Effective Separation of Prime Olefins from Gas Stream Using Anion Pillared Metal Organic Frameworks: Ideal Adsorbed Solution Theory Studies, Cyclic Application and Stability
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Adsorbent Synthesis
2.2. Sample Characterization:
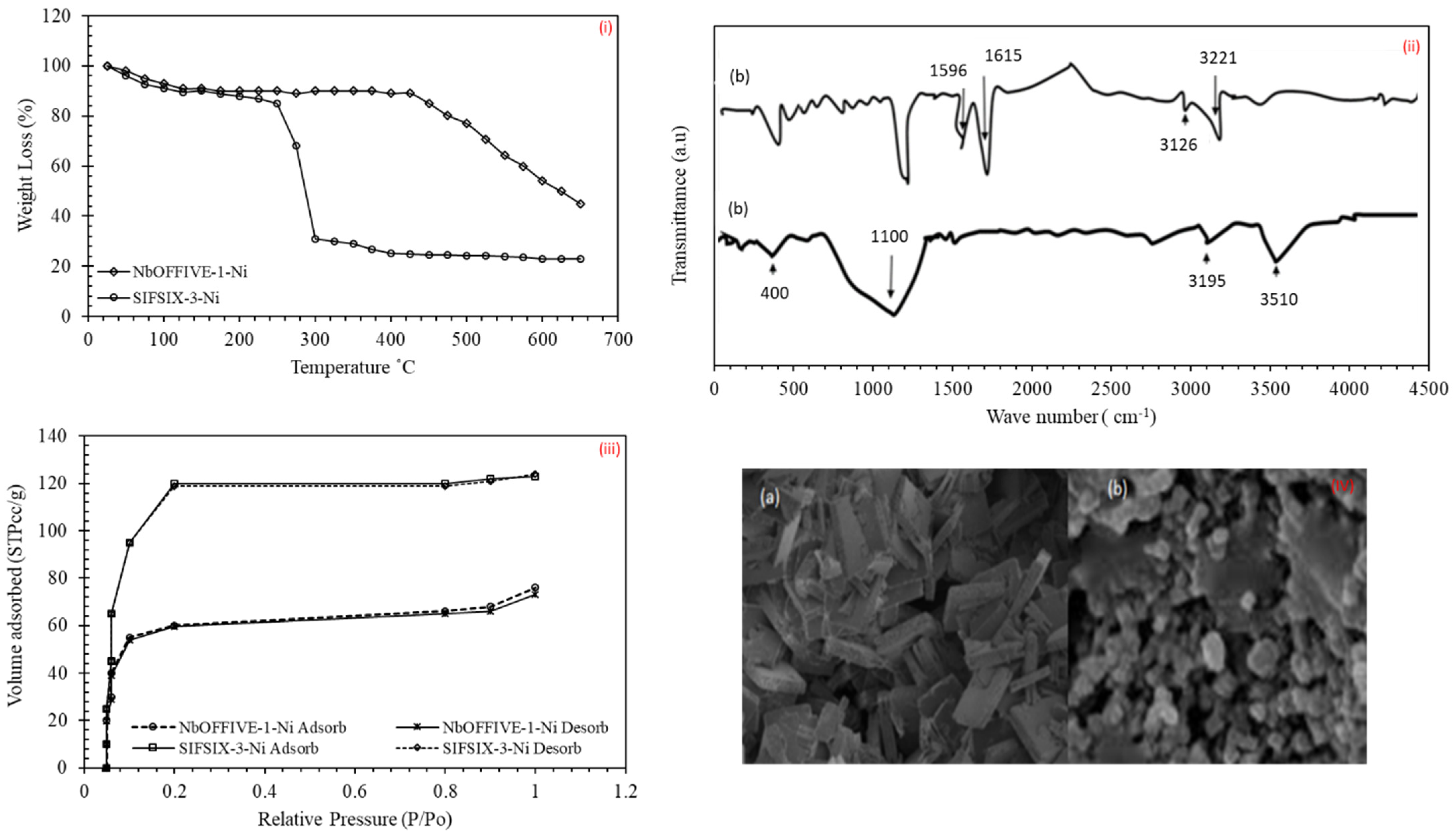
2.2.1. Brunauer-Emmett-Teller (BET) Analysis
2.2.2. Thermogravimetric Analysis (TGA), SEM and FTIR
2.3. Gas Uptake
Equilibrium and Kinetic Breakthrough Gas Adsorption Studies
3. Theoretical Modeling
3.1. Isotherm and Kinetic Models
3.2. Isosteric Heats of Adsorption, IAST Selectivity and Adsorption Kinetics
4. Results and Discussion
4.1. Adsorbent Characterization
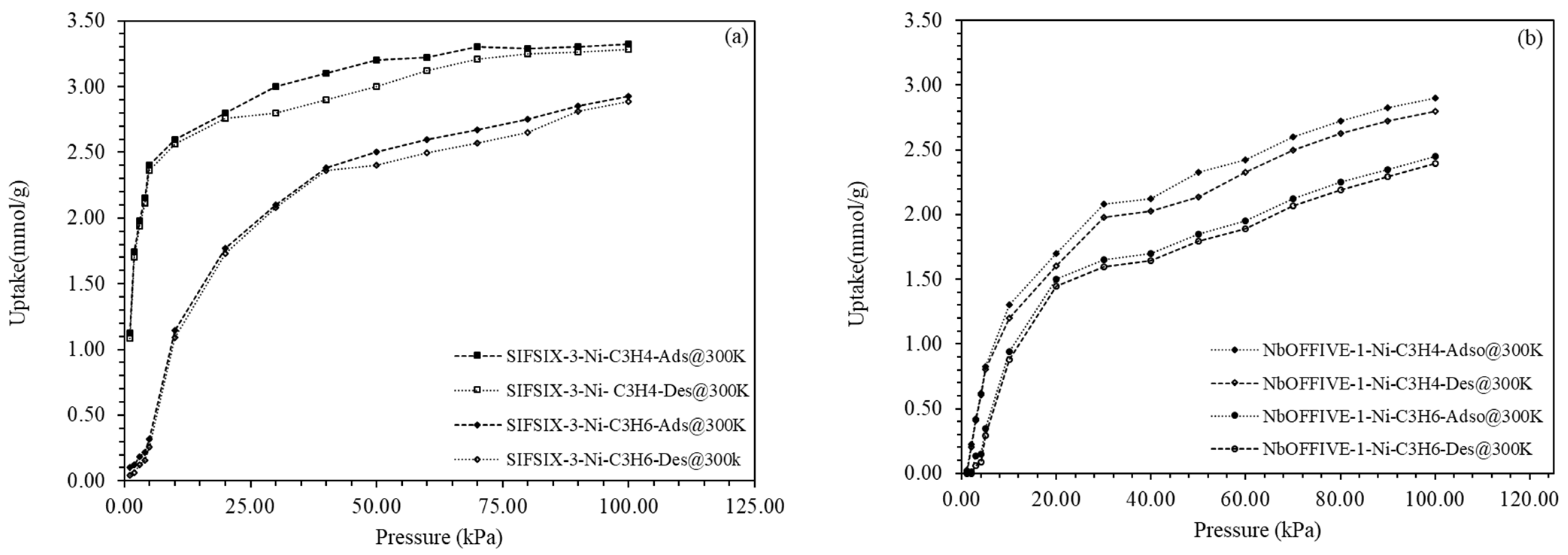
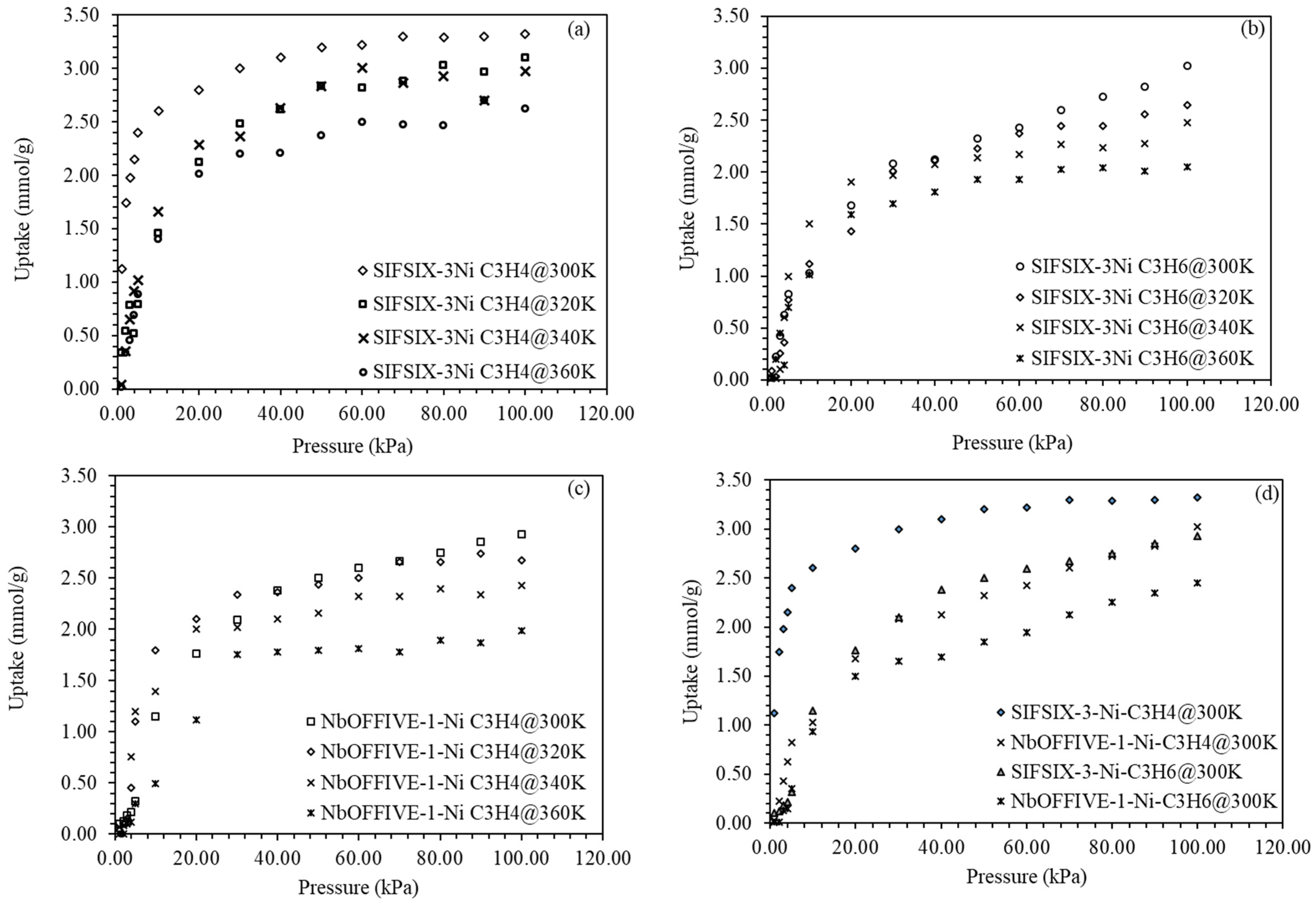
4.2. C3H4/C3H6 Uptake and Separation
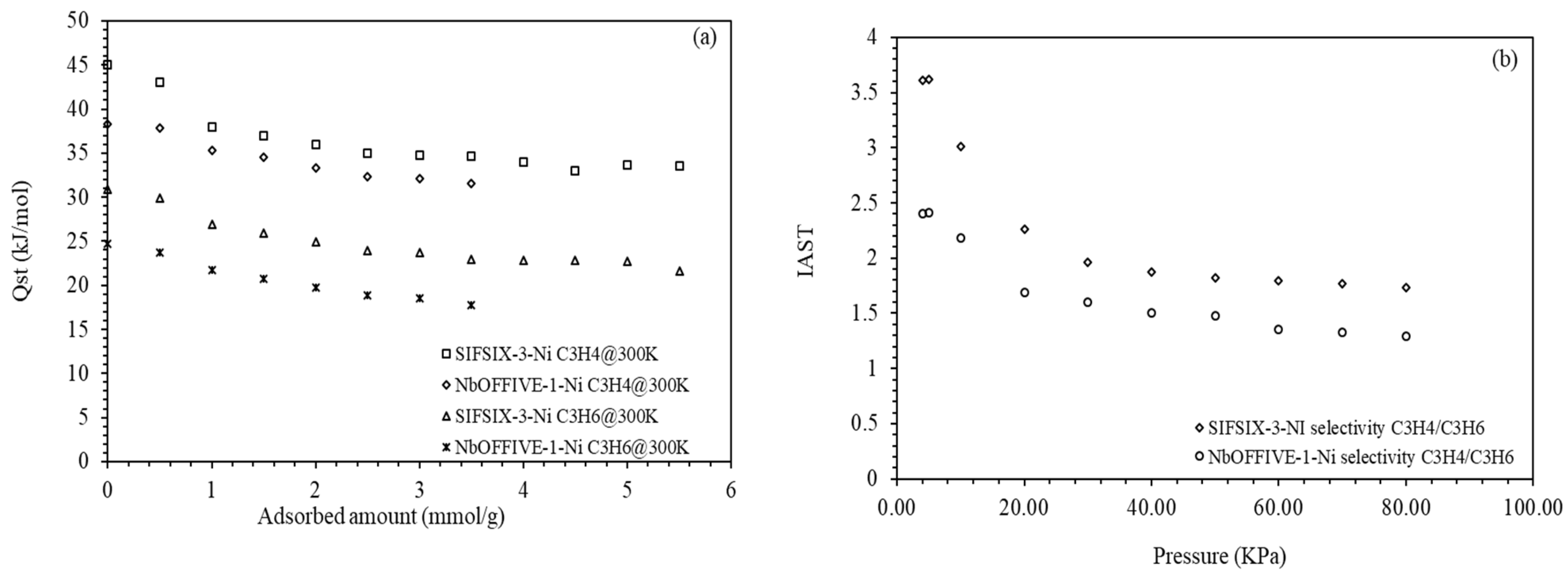
4.3. IAST Selectivity and Isosteric Heats of Adsorption
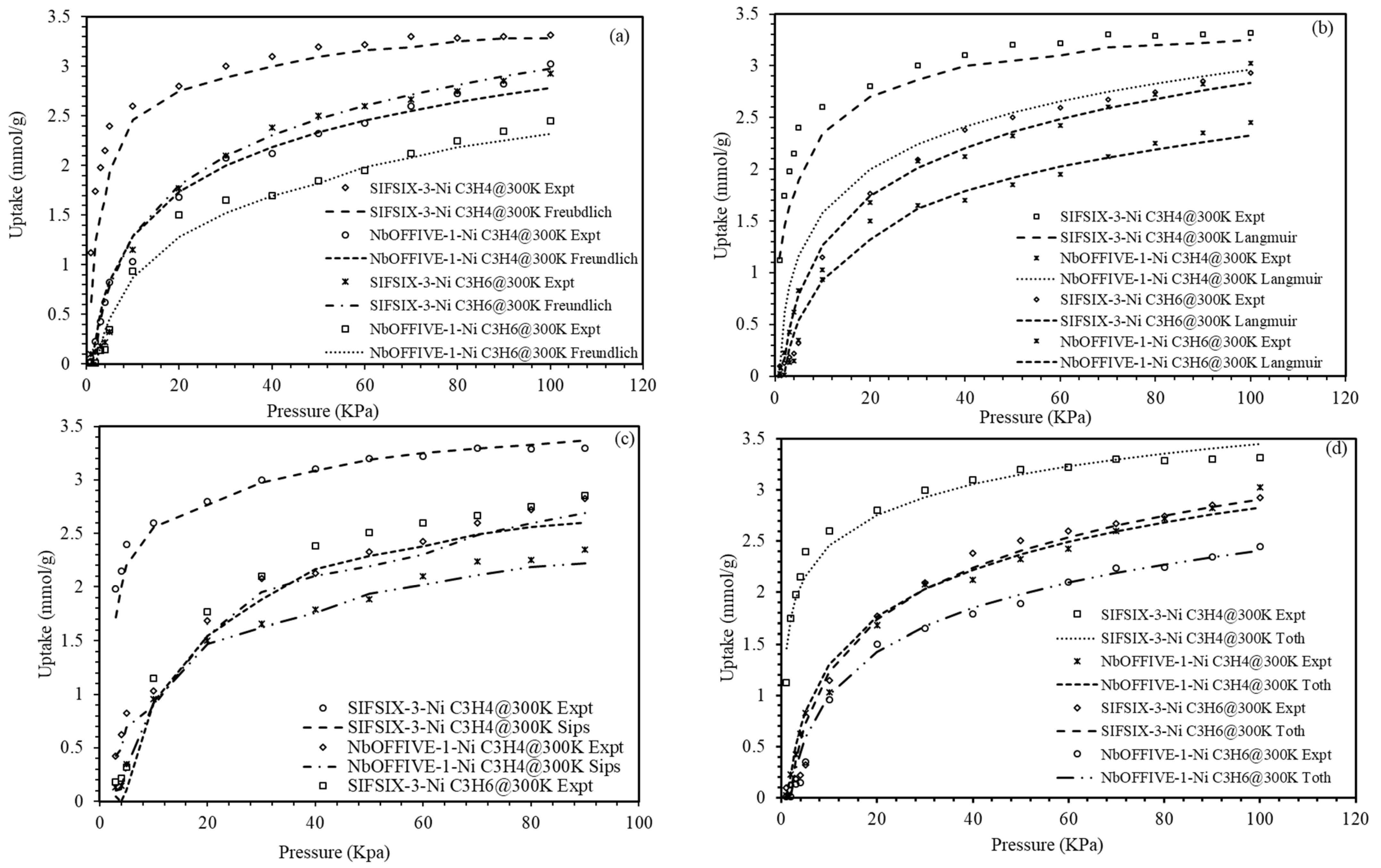
4.4. Modeling of the Equilibrium and Kinetic Adsorption for C3H4/C3H6 on Metal Organic Frameworks
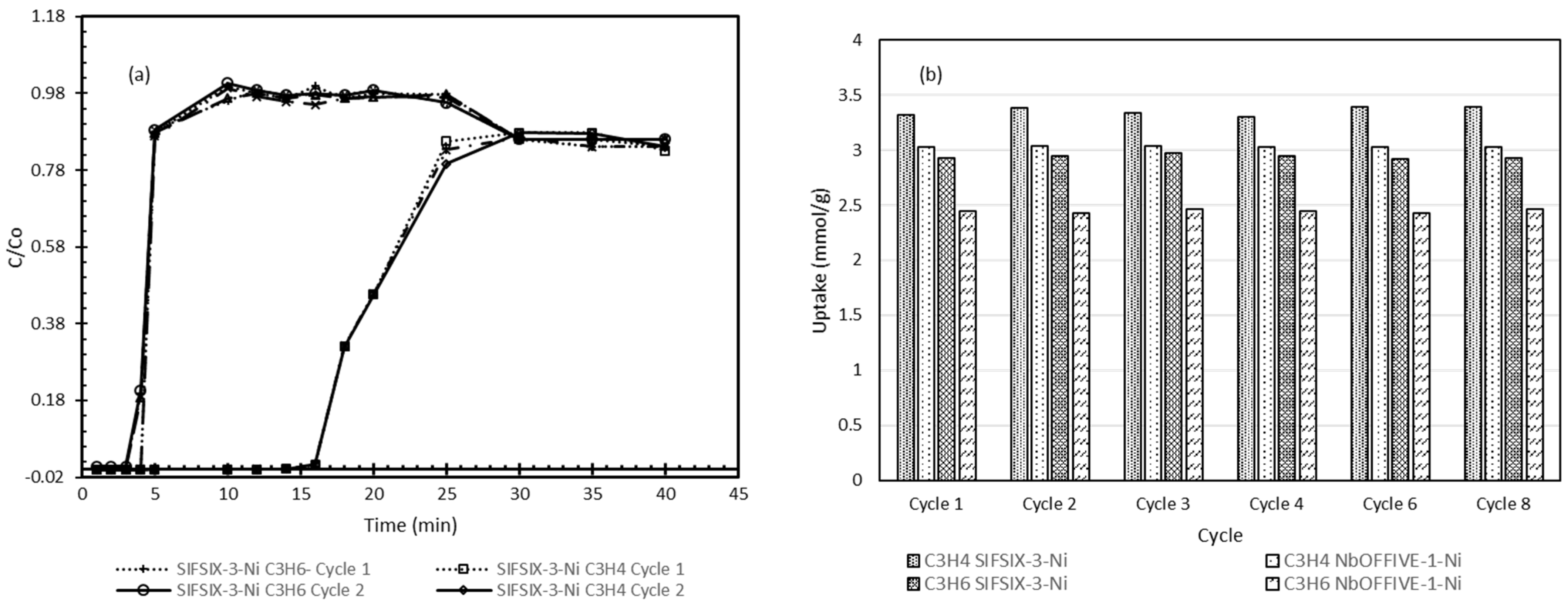
4.5. Breakthrough and Cyclic Breakthrough Experiments
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Zhao, D. Beyond Equilibrium: Metal–Organic Frameworks for Molecular Sieving and Kinetic Gas Separation. Cryst. Growth Des. 2017, 17, 2291–2308. [Google Scholar] [CrossRef]
- Da Silva, F.A.; Rodrigues, A.E. Adsorption equilibria and kinetics for propylene and propane over 13X and 4A zeolite pellets. Ind. Eng. Chem. Res. 1999, 38, 2051–2057. [Google Scholar] [CrossRef]
- Li, L.; Guo, L.; Zheng, F.; Zhang, Z.; Yang, Q.; Yang, Y.; Ren, Q.; Bao, Z. Calcium-Based Metal–Organic Framework for Simultaneous Capture of Trace Propyne and Propadiene from Propylene. ACS Appl. Mater. Interfaces 2020, 12, 17147–17154. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wen, H.-M.; He, C.; Lin, R.-B.; Krishna, R.; Wu, H.; Zhou, W.; Li, J.; Li, B.; Chen, B. A Metal–Organic Framework with Suitable Pore Size and Specific Functional Sites for the Removal of Trace Propyne from Propylene. Angew. Chem. 2018, 130, 15403–15408. [Google Scholar] [CrossRef]
