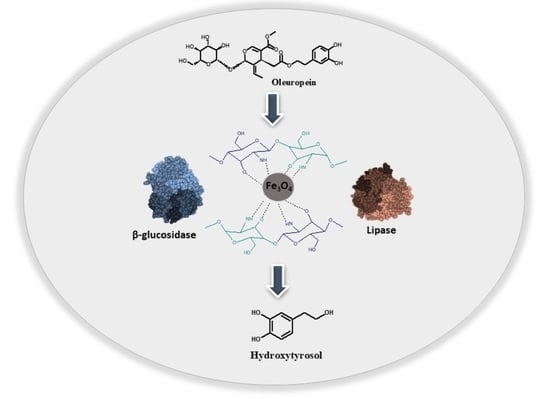
Development of a Novel Bi-Enzymatic Nanobiocatalyst for the Efficient Bioconversion of Oleuropein to Hydroxytyrosol
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of the Chitosan Magnetic Nanoparticles
Atomic Force Microscopy (AFM)
2.2. Preparation of the Bi-Enzymatic Magnetic Nanobiocatalyst
2.3. Characterization of the Bio-Nanoconjugates
2.3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.2. Circular Dichroism Spectroscopy (CD)
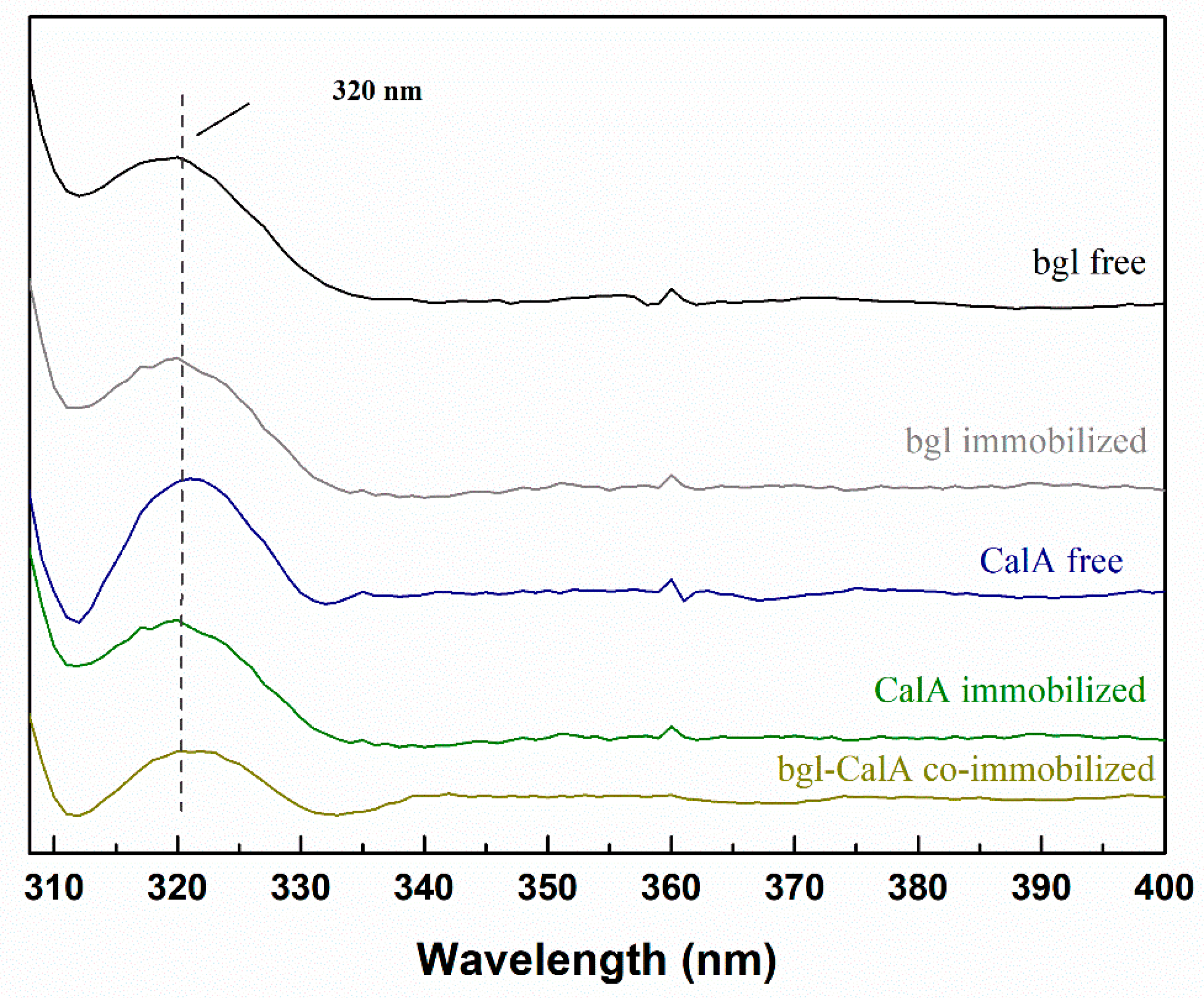
2.3.3. Fluorescence Spectroscopy
2.4. Thermal Stability of the Bi-Enzymatic Nanobiocatalyst
2.5. Kinetic Studies of Free, Individually Immobilized and Co-Immobilized Biocatalysts
2.6. Application of the Bi-Enzymatic Nanobiocatalyst to the Bioconversion of Oleuropein to Hydroxytyrosol
2.7. Reusability of the Bi-Enzymatic Nanobiocatalyst
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Functionalization of Iron Oxide Magnetic Nanoparticles (Fe3O4) with Chitosan (CS-MNPs)
3.2.2. Characterization of the Chitosan-Functionalized Magnetic Nanoparticles
XRD
AFM
3.2.3. Preparation of Bi-Enzymatic Magnetic Nanobiocatalyst
3.2.4. Characterization of the Bi-Enzymatic Magnetic Nanobiocatalyst
Fourier-Transform Infrared Spectroscopy (FTIR)
Circular Dichroism Spectroscopy (CD)
Fluorescence Spectroscopy
3.2.5. Enzyme Assays
3.2.6. Thermal Stability Studies
3.2.7. Kinetic Studies of Free, Individually Immobilized and Co-Immobilized Biocatalysts
3.2.8. Hydrolysis of Oleuropein to Hydroxytyrosol by the Βi-Enzymatic Νanobiocatalyst High-Performance Liquid Chromatography (HPLC) Analysis
3.2.9. Nuclear Magnetic Resonance (NMR) Analysis
3.2.10. Reusability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a Bioactive Compound from Olea europaea L., as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.-J.; Wang, C.-Z.; Ye, J.-Z.; Tao, R.; Zhang, Y.-S. Enzymatic Hydrolysis of Oleuropein from Olea europea (Olive) Leaf Extract and Antioxidant Activities. Molecules 2015, 20, 2903–2921. [Google Scholar] [CrossRef]
- Liu, M.; Yong, Q.; Yu, S. Efficient bioconversion of oleuropein from olive leaf extract to antioxidant hydroxytyrosol by enzymatic hydrolysis and high-temperature degradation. Biotechnol. Appl. Biochem. 2018, 65, 680–689. [Google Scholar] [CrossRef]
- De Leonardis, A.; Aretini, A.; Alfano, G.; Macciola, V.; Ranalli, G. Isolation of a hydroxytyrosol-rich extract from olive leaves (Olea Europaea L.) and evaluation of its antioxidant properties and bioactivity. Eur. Food Res. Technol. 2008, 226, 653–659. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Zheng, M.; Xi, X.; Zhang, X.; Han, C. Structure Properties, Acquisition Protocols, and Biological Activities of Oleuropein Aglycone. Front. Chem. 2018, 6, 239. [Google Scholar] [CrossRef] [PubMed]
