Continuous 2-Methyl-3-butyn-2-ol Selective Hydrogenation on Pd/γ-Al2O3 as a Green Pathway of Vitamin A Precursor Synthesis
Abstract
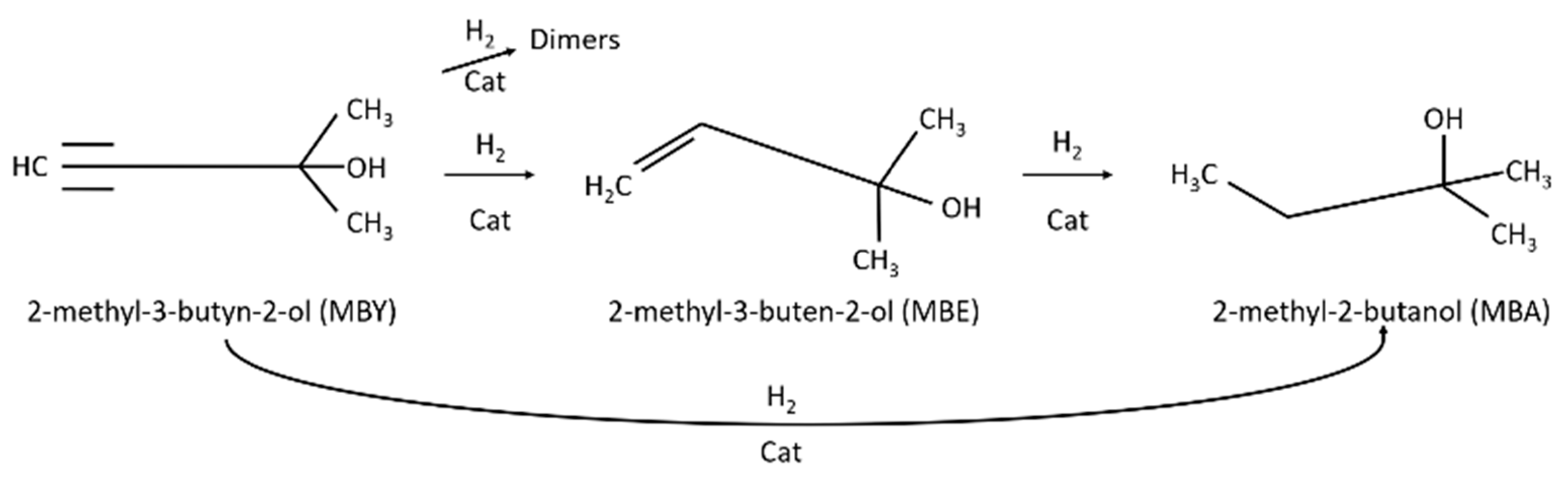
1. Introduction
2. Results and Discussion
2.1. Characterization Results
2.1.1. Physisorption and Chemisorption Results
2.1.2. X-ray Diffraction Results
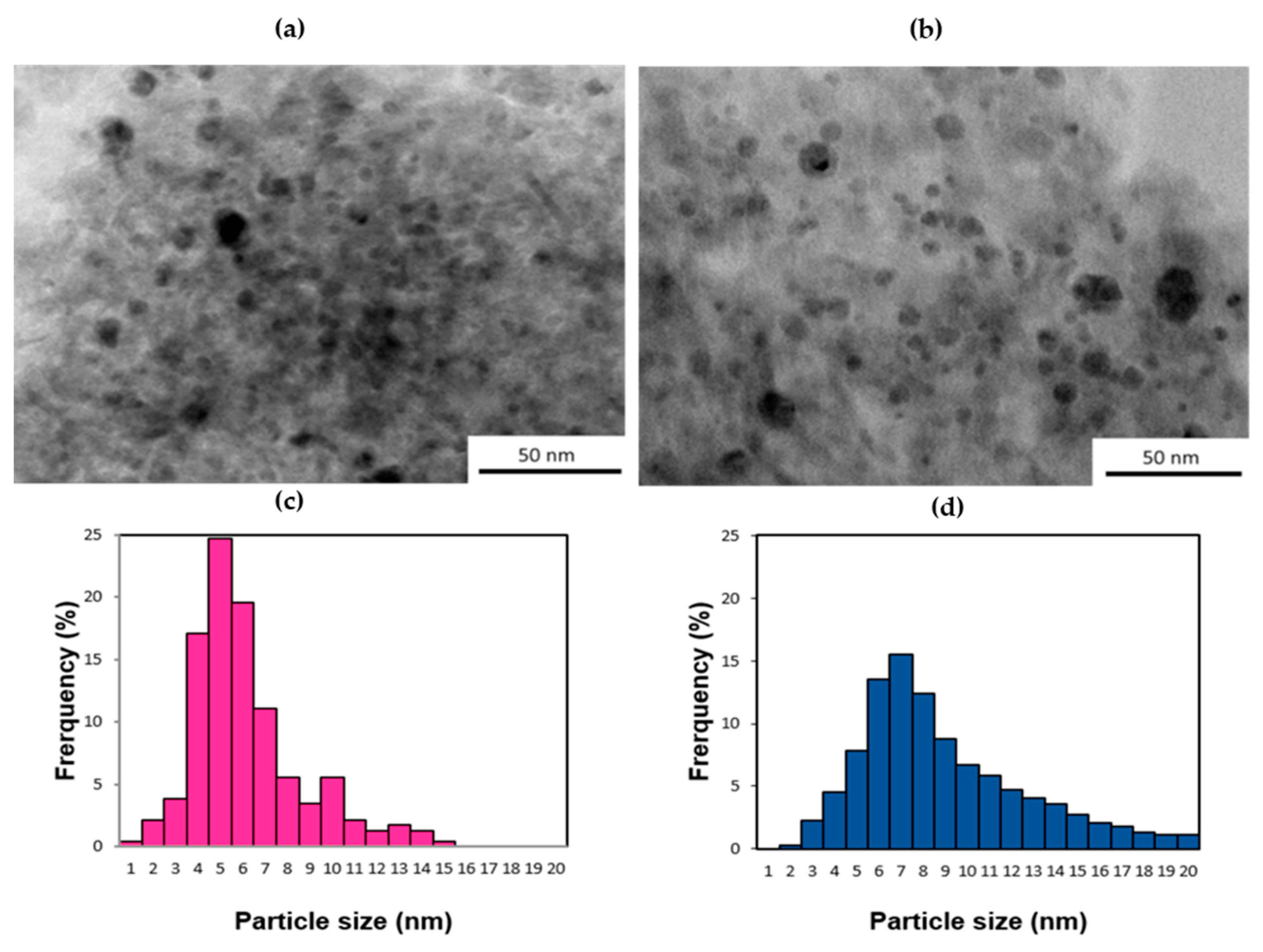
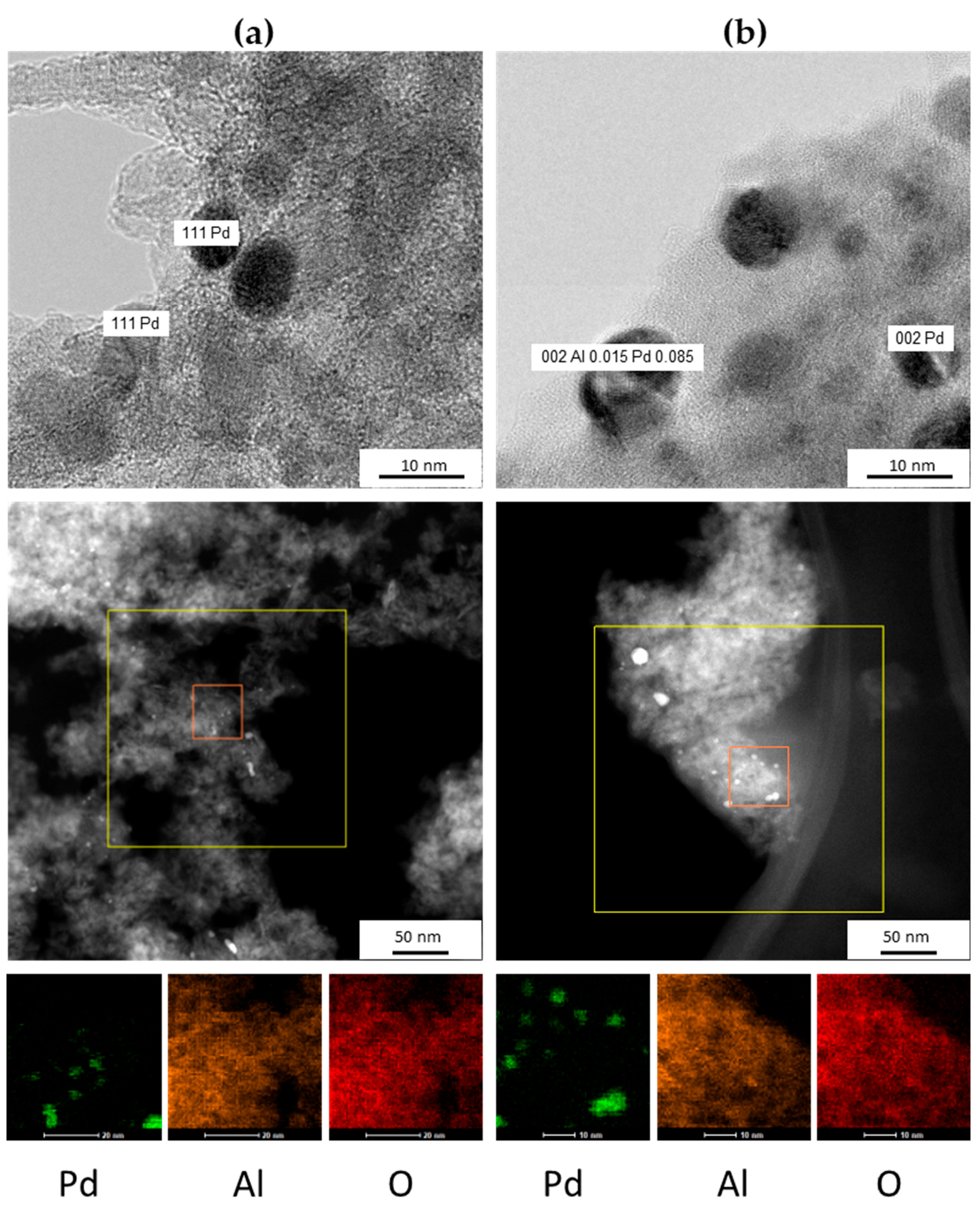
2.1.3. Transmission Electron Microscopy Results
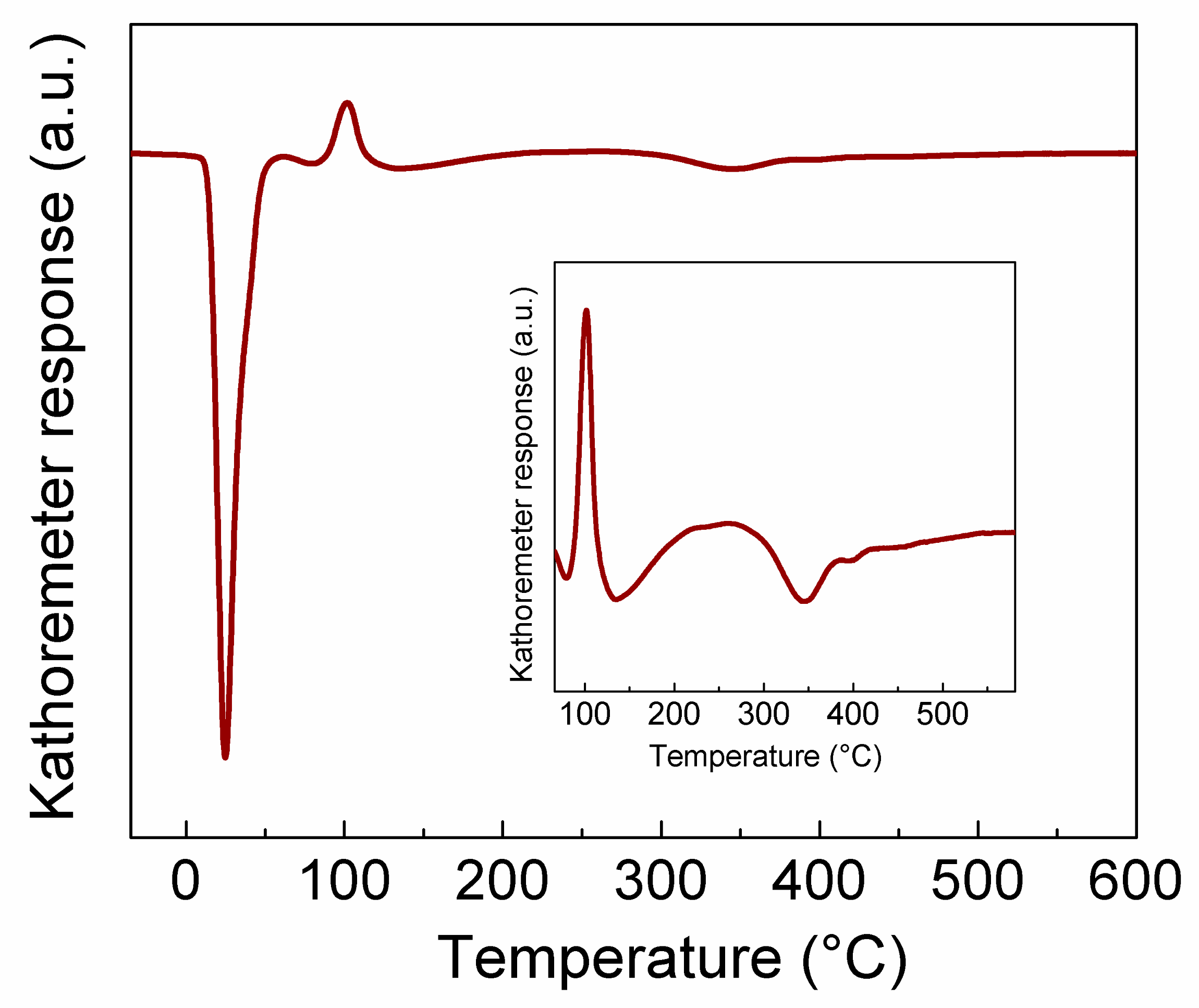
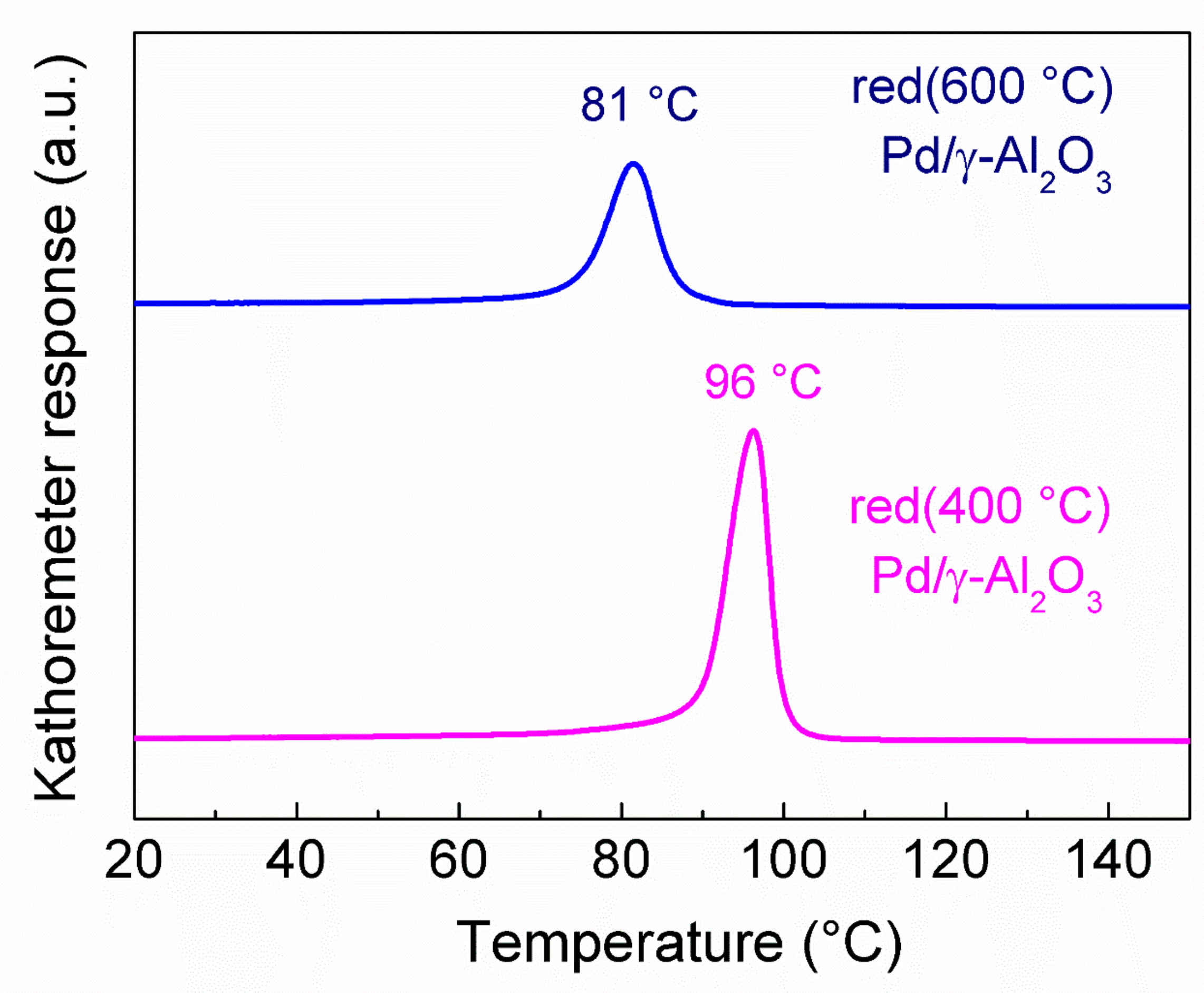
2.1.4. Temperature Programmed Studies Results
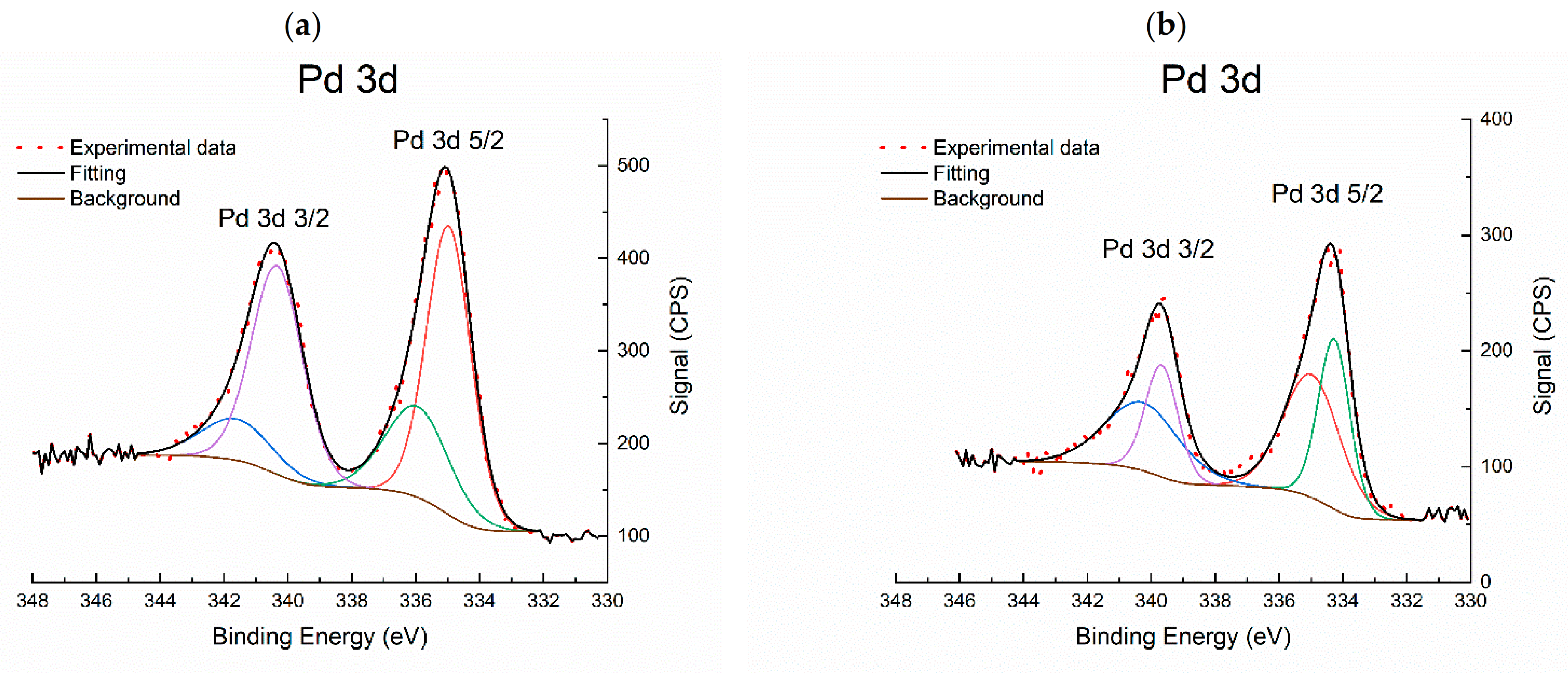
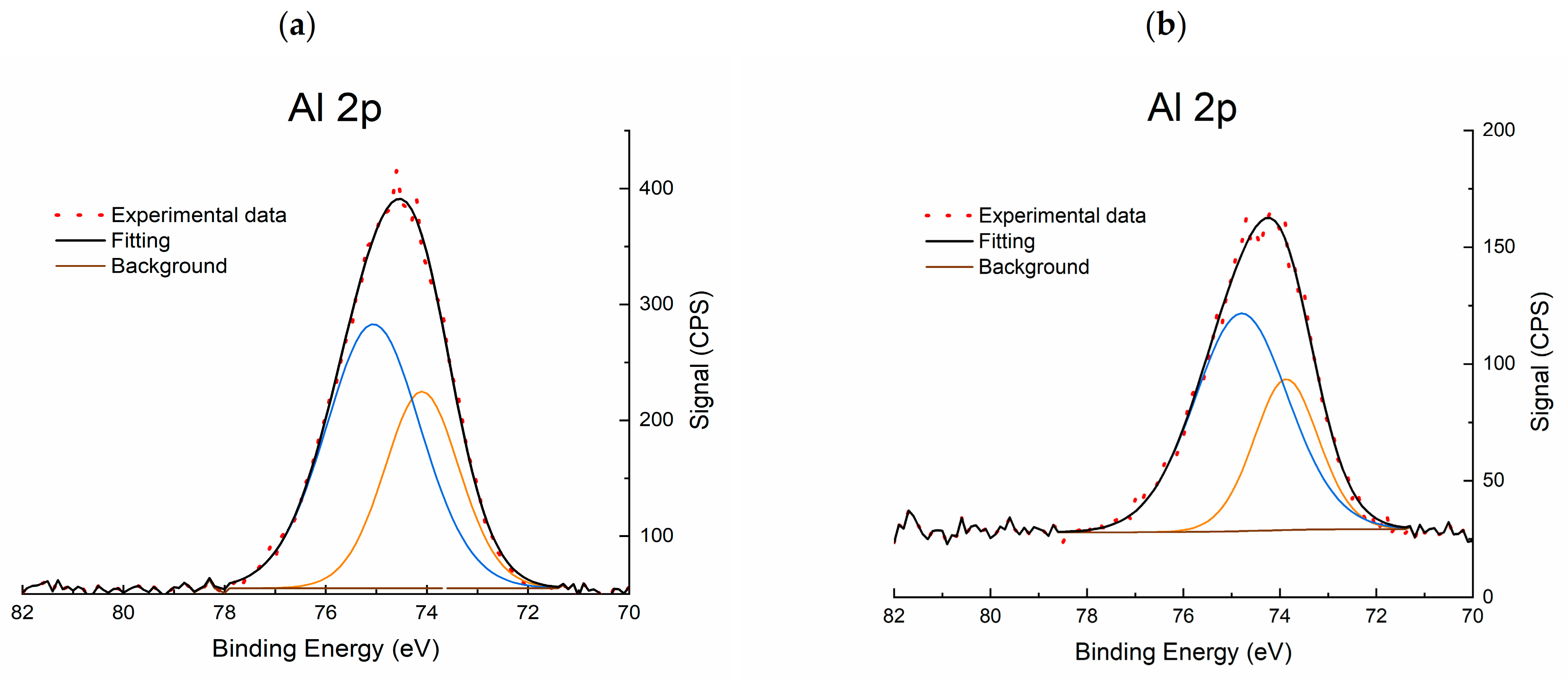
2.1.5. X-ray Photoelectron Spectroscopy (XPS)
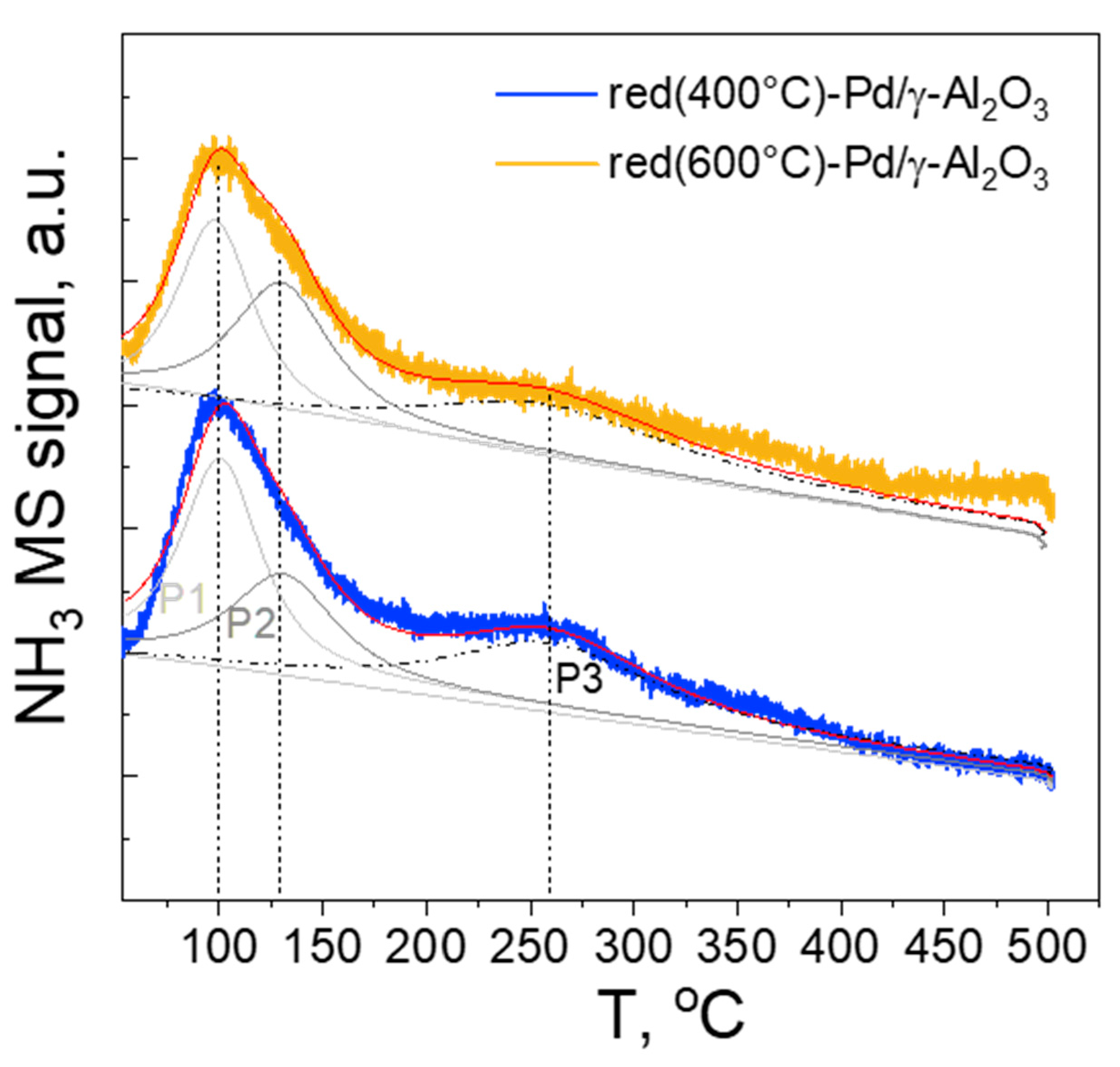
2.1.6. NH3 Temperature-Programmed Desorption (NH3-TPD)
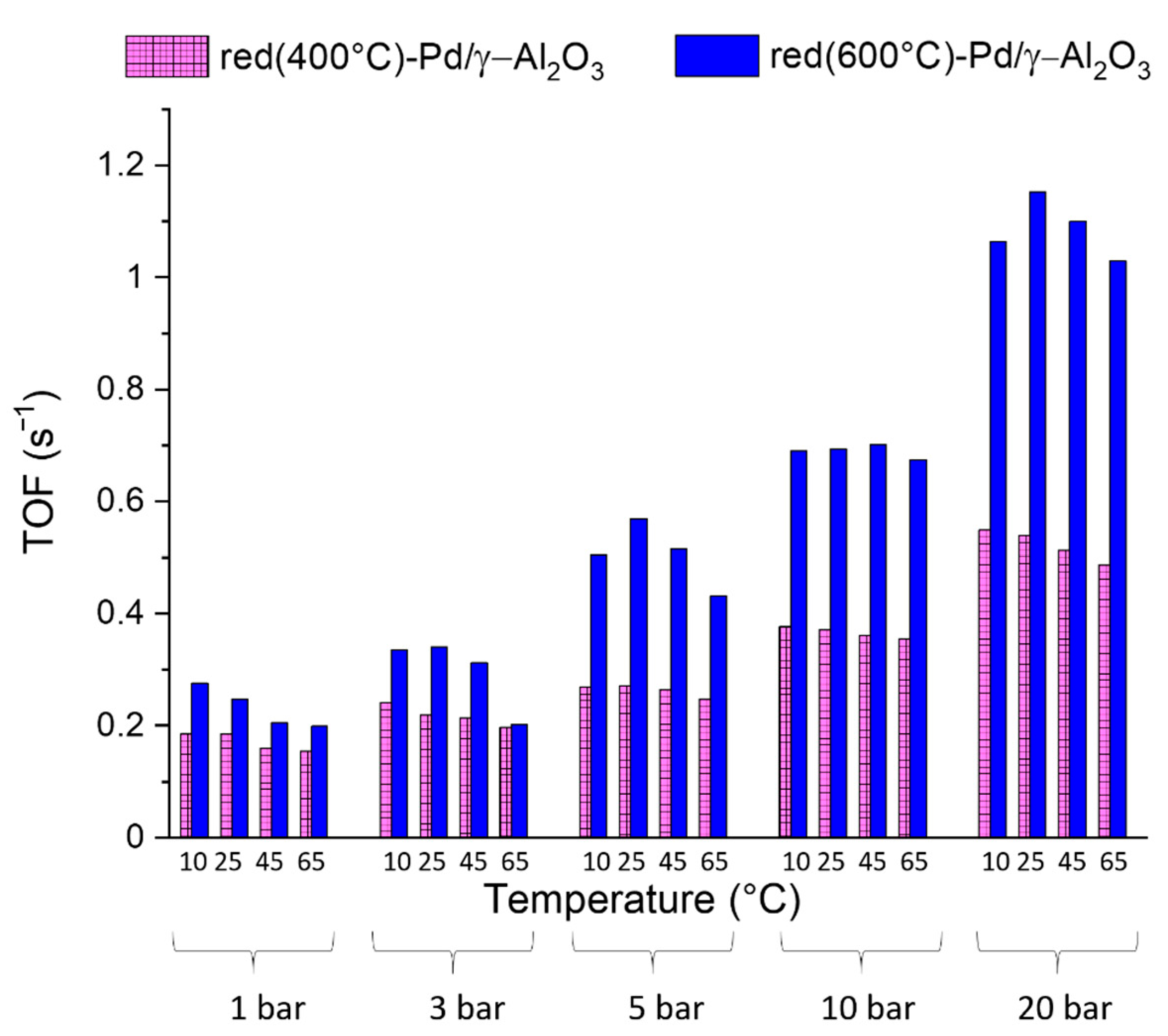
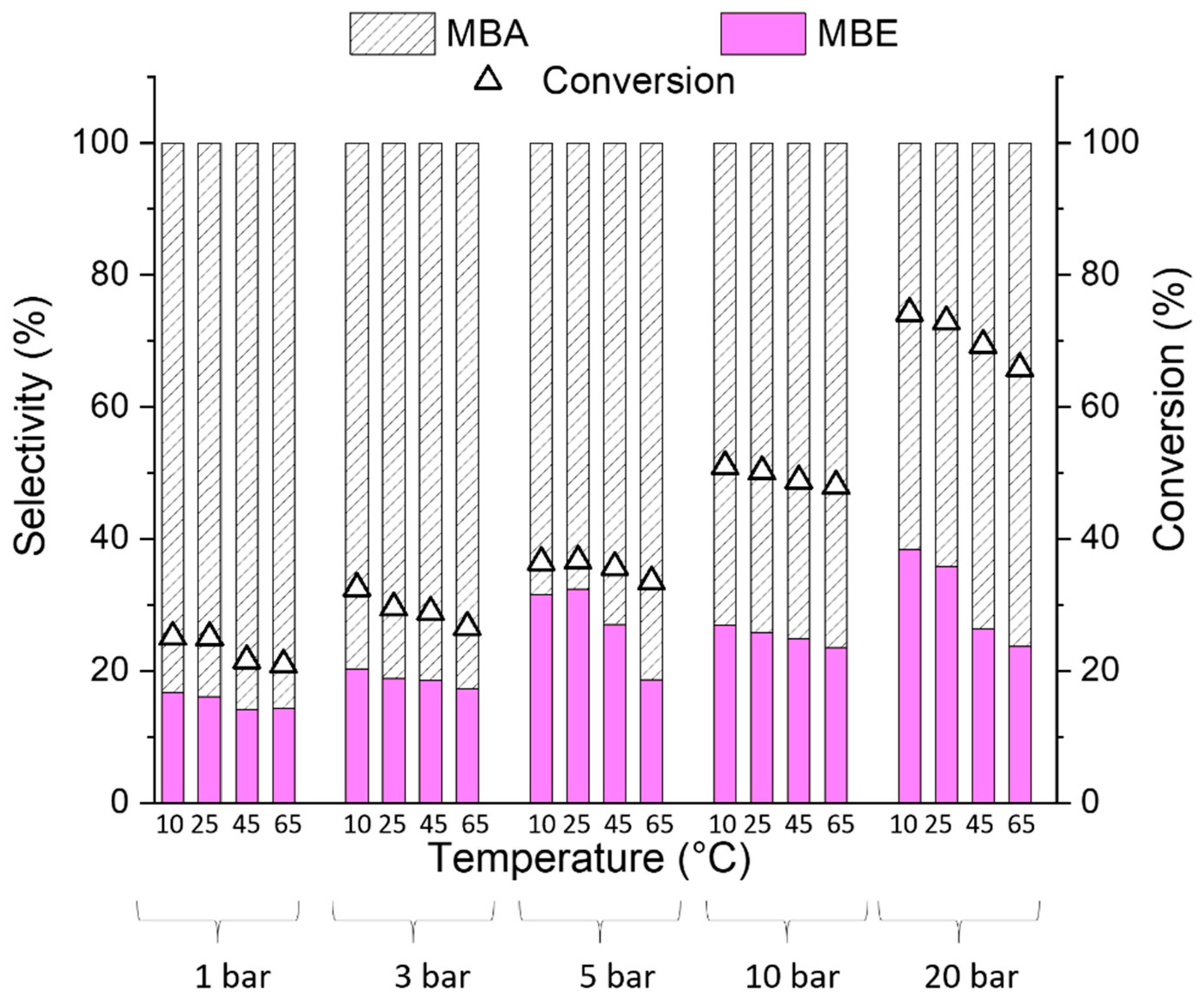
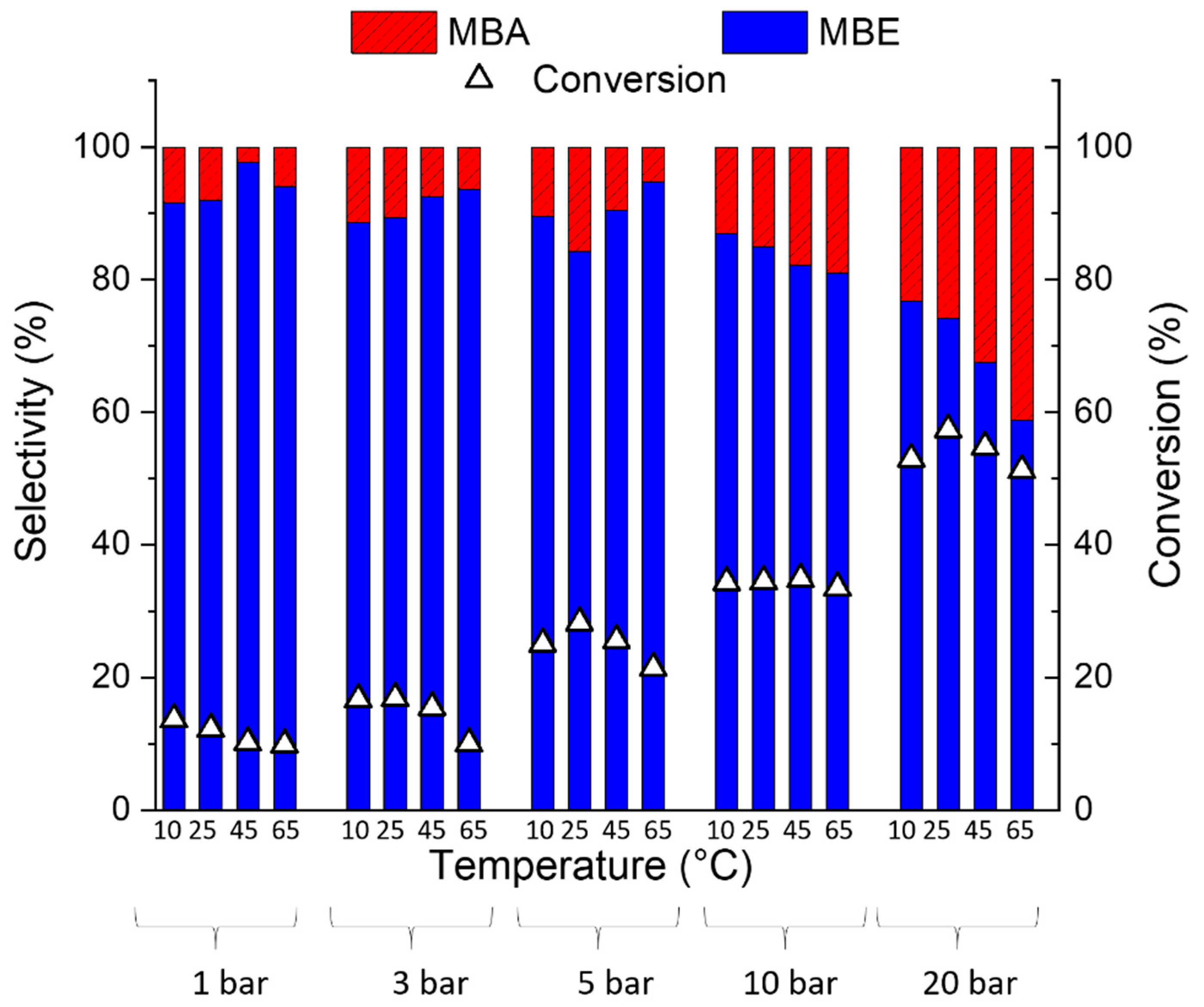
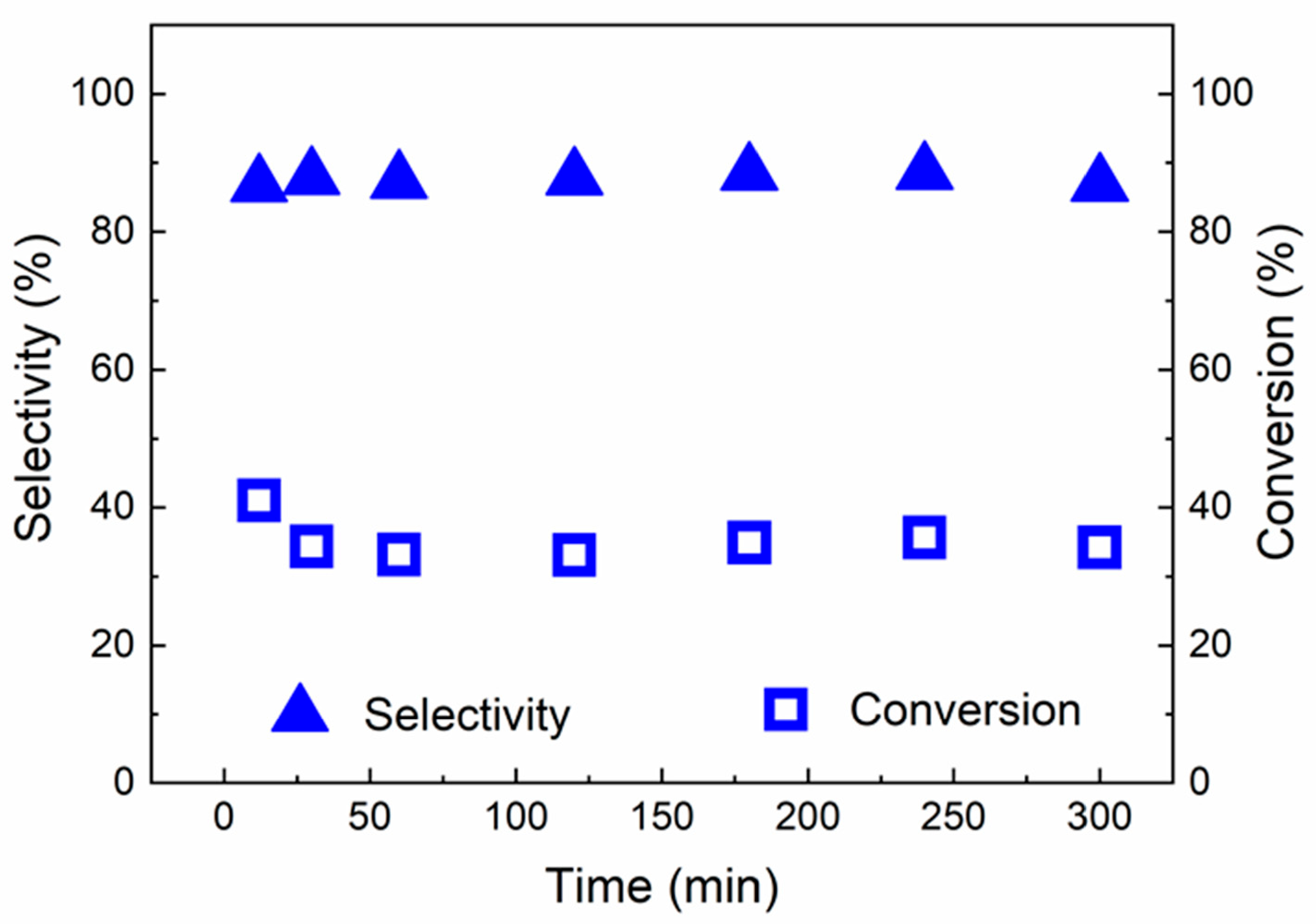
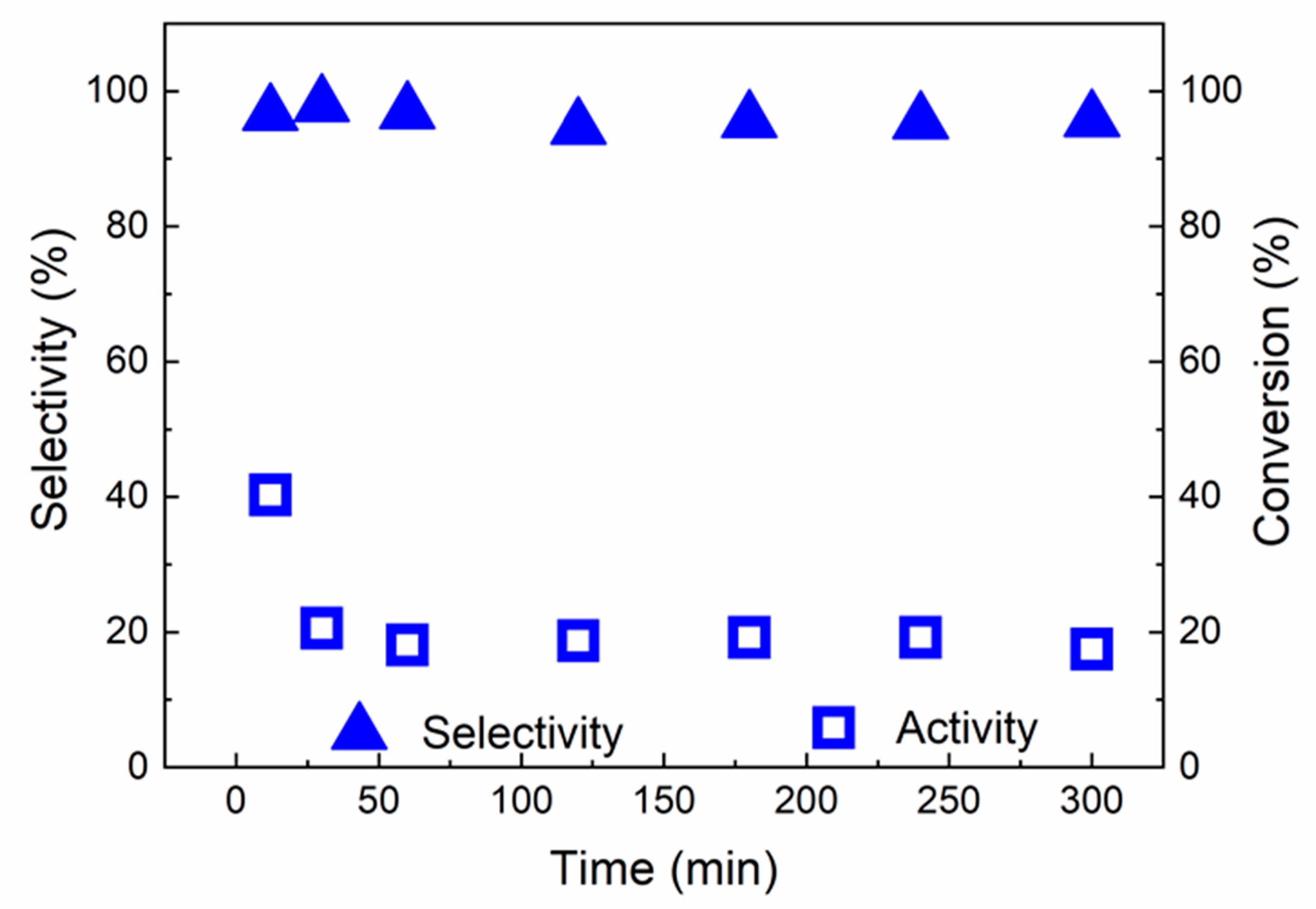
2.2. Catalytic Results
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalysts Characterization
3.2.1. Physisorption
3.2.2. Chemisorption
3.2.3. X-ray Diffraction
3.2.4. Temperature Programmed Studies
3.2.5. TEM Measurements