- Cui, W.; Hu, T.; Bu, X. Metal–Organic Framework Materials for the Separation and Purification of Light Hydrocarbons. Adv. Mater. 2020, 32, e1806445. [Google Scholar] [CrossRef]
- Li, L.; Duan, Y.; Liao, S.; Ke, Q.; Qiao, Z.; Wei, Y. Adsorption and separation of propane/propylene on various ZIF-8 poly-morphs: Insights from GCMC simulations and the ideal adsorbed solution theory (IAST). Chem. Eng. J. 2020, 386, 123945. [Google Scholar] [CrossRef]
- Martins, V.F.D.; Ribeiro, A.M.; Plaza, M.G.; Santos, J.C.; Loureiro, J.M.; Ferreira, A.F.P. Gas-phase simulated moving bed: Pro-pane/propylene separation on 13X zeolite. J. Chromatogr. A 2015, 1423, 136–148. [Google Scholar] [CrossRef]
- Mundstock, A.; Wang, N.; Friebe, S.; Caro, J. Propane/propene permeation through Na-X membranes: The interplay of separa-tion performance and pre-synthetic support functionalization. Microporous Mesoporous Mater. 2015, 215, 20–28. [Google Scholar] [CrossRef]
- Kim, J.; Hong, S.H.; Park, D.; Chung, K.; Lee, C. Separation of propane and propylene by desorbent swing adsorption using zeolite 13X and carbon dioxide. Chem. Eng. J. 2021, 410, 128276. [Google Scholar] [CrossRef]
- Abedini, H.; Shariati, A.; Khosravi-Nikou, M.R. Adsorption of propane and propylene on M-MOF-74 (M = Cu, Co): Equilibrium and kinetic study. Chem. Eng. Res. Des. 2020, 153, 96–106. [Google Scholar] [CrossRef]
- Dobladez, J.A.D.; Maté, V.I.Á.; Torrellas, S.Á.; Larriba, M. Separation of the propane propylene mixture with high recovery by a dual PSA process. Comput. Chem. Eng. 2020, 136, 106717. [Google Scholar] [CrossRef]
