Influence of Phase Composition and Pretreatment on the Conversion of Iron Oxides into Iron Carbides in Syngas Atmospheres
Abstract
:1. Introduction
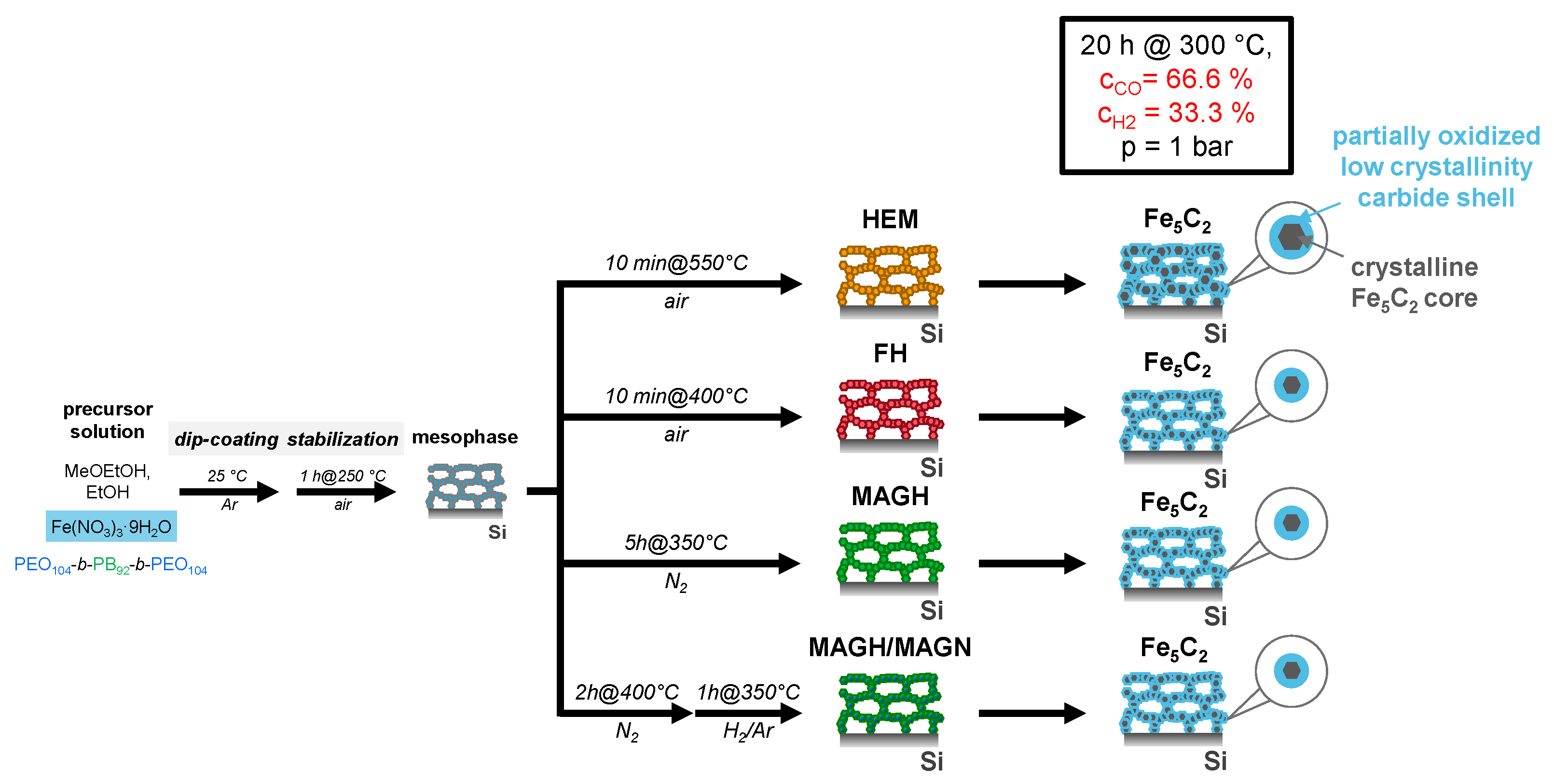
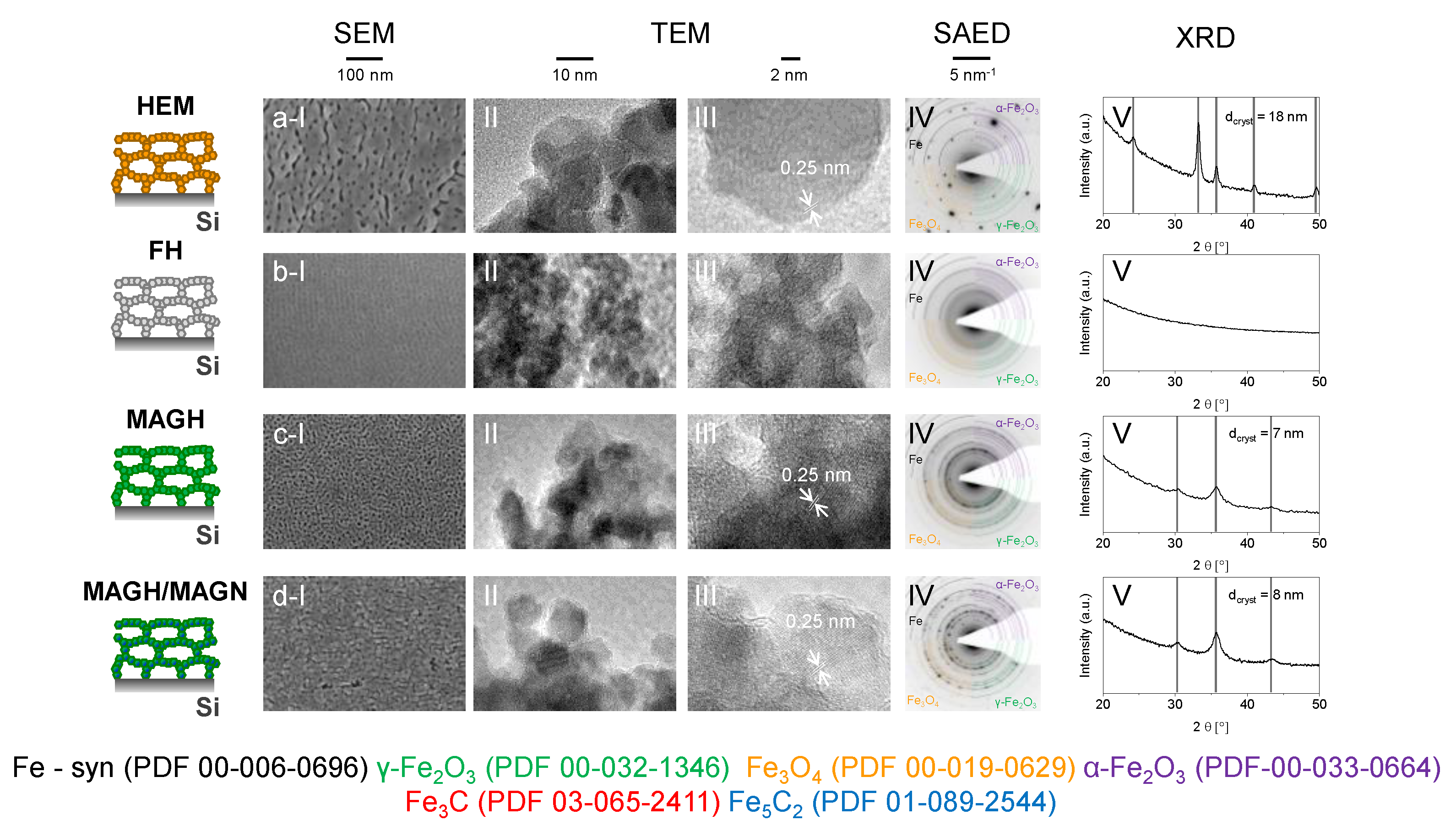
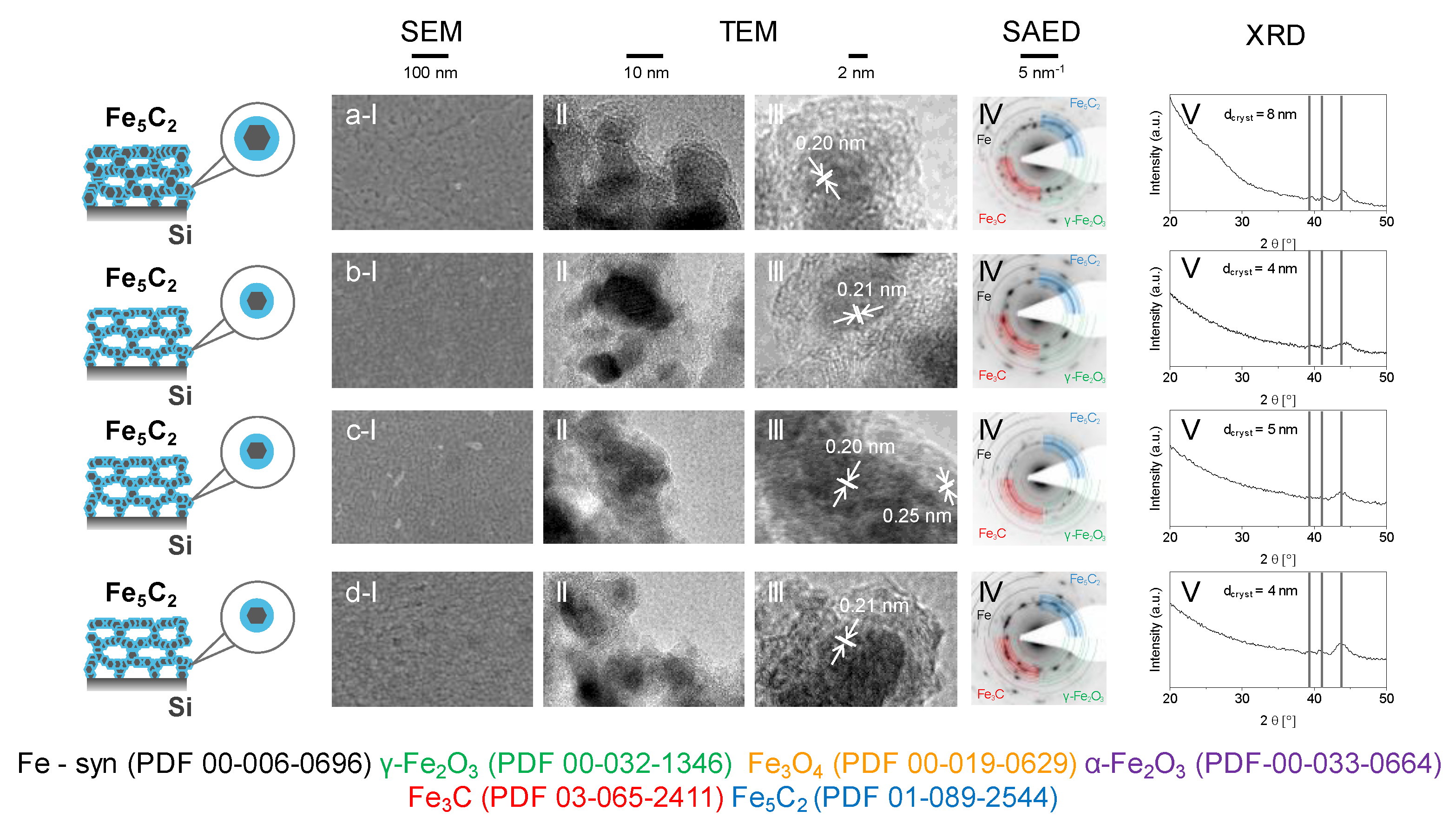
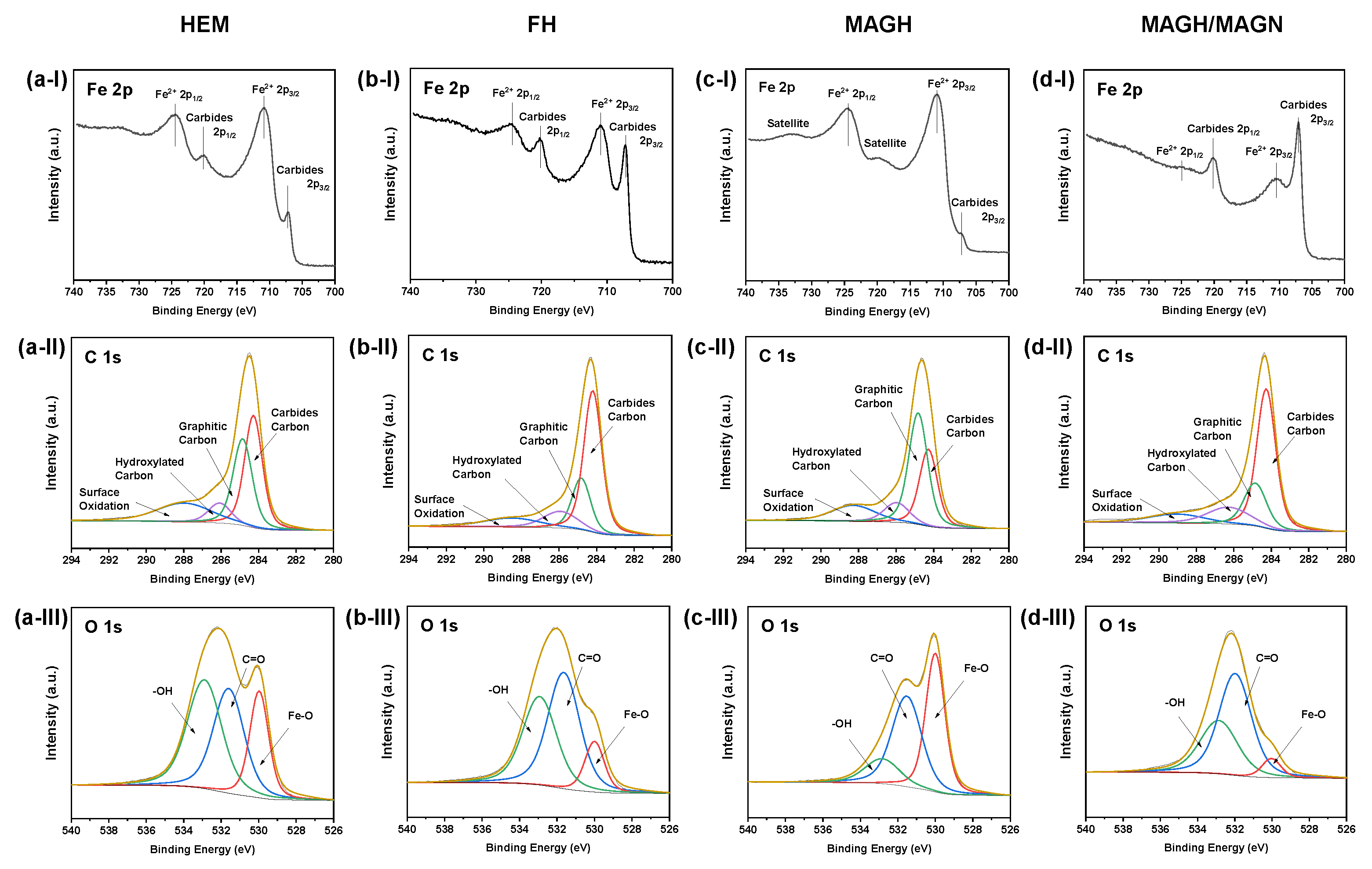
2. Results
3. Conclusions
4. Materials and Methods
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, T.R.; Hawkins, E.; Jones, P.D. CO2, the greenhouse effect and global warming: From the pioneering work of Arrhenius and Callendar to today’s Earth System Models. Endeavour 2016, 40, 178–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, G.P.; Andrew, R.M.; Canadell, J.G.; Friedlingstein, P.; Jackson, R.B.; Korsbakken, J.I.; le Quéré, C.; Peregon, A. Carbon dioxide emissions continue to grow amidst slowly emerging climate policies. Nat. Clim. Chang. 2020, 10, 3–6. [Google Scholar] [CrossRef]
- Kemper, J. Biomass and carbon dioxide capture and storage: A review. Int. J. Greenh. Gas Control 2015, 40, 401–430. [Google Scholar] [CrossRef]
- Gulzar, A.; Gulzar, A.; Ansari, M.B.; He, F.; Gai, S.; Yang, P. Carbon dioxide utilization: A paradigm shift with CO2 economy. Chem. Eng. J. Adv. 2020, 3, 100013. [Google Scholar] [CrossRef]
- Schoedel, A.; Ji, Z.; Yaghi, O.M. The role of metal–organic frameworks in a carbon-neutral energy cycle. Nat. Energy 2016, 1, 16034. [Google Scholar] [CrossRef]
- Jiang, X.; Nie, X.; Guo, X.; Song, C.; Chen, J.G. Recent Advances in Carbon Dioxide Hydrogenation to Methanol via Heterogeneous Catalysis. Chem. Rev. 2020, 120, 7984–8034. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábal, G.O.; Pérez–Ramírez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112. [Google Scholar] [CrossRef] [Green Version]
