Selective and Efficient Olefin Epoxidation by Robust Magnetic Mo Nanocatalysts
Abstract
1. Introduction
2. Results and Discussion
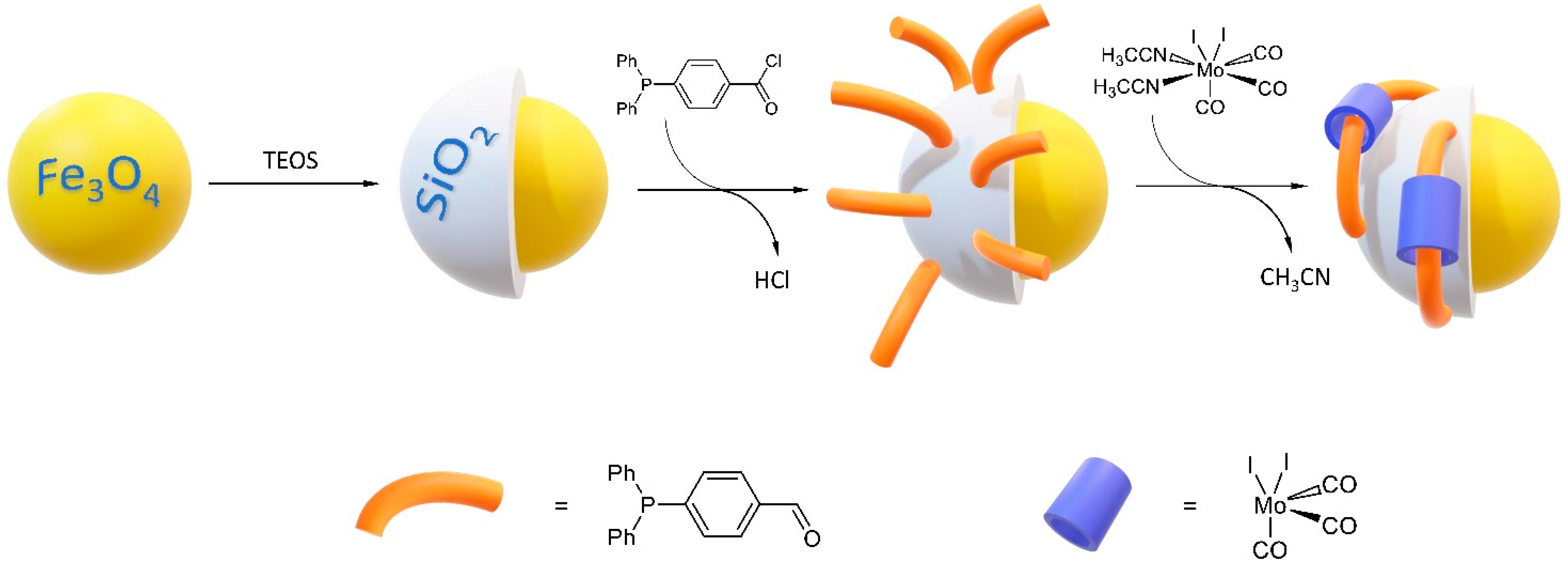
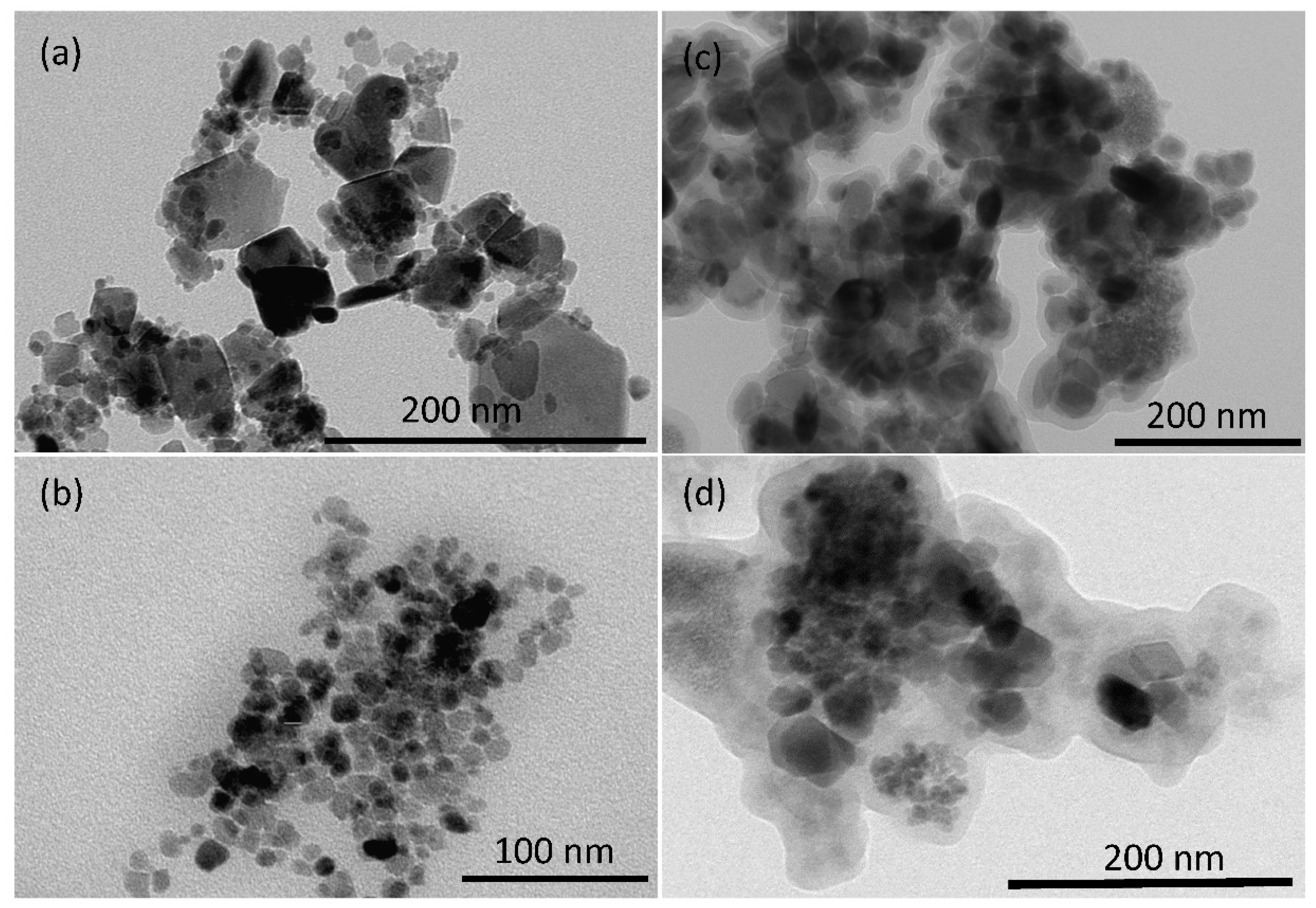
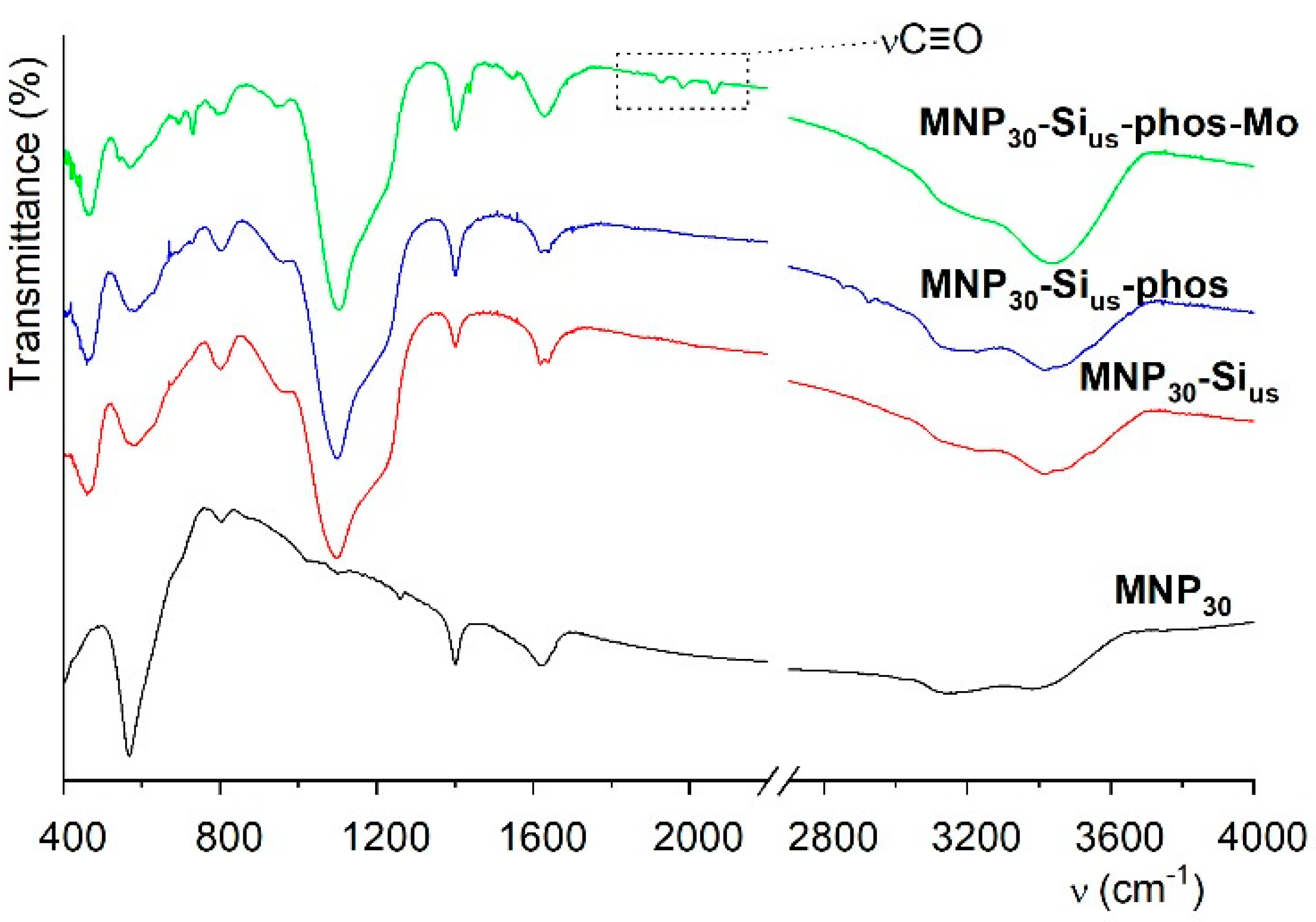
2.1. Synthesis and Characterization of Magnetic Nanoparticles
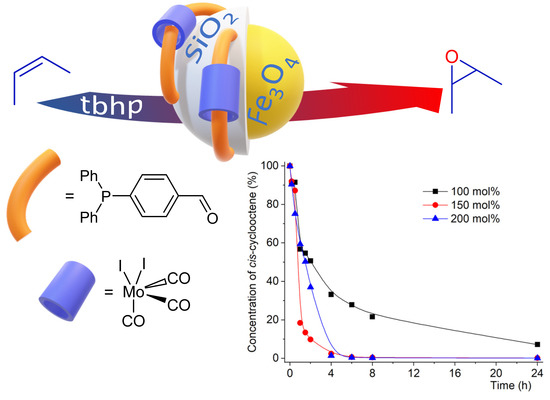
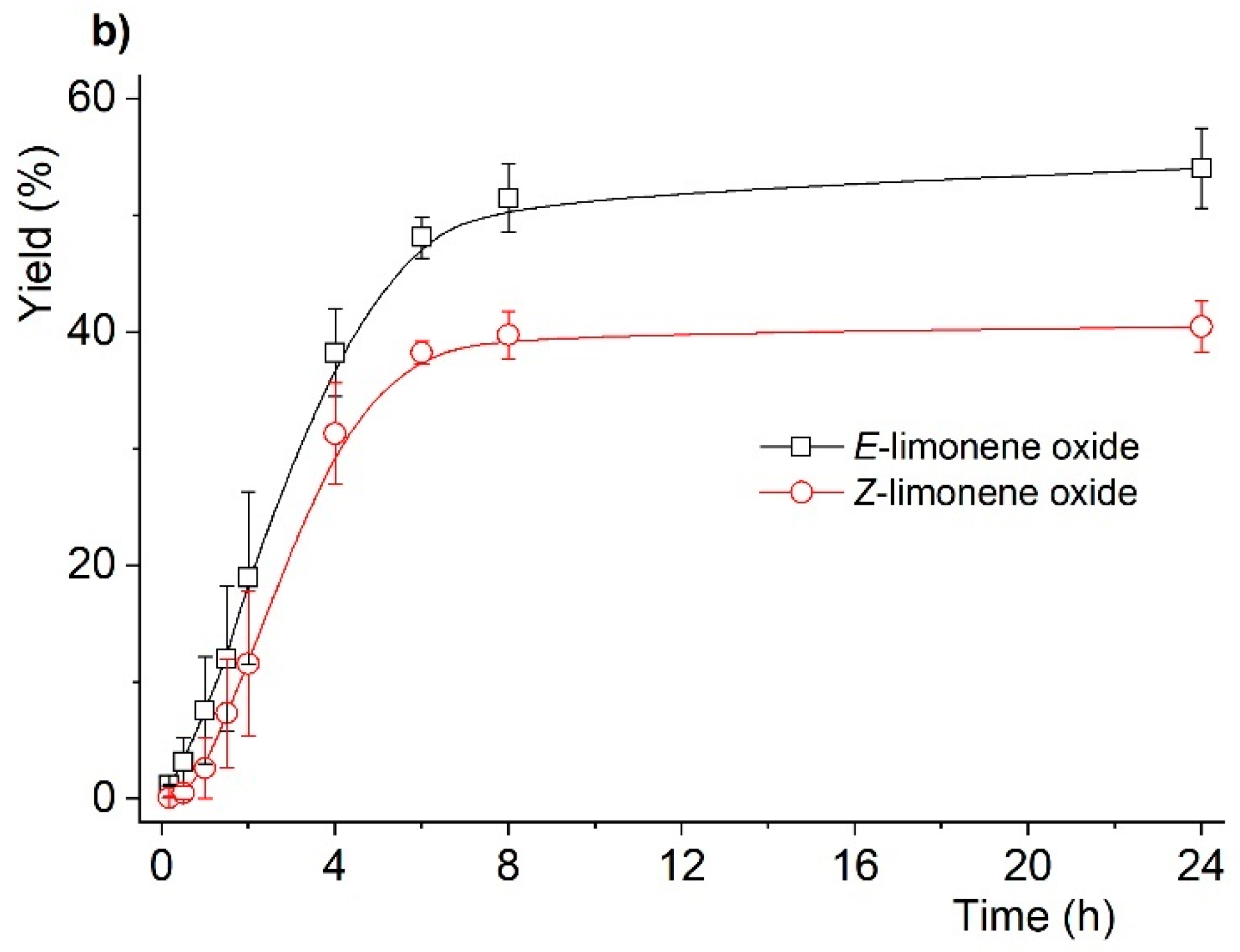
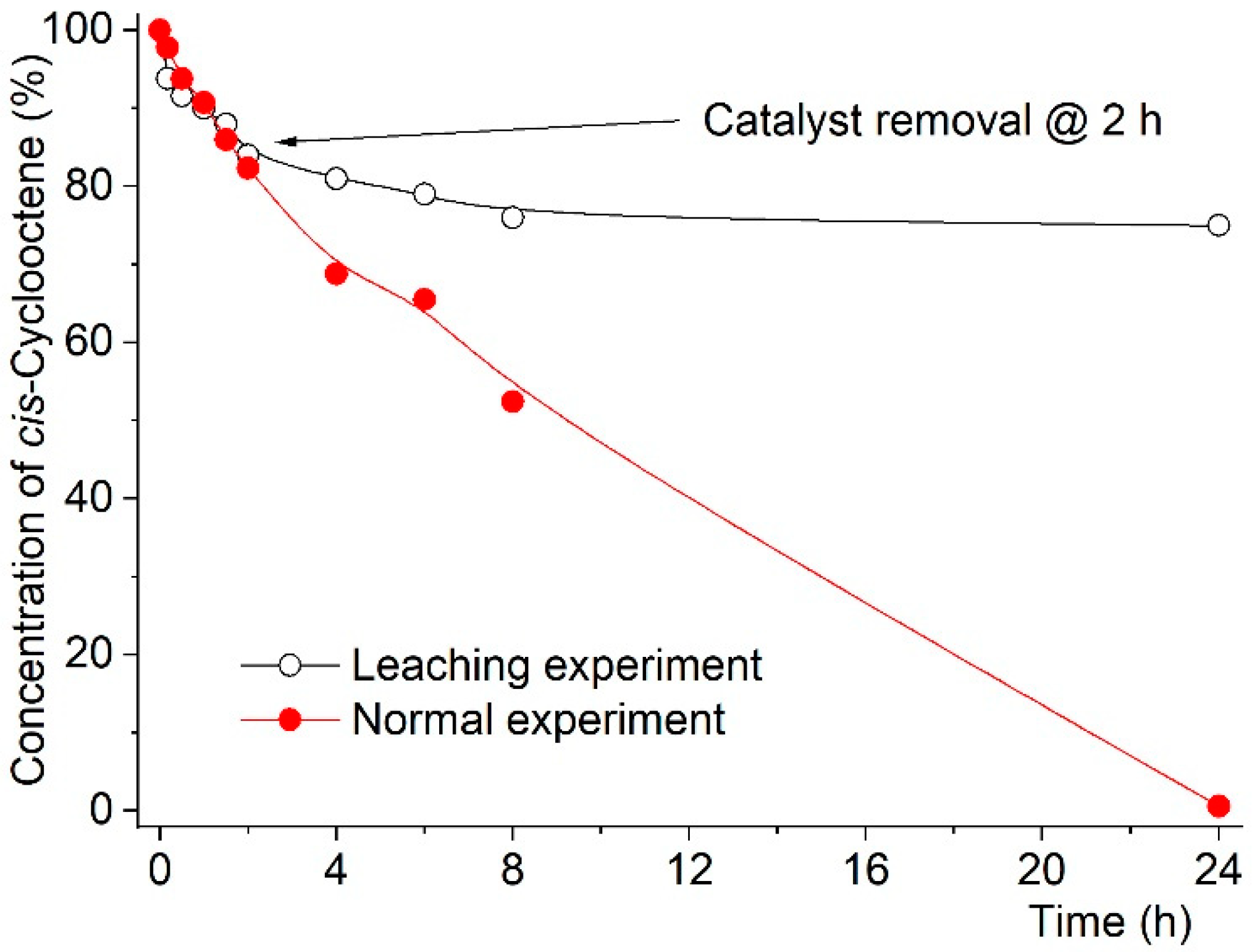
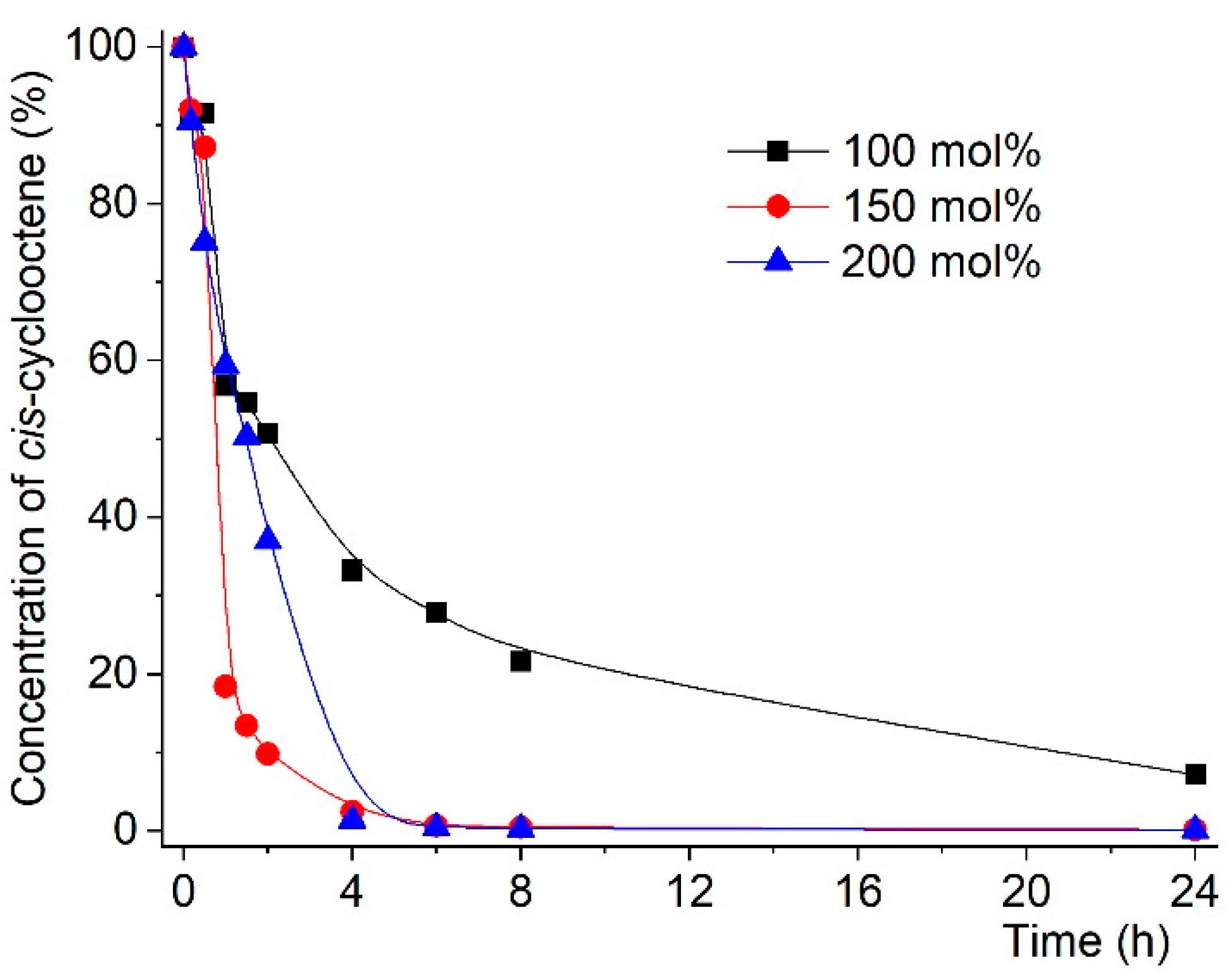
2.2. Catalytic Studies
3. Materials and Methods
3.1. General
3.1.1. Methods
Synthesis of 4-(diphenylphosphino)benzoyl Chloride (phosCl)
Preparation of MNP30-Sius Material
Preparation of MNP30-Si-phos, MNP30-Sius-phos and MNP11-Si-phos Materials
Preparation of MNP30-Si-phos-Mo, MNP30-Sius-phos-Mo, and MNP11-Si-phos-Mo Materials
3.2. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ganesh, M.; Ramakrishna, J. Synthetic Organic Transformations of Transition-Metal Nanoparticles as Propitious Catalysts: A Review. Asian J. Org. Chem. 2020, 9, 1341–1376. [Google Scholar] [CrossRef]
- Duan, M.; Shpter, J.G.; Qi, W.; Yang, S.; Gao, G. Recent progress in magnetic nanoparticles: Synthesis, properties, and applications. Nanotechnology 2018, 29, 452001. [Google Scholar] [CrossRef]
- Arndt, D.; Gesing, T.M.; Bäumer, M. Surface Functionalization of Iron Oxide Nanoparticles and their Stability in Different Media. ChemPlusChem 2012, 77, 576–583. [Google Scholar] [CrossRef]
- Kim, D.; Shin, K.; Kwon, S.G.; Hyeon, T. Synthesis and Biomedical Applications of Multifunctional Nanoparticles. Adv. Mater. 2018, 30, 1802309–1802334. [Google Scholar] [CrossRef]
- Fernandes Cardoso, V.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lancero-Mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Healthcare Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, X.; Guan, J. Applications of Magnetic Nanomaterials in Heterogeneous Catalysis. ACS Appl. Nano Mater. 2019, 2, 4681–4697. [Google Scholar] [CrossRef]
- Farooqi, Z.H.; Begum, R.; Nassim, K.; Wu, W.; Irfan, A. Zero valent iron nanoparticles as sustainable nanocatalysts for reduction reactions. Catal. Rev. 2020. [Google Scholar] [CrossRef]
- Chiozzi, V.; Rossi, F. Inorganic–organic core/shell nanoparticles: Progress and applications. Nanoscale Adv. 2020, 2, 5090–5105. [Google Scholar] [CrossRef]