- Grande, C.A.; Gascon, J.; Kapteijn, F.; Rodrigues, A.E. Propane/propylene separation with Li-exchanged zeolite 13X. Chem. Eng. J. 2010, 160, 207–214. [Google Scholar] [CrossRef]
- Jorge, M.; Lamia, N.; Rodrigues, A.E. Molecular simulation of propane/propylene separation on the metal–organic framework CuBTC. Colloids Surf. A Physicochem. Eng. Asp. 2010, 357, 27–34. [Google Scholar] [CrossRef]
- Lamia, N.; Wolff, L.; Leflaive, P.; Gomes, P.S.; Grande, C.A.; Rodrigues, A.E. Propane/Propylene Separation by Simulated Moving Bed I. Adsorption of Propane, Propylene and Isobutane in Pellets of 13X Zeolite. Sep. Sci. Technol. 2007, 42, 2539–2566. [Google Scholar] [CrossRef]
- Li, L.; Lin, R.-B.; Wang, X.; Zhou, W.; Jia, L.; Li, J. Kinetic separation of propylene over propane in a microporous metal-organic framework. Chem. Eng. J. 2018, 354, 977–982. [Google Scholar] [CrossRef]
- Plaza, M.; Ribeiro, A.; Ferreira, A.; Santos, J.; Lee, U.-H.; Chang, J.-S. Propylene/propane separation by vacuum swing adsorption using Cu-BTC spheres. Sep. Purif. Technol. 2012, 90, 109–119. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Tang, Y.; Wen, Y.; Lv, Z.; Liu, S.; Li, X.; Zhou, X. Propane-selective design of zirconium-based MOFs for propylene purification. Chem. Eng. Sci. 2020, 219. [Google Scholar] [CrossRef]
- Yang, L.; Cui, X.; Yang, Q.; Qian, S.; Wu, H.; Bao, Z. A Single-Molecule Propyne Trap: Highly Efficient Removal of Propyne from Propylene with Anion-Pillared Ultramicroporous Materials. Adv. Mater. 2018, 30, 1705374. [Google Scholar] [CrossRef]
- Yang, L.; Cui, X.; Zhang, Y.; Yang, Q.; Xing, H. A highly sensitive flexible metal–organic framework sets a new benchmark for separating propyne from propylene. J. Mater. Chem. A 2018, 6, 24452–24458. [Google Scholar] [CrossRef]
