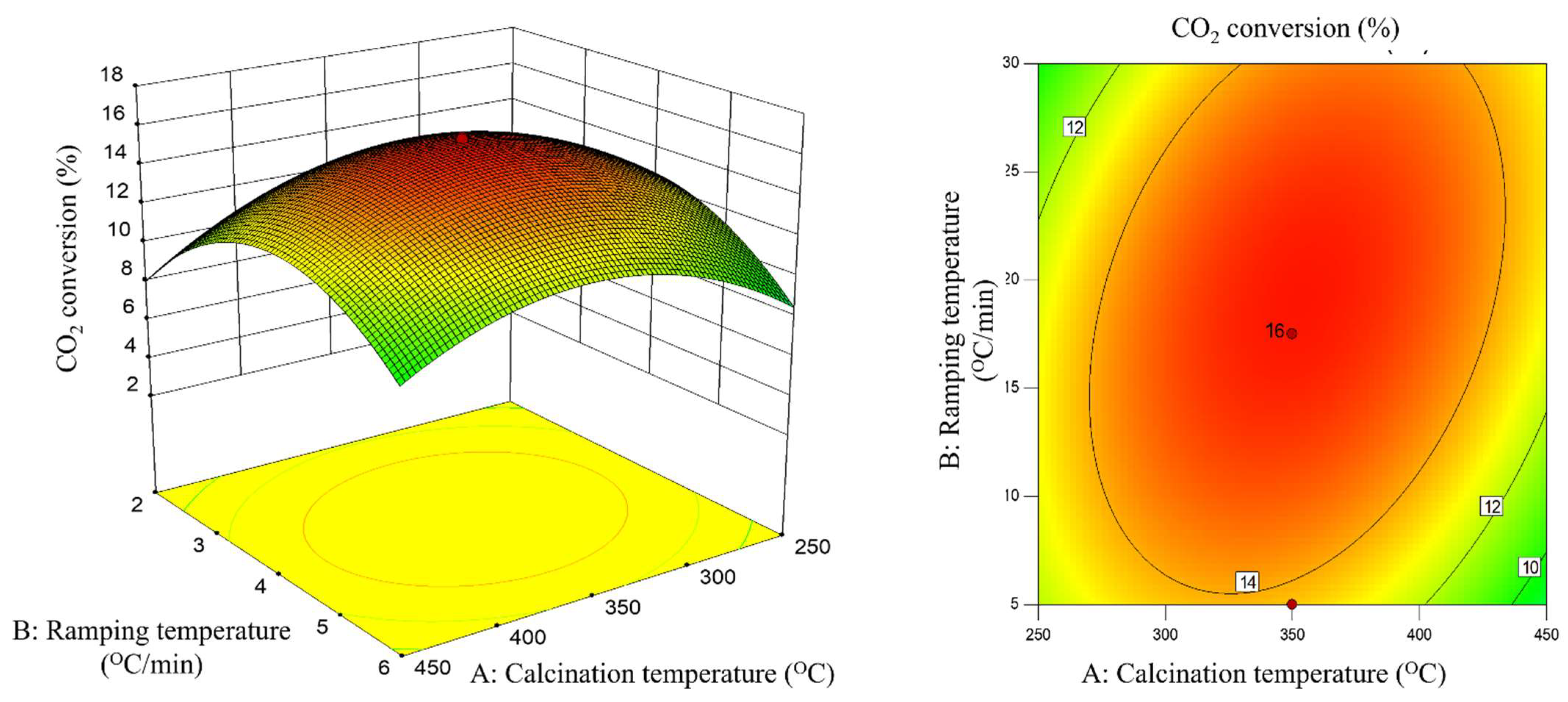
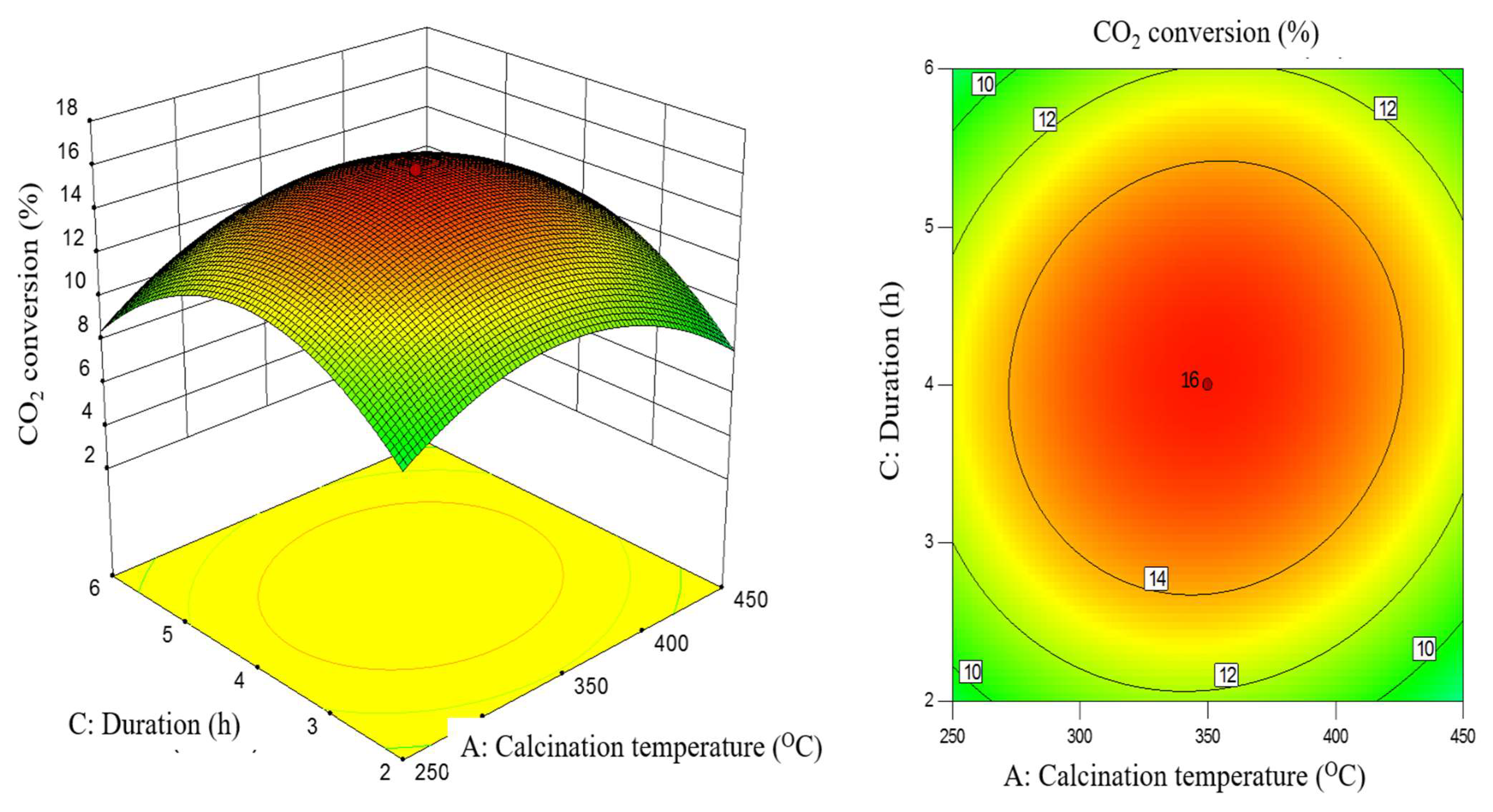
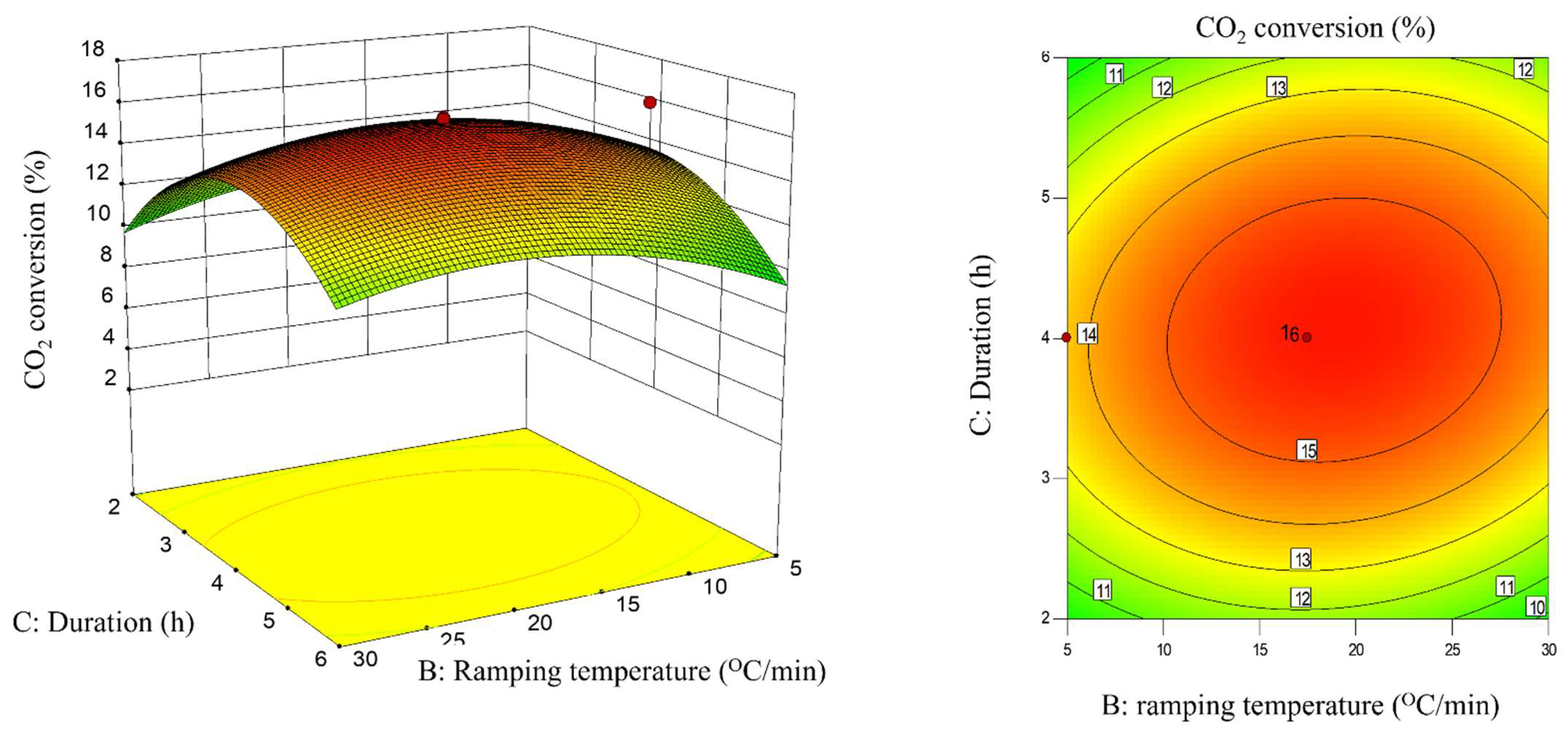
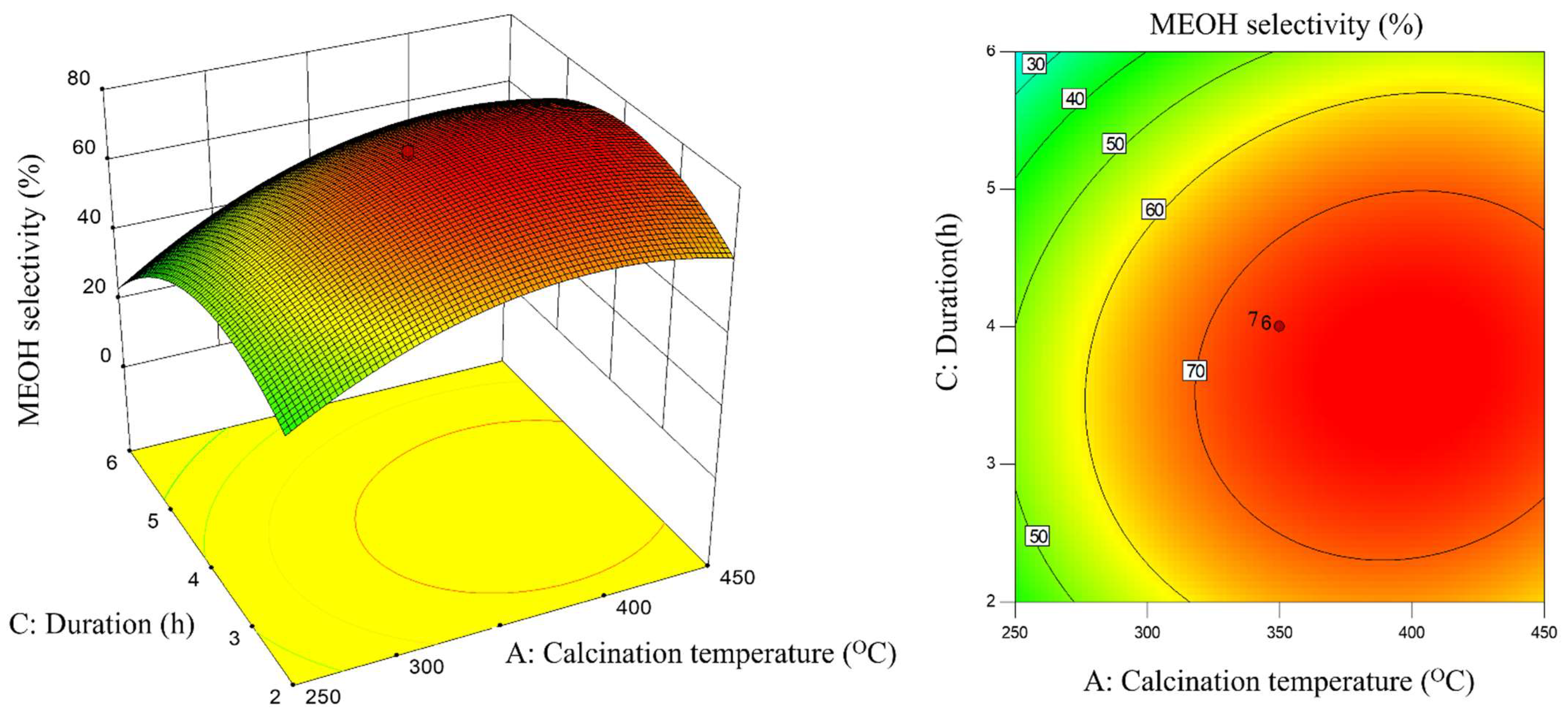
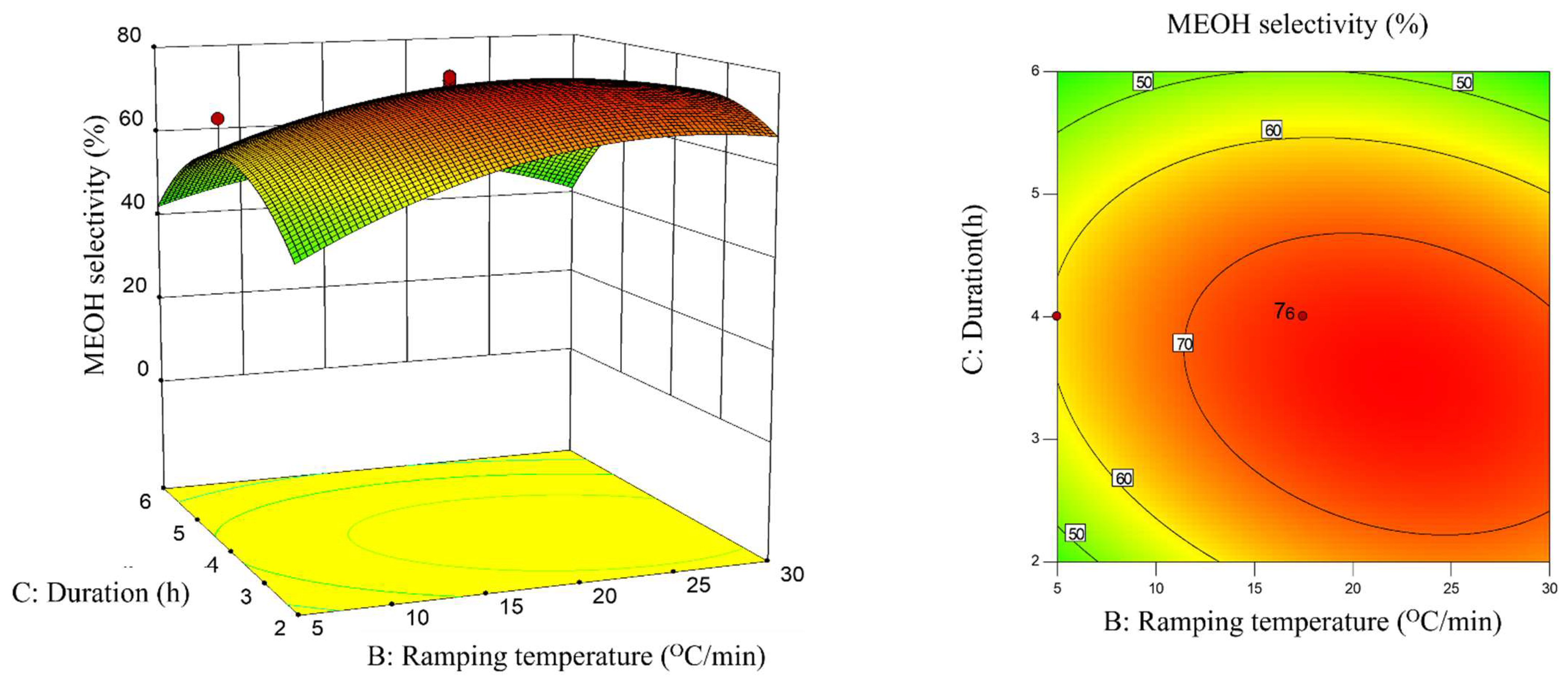
2.3. Interaction Effect of Calcination Condition of Catalyst (MNCZAZ)
- temperature and ramping rate (AB)
- temperature and duration (AC)
- ramping rate and duration (BC)
2.4. Diagnostic Plots of the Calcination Parameters to XCO2 and SMEOH Responses
2.5. RSM Optimizations of Calcination Parameters
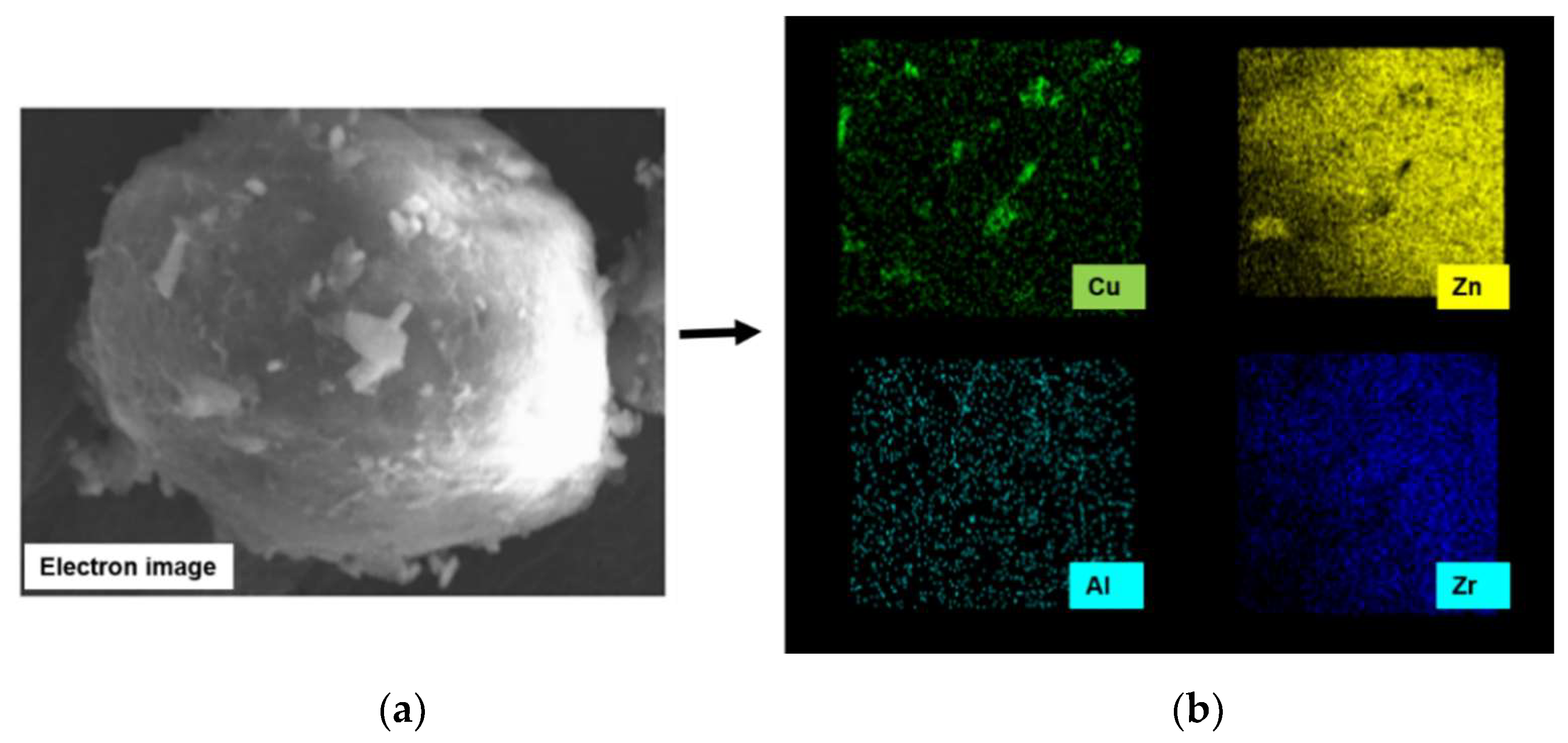
2.6. Textural and Morphological Properties
3. Materials and Methods
3.1. Experimental Design
3.2. Preparation and Characterization of MNCZAZ Catalyst
3.3. Catalytic Performance Evaluation of MNCZAZ Catalyst
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atsbha, T.A.; Yoon, T.; Seongho, P.; Lee, C.J. A review on the catalytic conversion of CO2 using H2 for synthesis of CO, methanol, and hydrocarbons. J. CO2 Util. 2021, 44, 101413. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Liu, W.; Yuan, F.; Wang, J.; Zhu, J.; Jiang, X.; Zhang, A.; Ding, F.; Song, C.; et al. Reaction-driven surface reconstruction of ZnAl2O4 boosts the methanol selectivity in CO2 catalytic hydrogenation. Appl. Catal. B Environ. 2021, 284, 119700. [Google Scholar] [CrossRef]