- Hou, Z.; Liu, Y.; Xu, J.; Zhu, J. Surface engineering of magnetic iron oxide nanoparticles by polymer grafting: Synthesis progress and biomedical applications. Nanoscale 2020, 12, 14957–14975. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Bhateria, R. Core–shell nanostructures: A simplest two-component system with enhanced properties and multiple applications. Environ. Geochem. Health 2020. [Google Scholar] [CrossRef]
- Plumeré, N.; Ruff, A.; Speiser, B.; Felbmann, V.; Mayer, H.A. Stöber silica particles as basis for redox modifications: Particle shape, size, polydispersity, and porosity. J. Colloid Interface Sci. 2012, 368, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, S.; Shao, Y.; Liu, J.; Xu, Z.; Zhu, D. Amino-functionalized Fe3O4@SiO2 core-shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J. Colloid Interface Sci. 2010, 349, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, F.; Sun, L.; Wang, Q.; Ding, Y. Well-Dispersed Fe3O4/SiO2 Nanoparticles Synthesized by a Mechanical Stirring and Ultrasonication Assisted Stöber Method. J. Inorg. Organomet. Polym. 2012, 22, 311–315. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Piao, Y.; Kim, J.; Jan, Y.; Hyeon, T. A magnetically recyclable nanocomposite catalyst for olefin epoxidation. Angew. Chem. Int. Ed. Engl. 2007, 46, 7039–7043. [Google Scholar] [CrossRef]
- Pan, Z.; Hua, L.; Qiao, Y.; Yang, H.; Zhao, X.; Feng, B.; Zhu, W. Nanostructured Maghemite-Supported Silver Catalysts for Styrene Epoxidation. Chin. J. Catal. 2011, 32, 428–435. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, P.; Wai, P.T.; Gu, Q.; Zhang, W. Recent Progress in Application of Molybdenum-Based Catalysts for Epoxidation of Alkenes. Catalysts 2019, 9, 31. [Google Scholar] [CrossRef]
- Vasconcellos-Dias, M.; Nunes, C.D.; Vaz, P.D.; Ferreira, P.; Brandão, P.; Felix, V.; Calhorda, M.J. Heptacoordinate tricarbonyl Mo(II) complexes as highly selective oxidation homogeneous and heterogeneous catalysts. J. Catal. 2008, 256, 301–311. [Google Scholar] [CrossRef]
- Fernandes, C.I.; Stenning, G.B.G.; Taylor, J.D.; Nunes, C.D.; Vaz, P.D. Helical channel mesoporous materials with embedded magnetic iron nanoparticles: Chiral recognition and implications in asymmetric olefin epoxidation. Adv. Synth. Catal. 2015, 357, 3127–3140. [Google Scholar] [CrossRef]
