Improving the Performance of Gd Addition on Catalytic Activity and SO2 Resistance over MnOx/ZSM-5 Catalysts for Low-Temperature NH3-SCR
Abstract
1. Introduction
2. Results
2.1. Low-Temperature NH3-SCR Performance
2.2. Physicochemical Properties of the Fresh Catalysts
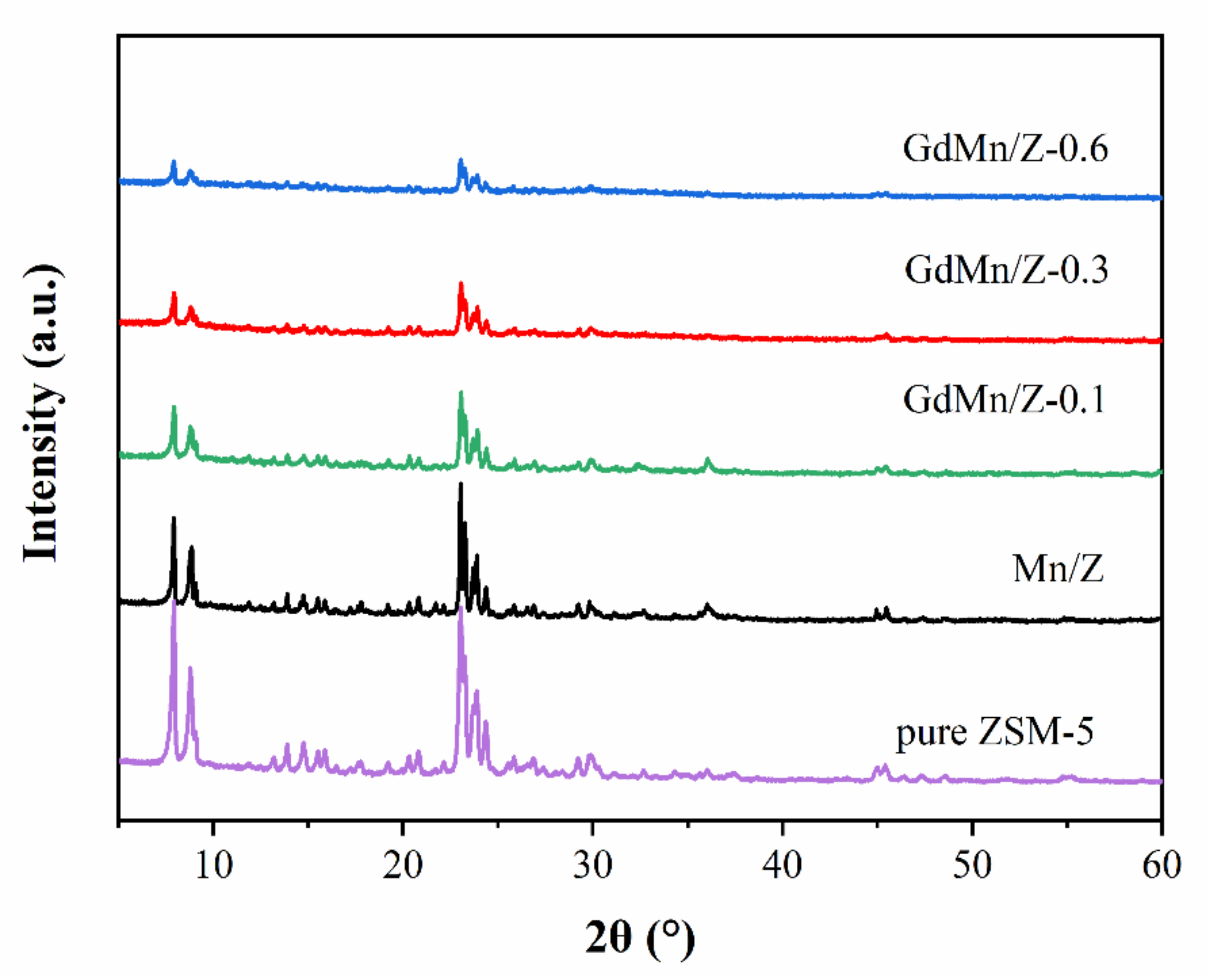
2.2.1. X-ray Diffraction (XRD) and Brunauer–Emmett–Teller (BET) Analyses
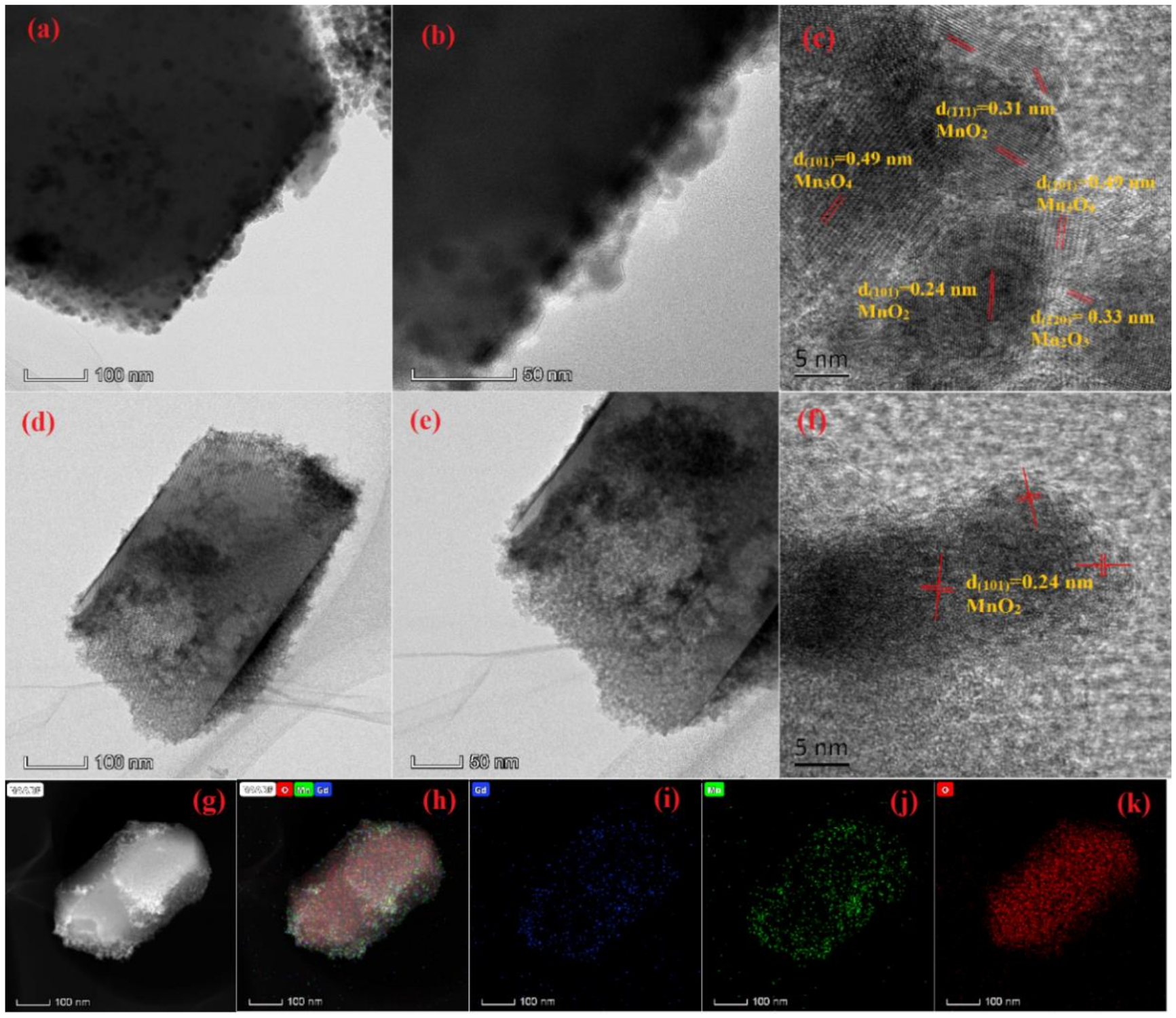
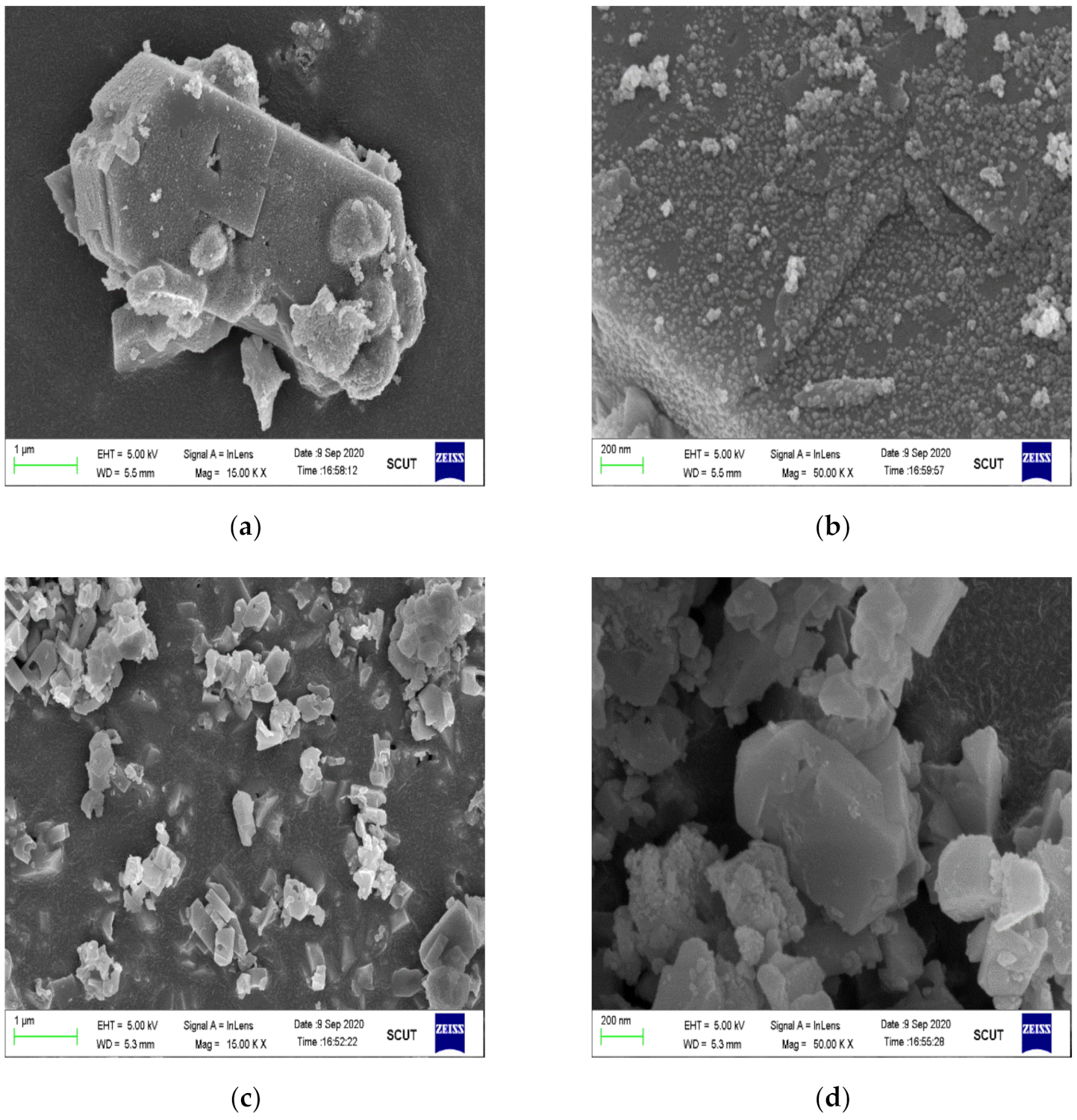
2.2.2. Microstructure Analysis
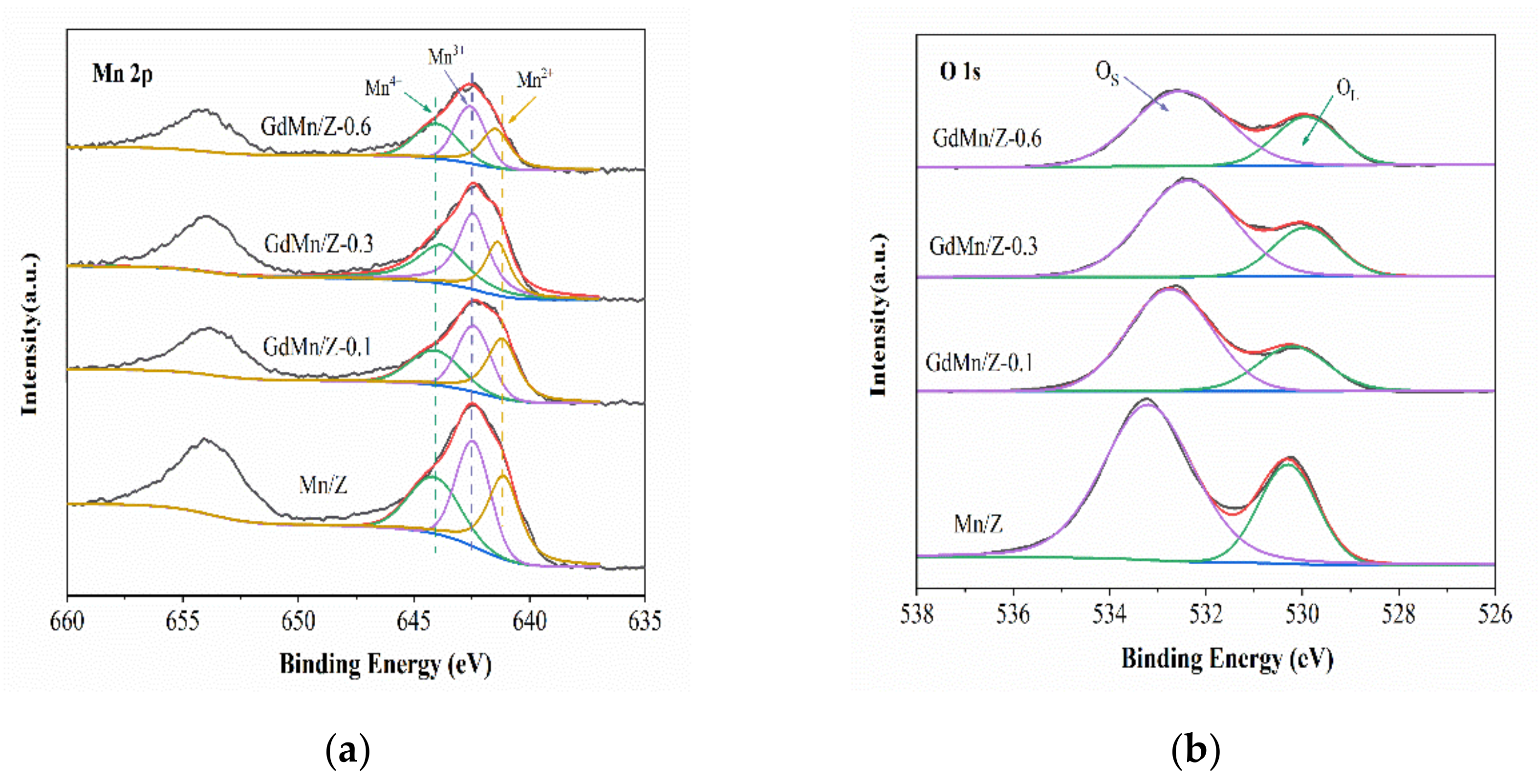
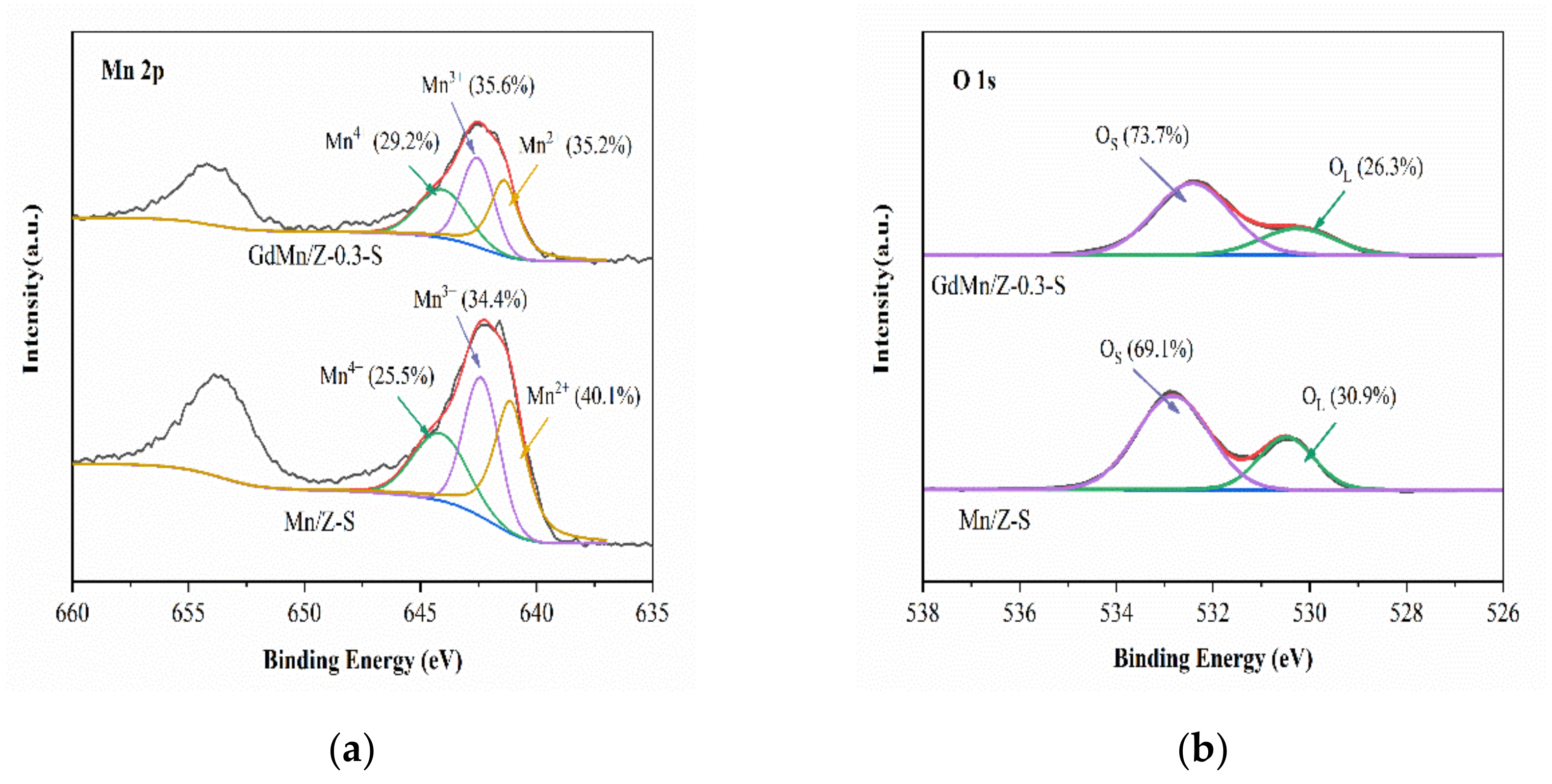
2.2.3. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.2.4. Temperature-Programmed Reduction of H2 (H2-TPR) and Temperature-Programmed Desorption of NH3 (NH3-TPD) Analyses
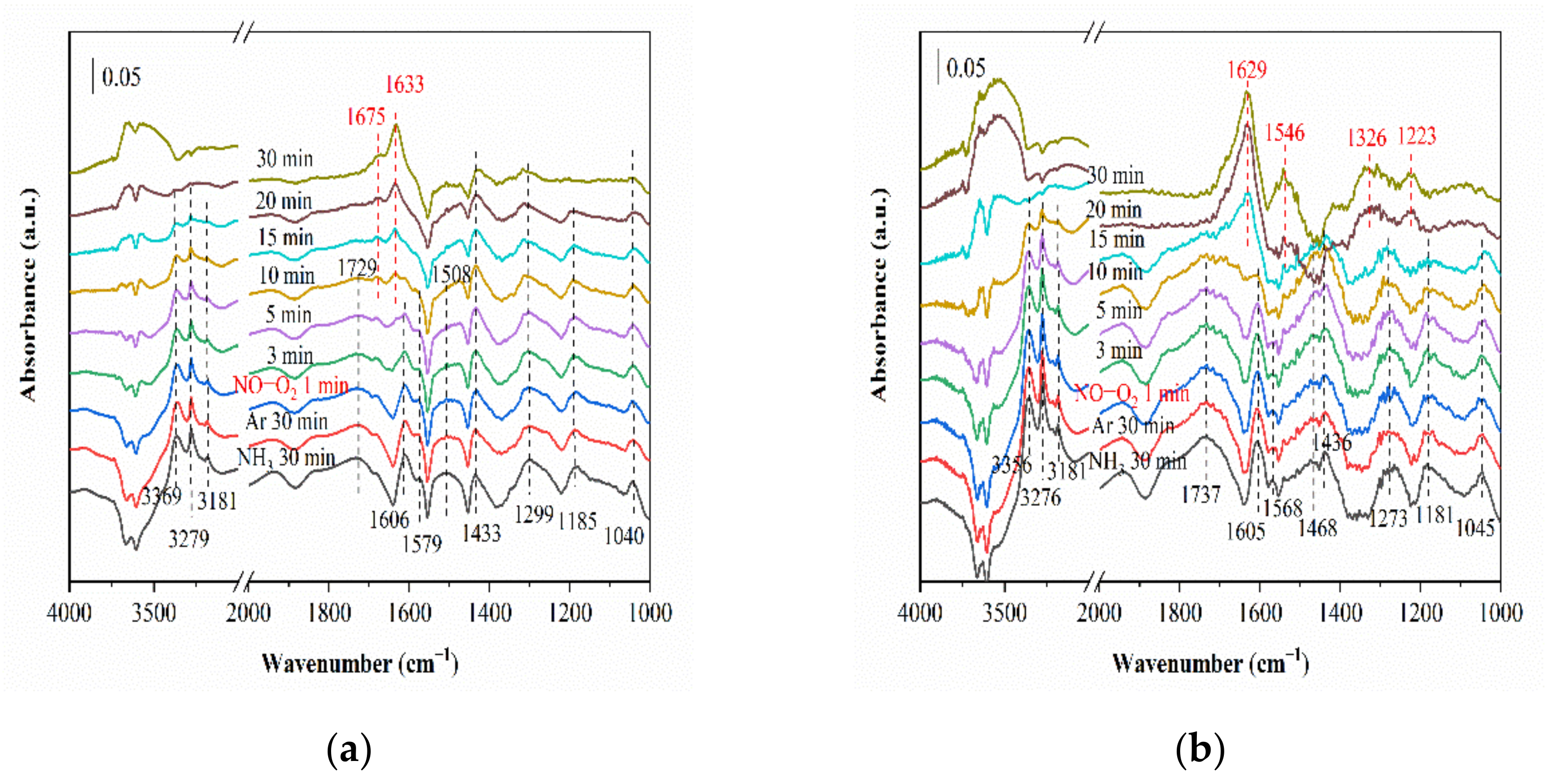
2.3. In Situ DRIFTS Studies for NH3-SCR
2.3.1. Adsorption of NH3
2.3.2. Co-Adsorption of NO and O2
2.3.3. Reaction between NO and O2 and Pre-Adsorbed NH3
2.3.4. Reaction between NH3 and Pre-Adsorbed NO and O2
2.3.5. In Situ DRIFTS of Standard NH3-SCR Reaction on the Catalysts
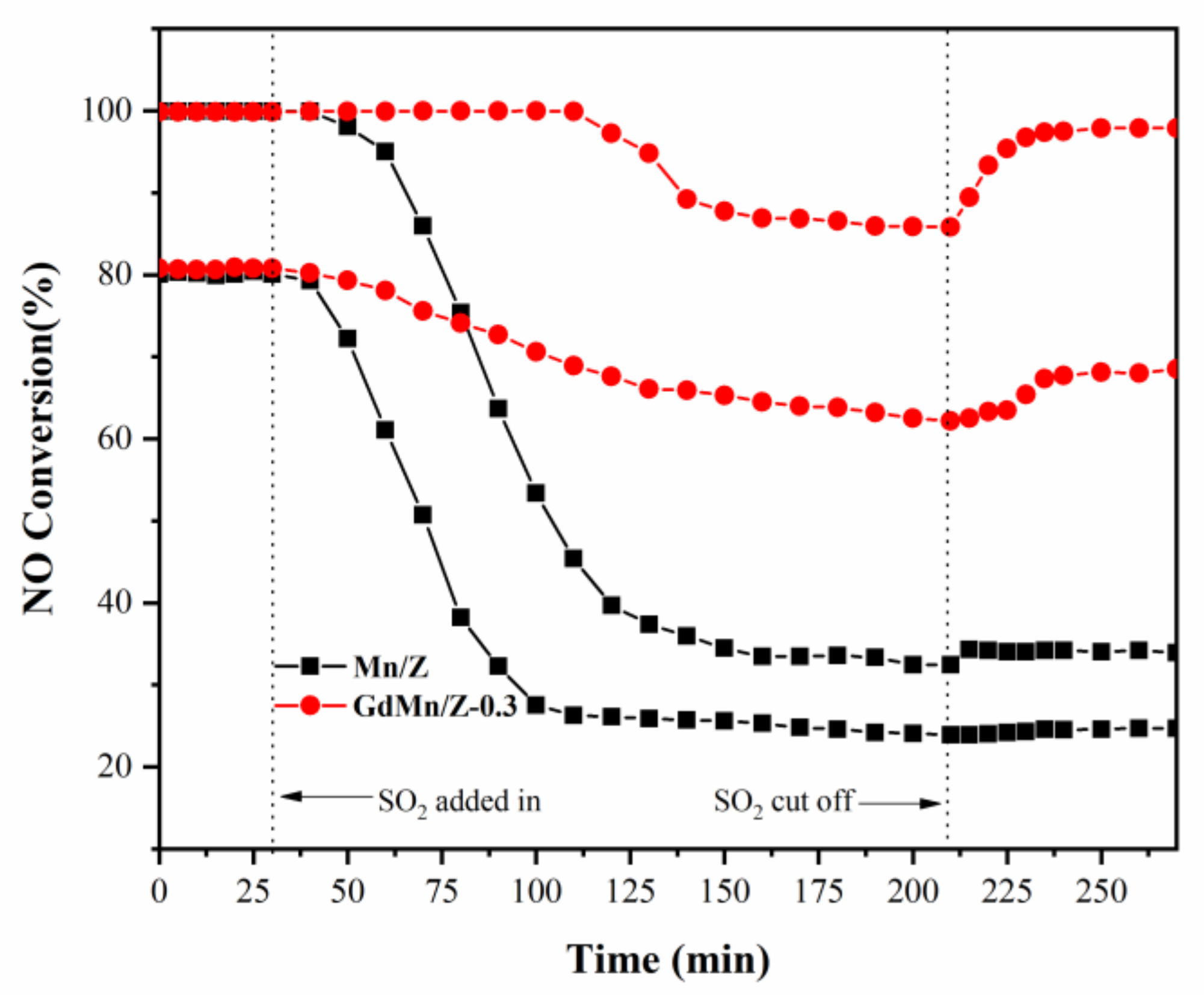
2.4. SO2 Resistance Studies
2.5. XRD, SEM and XPS Analyses of the Used Samples
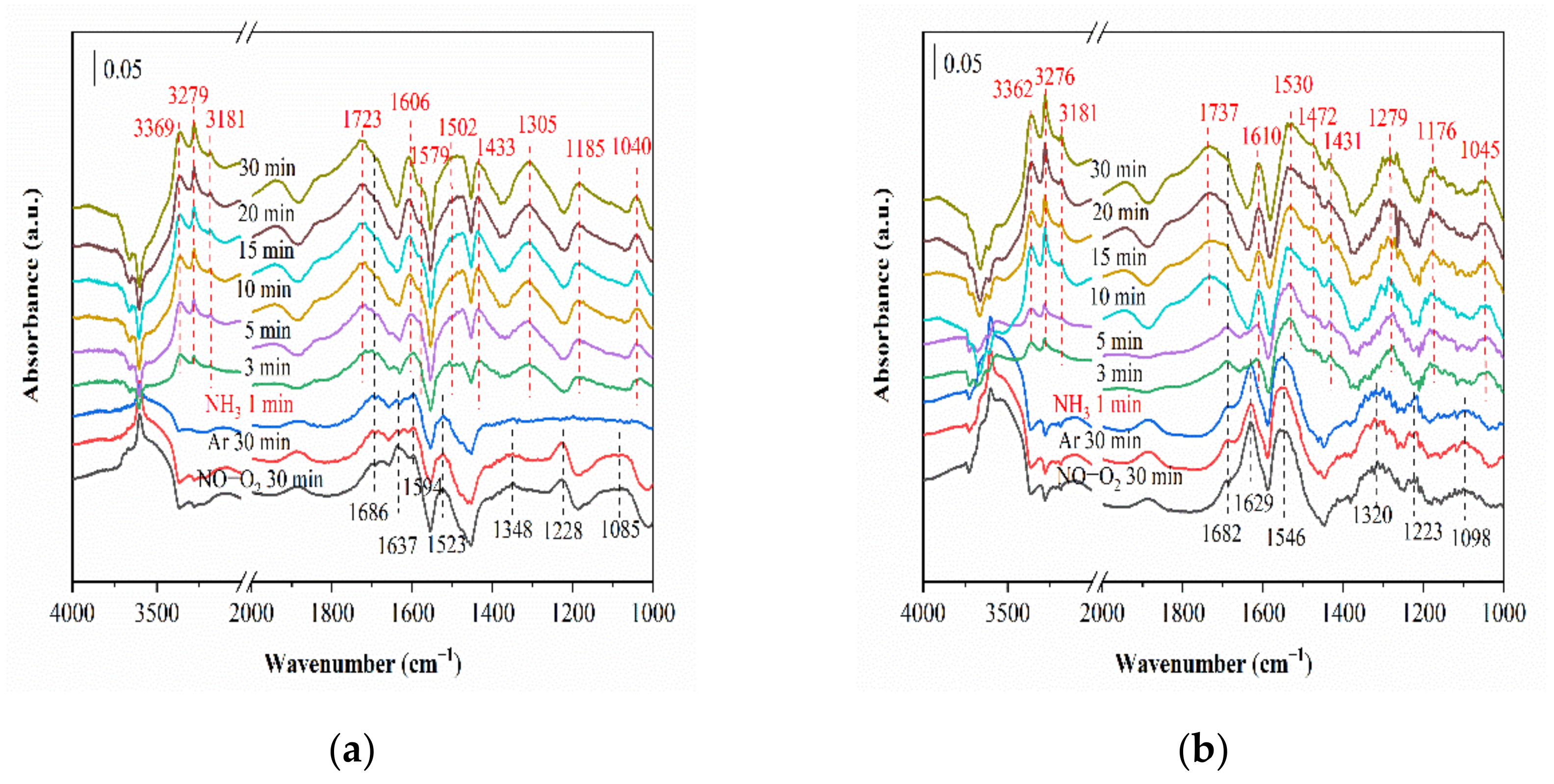
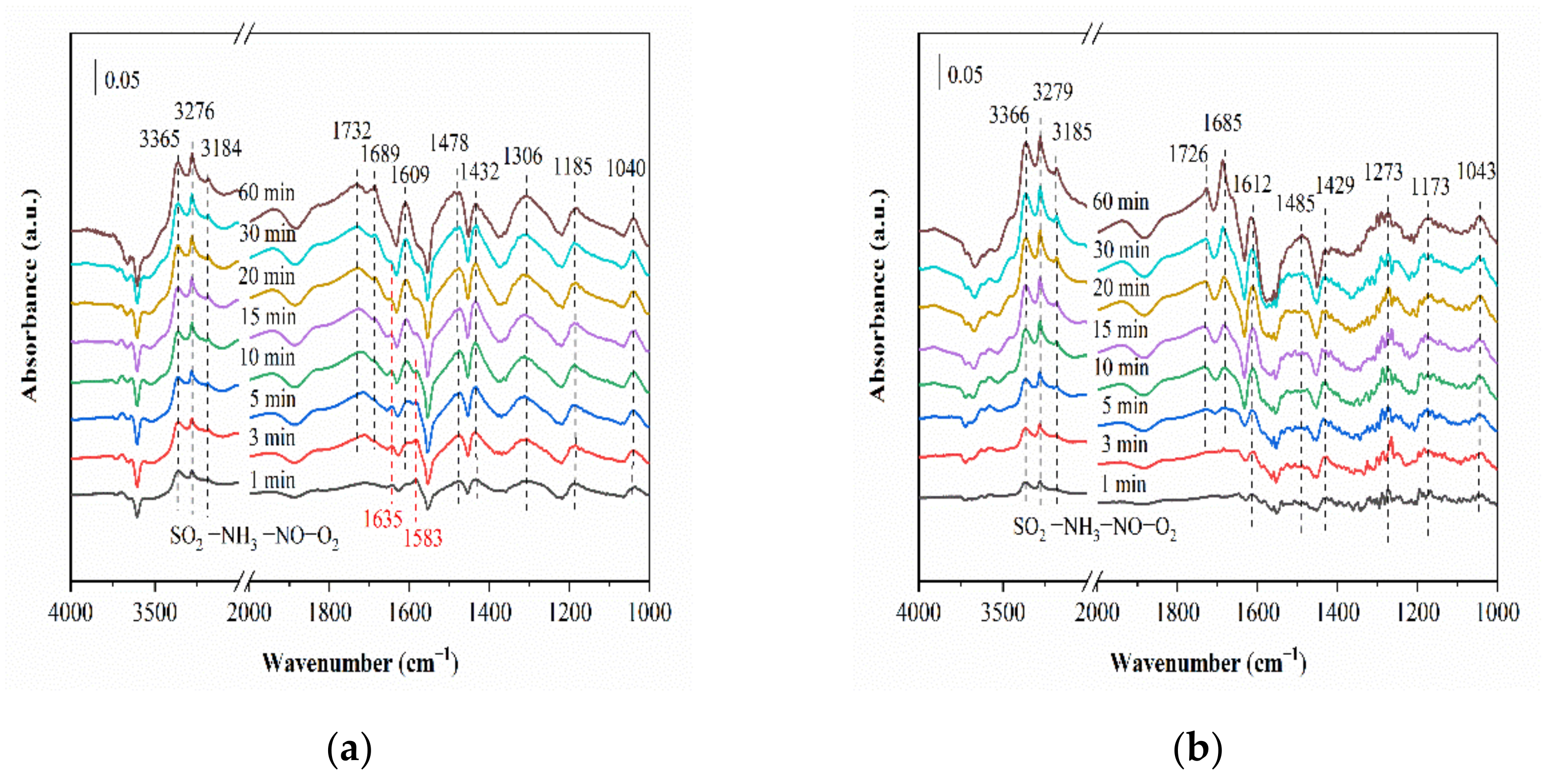
2.6. In Situ DRIFTS Studies for NH3-SCR with SO2
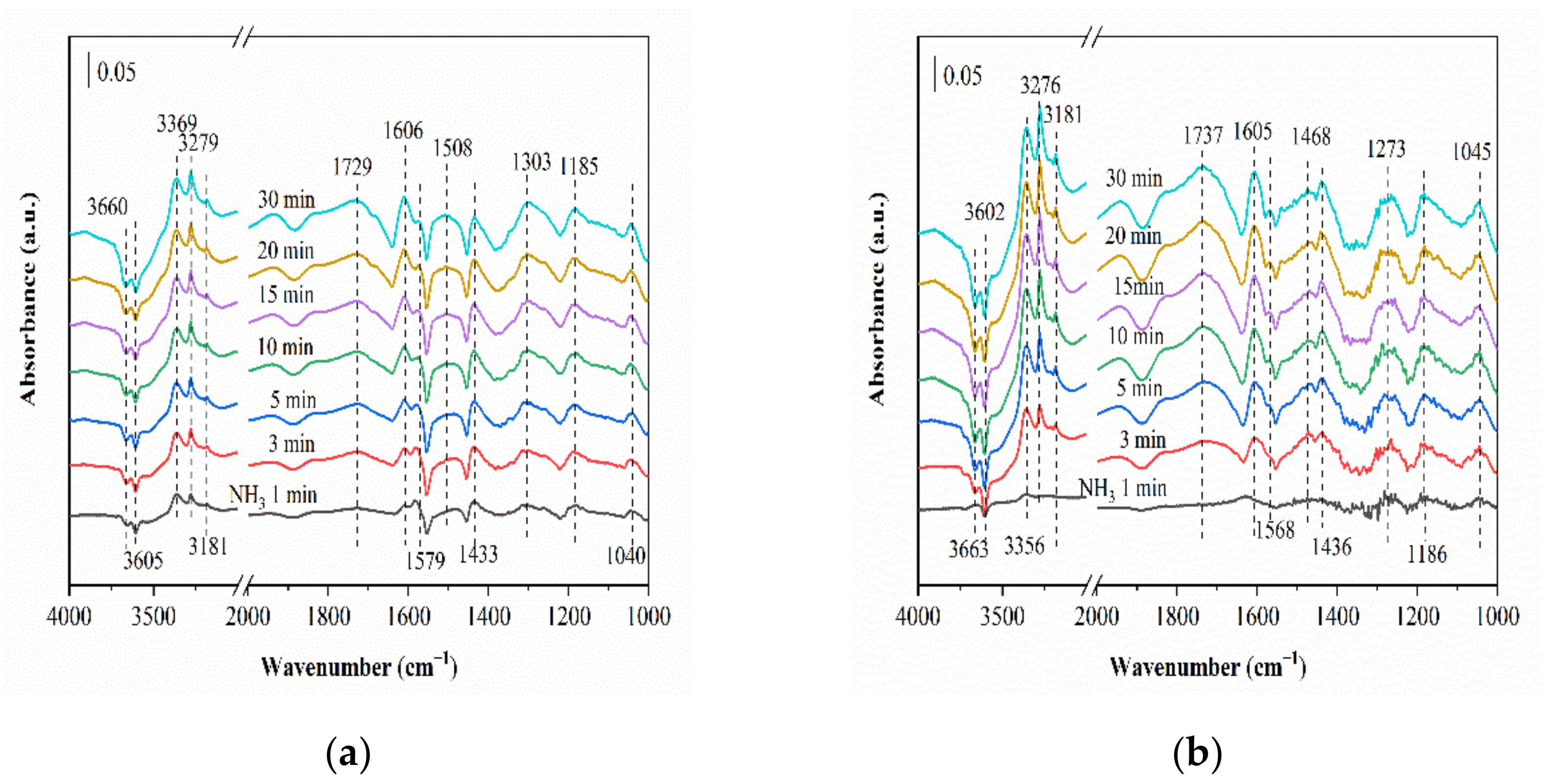
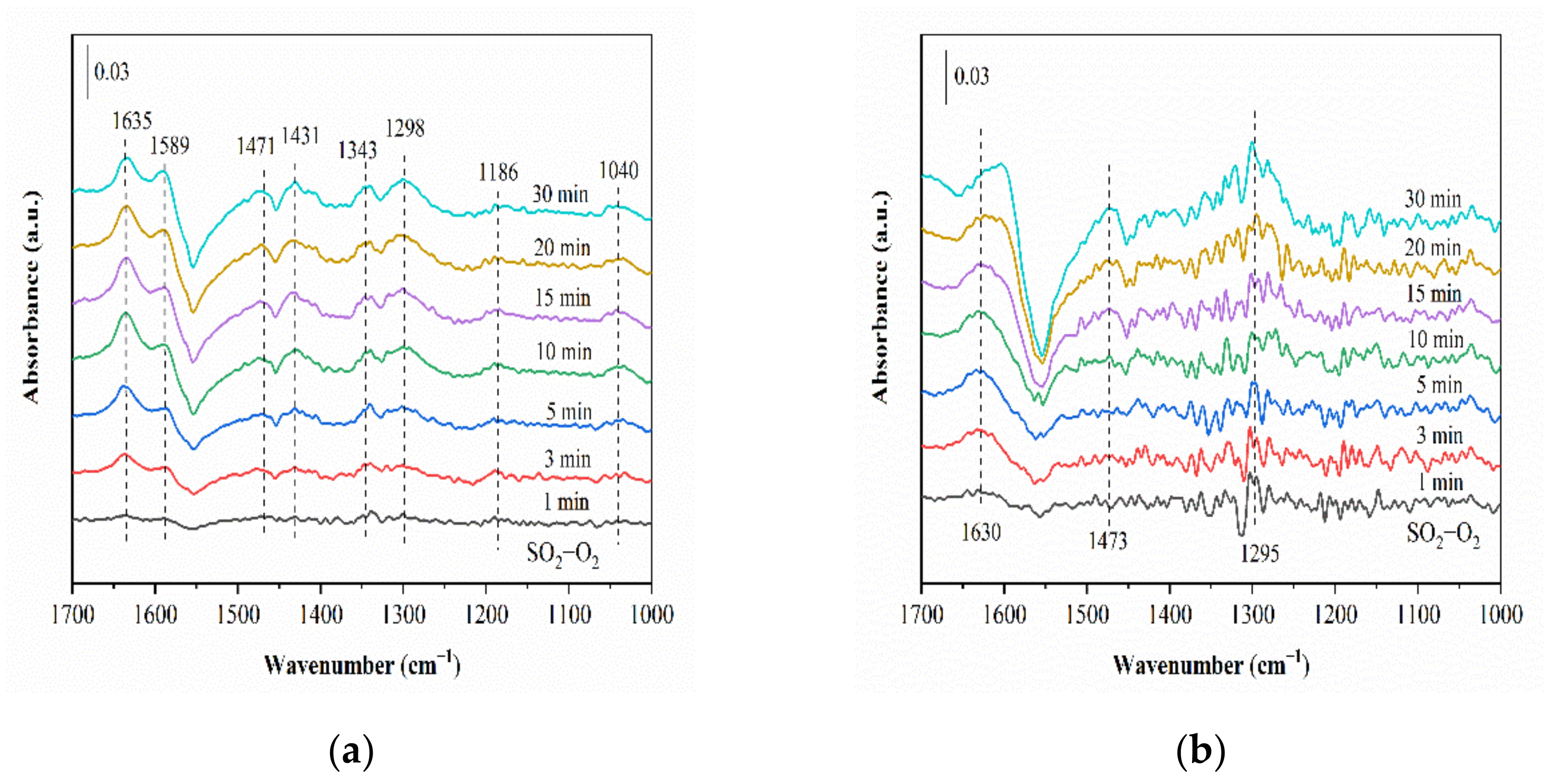
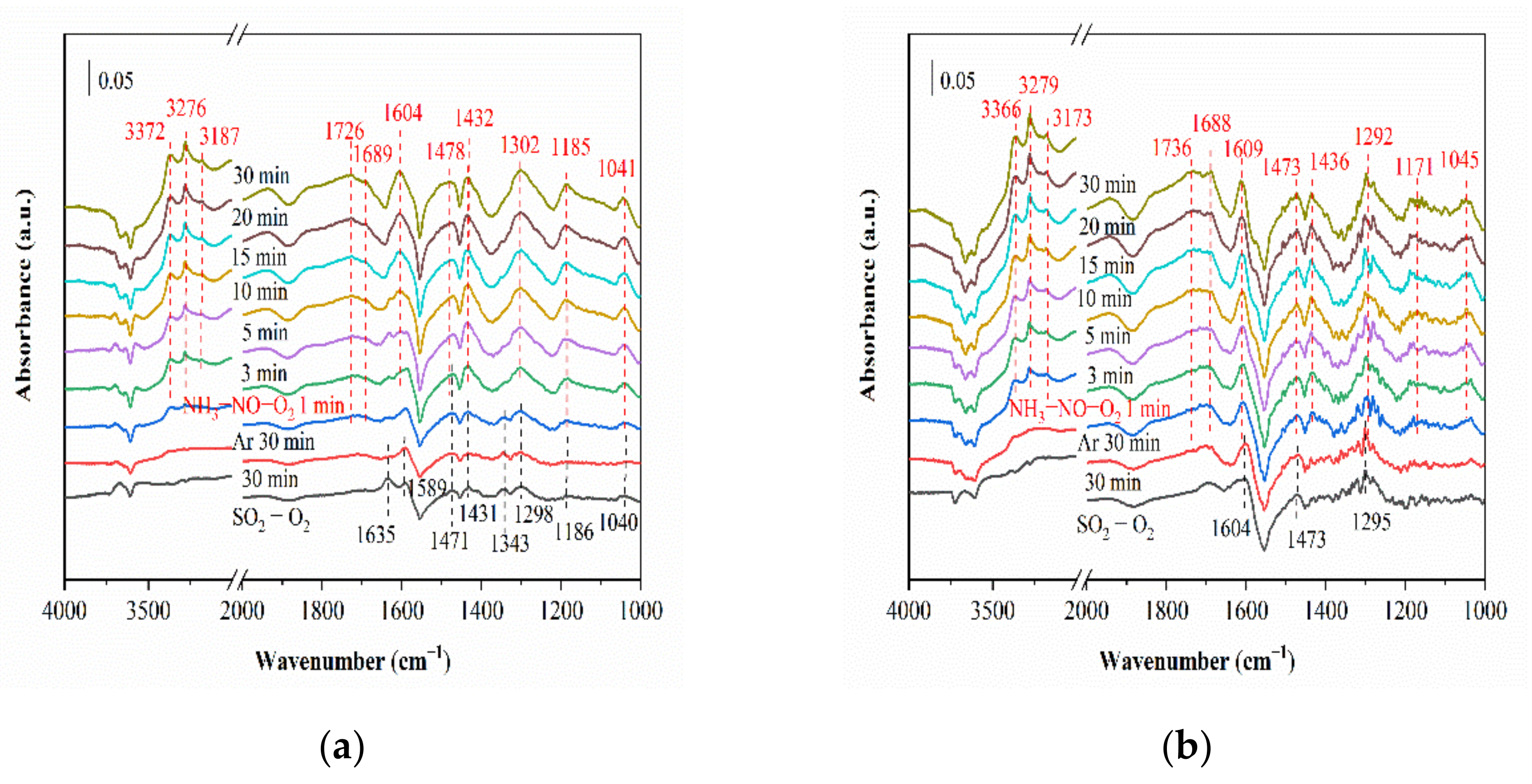
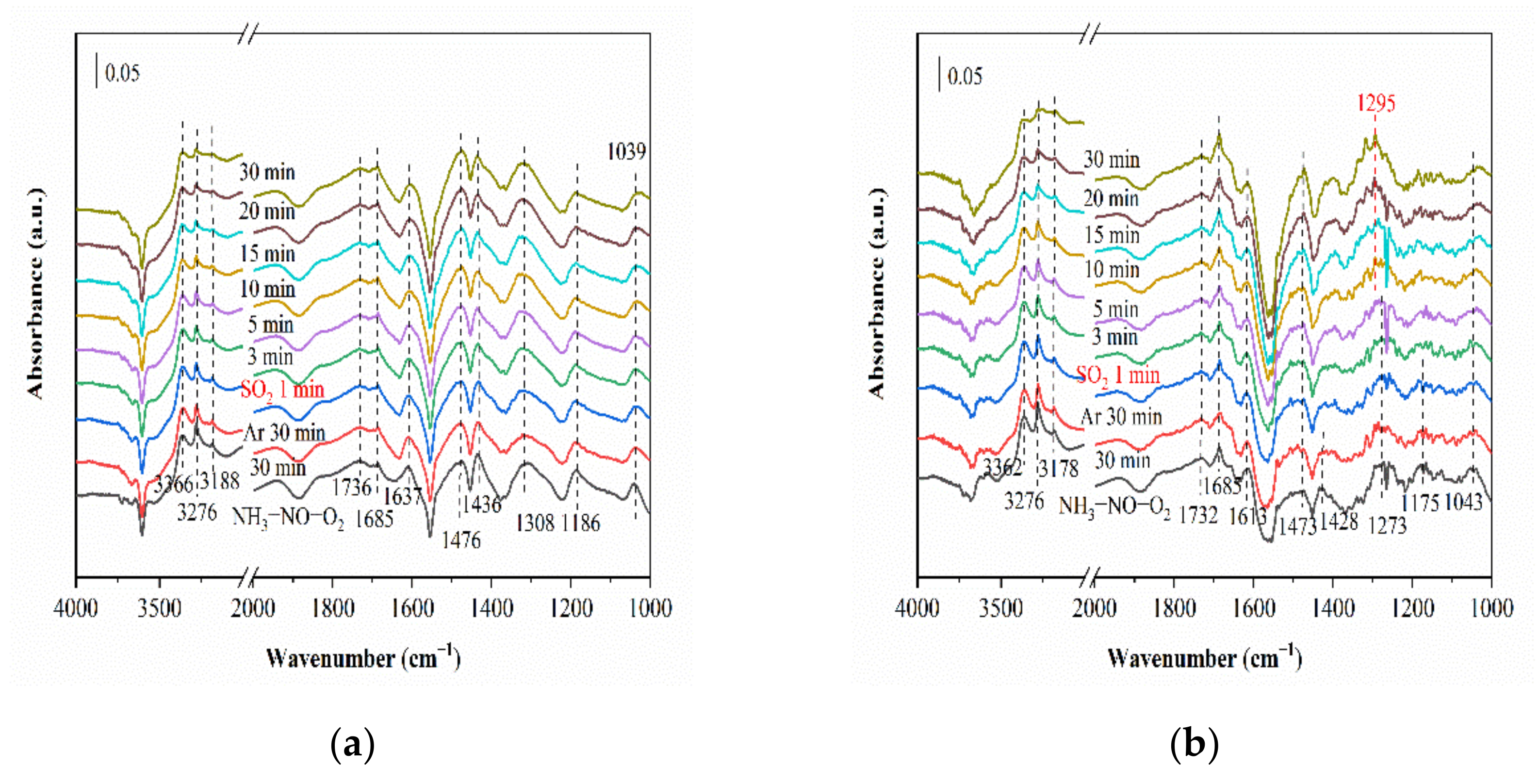
2.6.1. SO2 Adsorption
2.6.2. Reaction between NH3, NO and O2 (SO2) and Pre-Adsorbed SO2 (NH3, NO and O2)
2.6.3. Co-Adsorption of SO2, NH3, NO and O2
3. Materials and Methods
3.1. Catalyst Preparation
3.2. NH3-SCR Activity Test
3.3. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kwak, J.H.; Tonkyn, R.G.; Kim, D.H.; Szanyi, J.; Peden, C.H.F. Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J. Catal. 2010, 275, 187–190. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Q.; Wang, H.; Chen, Y.; Zhang, M. Temperature effect on the morphology and catalytic performance of Co-MOF-74 in low-temperature NH3-SCR process. Catal. Commun. 2016, 80, 24–27. [Google Scholar] [CrossRef]
