Photocatalytic and Antibacterial Potency of Titanium Dioxide Nanoparticles: A Cost-Effective and Environmentally Friendly Media for Treatment of Air and Wastewater
Abstract
1. Introduction
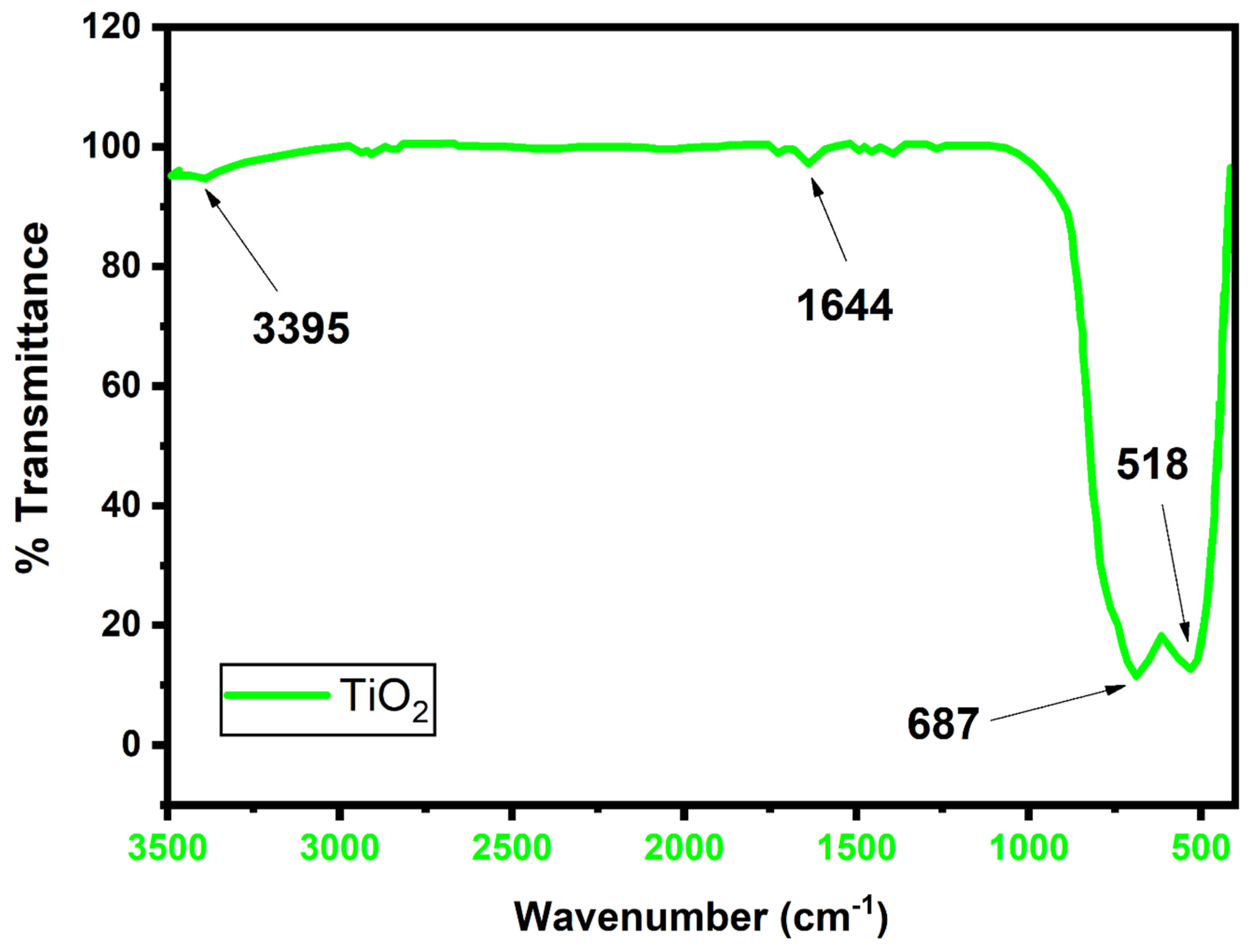
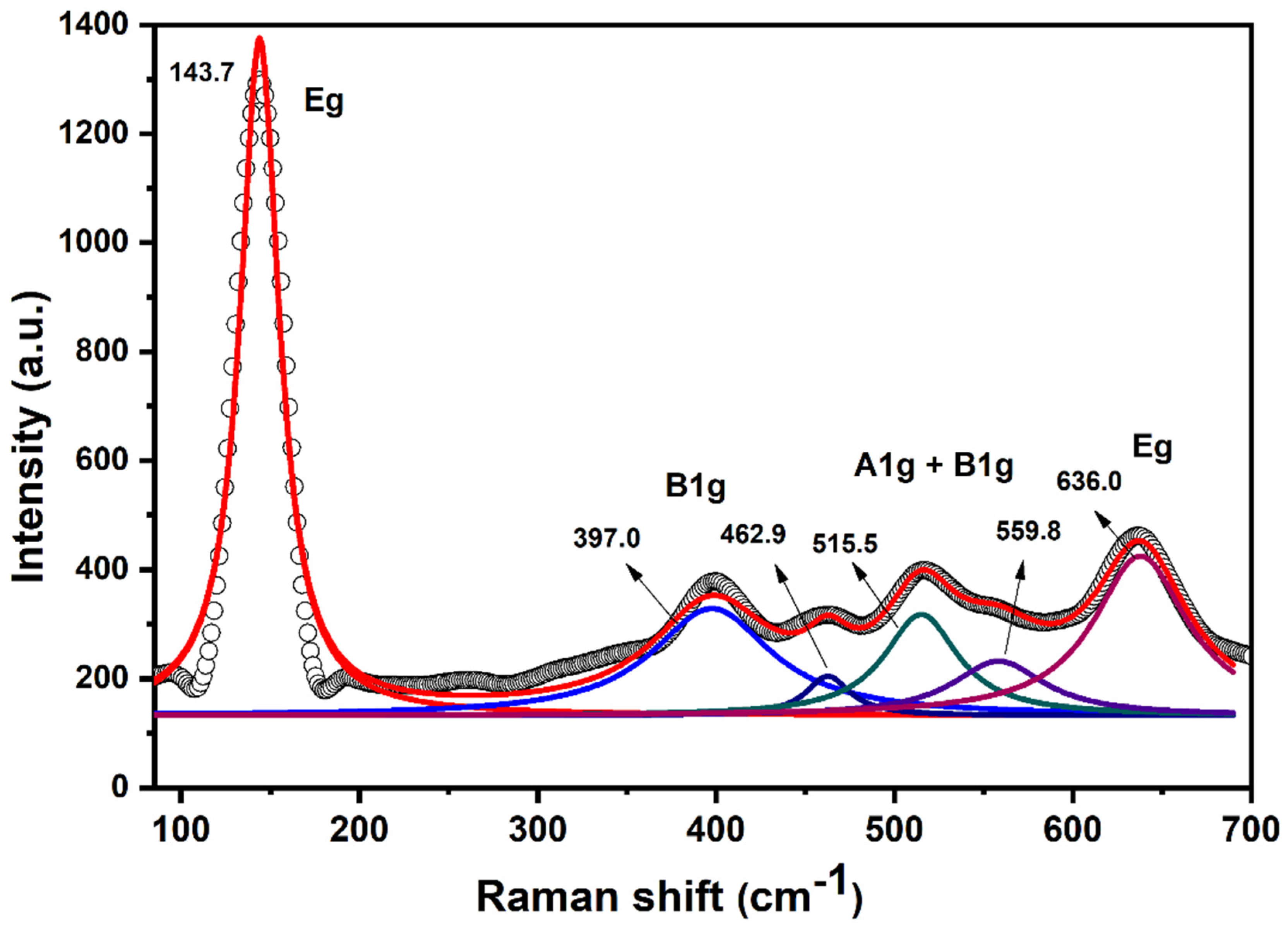
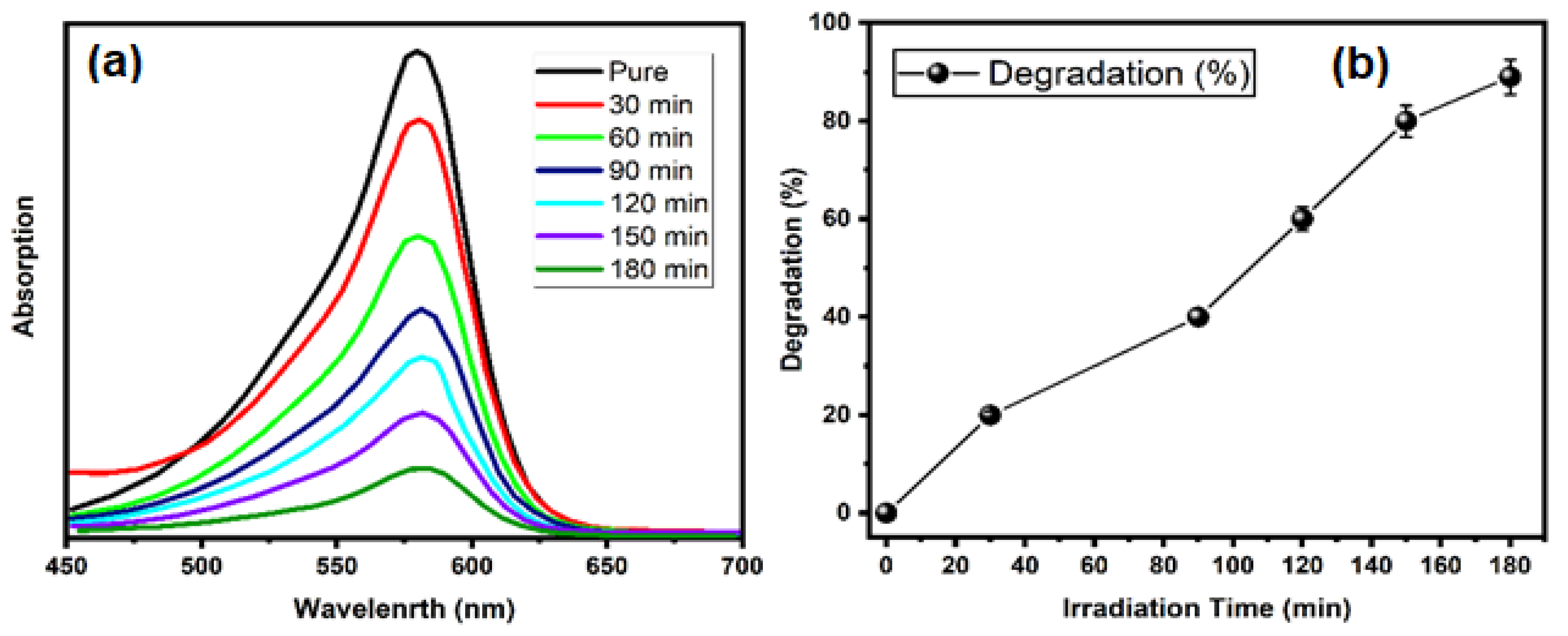
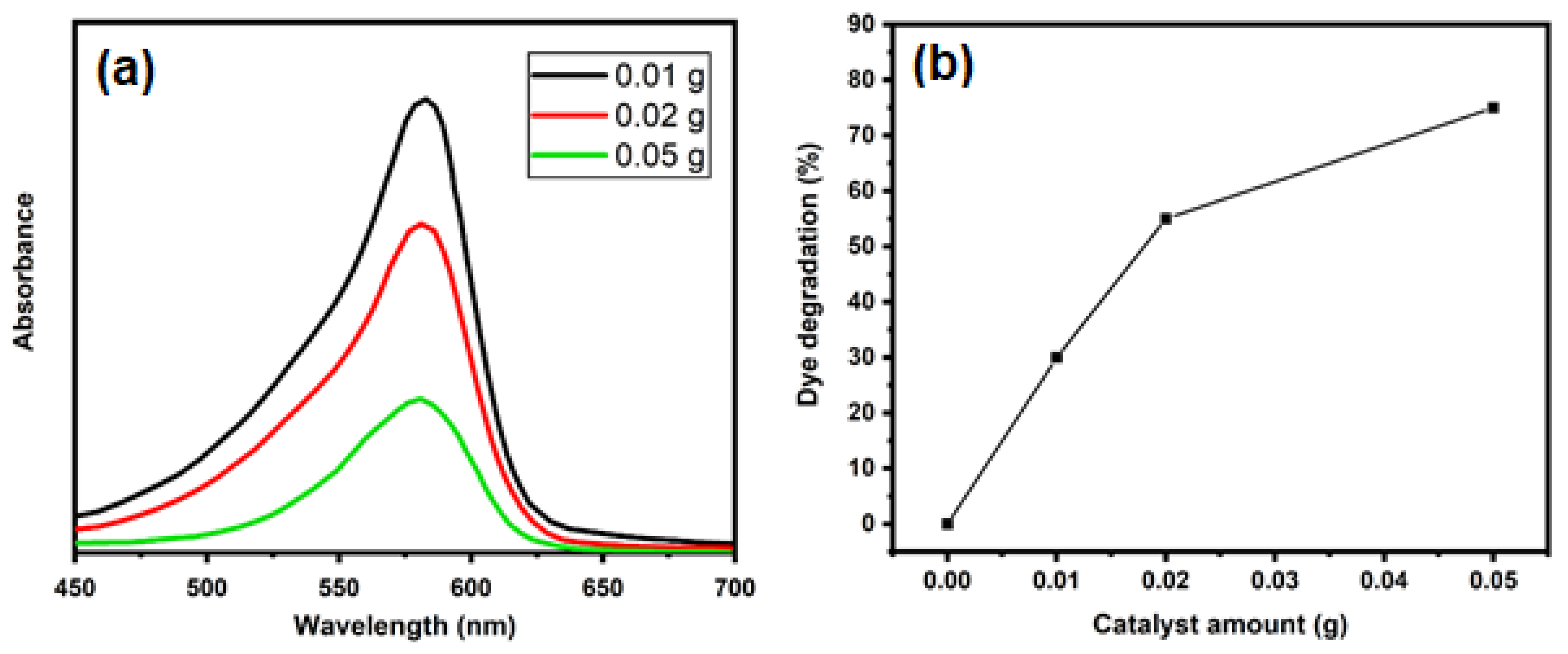
2. Results and Discussion
3. Materials and Methods
3.1. Sample Preparation
3.2. Characterization
3.3. Antibacterial Activity of Synthesized TiO2-NPs
3.4. Catalytic Activity of TiO2-NPs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandey, R.K.; Prajapati, V.K. Molecular and immunological toxic effects of nanoparticles. Int. J. Biol. Macromol. 2018, 107, 1278–1293. [Google Scholar] [CrossRef]
- Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Titanium dioxide (anatase and rutile): Surface chemistry, liquid–solid interface chemistry, and scientific synthesis of supported catalysts. Chem. Rev. 2014, 114, 9754–9823. [Google Scholar] [CrossRef]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; Von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Swidwinska-Gajewska, A.M.; Czerczak, S. Nanoczastki ditlenku tytanu-dziatanie biologiczne/titanium dioxide nanoparticles-biological effects. Med. Pr. 2014, 65, 651. [Google Scholar]
- Chen, X.X.; Cheng, B.; Yang, Y.X.; Cao, A.; Liu, J.H.; Du, L.J.; Liu, Y.; Zhao, Y.; Wang, H. Characterization and preliminary toxicity assay of nano-titanium dioxide additive in sugar-coated chewing gum. Small 2013, 9, 1765–1774. [Google Scholar] [CrossRef]
- Winkler, H.C.; Notter, T.; Meyer, U.; Naegeli, H. Critical review of the safety assessment of titanium dioxide additives in food. J. Nanobiotechnol. 2018, 16, 51. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Khandaker, M.U.; Amin, Y.M.; Khan, G.; Ramay, S.M.; Mahmood, A.; Amin, M.; Muhammad, N. Catalytic growth of vertically aligned neutron sensitive 10 Boron nitride nanotubes. J. Nanopart. Res. 2016, 18, 25. [Google Scholar] [CrossRef]
- Irshad, M.A.; Nawaz, R.; ur Rehman, M.Z.; Imran, M.; Ahmad, M.J.; Ahmad, S.; Inaam, A.; Razzaq, A.; Rizwan, M.; Ali, S. Synthesis and characterization of titanium dioxide nanoparticles by chemical and green methods and their antifungal activities against wheat rust. Chemosphere 2020, 258, 127352. [Google Scholar] [CrossRef]