- Alsuhaibani, A.S.; Afzal, S.; Challiwala, M.; Elbashir, N.O.; El-Halwagi, M.M. The impact of the development of catalyst and reaction system of the methanol synthesis stage on the overall profitability of the entire plant: A techno-economic study. Catal. Today 2020, 343, 191–198. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Pino, L.; Laganà, M.; Ferraro, M.; Antonucci, V. High-temperature CO2 methanation over structured Ni/GDC catalysts: Performance and scale-up for Power-to-Gas application. Fuel Process. Technol. 2020, 202, 106365. [Google Scholar] [CrossRef]
- Ren, S.; Fan, X.; Shang, Z.; Shoemaker, W.R.; Ma, L.; Wu, T.; Li, S.; Klinghoffer, N.B.; Yu, M.; Liang, X. Enhanced catalytic performance of Zr modified CuO/ZnO/Al2O3 catalyst for methanol and DME synthesis via CO2 hydrogenation. J. CO2 Util. 2020, 36, 82–95. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.G. CO2 Hydrogenation to Methanol over ZrO2-Containing Catalysts: Insights into ZrO2 Induced Synergy. ACS Catal. 2019, 9, 7840–7861. [Google Scholar] [CrossRef]
- Marcos, F.C.; Lin, L.; Betancourt, L.E.; Senanayake, S.D.; Rodriguez, J.A.; Assaf, J.M.; Giudici, R.; Assaf, E.M. Insights into the methanol synthesis mechanism via CO2 hydrogenation over Cu-ZnO-ZrO2 catalysts: Effects of surfactant/Cu-Zn-Zr molar ratio. J. CO2 Util. 2020, 41, 101215. [Google Scholar] [CrossRef]
