Do Gas Nanobubbles Enhance Aqueous Photocatalysis? Experiment and Analysis of Mechanism
Abstract
1. Introduction
2. Results and Discussion
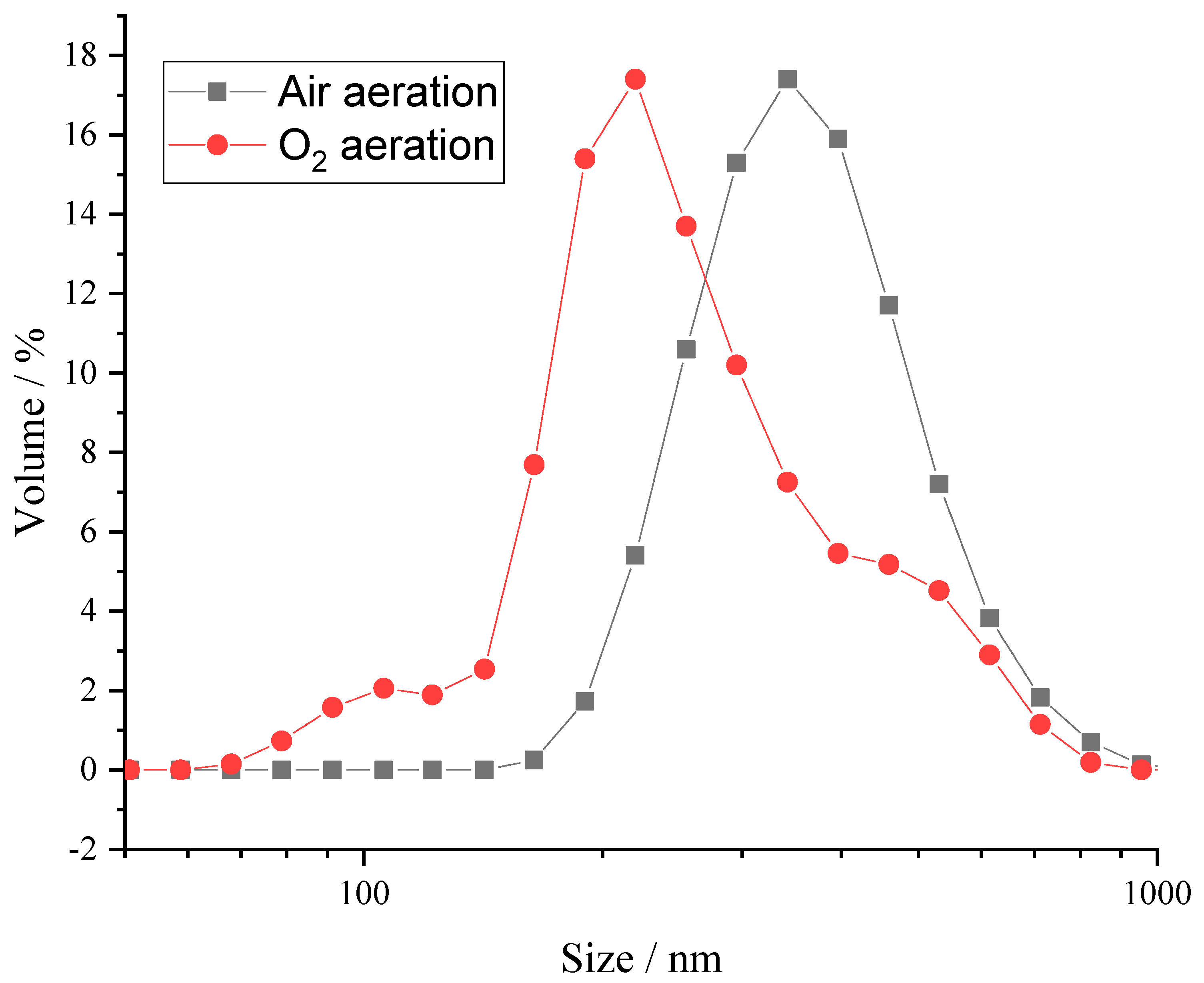
2.1. Bubble Size Distribution and DO Level
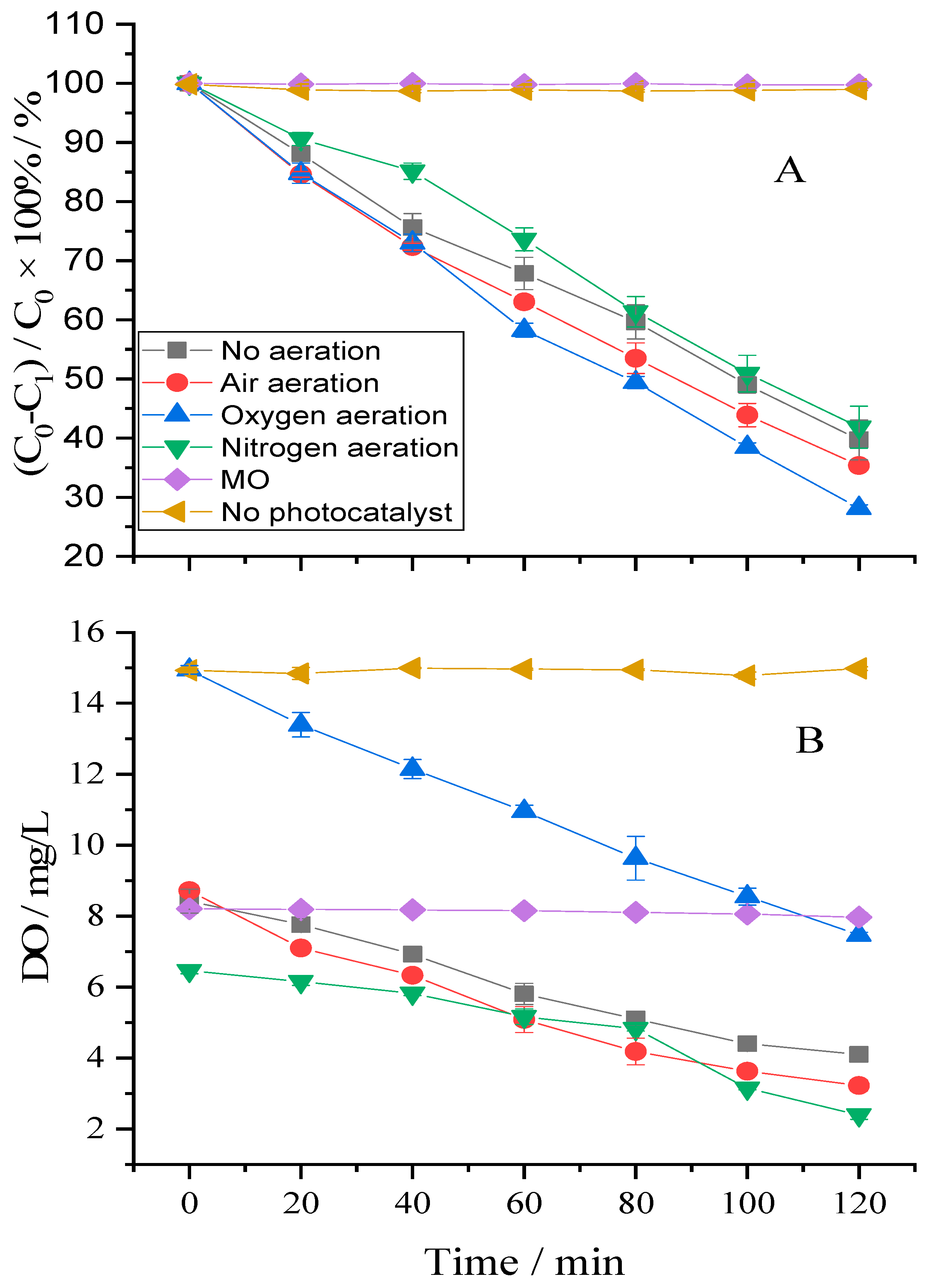
2.2. Effect of Applied Gasses on the Degradation
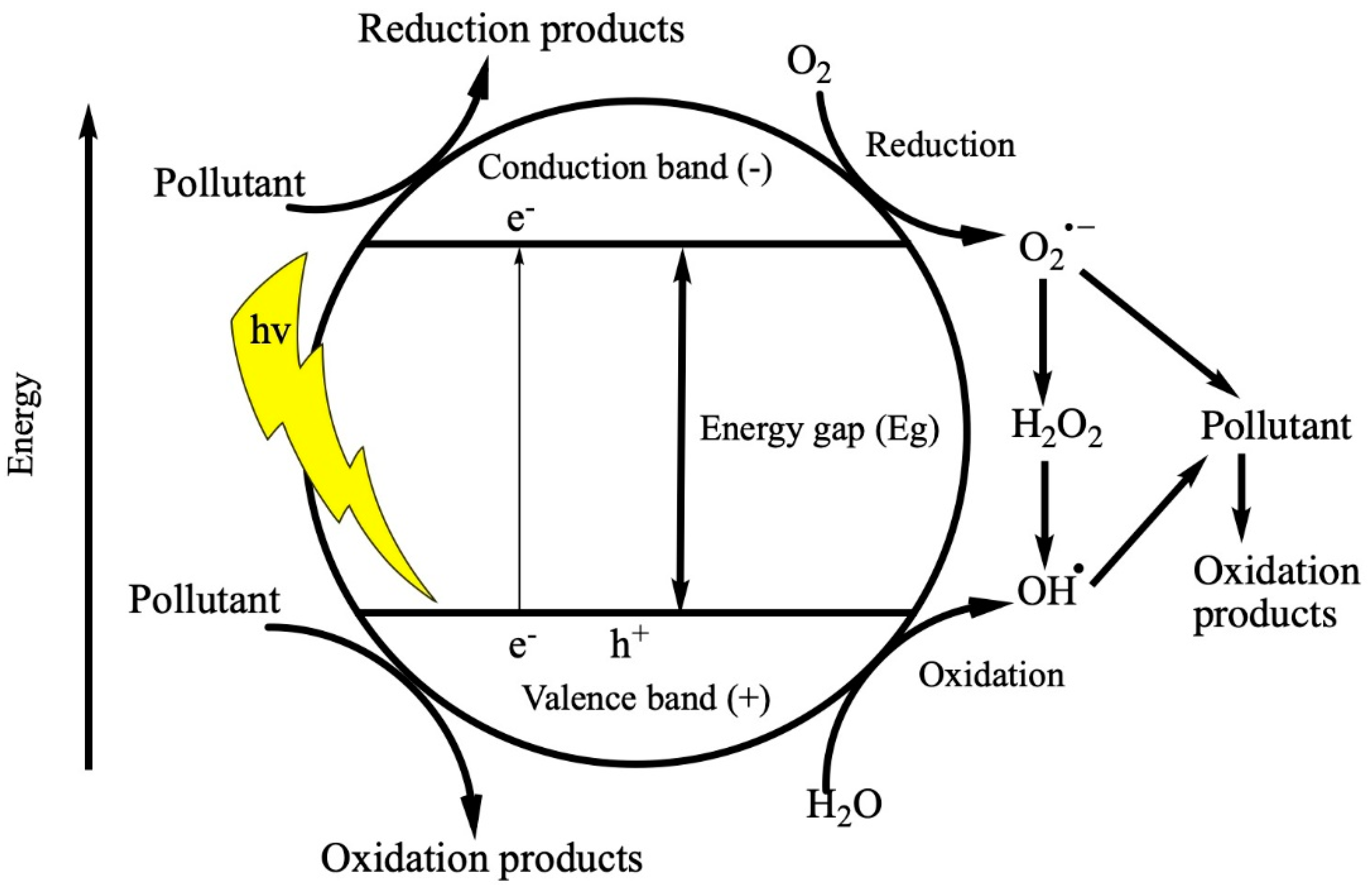
2.3. The Role of Reactive Species in the Degradation Mechanisms
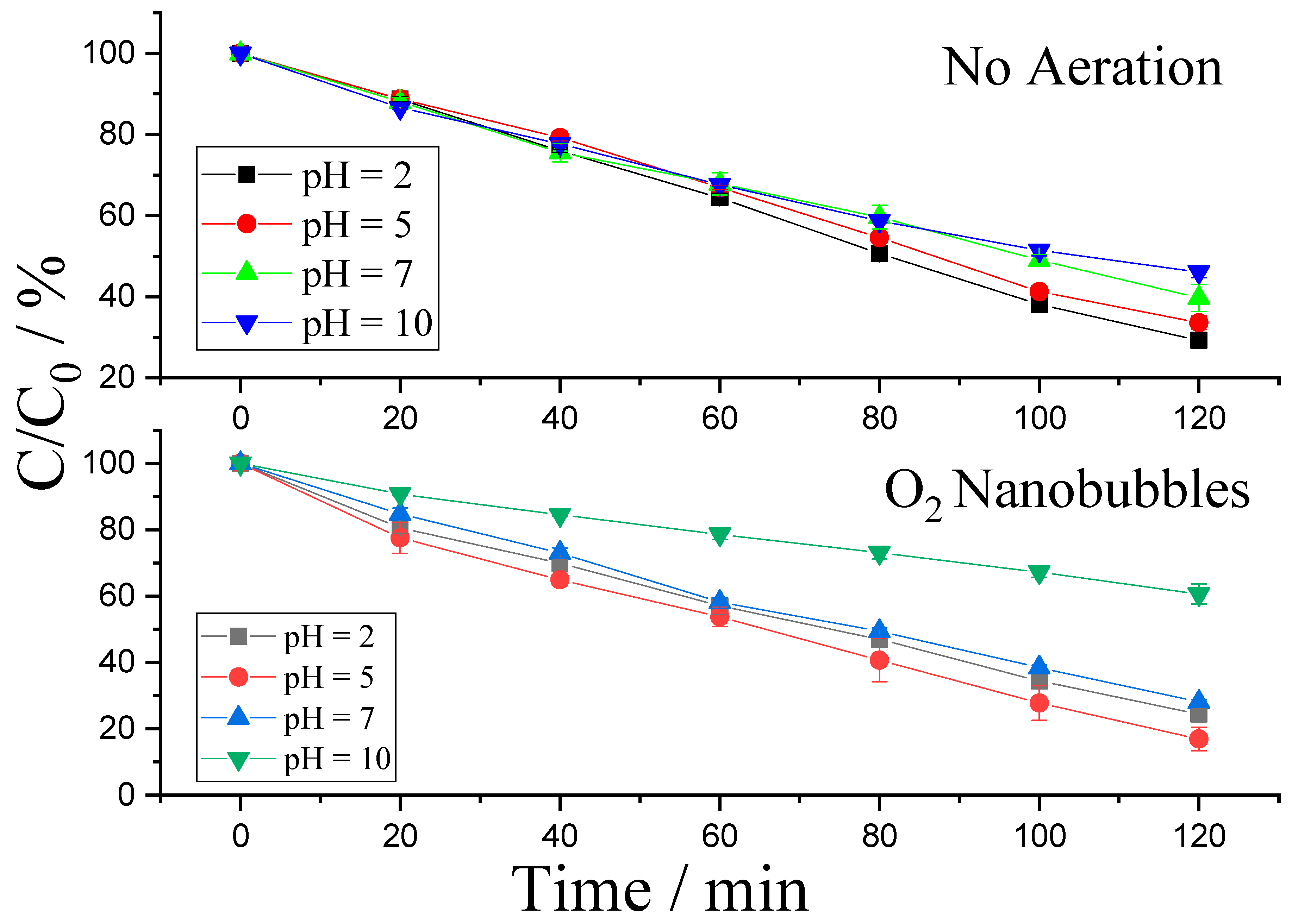
2.4. Effect of pH
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Sample Characterization
3.3. Experiment Setup
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Awfa, D.; Ateia, M.; Fujii, M.; Johnson, M.S.; Yoshimura, C. Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2 composites: A critical review of recent literature. Water Res. 2018, 142, 26–45. [Google Scholar] [CrossRef]
- Zhao, B.; Lv, M.; Zhou, L. Photocatalytic degradation of perfluorooctanoic acid with β-Ga2O3 in anoxic aqueous solution. J. Environ. Sci. 2012, 24, 774–780. [Google Scholar] [CrossRef]
- Ikeda, S.; Sugiyama, N.; Pal, B.; Marcí, G.; Palmisano, L.; Noguchi, H.; Uosaki, K.; Ohtani, B. Photocatalytic activity of transition-metal-loaded titanium (IV) oxide powders suspended in aqueous solutions: Correlation with electron–hole recombination kinetics. Phys. Chem. Chem. Phys. 2001, 3, 267–273. [Google Scholar] [CrossRef]
- Serpone, N.; Lawless, D.; Khairutdinov, R.; Pelizzetti, E. Subnanosecond relaxation dynamics in TiO2 colloidal sols (particle sizes Rp= 1.0−13.4 nm). Relevance to heterogeneous photocatalysis. J. Phys. Chem. 1995, 99, 16655–16661. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Sun, S.; Wang, L. Enhancing visible-light-induced photocatalytic activity by coupling with wide-band-gap semiconductor: A case study on Bi2WO6/TiO2. Appl. Catal. B Environ. 2012, 111, 126–132. [Google Scholar] [CrossRef]
- Xing, C.; Zhang, Y.; Yan, W.; Guo, L. Band structure-controlled solid solution of Cd1-x ZnxS photocatalyst for hydrogen production by water splitting. Int. J. Hydrogen Energy 2006, 31, 2018–2024. [Google Scholar] [CrossRef]