- France, L.J.; Yang, Q.; Li, W.; Chen, Z.H.; Guang, J.Y.; Guo, D.W.; Wang, L.F.; Li, X.H. Ceria modified FeMnOx-Enhanced performance and sulphur resistance for low-temperature SCR of NOx. Appl. Catal. B Environ. 2017, 206, 203–215. [Google Scholar] [CrossRef]
- Gholami, F.; Tomas, M.; Gholami, Z.; Vakili, M. Technologies for the nitrogen oxides reduction from flue gas: A review. Sci. Total Environ. 2020, 714. [Google Scholar] [CrossRef] [PubMed]
- Heck, R.M. Catalytic abatement of nitrogen oxides-stationary applications. Catal. Today 1999, 53, 519–523. [Google Scholar] [CrossRef]
- Roy, S.; Baiker, A. NOx storage-reduction catalysis: From mechanism and materials properties to storage-reduction performance. Chem. Rev. 2009, 109, 4054–4091. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Xie, J.; Hu, H.; Yang, H.; He, F.; Fu, Z. Identification of MnOx species and Mn valence states in MnOx/TiO2 catalysts for low temperature SCR. Chem. Eng. J. 2015, 271, 23–30. [Google Scholar] [CrossRef]
- Yu, C.; Huang, B.; Dong, L.; Chen, F.; Liu, X. In situ FT-IR study of highly dispersed MnOx/SAPO-34 catalyst for low-temperature selective catalytic reduction of NOx by NH3. Catal. Today 2017, 281, 610–620. [Google Scholar] [CrossRef]
- Lou, X.; Liu, P.; Li, J.; Li, Z.; He, K. Effects of calcination temperature on Mn species and catalytic activities of Mn/ZSM-5 catalyst for selective catalytic reduction of NO with ammonia. Appl. Surf. Sci. 2014, 307, 382–387. [Google Scholar] [CrossRef]
- Xie, J.L.; Fang, D.; He, F.; Chen, J.F.; Fu, Z.B.; Chen, X.L. Performance and mechanism about MnOx species included in MnOx/TiO2 catalysts for SCR at low temperature. Catal. Commun. 2012, 28, 77–81. [Google Scholar] [CrossRef]
- Wei, L.; Cui, S.P.; Guo, H.X.; Ma, X.Y.; Wan, Y.Q.; Yu, S.S. The mechanism of the deactivation of MnOx/TiO2 catalyst for low-temperature SCR of NO. Appl. Surf. Sci. 2019, 483, 391–398. [Google Scholar] [CrossRef]
- Kijlstra, W.S.; Biervliet, M.; Poels, E.K.; Bliek, A. Deactivation by SO2 of MnOx/Al2O3 catalysts used for the selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 1998, 16, 327–337. [Google Scholar] [CrossRef]