- Wu, J.; Saito, M.; Takeuchi, M.; Watanabe, T. The stability of Cu/ZnO-based catalysts in methanol synthesis from a CO2-rich feed and from a CO-rich feed. Appl. Catal. A Gen. 2001, 218, 235–240. [Google Scholar] [CrossRef]
- Din, I.U.; Usman, M.; Khan, S.; Helal, A.; Alotaibi, M.A.; Alharthi, A.I.; Centi, G. Prospects for a green methanol thermo-catalytic process from CO2 by using MOFs based materials: A mini-review. J. CO2 Util. 2021, 43, 101361. [Google Scholar] [CrossRef]
- Mureddu, M.; Lai, S.; Atzori, L.; Rombi, E.; Ferrara, F.; Pettinau, A.; Cutrufello, M.G. Ex-LDH-based catalysts for CO2 conversion to methanol and dimethyl ether. Catalysts 2021, 11, 615. [Google Scholar] [CrossRef]
- Duyar, M.S.; Gallo, A.; Regli, S.K.; Snider, J.L.; Singh, J.A.; Valle, E.; McEnaney, J.; Bent, S.F.; Rønning, M.; Jaramillo, T.F. Understanding selectivity in CO2 hydrogenation to methanol for mop nanoparticle catalysts using in situ techniques. Catalysts 2021, 11, 143. [Google Scholar] [CrossRef]
- Bibi, M.; Ullah, R.; Sadiq, M.; Sadiq, S.; Khan, I.; Saeed, K.; Zia, M.A.; Iqbal, Z.; Ullah, I.; Iqbal, Z.; et al. Catalytic hydrogenation of carbon dioxide over magnetic nanoparticles: Modification in fixed-bed reactor. Catalysts 2021, 11, 592. [Google Scholar] [CrossRef]
- Lu, Z.; Sun, K.; Wang, J.; Zhang, Z.; Liu, C. A Highly Active Au/In2O3-ZrO2 Catalyst for Selective Hydrogenation of CO2 to Methanol. Catalysts 2020, 11, 1360. [Google Scholar] [CrossRef]