- Tokudome, Y.; Morimoto, T.; Tarutani, N.; Vaz, P.D.; Nunes, C.D.; Prevot, V.; Stenning, G.B.G.; Takahashi, M. Layered Double Hydroxide Nanoclusters: Aqueous, Concentrated, Stable, and Catalytically Active Colloids toward Green Chemistry. ACS Nano 2016, 10, 5550–5559. [Google Scholar] [CrossRef]
- Fernandes, C.I.; Carvalho, M.D.; Ferreira, L.P.; Nunes, C.D.; Vaz, P.D. Organometallic Mo complex anchored to magnetic iron oxide nanoparticles as highly recyclable epoxidation catalyst. J. Organomet. Chem. 2014, 760, 2–10. [Google Scholar] [CrossRef]
- Shylesh, S.; Schweizer, J.; Demeshko, S.; Schunemann, V.; Ernst, S.; Thiel, W.R. Nanoparticle Supported, Magnetically Recoverable Oxodiperoxo Molybdenum Complexes: Efficient Catalysts for Selective Epoxidation Reactions. Adv. Synth. Catal. 2009, 351, 1789–1795. [Google Scholar] [CrossRef]
- Bui, T.Q.; Ngo, H.T.M.; Tran, H.T. Surface-protective assistance of ultrasound in synthesis of superparamagnetic magnetite nanoparticles and in preparation of mono-core magnetite-silica nanocomposites. J. Sci. Adv. Mater. Dev. 2018, 3, 323–330. [Google Scholar] [CrossRef]
- Montaño-Priede, J.L.; Coelho, J.P.; Guerrero-Martínez, A.; Peña-Rodriguez, O.; Pal, U. Fabrication of Monodispersed Au@SiO2 Nanoparticles with Highly Stable Silica Layers by Ultrasound-Assisted Stöber Method. J. Phys. Chem. C 2017, 121, 9543–9551. [Google Scholar] [CrossRef]
- Ventura, A.C.; Fernandes, C.I.; Saraiva, M.S.; Nunes, T.G.; Vaz, P.D.; Nunes, C.D. Tuning the Surface of Mesoporous Materials Towards Hydrophobicity-Effects in Olefin Epoxidation. Curr. Inorg. Chem. 2011, 1, 156–165. [Google Scholar] [CrossRef]
- Arzoumanian, H.; Castellanos, N.J.; Martínez, F.O.; Páez-Mozo, E.A.; Ziarelli, F. Silicon-Assisted Direct Covalent Grafting on Metal Oxide Surfaces: Synthesis and Characterization of Carboxylate N,N′-Ligands on TiO2. Eur. J. Inorg. Chem. 2010, 1633–1641. [Google Scholar] [CrossRef]
- Vaz, P.D.; Nunes, C.D.; Vasconcellos-Dias, M.; Nolasco, M.M.; Ribeiro-Claro, P.J.A.; Calhorda, M.J. Vibrational Study on the Local Structure of Post-Synthesis and Hybrid Mesoporous Materials: Are There Fundamental Distinctions? Chem. Eur. J. 2007, 13, 7874–7882. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.K. Seven-coordinate halocarbonyl complexes of the type [MXY(CO)3(NCMe)2] (M = Mo, W.; X, Y = halide, pseudo halide) as highly versatile starting materials. Chem. Soc. Rev. 1998, 27, 125–132. [Google Scholar] [CrossRef]