- Su, Y.X.; Fan, B.X.; Wang, L.S.; Liu, Y.F.; Huang, B.C.; Fu, M.L.; Chen, L.M.; Ye, D.Q. MnOx supported on carbon nanotubes by different methods for the SCR of NO with NH3. Catal. Today 2013, 201, 115–121. [Google Scholar] [CrossRef]
- Carja, G.; Kameshima, Y.; Okada, K.; Madhusoodana, C.D. Mn–Ce/ZSM5 as a new superior catalyst for NO reduction with NH3. Appl. Catal. B Environ. 2007, 73, 60–64. [Google Scholar] [CrossRef]
- Lv, G.; Bin, F.; Song, C.L.; Wang, K.P.; Song, J.N. Promoting effect of zirconium doping on Mn/ZSM-5 for the selective catalytic reduction of NO with NH3. Fuel 2013, 107, 217–224. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, G.; Chen, H.; Zhang, G.; Han, Y.; Chang, Y.; Gong, P. Mn/beta and Mn/ZSM-5 for the low-temperature selective catalytic reduction of NO with ammonia: Effect of manganese precursors. Chin. J. Catal. 2018, 39, 118–127. [Google Scholar] [CrossRef]
- Chen, P.; Jabłońska, M.; Weide, P.; Caumanns, T.; Weirich, T.; Muhler, M.; Moos, R.; Palkovits, R.; Simon, U. Formation and Effect of NH4+ Intermediates in NH3–SCR over Fe-ZSM-5 Zeolite Catalysts. ACS Catal. 2016, 6, 7696–7700. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Y.; Fan, Z.; Chen, M.; Zhang, Z.; Shangguan, W. Widened Active Temperature Window of a Fe-ZSM-5 Catalyst by an Impregnation Solvent for NH3-SCR of NO. Ind. Eng. Chem. Res. 2018, 57, 13703–13712. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kwon, H.J.; Heo, I.; Nam, I.-S.; Cho, B.K.; Choung, J.W.; Cha, M.-S.; Yeo, G.K. Mn–Fe/ZSM5 as a low-temperature SCR catalyst to remove NOx from diesel engine exhaust. Appl. Catal. B Environ. 2012, 126, 9–21. [Google Scholar] [CrossRef]
- Han, L.P.; Cai, S.X.; Gao, M.; Hasegawa, J.; Wang, P.L.; Zhang, J.P.; Shi, L.Y.; Zhang, D.S. Selective Catalytic Reduction of NOx with NH3 by Using Novel Catalysts: State of the Art and Future Prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, L.; Pantaleo, G.; Ivanov, I.; Nedyalkova, R.; Venezia, A.M.; Andreeva, D. NO reduction by CO over gold based on ceria, doped by rare earth metals. Catal. Today 2008, 139, 168–173. [Google Scholar] [CrossRef]