3.2.6. XPS Measurements
3.2.7. NH3-TPD Measurements
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonrath, W.; Netscher, T. Catalytic processes in vitamins synthesis and production. Appl. Catal. A Gen. 2005, 280, 55–73. [Google Scholar] [CrossRef]
- Ricciardi, R.; Huskens, J.; Verboom, W. Nanocatalysis in Flow. ChemSusChem 2015, 8, 2586–2605. [Google Scholar] [CrossRef] [PubMed]
- Baxendale, I.R. The integration of flow reactors into synthetic organic chemistry. J. Chem. Technol. Biotechnol. 2013, 88, 519–552. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2009, 39, 301–312. [Google Scholar] [CrossRef]
- González-Fernández, A.; Berenguer-Murcia, Á.; Cazorla-Amoros, D.; Cárdenas-Lizana, F. Zn-Promoted Selective Gas-Phase Hydrogenation of Tertiary and Secondary C4 Alkynols over Supported Pd. ACS Appl. Mater. Interfaces 2020, 12, 28158–28168. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Laudert, D.; Létinois, U.; McClymont, T.; Medlock, J.; Netscher, T.; Bonrath, W. One Hundred Years of Vitamins-A Success Story of the Natural Sciences. Angew. Chem. Int. Ed. 2012, 51, 12960–12990. [Google Scholar] [CrossRef]
