Eco-Friendly Colloidal Aqueous Sol-Gel Process for TiO2 Synthesis: The Peptization Method to Obtain Crystalline and Photoactive Materials at Low Temperature
Abstract
:1. Introduction
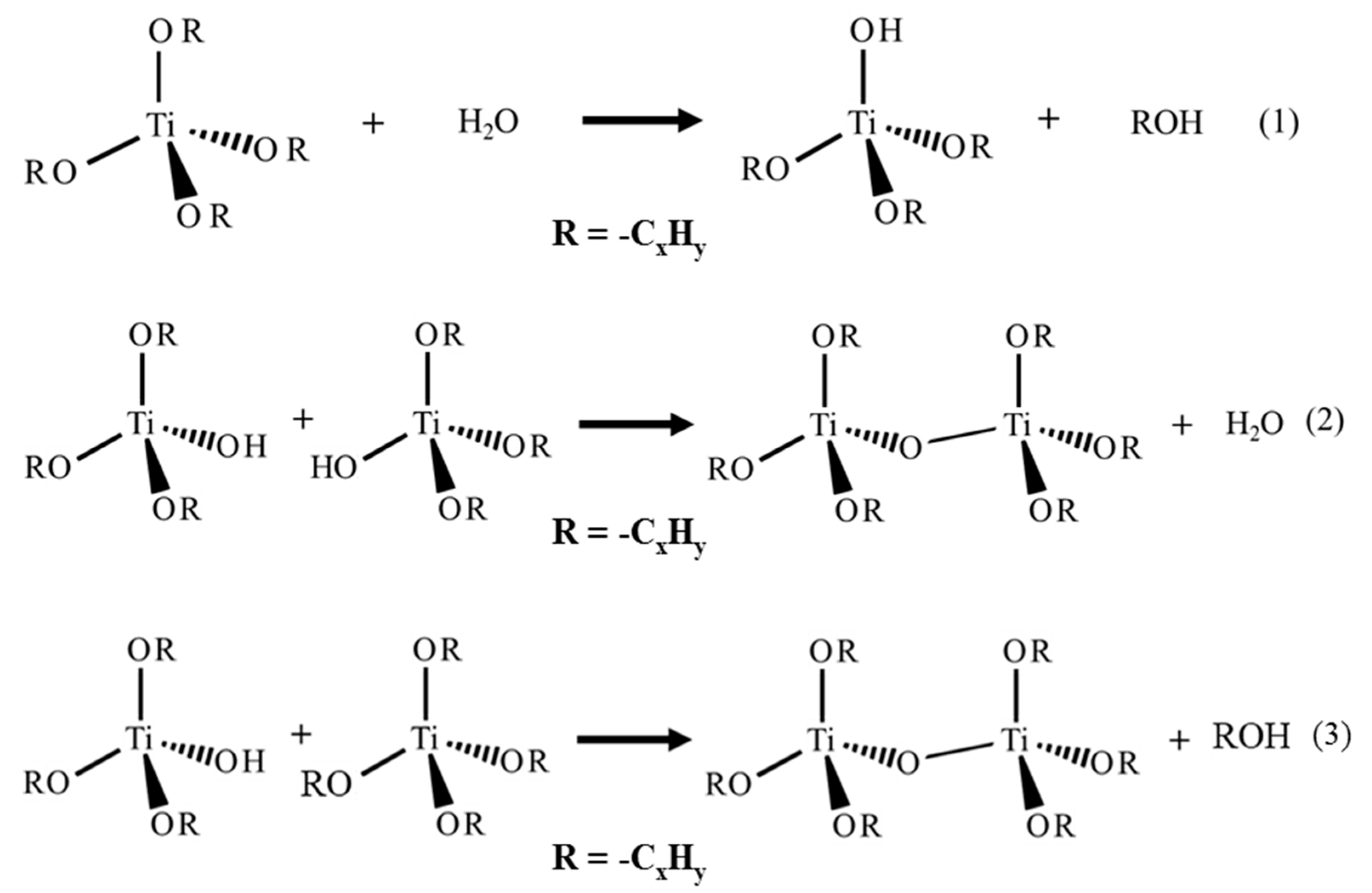
2. Synthesis of TiO2 with PA in Water
3. Crystallinity
3.1. As-Synthesized Aqueous TiO2
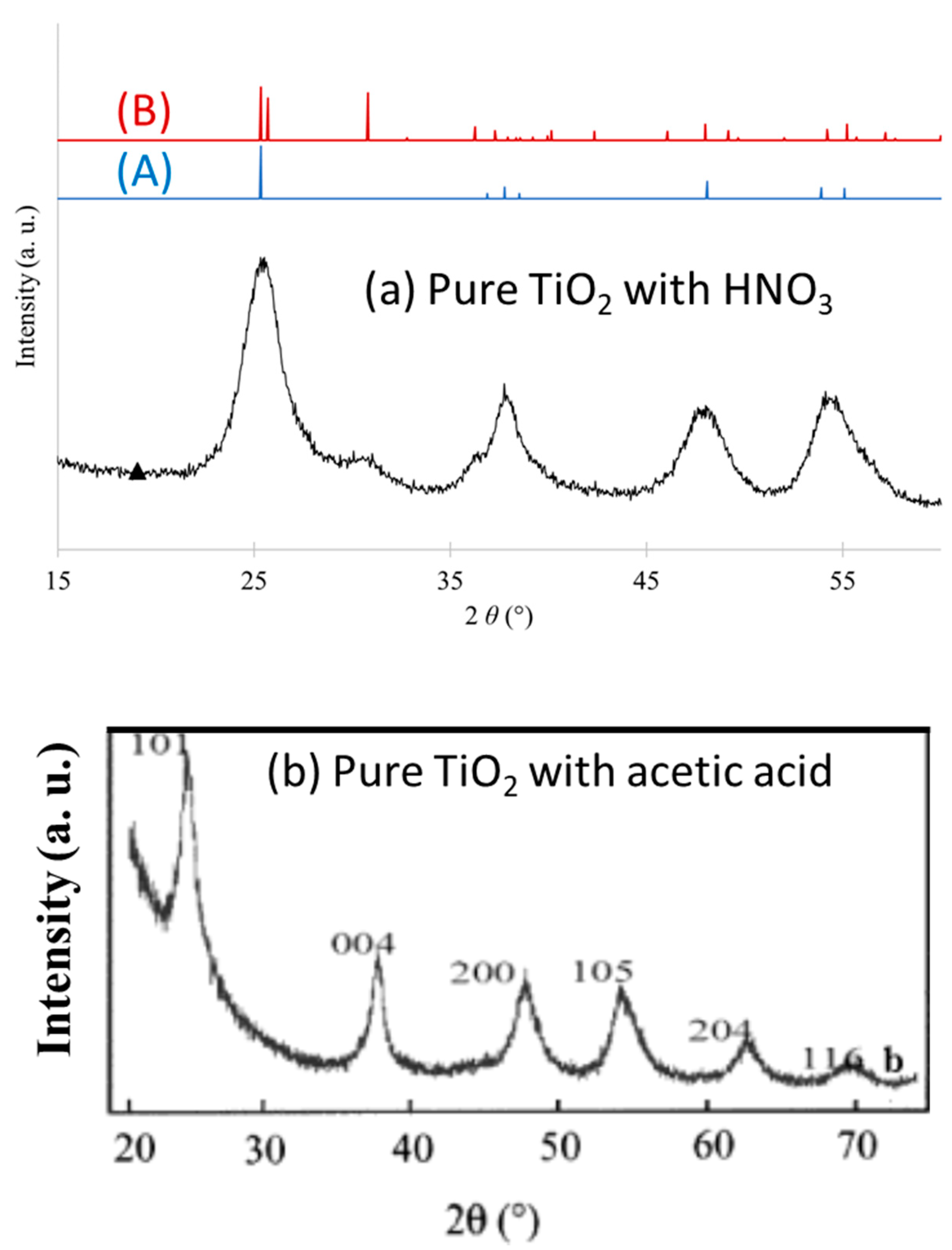
3.1.1. Acid Peptizing Agent
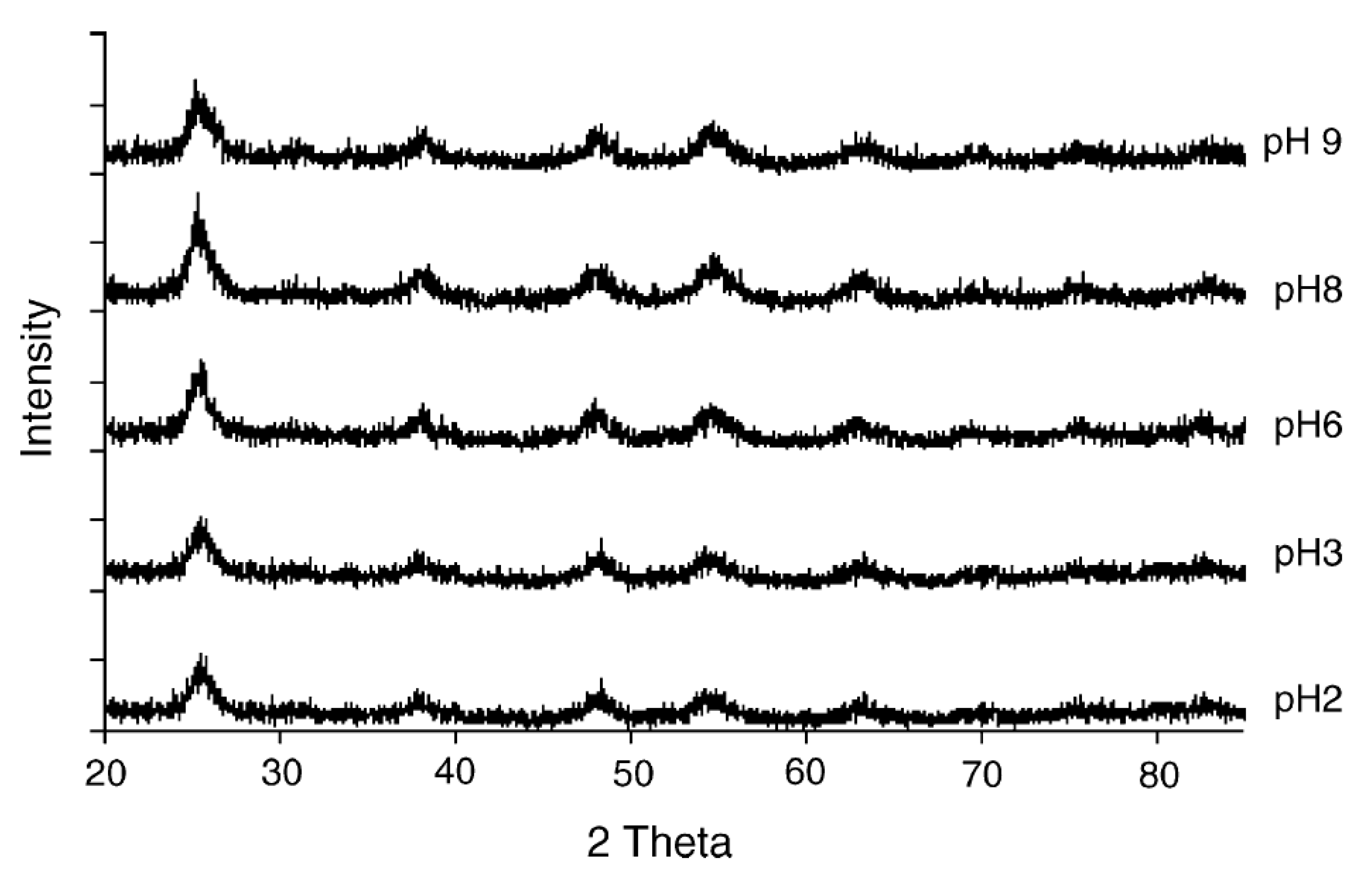
3.1.2. Basic Peptizing Agent
3.2. Aqueous TiO2 after a Calcination Treatment
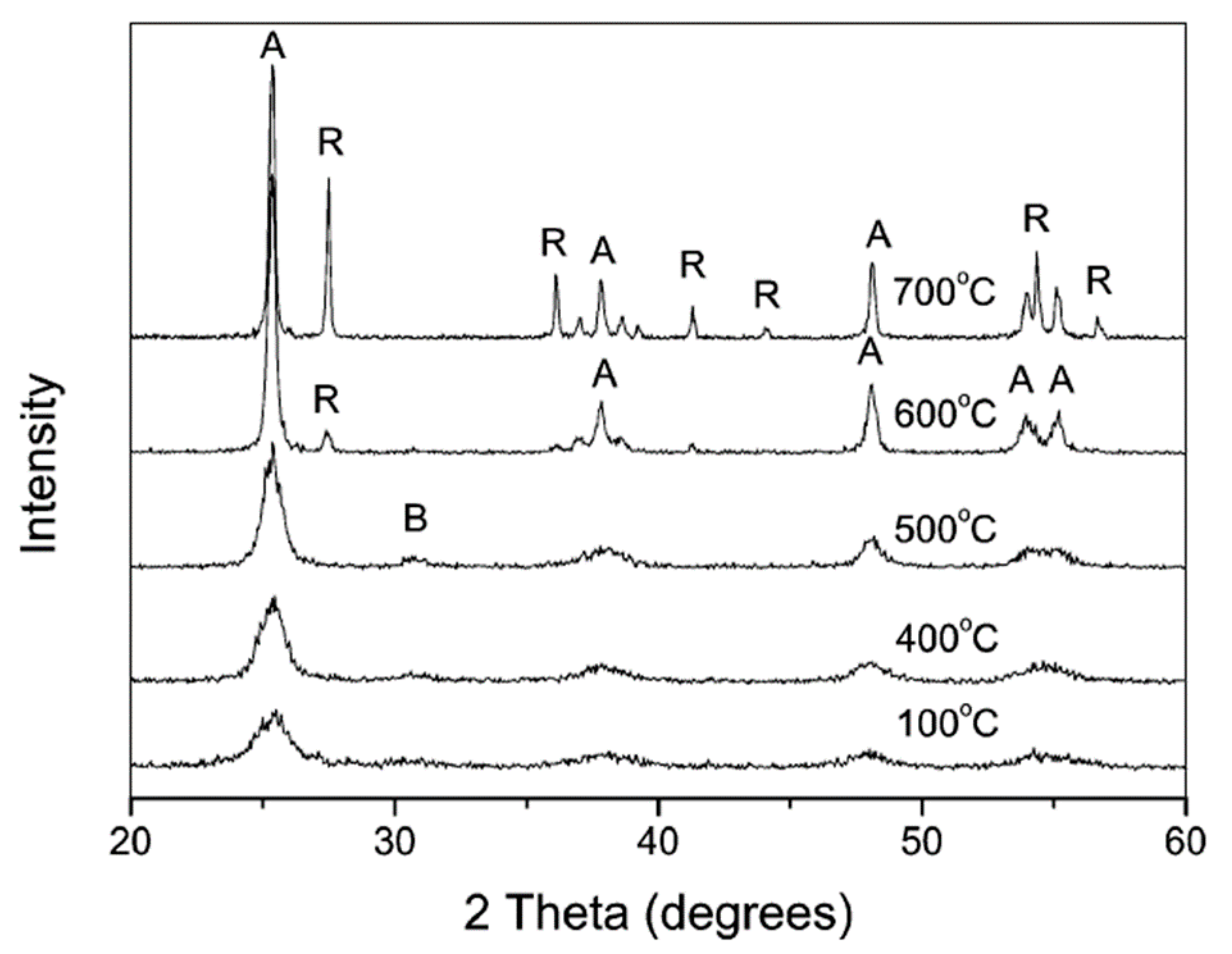
3.2.1. Calcination after Acidic Peptization
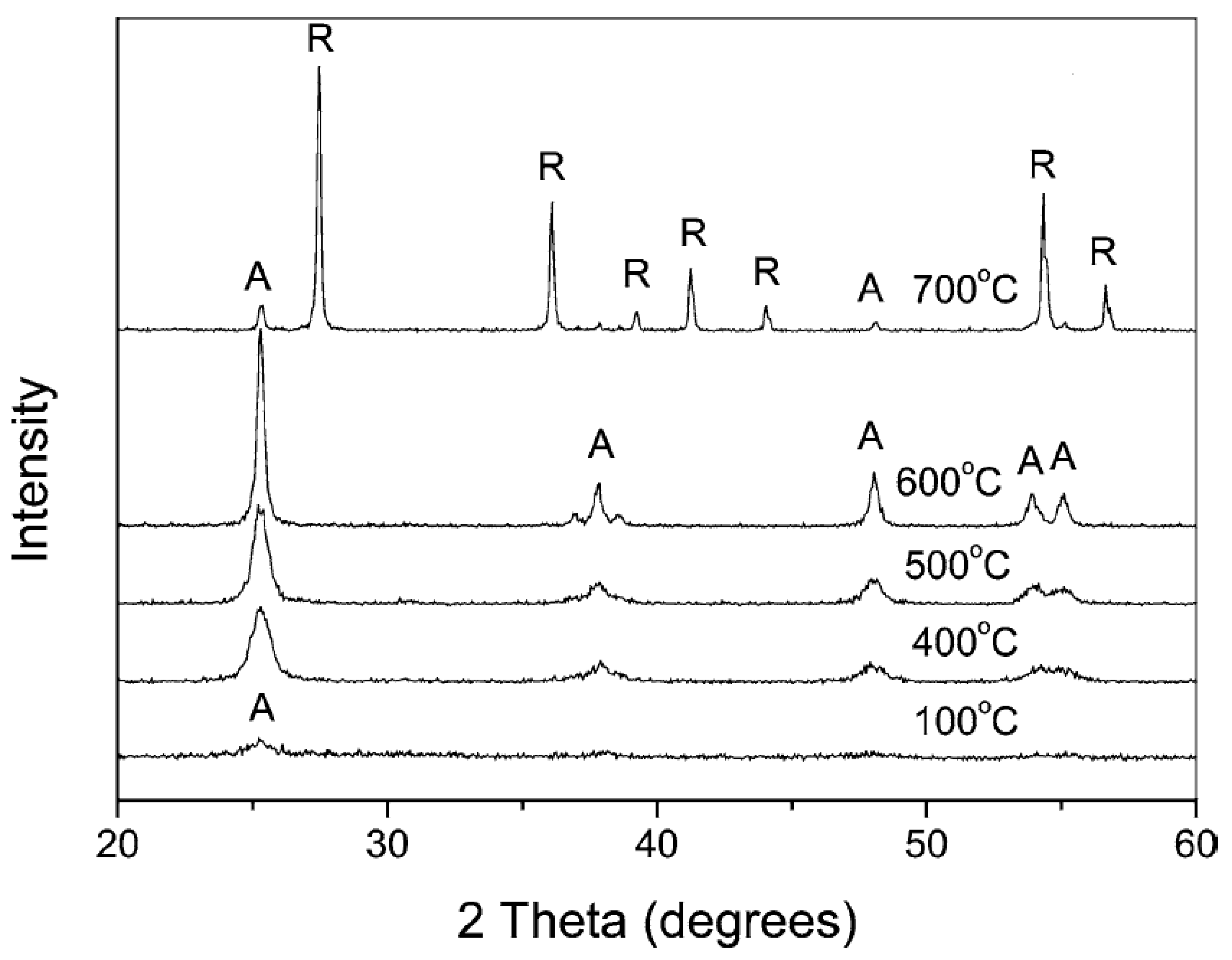
3.2.2. Basic Peptization Followed by Calcination
3.3. Aqueous TiO2 after Hydrothermal Treatment
4. Morphology
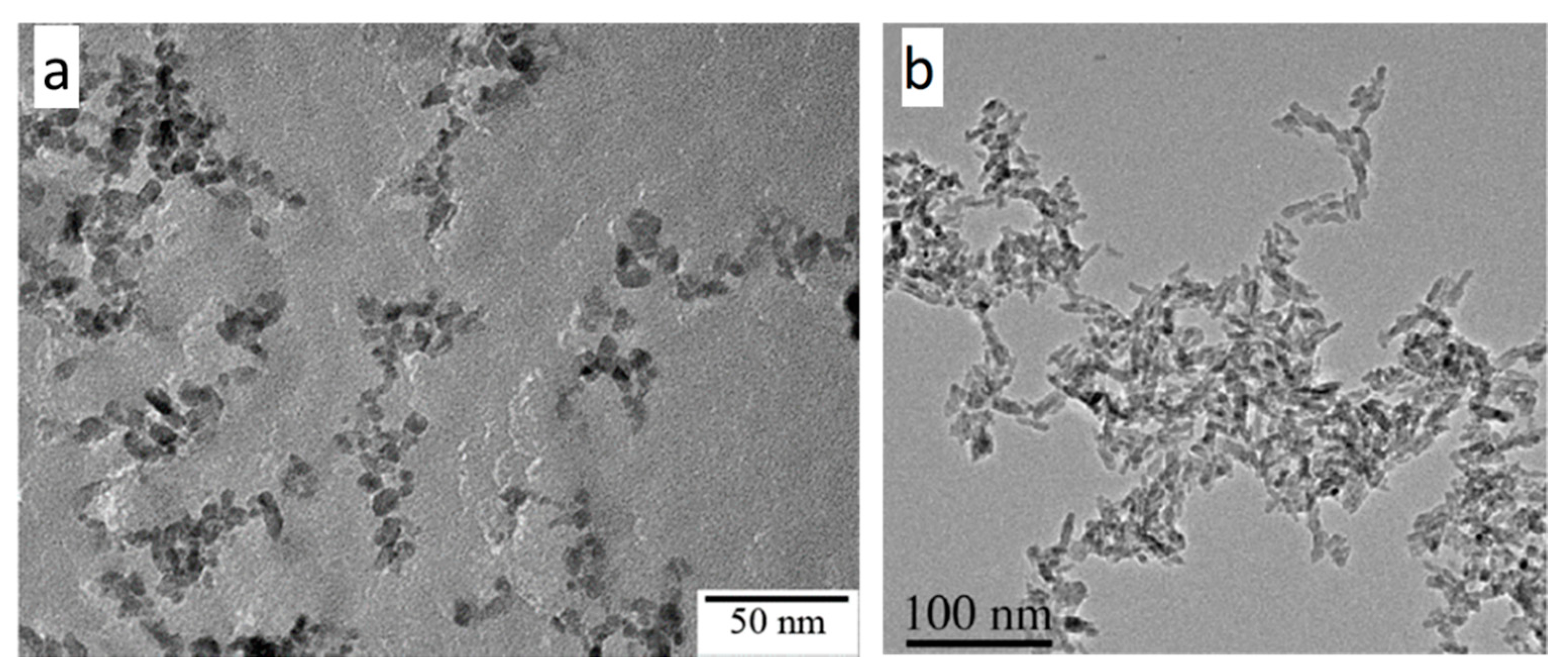
4.1. Morphology of As-Synthesized Aqueous TiO2
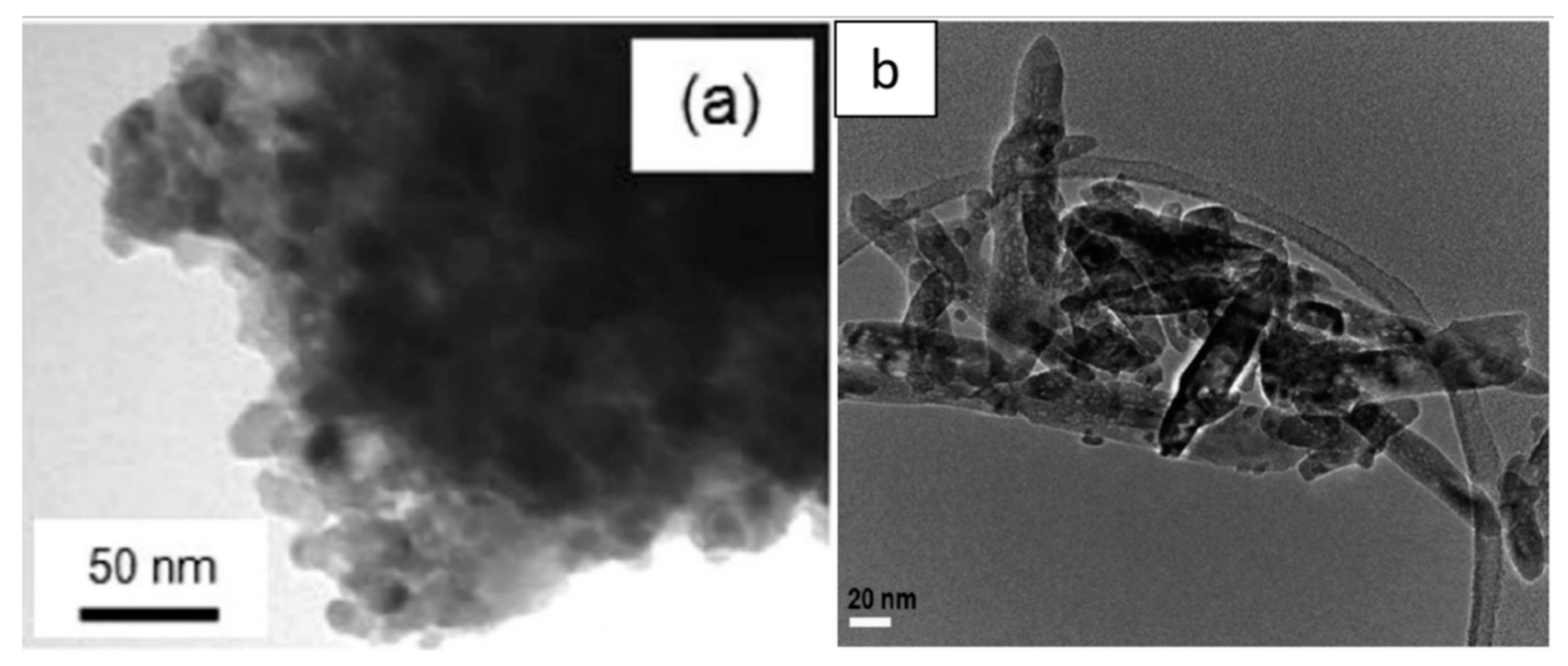
4.2. Morphology of Aqueous TiO2 after Calcination Treatment
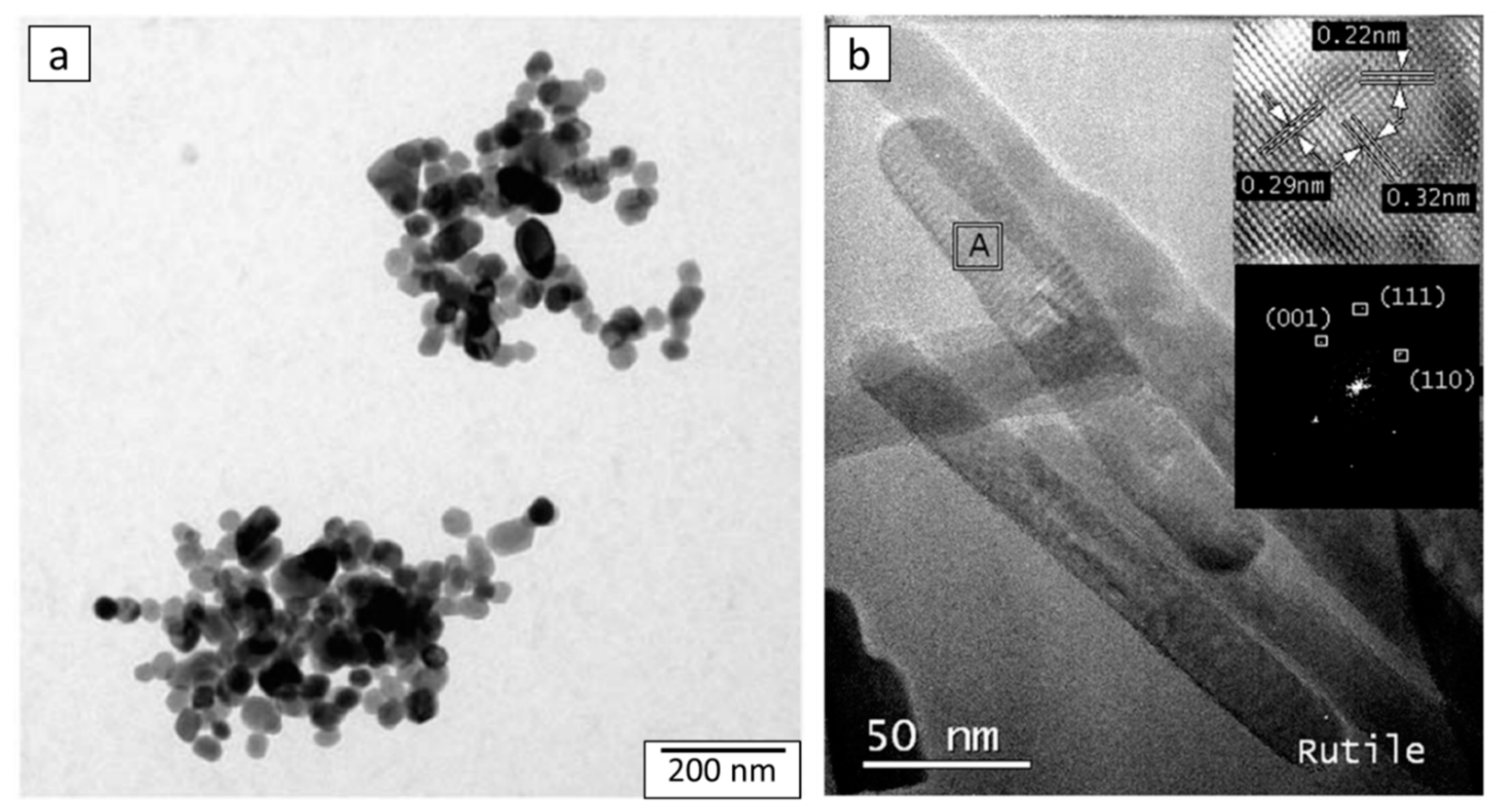
4.3. Morphology of Aqueous TiO2 after Hydrothermal Treatment
5. Doping and Additives
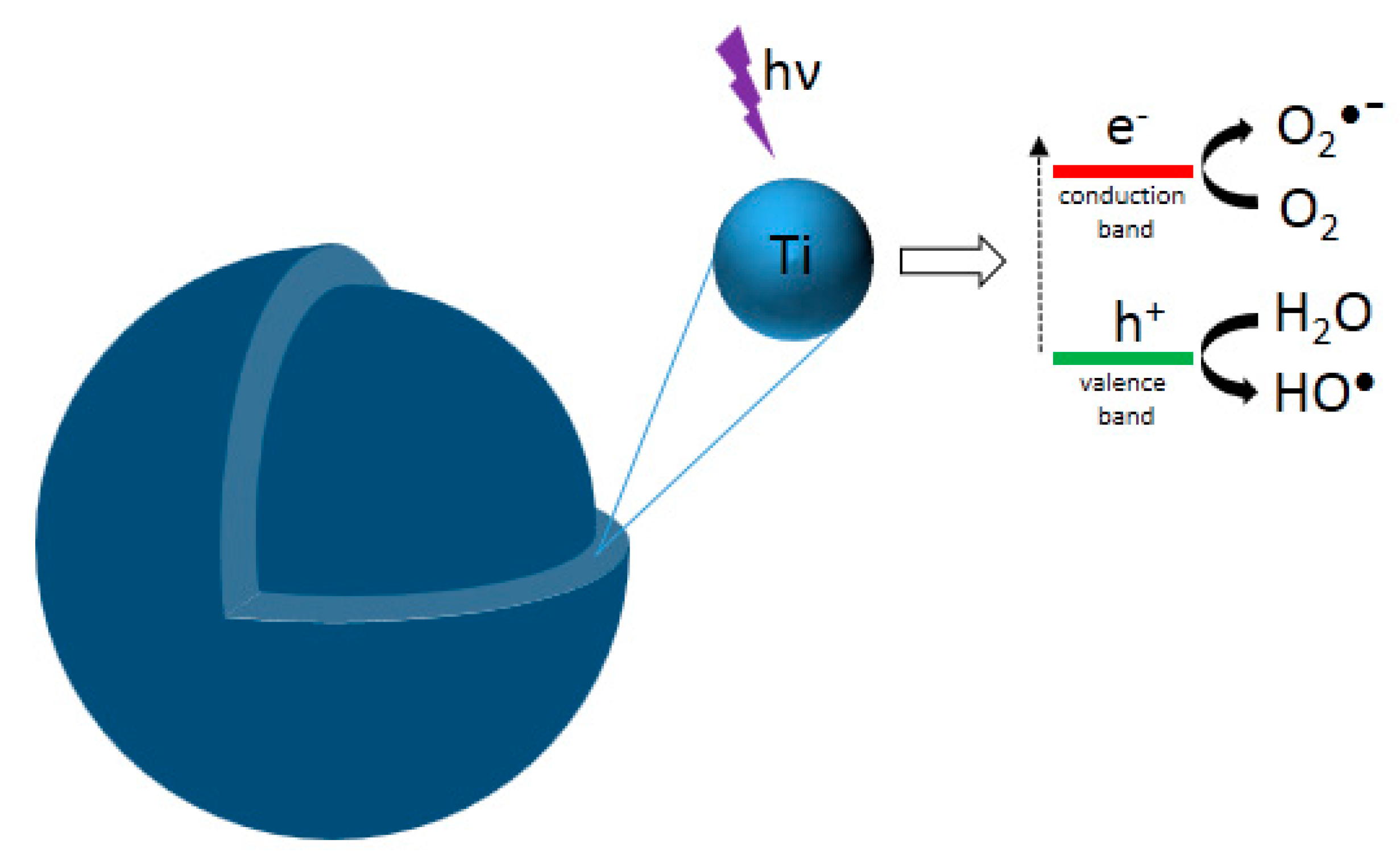
6. Photocatalytic Properties
6.1. Photoactivity of As-Synthesized Aqueous TiO2
6.2. Photoactivity of Aqueous TiO2 after a Calcination Treatment
6.3. Photoactivity of Aqueous TiO2 after Hydrothermal Treatment
7. Addition Features for Aqueous Sol–Gel TiO2
8. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Hermawan, A.; Hanindriyo, A.T.; Ramadhan, E.R.; Asakura, Y.; Hasegawa, T.; Hongo, K.; Inada, M.; Maezono, R.; Yin, S. Octahedral morphology of NiO with (111) facet synthesized from the transformation of NiOHCl for the NOx detection and degradation: Experiment and DFT calculation. Inorg. Chem. Front. 2020, 7, 3431–3442. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, S.; Wen, T.; Gu, P.; Li, L.; Zhao, G.; Niu, F.; Huang, Q.; Tang, Z.; Wang, X. A critical review on visible-light-response CeO2-based photocatalysts with enhanced photooxidation of organic pollutants. Catal. Today 2019, 335, 20–30. [Google Scholar] [CrossRef]
- Chiam, S.-L.; Pung, S.-Y.; Yeoh, F.-Y. Recent developments in MnO2-based photocatalysts for organic dye removal: A review. Environ. Sci. Pollut. Res. 2020, 27, 5759–5778. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.; Hamilton, J.W.; Byrne, J.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Malengreaux, C.M.; Douven, S.; Poelman, D.; Heinrichs, B.; Bartlett, J.R. An ambient temperature aqueous sol–gel processing of efficient nanocrystalline doped TiO2-based photocatalysts for the degradation of organic pollutants. J. Sol Gel Sci. Technol. 2014, 71, 557–570. [Google Scholar] [CrossRef]
- Espino-Estévez, M.; Fernández-Rodríguez, C.; González-Díaz, O.M.; Araña, J.; Espinós, J.; Ortega-Méndez, J.; Doña-Rodríguez, J.M. Effect of TiO2–Pd and TiO2–Ag on the photocatalytic oxidation of diclofenac, isoproturon and phenol. Chem. Eng. J. 2016, 298, 82–95. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Sannino, D.; Murcia, J.J.; Hidalgo, M.C.; Ciambelli, P.; Navío, J.A. Photocatalytic removal of patent blue V dye on Au-TiO2 and Pt-TiO2 catalysts. Appl. Catal. B Environ. 2016, 188, 134–146. [Google Scholar] [CrossRef]
- Di Paola, A.; Marci, G.; Palmisano, L.; Schiavello, M.; Uosaki, K.; Ikeda, A.S.; Ohtani, B. Preparation of Polycrystalline TiO2Photocatalysts Impregnated with Various Transition Metal Ions: Characterization and Photocatalytic Activity for the Degradation of 4-Nitrophenol. J. Phys. Chem. B 2002, 106, 637–645. [Google Scholar] [CrossRef] [Green Version]
- Rauf, M.; Meetani, M.; Hisaindee, S. An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 2011, 276, 13–27. [Google Scholar] [CrossRef]
- Bodson, C.J.; Heinrichs, B.; Tasseroul, L.; Bied, C.; Mahy, J.G.; Man, M.W.C.; Lambert, S.D. Efficient P- and Ag-doped titania for the photocatalytic degradation of waste water organic pollutants. J. Alloys Compd. 2016, 682, 144–153. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Giamello, E. Characterization of Paramagnetic Species in N-Doped TiO2 Powders by EPR Spectroscopy and DFT Calculations. J. Phys. Chem. B 2005, 109, 11414–11419. [Google Scholar] [CrossRef]
- Gilma, G.O.; Carlos, A.P.M.; Fernando, M.O.; Edgar, A.P.-M. Photocatalytic degradation of phenol on TiO2 and TiO2/Pt sensitized with metallophthalocyanines. Catal. Today 2005, 107–108, 589–594. [Google Scholar] [CrossRef]
- Mahy, J.G.; Paez, C.A.; Carcel, C.; Bied, C.; Tatton, A.S.; Damblon, C.; Heinrichs, B.; Man, M.W.C.; Lambert, S.D. Porphyrin-based hybrid silica-titania as a visible-light photocatalyst. J. Photochem. Photobiol. A Chem. 2019, 373, 66–76. [Google Scholar] [CrossRef]
- Xie, H.; Gao, G.; Tian, Z.; Bing, N.; Wang, L. Synthesis of TiO2 nanoparticles by propane/air turbulent flame CVD process. Particuology 2009, 7, 204–210. [Google Scholar] [CrossRef]
- Djenadic, R.; Winterer, M. Chemical Vapor Synthesis of Nanocrystalline Oxides. In 2D Nanoelectronics; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2012; pp. 49–76. [Google Scholar]
- Inturi, S.N.R.; Boningari, T.; Suidan, M.; Smirniotis, P.G. Flame Aerosol Synthesized Cr Incorporated TiO2for Visible Light Photodegradation of Gas Phase Acetonitrile. J. Phys. Chem. C 2013, 118, 231–242. [Google Scholar] [CrossRef]
- Dar, M.I.; Chandiran, A.K.; Graetzel, M.; Nazeeruddin, M.K.; Shivashankar, S.A. Controlled synthesis of TiO2 nanoparticles and nanospheres using a microwave assisted approach for their application in dye-sensitized solar cells. J. Mater. Chem. A 2013, 2, 1662–1667. [Google Scholar] [CrossRef]