- Chang, H.Z.; Chen, X.Y.; Li, J.H.; Ma, L.; Wang, C.Z.; Liu, C.X.; Schwank, J.W.; Hao, J.M. Improvement of Activity and SO2 Tolerance of Sn-Modified MnOx-CeO2 Catalysts for NH3-SCR at Low Temperatures. Environ. Sci. Technol. 2013, 47, 5294–5301. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Liu, Y.; Wang, Y.; Cen, W.; Wu, Z.; Wang, H.; Weng, X. The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature. Appl. Catal. B Environ. 2014, 148–149, 582–588. [Google Scholar] [CrossRef]
- Meng, D.; Zhan, W.; Guo, Y.; Guo, Y.; Wang, L.; Lu, G. A Highly Effective Catalyst of Sm-MnOx for the NH3-SCR of NOx at Low Temperature: Promotional Role of Sm and Its Catalytic Performance. ACS Catal. 2015, 5, 5973–5983. [Google Scholar] [CrossRef]
- You, X.C.; Sheng, Z.Y.; Yu, D.Q.; Yang, L.; Xiao, X.; Wang, S. Influence of Mn/Ce ratio on the physicochemical properties and catalytic performance of graphene supported MnOx-CeO2 oxides for NH3-SCR at low temperature. Appl. Surf. Sci. 2017, 423, 845–854. [Google Scholar] [CrossRef]
- Liu, J.; Guo, R.-t.; Li, M.-y.; Sun, P.; Liu, S.-m.; Pan, W.-g.; Liu, S.-w.; Sun, X. Enhancement of the SO2 resistance of Mn/TiO2 SCR catalyst by Eu modification: A mechanism study. Fuel 2018, 223, 385–393. [Google Scholar] [CrossRef]
- Yu, C.; Huang, B.; Dong, L.; Chen, F.; Yang, Y.; Fan, Y.; Yang, Y.; Liu, X.; Wang, X. Effect of Pr/Ce addition on the catalytic performance and SO2 resistance of highly dispersed MnOx/SAPO-34 catalyst for NH3-SCR at low temperature. Chem. Eng. J. 2017, 316, 1059–1068. [Google Scholar] [CrossRef]
- Liu, C.; Shi, J.-W.; Gao, C.; Niu, C. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A review. Appl. Catal. A Gen. 2016, 522, 54–69. [Google Scholar] [CrossRef]
- Panahi, P.N.; Salari, D.; Niaei, A.; Mousavi, S.M. NO reduction over nanostructure M-Cu/ZSM-5 (M: Cr, Mn, Co and Fe) bimetallic catalysts and optimization of catalyst preparation by RSM. J. Ind. Eng. Chem. 2013, 19, 1793–1799. [Google Scholar] [CrossRef]
- Zhao, X.; Mao, L.; Dong, G. Mn-Ce-V-WOx/TiO2 SCR Catalysts: Catalytic Activity, Stability and Interaction among Catalytic Oxides. Catalysts 2018, 8, 76. [Google Scholar] [CrossRef]
- Fan, Z.; Shi, J.-W.; Gao, C.; Gao, G.; Wang, B.; Wang, Y.; He, C.; Niu, C. Gd-modified MnOx for the selective catalytic reduction of NO by NH3: The promoting effect of Gd on the catalytic performance and sulfur resistance. Chem. Eng. J. 2018, 348, 820–830. [Google Scholar] [CrossRef]