- Fujita, S.I.; Moribe, S.; Kanamori, Y.; Kakudate, M.; Takezawa, N. Preparation of a coprecipitated Cu/ZnO catalyst for the methanol synthesis from CO2- effects of the calcination and reduction conditions on the catalytic performance. Appl. Catal. A Gen. 2001, 207, 121–128. [Google Scholar] [CrossRef]
- Zhang, Y.; Fei, J.; Yu, Y.; Zheng, X. Methanol synthesis from CO2 hydrogenation over Cu based catalyst supported on zirconia modified γ-Al2O3. Energy Convers. Manag. 2006, 47, 3360–3367. [Google Scholar] [CrossRef]
- Amin, N.A.S.; Zakaria, Z.Y. Optimization of Oxidative Coupling of Methane Using Response Surface Methodology. J. Teknol. 2012, 39, 35–51. [Google Scholar]
- Eze, F.C.; Ngonadi, L. Alphabetic Optimality Criteria for 2K Central Composite Design. App. Math. Sci. 2018, 4, 107–118. [Google Scholar]
- Aslan, N. Application of response surface methodology and central composite rotatable design for modeling and optimization of a multi-gravity separator for chromite concentration. Powder Technol. 2008, 185, 80–86. [Google Scholar] [CrossRef]
- Box, G.E.P.; Hunter, J.S. Multi-Factor Experimental Designs for Exploring Response Surfaces. Ann. Math. Stat. 1957, 28, 195–241. [Google Scholar] [CrossRef]