- Khalid, A.; Ahmad, P.; Alharthi, A.I.; Muhammad, S.; Khandaker, M.U.; Iqbal Faruque, M.R.; Din, I.U.; Alotaibi, M.A. Unmodified Titanium Dioxide Nanoparticles as a Potential Contrast Agent in Photon Emission Computed Tomography. Crystals 2021, 11, 171. [Google Scholar] [CrossRef]
- Ahmad, P.; Khandaker, M.U.; Amin, Y.M.; Muhammad, N.; Khan, G.; Khan, A.S.; Numan, A.; Rehman, M.A.; Ahmed, S.M.; Khan, A. Synthesis of hexagonal boron nitride fibers within two hour annealing at 500 C and two hour growth duration at 1000 C. Ceram. Int. 2016, 42, 14661–14666. [Google Scholar] [CrossRef]
- Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Cogliano, V.; WHO International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of Carbon Black, Titanium Dioxide and Talc; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Khalid, A.; Ahmad, P.; Alharthi, A.I.; Muhammad, S.; Khandaker, M.U.; Rehman, M.; Faruque, M.R.I.; Din, I.U.; Alotaibi, M.A.; Alzimami, K. Structural, Optical and Antibacterial Efficacy of Pure and Zinc-Doped Copper Oxide against Pathogenic Bacteria. Nanomaterials 2021, 11, 451. [Google Scholar] [CrossRef]
- Khalid, A.; Ahmad, P.; Alharthi, A.I.; Muhammad, S.; Khandaker, M.U.; Iqbal Faruque, M.R.; Din, I.U.; Alotaibi, M.A.; Khan, A. Synergistic effects of Cu-doped ZnO nanoantibiotic against Gram-positive bacterial strains. PLoS ONE 2021, 16, e0251082. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Khandaker, M.U.; Muhammad, N.; Khan, G.; Rehman, F.; Khan, A.S.; Ullah, Z.; Khan, A.; Ali, H.; Ahmed, S.M. Fabrication of hexagonal boron nitride quantum dots via a facile bottom-up technique. Ceram. Int. 2019, 45, 22765–22768. [Google Scholar] [CrossRef]
- Khalid, A.; Ahmad, P.; Alharthi, A.I.; Mohammad, S.; Khandaker, M.U.; Faruque, M.R.I.; Din, U.; Alotaibi, M.A. A practical method for incorporation of Fe (III) in Titania matrix for photocatalytic applications. Mater. Res. Express 2021, 8, 045006. [Google Scholar] [CrossRef]