- Skrypnik, A.S.; Yang, Q.; Matvienko, A.A.; Bychkov, V.Y.; Tulenin, Y.P.; Lund, H.; Petrov, S.A.; Kraehnert, R.; Arinchtein, A.; Weiss, J.; et al. Understanding reaction-induced restructuring of well-defined FexOyCz compositions and its effect on CO2 hydrogenation. Appl. Catal. B Environ. 2021, 291, 120121. [Google Scholar] [CrossRef]
- Herranz, T.; Rojas, S.; Pérez–Alonso, F.J.; Ojeda, M.; Terreros, P.; Fierro, J.L.G. Genesis of iron carbides and their role in the synthesis of hydrocarbons from synthesis gas. J. Catal. 2006, 243, 199–211. [Google Scholar] [CrossRef]
- Ding, M.; Yang, Y.; Xu, J.; Tao, Z.; Wang, H.; Wang, H.; Xiang, H.; Li, Y. Effect of reduction pressure on precipitated potassium promoted iron–manganese catalyst for Fischer–Tropsch synthesis. Appl. Catal. A Gen. 2008, 345, 176–184. [Google Scholar] [CrossRef]
- De Smit, E.; Weckhuysen, B.M. The renaissance of iron-based Fischer–Tropsch synthesis: On the multifaceted catalyst deactivation behaviour. Chem. Soc. Rev. 2008, 37, 2758–2781. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Wang, C.; Yue, J.; Zhang, X.; Ma, L. Effect of a potassium promoter on the Fischer–Tropsch synthesis of light olefins over iron carbide catalysts encapsulated in graphene-like carbon. Catal. Sci. Technol. 2019, 9, 2728–2741. [Google Scholar] [CrossRef]
- Shroff, M.D.; Kalakkad, D.S.; Coulter, K.E.; Kohler, S.D.; Harrington, M.S.; Jackson, N.B.; Sault, A.G.; Datye, A.K. Activation of Precipitated Iron Fischer-Tropsch Synthesis Catalysts. J. Catal. 1995, 156, 185–207. [Google Scholar] [CrossRef]