- Vernuccio, S.; Goy, R.; Von Rohr, P.R.; Medlock, J.; Bonrath, W. Hydrogenation of 2-methyl-3-butyn-2-ol over a Pd/ZnO catalyst: Kinetic model and selectivity study. React. Chem. Eng. 2016, 1, 445–453. [Google Scholar] [CrossRef]
- Lindlar, H. Ein neuer Katalysator für selektive Hydrierungen. Helv. Chim. Acta 1952, 35, 446–450. [Google Scholar] [CrossRef]
- Ao, I.; Sk, K. The Reduction of Alkynes over Pd-Based Catalyst Materials—A Pathway to Chemical Synthesis. J. Biosens. Bioelectron. 2017, 9, 1–15. [Google Scholar] [CrossRef]
- Crespo-Quesada, M.; Grasemann, M.; Semagina, N.; Renken, A.; Kiwi-Minsker, L. Kinetics of the solvent-free hydrogenation of 2-methyl-3-butyn-2-ol over a structured Pd-based catalyst. Catal. Today 2009, 147, 247–254. [Google Scholar] [CrossRef]
- Rebrov, E.V.; Klinger, E.A.; Berenguer-Murcia, A.; Sulman, E.M.; Schouten, J.C. Selective Hydrogenation of 2-Methyl-3-butyne-2-ol in a Wall-Coated Capillary Microreactor with a Pd25Zn75/TiO2Catalyst. Org. Process. Res. Dev. 2009, 13, 991–998. [Google Scholar] [CrossRef]
- Okhlopkova, L.B.; Kerzhentsev, M.A.; Ismagilov, Z.R. Capillary microreactor with a catalytic coating based on mesoporous titanium dioxide for the selective hydrogenation of 2-methyl-3-butyn-2-ol. Kinet. Catal. 2016, 57, 497–503. [Google Scholar] [CrossRef]