- García-Rodríguez, A.; Vila, L.; Cortés, C.; Hernández, A.; Marcos, R. Effects of differently shaped TiO2 NPs (nanospheres, nanorods and nanowires) on the in vitro model (Caco-2/HT29) of the intestinal barrier. Part. Fibre Toxicol. 2018, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Gosteva, I.; Morgalev, Y.; Morgaleva, T.; Morgalev, S. Effect of Al2O3 and TiO2 nanoparticles on aquatic organisms. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Tambov, Russia, 21–22 May 2015; IOP Publishing: Bristol, UK, 2015; p. 012007. [Google Scholar]
- Ahmed, S.; Kazi, S.; Khan, G.; Dahari, M.; Zubir, M.; Ahmad, P.; Montazer, E. Experimental investigation on momentum and drag reduction of Malaysian crop suspensions in closed conduit flow. In Proceedings of the IOP Conference Series: Materials Science and Engineering, University of Malaya, Kuala Lumpur, Malaysia, 5–6 April 2017; IOP Publishing: Bristol, UK, 2017; p. 012065. [Google Scholar]
- Wang, Y.; Ding, L.; Yao, C.; Li, C.; Xing, X.; Huang, Y.; Gu, T.; Wu, M. Toxic effects of metal oxide nanoparticles and their underlying mechanisms. Sci. China Mater. 2017, 60, 93–108. [Google Scholar] [CrossRef]
- Kandeil, M.A.; Mohammed, E.T.; Hashem, K.S.; Aleya, L.; Abdel-Daim, M.M. Moringa seed extract alleviates titanium oxide nanoparticles (TiO2-NPs)-induced cerebral oxidative damage, and increases cerebral mitochondrial viability. Environ. Sci. Pollut. Res. 2019, 27, 19169–19184. [Google Scholar]
- Li, J.; Ma, W.; Chen, C.; Zhao, J.; Zhu, H.; Gao, X. Photodegradation of dye pollutants on one-dimensional TiO2 nanoparticles under UV and visible irradiation. J. Mol. Catal. A Chem. 2007, 261, 131–138. [Google Scholar] [CrossRef]
- Yin, M.; Li, Z.; Kou, J.; Zou, Z. Mechanism investigation of visible light-induced degradation in a heterogeneous TiO2/Eosin Y/Rhodamine B system. Environ. Sci. Technol. 2009, 43, 8361–8366. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, S.; Zhou, X.; Lin, C.; Wang, Y.; Zhang, W. Enhanced photocatalytic activity for degradation of methyl orange over silica-titania. J. Nanomater. 2011. [Google Scholar] [CrossRef]
- Thapa, R.; Maiti, S.; Rana, T.; Maiti, U.; Chattopadhyay, K. Anatase TiO2 nanoparticles synthesis via simple hydrothermal route: Degradation of Orange II, Methyl Orange and Rhodamine B. J. Mol. Catal. A Chem. 2012, 363, 223–229. [Google Scholar] [CrossRef]