- Yao, B.; Xiao, T.; Makgae, O.A.; Jie, X.; Gonzalez–Cortes, S.; Guan, S.; Kirkland, A.I.; Dilworth, J.R.; Al–Megren, H.A.; Alshihri, S.M.; et al. Transforming carbon dioxide into jet fuel using an organic combustion-synthesized Fe-Mn-K catalyst. Nat. Commun. 2020, 11, 6395. [Google Scholar] [CrossRef]
- Galvis, H.M.T.; Bitter, J.H.; Davidian, T.; Ruitenbeek, M.; Dugulan, A.I.; de Jong, K.P. Iron Particle Size Effects for Direct Production of Lower Olefins from Synthesis Gas. J. Am. Chem. Soc. 2012, 134, 16207–16215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, M.A.; Cariem, M.J.; Claeys, M.; van Steen, E. A DFT perspective of potassium promotion of χ-Fe5C2(1 0 0). Appl. Catal. A Gen. 2015, 496, 64–72. [Google Scholar] [CrossRef]
- Galvis, H.M.T.; Koeken, A.C.J.; Bitter, J.H.; Davidian, T.; Ruitenbeek, M.; Dugulan, A.I.; de Jong, K.P. Effects of sodium and sulfur on catalytic performance of supported iron catalysts for the Fischer–Tropsch synthesis of lower olefins. J. Catal. 2013, 303, 22–30. [Google Scholar] [CrossRef]
- Davis, B.H. Fischer–Tropsch synthesis: Current mechanism and futuristic needs. Fuel Process. Technol. 2001, 71, 157–166. [Google Scholar] [CrossRef]
- Schulz, K.; Schmack, R.; Klemm, H.W.; Kabelitz, A.; Schmidt, T.; Emmerling, F.; Kraehnert, R. Mechanism and kinetics of hematite crystallization in air: Linking bulk and surface models via mesoporous films with defined nanostructure. Chem. Mater. 2017, 29, 1724–1734. [Google Scholar] [CrossRef]