- Fernandes, C.I.; Silva, N.U.; Vaz, P.D.; Nunes, T.G.; Nunes, C.D. Bio-inspired Mo(II) complexes as active catalysts in homogeneous and heterogeneous olefin epoxidation. Appl. Catal. A Gen. 2010, 384, 84–93. [Google Scholar] [CrossRef]
- Guo, W.C.; Wang, G.; Wang, Q.; Dong, W.J.; Yang, M.; Huang, X.B.; Yu, J.; Shi, Z. A hierarchical Fe3O4@P4VP@MoO2(acac)2 nanocomposite: Controlled synthesis and green catalytic application. J. Mol. Catal. A Chem. 2013, 378, 344–349. [Google Scholar] [CrossRef]
- Huang, X.B.; Guo, W.C.; Wang, G.; Yang, M.; Wang, Q.; Zhang, X.X.; Feng, Y.H.; Shi, Z.; Li, C.G. Synthesis of Mo–Fe3O4@SiO2@P4VP core–shell–shell structured magnetic microspheres for alkene epoxidation reactions. Mater. Chem. Phys. 2012, 135, 985–990. [Google Scholar] [CrossRef]
- Tong, J.; Cai, X.; Wang, H.; Zhang, Q. Improvement of catalytic activity in selective oxidation of styrene with H2O2 over spinel Mg–Cu ferrite hollow spheres in water. Mater. Res. Bull. 2014, 55, 205–211. [Google Scholar] [CrossRef]
- Wilson, K.; Lee, A.F. Catalyst design for biorefining. Philos. Trans. R. Soc. A 2016, 374, 20150081. [Google Scholar] [CrossRef]
- Pardeshi, S.K.; Pawar, R.Y. SrFe2O4 complex oxide an effective and environmentally benign catalyst for selective oxidation of styrene. J. Mol. Catal. A Chem. 2011, 334, 35–43. [Google Scholar] [CrossRef]
- Ma, N.; Yue, Y.; Hua, W.; Gao, Z. Selective oxidation of styrene over nanosized spinel-type MgxFe3−xO4 complex oxide catalysts. Appl. Catal. A Gen. 2003, 251, 39–47. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Z.; Tang, J.; Zhang, Y.; Kong, L.; Zhang, Y. Direct, efficient, and inexpensive formation of a-hydroxyketones from olefins by hydrogen peroxide oxidation catalyzed by the 12-tungstophosphoric acid/cetylpyridinium chloride system. Org. Biomol. Chem. 2006, 4, 1478–1482. [Google Scholar] [CrossRef]
- Farzaneh, F.; Asgharpour, Z. Synthesis of a new Schiff base oxovanadium complex with melamine and 2-hydroxynaphtaldehyde on modified magnetic nanoparticles as catalyst for allyl alcohols and olefins epoxidation. Appl. Organomet. Chem. 2019, 33, e489. [Google Scholar] [CrossRef]