- Zanjanchi, M.A.; Sajjadi, H.; Arvand, M.; Mohammad-Khah, A.; Ghalami-Choobar, B. Modification of MCM-41 with Anionic Surfactant: A Convenient Design for Efficient Removal of Cationic Dyes from Wastewater. CLEAN–Soil Air Water 2011, 39, 1007–1013. [Google Scholar] [CrossRef]
- Pang, Y.L.; Abdullah, A.Z. Effect of carbon and nitrogen co-doping on characteristics and sonocatalytic activity of TiO2 nanotubes catalyst for degradation of Rhodamine B in water. Chem. Eng. J. 2013, 214, 129–138. [Google Scholar] [CrossRef]
- Shi, X.; Yang, X.; Wang, S.; Wang, S.; Zhang, Q.; Wang, Y. Photocatalytic degradation of rhodamine B Dye with high purity anatase nano-TiO2 synthesized by a hydrothermal method. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2011, 26, 600–605. [Google Scholar] [CrossRef]
- Li, W.; Li, D.; Meng, S.; Chen, W.; Fu, X.; Shao, Y. Novel Approach To Enhance Photosensitized Degradation of Rhodamine B under Visible Light Irradiation by the ZnxCd1-xS/TiO2 Nanocomposites. Environ. Sci. Technol. 2011, 45, 2987–2993. [Google Scholar] [CrossRef] [PubMed]
- Melián, E.P.; Díaz, O.G.; Rodríguez, J.D.; Colón, G.; Navío, J.; Peña, J.P. Effect of hydrothermal treatment on structural and photocatalytic properties of TiO2 synthesized by sol–gel method. Appl. Catal. A Gen. 2012, 411, 153–159. [Google Scholar] [CrossRef]
- Juang, L.-C.; Semblante, G.U.; You, S.-J.; Hong, S.-H. Degradation of 2-chlorophenol using carbon nanotube/titanium oxide composite prepared by hydrothermal method. J. Taiwan Inst. Chem. Eng. 2013, 44, 432–437. [Google Scholar] [CrossRef]
- Grabowska, E.; Reszczyńska, J.; Zaleska, A. RETRACTED: Mechanism of phenol photodegradation in the presence of pure and modified-TiO2: A review. Water Res. 2012, 46, 5453–5471. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, P.; Stephan, D. Photodegradation of rhodamine B in aqueous solution via SiO2@ TiO2 nano-spheres. J. Photochem. Photobiol. A Chem. 2007, 185, 19–25. [Google Scholar] [CrossRef]
- Dong, W.; Lee, C.W.; Lu, X.; Sun, Y.; Hua, W.; Zhuang, G.; Zhang, S.; Chen, J.; Hou, H.; Zhao, D. Synchronous role of coupled adsorption and photocatalytic oxidation on ordered mesoporous anatase TiO2–SiO2 nanocomposites generating excellent degradation activity of RhB dye. Appl. Catal. B Environ. 2010, 95, 197–207. [Google Scholar] [CrossRef]