- Chen, H.F.; Xia, Y.; Fang, R.Y.; Huang, H.; Gan, Y.P.; Liang, C.; Zhang, J.; Zhang, W.K.; Liu, X.S. Effects of Nd-modification on the activity and SO2 resistance of MnOx/TiO2 catalysts for low-temperature NH3-SCR. New J. Chem. 2018, 42, 12845–12852. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, H.T.; Luo, X.; Shi, K.Q.; Zhao, H.B.; Wang, W.K.; Chen, Q.H.; Fan, H.; Wu, T. Promotion effect and mechanism of the addition of Mo on the enhanced low temperature SCR of NOx by NH3 over MnOx/gamma-Al2O3 catalysts. Appl. Catal. B Environ. 2019, 245, 743–752. [Google Scholar] [CrossRef]
- Liu, Z.; Yi, Y.; Zhang, S.; Zhu, T.; Zhu, J.; Wang, J. Selective catalytic reduction of NOx with NH3 over Mn-Ce mixed oxide catalyst at low temperatures. Catal. Today 2013, 216, 76–81. [Google Scholar] [CrossRef]
- Tang, W.; Deng, Y.; Li, W.; Li, S.; Wu, X.; Chen, Y. Restrictive nanoreactor for growth of transition metal oxides (MnO2, Co3O4, NiO) nanocrystal with enhanced catalytic oxidation activity. Catal. Commun. 2015, 72, 165–169. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Li, K.; Farrauto, R. Enhanced propane and carbon monoxide oxidation activity by structural interactions of CeO2 with MnOx/Nb2O5-x catalysts. Appl. Catal. B Environ. 2020, 267. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, M.; Ma, X.; Wu, S.; Ge, M.; Yu, X. Insights into the transformations of Mn species for peroxymonosulfate activation by tuning the Mn3O4 shapes. Chem. Eng. J. 2021, 404. [Google Scholar] [CrossRef]
- Yang, S.; Wang, C.; Li, J.; Yan, N.; Ma, L.; Chang, H. Low temperature selective catalytic reduction of NO with NH3 over Mn-Fe spinel: Performance, mechanism and kinetic study. Appl. Catal. B Environ. 2011, 110, 71–80. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Q.; Diao, Q.; Wang, J.; Xiao, K.; Yang, B.; Wu, X. Performance study for NH3-SCR at low temperature based on different methods of Mnx/SEP catalyst. Chem. Eng. J. 2019, 370, 364–371. [Google Scholar] [CrossRef]
