Carbon-Encapsulated Iron Nanoparticles as a Magnetic Modifier of Bioanode and Biocathode in a Biofuel Cell and Biobattery
Abstract
1. Introduction
2. Results and Discussion
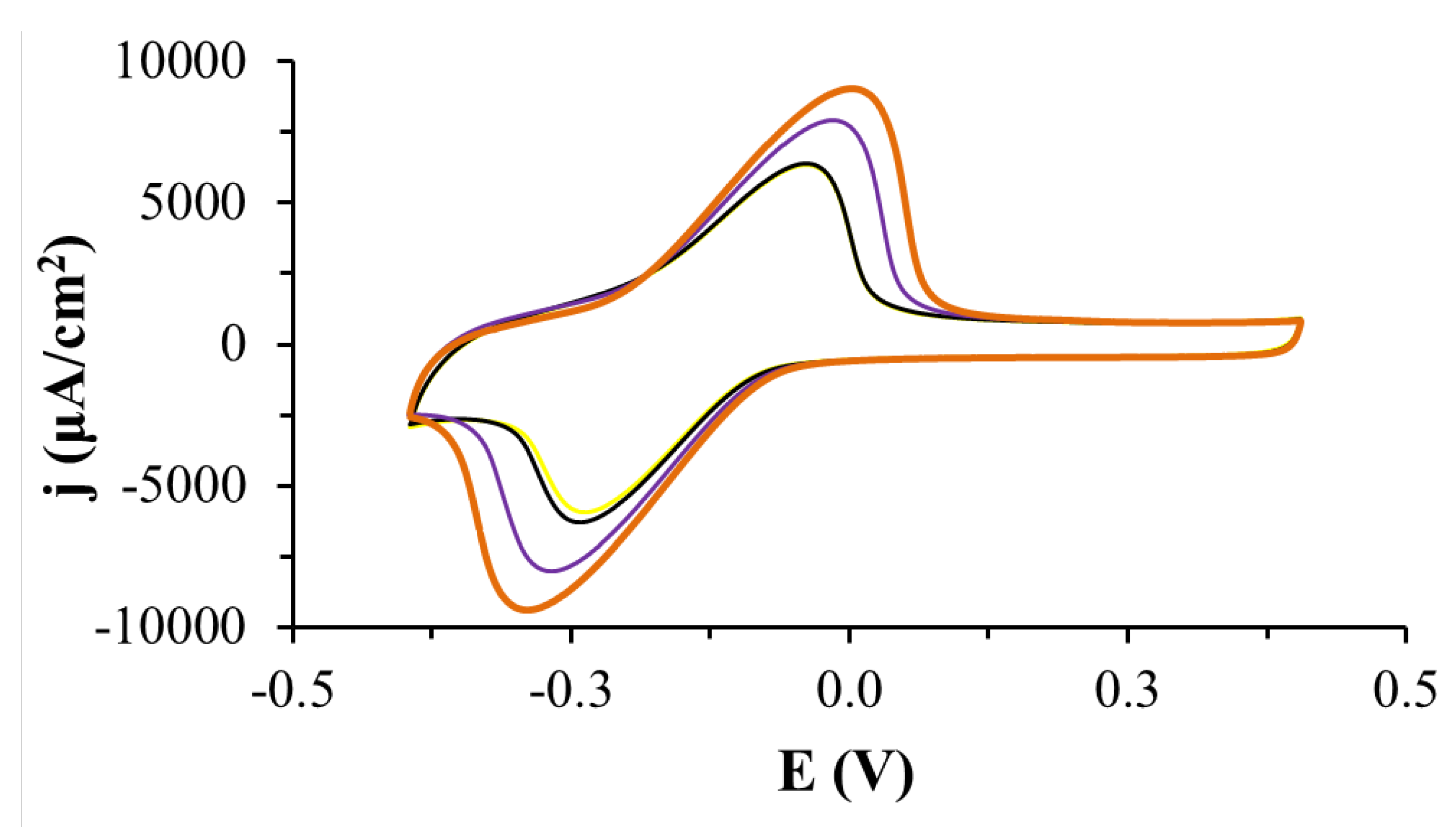
2.1. Electrode Modified with CEINs and Laccase
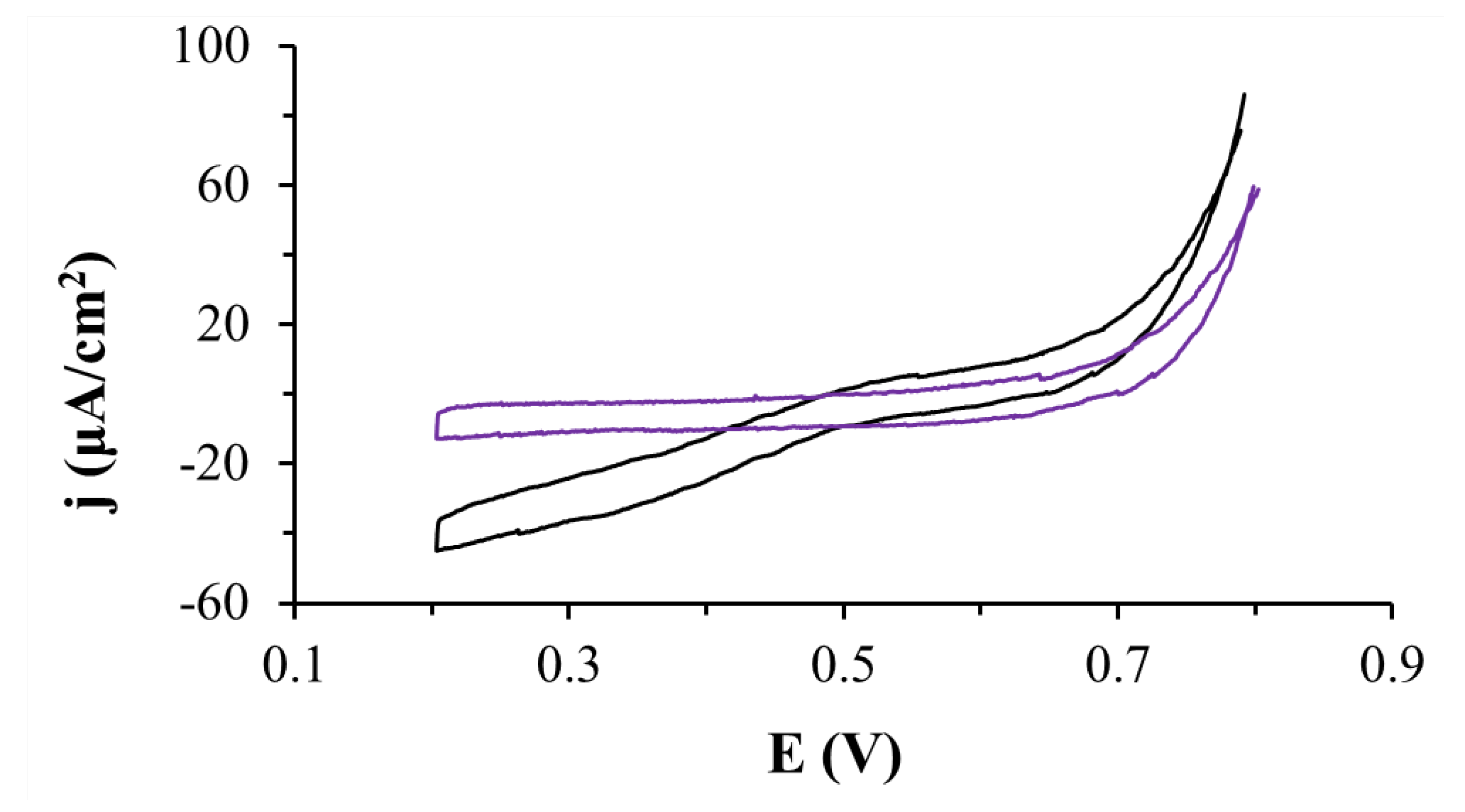
2.2. Electrode Modified with CEINs and Fructose Dehydrogenase
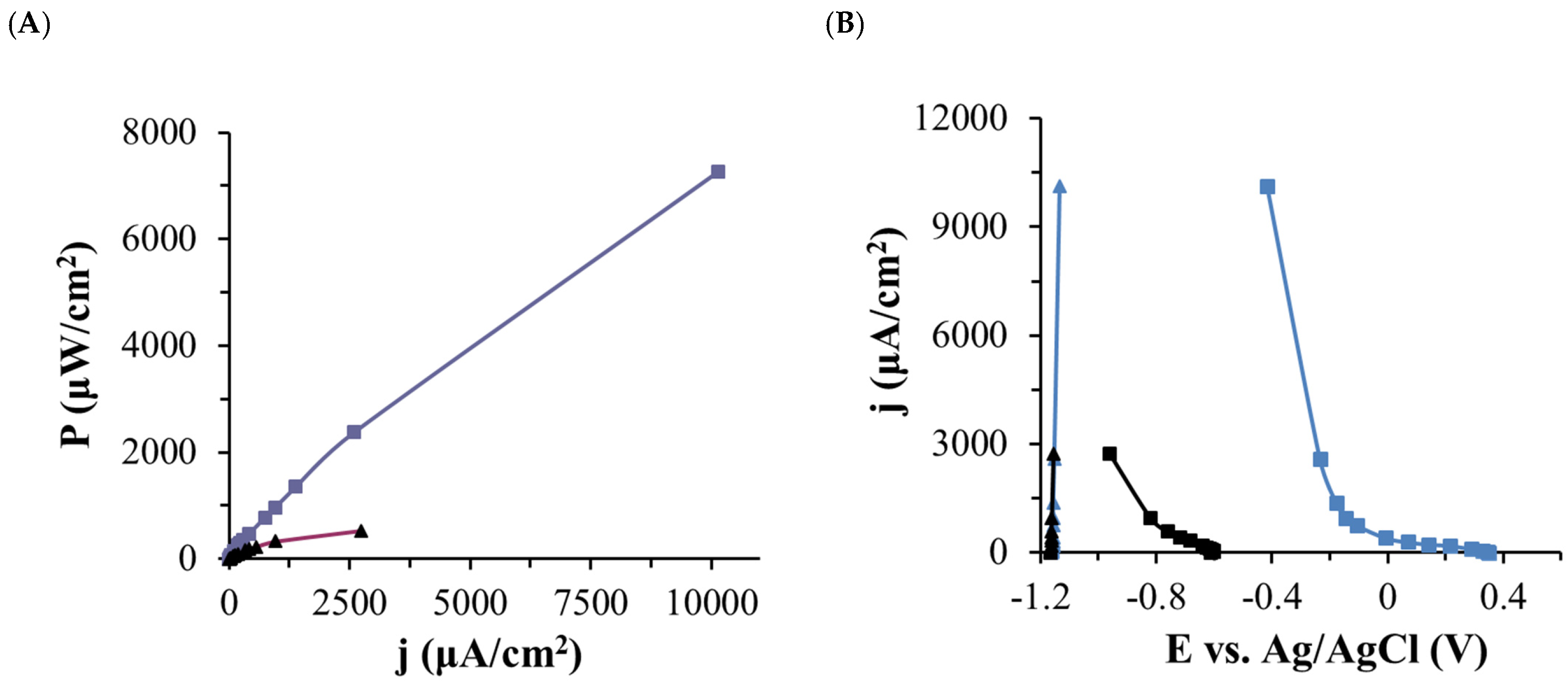
2.3. Biobattery Studies
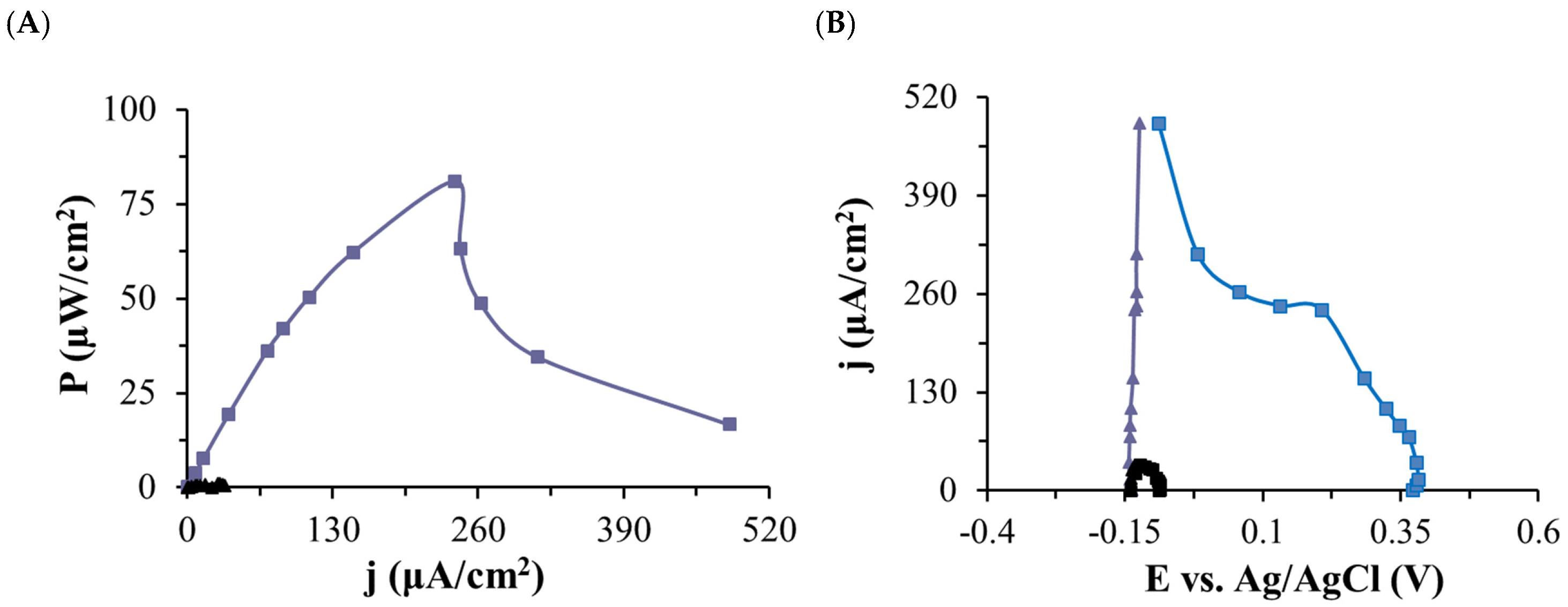
2.4. Biofuel Cell Studies
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Electrochemical Instrumentation and Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Falk, M.; Blum, Z.; Shleev, S. Direct electron transfer based enzymatic fuel cells. Electrochim. Acta 2012, 82, 191–202. [Google Scholar] [CrossRef]
- Luz, R.A.S.; Pereira, A.R.; de Souza, J.C.P.; Sales, F.C.P.F.; Crespilho, F.N. Enzyme Biofuel Cells: Thermodynamics, Kinetics and Challenges in Applicability. ChemElectroChem 2014, 1, 1751–1777. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Zebda, A.; Alcaraz, J.-P.P.; Vadgama, P.; Shleev, S.; Minteer, S.D.; Boucher, F.; Cinquin, P.; Martin, D.K. Challenges for successful implantation of biofuel cells. Bioelectrochemistry 2018, 124, 57–72. [Google Scholar] [CrossRef]
- Kamitaka, Y.; Tsujimura, S.; Setoyama, N.; Kajino, T.; Kano, K. Fructose/dioxygen biofuel cell based on direct electron transfer-type bioelectrocatalysis. Phys. Chem. Chem. Phys. 2007, 9, 1793–1801. [Google Scholar] [CrossRef]
- Ivanov, I.; Vidaković-Koch, T.; Sundmacher, K. Recent advances in enzymatic fuel cells: Experiments and modeling. Energies 2010, 3, 803. [Google Scholar] [CrossRef]