- Das, M.C.; Guo, Q.; He, Y.; Kim, J.; Zhao, C.-G.; Hong, K.; Xiang, S.; Zhang, Z.; Thomas, K.M.; Krishna, R.; et al. Interplay of Metalloligand and Organic Ligand to Tune Micropores within Isostructural Mixed-Metal Organic Frameworks (M′MOFs) for Their Highly Selective Separation of Chiral and Achiral Small Molecules. J. Am. Chem. Soc. 2012, 134, 8703–8710. [Google Scholar] [CrossRef]
- Li, L.; Lin, R.-B.; Krishna, R.; Wang, X.; Li, B.; Wu, H. Flexible–robust metal–organic framework for efficient removal of propyne from propylene. J. Am. Chem. Soc. 2017, 139, 7733–7736. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Krishna, R.; Li, L.; Wang, B.; He, T.; Zhang, Y.-Z.; Li, J.-R.; Li, J. Guest-dependent pressure induced gate-opening effect enables effective separation of propene and propane in a flexible MOF. Chem. Eng. J. 2018, 346, 489–496. [Google Scholar] [CrossRef]
- Bulánek, R.; Koudelková, E.; de Oliveira Ramos, F.S.; Trachta, M.; Bludský, O.; Rubeš, M. Experimental and theoretical study of propene adsorption on alkali metal exchanged FER zeolites. Microporous Mesoporous Mater. 2019, 280, 203–210. [Google Scholar] [CrossRef]
- Li, X.; Shen, W.; Zheng, A. The influence of acid strength and pore size effect on propene elimination reaction over zeolites: A theoretical study. Microporous Mesoporous Mater. 2019, 278, 121–129. [Google Scholar] [CrossRef]
- Liu, G.; Labreche, Y.; Chernikova, V.; Shekhah, O.; Zhang, C.; Belmabkhout, Y.; Eddaoudi, M.; Koros, W.J. Zeolite-like MOF nanocrystals incorporated 6FDA-polyimide mixed-matrix membranes for CO2/CH4 separation. J. Membr. Sci. 2018, 565, 186–193. [Google Scholar] [CrossRef]
- Yilmaz, G.; Keskin, S. Molecular modeling of MOF and ZIF-filled MMMs for CO2/N2 separations. J. Membr. Sci. 2014, 454, 407–417. [Google Scholar] [CrossRef]