- Zhang, X.; Huo, K.; Wang, H.; Zhang, W.; Chu, P.K. Influence of structure parameters and crystalline phase on the photocatalytic activity of TiO2 nanotube arrays. J. Nanosci. Nanotechnol. 2011, 11, 11200–11205. [Google Scholar] [CrossRef]
- Dlamini, L.; Krause, R.; Kulkarni, G.; Durbach, S. Photodegradation of bromophenol blue with fluorinated TiO2 composite. Appl. Water Sci. 2011, 1, 19–24. [Google Scholar] [CrossRef]
- Su, Y.; Yang, Y.; Zhang, H.; Xie, Y.; Wu, Z.; Jiang, Y.; Fukata, N.; Bando, Y.; Wang, Z.L. Enhanced photodegradation of methyl orange with TiO2 nanoparticles using a triboelectric nanogenerator. Nanotechnology 2013, 24, 295401. [Google Scholar] [CrossRef]
- Sahu, K.; Murty, V. Novel sol gel method of synthesis of pure and Aluminium doped TiO2 nano particles useful for dye sensitized solar cell applications. Int. Res. J. Eng. Technol. 2015, 2, 567–571. [Google Scholar]
- Muilenberg, G. Handbook of X-ray photoelectron spectroscopy. Perkin-Elmer Corp. 1979, 64, 1–261. [Google Scholar]
- Göpel, W.; Anderson, J.A.; Frankel, D.; Jaehnig, M.; Phillips, K.; Schäfer, J.A.; Rocker, G. Surface defects of TiO2(110): A combined XPS, XAES AND ELS study. Surf. Sci 1984, 139, 333–346. [Google Scholar] [CrossRef]
- Warren, D.S.; Shapira, Y.; Kisch, H.; McQuillan, A.J. Apparent semiconductor type reversal in anatase TiO2 nanocrystalline films. J. Phys. Chem. C 2007, 111, 14286–14289. [Google Scholar] [CrossRef][Green Version]
- Wang, L.-Q.; Baer, D.R.; Engelhard, M.H.; Shultz, A.N. The adsorption of liquid and vapor water on TiO2 (110) surfaces: The role of defects. Surf. Sci. 1995, 344, 237–250. [Google Scholar] [CrossRef]
- Patthey, L.; Bullock, E.; Steinemann, S. Clean and hydroxylated rutile TiO2 (110) surfaces studied by photoelectron spectroscopy. Surf. Sci. 1996, 504, 352–354. [Google Scholar]
- Choudhury, B.; Dey, M.; Choudhury, A. Defect generation, d-d transition, and band gap reduction in Cu-doped TiO2 nanoparticles. Int. Nano Lett. 2013, 3, 25. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Room temperature ferromagnetism in defective TiO2 nanoparticles: Role of surface and grain boundary oxygen vacancies. J. Appl. Phys. 2013, 114, 203906. [Google Scholar] [CrossRef]