- Yan, X.; Tang, J.; Tanner, D.; Ulstrup, J.; Xiao, X. Direct electrochemical enzyme electron transfer on electrodes modified by self-assembled molecular monolayers. Catalysts 2020, 10, 1458. [Google Scholar] [CrossRef]
- Zhou, M. Recent Progress on the Development of Biofuel Cells for Self-Powered Electrochemical Biosensing and Logic Biosensing: A Review. Electroanalysis 2015, 27, 1786–1810. [Google Scholar] [CrossRef]
- Zelechowska, K.; Stolarczyk, K.; Łyp, D.; Rogalski, J.; Roberts, K.P.; Bilewicz, R.; Biernat, J.F. Aryl and N-arylamide carbon nanotubes for electrical coupling of laccase to electrodes in biofuel cells and biobatteries. Biocybern. Biomed. Eng. 2013, 33, 235–245. [Google Scholar] [CrossRef]
- Majdecka, D.; Draminska, S.; Stolarczyk, K.; Kizling, M.; Krysinski, P.; Golimowski, J.; Biernat, J.F.; Bilewicz, R. Sandwich biobattery with enzymatic cathode and Zinc anode integrated with sensor. J. Electrochem. Soc. 2015, 162. [Google Scholar] [CrossRef]
- Kizling, M.; Biedul, P.; Zabost, D.; Stolarczyk, K.; Bilewicz, R. Application of Hydroxyethyl Methacrylate and Ethylene Glycol Methacrylate Phosphate Copolymer as Hydrogel Electrolyte in Enzymatic Fuel Cell. Electroanalysis 2016, 28. [Google Scholar] [CrossRef]
- Kizling, M.; Dzwonek, M.; Olszewski, B.; Bącal, P.; Tymecki, Ł.; Więckowska, A.; Stolarczyk, K.; Bilewicz, R. Reticulated vitreous carbon as a scaffold for enzymatic fuel cell designing. Biosens. Bioelectron. 2017, 95, 1–7. [Google Scholar] [CrossRef]