- Elsaidi, S.K.; Mohamed, M.H.; Banerjee, D.; Thallapally, P.K. Flexibility in Metal–Organic Frameworks: A fundamental under-standing. Coord. Chem. Rev. 2018, 358, 125–152. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Lin, R.-B.; Zhou, W.; Zhang, Z.; Xiang, S.; Chen, B. Porous metal-organic frameworks for gas storage and separation: Status and challenges. EnergyChem 2019, 1, 100006. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z. Metal-organic frameworks as stationary phase for application in chromatographic separation. J. Chromatogr. A 2017, 1530, 1–18. [Google Scholar] [CrossRef]
- Khraisheh, M.; Mukherjee, S.; Kumar, A.; Al Momani, F.; Walker, G.; Zaworotko, M.J. An overview on trace CO2 removal by advanced physisorbent materials. J. Environ. Manag. 2020, 255, 109874. [Google Scholar] [CrossRef]
- Madden, D.; Albadarin, A.B.; O’Nolan, D.; Cronin, P.; Perry, I.V.J.J.; Solomon, S. Metal-Organic Material Polymer Coatings for Enhanced Gas Sorption Performance and Hydrolytic Stability Under Humid Conditions. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef] [PubMed]
- Madden, D.G.; O’Nolan, D.; Chen, K.-J.; Hua, C.; Kumar, A.; Pham, T.; Forrest, K.A.; Space, B.; Perry, J.J.; Khraisheh, M.; et al. Highly selective CO2 removal for one-step liquefied natural gas processing by physisorbents. Chem. Commun. 2019, 55, 3219–3222. [Google Scholar] [CrossRef]
- Cadiau, A.; Adil, K.; Bhatt, P.; Belmabkhout, Y.; Eddaoudi, M. A metal-organic framework–based splitter for separating propyl-ene from propane. Science 2016, 353, 137–140. [Google Scholar] [CrossRef]
- Khraisheh, M.; Almomani, F.; Walker, G. Solid Sorbents as a Retrofit Technology for CO2 Removal from Natural Gas Under High Pressure and Temperature Conditions. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Ullah, S.; Bustam, M.A.; Al-Sehemi, A.G.; Assiri, M.A.; Abdul Kareem, F.A.; Mukhtar, A. Influence of post-synthetic graphene oxide (GO) functionalization on the selective CO2/CH4 adsorption behavior of MOF-200 at different temperatures; an experimental and adsorption isotherms study. Microporous Mesoporous Mater. 2020, 296, 110002. [Google Scholar] [CrossRef]