- Kraffert, K.; Kabelitz, A.; Siemensmeyer, K.; Schmack, R.; Bernsmeier, D.; Emmerling, F.; Kraehnert, R. Nanocasting of Superparamagnetic Iron Oxide Films with Ordered Mesoporosity. Adv. Mater. Interfaces 2018, 5, 1700960. [Google Scholar] [CrossRef]
- Arinchtein, A.; Schmack, R.; Kraffert, K.; Radnik, J.; Dietrich, P.; Sachse, R.; Kraehnert, R. Role of Water in Phase Transformations and Crystallization of Ferrihydrite and Hematite. ACS Appl. Mater. Interfaces 2020, 12, 38714–38722. [Google Scholar] [CrossRef] [PubMed]
- Grosso, D.; Cagnol, F.; Soler-Illia, G.J.d.A.; Crepaldi, E.L.; Amenitsch, H.; Brunet-Bruneau, A.; Bourgeois, A.; Sanchez, C. Fundamentals of Mesostructuring through Evaporation-Induced Self-Assembly. Adv. Funct. Mater. 2004, 14, 309–322. [Google Scholar] [CrossRef]
- Yan, M.; Henderson, M.J.; Gibaud, A. Grating induced micelle alignment of mesostructured silica films. Appl. Phys. Lett. 2007, 91, 023104. [Google Scholar] [CrossRef]
- Strasser, P.; Polte, J.; Kraehnert, R.; Görke, O.; Ortel, E.; Bernsmeier, D.; Eckhardt, B. Micelle-Templated Mesoporous Films of Magnesium Carbonate and Magnesium Oxide. Adv. Mater. 2012, 24, 3115–3119. [Google Scholar]
- Frisch, M.; Laun, J.; Marquardt, J.; Arinchtein, A.; Bauerfeind, K.; Bernsmeier, D.; Bernicke, M.; Bredow, T.; Kraehnert, R. Bridging experiment and theory: Enhancing the electrical conductivities of soft-templated niobium-doped mesoporous titania films. Phys. Chem. Chem. Phys. 2021, 23, 3219–3224. [Google Scholar] [CrossRef]