- Kundra, M.; Sultan, B.B.M.; Ng, D.; Wang, Y.; Alexander, D.L.; Nguyen, X.; Xie, Z.; Hornung, C.H. Continuous flow semi-hydrogenation of alkynes using 3D printed catalytic static mixers. Chem. Eng. Process. Process. Intensif. 2020, 154, 108018. [Google Scholar] [CrossRef]
- Prestianni, A.; Crespo-Quesada, M.; Cortese, R.; Ferrante, F.; Kiwi-Minsker, L.; Duca, D. Structure Sensitivity of 2-Methyl-3-butyn-2-ol Hydrogenation on Pd: Computational and Experimental Modeling. J. Phys. Chem. C 2014, 118, 3119–3128. [Google Scholar] [CrossRef]
- Albani, D.; Vilé, G.; Toro, M.A.B.; Kaufmann, R.; Mitchell, S.; Pérez-Ramírez, J. Structuring hybrid palladium nanoparticles in metallic monolithic reactors for continuous-flow three-phase alkyne hydrogenation. React. Chem. Eng. 2016, 1, 454–462. [Google Scholar] [CrossRef]
- Vilé, G.; Albani, D.; Nachtegaal, M.; Chen, Z.; Dontsova, D.; Antonietti, M.; López, N.; Pérez-Ramírez, J. A Stable Single-Site Palladium Catalyst for Hydrogenations. Angew. Chem. Int. Ed. 2015, 54, 11265–11269. [Google Scholar] [CrossRef]
- Radlik, M.; Małolepszy, A.; Matus, K.; Śrębowata, A.; Juszczyk, W.; Dłużewski, P.; Karpiński, Z. Alkane isomerization on highly reduced Pd/Al2O3 catalysts. The crucial role of Pd-Al species. Catal. Commun. 2019, 123, 17–22. [Google Scholar] [CrossRef]