- Wang, T.; Wan, Z.; Yang, X.; Zhang, X.; Niu, X.; Sun, B. Promotional effect of iron modification on the catalytic properties of Mn-Fe/ZSM-5 catalysts in the Fast SCR reaction. Fuel Process. Technol. 2018, 169, 112–121. [Google Scholar] [CrossRef]
- Chang, H.Z.; Li, J.H.; Yuan, J.; Chen, L.; Dai, Y.; Arandiyan, H.; Xu, J.Y.; Hao, J.M. Ge, Mn-doped CeO2-WO3 catalysts for NH3-SCR of NOx: Effects of SO2 and H-2 regeneration. Catal. Today 2013, 201, 139–144. [Google Scholar] [CrossRef]
- Meng, D.; Zhan, W.; Guo, Y.; Guo, Y.; Wang, Y.; Wang, L.; Lu, G. A highly effective catalyst of Sm-Mn mixed oxide for the selective catalytic reduction of NOx with ammonia: Effect of the calcination temperature. J. Mol. Catal. A Chem. 2016, 420, 272–281. [Google Scholar] [CrossRef]
- Gao, C.; Shi, J.-W.; Fan, Z.; Yu, Y.; Chen, J.; Li, Z.; Niu, C. Eu-Mn-Ti mixed oxides for the SCR of NOx with NH3: The effects of Eu-modification on catalytic performance and mechanism. Fuel Process. Technol. 2017, 167, 322–333. [Google Scholar] [CrossRef]
- Liu, D.J.; Zhou, W.G.; Wu, J. Effect of Ce and La on the activity of CuO/ZSM-5 and MnOx/ZSM-5 composites for elemental mercury removal at low temperature. Fuel 2017, 194, 115–122. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Shi, L.; Fang, C.; Li, H.; Gao, R.; Huang, L.; Zhang, J. In situ supported MnOx-CeOx on carbon nanotubes for the low-temperature selective catalytic reduction of NO with NH3. Nanoscale 2013, 5, 1127–1136. [Google Scholar] [CrossRef]
- Shao, J.; Cheng, S.; Li, Z.; Huang, B. Enhanced Catalytic Performance of Hierarchical MnOx/ZSM-5 Catalyst for the Low-Temperature NH3-SCR. Catalysts 2020, 10, 311. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Zhong, B.C.; Wang, W.H.; Guan, X.J.; Huang, B.C.; Ye, D.Q.; Wu, H.J. In situ DRIFTS study of NO reduction by NH3 over Fe-Ce-Mn/ZSM-5 catalysts. Catal. Today 2011, 175, 157–163. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, Y.; Li, Z.; Shan, Y.; Shan, W.; Shi, X.; Yu, Y.; Zhang, C.; Li, K.; Ning, P.; et al. Promoting effect of acid sites on NH3-SCO activity with water vapor participation for Pt-Fe/ZSM-5 catalyst. Catal. Today 2020. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Reaction Mechanism of Selective Catalytic Reduction of NO with NH3 over Fe-ZSM-5 Catalyst. J. Catal. 2002, 207, 224–231. [Google Scholar] [CrossRef]