- Frank, O.; Zukalova, M.; Laskova, B.; Kürti, J.; Koltai, J.; Kavan, L. Raman spectra of titanium dioxide (anatase, rutile) with identified oxygen isotopes (16, 17, 18). Phys. Chem. Chem. Phys. 2012, 14, 14567–14572. [Google Scholar] [CrossRef]
- Li, F.; Gu, Y. Improvement of performance of dye-sensitized solar cells by doping Er2O3 into TiO2 electrodes. Mater. Sci. Semicond. Process. 2012, 15, 11–14. [Google Scholar] [CrossRef]
- Subhapriya, S.; Gomathipriya, P. Green synthesis of titanium dioxide (TiO2) nanoparticles by Trigonella foenum-graecum extract and its antimicrobial properties. Microb. Pathog. 2018, 116, 215–220. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Bama, K.; Bhavani, M.; Jegatheeswaran, S.; Ambika, S.; Sangili, A.; Nithya, P.; Sumathi, R. Obtaining titanium dioxide nanoparticles with spherical shape and antimicrobial properties using M. citrifolia leaves extract by hydrothermal method. J. Photochem. Photobiol. B Biol. 2017, 171, 117–124. [Google Scholar]
- Ahmad, R.; Mohsin, M.; Ahmad, T.; Sardar, M. Alpha amylase assisted synthesis of TiO2 nanoparticles: Structural characterization and application as antibacterial agents. J. Hazard. Mater. 2015, 283, 171–177. [Google Scholar] [CrossRef]
- Priyanka, K.P.; Sukirtha, T.H.; Balakrishna, K.M.; Varghese, T. Microbicidal activity of TiO2 nanoparticles synthesised by sol–gel method. IET Nanobiotechnol. 2016, 10, 81–86. [Google Scholar] [CrossRef]
- Verdier, T.; Coutand, M.; Bertron, A.; Roques, C. Antibacterial activity of TiO2 photocatalyst alone or in coatings on E. coli: The influence of methodological aspects. Coatings 2014, 4, 670–686. [Google Scholar] [CrossRef]
- Khashan, K.S.; Sulaiman, G.M.; Abdulameer, F.A.; Albukhaty, S.; Ibrahem, M.A.; Al-Muhimeed, T.; AlObaid, A.A. Antibacterial Activity of TiO2 Nanoparticles Prepared by One-Step Laser Ablation in Liquid. Appl. Sci. 2021, 11, 4623. [Google Scholar] [CrossRef]
- Manjunath, K.; Yadav, L.S.R.; Jayalakshmi, T.; Reddy, V.; Rajanaika, H.; Nagaraju, G. Ionic liquid assisted hydrothermal synthesis of TiO2 nanoparticles: Photocatalytic and antibacterial activity. J. Mater. Res. Technol. 2018, 7, 7–13. [Google Scholar] [CrossRef]