- Skotak, M.; Łomot, D.; Karpiński, Z. Catalytic conversion of C6-alkanes over Pd/Al2O3 catalysts. Appl. Catal. A Gen. 2002, 229, 103–115. [Google Scholar] [CrossRef]
- Skotak, M.; Karpiński, Z. C6-alkane conversion over γ-alumina supported palladium and platinum catalysts. Chem. Eng. J. 2002, 90, 89–96. [Google Scholar] [CrossRef]
- Skotak, M.; Karpinski, Z.; Juszczyk, W.; Pielaszek, J.; Kepiński, L.; Kazachkin, D.; Kovalchuk, V.; Ditri, J. Characterization and catalytic activity of differently pretreated Pd/Al2O3 catalysts: The role of acid sites and of palladium–alumina interactions. J. Catal. 2004, 227, 11–25. [Google Scholar] [CrossRef]
- Bronstein, L.M.; Chernyshov, D.M.; Karlinsey, R.; Zwanziger, J.W.; Matveeva, V.G.; Sulman, E.M.; Demidenko, G.N.; Hentze, H.-P.; Antonietti, M. Mesoporous Alumina and Aluminosilica with Pd and Pt Nanoparticles: Structure and Catalytic Properties. Chem. Mater. 2003, 15, 2623–2631. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Penner, S.; Jenewein, B.; Hayek, K. Pd–Al interaction at elevated temperatures: A TEM and SAED study. Catal. Lett. 2007, 113, 65–72. [Google Scholar] [CrossRef]