- Olszewski, B.; Stolarczyk, K. Laccase-catalyzed reduction of oxygen at electrodes modified by carbon nanotubes with adsorbed promazine or acetosyringone. Catalysts 2018, 8, 414. [Google Scholar] [CrossRef]
- Nowicka, A.M.; Kowalczyk, A.; Donten, M.L.; Donten, M.; Bystrzejewski, M.; Stojek, Z. Carbon-encapsulated iron nanoparticles as ferromagnetic matrix for oxygen reduction in absence and presence of immobilized laccase. Electrochim. Acta 2014, 126, 115–121. [Google Scholar] [CrossRef]
- Matysiak, E.; Wagner, B.; Bystrzejewski, M.; Grudzinski, I.P.; Nowicka, A.M. Direct voltammetric detection of ceruloplasmin in blood in presence of other paramagnetic species. Sens. Actuators B Chem. 2015, 221. [Google Scholar] [CrossRef]
- Matysiak, E.; Donten, M.; Kowalczyk, A.; Bystrzejewski, M.; Grudzinski, I.P.; Nowicka, A.M. A novel type of electrochemical sensor based on ferromagnetic carbon-encapsulated iron nanoparticles for direct determination of hemoglobin in blood samples. Biosens. Bioelectron. 2015, 64, 554–559. [Google Scholar] [CrossRef] [PubMed]
- del Barrio, M.; Moros, M.; Puertas, S.; de la Fuente, J.M.; Grazú, V.; Cebolla, V.; de Marcos, S.; Galbán, J. Glucose oxidase immobilized on magnetic nanoparticles: Nanobiosensors for fluorescent glucose monitoring. Microchim. Acta 2017, 184, 1325–1333. [Google Scholar] [CrossRef]
- Kizling, M.; Rekorajska, A.; Krysinski, P.; Bilewicz, R. Magnetic-field-induced orientation of fructose dehydrogenase on iron oxide nanoparticles for enhanced direct electron transfer. Electrochem. Commun. 2018, 93, 66–70. [Google Scholar] [CrossRef]