- Zhang, D.; Qi, L.; Ma, J.; Cheng, H. Formation of crystalline nanosized titania in reverse micelles at room temperature. J. Mater. Chem. 2002, 12, 3677–3680. [Google Scholar] [CrossRef]
- Nian, J.-N.; Teng, H. Hydrothermal Synthesis of Single-Crystalline Anatase TiO2Nanorods with Nanotubes as the Precursor. J. Phys. Chem. B 2006, 110, 4193–4198. [Google Scholar] [CrossRef]
- Simon, P.; Pignon, B.; Miao, B.; Coste-Leconte, S.; Leconte, Y.; Marguet, S.; Jegou, P.; Bouchet-Fabre, B.; Reynaud, C.; Herlin-Boime, N. N-Doped Titanium Monoxide Nanoparticles with TiO Rock-Salt Structure, Low Energy Band Gap, and Visible Light Activity. Chem. Mater. 2010, 22, 3704–3711. [Google Scholar] [CrossRef]
- Gratzel, M. Sol-Gel Processed TiO2 Films for Photovoltaic Applications. J. Sol Gel Sci. Technol. 2001, 22, 7–13. [Google Scholar] [CrossRef]
- Carp, O. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Huang, T.; Huang, W.; Zhou, C.; Situ, Y.; Huang, H. Superhydrophilicity of TiO2/SiO2 thin films: Synergistic effect of SiO2 and phase-separation-induced porous structure. Surf. Coat. Technol. 2012, 213, 126–132. [Google Scholar] [CrossRef]
- Guan, K. Relationship between photocatalytic activity, hydrophilicity and self-cleaning effect of TiO2/SiO2 films. Surf. Coat. Technol. 2005, 191, 155–160. [Google Scholar] [CrossRef]
- Antonelli, D.M.; Ying, J. Synthesis of Hexagonally Packed Mesoporous TiO2 by a Modified Sol–Gel Method. Angew. Chem. Int. Ed. 1995, 34, 2014–2017. [Google Scholar] [CrossRef]
- Braconnier, B.; Páez, C.A.; Lambert, S.; Alié, C.; Henrist, C.; Poelman, D.; Pirard, J.-P.; Cloots, R.; Heinrichs, B. Ag- and SiO2-doped porous TiO2 with enhanced thermal stability. Microporous Mesoporous Mater. 2009, 122, 247–254. [Google Scholar] [CrossRef]
- Anderson, C.; Bard, A.J. An Improved Photocatalyst of TiO2/SiO2 Prepared by a Sol-Gel Synthesis. J. Phys. Chem. 1995, 99, 9882–9885. [Google Scholar] [CrossRef]
- Brinker, G.W.; Jeffrey, C.S. Sol-gel science. In The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Schubert, U. Chemical modification of titanium alkoxides for sol–gel processing. J. Mater. Chem. 2005, 15, 3701–3715. [Google Scholar] [CrossRef]
- Jan, W.G. Encyclopedic Dictionary of Polymers. Encycl. Dict. Polym. 2011. [Google Scholar] [CrossRef]
- Mahmoud, H.A.; Narasimharao, K.; Ali, T.T.; Khalil, K.M.S. Acidic Peptizing Agent Effect on Anatase-Rutile Ratio and Photocatalytic Performance of TiO2 Nanoparticles. Nanoscale Res. Lett. 2018, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S.; Nishihara, T.; Hattori, M.; Suzuki, Y. Preparation and properties of titania pillared clay. Mater. Chem. Phys. 1987, 17, 87–101. [Google Scholar] [CrossRef]
- Anderson, M.A.; Gieselmann, M.J.; Xu, Q. Titania and alumina ceramic membranes. J. Membr. Sci. 1988, 39, 243–258. [Google Scholar] [CrossRef]
- Doeuff, S.; Henry, M.; Sanchez, C.; Livage, J. Hydrolysis of titanium alkoxides: Modification of the molecular precursor by acetic acid. J. Non Cryst. Solids 1987, 89, 206–216. [Google Scholar] [CrossRef]
- Mahshid, S.; Askari, M.; Ghamsari, M.S. Synthesis of TiO2 nanoparticles by hydrolysis and peptization of titanium isopropoxide solution. J. Mater. Process. Technol. 2007, 189, 296–300. [Google Scholar] [CrossRef]
- Bischoff, B.L.; Anderson, M.A. Peptization Process in the Sol-Gel Preparation of Porous Anatase (TiO2). Chem. Mater. 1995, 7, 1772–1778. [Google Scholar] [CrossRef]
- Matijevic, E. Monodispersed metal (hydrous) oxides—A fascinating field of colloid science. Acc. Chem. Res. 1981, 14, 22–29. [Google Scholar] [CrossRef]
- Mahy, J.G.; Deschamps, F.; Collard, V.; Jérôme, C.; Bartlett, J.; Lambert, S.D.; Heinrichs, B. Acid acting as redispersing agent to form stable colloids from photoactive crystalline aqueous sol–gel TiO2 powder. J. Sol Gel Sci. Technol. 2018, 87, 568–583. [Google Scholar] [CrossRef]
- Douven, S.; Mahy, J.G.; Wolfs, C.; Reyserhove, C.; Poelman, D.; Devred, F.; Gaigneaux, E.M.; Lambert, S.D. Efficient N, Fe Co-Doped TiO2 Active under Cost-Effective Visible LED Light: From Powders to Films. Catalysts 2020, 10, 547. [Google Scholar] [CrossRef]
- Cesconeto, F.R.; Borlaf, M.; Nieto, M.I.; de Oliveira, A.P.N.; Moreno, R. Synthesis of CaTiO3 and CaTiO3/TiO2 nanoparticulate compounds through Ca2+/TiO2 colloidal sols: Structural and photocatalytic characterization. Ceram. Int. 2018, 44, 301–309. [Google Scholar] [CrossRef]
- Cano-Franco, J.C.; Álvarez-Láinez, M. Effect of CeO2 content in morphology and optoelectronic properties of TiO2-CeO2 nanoparticles in visible light organic degradation. Mater. Sci. Semicond. Process. 2019, 90, 190–197. [Google Scholar] [CrossRef]
- Colomer, M.T.; Guzmán, J.; Moreno, R. Determination of Peptization Time of Particulate Sols Using Optical Techniques: Titania As a Case Study. Chem. Mater. 2008, 20, 4161–4165. [Google Scholar] [CrossRef]
- Colomer, M.T.; Guzmã¡n, J.; Moreno, R. Peptization of Nanoparticulate Titania Sols Prepared Under Different Water–Alkoxide Molar Ratios. J. Am. Ceram. Soc. 2009, 93, 59–64. [Google Scholar] [CrossRef]
- Ghamsari, M.S.; Gaeeni, M.R.; Han, W.; Park, H.-H. Highly stable colloidal TiO2 nanocrystals with strong violet-blue emission. J. Lumin. 2016, 178, 89–93. [Google Scholar] [CrossRef]
- Haq, S.; Rehman, W.; Waseem, M. Adsorption Efficiency of Anatase TiO2 Nanoparticles Against Cadmium Ions. J. Inorg. Organomet. Polym. Mater. 2018, 29, 651–658. [Google Scholar] [CrossRef]