- Hu, S.; Zhou, F.; Wang, L.; Zhang, J. Preparation of Cu2O/CeO2 heterojunction photocatalyst for the degradation of Acid Orange 7 under visible light irradiation. Catal. Commun. 2011, 12, 794–797. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Yoshimura, C. Novel magnetic carbon nanotube-TiO2 composites for solar light photocatalytic degradation of pharmaceuticals in the presence of natural organic matter. J. Water Process Eng. 2019, 31, 100836. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Yoshimura, C. Photocatalytic degradation of organic micropollutants: Inhibition mechanisms by different fractions of natural organic matter. Water Res. 2020, 174, 115643. [Google Scholar] [CrossRef]
- Xiong, J.; Di, J.; Xia, J.; Zhu, W.; Li, H. Surface defect engineering in 2D nanomaterials for photocatalysis. Adv. Funct. Mater. 2018, 28, 1801983. [Google Scholar] [CrossRef]
- Kong, L.; Wang, C.; Zheng, H.; Zhang, X.; Liu, Y. Defect-induced yellow color in Nb-doped TiO2 and its impact on visible-light photocatalysis. J. Phys. Chem. C 2015, 119, 16623–16632. [Google Scholar] [CrossRef]
- Neppolian, B.; Bruno, A.; Bianchi, C.L.; Ashokkumar, M. Graphene oxide based Pt-TiO2 photocatalyst: Ultrasound assisted synthesis, characterization and catalytic efficiency. Ultrason. Sonochem. 2012, 19, 9–15. [Google Scholar] [CrossRef]
- Fotiou, T.; Triantis, T.M.; Kaloudis, T.; Hiskia, A. Evaluation of the photocatalytic activity of TiO2 based catalysts for the degradation and mineralization of cyanobacterial toxins and water off-odor compounds under UV-A, solar and visible light. Chem. Eng. J. 2015, 261, 17–26. [Google Scholar] [CrossRef]
- Wöhrle, D.; Suvorova, O.; Gerdes, R.; Bartels, O.; Lapok, L.; Baziakina, N.; Makarov, S.; Slodek, A. Efficient oxidations and photooxidations with molecular oxygen using metal phthalocyanines as catalysts and photocatalysts. J. Porphyr. Phthalocyanines 2004, 8, 1020–1041. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Q.; Chen, C.; Zang, L.; Ma, W.; Zhao, J. Oxygen atom transfer in the photocatalytic oxidation of alcohols by TiO2: Oxygen isotope studies. Angew. Chem. Int. Ed. Engl. 2009, 48, 6081–6084. [Google Scholar] [CrossRef] [PubMed]