- Ullah, S.; Bustam, M.A.; Assiri, M.A.; Al-Sehemi, A.G.; Gonfa, G.; Mukhtar, A.; Kareem, F.A.A.; Ayoub, M.; Saqib, S.; Mellon, N.B. Synthesis and characterization of mesoporous MOF UMCM-1 for CO2/CH4 adsorption; an experimental, isotherm modeling and thermodynamic study. Microporous Mesoporous Mater. 2020, 294, 109844. [Google Scholar] [CrossRef]
- Weston, M.H.; Colón, Y.J.; Bae, Y.-S.; Garibay, S.J.; Snurr, R.Q.; Farha, O.K.; Hupp, J.T.; Nguyen, S.T. High propylene/propane adsorption selectivity in a copper(catecholate)-decorated porous organic polymer. J. Mater. Chem. A 2013, 2, 299–302. [Google Scholar] [CrossRef]
- Kim, A.-R.; Yoon, T.-U.; Kim, E.-J.; Yoon, J.W.; Kim, S.-Y.; Yoon, J.W. Facile loading of Cu (I) in MIL-100 (Fe) through redox-active Fe (II) sites and remarkable propylene/propane separation performance. Chem. Eng. J. 2018, 331, 777–784. [Google Scholar] [CrossRef]
- Bhatt, P.M.; Belmabkhout, Y.; Cadiau, A.; Adil, K.; Shekhah, O.; Shkurenko, A.; Barbour, L.J.; Eddaoudi, M. A Fine-Tuned Fluorinated MOF Addresses the Needs for Trace CO2 Removal and Air Capture Using Physisorption. J. Am. Chem. Soc. 2016, 138, 9301–9307. [Google Scholar] [CrossRef]
- Kumar, A.; Hua, C.; Madden, D.G.; O’Nolan, D.; Chen, K.-J.; Keane, L.-A.J.; Perry, J.J.; Zaworotko, M.J. Hybrid ultramicroporous materials (HUMs) with enhanced stability and trace carbon capture performance. Chem. Commun. 2017, 53, 5946–5949. [Google Scholar] [CrossRef]
- Ullah, S.; Bustam, M.A.; Assiri, M.A.; Al-Sehemi, A.G.; Sagir, M.; Abdul Kareem, F.A. Synthesis, and characterization of metal-organic frameworks -177 for static and dynamic adsorption behavior of CO2 and CH4. Microporous Mesoporous Mater. 2019, 288, 109569. [Google Scholar] [CrossRef]