- Haque, F.Z.; Nandanwar, R.; Singh, P. Evaluating photodegradation properties of anatase and rutile TiO2 nanoparticles for organic compounds. Optik 2017, 128, 191–200. [Google Scholar] [CrossRef]
- Hore, S.; Palomares, E.; Smit, H.; Bakker, N.J.; Comte, P.; Liska, P.; Thampi, K.R.; Kroon, J.M.; Hinsch, A.; Durrant, J.R. Acid versus base peptization of mesoporous nanocrystalline TiO2 films: Functional studies in dye sensitized solar cells. J. Mater. Chem. 2004, 15, 412–418. [Google Scholar] [CrossRef]
- Huang, B.-S.; Tseng, H.-H.; Su, E.-C.; Chiu, I.-C.; Wey, M.-Y. Characterization and photoactivity of Pt/N-doped TiO2 synthesized through a sol–gel process at room temperature. J. Nanoparticle Res. 2015, 17, 282. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Sreekantan, S. Effect of pH on TiO2 Nanoparticles via Sol-Gel Method. Adv. Mater. Res. 2010, 173, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Khalil, K.M.; El-Khatib, R.M.; Ali, T.T.; Mahmoud, H.A.; Elsamahy, A.A. Titania nanoparticles by acidic peptization of xerogel formed by hydrolysis of titanium(IV) isopropoxide under atmospheric humidity conditions. Powder Technol. 2013, 245, 156–162. [Google Scholar] [CrossRef]
- Kaplan, R.; Erjavec, B.; Dražić, G.; Grdadolnik, J.; Pintar, A. Simple synthesis of anatase/rutile/brookite TiO2 nanocomposite with superior mineralization potential for photocatalytic degradation of water pollutants. Appl. Catal. B Environ. 2016, 181, 465–474. [Google Scholar] [CrossRef]
- Kashyout, A.; Soliman, M.; Fathy, M. Effect of preparation parameters on the properties of TiO2 nanoparticles for dye sensitized solar cells. Renew. Energy 2010, 35, 2914–2920. [Google Scholar] [CrossRef]
- Léonard, G.L.-M.; Remy, S.; Heinrichs, B. Doping TiO2 films with carbon nanotubes to simultaneously optimise antistatic, photocatalytic and superhydrophilic properties. J. Sol Gel Sci. Technol. 2016, 79, 413–425. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; Toxqui-Teran, A.; Vega-Becerra, O.; Miki-Yoshida, M.; Rojas-Villalobos, M.; García-Guaderrama, M.; Aguilar-Martínez, J. Low-temperature synthesis and characterization of anatase TiO2 nanoparticles by an acid assisted sol–gel method. J. Alloys Compd. 2015, 647, 627–636. [Google Scholar] [CrossRef]
- Lim, C.S. Effect of pH on the Microstructural Morphology and Phase Transformation of TiO2 Nanopowders Prepared by Sol-Gel Method. Asian J. Chem. 2014, 26, 1843–1847. [Google Scholar] [CrossRef]
- Mahata, S.; Mahato, S.S.; Nandi, M.M.; Mondal, B. Synthesis of TiO[sub 2] nanoparticles by hydrolysis and peptization of titanium isopropoxide solution. AIP Conf. Proc. 2011, 1461, 225–228. [Google Scholar] [CrossRef]
- Mahshid, S.; Askari, M.; Ghamsari, M.S.; Afshar, N.; Lahuti, S. Mixed-phase TiO2 nanoparticles preparation using sol–gel method. J. Alloys Compd. 2009, 478, 586–589. [Google Scholar] [CrossRef]
- Mahy, J.G.; Lambert, S.D.; Léonard, G.L.-M.; Zubiaur, A.; Olu, P.-Y.; Mahmoud, A.; Boschini, F.; Heinrichs, B. Towards a large scale aqueous sol-gel synthesis of doped TiO2: Study of various metallic dopings for the photocatalytic degradation of p-nitrophenol. J. Photochem. Photobiol. A Chem. 2016, 329, 189–202. [Google Scholar] [CrossRef]
- Mahy, J.G.; Léonard, G.L.-M.; Pirard, S.; Wicky, D.; Daniel, A.; Archambeau, C.; Liquet, D.; Heinrichs, B. Aqueous sol–gel synthesis and film deposition methods for the large-scale manufacture of coated steel with self-cleaning properties. J. Sol Gel Sci. Technol. 2017, 81, 27–35. [Google Scholar] [CrossRef]
- Mahy, J.G.; Cerfontaine, V.; Poelman, D.; Devred, F.; Gaigneaux, E.M.; Heinrichs, B.; Lambert, S.D. Highly Efficient Low-Temperature N-Doped TiO2 Catalysts for Visible Light Photocatalytic Applications. Materials 2018, 11, 584. [Google Scholar] [CrossRef] [Green Version]
- Mahy, J.G.; Tilkin, R.G.; Douven, S.; Lambert, S.D. TiO2 nanocrystallites photocatalysts modified with metallic species: Comparison between Cu and Pt doping. Surf. Interfaces 2019, 17, 100366. [Google Scholar] [CrossRef]
- Mahy, J.G.; Lambert, S.D.; Tilkin, R.G.; Wolfs, C.; Poelman, D.; Devred, F.; Gaigneaux, E.M.; Douven, S. Ambient temperature ZrO2-doped TiO2 crystalline photocatalysts: Highly efficient powders and films for water depollution. Mater. Today Energy 2019, 13, 312–322. [Google Scholar] [CrossRef]
- Malengreaux, C.M.; Pirard, S.L.; Léonard, G.; Mahy, J.G.; Herlitschke, M.; Klobes, B.; Hermann, R.; Heinrichs, B.; Bartlett, J.R. Study of the photocatalytic activity of Fe3+, Cr3+, La3+ and Eu3+ single-doped and co-doped TiO2 catalysts produced by aqueous sol-gel processing. J. Alloys Compd. 2017, 691, 726–738. [Google Scholar] [CrossRef] [Green Version]
- Maver, K.; Štangar, U.L.; Černigoj, U.; Gross, S.; Korošec, R.C. Low-temperature synthesis and characterization of TiO2 and TiO2–ZrO2 photocatalytically active thin films. Photochem. Photobiol. Sci. 2009, 8, 657–662. [Google Scholar] [CrossRef]
- Mohammadi, M.; Fray, D.; Mohammadi, A. Sol–gel nanostructured titanium dioxide: Controlling the crystal structure, crystallite size, phase transformation, packing and ordering. Microporous Mesoporous Mater. 2008, 112, 392–402. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Cordero-Cabrera, M.C.; Ghorbani, M.; Fray, D.J. Synthesis of high surface area nanocrystalline anatase-TiO2 powders derived from particulate sol-gel route by tailoring processing parameters. J. Sol Gel Sci. Technol. 2006, 40, 15–23. [Google Scholar] [CrossRef]