- Shu, Y.; Sun, H.; Quan, X.; Chen, S. Enhancement of Catalytic Activity over the Iron-Modified Ce/TiO2 Catalyst for Selective Catalytic Reduction of NOx with Ammonia. J. Phys. Chem. C 2012, 116, 25319–25327. [Google Scholar] [CrossRef]
- Sun, C.; Chen, W.; Jia, X.; Liu, A.; Gao, F.; Feng, S.; Dong, L. Comprehensive understanding of the superior performance of Sm-modified Fe2O3 catalysts with regard to NO conversion and H2O/SO2 resistance in the NH3-SCR reaction. Chin. J. Catal. 2021, 42, 417–430. [Google Scholar] [CrossRef]
- Mu, J.; Li, X.; Sun, W.; Fan, S.; Wang, X.; Wang, L.; Qin, M.; Gan, G.; Yin, Z.; Zhang, D. Inductive Effect Boosting Catalytic Performance of Advanced Fe1–xVxOδ Catalysts in Low-Temperature NH3 Selective Catalytic Reduction: Insight into the Structure, Interaction, and Mechanisms. ACS Catal. 2018, 8, 6760–6774. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Liu, L.; Mi, L.; Wang, X. In situ DRIFTS studies on MnO nanowires supported by activated semi-coke for low temperature selective catalytic reduction of NO with NH3. Appl. Surf. Sci. 2016, 366, 139–147. [Google Scholar] [CrossRef]
- Kijlstra, W.S.; Brands, D.S.; Poels, E.K.; Bliek, A. Mechanism of the selective catalytic reduction of NO by NH3 over MnOx/Al2O3. 1. Adsorption and desorption of the single reaction components. J. Catal. 1997, 171, 208–218. [Google Scholar] [CrossRef]
- Fei, Z.; Yang, Y.; Wang, M.; Tao, Z.; Liu, Q.; Chen, X.; Cui, M.; Zhang, Z.; Tang, J.; Qiao, X. Precisely fabricating Ce-O-Ti structure to enhance performance of Ce-Ti based catalysts for selective catalytic reduction of NO with NH3. Chem. Eng. J. 2018, 353, 930–939. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, X.; Shan, Y.; Du, J.; Liu, K.; He, H. Hydrothermal Stability Enhancement of Al-Rich Cu-SSZ-13 for NH3 Selective Catalytic Reduction Reaction by Ion Exchange with Cerium and Samarium. Ind. Eng. Chem. Res. 2020, 59, 6416–6423. [Google Scholar] [CrossRef]
- Dong, L.; Fan, Y.; Ling, W.; Yang, C.; Huang, B. Effect of Ce/Y Addition on Low-Temperature SCR Activity and SO2 and H2O Resistance of MnOx/ZrO2/MWCNTs Catalysts. Catalysts 2017, 7, 181. [Google Scholar] [CrossRef]