- Silva, N.U.; Fernandes, C.I.; Nunes, T.G.; Saraiva, M.S.; Nunes, C.D.; Vaz, P.D. Performance evaluation of mesoporous host materials in olefin epoxidation using Mo(II) and Mo(VI) active species—Inorganic vs. hybrid matrix. Appl. Catal. A Gen. 2011, 408, 105–116. [Google Scholar] [CrossRef]
- Al-Ajlouni, A.; Valente, A.A.; Nunes, C.D.; Pillinger, M.; Santos, A.M.; Zhao, J.; Romão, C.C.; Gonçalves, I.S.; Kühn, F.E. Kinetics of Cyclooctene Epoxidation with tert-Butyl Hydroperoxide in the Presence of [MoO2X2L]-Type Catalysts (L = Bidentate Lewis Base). Eur. J. Inorg. Chem. 2005, 9, 1716–1723. [Google Scholar] [CrossRef]
- Rayati, S.; Moradi, D.; Nejabat, F. Magnetically recoverable porphyrin-based nanocatalysts for the effective oxidation of olefins with hydrogen peroxide: A comparative study. New J. Chem. 2020, 44, 19385–19392. [Google Scholar] [CrossRef]
- Mortazavi-Manesh, A.; Bagherzadeh, M. Synthesis and characterization of molybdenum (VI) complex immobilized on polymeric Schiff base-coated magnetic nanoparticles as an efficient and retrievable nanocatalyst in olefin epoxidation reactions. Appl. Organomet. Chem. 2020, 34, e5410. [Google Scholar] [CrossRef]
- Niakan, M.; Asadi, Z.; Masteri-Farahani, M. Immobilization of salen molybdenum complex on dendrimer functionalized magnetic nanoparticles and its catalytic activity for the epoxidation of olefins. Appl. Surf. Sci. 2019, 481, 394–403. [Google Scholar] [CrossRef]
- Fakhimi, P.; Bezaatpour, A.; Amiri, M.; Szunerits, S.; Boukherroub, R.; Eskandari, H. Manganese Ferrite Nanoparticles Modified by Mo(VI) Complex: Highly Efficient Catalyst for Sulfides and Olefins Oxidation Under Solvent-less Condition. ChemistrySelect 2019, 4, 7116–7122. [Google Scholar] [CrossRef]
- Gimenez, G.; Nunes, C.D.; Vaz, P.D.; Valente, A.A.; Ferreira, P.; Calhorda, M.J. Hepta-coordinate halocarbonyl molybdenum(II) and tungsten(II) complexes as heterogeneous polymerization catalysts. J. Mol. Catal. A Chem. 2006, 256, 90–98. [Google Scholar] [CrossRef]

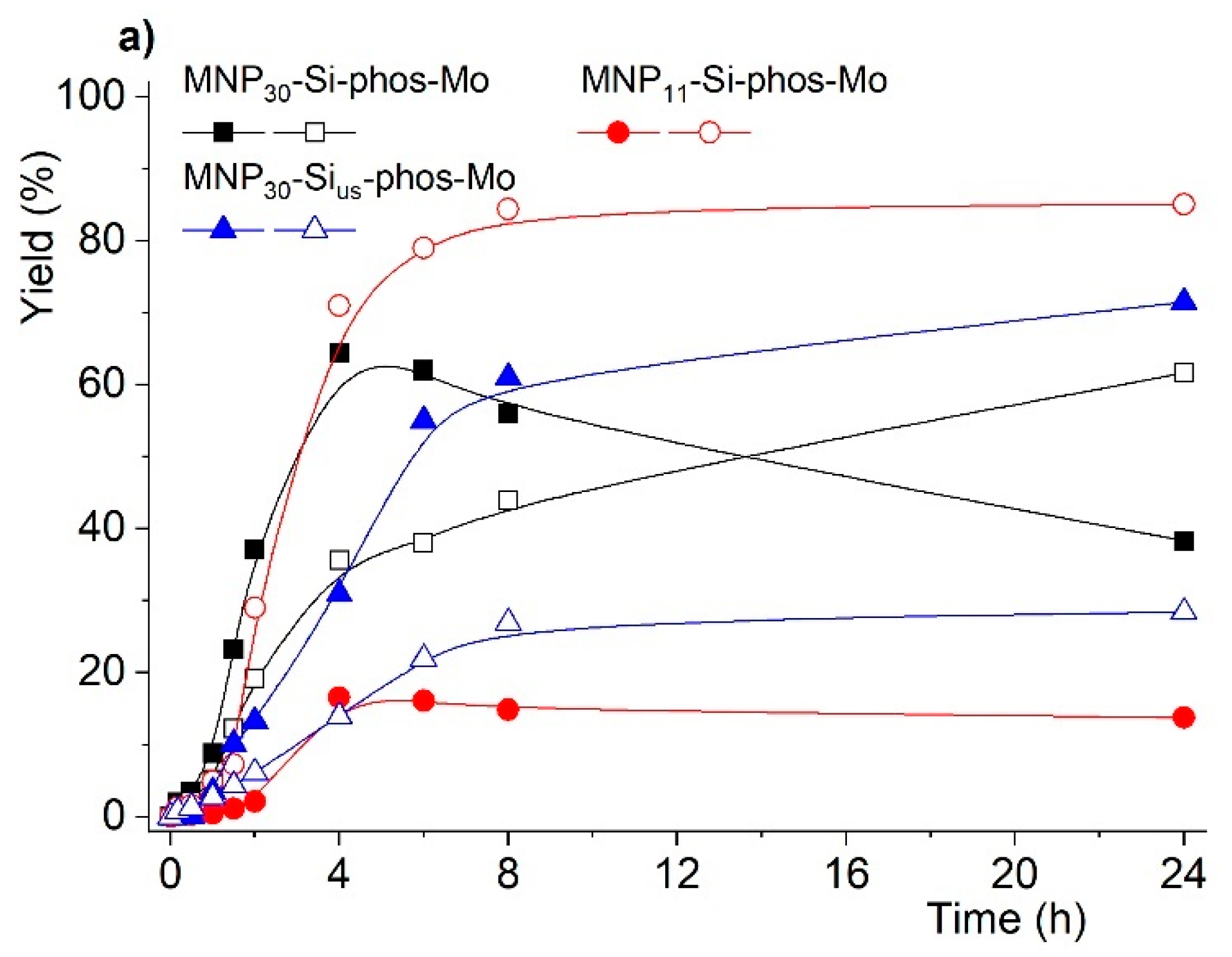

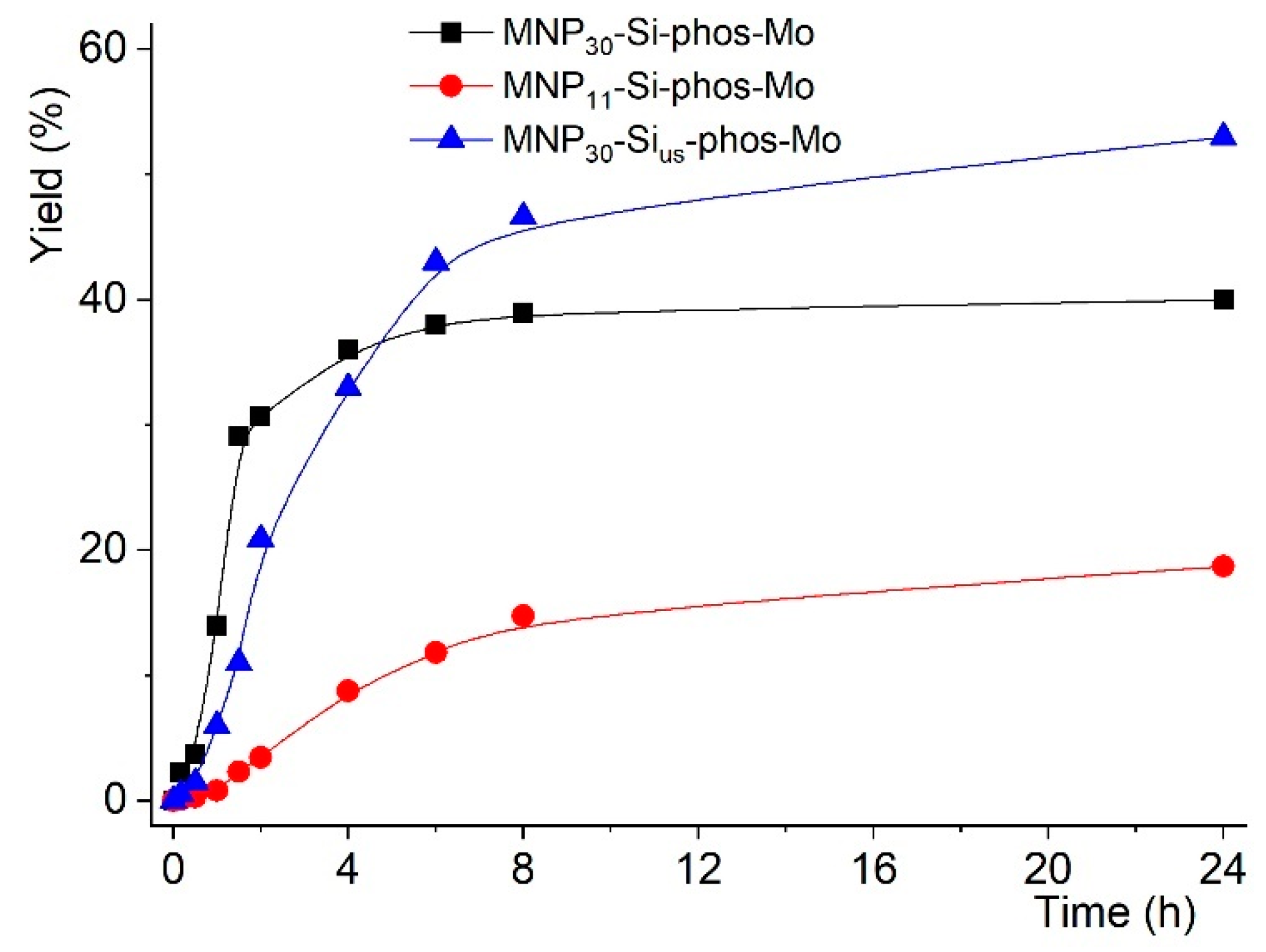
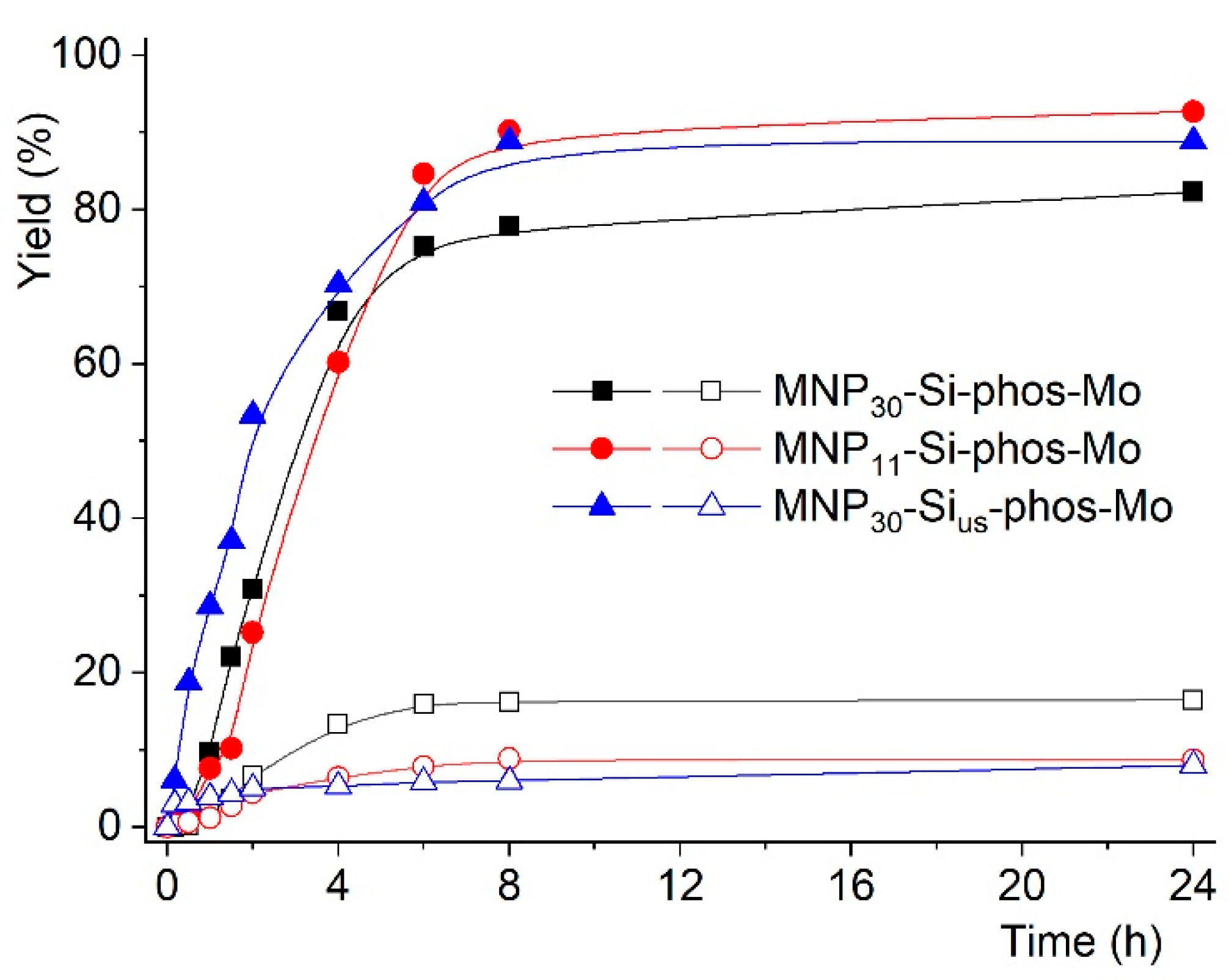
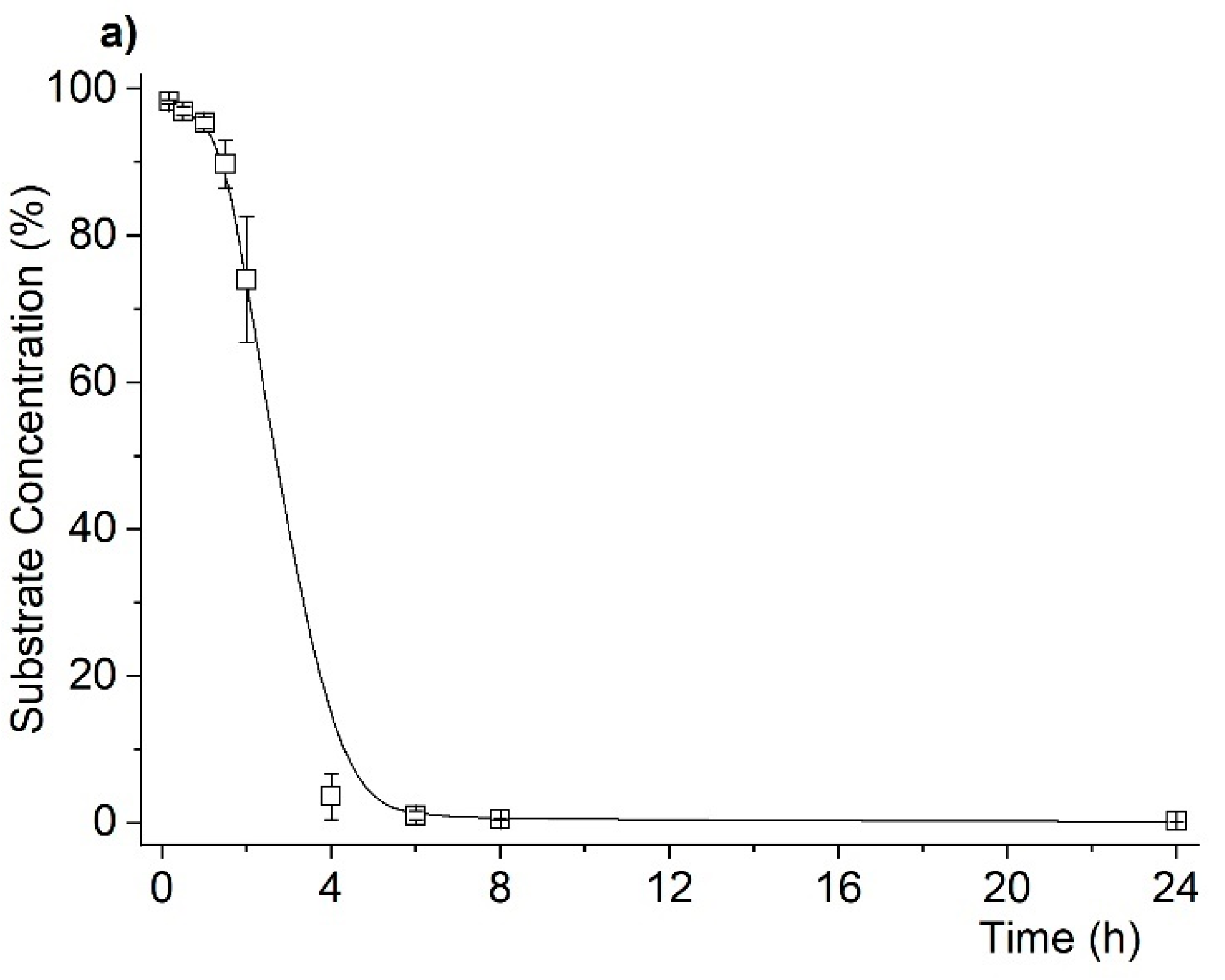

| Entry | Reaction [a] | Catalyst | Solvent | Temp. (K) | Conv. [b] (%) | Yield [b] (%) | Select. [c] (%) |
|---|---|---|---|---|---|---|---|
| 1 |  | MNP30-Si-phos-Mo | CH3CN | 353 | 97 | 97 | 100 |
| 2 | Toluene | 353 | 97 | 97 | 100 | ||
| 3 | Toluene | 383 | 75 | 75 | 100 | ||