- Mutuma, B.K.; Shao, G.; Kim, W.D.; Kim, H.T. Sol–gel synthesis of mesoporous anatase–brookite and anatase–brookite–rutile TiO2 nanoparticles and their photocatalytic properties. J. Colloid Interface Sci. 2015, 442, 1–7. [Google Scholar] [CrossRef]
- Okunaka, S.; Tokudome, H.; Hitomi, Y.; Abe, R. Facile preparation of stable aqueous titania sols for fabrication of highly active TiO 2 photocatalyst films. J. Mater. Chem. A 2014, 3, 1688–1695. [Google Scholar] [CrossRef]
- Papiya, F.; Pattanayak, P.; Kumar, V.; Das, S.; Kundu, P.P. Sulfonated graphene oxide and titanium dioxide coated with nanostructured polyaniline nanocomposites as an efficient cathode catalyst in microbial fuel cells. Mater. Sci. Eng. C 2020, 108, 110498. [Google Scholar] [CrossRef]
- Periyat, P.; Saeed, P.; Ullattil, S. Anatase titania nanorods by pseudo-inorganic templating. Mater. Sci. Semicond. Process. 2015, 31, 658–665. [Google Scholar] [CrossRef]
- Qi, K.; Xin, J.H. Room-Temperature Synthesis of Single-Phase Anatase TiO2 by Aging and its Self-Cleaning Properties. ACS Appl. Mater. Interfaces 2010, 2, 3479–3485. [Google Scholar] [CrossRef]
- Qiu, X.; Zhao, Y.; Burda, C. Synthesis and Characterization of Nitrogen-Doped Group IVB Visible-Light-Photoactive Metal Oxide Nanoparticles. Adv. Mater. 2007, 19, 3995–3999. [Google Scholar] [CrossRef]
- Quintero, Y.; Mosquera, E.; Diosa, J.; García, A. Ultrasonic-assisted sol–gel synthesis of TiO2 nanostructures: Influence of synthesis parameters on morphology, crystallinity, and photocatalytic performance. J. Sol Gel Sci. Technol. 2020, 94, 477–485. [Google Scholar] [CrossRef]
- Ropero-Vega, J.L.; Candal, R.J.; Pedraza-Avella, J.A.; Niño-Gómez, M.E.; Bilmes, S.A. Enhanced visible light photoelectrochemical performance of β-Bi2O3-TiO2/ITO thin films prepared by aqueous sol-gel. J. Solid State Electrochem. 2019, 23, 1757–1765. [Google Scholar] [CrossRef]
- Ryu, D.H.; Kim, S.C.; Koo, S.M.; Kim, D.P. Deposition of Titania Nanoparticles on Spherical Silica. J. Sol Gel Sci. Technol. 2003, 26, 489–493. [Google Scholar] [CrossRef]
- Salahuddin, N.; Abdelwahab, M.; Gaber, M.; Elneanaey, S. Synthesis and Design of Norfloxacin drug delivery system based on PLA/TiO2 nanocomposites: Antibacterial and antitumor activities. Mater. Sci. Eng. C 2020, 108, 110337. [Google Scholar] [CrossRef]
- Ghamsari, M.S.; Radiman, S.; Hamid, M.A.A.; Mahshid, S.; Rahmani, S. Room temperature synthesis of highly crystalline TiO2 nanoparticles. Mater. Lett. 2013, 92, 287–290. [Google Scholar] [CrossRef]
- Shinozaki, K.; Zack, J.W.; Richards, R.M.; Pivovar, B.S.; Kocha, S.S. Oxygen Reduction Reaction Measurements on Platinum Electrocatalysts Utilizing Rotating Disk Electrode Technique. J. Electrochem. Soc. 2015, 162, F1144–F1158. [Google Scholar] [CrossRef]
- Shin, H.; Jung, H.S.; Hong, K.S.; Lee, J.-K. Crystallization Process of TiO2Nanoparticles in an Acidic Solution. Chem. Lett. 2004, 33, 1382–1383. [Google Scholar] [CrossRef]
- Sugimoto, T.; Zhou, X.; Muramatsu, A. Synthesis of uniform anatase TiO2 nanoparticles by gel–sol method 4. Shape control. J. Colloid Interface Sci. 2003, 259, 53–61. [Google Scholar] [CrossRef]
- Sung-Suh, H.M.; Choi, J.R.; Hah, H.J.; Koo, S.M.; Bae, Y.C. Comparison of Ag deposition effects on the photocatalytic activity of nanoparticulate TiO2 under visible and UV light irradiation. J. Photochem. Photobiol. A Chem. 2004, 163, 37–44. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Pullar, R.; Binions, R.; Jorge, A.B.; McMillan, P.F.; Saeli, M.; Seabra, M.P.; Labrincha, J.A. Influence of sol counter-ions on the visible light induced photocatalytic behaviour of TiO2 nanoparticles. Catal. Sci. Technol. 2014, 4, 2134–2146. [Google Scholar] [CrossRef]
- Uchiyama, H.; Bando, T.; Kozuka, H. Effect of the amount of H2O and HNO3 in Ti(OC3H7)4 solutions on the crystallization of sol-gel-derived TiO2 films. Thin Solid Film 2019, 669, 157–161. [Google Scholar] [CrossRef]
- Vinogradov, A.V.; Vinogradov, V.V. Effect of Acidic Peptization on Formation of Highly Photoactive TiO2 Films Prepared without Heat Treatment. J. Am. Ceram. Soc. 2014, 97, 290–294. [Google Scholar] [CrossRef]
- Xu, Q.; Anderson, M.A. Synthesis of porosity controlled ceramic membranes. J. Mater. Res. 1991, 6, 1073–1081. [Google Scholar] [CrossRef]
- Yamazaki, S.; Fujinaga, N.; Araki, K. Effect of sulfate ions for sol–gel synthesis of Titania photocatalyst. Appl. Catal. A Gen. 2001, 210, 97–102. [Google Scholar] [CrossRef]
- Yang, J.; Mei, S.; Ferreira, J.M.; Norby, P.; Quaresmâ, S. Fabrication of rutile rod-like particle by hydrothermal method: An insight into HNO3 peptization. J. Colloid Interface Sci. 2005, 283, 102–106. [Google Scholar] [CrossRef]
- Yu, J.; Leung, M.K.-P.; Ho, W.; Cheng, B.; Zhao, X. Effects of acidic and basic hydrolysis catalysts on the photocatalytic activity and microstructures of bimodal mesoporous Titania. J. Catal. 2003, 220, 69–78. [Google Scholar] [CrossRef]
- Yun, Y.J.; Chung, J.S.; Kim, S.; Hahn, S.H.; Kim, E.J. Low-temperature coating of sol–gel anatase thin films. Mater. Lett. 2004, 58, 3703–3706. [Google Scholar] [CrossRef]
- Borlaf, M.; Poveda, J.M.; Moreno, R.; Colomer, M.T. Synthesis and characterization of TiO2/Rh3+ nanoparticulate sols, xerogels and cryogels for photocatalytic applications. J. Sol Gel Sci. Technol. 2012, 63, 408–415. [Google Scholar] [CrossRef]