- Obeng, D.P.; Morrell, S.; Napier-Munn, T.J. Application of central composite rotatable design to modelling the effect of some operating variables on the performance of the three-product cyclone. Int. J. Miner. Process. 2005, 76, 181–192. [Google Scholar] [CrossRef]
- Anuar, M.A.M.; Amran, N.A.; Ruslan, M.S.H. Optimization of Progressive Freezing for Residual Oil Recovery from a Palm Oil − Water Mixture (POME Model). ACS Omega 2020, 6, 2707–2716. [Google Scholar] [CrossRef] [PubMed]
- Saafie, N.; Samsudin, M.R.; Sufian, S. Optimization of methylene blue adsorption via functionalized activated carbon using response surface methodology with central composite design. Key Eng. Mater. 2020, 841, 220–224. [Google Scholar] [CrossRef]
- Tasfy, S.F.H.; Mohd Zabidi, N.A.; Shaharun, M.S.; Subbarao, D. Effect of Mn and Pb Promoters on the Performance of Cu/ZnO-Catalyst in CO2 Hydrogenation to Methanol. Appl. Mech. Mater. 2014, 625, 289–292. [Google Scholar] [CrossRef]



| Variables | Unit | Levels | ||||
|---|---|---|---|---|---|---|
| −α Min | −1 Low | 0 Mid | 1 High | +α Max | ||
| A: Calcination temperature | °C | 181 | 250 | 350 | 450 | 518 |
| B: Ramping rate | °C/min | 1 | 5 | 17.5 | 30 | 40 |
| C: Duration (h) | h | 1 | 2 | 4 | 6 | 7 |
| Run | A | B | C | Response 1: XCO2 | Response 2: SMEOH | YieldMEOH |
|---|---|---|---|---|---|---|
| 1 | 350 | 17.5 | 4 | 16.02 | 75.86 | 12.15 |
| 2 | 518 | 17.5 | 4 | 7.19 | 58.35 | 4.19 |