| 4 | Decane | 393 | 53 | 53 | 100 | ||
| 5 | MNP11-Si-phos-Mo | CH3CN | 353 | 92 | 92 | 100 | |
| 6 | Toluene | 353 | 99 | 99 | 100 | ||
| 7 | Toluene | 383 | 99 | 99 | 100 | ||
| 8 | Decane | 393 | 85 | 85 | 100 | ||
| 9 | MNP30-Sius-phos-Mo | CH3CN | 353 | 83 | 83 | 100 | |
| 10 | Toluene | 353 | 99 | 99 | 100 | ||
| 11 | Toluene | 383 | 87 | 87 | 100 | ||
| 12 | Decane | 393 | 52 | 52 | 100 | ||
| 13 | 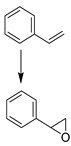 | MNP30-Si-phos-Mo | CH3CN | 353 | 100 | 38 | 38 [d] |
| 14 | Toluene | 353 | 99 | 12 | 12 [d] | ||
| 15 | Toluene | 383 | 100 | 39 | 39 [d] | ||
| 16 | Decane | 393 | 100 | 27 | 27 [d] | ||
| 17 | MNP11-Si-phos-Mo | CH3CN | 353 | 100 | 17 | 17 [d] | |
| 18 | Toluene | 353 | 100 | 5 | 5 [d] | ||
| 19 | Toluene | 383 | 100 | 19 | 19 [d] | ||
| 20 | Decane | 393 | 100 | 5 | 5 [d] | ||
| 21 | MNP30-Sius-phos-Mo | CH3CN | 353 | 100 | 72 | 72 [d] | |
| 22 | Toluene | 353 | 96 | 7 | 8 [d] | ||
| 23 | Toluene | 383 | 100 | 54 | 54 [d] | ||
| 24 | Decane | 393 | 99 | 26 | 26 [d] |
| Entry | Reaction [a] | Catalyst | Solvent | Temp. (K) | Conv. [b] (%) | Yield [b] (%) | Select. [c] (%) |
|---|---|---|---|---|---|---|---|
| 1 | 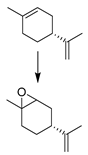 | MNP30-Si-phos-Mo | CH3CN | 353 | 99 | 65 | 65 [d] |
| 2 | Toluene | 353 | 99 | 74 | 74 [d] | ||
| 3 | Toluene | 383 | 100 | 96 | 99 [d] | ||
| 4 | Decane | 393 | 99 | 86 | 87 [d] | ||
| 5 | MNP11-Si-phos-Mo | CH3CN | 353 | 100 | 78 | 78 [d] | |
| 6 | Toluene | 353 | 100 | 87 | 87 [d] | ||
| 7 | Toluene | 383 | 100 | 88 | 88 [d] | ||
| 8 | Decane | 393 | 99 | 88 | 88 [d] | ||
| 9 | MNP30-Sius-phos-Mo | CH3CN | 353 | 88 | 75 | 85 [d] | |
| 10 | Toluene | 353 | 81 | 73 | 90 [d] | ||
| 11 | Toluene | 383 | 97 | 92 | 95 [d] | ||
| 12 | Decane | 393 | 76 | 63 | 83 [d] | ||
| 13 |  | MNP30-Si-phos-Mo | CH3CN | 353 | 95 | 65 | 70 [e] |
| 14 | Toluene | 353 | 97 | 78 | 80 [e] | ||
| 15 | Toluene | 383 | 99 | 82 | 83 [e] | ||
| 16 | Decane | 393 | 95 | 76 | 80 [e] | ||
| 17 | MNP11-Si-phos-Mo | CH3CN | 353 | 86 | 56 | 65 [e] | |
| 18 | Toluene | 353 | 98 | 85 | 86 [e] | ||
| 19 | Toluene | 383 | 100 | 93 | 93 [e] | ||
| 20 | Decane | 393 | 99 | 84 | 85 [e] | ||
| 21 | MNP30-Sius-phos-Mo | CH3CN | 353 | 100 | 50 | 50 [e] | |
| 22 | Toluene | 353 | 100 | 50 | 50 [e] | ||
| 23 | Toluene | 383 | 96 | 89 | 93 [e] | ||