- Bugakova, D.; Slabov, V.; Sergeeva, E.; Zhukov, M.; Vinogradov, A. Comprehensive characterization of TiO2 inks and their application for inkjet printing of microstructures. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124146. [Google Scholar] [CrossRef]
- Chen, X.; Lou, Y.; Samia, A.C.S.; Burda, C.; Gole, J.L. Formation of Oxynitride as the Photocatalytic Enhancing Site in Nitrogen-Doped Titania Nanocatalysts: Comparison to a Commercial Nanopowder. Adv. Funct. Mater. 2005, 15, 41–49. [Google Scholar] [CrossRef]
- Vorkapic, D.; Matsoukas, T. Effect of Temperature and Alcohols in the Preparation of Titania Nanoparticles from Alkoxides. J. Am. Ceram. Soc. 2005, 81, 2815–2820. [Google Scholar] [CrossRef]
- Hu, Y.; Yuan, C. Low-temperature preparation of photocatalytic TiO2 thin films from anatase sols. J. Cryst. Growth 2005, 274, 563–568. [Google Scholar] [CrossRef]
- Su, C.; Hong, B.-Y.; Tseng, C.-M. Sol–gel preparation and photocatalysis of titanium dioxide. Catal. Today 2004, 96, 119–126. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Liu, X.; Li, X.; Xu, P.; Han, X. Formation of Ag nanoparticles on water-soluble anatase TiO2 clusters and the activation of photocatalysis. Catal. Commun. 2009, 10, 1052–1056. [Google Scholar] [CrossRef]
- Wang, J.; Han, X.; Liu, C.; Zhang, W.; Cai, R.; Liu, Z. Adjusting the Crystal Phase and Morphology of Titania via a Soft Chemical Process. Cryst. Growth Des. 2010, 10, 2185–2191. [Google Scholar] [CrossRef]
- Yang, J.; Mei, S.; Ferreira, J.M. In situ preparation of weakly flocculated aqueous anatase suspensions by a hydrothermal technique. J. Colloid Interface Sci. 2003, 260, 82–88. [Google Scholar] [CrossRef]
- Yang, J.; Mei, S.; Ferreira, J.M.F. Hydrothermal Synthesis of Nanosized Titania Powders: Influence of Peptization and Peptizing Agents on the Crystalline Phases and Phase Transitions. J. Am. Ceram. Soc. 2000, 83, 1361–1368. [Google Scholar] [CrossRef]
- Yang, J.; Mei, S.; Ferreira, J.M.F. Hydrothermal Synthesis of Nanosized Titania Powders: Influence of Tetraalkyl Ammonium Hydroxides on Particle Characteristics. J. Am. Ceram. Soc. 2004, 84, 1696–1702. [Google Scholar] [CrossRef]
- Cassaignon, S.; Koelsch, M.; Jolivet, J.-P. From TiCl3 to TiO2 nanoparticles (anatase, brookite and rutile): Thermohydrolysis and oxidation in aqueous medium. J. Phys. Chem. Solids 2007, 68, 695–700. [Google Scholar] [CrossRef]
- Molea, A.; Popescu, V.; Rowson, N.; Dinescu, A.M. Influence of pH on the formulation of TiO2 nano-crystalline powders with high photocatalytic activity. Powder Technol. 2014, 253, 22–28. [Google Scholar] [CrossRef]
- Bazrafshan, H.; Tesieh, Z.A.; Dabirnia, S.; Naderifar, A. Low Temperature Synthesis of TiO 2 Nanoparticles with High Photocatalytic Activity and Photoelectrochemical Properties through Sol-Gel Method. Mater. Manuf. Process. 2015, 31, 119–125. [Google Scholar] [CrossRef]
- Kanna, M.; Wongnawa, S. Mixed amorphous and nanocrystalline TiO2 powders prepared by sol–gel method: Characterization and photocatalytic study. Mater. Chem. Phys. 2008, 110, 166–175. [Google Scholar] [CrossRef]
- Lee, J.H.; Yang, Y.S. Effect of HCl concentration and reaction time on the change in the crystalline state of TiO2 prepared from aqueous TiCl4 solution by precipitation. J. Eur. Ceram. Soc. 2005, 25, 3573–3578. [Google Scholar] [CrossRef]
- Xie, Y.; Yuan, C. Visible-light responsive cerium ion modified Titania sol and nanocrystallites for X-3B dye photodegradation. Appl. Catal. B Environ. 2003, 46, 251–259. [Google Scholar] [CrossRef]
- Xie, Y.; Yuan, C.; Li, X. Photocatalytic degradation of X-3B dye by visible light using lanthanide ion modified titanium dioxide hydrosol system. Colloids Surf. A Physicochem. Eng. Asp. 2005, 252, 87–94. [Google Scholar] [CrossRef]
- Xie, Y.; Yuan, C. Photocatalytic and photoelectrochemical performance of crystallized titanium dioxide sol with neodymium ion modification. J. Chem. Technol. Biotechnol. 2005, 80, 954–963. [Google Scholar] [CrossRef]
- Zeng, T.; Qiu, Y.; Chen, L.; Song, X. Microstructure and phase evolution of TiO2 precursors prepared by peptization-hydrolysis method using polycarboxylic acid as peptizing agent. Mater. Chem. Phys. 1998, 56, 163–170. [Google Scholar] [CrossRef]
- Zhang, Q.-H.; Gao, L.; Guo, J.-K. Preparation and characterization of nanosized TiO2 powders from aqueous TiCl4 solution. Nanostruct. Mater. 1999, 11, 1293–1300. [Google Scholar] [CrossRef]
- Kattoor, V.; Smitha, V.S.; Mohamed, A.P.; Hareesh, U.N.S.; Warrier, K.G. Temperature assisted acid catalyzed peptization of TiO2; facile sol–gel approach for thermally stable anatase phase. RSC Adv. 2014, 4, 21664–21671. [Google Scholar] [CrossRef]
- Li, Y.; Qin, Z.; Guo, H.; Yang, H.; Zhang, G.; Ji, S.; Zeng, T. Low-Temperature Synthesis of Anatase TiO2 Nanoparticles with Tunable Surface Charges for Enhancing Photocatalytic Activity. PLoS ONE 2014, 9, e114638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.-X.; Jiang, P.; Shao, W.-N.; Zhang, J.; Cao, W.-B. A novel approach for the synthesis of visible-light-active nanocrystalline N-doped TiO2 photocatalytic hydrosol. Solid State Sci. 2014, 33, 45–48. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, J.; Tian, B.; Chen, F.; Bao, S.; Anpo, M. Synthesis of visible light-driven Eu, N co-doped TiO2 and the mechanism of the degradation of salicylic acid. Res. Chem. Intermed. 2012, 38, 1947–1960. [Google Scholar] [CrossRef]
