2.1. Stability of Alkali-Activated Materials
Table 1 lists the conductivity values of aqueous solutions after 4 h at 150 °C under an N
2 atmosphere of 2 MPa and an AAM concentration of 4 g/dm
3.
The conductivity values of aqueous solutions after 4 h of experiments were 200–300 µS/cm; according to these values, the alkali activation of BFS stabilized the material. With the increase in the amount of NaOH in the sample, the conductivity of aqueous solutions decreased slightly. In addition, the curing temperature affected the conductivity, i.e., samples that were first cured at 60 °C for 24 h exhibited lower conductivity than those cured at room temperature.
Table 2 lists the concentrations of Ca, Si, and Al in aqueous solutions after 4 h at 150 °C, under an N
2 atmosphere of 2 MPa and an AAM concentration of 4 g/dm
3.
In addition to those of Ca, Si, and Al, Mg and Na concentrations also were analyzed from water samples by inductively coupled plasma–optical emission spectroscopy (ICP-OES). However, the magnesium concentration was less than the detection limit (≤0.1 mg/dm
3), and the maximum sodium concentration was 1 mg/dm
3 after 4 h at 150 °C and an N
2 atmosphere of 2 MPa. All of the samples exhibited almost the same Ca and Si concentrations. However, with the increase in the amount of NaOH in the samples, the leaching of aluminum increased. Clearly, alkali activation immobilized Al in the inorganic matrix, but basicity enhanced its dissolution [
41]. Furthermore, curing at room temperature led to the enhanced dissolution of Ca and Al from AAMs. A curing temperature of 60 °C has been found to be favorable for geopolymer preparation. Muñiz-Villarreal et al. [
42] have reported that the optimum dissolution and formation of hydroxy species and oligomers, as well as further polymerization or condensation, occur at 60 °C. Therefore, with the increase in the curing temperature of the BFS-based AAMs, the leaching of Ca, Si, and Al decreased. Thus, based on these stability tests, AAMs that are first cured at 60 °C for 24 h are further characterized by XRD, diffuse-reflectance infrared Fourier transform spectroscopy (DRIFTS), field emission scanning electron microscope with energy-dispersive X-ray spectroscopy (FESEM-EDS), ICP-OES, and surface area techniques, as well as being examined for the CWPO of a BPA aqueous solution.
2.2. Characterization of AAMs
Table 3 lists the results of the Brunauer–Emmett–Teller (BET) surface areas of the prepared AAMs.
The specific surface area of BFS was negligible, while alkali activation led to the increased surface area of all samples (
Table 3). Samples prepared by using the highest amount of NaOH exhibited the highest specific surface area, as well as the highest pore volume. Clearly, a low Si/Na ratio favored the formation of a porous structure in the samples. Sindhunata et al. [
43] have reported the highest pore volume for fly-ash-based geopolymers at a SiO
2/Na
2O ratio < 1. Moreover, the samples cured at 60 °C for 24 h exhibited a slightly higher specific surface area, and hence a higher pore volume, than those prepared at room temperature. The higher curing temperature promoted the removal of excess water from the material structure, which in turn increased the porosity of samples further. Furthermore, higher curing temperatures (>50 °C) have been reported to particularly increase the amount of mesopores in the material [
43].
As can be observed from the surface area results, no significant differences between the AAMs were observed. Therefore, to examine the effect of the Na concentration of samples on the catalytic behavior, samples with the lowest and highest Na concentration (BFS17.5-60 and BFS30-60) were selected as support materials for Fe catalysts. Surprisingly, the surface areas of the Fe catalysts were greater than those of the BFS17.5-60 and BFS30-60 pure supports (
Table 3). This result was related to the calcination performed for Fe catalysts. During heat treatment, excess water and carbon dioxide of the support material, and well as traces of Fe salt, evaporated from the AAM structure, enabling the increase in the specific surface area [
44]. In addition, the calcination of Fe catalysts led to the decomposition of hydrotalcite (
Figure 1, XRD results), which also affected the surface area of materials [
44]. Furthermore, as-prepared AAMs mainly exhibited a mesoporous structure (i.e., pore diameter between 2 and 50 nm), with ~10% of pores exhibiting a diameter of less than 2 nm. However, by the addition of Fe to BFS17.5-60 and BFS30-60 via ion exchange, the number of mesopores decreased to 80%, while micropores accounted for only a small percentage of the total pore volume. Moreover, macropores accounted for only ~15% of the total pore volume in Fe/BFS17.5-60 and Fe/BFS30-60, while before Fe ion exchange, pores greater than 50 nm were not detected (i.e., heat treatment enhanced the formation of large pores).