- McKeehan, L.W. The Crystal Structure of Silver-Palladium and Silver-Gold Alloys. Phys. Rev. 1922, 20, 424–432. [Google Scholar] [CrossRef]
- Bergerhoff, G.; Brown, I.D. Crystallographic Databases; Allen, F.H., Bergerhoff, G., Sievers, R., Eds.; International Union of Crystallography: Chester, UK, 1987. [Google Scholar]
- Ellner, M. Zusammenhang zwischen strukturellen und thermodynamischen eigenschaften bei phasen der Cu-Familie in den T10-B3-systemen. J. Less Common Met. 1978, 60, P15–P39. [Google Scholar] [CrossRef]
- Vegard, L. Die Konstitution der Mischkristalle und die Raumfüllung der Atome. Eur. Phys. J. A 1921, 5, 17–26. [Google Scholar] [CrossRef]
- Denton, A.R.; Ashcroft, N.W. Vegard’s law. Phys. Rev. A 1991, 43, 3161–3164. [Google Scholar] [CrossRef]
- Lieske, H.; Voelter, J. Palladium redispersion by spreading of palladium(II) oxide in oxygen treated palladium/alumina. J. Phys. Chem. 1985, 89, 1841–1842. [Google Scholar] [CrossRef]
- Matam, S.K.; Otal, E.; Aguirre, M.; Winkler, A.; Ulrich, A.; Rentsch, D.; Weidenkaff, A.; Ferri, D. Thermal and chemical aging of model three-way catalyst Pd/Al2O3 and its impact on the conversion of CNG vehicle exhaust. Catal. Today 2012, 184, 237–244. [Google Scholar] [CrossRef]