- Ateia, M.; Alalm, M.G.; Awfa, D.; Johnson, M.S.; Yoshimura, C. Modeling the degradation and disinfection of water pollutants by photocatalysts and composites: A critical review. Sci. Total Environ. 2020, 698, 134197. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, A.J.; Apul, O.G.; Schneider, O.; Garcia-Segura, S.; Westerhoff, P. Nanobubble Technologies Offer Opportunities to Improve Water Treatment. Acc. Chem. Res. 2019, 52, 1196–1205. [Google Scholar] [CrossRef]
- Thomson, W. LX. On the equilibrium of vapour at a curved surface of liquid. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1871, 42, 448–452. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, H.; Qi, N.; Qin, Y.; Zhang, X.; Li, Y. Generation and Stability of Size-Adjustable Bulk Nanobubbles Based on Periodic Pressure Change. Sci. Rep. 2019, 9, 1118. [Google Scholar] [CrossRef]
- Matsuki, N.; Ishikawa, T.; Ichiba, S.; Shiba, N.; Ujike, Y.; Yamaguchi, T. Oxygen supersaturated fluid using fine micro/nanobubbles. Int. J. Nanomed. 2014, 9, 4495. [Google Scholar] [CrossRef]
- Chu, L.-B.; Xing, X.-H.; Yu, A.-F.; Zhou, Y.-N.; Sun, X.-L.; Jurcik, B. Enhanced ozonation of simulated dyestuff wastewater by microbubbles. Chemosphere 2007, 68, 1854–1860. [Google Scholar] [CrossRef]
- Temesgen, T.; Bui, T.T.; Han, M.; Kim, T.-I.; Park, H. Micro and nanobubble technologies as a new horizon for water-treatment techniques: A review. Adv. Colloid Interface Sci. 2017, 246, 40–51. [Google Scholar] [CrossRef]
- Ushikubo, F.Y.; Furukawa, T.; Nakagawa, R.; Enari, M.; Makino, Y.; Kawagoe, Y.; Shiina, T.; Oshita, S. Evidence of the existence and the stability of nano-bubbles in water. Colloids Surf. A Physicochem. Eng. Asp. 2010, 361, 31–37. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Aluthgun Hewage, S.; Batagoda, J.H. Stability of nanobubbles. Environ. Eng. Sci. 2018, 35, 1216–1227. [Google Scholar] [CrossRef]