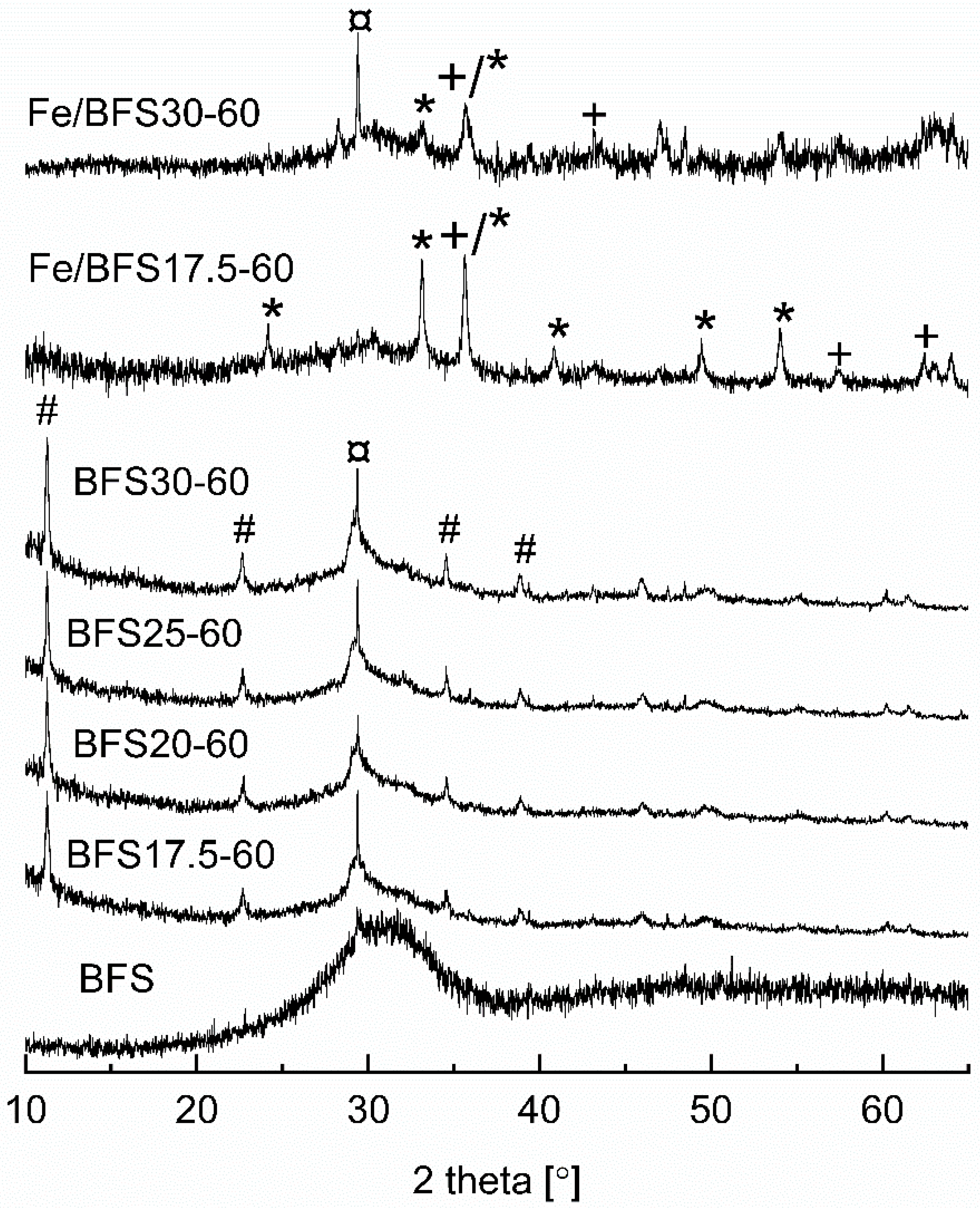
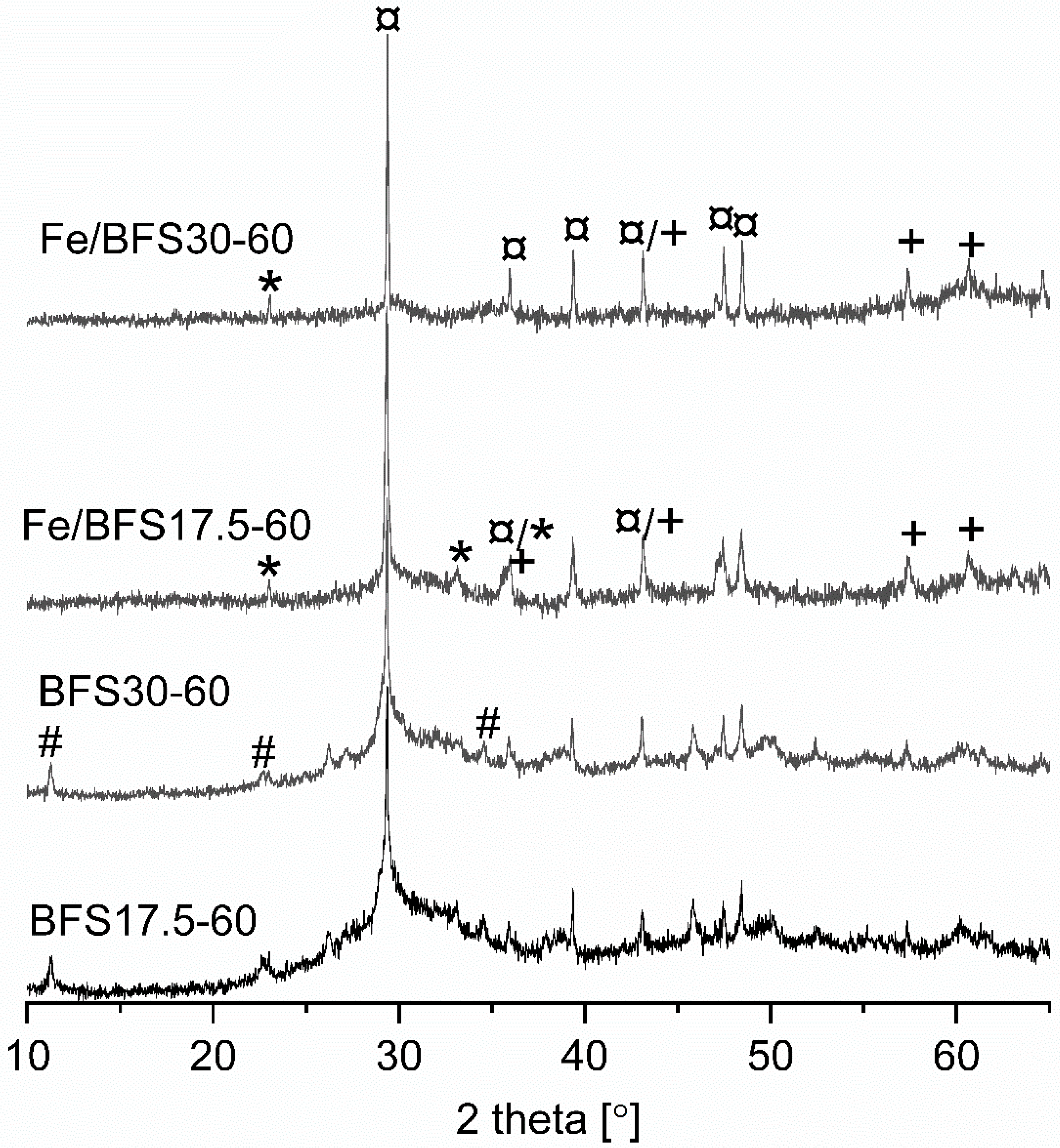
Figure 1 shows the X-ray diffractograms of the BFS raw material; BFS17.5-60, BFS20-60, BFS25-60, and BFS30-60 supports; and Fe/BFS17.5-60 and Fe/BFS30-60 catalysts. In the X-ray diffractogram of BFS, crystal peaks were not observed, but only one wide halo at 2θ between 22° and 40° was observed, which is characteristic of an amorphous material. After the alkali activation of BFS with NaOH, peaks were observed at 2θ values of 11.3°, 22.8°, 34.5°, and 38.6° (denoted with #), corresponding to hydrotalcite (Mg
6Al
2CO
3(OH)
16·4H
2O (ICDD file 00-022-0700)), and the high-intensity peak at ~29.4° corresponded to CaCO
3 (ICDD file 01-083-4609). However, the broad “hump” observed at 2θ of 28–35° was still present in the X-ray diffractograms of all supports, indicative of a partly amorphous structure. After the ion exchange of BFS17.5-60 and BFS30-60 with the Fe solution, the peaks observed at 2θ values of 24.1°, 33.2°, 35.6°, 40.9°, 49.5°, and 54.1° (denoted by *) and at 35.7°, 43.4°, 57.4°, and 63.0° (denoted by +) revealed the presence of Fe
2O
3 (ICDD file 04-015-7029) and Fe
3O
4 (ICDD file 04-008-8146) phases, respectively. Owing to the heat treatment of Fe catalysts, hydrotalcite was decomposed [
44], and peaks corresponding to hydrotalcite were not observed in the X-ray diffractograms of Fe/BFS17.5-60 and Fe/BFS30-60.