- Bonarowska, M.; Zieliński, M.; Matus, K.; Sá, J.; Śrębowata, A. Influence of microwave activation on the catalytic behavior of Pd-Au/C catalysts employed in the hydrodechlorination of tetrachloromethane. React. Kinet. Mech. Catal. 2018, 124, 375–388. [Google Scholar] [CrossRef]
- Baker, R.; Prestridge, E.; McVicker, G. The Interaction of palladium with alumina and titanium oxide supports. J. Catal. 1984, 89, 422–432. [Google Scholar] [CrossRef]
- Bonarowska, M.; Kaszkur, Z.; Łomot, D.; Rawski, M.; Karpiński, Z. Effect of gold on catalytic behavior of palladium catalysts in hydrodechlorination of tetrachloromethane. Appl. Catal. B Environ. 2015, 162, 45–56. [Google Scholar] [CrossRef]
- Hubkowska, K.; Łukaszewski, M.; Czerwinski, A. Thermodynamics of hydride formation and decomposition in electrodeposited Pd-rich Pd–Ru alloys. Electrochem. Commun. 2014, 48, 40–43. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., King, R.C., Jr., Eds.; Physical Electronics, Inc.: Chanhassen, MN, USA, 1995. [Google Scholar]
- NIST X-ray Photoelectron Spectroscopy Database; Version 4.1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012. Available online: http://srdata.nist.gov/xps/ (accessed on 29 January 2021).