- Nikolaivits, E.; Termentzi, A.; Skaltsounis, A.-L.; Fokialakis, N.; Topakas, E. Enzymatic tailoring of oleuropein from Olea europaea leaves and product identification by HRMS/MS spectrometry. J. Biotechnol. 2017, 253, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Soler-Rivas, C.; Espín, J.C.; Wichers, H.J. Oleuropein and related compounds. J. Sci. Food Agric. 2000, 80, 1013–1023. [Google Scholar] [CrossRef]
- Ramírez, E.; Brenes, M.; García, P.; Medina, E.; Romero, C. Oleuropein hydrolysis in natural green olives: Importance of the endogenous enzymes. Food Chem. 2016, 206, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Chatzikonstantinou, A.V.; Gkantzou, E.; Thomou, E.; Chalmpes, N.; Lyra, K.-M.; Kontogianni, V.G.; Spyrou, K.; Patila, M.; Gournis, D.; Stamatis, H.; et al. Enzymatic Conversion of Oleuropein to Hydroxytyrosol Using Immobilized β-Glucosidase on Porous Carbon Cuboids. Nanomaterials 2019, 9, 1166. [Google Scholar] [CrossRef]
- Giannakopoulou, A.; Gkantzou, E.; Polydera, A.; Stamatis, H. Multienzymatic Nanoassemblies: Recent Progress and Applications. Trends Biotechnol. 2020, 38, 202–216. [Google Scholar] [CrossRef]
- Ren, S.; Liu, X.; Jiao, X.; Jia, S.; Jiang, Y.; Bilal, M.; Cui, J. Recent progress in multienzymes co-immobilization and multienzyme system applications. Chem. Eng. J. 2019, 373, 1254–1278. [Google Scholar] [CrossRef]
- Filice, M.; Palomo, J.M. Cascade Reactions Catalyzed by Bionanostructures. ACS Catal. 2014, 4, 1588–1598. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Lima, P.J.M.; Pinheiro, B.; Freire, T.M.; Dutra, L.M.U.; Fechine, L.; Gonçalves, L.R.B.; De Souza, M.C.M.; Dos Santos, J.C.S.; Fernandez-Lafuente, R. Immobilization of Lipase A from Candida antarctica onto Chitosan-Coated Magnetic Nanoparticles. Int. J. Mol. Sci. 2019, 20, 4018. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, X.; Chen, Y.; Xue, Z.; Guo, Q.; Ma, Q.; Chen, H. Preparation and characterization of a novel nanocomposite with double enzymes immobilized on magnetic Fe3O4-chitosan-sodium tripolyphosphate. Colloids Surfaces B Biointerfaces 2018, 169, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, A.M.; Patel, G.R. Analysis of variability in tensile ductility of a semi-solid metal cast A356 Al-alloy. Mater. Sci. Eng. A 2005, 392, 184–190. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Luo, G.; Dai, Y. In situ preparation of magnetic Fe3O4-chitosan nanoparticles for lipase immobilization by cross-linking and oxidation in aqueous solution. Bioresour. Technol. 2009, 100, 3459–3464. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Díaz-Hernández, A.; Gracida, J.; García-Almendárez, B.E.; Regalado, C.; Núñez, R.; Amaro-Reyes, A. Characterization of Magnetic Nanoparticles Coated with Chitosan: A Potential Approach for Enzyme Immobilization. J. Nanomater. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Assa, F.; Jafarizadeh-Malmiri, H.; Ajamein, H.; Vaghari, H.; Anarjan, N.; Ahmadi, O.; Berenjian, A. Chitosan magnetic nanoparticles for drug delivery systems. Crit. Rev. Biotechnol. 2016, 37, 492–509. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.T.R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Monteiro, R.R.; Virgen-Ortiz, J.J.; Berenguer-Murcia, Á; da Rocha, T.N.; dos Santos, J.C.; Alcántara, A.R.; Fernandez-Lafuente, R. Biotechnological relevance of the lipase A from Candida antarctica. Catal. Today 2021, 362, 141–154. [Google Scholar] [CrossRef]
- Akın, D.; Yakar, A.; Gündüz, U. Synthesis of Magnetic Fe3O4-Chitosan Nanoparticles by Ionic Gelation and Their Dye Removal Ability. Water Environ. Res. 2015, 87, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Xiao, L.; Chen, C.; Shi, X.; Cao, Q.; Gao, L. In situ preparation of magnetic Fe3O4/chitosan nanoparticles via a novel reduction–precipitation method and their application in adsorption of reactive azo dye. Powder Technol. 2014, 260, 90–97. [Google Scholar] [CrossRef]
- López-Gallego, F.; Guisán, J.M.; Betancor, L. Glutaraldehyde-Mediated Protein Immobilization. Methods Mol. Biol. 2013, 1051, 33–41. [Google Scholar] [CrossRef]
- Giannakopoulou, A.; Patila, M.; Spyrou, K.; Chalmpes, N.; Zarafeta, D.; Skretas, G.; Gournis, D.; Stamatis, H. Development of a Four-Enzyme Magnetic Nanobiocatalyst for Multi-Step Cascade Reactions. Catalysts 2019, 9, 995. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, D.; Wu, C.; Yao, K.; Li, Z.; Shi, N.; Wen, F.; Gates, I.D. Co-immobilization of cellulase and lysozyme on amino-functionalized magnetic nanoparticles: An activity-tunable biocatalyst for extraction of lipids from microalgae. Bioresour. Technol. 2018, 263, 317–324. [Google Scholar] [CrossRef]
- Sojitra, U.V.; Nadar, S.; Rathod, V.K. A magnetic tri-enzyme nanobiocatalyst for fruit juice clarification. Food Chem. 2016, 213, 296–305. [Google Scholar] [CrossRef]
- Muley, A.B.; Thorat, A.S.; Singhal, R.S.; Babu, K.H. A tri-enzyme co-immobilized magnetic complex: Process details, kinetics, thermodynamics and applications. Int. J. Biol. Macromol. 2018, 118, 1781–1795. [Google Scholar] [CrossRef]
- Pavlidis, I.V.; Vorhaben, T.; Gournis, D.; Papadopoulos, G.K.; Bornscheuer, U.T.; Stamatis, H. Regulation of catalytic behaviour of hydrolases through interactions with functionalized carbon-based nanomaterials. J. Nanopart. Res. 2012, 14, 1–10. [Google Scholar] [CrossRef]
- Tzialla, A.A.; Pavlidis, I.V.; Felicissimo, M.P.; Rudolf, P.; Gournis, D.; Stamatis, H. Lipase immobilization on smectite nanoclays: Characterization and application to the epoxidation of α-pinene. Bioresour. Technol. 2010, 101, 1587–1594. [Google Scholar] [CrossRef]