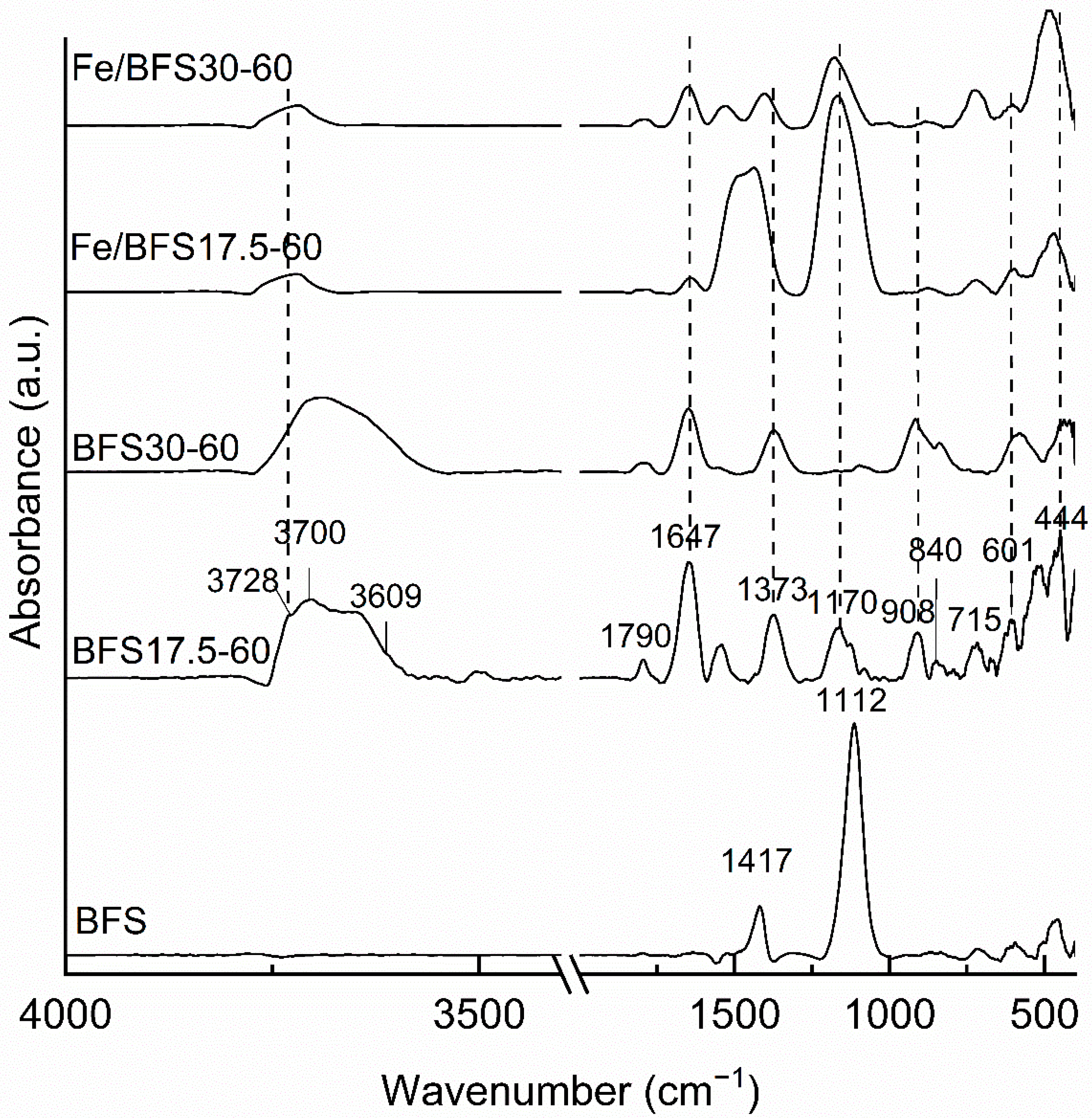
Figure 2 shows the DRIFT spectra of BFS, BFS17.5-60, BFS30-60, Fe/BFS17.5-60, and Fe/BFS30-60. In the DRIFT spectrum of the BFS raw material, only a few peaks were observed. The band at ~1420 cm
−1 corresponded to Na
2CO
3 [
45], and the strong peak at ~1110 cm
−1 corresponded to pure silica [
46].
Alkali-activated samples exhibited several peaks in the analyzed region. The peak at 3730 cm
−1 for BFS17.5-60 corresponded to silanol groups, which interact with other atoms—for example, in silanol nests [
47]—and the absorption peak at ~3700 cm
−1 revealed the presence of four coordinated Al [
48]. Moreover, the band at 3610 cm
−1 for BFS17.5-60 corresponded to the bridging hydroxyl groups [
49]. In the DRIFT spectra of Fe/BFS17.5-60 and Fe/BFS30-60, the peak centers were shifted to higher wavenumbers than those for the samples without iron, probably due to calcination, and the absorption band corresponding to the silanol groups (3730 cm
−1) disappeared by the introduction of iron into AAMs [
47].