- Kim, Y.T.; Park, Y.S.; Myung, H.; Chae, H.K. A chelate-assisted route to anatase TiO2 nanoparticles in acidic aqueous media. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313-314, 260–263. [Google Scholar] [CrossRef]
- Liu, T.-X.; Li, F.-B.; Li, X.-Z. Effects of peptizing conditions on nanometer properties and photocatalytic activity of TiO2 hydrosols prepared by H2TiO3. J. Hazard. Mater. 2008, 155, 90–99. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.-X.; Li, X.-Z.; Li, F.-B. Enhanced photocatalytic activity of Ce3+-TiO2 hydrosols in aqueous and gaseous phases. Chem. Eng. J. 2010, 157, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Šuligoj, A.; Štangar, U.L.; Ristić, A.; Mazaj, M.; Verhovšek, D.; Tušar, N.N. TiO2–SiO2 films from organic-free colloidal TiO2 anatase nanoparticles as photocatalyst for removal of volatile organic compounds from indoor air. Appl. Catal. B Environ. 2016, 184, 119–131. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, L. Effect of peptization on phase transformation of TiO2 nanoparticles. Mater. Res. Bull. 2001, 36, 1957–1965. [Google Scholar] [CrossRef]
- Alcober, C.; Alvarez, F.; Bilmes, S.A.; Candal, R.J. Photochromic W-TiO2 membranes. J. Mater. Sci. Lett. 2002, 21, 501–504. [Google Scholar] [CrossRef]
- Belet, A.; Wolfs, C.; Mahy, J.G.; Poelman, D.; Vreuls, C.; Gillard, N.; Lambert, S.D. Sol-gel Syntheses of Photocatalysts for the Removal of Pharmaceutical Products in Water. Nanomaterials 2019, 9, 126. [Google Scholar] [CrossRef] [Green Version]
- Bergamonti, L.; Alfieri, I.; Lorenzi, A.; Montenero, A.; Predieri, G.; Di Maggio, R.; Girardi, F.; Lazzarini, L.; Lottici, P.P. Characterization and photocatalytic activity of TiO2 by sol–gel in acid and basic environments. J. Sol Gel Sci. Technol. 2014, 73, 91–102. [Google Scholar] [CrossRef]
- Borlaf, M.; Moreno, R.; Ortiz, A.L.; Colomer, M.T. Synthesis and photocatalytic activity of Eu3+-doped nanoparticulate TiO2 sols and thermal stability of the resulting xerogels. Mater. Chem. Phys. 2014, 144, 8–16. [Google Scholar] [CrossRef]
- Hu, L.; Wang, J.; Zhang, J.; Zhang, Q.; Liu, Z. An N-doped anatase/rutile TiO2hybrid from low-temperature direct nitridization: Enhanced photoactivity under UV-/visible-light. RSC Adv. 2014, 4, 420–427. [Google Scholar] [CrossRef]
- Jiang, J.; Long, M.; Wu, D.; Cai, W. Alkoxyl-derived visible light activity of TiO2 synthesized at low temperature. J. Mol. Catal. A Chem. 2011, 335, 97–104. [Google Scholar] [CrossRef]
- Khan, H. Sol–gel synthesis of TiO2 from TiOSO4: Characterization and UV photocatalytic activity for the degradation of 4-chlorophenol. React. Kinet. Mech. Catal. 2017, 121, 811–832. [Google Scholar] [CrossRef]
- Mao, L.; Li, Q.; Dang, H.; Zhang, Z. Synthesis of nanocrystalline TiO2 with high photoactivity and large specific surface area by sol–gel method. Mater. Res. Bull. 2005, 40, 201–208. [Google Scholar] [CrossRef]
- Yan, Q.; Wang, J.; Han, X.; Liu, Z. Soft-chemical method for fabrication of SnO–TiO2 nanocomposites with enhanced photocatalytic activity. J. Mater. Res. 2013, 28, 1862–1869. [Google Scholar] [CrossRef]
- Al-Maliki, F.J.; Al-Lamey, N.H. Synthesis of Tb-doped titanium dioxide nanostructures by sol–gel method for environmental photocatalysis applications. J. Sol Gel Sci. Technol. 2016, 81, 276–283. [Google Scholar] [CrossRef]
- Gole, J.L.; Stout, J.D.; Burda, C.; Lou, Y.; Chen, X. Highly Efficient Formation of Visible Light Tunable TiO 2-x N x Photocatalysts and Their Transformation at the Nanoscale. J. Phys. Chem. B. 2004, 108, 1230–1240. [Google Scholar] [CrossRef]
- Chung, W.; Kim, S.; Chang, S. A Study of the Correlation Between the Physical Characteristics and Efficiency of TiO2 Photocatalyst Prepared with the Sol–Gel Method. J. Nanosci. Nanotechnol. 2016, 16, 11040–11045. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, P.; Wang, Z.; Rao, Y.; Cao, J.-J.; Pu, S.; Ho, W.; Lee, S.-C. Protonated g-C3N4/Ti3+ self-doped TiO2 nanocomposite films: Room-temperature preparation, hydrophilicity, and application for photocatalytic NO removal. Appl. Catal. B Environ. 2019, 240, 122–131. [Google Scholar] [CrossRef]
- Look, J.L.; Zukoski, C.F. Alkoxide-Derived Titania Particles: Use of Electrolytes to Control Size and Agglomeration Levels. J. Am. Ceram. Soc. 1992, 75, 1587–1595. [Google Scholar] [CrossRef]
- Look, J.-L.; Zukoski, C.F. Colloidal Stability and Titania Precipitate Morphology: Influence of Short-Range Repulsions. J. Am. Ceram. Soc. 1995, 78, 21–32. [Google Scholar] [CrossRef]
- Sharma, B.; Agarwal, R.; Jassal, M.; Agrawal, A.K. Stabilizer-free low-acid rapid synthesis of highly stable transparent aqueous titania nano sol and its photocatalytic activity. J. Mol. Liq. 2020, 305, 112842. [Google Scholar] [CrossRef]
- Antonello, A.; Brusatin, G.; Guglielmi, M.; Bello, V.; Mattei, G.; Zacco, G.; Martucci, A. Nanocomposites of titania and hybrid matrix with high refractive index. J. Nanoparticle Res. 2011, 13, 1697–1708. [Google Scholar] [CrossRef]
- Bi-Tao, X.; Bao-Xue, Z.; Long-Hai, L.; Jun, C.; Yan-Biao, L.; Wei-Min, C. Preparation of nanocrystalline anatase TiO2 using basic sol-gel method. Chem. Pap. 2008, 62, 382–387. [Google Scholar] [CrossRef]
- Li, H.; Afanasiev, P. On the selective growth of titania polymorphs in acidic aqueous medium. Mater. Res. Bull. 2011, 46, 2506–2514. [Google Scholar] [CrossRef]