- Wang, L.; Ali, J.; Wang, Z.; Oladoja, N.; Cheng, R.; Zhang, C.; Mailhot, G.; Pan, G. Oxygen nanobubbles enhanced photodegradation of oxytetracycline under visible light: Synergistic effect and mechanism. Chem. Eng. J. 2020, 388, 124227. [Google Scholar] [CrossRef]
- Fan, W.; Zhou, Z.; Wang, W.; Huo, M.; Zhang, L.; Zhu, S.; Yang, W.; Wang, X. Environmentally friendly approach for advanced treatment of municipal secondary effluent by integration of micro-nano bubbles and photocatalysis. J. Clean. Prod. 2019, 237, 117828. [Google Scholar] [CrossRef]
- Yang, L.; Liya, E.Y.; Ray, M.B. Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water Res. 2008, 42, 3480–3488. [Google Scholar] [CrossRef] [PubMed]
- Shan, R.; Lu, L.; Gu, J.; Zhang, Y.; Yuan, H.; Chen, Y.; Luo, B. Photocatalytic degradation of methyl orange by Ag/TiO2/biochar composite catalysts in aqueous solutions. Mater. Sci. Semicond. Process. 2020, 114, 105088. [Google Scholar] [CrossRef]
- Li, P.; Takahashi, M.; Chiba, K. Enhanced free-radical generation by shrinking microbubbles using a copper catalyst. Chemosphere 2009, 77, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Minamikawa, K.; Takahashi, M.; Makino, T.; Tago, K.; Hayatsu, M. Irrigation with oxygen-nanobubble water can reduce methane emission and arsenic dissolution in a flooded rice paddy. Environ. Res. Lett. 2015, 10, 084012. [Google Scholar] [CrossRef]
- Liu, S.; Oshita, S.; Kawabata, S.; Makino, Y.; Yoshimoto, T. Identification of ROS produced by nanobubbles and their positive and negative effects on vegetable seed germination. Langmuir 2016, 32, 11295–11302. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Kanematsu, W. High temperature and pressure inside a dissolving oxygen nanobubble. Ultrason. Sonochem. 2019, 55, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Heu, R.; Ateia, M.; Awfa, D.; Punyapalakul, P.; Yoshimura, C. Photocatalytic Degradation of Organic Micropollutants in Water by Zr-MOF/GO Composites. J. Compos. Sci. 2020, 4, 54. [Google Scholar] [CrossRef]