- Kumar, P.S.M.; Francis, A.P.; Devasena, T. Biosynthesized and chemically synthesized titania nanoparticles: Comparative analysis of antibacterial activity. J. Environ. Nanotechnol. 2014, 3, 73–81. [Google Scholar]
- Yadav, L.; Manjunath, K.; Kavitha, C.; Nagaraju, G. Investigation of hydrogen generation and antibacterial activity by ionic liquid aided synthesis of TiO2 nanoparticles. J. Sci. Advan. Mat. Dev. 2018, 21, 20–25. [Google Scholar]
- Abdulraheem, G.; Peter Obinna, N.; Kasali Ademola, B.; Kasali Ademola, K. Photocatalytic decolourization and degradation of CI Basic Blue 41 using TiO2 nanoparticles. J. Environ. Prot. 2012, 3. [Google Scholar] [CrossRef]
- Muneer, M.; Philip, R.; Das, S. Photocatalytic degradation of waste water pollutants. Titanium dioxidemediated oxidation of a textile dye, Acid Blue 40. Res. Chem. Intermed. 1997, 23, 233–246. [Google Scholar] [CrossRef]
- Neppolian, B.; Choi, H.; Sakthivel, S.; Arabindoo, B.; Murugesan, V. Solar/UV-induced photocatalytic degradation of three commercial textile dyes. J. Hazard. Mater. 2002, 89, 303–317. [Google Scholar] [CrossRef]
- Kansal, S.K.; Kaur, N.; Singh, S. Photocatalytic degradation of two commercial reactive dyes in aqueous phase using nanophotocatalysts. Nanoscale Res. Lett. 2009, 4, 709. [Google Scholar] [CrossRef]
- Saeed, K.; Khan, I.; Gul, T.; Sadiq, M. Efficient photodegradation of methyl violet dye using TiO2/Pt and TiO2/Pd photocatalysts. Appl. Water Sci. 2017, 7, 3841–3848. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Lakshmi, S.; Renganathan, R.; Fujita, S. Study on TiO2-mediated photocatalytic degradation of methylene blue. J. Photochem. Photobiol. A Chem. 1995, 88, 163–167. [Google Scholar] [CrossRef]
- Pellegrino, F.; Pellutiè, L.; Sordello, F.; Minero, C.; Ortel, E.; Hodoroaba, V.-D.; Maurino, V. Influence of agglomeration and aggregation on the photocatalytic activity of TiO2 nanoparticles. Appl. Catal. B Environ. 2017, 216, 80–87. [Google Scholar] [CrossRef]