- Brezesinski, T.; Fattakhova Rohlfing, D.; Sallard, S.; Antonietti, M.; Smarsly, B.M. Highly Crystalline WO3 Thin Films with Ordered 3D Mesoporosity and Improved Electrochromic Performance. Small 2006, 2, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Bernicke, M.; Eckhardt, B.; Lippitz, A.; Ortel, E.; Bernsmeier, D.; Schmack, R.; Kraehnert, R. Synthesis and OER activity of NiO coatings with micelle–templated mesopore structure. ChemistrySelect 2016, 1, 482–489. [Google Scholar] [CrossRef]
- Bernsmeier, D.; Ortel, E.; Polte, J.; Eckhardt, B.; Nowag, S.; Haag, R.; Kraehnert, R. Versatile control over size and spacing of small mesopores in metal oxide films and catalytic coatings via templating with hyperbranched core–multishell polymers. J. Mater. Chem. A 2014, 2, 13075–13082. [Google Scholar] [CrossRef] [Green Version]
- Sakatani, Y.; Grosso, D.; Nicole, L.; Boissière, C.; Soler-Illia, G.J.D.A.A.; Sanchez, C. Optimised photocatalytic activity of grid-like mesoporous TiO2 films: Effect of crystallinity, pore size distribution, and pore accessibility. J. Mater. Chem. 2006, 16, 77–82. [Google Scholar]
- Brezesinski, T.; Groenewolt, M.; Pinna, N.; Amenitsch, H.; Antonietti, M.; Smarsly, B. Surfactant-Mediated Generation of Iso-Oriented Dense and Mesoporous Crystalline Metal-Oxide Layers. Adv. Mater. 2006, 18, 1827–1831. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, H.; Cao, T.; Qian, L.; Wang, Y.; Zhao, G. Efficient degradation of high concentration azo-dye wastewater by heterogeneous Fenton process with iron-based metal-organic framework. J. Mol. Catal. A Chem. 2015, 400, 81–89. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Wang, W.; Niu, Q.; Zeng, G.; Zhang, C.; Huang, D.; Shao, B.; Zhou, C.; Yang, Y.; Liu, Y.; Guo, H.; et al. 1D porous tubular g-C3N4 capture black phosphorus quantum dots as 1D/0D metal-free photocatalysts for oxytetracycline hydrochloride degradation and hexavalent chromium reduction. Appl. Catal. B Environ. 2020, 273, 119051. [Google Scholar] [CrossRef]