- Deng, W.; Zhao, H.; Pan, F.; Feng, X.; Jung, B.; Abdel-Wahab, A.; Batchelor, B.; Li, Y. Visible-Light-Driven Photocatalytic Degradation of Organic Water Pollutants Promoted by Sulfite Addition. Environ. Sci. Technol. 2017, 51, 13372–13379. [Google Scholar] [CrossRef] [PubMed]
- Ziylan-Yavaş, A.; Ince, N.H. Enhanced photo-degradation of paracetamol on n-platinum-loaded TiO2: The effect of ultrasound and OH/hole scavengers. Chemosphere 2016, 162, 324–332. [Google Scholar] [CrossRef]
- Deng, X.; Li, Z.; Garcia, H. Visible Light Induced Organic Transformations Using Metal-Organic-Frameworks (MOFs). Chemistry 2017, 23, 11189–11209. [Google Scholar] [CrossRef]
- Pelaez, M.; Falaras, P.; Likodimos, V.; O’Shea, K.; Armah, A.; Dunlop, P.S.; Byrne, J.A.; Dionysiou, D.D. Use of selected scavengers for the determination of NF-TiO2 reactive oxygen species during the degradation of microcystin-LR under visible light irradiation. J. Mol. Catal. A Chem. 2016, 425, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, D.; Lin, Y.; Wang, P.; Chen, W.; Fu, X.; Shao, Y. Evidence for the active species involved in the photodegradation process of methyl orange on TiO2. J. Phys. Chem. C 2012, 116, 3552–3560. [Google Scholar] [CrossRef]
- Xue, B.; Sun, T.; Wu, J.K.; Mao, F.; Yang, W. AgI/TiO2 nanocomposites: Ultrasound-assisted preparation, visible-light induced photocatalytic degradation of methyl orange and antibacterial activity. Ultrason. Sonochem. 2015, 22, 1–6. [Google Scholar] [CrossRef]
- Lu, L.; Shan, R.; Shi, Y.; Wang, S.; Yuan, H. A novel TiO2/biochar composite catalysts for photocatalytic degradation of methyl orange. Chemosphere 2019, 222, 391–398. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, B.; Cui, S.; Yang, S.; Tang, X. Fabrication of SnSO4-modified TiO2 for enhance degradation performance of methyl orange (MO) and antibacterial activity. Appl. Surf. Sci. 2021, 149419. [Google Scholar] [CrossRef]
- Mahalakshmi, G.; Rajeswari, M.; Ponnarasi, P. Synthesis of few-layer g-C3N4 nanosheets-coated MoS2/TiO2 heterojunction photocatalysts for photo-degradation of methyl orange (MO) and 4-nitrophenol (4-NP) pollutants. Inorg. Chem. Commun. 2020, 120, 108146. [Google Scholar] [CrossRef]