- Wettstein, C.; Kano, K.; Schäfer, D.; Wollenberger, U.; Lisdat, F. Interaction of Flavin-Dependent Fructose Dehydrogenase with Cytochrome c as Basis for the Construction of Biomacromolecular Architectures on Electrodes. Anal. Chem. 2016, 88, 6382–6389. [Google Scholar] [CrossRef]
- Tominaga, M.; Shirakihara, C.; Taniguchi, I. Direct heterogeneous electron transfer reactions and molecular orientation of fructose dehydrogenase adsorbed onto pyrolytic graphite electrodes. J. Electroanal. Chem. 2007, 610, 1–8. [Google Scholar] [CrossRef]
- Kawai, S.; Yakushi, T.; Matsushita, K.; Kitazumi, Y.; Shirai, O.; Kano, K. Electrochemistry Communications The electron transfer pathway in direct electrochemical communication of fructose dehydrogenase with electrodes. Electrochem. Commun. 2014, 38, 28–31. [Google Scholar] [CrossRef]
- Kizling, M.; Bilewicz, R. Fructose Dehydrogenase Electron Transfer Pathway in Bioelectrocatalytic Reactions. ChemElectroChem 2018, 5, 166–174. [Google Scholar] [CrossRef]
- Adachi, T.; Kaida, Y.; Kitazumi, Y.; Shirai, O.; Kano, K. Bioelectrocatalytic performance of D-fructose dehydrogenase. Bioelectrochemistry 2019, 129, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial sources, production, purification, and potential biotechnological applications. Enzyme Res. 2011, 2011. [Google Scholar] [CrossRef]
- Giroud, F.; Milton, R.D.; Tan, B.-X.; Minteer, S. Simplifying Enzymatic Biofuel Cells: Immobilized Naphthoquinone as a Biocathodic Orientational Moiety and Bioanodic Electron Mediator. ACS Catal. 2015, 5, 1240–1244. [Google Scholar] [CrossRef]