The absorbance bands for BFS17.5-60 and BFS30-60 were observed at 715, 840, 1373, and 1790 cm
−1, corresponding to CO
32−-containing compounds [
46]. Bands at 840 and 1790 cm
−1 connected to Na
2CO
3, and the band at 715 cm
−1 corresponded to CaCO
3 [
46], while that observed at 1373 cm
−1 corresponded to hydrotalcite [
50], which was also detected in the X-ray diffractograms of these samples (
Figure 1). In the DRIFT spectra of Fe/BFS17.5-60 and Fe/BFS30-60, these peaks were slightly shifted to higher wavenumbers, especially for the band corresponding to hydrotalcite, indicative of its decomposition as a result of heat treatment. Furthermore, the peak at ~1650 cm
−1 observed in all samples corresponded to the H–OH stretching vibrations characteristic of absorbed water [
51], the intensity of which slightly decreased due to the heat treatment of iron-containing samples.
All AAMs exhibited several bands corresponding to the Al and Si bonds. The bands at 435–483 cm
−1 corresponded to the Si–O–Si and O–Si–O bending vibrations [
52], while the absorption peak at ~600 cm
−1 revealed the presence of Si–O–Si and Al–O–Si symmetric stretching vibrations [
53]. The band at ~900 cm
−1 in the spectra of BFS17.5-60 and BFS30-60 corresponded to the Si–O stretching and Si–OH bending modes [
53]. Moreover, the band at ~1170 cm
−1 corresponded to the Si–O–Si and Al–O–Si asymmetric stretching vibrations [
53], and this band was broadened in the spectra of Fe/BFS17.5-60 and Fe/BFS30-60, due to the calcination of these samples [
45]. According to [
54,
55,
56], Fe
2O
3 and Fe
3O
4 species should exhibit IR vibrations at 550 and 780 cm
−1 and 571 and 590 cm
−1, respectively. However, owing to the overlap of the Si and Al vibrations in this wavenumber region, peaks corresponding to Fe cannot be observed in the DRIFT spectra of the prepared samples.
Table 4 lists the concentrations (as wt %) of Ca, Si, Al, Mg, Fe, and Na of BFS17.5-60, BFS30-60, Fe/BFS17.5-60, and Fe/BFS30-60, as determined by ICP-OES analysis.
The Ca concentrations of the prepared samples were several times lower than those in BFS, while the Si, Al, Mg, and Na concentrations were about the same as those in the raw material (
Table 5, experimental). The leaching of Ca probably occurred during the washing of the AAMs using deionized water. BFS contained ~0.5 wt % iron, and ion exchange led to the increase in the iron concentration to 5–7 wt % for Fe/BFS30-60 and Fe/BFS17.5-60, respectively. The theoretical amount of iron by the employed impregnation method was 5.3 wt %, indicating that ion exchange between BFS17.5-60 and the iron salt is slightly better than that between BFS30-60 and the iron salt.
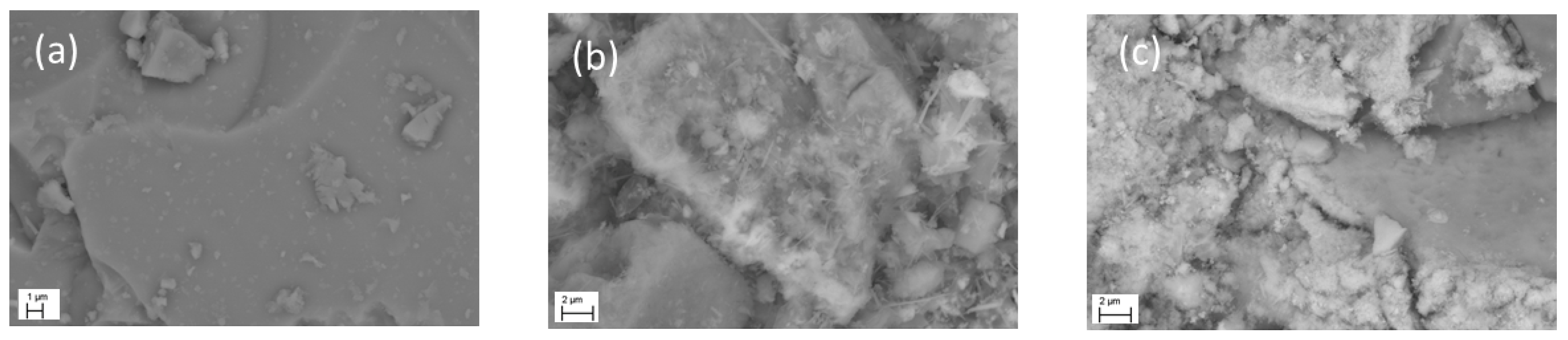
Figure 3 shows the FESEM images of BFS, Fe/BFS17.5-60, and Fe/BFS30-60. AAMs clearly exhibited an irregular, non-crystalline shape (
Figure 3b,c). According to EDS analysis, the Al and Mg concentrations were ~5 wt %, while on the Fe catalyst surface, the Si and Ca concentrations were a few percent less than those in the bulk, as determined by ICP-OES (
Table 4).