- Barka, N.; Assabbane, A.; Nounah, A.; Dussaud, J.; Ichou, Y.A.J.P.C.N. Photocatalytic degradation of methyl orange with immobilized TiO2 nanoparticles: Effect of pH and some inorganic anions. Phys. Chem. News 2008, 41, 85–88. [Google Scholar]
- Grover, I.S.; Singh, S.; Pal, B. The preparation, surface structure, zeta potential, surface charge density and photocatalytic activity of TiO2 nanostructures of different shapes. Appl. Surf. Sci. 2013, 280, 366–372. [Google Scholar] [CrossRef]
- Harnung, S.E.; Johnson, M.S. Chemistry and the Environment; Cambridge University Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Bouanimba, N.; Laid, N.; Zouaghi, R.; Sehili, T. Effect of pH and inorganic salts on the photocatalytic decolorization of methyl orange in the presence of TiO2 P25 and PC500. Desalination Water Treat. 2015, 53, 951–963. [Google Scholar]
- Nirmalkar, N.; Pacek, A.W.; Barigou, M. On the Existence and Stability of Bulk Nanobubbles. Langmuir 2018, 34, 10964–10973. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Ng, W.J.; Liu, Y. Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere 2011, 84, 1175–1180. [Google Scholar] [CrossRef]
- Niu, P.; Hao, J. Fabrication of Titanium Dioxide and Tungstophosphate Nanocomposite Films and Their Photocatalytic Degradation for Methyl Orange. Langmuir 2011, 27, 13590–13597. [Google Scholar] [CrossRef]
- Bahrudin, N.N.; Nawi, M.A.; Zainal, Z. Insight into the synergistic photocatalytic-adsorptive removal of methyl orange dye using TiO2/chitosan based photocatalyst. Int. J. Biol. Macromol. 2020, 165, 2462–2474. [Google Scholar] [CrossRef]
- Gomathi Devi, L.; Mohan Reddy, K. Enhanced photocatalytic activity of silver metallized TiO2 particles in the degradation of an azo dye methyl orange: Characterization and activity at different pH values. Appl. Surf. Sci. 2010, 256, 3116–3121. [Google Scholar] [CrossRef]
- Azevedo, A.; Etchepare, R.; Calgaroto, S.; Rubio, J. Aqueous dispersions of nanobubbles: Generation, properties and features. Miner. Eng. 2016, 94, 29–37. [Google Scholar] [CrossRef]