- Milton, R.D.; Hickey, D.P.; Abdellaoui, S.; Lim, K.; Wu, F.; Tan, B.; Minteer, S.D. Rational design of quinones for high power density biofuel cells. Chem. Sci. 2015, 6, 4867–4875. [Google Scholar] [CrossRef]
- Hou, C.; Lang, Q.; Liu, A. Electrochimica Acta Tailoring 1, 4-naphthoquinone with electron-withdrawing group: Toward developing redox polymer and FAD-GDH based hydrogel bioanode for ef fi cient electrocatalytic glucose oxidation. Electrochim. Acta 2016, 211, 663–670. [Google Scholar] [CrossRef]
- Kobayashi, S.; Higashimura, H. Oxidative polymerization of phenols revisited. Prog. Polym. Sci. 2003, 28, 1015–1048. [Google Scholar] [CrossRef]
- Bao, L.; Xiong, R.; Wei, G. Electrochemical polymerization of phenol on 304 stainless steel anodes and subsequent coating structure analysis. Electrochim. Acta 2010, 55, 4030–4038. [Google Scholar] [CrossRef]
- Stolarczyk, K.; Kizling, M.; Majdecka, D.; Zelechowska, K.; Biernat, J.F.; Rogalski, J.; Bilewicz, R. Biobatteries and biofuel cells with biphenylated carbon nanotubes. J. Power Sources 2014, 249, 263–269. [Google Scholar] [CrossRef]
- Bystrzejewski, M.; Łabedź, O.; Kaszuwara, W.; Huczko, A.; Lange, H. Controlling the diameter and magnetic properties of carbon-encapsulated iron nanoparticles produced by carbon arc discharge. Powder Technol. 2013, 246, 7–15. [Google Scholar] [CrossRef]
- Rola, B.; Karaśkiewicz, M.; Majdecka, D.; Mazur, I.; Bilewicz, R.; Rogalski, J.; Ohga, S. Scale up of cerrena unicolor lacease production. J. Fac. Agric. Kyushu Univ. 2013, 58, 231–238. [Google Scholar] [CrossRef]


| Cell | Biobattery | Biofuel Cell | ||
|---|---|---|---|---|