2.3. Oxidation Experiments with AAMs
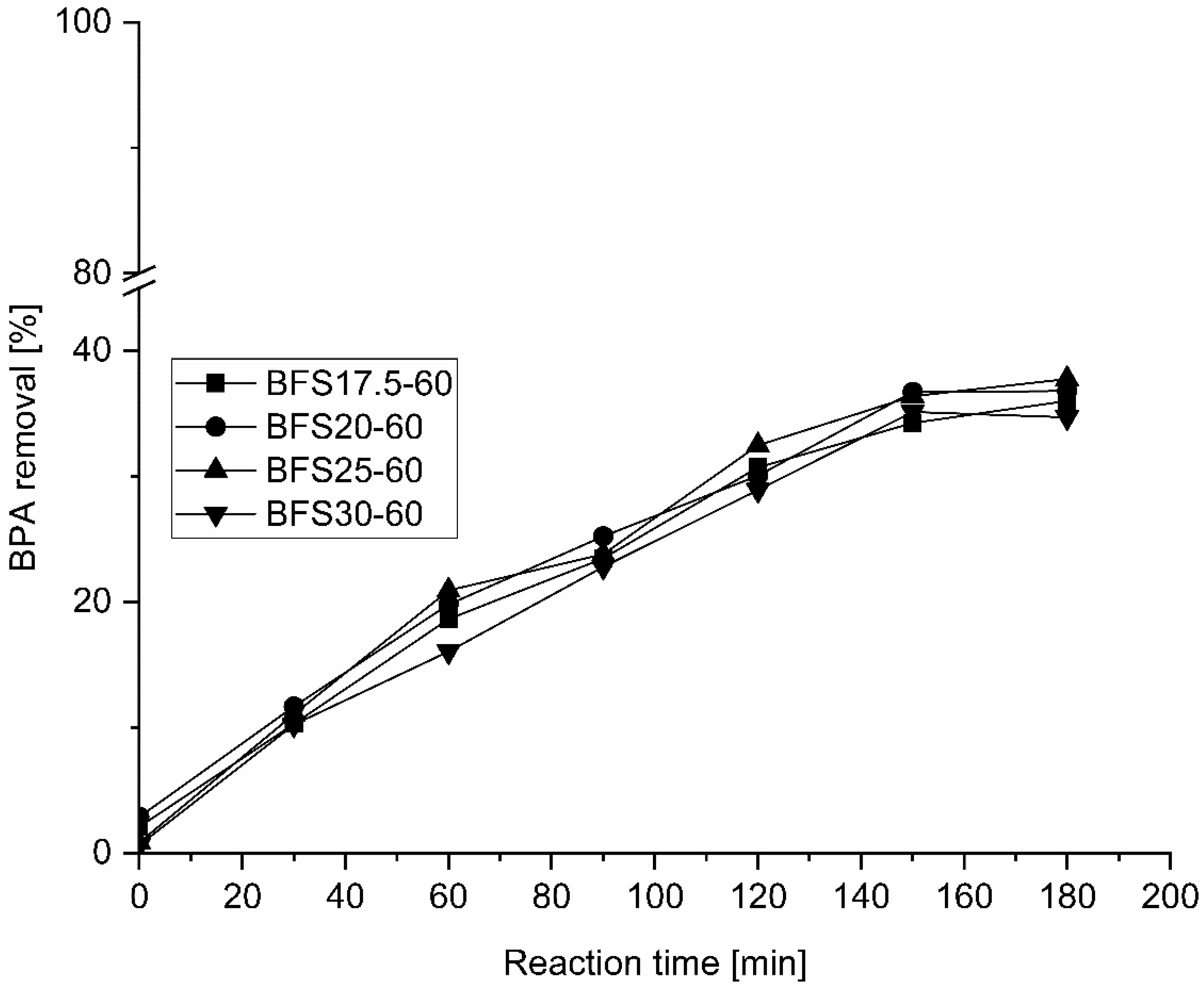
The prepared AAMs, namely BFS17.5-60, BFS20-60, BFS25-60, and BFS30-60, which were first cured at 60 °C for 24 h, were examined for the CWPO of a BPA aqueous solution.
Figure 4 shows the results of these experiments.
Oxidation reactions were performed at 50 °C at an initial pH of 6–7, a catalyst concentration of 4 g/dm
3, and a H
2O
2 concentration of 1.5 g/dm
3. In the absence of a catalyst (not shown), only ~10% of BPA removal was observed, while in the presence of AAMs, BPA removal of 35–39% after 180 min oxidation was observed. Oxidation proceeded during 2.5 h for all samples and stabilized for 3 h. The oxidant H
2O
2 was added in batches; hence, the final addition was performed at 2 h sampling. The total organic carbon (TOC) was measured from the initial and final samples, and 27–31% of organics were removed. The dissolved oxygen (DO) concentration of the BPA samples changed from ~9.5 mg O
2/dm
3 to 8.1 mg O
2/dm
3 during 180 min oxidation, revealing that at the end of the run, oxygen is still present in the samples. Probably, the used reaction temperature (50 °C) was not sufficiently high for the effective decomposition of H
2O
2 to form active ∙OH radicals. In several studies, a higher reaction temperature has been reported to enhance the degradation of H
2O
2, thereby enhancing pollutant removal [
57,
58,
59].
As all of the AAMs exhibited similar activities for the removal of BPA, samples with the lowest and highest NaOH concentration were selected for further research. Iron was impregnated onto BFS17.5-60 and BFS30-60 samples by ion exchange (
Section 3.1), and the prepared Fe catalysts were examined under different reaction conditions.