| 3 | 450 | 5 | 6 | 3.35 | 52.39 | 1.75 |
| 4 | 250 | 30 | 6 | 5.55 | 25.43 | 1.41 |
| 5 | 250 | 5 | 2 | 8.17 | 20.91 | 1.70 |
| 6 | 250 | 30 | 2 | 7.97 | 47.59 | 3.79 |
| 7 | 450 | 30 | 2 | 7.43 | 63.83 | 4.74 |
| 8 | 350 | 17.5 | 1 | 5.55 | 47.76 | 2.65 |
| 9 | 250 | 5 | 6 | 7.87 | 5.57 | 0.43 |
| 10 | 350 | 1 | 4 | 15.81 | 68.77 | 10.87 |
| 11 | 350 | 17.5 | 4 | 13.6 | 63.1 | 8.58 |
| 12 | 350 | 17.5 | 4 | 15.9 | 74 | 11.76 |
| 13 | 350 | 17.5 | 4 | 16.01 | 75 | 12.00 |
| 14 | 350 | 40 | 4 | 9.63 | 52.54 | 5.05 |
| 15 | 350 | 17.5 | 4 | 16.04 | 76.1 | 12.2 |
| 16 | 450 | 30 | 6 | 11.28 | 37.34 | 4.21 |
| 17 | 350 | 17.5 | 4 | 16.1 | 75.1 | 12.09 |
| 18 | 181 | 17.5 | 4 | 6.47 | 19.52 | 1.26 |
| 19 | 350 | 17.5 | 7 | 7.91 | 24.63 | 1.94 |
| 20 | 450 | 5 | 2 | 5.75 | 42.46 | 2.44 |
| XCO2 | SMEOH | |
|---|---|---|
| Model | Quadratic Model | Quadratic Model |
| Standard deviation | 1.59 | 5.84 |
| R2 | 0.93 | 0.96 |
| Adjusted R2 | 0.87 | 0.93 |
| Adequate precision | 9.75 | 15.36 |
| p-value | <0.0001 | <0.0001 |
| F-value | 15.31 | 28.86 |
| LOF | 0.0711 | 0.2813 |
| Source | Coefficient Estimate | F-Value | Prob. > F | Remarks |
|---|---|---|---|---|
| Quadratic model | 15.31 | <0.0001 | significant | |
| A | −0.052 | 0.015 | 0.9063 | insignificant |
| B | 0.38 | 0.61 | 0.4545 | insignificant |