- Lin, Z.-T.; Liu, Q.-Y.; Yang, L.; He, C.-T.; Li, L.; Wang, Y.-L. Fluorinated Biphenyldicarboxylate-Based Metal–Organic Framework Exhibiting Efficient Propyne/Propylene Separation. Inorg. Chem. 2020, 59, 4030–4036. [Google Scholar] [CrossRef]
- Li, L.; Krishna, R.; Wang, Y.; Yang, J.; Wang, X.; Li, J. Exploiting the gate opening effect in a flexible MOF for selective adsorption of propyne from C1/C2/C3 hydrocarbons. J. Mater. Chem. A 2016, 4, 751–755. [Google Scholar] [CrossRef]
- Wu, H.; Yuan, Y.; Chen, Y.; Xu, F.; Lv, D.; Wu, Y. Efficient adsorptive separation of propene over propane through a pillar-layer cobalt-based metal–organic framework. AIChE J. 2020, 66, e16858. [Google Scholar] [CrossRef]
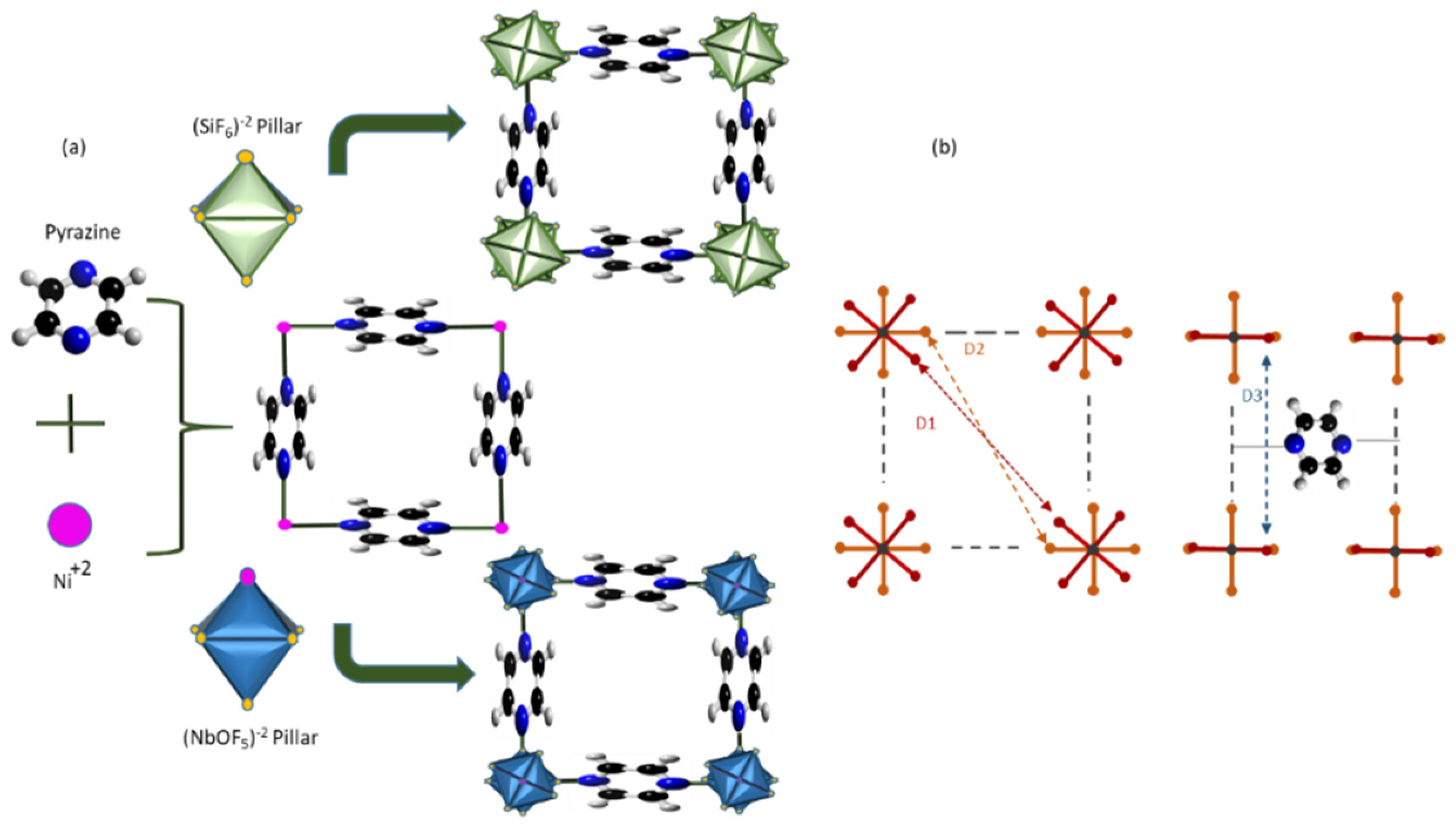

| Sorbent | BET (m2 g−1) | Pore Size (nm) | Pore Volume (cm3 g−1) | Ref | Size (Å3) * | References |
| NbOFFIVE-1-Ni | 248 | 0.139 | 0.095 | This study, ref. [34] | D1:4.66, D2:3.21, D3:4.9 D2:3.047 | [1,18] [1] |
| SIFSIX-3-Ni | 368 | 0.36 | 0.167 | This study, ref. [34] | D1:5.03, D2:3.75, D3:4.6 D2:4.2 D1:5.047 | [18] [4] [1] |
| Hydrocarbon | Structure | Size (Å3) * | References | |||
| C3H4 |  | 4.16 × 4.01 × 6.51 | [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18] | |||
| C3H6 | 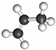 | 5.25 × 4.16 × 6.44 | [3,4,5,18] | |||
| Material | Adsorption Uptake (mmol g−1) | Ref. |
|---|---|---|
| C3H4 from C3H4/C3H6 | ||
| SIFSIX-3-Ni | 3.2 | This study |
| NbOFFIVE-1-Ni | 2.9 | This study |
| SIF-Six-2-Cu-i | 1.73 | [3] |
| SIFSIX-3-Ni | 2.7 | [3] |
| SIFSIX-1-Cu | 0.19 | [18] |
| SIFSIX-2-Cu-i | 0.2 | [18] |
| SIFSIX-3-Ni | 2.65 | [18] |
| [Cu(dhbc)2(4,4′-bipy)] | 0.25 | [43] |
| NK-MOF-Ni | 1.83 | [3] |
| NK-MOF-Cu | 1.76 | [3] |
| SIFSIX-3-Ni | NbOFFIVE-1-Ni | |||
|---|---|---|---|---|
| C3H4 | C3H8 | C3H4 | C3H8 | |
| Langmuir | ||||
| qsat (mmol/g) | 3.32 | 2.93 | 3.03 | 2.45 |
| kl | 0.23 | 0.21 | 0.034 | 0.0022 |
| Rl | 0.82 | 0.65 | 0.74 | 0.62 |