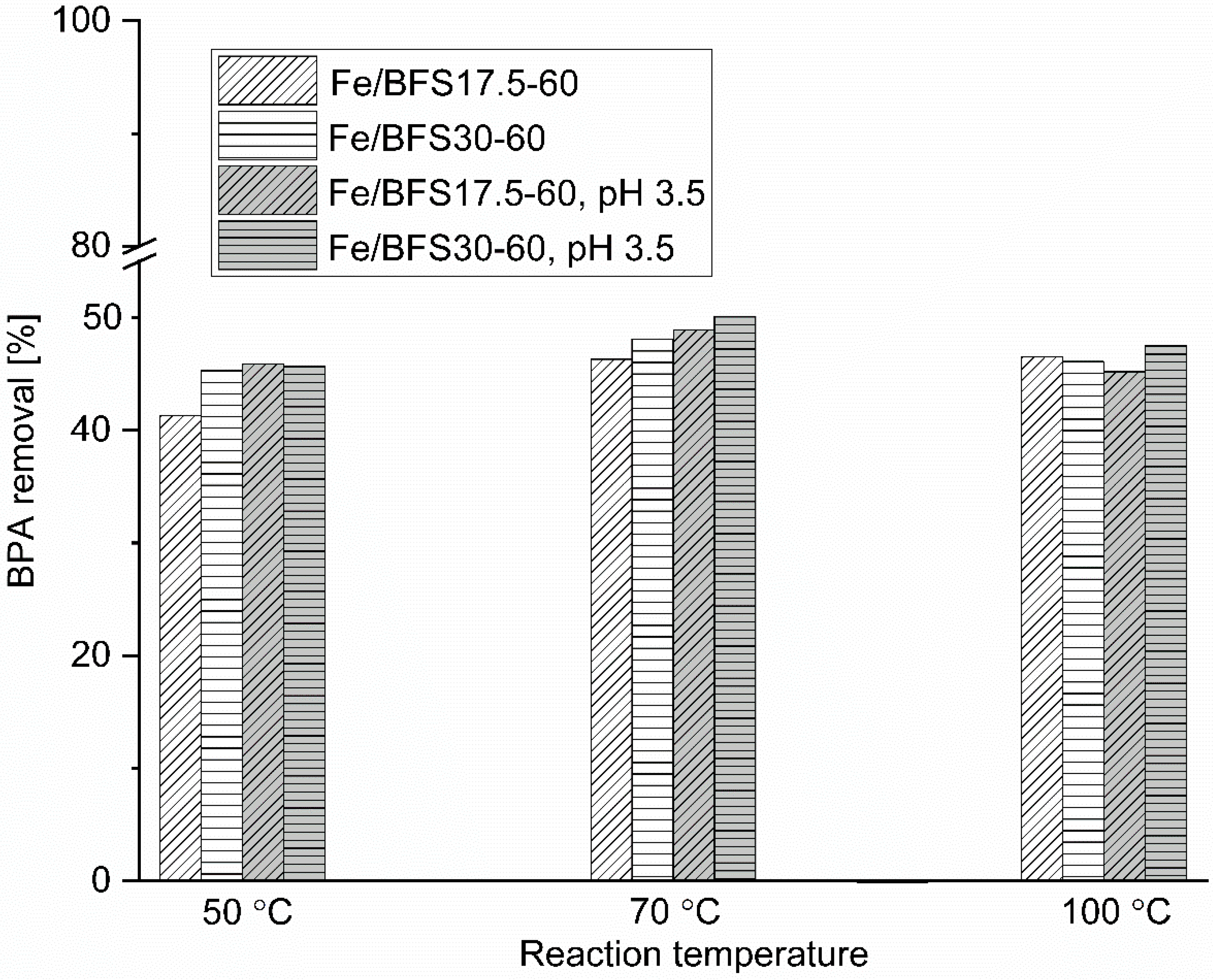
First, the effect of the addition of the active metal on BFS17.5-60 and BFS30-60 was examined at 50 °C at the initial pH, and a catalyst loading of 4 g/dm
3. After 3 h oxidation, BPA removal of 42% and 45% for Fe/BFS17.5-60 and Fe/BFS30-60 were observed, respectively (
Figure 5). Using the comparison of BPA removal over AAMs without the active metal (
Figure 4), the addition of Fe led to the increased activity of both catalysts, namely BPA removal of 6% and 10% for BFS17.5-60 and BFS30-60, respectively. TOC removal after 3 h oxidation was at the same level for both catalysts compared to that over the pure supports (30% and 33% for Fe/BFS17.5-60 and Fe/BFS30-60, respectively). During oxidation, the DO concentration decreased slightly from ~9 mg/O
2 dm
3 to 6.2–6.6 mg/O
2 dm
3, indicating that hydrogen peroxide is not consumed completely in the runs. Therefore, Fe/BFS17.5-60 and Fe/BFS30-60 were further examined at higher reaction temperatures.
To investigate the effect of temperature on the CWPO of BPA over Fe/BFS17.5-60 and Fe/BFS30-60, oxidation experiments were performed at 70 °C and 100 °C at the initial pH of the BPA aqueous solution. Typically, with the increase in the reaction temperature, the oxidation rate increases. Furthermore, the decomposition rate of H
2O
2 to active hydroxyl radicals also increases. A higher reaction temperature led to the improved degradation of BPA during 3 h oxidation, with the maximum of 5% over Fe/BFS17.5-60 at 70 °C (
Figure 5). The increase in the reaction temperature to 100 °C did not affect BPA removal. During oxidation, the DO concentration decreased from 8.0 mg/O
2 dm
3 to 5.7 mg/O
2 dm
3 and from ~10.0 mg/O
2 dm
3 to 4.2 mg/O
2 dm
3 at 70 °C and 100 °C, respectively, revealing that hydrogen peroxide is consumed in the reaction. However, owing to the low degradation level of BPA at 100 °C, hydrogen peroxide was probably decomposed directly to H
2O without the formation of hydroxyl radicals.
Typically, homogeneous iron catalysts for CWPO (Fenton process) are used at a pH of ~3, which is known to be optimum for the decomposition of organic compounds [
60]. The effect of pH on the degradation level of BPA was investigated at pH 3.5, in addition to the initial pH (6–7) by using Fe/BFS17.5-60 and Fe/BFS30-60 catalysts. The effect of pH was examined at 50 °C, 70 °C, and 100 °C. The pH of the BPA solution was adjusted to 3.5 using 2.0 M HNO
3 before oxidation. At 50 °C and pH 3.5, BPA removal increased by 5% over Fe/BFS17.5-60, while over Fe/BFS30-60, it was almost the same after 3 h oxidation compared to experiments performed at the initial pH of BPA (
Figure 5). The DO concentration of the liquid samples was considerably higher (at the end of the run for Fe/BFS17.5-60, 14 mg/O
2 dm
3) than that after oxidation at the initial pH. Therefore, acidic pH promotes the formation of hydroxyl radicals during the reaction. However, Fe catalysts did not exhibit considerably higher activity for BPA removal than that at the initial pH, probably due to the basic surfaces of Fe/BFS17.5-60 and Fe/BFS30-60.