| A: Zn C: CEINs Lc Nf | A: CEINs NQ FDH Nf C: CEINs Lc Nf | |||
| Electrolyte | oxygenated McIlvaine buffer pH 6.0 | deoxygenated McIlvaine buffer pH 6.0 | oxygenated McIlvaine buffer pH 6.0, 100 mM fructose | deoxygenated McIlvaine buffer pH 6.0, 0 mM fructose |
| P (µW/cm2) | 7147.60 ± 160.68 | 524.97 ± 8.09 | 78.37 ± 3.83 | 0.96 ± 0.13 |
| j (µW/cm2) | 10,054.46 ± 113.02 | 2724.90 ± 21.01 | 235.40 ± 5.75 | 26.06 ± 1.75 |
| E (V) | 0.71 ± 0.01 | 0.19 ± 0.01 | 0.33 ± 0.01 | 0.04 ± 0.01 |
| R (kΩ) | 1 | 1 | 20 | 20 |
| OCV (V) | 1.51 ± 0.01 | 0.55 ± 0.01 | 0.49 ± 0.03 | 0.05 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chomicz, R.; Bystrzejewski, M.; Stolarczyk, K. Carbon-Encapsulated Iron Nanoparticles as a Magnetic Modifier of Bioanode and Biocathode in a Biofuel Cell and Biobattery. Catalysts 2021, 11, 705. https://doi.org/10.3390/catal11060705
Chomicz R, Bystrzejewski M, Stolarczyk K. Carbon-Encapsulated Iron Nanoparticles as a Magnetic Modifier of Bioanode and Biocathode in a Biofuel Cell and Biobattery. Catalysts. 2021; 11(6):705. https://doi.org/10.3390/catal11060705
Chicago/Turabian StyleChomicz, Roman, Michał Bystrzejewski, and Krzysztof Stolarczyk. 2021. "Carbon-Encapsulated Iron Nanoparticles as a Magnetic Modifier of Bioanode and Biocathode in a Biofuel Cell and Biobattery" Catalysts 11, no. 6: 705. https://doi.org/10.3390/catal11060705
APA StyleChomicz, R., Bystrzejewski, M., & Stolarczyk, K. (2021). Carbon-Encapsulated Iron Nanoparticles as a Magnetic Modifier of Bioanode and Biocathode in a Biofuel Cell and Biobattery. Catalysts, 11(6), 705. https://doi.org/10.3390/catal11060705