| AARD (%) | 13.7 | 12.2 | 10.3 | 9.7 |
| Freundlich | ||||
| n | 0.298 | 0.228 | 0.265 | 0.2007 |
| kf | 0.342 | 0.432 | 0.665 | 0.453 |
| AARD (%) | 9.2 | 8.5 | 6.8 | 6.3 |
| Sips | ||||
| qsat (mmol/g) | 0.047 | 0.837 | 1.179 | 1.231 |
| Ks (mmol/gbar) | 0.0087 | 0.0076 | 0.0061 | 0.0052 |
| m | 0.067 | 0.025 | 0.0289 | 0.088 |
| AARD (%) | 2.3 | 1.7 | 3.2 | 2.1 |
| Toth | ||||
| qsat (mmol/g) | 4.09 | 3.98 | 3.54 | 2.48 |
| kt | 0.042 | 0.076 | 0.0342 | 0.066 |
| n | 0.203 | 0.019 | 0.187 | 0.0172 |
| AARD(%) | 0.03 | 0.04 | 0.05 | 0.04 |
| Qst (J/mol) | 45.0 | 38.3 | 30.8 | 24.7 |
| Dc/r2c (s−1) | 9.34 × 10−3 | 5.23 × 10−3 | 6.14 × 10−3 | 4.12 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khraisheh, M.; Almomani, F.; Walker, G. Effective Separation of Prime Olefins from Gas Stream Using Anion Pillared Metal Organic Frameworks: Ideal Adsorbed Solution Theory Studies, Cyclic Application and Stability. Catalysts 2021, 11, 510. https://doi.org/10.3390/catal11040510
Khraisheh M, Almomani F, Walker G. Effective Separation of Prime Olefins from Gas Stream Using Anion Pillared Metal Organic Frameworks: Ideal Adsorbed Solution Theory Studies, Cyclic Application and Stability. Catalysts. 2021; 11(4):510. https://doi.org/10.3390/catal11040510
Chicago/Turabian StyleKhraisheh, Majeda, Fares Almomani, and Gavin Walker. 2021. "Effective Separation of Prime Olefins from Gas Stream Using Anion Pillared Metal Organic Frameworks: Ideal Adsorbed Solution Theory Studies, Cyclic Application and Stability" Catalysts 11, no. 4: 510. https://doi.org/10.3390/catal11040510
APA StyleKhraisheh, M., Almomani, F., & Walker, G. (2021). Effective Separation of Prime Olefins from Gas Stream Using Anion Pillared Metal Organic Frameworks: Ideal Adsorbed Solution Theory Studies, Cyclic Application and Stability. Catalysts, 11(4), 510. https://doi.org/10.3390/catal11040510