- Gupta, A.; Mittal, M.; Singh, M.K.; Suib, S.L.; Pandey, O.P. Low temperature synthesis of NbC/C nano-composites as visible light photoactive catalyst. Sci. Rep. 2018, 8, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Bourlier, Y.; Bouttemy, M.; Patard, O.; Gamarra, P.; Piotrowicz, S.; Vigneron, J.; Aubry, R.; Delage, S.; Etcheberry, A. Investigation of InAlN Layers Surface Reactivity after Thermal Annealings: A Complete XPS Study for HEMT. ECS J. Solid State Sci. Technol. 2018, 7, P329–P338. [Google Scholar] [CrossRef] [Green Version]
- Kwan, Y.C.G.; Ng, G.M.; Huan, C.H.A. Identification of functional groups and determination of carboxyl formation temperature in graphene oxide using the XPS O 1s spectrum. Thin Solid Films 2015, 590, 40–48. [Google Scholar] [CrossRef]
- Miller, D.J.; Biesinger, M.C.; McIntyre, N.S. Interactions of CO2 and CO at fractional atmosphere pressures with iron and iron oxide surfaces: One possible mechanism for surface contamination? Surf. Interface Anal. 2002, 33, 299–305. [Google Scholar] [CrossRef]
- Oschatz, M.; Krans, N.; Xie, J.; de Jong, K.P. Systematic variation of the sodium/sulfur promoter content on carbon-supported iron catalysts for the Fischer-Tropsch to olefins reaction. J. Energy Chem. 2016, 25, 985–993. [Google Scholar] [CrossRef]
- Janbroers, S.; Louwen, J.N.; Zandbergen, H.W.; Kooyman, P.J. Insights into the nature of iron-based Fischer–Tropsch catalysts from quasi in situ TEM-EELS and XRD. J. Catal. 2009, 268, 235–242. [Google Scholar] [CrossRef]