| 24 | Decane | 393 | 98 | 97 | 99 [e] |
| Entry | Reaction [a] | Catalyst | Solvent | Temp. (K) | Conv. [b,c] (%) |
|---|---|---|---|---|---|
| 1 |  | MNP30-Si-phos-Mo | CH3CN | 353 | 97/85/70 |
| 2 | Toluene | 353 | 97/96/96 | ||
| 3 | Toluene | 383 | 75/64/53 | ||
| 4 | Decane | 393 | 53/36/18 | ||
| 5 | MNP11-Si-phos-Mo | CH3CN | 353 | 92/72/70 | |
| 6 | Toluene | 353 | 99/82/78 | ||
| 7 | Toluene | 383 | 99/99/98 | ||
| 8 | Decane | 393 | 85/73/68 | ||
| 9 | MNP30-Sius-phos-Mo | CH3CN | 353 | 83/86/40 | |
| 10 | Toluene | 353 | 99/99/81 | ||
| 11 | Toluene | 383 | 87/49/35 | ||
| 12 | Decane | 393 | 52/39/17 | ||
| 13 | 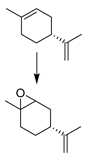 | MNP30-Si-phos-Mo | CH3CN | 353 | 99/90/72 |
| 14 | Toluene | 353 | 99/97/37 | ||
| 15 | Toluene | 383 | 100/100/94 | ||
| 16 | Decane | 393 | 99/31/27 | ||
| 17 | MNP11-Si-phos-Mo | CH3CN | 353 | 100/99/99 | |
| 18 | Toluene | 353 | 100/99/99 | ||
| 19 | Toluene | 383 | 100/100/100 | ||
| 20 | Decane | 393 | 99/99/99 | ||
| 21 | MNP30-Sius-phos-Mo | CH3CN | 353 | 88/53/13 | |
| 22 | Toluene | 353 | 81/45/10 | ||
| 23 | Toluene | 383 | 97/49/47 | ||
| 24 | Decane | 393 | 76/63/29 | ||
| 25 | 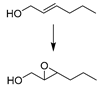 | MNP30-Si-phos-Mo | CH3CN | 353 | 95/95/86 |
| 26 | Toluene | 353 | 97/95/89 | ||
| 27 | Toluene | 383 | 99/96/93 | ||
| 28 | Decane | 393 | 95/92/91 | ||
| 29 | MNP11-Si-phos-Mo | CH3CN | 353 | 86/67/34 | |
| 30 | Toluene | 353 | 98/87/30 | ||
| 31 | Toluene | 383 | 100/100/63 | ||
| 32 | Decane | 393 | 99/99/79 | ||
| 33 | MNP30-Sius-phos-Mo | CH3CN | 353 | 100/68/73 | |
| 34 | Toluene | 353 | 100/99/44 | ||
| 35 | Toluene | 383 | 96/98/85 | ||
| 36 | Decane | 393 | 98/98/96 |
| Entry | Reaction [a] | Catalyst | Temp. (K) | Conv. [b] (%) | Select. [b,c] (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 100 [d] | 150 [d] | 200 [d] | 100 [d] | 150 [d] | 200 [d] | ||||
| 1 | 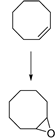 | MNP30-Si-phos-Mo | 353 | 99 | 99 | 97 | 100 | 100 | 100 |
| 2 | 383 | 99 | 99 | 75 | 100 | 100 | 100 | ||
| 3 | MNP11-Si-phos-Mo | 353 | 54 | 28 | 99 | 100 | 100 | 100 | |
| 4 | 383 | 93 | 100 | 99 | 100 | 100 | 100 | ||
| 5 | MNP30-Sius-phos-Mo | 353 | 37 | 37 | 99 | 100 | 100 | 100 | |
| 6 | 383 | 84 | 86 | 87 | 100 | 100 | 100 | ||