At pH 3.5 and 70 °C (
Figure 5), the DO concentration was the same during tests compared to that in experiments at the initial pH, and the pH change of the BPA solution led to an increase in BPA removal by only 3% and 2% over Fe/BFS17.5-60 and Fe/BFS30-60, respectively. At 100 °C and pH 3.5, BPA removal after 3 h was around the same for both Fe catalysts compared with that observed at 100°C and at the initial pH. However, notably, owing to the basicity of Fe catalysts, the pH of the BPA solution changed to basic during runs in all experiments. The decomposition of H
2O
2 to ∙OH radicals is the key step in CWPO. However, under a basic reaction pH, the generation of hydroxyl radicals was restricted, thereby further decreasing the degradation of BPA [
61]. Thus, the change in pH marginally affects BPA removal.
The adsorption capacity of the Fe catalysts was examined under the severest reaction conditions in this study, i.e., pH of 3.5, a reaction temperature of 100 °C, in the absence of the oxidant, and a catalyst concentration of 4 g/dm
3. For Fe/BFS17.5-60 and Fe/BFS30-60, during the 3 h experiment, 12% and 17% of BPA was adsorbed, respectively, revealing that Fe/BFS17.5-60 is catalytically more active than Fe/BFS30-60. The higher adsorption capacity of Fe/BFS30-60 was related to the higher specific surface area of this sample (
Table 3).
2.4. Stability of the Used Catalysts
The possible leaching of the elements from the prepared AAMs was examined by ICP-OES in detail, in addition to the leaching tests (
Section 2.1) after oxidation. The oxidized water samples were immediately filtered after 3 h CWPO using a 0.45 µm cellulose nitrate filter to remove the solid catalysts. The Al, Si, and Ca concentrations were determined from the oxidized water samples catalyzed by BFS17.5-60, BFS20-60, BFS25-60, and BFS30-60, and in addition to these elements, Fe was analyzed from the filtered samples catalyzed by Fe/BFS17.5-60 and Fe/BFS30-60. According to the results, the leaching of Ca and Si was observed under all of the utilized reaction conditions with all catalysts. In all of the oxidized water samples, the Ca concentration was 25–50 mg/dm
3, and the Si concentration was 11–19 mg/dm
3. However, notably, the Ca concentration was slightly lower in the water samples catalyzed by AAMs without iron. Therefore, the heat treatment of Fe catalysts (
Section 3.1) led to the increased dissolution of Ca in the water phase during oxidation treatment. The leaching of Ca was around the same level as that detected in stability tests (
Section 2.1) with BFS17.5-60, BFS20-60, BFS25-60, and BFS30-60, revealing that these samples also can be used at reaction temperatures > 100 °C and pressures ≥ 2 MPa.
The Al concentration of aqueous BPA samples oxidized at the initial pH was 1.0–1.4 mg/dm
3. The dissolution of Al was slightly higher at 150 °C and 2.0 MPa (
Table 1), i.e., under conditions of the stability test, than that under CWPO reaction conditions. However, in the case of oxidation experiments performed at a pH of 3.5, and at temperatures 50 °C, 70 °C, and 100 °C over that of Fe/BFS17.5-60 and Fe/BFS30-60, 0.6–1.2 mg/dm
3 of Al was leached from the catalysts in the obtained effluents. Therefore, the dissolution of Al from the prepared AAMs is more dominant in the CWPO of BPA, which is conducted at the initial pH. Onisei et al. [
41] have investigated the leaching behavior of several elements (e.g., Si, Pb, Ca, Zn, Al) from fly ash-based geopolymers. The study was performed in the pH range of 6–13. According to their results, the leaching of Al increased slightly in the pH range of 10.5–13.0. In the CWPO of BPA, the initial pH of the BPA solution was 6–7. However, at the end of the run, the effluent pH was ~11, due to the basic character of the Fe catalysts. Moreover, in CWPO experiments, which were started at a pH of 3.5, the pH of the BPA solution was ~10 in the oxidized water sample. Therefore, the adjustment of the pH at the start of the CWPO of BPA did not considerably affect the removal of BPA, but it decreased the leaching of Al from the Fe catalysts.
However, the leaching of Ca, Si, and Al was not related to the removal of BPA, because those elements were not active in CWPO. The stability of the material is a key characteristic of the catalyst; therefore, the preparation method of AAM-based catalysts should be carefully considered. Moreover, the leaching of iron was rather negligible (maximum of 0.2 mg/dm3 at 70 °C and at the initial pH) using Fe/BFS17.5-60 and Fe/BFS30-60 at the employed reaction temperatures and pH. Therefore, the CWPO of BPA with these catalysts proceeded via a heterogeneous reaction.