- Shroff, M.D.; Datye, A.K. The importance of passivation in the study of iron Fischer-Tropsch catalysts. Catal. Lett. 1996, 37, 101–106. [Google Scholar] [CrossRef]
- Brezesinski, K.; Haetge, J.; Wang, J.; Mascotto, S.; Reitz, C.; Rein, A.; Tolbert, S.H.; Perlich, J.; Dunn, B.; Brezesinski, T. Ordered Mesoporous α-Fe2O3 (Hematite) Thin-Film Electrodes for Application in High Rate Rechargeable Lithium Batteries. Small 2011, 7, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arinchtein, A.; Ye, M.-Y.; Geske, M.; Frisch, M.; Kraehnert, R. Influence of Phase Composition and Pretreatment on the Conversion of Iron Oxides into Iron Carbides in Syngas Atmospheres. Catalysts 2021, 11, 773. https://doi.org/10.3390/catal11070773
Arinchtein A, Ye M-Y, Geske M, Frisch M, Kraehnert R. Influence of Phase Composition and Pretreatment on the Conversion of Iron Oxides into Iron Carbides in Syngas Atmospheres. Catalysts. 2021; 11(7):773. https://doi.org/10.3390/catal11070773
Chicago/Turabian StyleArinchtein, Aleks, Meng-Yang Ye, Michael Geske, Marvin Frisch, and Ralph Kraehnert. 2021. "Influence of Phase Composition and Pretreatment on the Conversion of Iron Oxides into Iron Carbides in Syngas Atmospheres" Catalysts 11, no. 7: 773. https://doi.org/10.3390/catal11070773
APA StyleArinchtein, A., Ye, M.-Y., Geske, M., Frisch, M., & Kraehnert, R. (2021). Influence of Phase Composition and Pretreatment on the Conversion of Iron Oxides into Iron Carbides in Syngas Atmospheres. Catalysts, 11(7), 773. https://doi.org/10.3390/catal11070773