- Verma, M.L.; Rao, N.M.; Tsuzuki, T.; Barrow, C.J.; Puri, M. Suitability of Recombinant Lipase Immobilised on Functionalised Magnetic Nanoparticles for Fish Oil Hydrolysis. Catalysts 2019, 9, 420. [Google Scholar] [CrossRef]
- Verma, M.L.; Naebe, M.; Barrow, C.; Puri, M. Enzyme Immobilisation on Amino-Functionalised Multi-Walled Carbon Nanotubes: Structural and Biocatalytic Characterisation. PLoS ONE 2013, 8, e73642. [Google Scholar] [CrossRef]
- Chatzikonstantinou, A.V.; Polydera, A.C.; Thomou, E.; Chalmpes, N.; Baroud, T.N.; Enotiadis, A.; Estevez, L.; Patila, M.; Hammami, M.A.; Spyrou, K.; et al. Lipase immobilized on magnetic hierarchically porous carbon materials as a versatile tool for the synthesis of bioactive quercetin derivatives. Bioresour. Technol. Rep. 2020, 9, 100372. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Yan, B.; Yu, S. Preparation of a reversible soluble-insoluble β-d-Glucosidase with perfect stability and activity. J. Biotechnol. 2019, 291, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Girard-Egrot, A.P.; Godoy, S.; Blum, L.J. Enzyme association with lipidic Langmuir–Blodgett films: Interests and applications in nanobioscience. Adv. Colloid Interface Sci. 2005, 116, 205–225. [Google Scholar] [CrossRef] [PubMed]
- Paudyal, S.; Sharma, S.K.; da Silva, R.L.; Mintz, K.J.; Liyanage, P.Y.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. Tyrosinase enzyme Langmuir monolayer: Surface chemistry and spectroscopic study. J. Colloid Interface Sci. 2020, 564, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, F.D.P.; Caseli, L. Controlling the molecular architecture of lactase immobilized in Langmuir-Blodgett films of phospholipids to modulate the enzyme activity. Colloids Surfaces B Biointerfaces 2017, 150, 8–14. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial Applications of Enzymes: Recent Advances, Techniques, and Outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Gupta, P.; Verma, S.; Vakhlu, J. Comparative Analysis of Β- Glucosidases Thermostability: Differences in Amino Acids Com-position and Distribution among Mesostable and Thermostable Β-Glucosidases. J. Adv. Bioinform. Appl. Res. 2014, 5, 215–227. [Google Scholar]
- Shahrestani, H.; Taheri-Kafrani, A.; Soozanipour, A.; Tavakoli, O. Enzymatic clarification of fruit juices using xylanase immobilized on 1,3,5-triazine-functionalized silica-encapsulated magnetic nanoparticles. Biochem. Eng. J. 2016, 109, 51–58. [Google Scholar] [CrossRef]
- Alnadari, F.; Xue, Y.; Zhou, L.; Hamed, Y.S.; Taha, M.; Foda, M.F. Immobilization of β-Glucosidase from Thermatoga maritima on Chitin-functionalized Magnetic Nanoparticle via a Novel Thermostable Chitin-binding Domain. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Whitmore, L.; Wallace, B.A. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004, 32, W668–W673. [Google Scholar] [CrossRef] [PubMed]