- Mahy, J.G.; Lambert, S.D.; Geens, J.; Daniel, A.; Wicky, D.; Archambeau, C.; Heinrichs, B. Large scale production of photocatalytic TiO2 coating for volatile organic compound (VOC) air remediation. AIMS Mater. Sci. 2018, 5, 945–956. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Yu, J.-X.; Liu, Z.-H.; He, Z.-K.; Cai, R.-X. A simple new way to prepare anatase TiO2 hydrosol with high photocatalytic activity. Semicond. Sci. Technol. 2005, 20, L36–L39. [Google Scholar] [CrossRef]
- Nie, X.; Zhuo, S.; Maeng, G.; Sohlberg, K. Doping ofTiO2Polymorphs for Altered Optical and Photocatalytic Properties. Int. J. Photoenergy 2009, 2009, 294042. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Zhang, J.; Feng, Z.; Ma, Y.; Wang, X.; Li, C. Surface Structural Transformation and the Phase Transition Kinetics of Brookite TiO2. Chem. Asian J. 2010, 5, 2158–2161. [Google Scholar] [CrossRef] [PubMed]
- Bakardjieva, S.; Štengl, V.; Szatmary, L.; Subrt, J.; Lukac, J.; Murafa, N.; Niznansky, D.; Cizek, K.; Jirkovsky, J.; Petrova, N. Transformation of brookite-type TiO2 nanocrystals to rutile: Correlation between microstructure and photoactivity. J. Mater. Chem. 2006, 16, 1709–1716. [Google Scholar] [CrossRef]
- Balaganapathi, T.; Kaniamuthan, B.; Vinoth, S.; Arun, T.; Thilakan, P. Controlled synthesis of brookite and combined brookite with rutile phases of titanium di-oxide and its characterization studies. Ceram. Int. 2017, 43, 2438–2440. [Google Scholar] [CrossRef]
- Li, J.-G.; Ishigaki, T. Brookite rutile phase transformation of TiO2 studied with monodispersed particles. Acta Mater. 2004, 52, 5143–5150. [Google Scholar] [CrossRef]
- Lin, Y.; Cai, Y.; Qiu, M.; Drioli, E.; Fan, Y. Environment-benign preparation of Ag toughening TiO2/Ti tight ultrafiltration membrane via aqueous sol–gel route. J. Mater. Sci. 2015, 50, 5307–5317. [Google Scholar] [CrossRef]
- Fallet, M.; Permpoon, S.; Deschanvres, J.-L.; Langlet, M. Influence of physico-structural properties on the photocatalytic activity of sol-gel derived TiO2 thin films. J. Mater. Sci. 2006, 41, 2915–2927. [Google Scholar] [CrossRef]
- Mamadou, S.D.; Neil, A.F.; Myung, S.J. Nanotechnology for Sustainable Development. Nanotechnol. Sustain. Dev. 2014, 14, 101–111. [Google Scholar] [CrossRef]
- Sugimoto, T.; Zhou, X.; Muramatsu, A. Synthesis of uniform anatase TiO2 nanoparticles by gel–sol method 3. Formation process and size control. J. Colloid Interface Sci. 2003, 259, 43–52. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lin, C.-Y.; Hsu, M.-H. Enhanced photocatalytic activity of Ce-doped ZnO nanorods under UV and visible light. J. Taiwan Inst. Chem. Eng. 2014, 45, 1954–1963. [Google Scholar] [CrossRef]
- Vaiano, V.; Lara, M.; Iervolino, G.; Matarangolo, M.; Navio, J.; Hidalgo, M.C. Photocatalytic H2 production from glycerol aqueous solutions over fluorinated Pt-TiO2 with high {001} facet exposure. J. Photochem. Photobiol. A Chem. 2018, 365, 52–59. [Google Scholar] [CrossRef]
- Cao, D.; Wang, Y.; Zhao, X. Combination of photocatalytic and electrochemical degradation of organic pollutants from water. Curr. Opin. Green Sustain. Chem. 2017, 6, 78–84. [Google Scholar] [CrossRef]
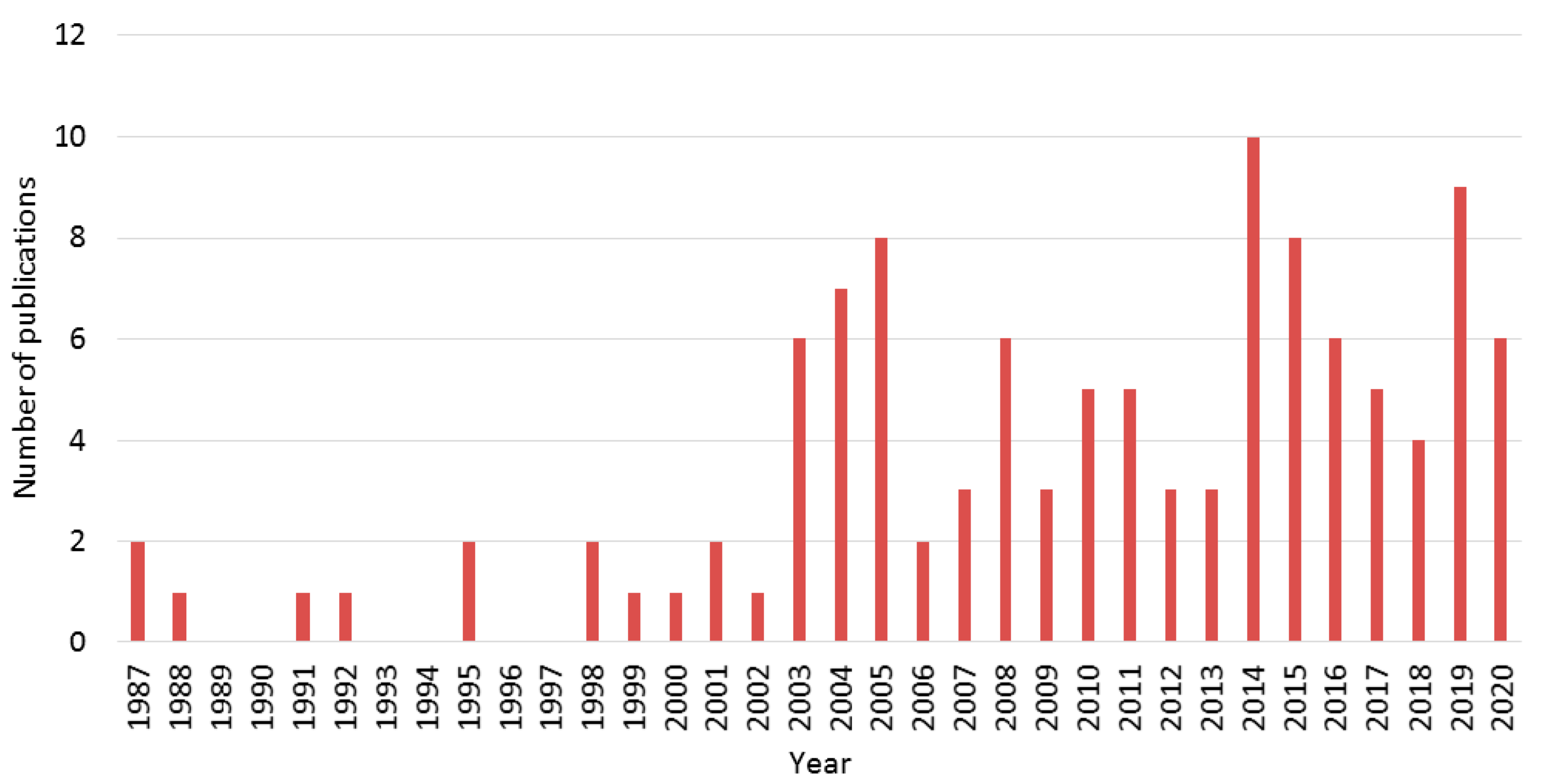
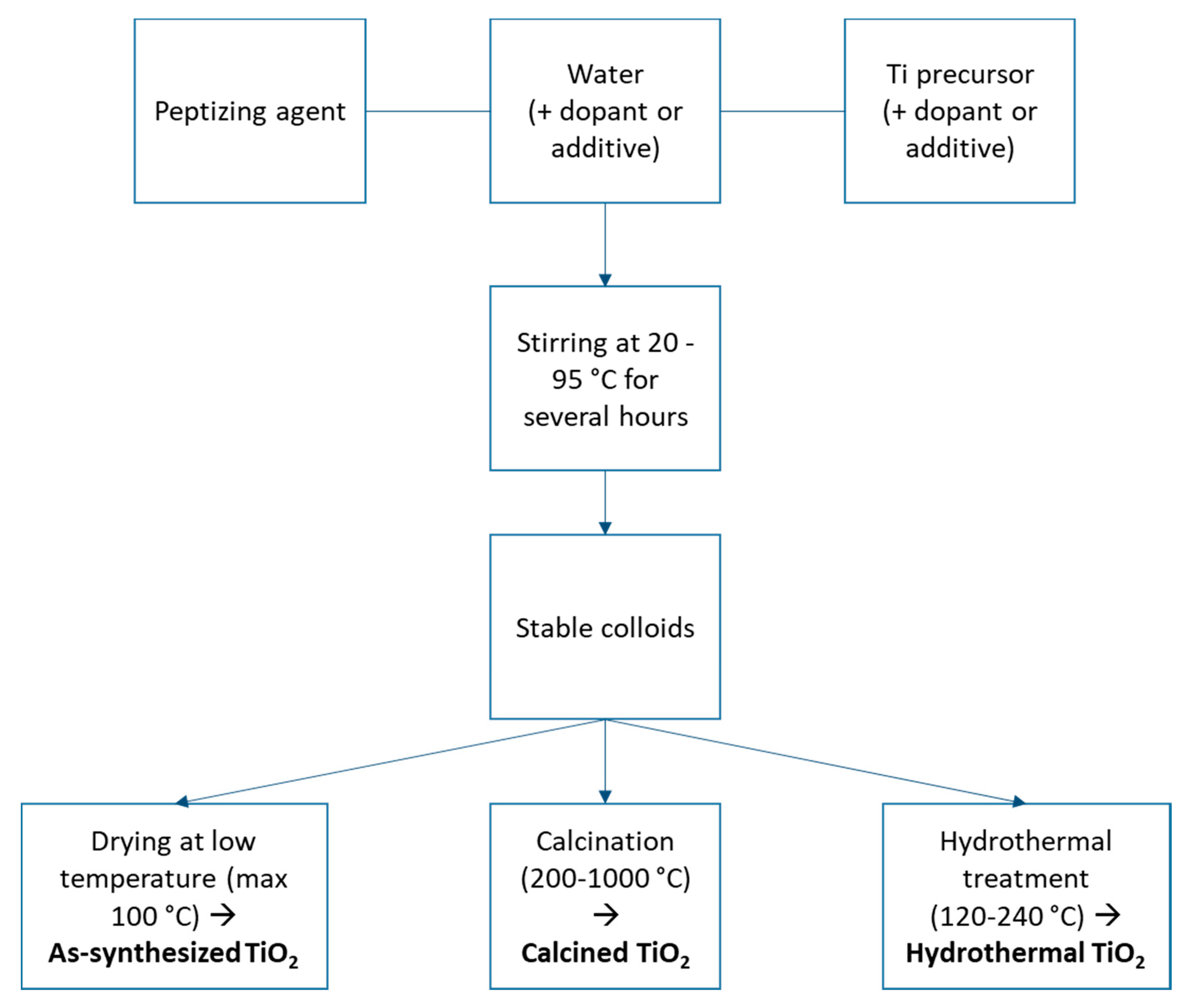


| Synthesis Parameters | Corresponding Parameters Collected in the Literature (Variants) |
|---|---|
| Ti precursor | Ti isopropoxide [8,16,34,35,38,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95], Ti ethoxide [39,96], Ti butoxide [37,97,98,99,100,101,102,103], Ti trichloride [104,105], Ti tetrachloride [106,107,108,109,110,111,112,113], Titanyl sulfate and disulfate [114,115,116,117], Titanium(IV) bis(acetylacetonate) diisopropoxide [118], metatitanic acid [119,120,121,122], Ti propoxide [96]. |
| Peptizing agent | Nitric acid [8,16,36,39,41,42,43,45,46,53,56,59,61,62,64,66,67,70,73,76,77,78,79,82,86,93,96,100,106,107,109,114,117,119,120,123,124,125,126,127,128,129,130,131], acetic acid [37,44,87,125,132,133], hydrochloric acid [39,49,54,68,72,87,104,108,121,134,135,136,137,138,139], malonic acid [125], sulfuric acid [39,53,89,107], tetramethylammonium hydroxide [50,101,140], sodium hydroxide [52,54], phosphoric acid [54,107], perchloric acid [83,141], ammonium hydroxide [38,58,91], hydrogen peroxide [105,116], lactic acid [71], citric acid [138], boric acid [85] |
| Temperature range of reaction | 20–95 °C |
| Trace of organic solvent | Isopropanol, ethanol, methanol |
| Additive or dopant | Other metallic alkoxides, metallic salts, carbon materials, nitrogen compounds |
| Thermal treatment | Ambient drying, calcination in the range 200–1000 °C, hydrothermal treatment |
| Shaping | Powder, coating, colloid |
| Paper | Photocatalyst and Shape (Concentration) | Pollutant (Concentration) | Illumination and Time | Best Degradation Results |
|---|---|---|---|---|
| Bazrafshan et al., 2015 [106] |
| Reactive orange dye (200 ppm) | Xenon lamp—40 min | 100% |
| Belet et al., 2019 [124] |
|
| 254 nm—4 h |
|
| Bergamonti et al., 2014 [125] |
|
| 365 nm—160 min | 100% on both |
| Borlaf et al., 2014 [126] |
| MB (0.33×10−2 M) | 254 or 312 or 365 nm—40 min | Only kinetic constants given |
| Gole et al., 2004 [133] |
| MB (--) |
|
|
| Chen et al., 2005 [95] |
| MB (--) |
|
|
| Douven et al., 2020 [42] |
|
|
|
|
| Hu et al., 2005 [97] |
| Reactive brilliant red dye XB3 (50 mg/L) | 365 nm—120 min | 100% |
| Hu et al., 2014 [127] |
| MB (20 µM) |
|
|
| Huang et al., 2019 [135] |
| NOx (gas phase- 400 ppb) | Visible—cycle of 30 min | 25% for one cycle |