| Gas | dbubble/nm | DO/mg/L | Reference |
|---|---|---|---|
| Air | 140–350 | 10.7 | [23] |
| O2 | 137–380 | 39.6 | |
| Air | 20–204 | - | [24] |
| O2 | 67–159 | - | |
| N2 | 38–166 | - | |
| O2 | 100–500 | 30.0 | [25] |
| Air | 400–6990 | 9.3 | [26] |
| Air | 200–700 | 8.7 | This study |
| O2 | 40–700 | 14.9 |
| Target Reactive Species | k1 for No Aeration/min−1 | k1 for Air Aeration/min−1 | k1 for N2 Aeration/min−1 | |
|---|---|---|---|---|
| Without scavengers | 7.5 × 10−3 | 8.5 × 10−3 | 7.3 × 10−3 | |
| KI | h+ and •OH | 2.8 × 10−3 | 4.2 × 10−3 | 2.9 × 10−3 |
| TBA | •OH | 5.1 × 10−3 | 6.6 × 10−3 | 4.4 × 10−3 |
| NaNO3 | e−cb | 3.5 × 10−3 | 2.6 × 10−3 | 6.3 × 10−3 |
| pBQ | O2 − | 3.6 × 10−3 | 1.7 × 10−3 | 6.4 × 10−3 |
| Contaminant | Photocatalyst | Scavenger | Target Reactive Species | Removal Efficiency (%) | Conditions | References |
|---|---|---|---|---|---|---|
| Methyl orange (20 mg/L) | P25 TiO2 (2 g/L) | - | - | 60.3 ± 3.4 | 120 min 14.7 W UVA irradiation, without bubbling, 2 L solution | this study |
| KI | h+ and •OH | 28.2 | ||||
| tert-butyl alcohol (TBA) | •OH | 47.0 | ||||
| NaNO3 | e−cb | 33.75 | ||||
| pBQ | O2•− | 36.3 | ||||
| Methyl orange (6.1 × 10−5 M) | P25 TiO2 (0.53 g/L) | - | - | 95.0 (air-equilibrated) | 4 W UV lamps (centered at 365 nm), 120 min irradiation, 150 mL solution | [38] |
| benzoquinone (BQ) | O2•− | 13.0 | ||||
| AgNO3 | e−cb | 55.0 | ||||
| TBA | •OH | 91.0 | ||||
| ammonium oxalate | h+ | 64.0 | ||||
| Methyl orange (20 mg/L) | 5% SnSO4-modified TiO2 (0.2 g/L) | - | - | ≈92.0 | 14 h visible light irradiation, 150 mL solution | [41] |
| 2 mM BQ | O2•− | ≈19.3 | ||||
| 2 mM KI | h+ and •OH | ≈28.3 | ||||
| 40 mM isopropanol | •OH | ≈49.1 | ||||
| Methyl orange (1 × 10−5 M) | g-C3N4@MoS2/TiO2 (1 g/L) | - | - | 88.0 | 60 min visible light irradiation, 50 mL solution | [42] |
| TBA | •OH | 70.0 | ||||
| BQ | O2•− | 10.0 |
| pH | Methyl Orange Degradation Efficiency without Aeration/% | Methyl Orange Photodegradation Efficiency with O2 Bubbling/% | Methyl Orange Adsorption Efficiency (%) |