| C | 0.18 | 0.16 | 0.6949 | insignificant |
| AB | 1.52 | 7.27 | 0.0224 | significant |
| AC | 0.52 | 0.86 | 0.3758 | insignificant |
| BC | 0.52 | 0.84 | 0.3802 | insignificant |
| A2 | −3.10 | 55.75 | <0.0001 | significant |
| B2 | −1.80 | 13.41 | 0.0044 | significant |
| C2 | −3.90 | 62.38 | <0.0001 | significant |
| Source | Coefficient Estimate | F-Value | Prob. > F | Remarks |
|---|---|---|---|---|
| Quadratic model | 28.86 | <0.0001 | significant | |
| A | 11.79 | 55.74 | <0.0001 | significant |
| B | 4.92 | 7.51 | 0.0208 | significant |
| C | −7.10 | 18.48 | 0.0016 | significant |
| AB | −5.03 | 5.93 | 0.0352 | significant |
| AC | 2.62 | 1.61 | 0.2336 | insignificant |
| BC | −5.40 | 6.85 | 0.0257 | significant |
| A2 | −12.25 | 64.72 | <0.0001 | significant |
| B2 | −8.45 | 21.84 | 0.0009 | significant |
| C2 | −16.67 | 83.45 | <0.0001 | significant |
| Variables | Units | Optimum Conditions |
|---|---|---|
| A | °C | 342 |
| B | °C/min | 17.9 |
| C | h | 4 |
| XCO2 activity, calculated | % | 16.2 |
| XCO2 activity, experimental | % | 15.5 |
| Percentage error, XCO2 | % | 4.32 |
| SMEOH activity, calculated | % | 74.7 |
| SMEOH activity, experimental | % | 72.6 |
| Percentage error, SMEOH | % | 2.81 |
| Standard deviation | % | 1.01 |
| Weight (%) | |||
|---|---|---|---|
| Cu | Zn | Mn | Nb |
| 11.27 | 3.72 | 0.05 | 0.04 |