- Lobley, A.; Whitmore, L.; Wallace, B.A. DICHROWEB: An interactive website for the analysis of protein secondary structure from circular dichroism spectra. Bioinformatics 2002, 18, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Bowers, G.N., Jr.; McComb, R.B.; Christensen, R.G.; Schaffer, R. High-purity 4-nitrophenol: Purification, characterization, and speci-fications for use as a spectrophotometric reference material. Clin. Chem. 1980, 26, 724–729. [Google Scholar] [CrossRef]









| Sample | α-Helix | β-Sheet | Other | |
|---|---|---|---|---|
| bgl | Buffer CS-MNPs | 28 25 | 19 23 | 53 52 |
| CalA | Buffer CS-MNPs | 32 31 | 17 22 | 51 47 |
| Sample | Half-Life Time (h) |
|---|---|
| Free CalA | 7.8 |
| Co-immobilized CalA | 44.2 |
| Individually immobilized CalA | 51.8 |
| Forms | Km (mM) | Vmax (μmol/min) |
|---|---|---|
| Free bgl | 0.72 | 9.27 |
| Co-immobilized bgl | 0.85 | 5.60 |
| Individually immobilized bgl | 1.20 | 7.64 |
| Forms | Km (mM) | Vmax (mol/min) |
|---|---|---|
| Free CalA | 0.07 | 0.07 |
| Co-immobilized CalA | 0.17 | 0.04 |
| Individually immobilized CalA | 0.22 | 0.05 |
| Sample | Initial Reaction Rate (mM h−1 mg−1 Nanobiocatalyst) | % Conversion Yield of Oleuropein | Hydroxytyrosol (mg mL−1) |
|---|---|---|---|
| Individually immobilized bgl | 0.038 | 100 | 0.150 |
| Individually immobilized CalA | 0.021 | 40 | 0.880 |
| Individually immobilized bgl and CalA | 0.032 | 90 | 1.210 |
| Co-Immobilized bgl-CalA | 0.046 | 100 | 2.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakopoulou, A.; Chatzikonstantinou, A.V.; Chalmpes, N.; Tsapara, G.; Gournis, D.; Polydera, A.C.; Stamatis, H. Development of a Novel Bi-Enzymatic Nanobiocatalyst for the Efficient Bioconversion of Oleuropein to Hydroxytyrosol. Catalysts 2021, 11, 749. https://doi.org/10.3390/catal11060749
Giannakopoulou A, Chatzikonstantinou AV, Chalmpes N, Tsapara G, Gournis D, Polydera AC, Stamatis H. Development of a Novel Bi-Enzymatic Nanobiocatalyst for the Efficient Bioconversion of Oleuropein to Hydroxytyrosol. Catalysts. 2021; 11(6):749. https://doi.org/10.3390/catal11060749
Chicago/Turabian StyleGiannakopoulou, Archontoula, Alexandra V. Chatzikonstantinou, Nikolaos Chalmpes, Georgia Tsapara, Dimitrios Gournis, Angeliki C. Polydera, and Haralambos Stamatis. 2021. "Development of a Novel Bi-Enzymatic Nanobiocatalyst for the Efficient Bioconversion of Oleuropein to Hydroxytyrosol" Catalysts 11, no. 6: 749. https://doi.org/10.3390/catal11060749
APA StyleGiannakopoulou, A., Chatzikonstantinou, A. V., Chalmpes, N., Tsapara, G., Gournis, D., Polydera, A. C., & Stamatis, H. (2021). Development of a Novel Bi-Enzymatic Nanobiocatalyst for the Efficient Bioconversion of Oleuropein to Hydroxytyrosol. Catalysts, 11(6), 749. https://doi.org/10.3390/catal11060749






_Stamatis.png)