|---|---|---|---|
| 2 | 70.8 ± 0.4 | 75.6 ± 1.0 | 29.4 ± 2.1 |
| 5 | 66.4 ± 0.7 | 83.1 ± 2.3 | 31.0 ± 4.2 |
| 7 | 60.1 ± 0.4 | 71.9 ± 0.6 | 18.3 ± 2.5 |
| 10 | 53.9 ± 0.5 | 39.4 ± 1.0 | −0.4 ± 1.3 |
| Contaminant | Photocatalyst | pH | Removal Efficiency/% | Conditions | Reference |
|---|---|---|---|---|---|
| Methyl orange 20 mg/L | P25 TiO2 (2 g/L) | 2 | 70.8 ± 0.4 | 120 min 14.7 W UVA irradiation, without bubbling, 2 L solution | this study |
| 5 | 66.4 ± 0.7 | ||||
| 7 | 60.1 ± 0.4 | ||||
| 10 | 53.9 ± 0.5 | ||||
| Methyl orange 10 mg/L | TiO2/PW12 | 2 | 93.2 | 300 W medium-pressure mercury lamp (MPML, λmax = 365 nm), 60 min irradiation, 20 mL solution | [49] |
| 4 | 54.5 | ||||
| 6.4 | 10.0 | ||||
| Methyl orange 20 mg/L | TiO2/Cs–MT | 3 | 93.7 | 45 W fluorescent lamp, 60 min irradiation, 20 mL solution | [50] |
| 6.3 | 98.0 | ||||
| 9 | 94.3 | ||||
| Methyl orange 10 mg/L | Ag-DP25 (0.16 g/L) | 3 | 43.0 | medium-pressure mercury vapor lamp (350–400 nm), 60 min irradiation, 250 mL solution | [51] |
| 5 | 72.0 | ||||
| 6.6 | 100.0 | ||||
| 9 | 51.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Chen, J.; Ateia, M.; Cates, E.L.; Johnson, M.S. Do Gas Nanobubbles Enhance Aqueous Photocatalysis? Experiment and Analysis of Mechanism. Catalysts 2021, 11, 511. https://doi.org/10.3390/catal11040511
Yu W, Chen J, Ateia M, Cates EL, Johnson MS. Do Gas Nanobubbles Enhance Aqueous Photocatalysis? Experiment and Analysis of Mechanism. Catalysts. 2021; 11(4):511. https://doi.org/10.3390/catal11040511
Chicago/Turabian StyleYu, Weijia, Jiaying Chen, Mohamed Ateia, Ezra L. Cates, and Matthew S. Johnson. 2021. "Do Gas Nanobubbles Enhance Aqueous Photocatalysis? Experiment and Analysis of Mechanism" Catalysts 11, no. 4: 511. https://doi.org/10.3390/catal11040511
APA StyleYu, W., Chen, J., Ateia, M., Cates, E. L., & Johnson, M. S. (2021). Do Gas Nanobubbles Enhance Aqueous Photocatalysis? Experiment and Analysis of Mechanism. Catalysts, 11(4), 511. https://doi.org/10.3390/catal11040511