- Zhao, Q.-E.; Wen, W.; Xia, Y.; Wu, J.-M. Photocatalytic activity of TiO2 nanorods, nanowires and nanoflowers filled with TiO2 nanoparticles. Thin Solid Film. 2018, 648, 103–107. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Alamdari, M.E.; Modirshahla, N. Investigation of the effect of heat treatment process on characteristics and photocatalytic activity of TiO2-UV100 nanoparticles. Environ. Prot. Eng. 2013, 39, 33–46. [Google Scholar]






| Bacteria | TiO2 | ||||
|---|---|---|---|---|---|
| 100 µg/mL | 500 µg/mL | 1 mg/mL | |||
| Gram negative | P. aeruginosa | Inhibition zone (mm) | 10 ± 0.19 | 11 ± 0.22 | 15 ± 0.27 |
| E. coli | 12 ± 0.11 | 15 ± 0.13 | 20 ± 0.21 | ||
| K. pneumoniae | 15 ± 0.16 | 20 ± 0.18 | 23 ± 0.19 | ||
| Gram positive | S. aureus | 12 ± 0.14 | 16 ± 0.12 | 19 ± 0.14 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razzaq, Z.; Khalid, A.; Ahmad, P.; Farooq, M.; Khandaker, M.U.; Sulieman, A.; Rehman, I.U.; Shakeel, S.; Khan, A. Photocatalytic and Antibacterial Potency of Titanium Dioxide Nanoparticles: A Cost-Effective and Environmentally Friendly Media for Treatment of Air and Wastewater. Catalysts 2021, 11, 709. https://doi.org/10.3390/catal11060709
Razzaq Z, Khalid A, Ahmad P, Farooq M, Khandaker MU, Sulieman A, Rehman IU, Shakeel S, Khan A. Photocatalytic and Antibacterial Potency of Titanium Dioxide Nanoparticles: A Cost-Effective and Environmentally Friendly Media for Treatment of Air and Wastewater. Catalysts. 2021; 11(6):709. https://doi.org/10.3390/catal11060709
Chicago/Turabian StyleRazzaq, Zohaib, Awais Khalid, Pervaiz Ahmad, Muhammad Farooq, Mayeen Uddin Khandaker, Abdelmoneim Sulieman, Ibad Ur Rehman, Sohail Shakeel, and Ajmal Khan. 2021. "Photocatalytic and Antibacterial Potency of Titanium Dioxide Nanoparticles: A Cost-Effective and Environmentally Friendly Media for Treatment of Air and Wastewater" Catalysts 11, no. 6: 709. https://doi.org/10.3390/catal11060709
APA StyleRazzaq, Z., Khalid, A., Ahmad, P., Farooq, M., Khandaker, M. U., Sulieman, A., Rehman, I. U., Shakeel, S., & Khan, A. (2021). Photocatalytic and Antibacterial Potency of Titanium Dioxide Nanoparticles: A Cost-Effective and Environmentally Friendly Media for Treatment of Air and Wastewater. Catalysts, 11(6), 709. https://doi.org/10.3390/catal11060709