| Temperature (°C) | Ramping Rate (°C/min) | Duration (h) | SBET (m2/g) | Vp (cm3/g) | DBJH (nm) | XCO2 (%) | SMeOH (%) |
|---|---|---|---|---|---|---|---|
| 518 | 17.5 | 4 | 137 | 0.31 | 8.9 | 7.19 | 58.35 |
| 450 | 5 | 6 | 143 | 0.31 | 8.6 | 3.35 | 52.39 |
| 350 | 10 | 4 | 140 | 0.31 | 6.2 | 15.81 | 68.77 |
| 350 | 17.5 | 4 | 147 | 0.31 | 8.1 | 16.02 | 75.86 |
| 250 | 5 | 6 | 123 | 0.24 | 7.6 | 7.87 | 5.57 |
| 181 | 17.5 | 4 | 167 | 0.31 | 7.4 | 6.47 | 19.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulkifli, N.I.; Berahim, N.H.; Mohd Zabidi, N.A.; Tasfy, S.F.H. Optimization of Calcination Conditions for Cu/ZnO/Al2O3-ZrO2 Catalyst. Catalysts 2021, 11, 871. https://doi.org/10.3390/catal11080871
Zulkifli NI, Berahim NH, Mohd Zabidi NA, Tasfy SFH. Optimization of Calcination Conditions for Cu/ZnO/Al2O3-ZrO2 Catalyst. Catalysts. 2021; 11(8):871. https://doi.org/10.3390/catal11080871
Chicago/Turabian StyleZulkifli, Nur Insyirah, Nor Hafizah Berahim, Noor Asmawati Mohd Zabidi, and Sara Faiz Hanna Tasfy. 2021. "Optimization of Calcination Conditions for Cu/ZnO/Al2O3-ZrO2 Catalyst" Catalysts 11, no. 8: 871. https://doi.org/10.3390/catal11080871
APA StyleZulkifli, N. I., Berahim, N. H., Mohd Zabidi, N. A., & Tasfy, S. F. H. (2021). Optimization of Calcination Conditions for Cu/ZnO/Al2O3-ZrO2 Catalyst. Catalysts, 11(8), 871. https://doi.org/10.3390/catal11080871