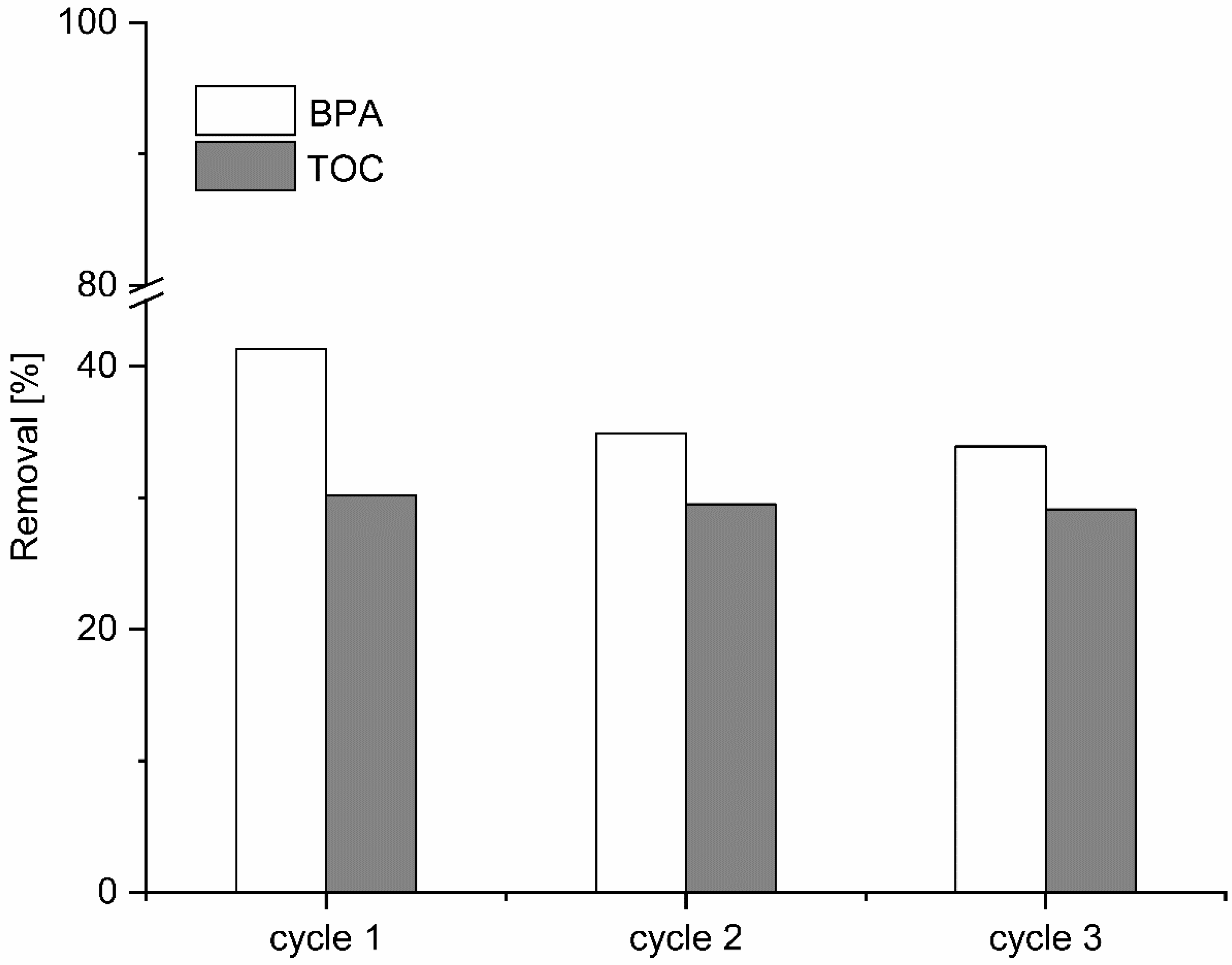
The activity and durability in consecutive tests and the effect of heat treatment as a regeneration method were examined using Fe/BFS7.5-60 at 50 °C, at the initial pH, and at H2O2 and catalyst concentrations of 1.5 g/dm3 and 4.0 g/dm3, respectively. To have sufficient material for consecutive tests and regeneration, 12 runs were performed in total, and the catalysts used in these experiments were collected and combined. Between consecutive experiments, the used catalyst was filtered from the effluent and dried at 105 °C for the subsequent runs.
After the first oxidation reaction, BPA removal of 41% was observed, which decreased to ~6% after the second run using the same catalyst (
Figure 6). Furthermore, BPA removal after the third experiment, which used Fe/BFS17.5-60 twice, was practically the same (34%) as that observed in the second run, indicative of the catalyst’s stability for multiple cycles in the CWPO of BPA. However, the removal of BPA after three cycles using Fe/BFS17.5-60 was around the same as that using BFS17.5-60 for one cycle, revealing that the addition of Fe does not significantly affect catalytic activity due to the basic reaction pH. Moreover, TOC results confirmed the reusability of Fe/BFS17.5-60, while BPA removal was the same during the three consecutive tests.
The regeneration of once-used Fe/BFS17.5-60 was examined by heat treatment at 250 °C and 500 °C. The procedure was performed by increasing the temperature at a rate of 1 °C/min to the reaction temperature, at which the catalyst was kept for 2 h. After oxidation, 34% and 32% BPA removal was observed at 250 °C and 500 °C, respectively, using Fe/BFS17.5-60. Therefore, the regeneration procedure is not effective at returning the activity of the catalysts to the original level. In addition, carbon deposition is confirmed to not be responsible for the activity decrease of Fe/BFS17.5-60, because heat treatment at elevated temperatures is a typical regeneration procedure for catalysts with carbon deactivation [
62].
2.5. Characterization of the Used Catalysts
Figure 7 shows the X-ray diffractograms of BFS17.5-60, BFS30-60, Fe/BFS17.5-60, and Fe/BFS30-60 after oxidation at the initial pH and at a reaction temperature of 50 °C. According to XRD analysis, the hydrotalcite phase (denoted by #, ICDD file 00-022-0700) was still present in BFS17.5-60 and BFS30-60, and the CaCO
3 phase (¤, ICDD file 01-083-4609) was observed in all samples. Moreover, in the X-ray diffractograms of Fe/BFS17.5-60 and Fe/BFS30-60, the Fe
3O
4 and Fe
2O
3 iron phases (denoted by +: ICDD file 04-008-8146 and *: ICDD file 04-015-7029, respectively) were still present, but the high Ca concentration of samples led to the overlap of the CaCO
3 peaks with those of Fe
3O
4 and Fe
2O
3 at 2θ of 36° and Fe
3O
4 at 2θ of 43°.
Furthermore, acidic pH and a higher reaction temperature did not affect the phase structure, and hydrotalcite was still observed in the X-ray diffractograms of BFS30-60 and BFS17.5-60 (results not shown).
The specific surface area of samples was analyzed after the oxidation of BPA at the initial pH and at reaction temperatures of 50 °C for BFS17.5-60 and BFS30-60, as well as reaction temperatures of 50 °C and 70 °C for Fe/BFS17.5-60 and Fe/BFS30-60. The BET results for used BFS17.5-60 and BFS30-60 revealed that the specific surface areas were 10.4 and 18.5 m
2/g, respectively, revealing that the surface area of BFS30-60 decreases to ~30%, while a rather negligible change in the surface area of BFS17.5-60 was observed (
Table 3). For Fe catalysts, the specific surface areas increased after oxidation. In case of Fe/BFS17.5-60, the surface area was ~45% higher, and in case of Fe/BFS30-60, it doubled compared to that of the fresh catalyst (
Table 3). Clearly, during oxidation, the Fe catalyst surface is refined by H
2O
2. For example, Han et al. [
63] and Liu et al. [
64] have used hydrogen peroxide to modify surface properties, i.e., to increase the surface area and porosity of materials. However, the larger specific surface area did not improve the removal of BPA in consecutive tests using Fe/BFS17.5-60 (
Figure 6); therefore, the CWPO of BPA is not a surface area-specific reaction, as is the case for the catalytic wet air oxidation of BPA [
65].