- Beamson, G.; Brighs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; John Wiley & Sons, Ltd.: Chichester, UK, 1992. [Google Scholar]
- Kung, H.; Kung, M.; Costello, C. Supported Au catalysts for low temperature CO oxidation. J. Catal. 2003, 216, 425–432. [Google Scholar] [CrossRef]
- Al-Daous, M.A.; Manda, A.A.; Hattori, H. Acid–base properties of γ-Al2O3 and MgO–Al2O3 supported gold nanoparticles. J. Mol. Catal. A Chem. 2012, 363-364, 512–520. [Google Scholar] [CrossRef]
- Lv, D.-M.; Xu, Z.-N.; Peng, S.-Y.; Wang, Z.-Q.; Chen, Q.-S.; Chen, Y.; Guo, G.-C. (Pd–CuCl2)/γ-Al2O3: A high-performance catalyst for carbonylation of methyl nitrite to dimethyl carbonate. Catal. Sci. Technol. 2015, 5, 3333–3339. [Google Scholar] [CrossRef]
- Wang, J.; Bokhimi, X.; Novaro, O.; López, T.; Tzompantzi, F.; Gómez, R.; Navarrete, J.; Llanos, M.; López-Salinas, E. Effects of structural defects and acid–basic properties on the activity and selectivity of isopropanol decomposition on nanocrystallite sol–gel alumina catalyst. J. Mol. Catal. A Chem. 1999, 137, 239–252. [Google Scholar] [CrossRef]
- Borodziński, A.; Bond, G.C. Selective Hydrogenation of Ethyne in Ethene-Rich Streams on Palladium Catalysts. Part 1. Effect of Changes to the Catalyst during Reaction. Catal. Rev. 2006, 48, 91–144. [Google Scholar] [CrossRef]
- Teschner, D.; Borsodi, J.; Wootsch, A.; Révay, Z.; Hävecker, M.; Knop-Gericke, A.; Jackson, S.D.; Schlögl, R. The Roles of Subsurface Carbon and Hydrogen in Palladium-Catalyzed Alkyne Hydrogenation. Science 2008, 320, 86–89. [Google Scholar] [CrossRef]
- Borodziński, A.; Bond, G.C. Selective Hydrogenation of Ethyne in Ethene-Rich Streams on Palladium Catalysts, Part 2: Steady-State Kinetics and Effects of Palladium Particle Size, Carbon Monoxide, and Promoters. Catal. Rev. 2008, 50, 379–469. [Google Scholar] [CrossRef]
- Kim, M.; Bertram, M.; Pollmann, M.; Von Oertzen, A.; Mikhailov, A.S.; Rotermund, H.H.; Ertl, G. Controlling Chemical Turbulence by Global Delayed Feedback: Pattern Formation in Catalytic CO Oxidation on Pt(110). Science 2001, 292, 1357–1360. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Kim, W.K.; Dzubiella, J. Coverage Fluctuations and Correlations in Nanoparticle-Catalyzed Diffusion-Influenced Bimolecular Reactions. J. Phys. Chem. C 2020, 124, 24204–24214. [Google Scholar] [CrossRef]
- Crespo-Quesada, M.; Yarulin, A.; Jin, M.; Xia, Y.; Kiwi-Minsker, L. Structure Sensitivity of Alkynol Hydrogenation on Shape- and Size-Controlled Palladium Nanocrystals: Which Sites Are Most Active and Selective? J. Am. Chem. Soc. 2011, 133, 12787–12794. [Google Scholar] [CrossRef]
- Khan, N.A.; Shaikhutdinov, S.; Freund, H.-J. Acetylene and Ethylene Hydrogenation on Alumina Supported Pd-Ag Model Catalysts. Catal. Lett. 2006, 108, 159–164. [Google Scholar] [CrossRef]
- Childers, D.; Saha, A.; Schweitzer, N.; Rioux, R.M.; Miller, J.T.; Meyer, R.J. Correlating Heat of Adsorption of CO to Reaction Selectivity: Geometric Effects vs Electronic Effects in Neopentane Isomerization over Pt and Pd Catalysts. ACS Catal. 2013, 3, 2487–2496. [Google Scholar] [CrossRef]
- Ke¸piński, L.; Wołcyrz, M.; Jabłoński, J. Effect of high-temperature reduction on carburization of alumina-supported palladium: Evidence for palladium-aluminium alloy formation. Appl. Catal. 1989, 54, 267–276. [Google Scholar] [CrossRef]
- Richards, N.; Carter, J.H.; Nowicka, E.; Parker, L.A.; Pattisson, S.; He, Q.; Dummer, N.F.; Golunski, S.; Hutchings, G.J. Structure-sensitivity of alumina supported palladium catalysts for N2O decomposition. Appl. Catal. B Environ. 2020, 264, 118501. [Google Scholar] [CrossRef]

| Samples | BET Specific Surface Area (m2 g−1) | Total Pore Volume (cm3 g−1) |
|---|---|---|
| γ-Al2O3 | 190 | 0.51 |
| Calcined-Pd/γ-Al2O3 | 210 | 0.56 |
| Samples | Particle Size (nm) * | Dispersion (%) |
|---|---|---|
| red(400 °C)-Pd/γ-Al2O3 | 10.2 | 11 |
| red(600 °C)-Pd/γ-Al2O3 | 24.9 | 4.5 |
| Samples | Pd | Al | O | C |
|---|---|---|---|---|
| red(400 °C)-Pd/γ-Al2O3, ex-situ red. | 5.7 | 61.7 | 16.6 | 16.0 |
| red(400 °C)-Pd/γ-Al2O3, in-situ red. | 5.2 | 59.9 | 29.2 | 5.7 |
| red(600 °C)-Pd/γ-Al2O3, ex-situ red. | 4.5 | 42.4 | 30.1 | 23.0 |
| red(600 °C)-Pd/γ-Al2O3, in-situ red. | 7.0 | 67.9 | 19.0 | 6.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Ropero, A.J.; Zawadzki, B.; Kowalewski, E.; Pieta, I.S.; Krawczyk, M.; Matus, K.; Lisovytskiy, D.; Śrębowata, A. Continuous 2-Methyl-3-butyn-2-ol Selective Hydrogenation on Pd/γ-Al2O3 as a Green Pathway of Vitamin A Precursor Synthesis. Catalysts 2021, 11, 501. https://doi.org/10.3390/catal11040501
Fernández-Ropero AJ, Zawadzki B, Kowalewski E, Pieta IS, Krawczyk M, Matus K, Lisovytskiy D, Śrębowata A. Continuous 2-Methyl-3-butyn-2-ol Selective Hydrogenation on Pd/γ-Al2O3 as a Green Pathway of Vitamin A Precursor Synthesis. Catalysts. 2021; 11(4):501. https://doi.org/10.3390/catal11040501
Chicago/Turabian StyleFernández-Ropero, Antonio J., Bartosz Zawadzki, Emil Kowalewski, Izabela S. Pieta, Mirosław Krawczyk, Krzysztof Matus, Dmytro Lisovytskiy, and Anna Śrębowata. 2021. "Continuous 2-Methyl-3-butyn-2-ol Selective Hydrogenation on Pd/γ-Al2O3 as a Green Pathway of Vitamin A Precursor Synthesis" Catalysts 11, no. 4: 501. https://doi.org/10.3390/catal11040501
APA StyleFernández-Ropero, A. J., Zawadzki, B., Kowalewski, E., Pieta, I. S., Krawczyk, M., Matus, K., Lisovytskiy, D., & Śrębowata, A. (2021). Continuous 2-Methyl-3-butyn-2-ol Selective Hydrogenation on Pd/γ-Al2O3 as a Green Pathway of Vitamin A Precursor Synthesis. Catalysts, 11(4), 501. https://doi.org/10.3390/catal11040501