| 7 | 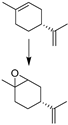 | MNP30-Si-phos-Mo | 353 | 46 | 60 | 99 | 91 [e] | 92 [e] | 74 [e] |
| 8 | 383 | 99 | 57 | 100 | 65 [e] | 92 [e] | 99 [e] | ||
| 9 | MNP11-Si-phos-Mo | 353 | 64 | 89 | 100 | 93 [e] | 90 [e] | 87 [e] | |
| 10 | 383 | 81 | 97 | 100 | 94 [e] | 93 [e] | 88 [e] | ||
| 11 | MNP30-Sius-phos-Mo | 353 | 27 | 41 | 81 | 87 [e] | 88 [e] | 90 [e] | |
| 12 | 383 | 100 | 76 | 97 | 87 [e] | 92 [e] | 95 [e] | ||
| Entry | Catalyst | Substrate | Oxidant | Temp (K) | Conv. (%) | Epoxide Select. (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Fe3O4@SiO2@PTMS@Mel-Naph-VO | cis-cyclooctene | tbhp | 353 | 80 | 100 (8 h) | [36] |
| 2 | styrene | 56 | 58 (3 h) | ||||
| 3 | Fe3O4/SiO2/NH2-MnTCPP(OAc) | cis-cyclooctene | H2O2 | 303 | 85 | 100 (3 h) | [39] |
| 4 | styrene | 74 | 78 (3 h) | ||||
| 5 | MNP@PMA-SB-Mo | cis-cyclooctene | tbhp | 357 | 98 | 100 (1 h) | [40] |
| 6 | styrene | 92 | 72 (3 h) | ||||
| 7 | Fe3O4@SiO2-dendrimer-Mo | cis-cyclooctene | tbhp | 323 | 96 | 99 (1 h) | [41] |
| 8 | styrene | 92 | 98 (2 h) | ||||
| 9 | MnFe2O4-Mo(VI) | cis-cyclooctene | tbhp | 368 | 99 | 100 (10 min) | [42] |
| 10 | styrene | 94 | 56 (15 min) | ||||
| 11 | MNP30-Si-phos-Mo | cis-cyclooctene | tbhp | 383 | 75 | 100 (24 h) | This work |
| 12 | styrene | 99 | 39 (24 h) | ||||
| 13 | MNP11-Si-phos-Mo | cis-cyclooctene | tbhp | 383 | 99 | 99 (24 h) | This work |
| 14 | styrene | 100 | 19 (24 h) | ||||
| 15 | MNP30-Sius-phos-Mo | cis-cyclooctene | tbhp | 383 | 87 | 100 (24 h) | This work |
| 16 | styrene | 100 | 54 (24 h) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, C.I.; Vaz, P.D.; Nunes, C.D. Selective and Efficient Olefin Epoxidation by Robust Magnetic Mo Nanocatalysts. Catalysts 2021, 11, 380. https://doi.org/10.3390/catal11030380
Fernandes CI, Vaz PD, Nunes CD. Selective and Efficient Olefin Epoxidation by Robust Magnetic Mo Nanocatalysts. Catalysts. 2021; 11(3):380. https://doi.org/10.3390/catal11030380
Chicago/Turabian StyleFernandes, Cristina I., Pedro D. Vaz, and Carla D. Nunes. 2021. "Selective and Efficient Olefin Epoxidation by Robust Magnetic Mo Nanocatalysts" Catalysts 11, no. 3: 380. https://doi.org/10.3390/catal11030380
APA StyleFernandes, C. I., Vaz, P. D., & Nunes, C. D. (2021). Selective and Efficient Olefin Epoxidation by Robust Magnetic Mo Nanocatalysts. Catalysts, 11(3), 380. https://doi.org/10.3390/catal11030380