| Kanna et al., 2008 [107] |
|
| 366 nm—3 h |
|
| Léonard et al., 2016 [56] |
| PNP (10−4 M) |
|
|
| Li et al., 2014 [115] |
|
| 365 nm—280 or 400 min |
|
| Liu et al., 2008 [119] |
|
|
|
|
| Liu et al., 2010 [120] |
|
|
|
|
| Mahy et al. [16,41,61,62,64,65] |
|
|
|
|
| Malengreaux et al. [8,66] |
| PNP (10−4 M) | UV-visible (300–800 nm)—7 h | 75% |
| Qi et al., 2010 [74] |
| Neolan Blue 2G (0.2 g/L) | 365 nm—2 h | 70% |
| Sharma et al., 2020 [138] |
| Solophenyl green (3.15 g/L) | 365 nm—350 min | 70% |
| Suligoj et al., 2016 [121] |
| Toluene (gas phase 49 ppmv) | 365 nm—100 min | 100% |
| Sung-Suh et al., 2004 [84] |
| RB (10−5 M) |
|
|
| Vinogradov et al., 2014 [87] |
| RB (40 mg/L) | UV—120 min | 95% |
| Wang et al., 2009 [99] |
| MB (30 µM) | UV—90 min | 55% |
| Wang et al., 2005 [143] |
| MB (0.016 g/L) | UV—25 min | 45% |
| Xie et al., 2005 [110] |
| X3B (100 mg/L) | 400–800 nm—120 min | 90% |
| Yan et al., 2013 [131] |
| MB (16 mg/L) | Visible (>420 nm)—100 min | 45% |
| Yun et al., 2004 [92] |
| Ethanol (gas phase 450 ppmv) | UV—50 min | 100% |
| Zhang et al., 2001 [122] |
| sodium benzenesulfate (12 mM) | UV—4 h | 100% |
| Paper | Photocatalyst and Shape (Concentration) | Pollutant (Concentration) | Illumination and Time | Best Degradation Results |
|---|---|---|---|---|
| Al-Maliki et al., 2017 [132] |
| KMnO4 (2 × 10−5 M) |
|
|
| Borlaf et al., 2012 [93] |
| MB (0.33 × 10−2 M) | 254 or 312 or 365 nm—40 min | Only kinetic constants given |
| Cano-Franco et al., 2019 [44] |
| MB (400 ppm) | Solar lamp (Xe lamp)—150 min | 98% |
| Cesconeto et al., 2018 [43] |
| MB (1.25 × 10−3 M) | 254 or 312 or 365 nm—40 min | Only kinetic constants given |
| Chung et al., 2016 [134] |
| Dye reactive orange 16 (RO16) (25 ppm) | UV—120 min | 100% |
| Haque et al., 2017 [49] |
| MB and MO (--) | Visible—120 min | 70% |
| Ibrahim et al., 2010 [52] |
| MO (30 ppm) | UV—5 h | 100% |
| Kattoor et al., 2014 [114] |
| MB (10−5 M) | UV-A—100 min | 85% |
| Khan et al., 2017 [129] |
| PNP (0.02 g/L) | 254 nm—30 min | 65% |
| Ma et al., 2012 [117] |
| Salicylic acid (50 mg/L) | Visible (>420 nm)—300 min | 88% |
| Mahmoud et al., 2018 [34] |
|
| UV—120 min | 100% |
| Mao et al., 2005 [130] |
| X3B (30 mg/L) | UV—40 min | 100% |
| Maver et al., 2009 [67] |
| PlasmocorinthB (40 mg/L) | UV-A—3000 s | 70% |
| Molea et al., 2014 [105] |
| MB (2.75 × 10−3 g/L) | 300–400 nm + 400–700 nm—300 min | 47% |
| Mutuma et al., 2015 [70] |
| MB (32 mg/L) | UV—70 min | 95% |
| Periyat et al., 2015 [73] |
| R6G (5 × 10−6 M) | 420–800 nm—20 min | 100% |
| Qiu et al., 2007 [75] |
| MB (--) | Visible (>400 nm)—350 min | 85% |
| Quintero et al., 2020 [76] |
| MB (5 ppm) | 365 nm—250 min | 90% |
| Ropero-Vega et al., 2019 [77] |
| Salicylic acid (0.1 mM) | UV-Visible (325–650 nm) —1 h | 10% |
| Su et al., 2004 [98] |
| Salicylic acid (4×10−4 M) | 254 nm—250 min | 65% |
| Tobaldi et al., 2014 [85] |
| MB (liquid phase—5 mg/L)NOx (gas phases—0.5 ppmv) | Solar light—7 hSolar light—40 min | 100%60% |
| Xie et al., 2005 [111] |
| X3B (100 mg/L) | 365 nm + 400–800 nm—120 min400–800 nm—120 min | 95%35% |
| Yamazaki et al., 2001 [89] |
| Ethylene (gas phase 160 ppmv) | 4W fluorescence black light bulbs—2 h | 100% |
| Yu et al., 2003 [91] |
| Acetone (gas phase—400 ppm) | 365 nm—60 min | Only kinetic constants given |
| Paper | Photocatalyst and Shape (Concentration) | Pollutant (Concentration) | Illumination and Time | Best Degradation Results |
|---|---|---|---|---|
| Fallet et al., 2006 [150] |
| Malic acid (3.7 × 10−4 M) | UV (>340 nm)—3 h | 90% |
| Jiang et al., 2011 [128] |
| MO (10 mg/L) | Visible (>400 nm)—100 min | 35% |
| Kaplan et al., 2016 [54] |
| Bisphenol A (BPA) (10 mg/L) | 365 nm—60 min | 100% |
| Liu et al., 2014 [116] |
| HCHO (gas phase—0.32 mg/m3) | Visible ()—24 h | 95% |
| Mahata et al., 2012 [59] |
| MO (--) | UV Visible—120 min | 85% |
| Saif et al., 2012 [151] |
| Real wastewater | Solar light—3 h | 57% mineralization |
| Xie et al., 2003 [109] |
| X3B (100 mg/L) | 400–800 nm—120 min | 95% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahy, J.G.; Lejeune, L.; Haynes, T.; Lambert, S.D.; Marcilli, R.H.M.; Fustin, C.-A.; Hermans, S. Eco-Friendly Colloidal Aqueous Sol-Gel Process for TiO2 Synthesis: The Peptization Method to Obtain Crystalline and Photoactive Materials at Low Temperature. Catalysts 2021, 11, 768. https://doi.org/10.3390/catal11070768
Mahy JG, Lejeune L, Haynes T, Lambert SD, Marcilli RHM, Fustin C-A, Hermans S. Eco-Friendly Colloidal Aqueous Sol-Gel Process for TiO2 Synthesis: The Peptization Method to Obtain Crystalline and Photoactive Materials at Low Temperature. Catalysts. 2021; 11(7):768. https://doi.org/10.3390/catal11070768
Chicago/Turabian StyleMahy, Julien G., Louise Lejeune, Tommy Haynes, Stéphanie D. Lambert, Raphael Henrique Marques Marcilli, Charles-André Fustin, and Sophie Hermans. 2021. "Eco-Friendly Colloidal Aqueous Sol-Gel Process for TiO2 Synthesis: The Peptization Method to Obtain Crystalline and Photoactive Materials at Low Temperature" Catalysts 11, no. 7: 768. https://doi.org/10.3390/catal11070768
APA StyleMahy, J. G., Lejeune, L., Haynes, T., Lambert, S. D., Marcilli, R. H. M., Fustin, C.-A., & Hermans, S. (2021). Eco-Friendly Colloidal Aqueous Sol-Gel Process for TiO2 Synthesis: The Peptization Method to Obtain Crystalline and Photoactive Materials at Low Temperature. Catalysts, 11(7), 768. https://doi.org/10.3390/catal11070768