- Jiang, B.Q.; Deng, B.Y.; Zhang, Z.Q.; Wu, Z.L.; Tang, X.J.; Yao, S.L.; Lu, H. Effect of Zr Addition on the Low-Temperature SCR Activity and SO2 Tolerance of Fe-Mn/Ti Catalysts. J. Phys. Chem. C 2014, 118, 14866–14875. [Google Scholar] [CrossRef]
- Yi, H.H.; Ma, C.B.; Tang, X.L.; Zhao, S.Z.; Yang, K.; Sani, Z.; Song, L.L.; Zhang, X.D.; Han, W. Synthesis of MgO@CeO2-MnOx core shell structural adsorbent and its application in reducing the competitive adsorption of SO2 and NOx in coal-fired flue gas. Chem. Eng. J. 2019, 372, 129–140. [Google Scholar] [CrossRef]
- Wei, L.; Cui, S.P.; Guo, H.X.; Ma, X.Y.; Zhang, L.J. DRIFT and DFT study of cerium addition on SO2 of Manganese-based Catalysts for low temperature SCR. J. Mol. Catal. A Chem. 2016, 421, 102–108. [Google Scholar] [CrossRef]
- Han, L.; Gao, M.; Hasegawa, J.Y.; Li, S.; Shen, Y.; Li, H.; Shi, L.; Zhang, D. SO2-Tolerant Selective Catalytic Reduction of NOx over Meso-TiO2@Fe2O3@Al2O3 Metal-Based Monolith Catalysts. Environ. Sci. Technol. 2019, 53, 6462–6473. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Shi, Y.; Gao, F.; Zhao, S.; Yi, H.; Xie, Z. Promotional role of Mo on Ce0.3FeOx catalyst towards enhanced NH3-SCR catalytic performance and SO2 resistance. Chem. Eng. J. 2020, 398. [Google Scholar] [CrossRef]
- Wang, F.M.; Shen, B.X.; Zhu, S.W.; Wang, Z. Promotion of Fe and Co doped Mn-Ce/TiO2 catalysts for low temperature NH3-SCR with SO2 tolerance. Fuel 2019, 249, 54–60. [Google Scholar] [CrossRef]




| Catalysts | Specific Surface Area (m2g−1) | Total Pore Volume (cm3g−1) | Average Pore Size (nm) |
|---|---|---|---|
| Mn/Z | 266.8 | 0.11 | 2.98 |
| GdMn/Z-0.1 | 241.6 | 0.20 | 3.27 |
| GdMn/Z-0.3 | 273.4 | 0.25 | 3.47 |
| GdMn/Z-0.6 | 259.2 | 0.19 | 3.40 |
| Samples | Surface Atomic Concentration (%) | Relative Concentration Ratios (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mn | O | Gd | others a | Mn4+ | Mn3+ | Mn2+ | OS | OL | |
| Mn/Z | 5.09 | 77.19 | — | 17.72 | 28.5 | 35.7 | 35.8 | 72.8 | 27.2 |
| GdMn/Z-0.1 | 4.47 | 64.59 | 1.24 | 29.70 | 27.2 | 34.6 | 38.1 | 71.9 | 28.1 |
| GdMn/Z-0.3 | 4.90 | 66.46 | 3.22 | 25.42 | 34.1 | 42.2 | 23.7 | 74.4 | 25.6 |
| GdMn/Z-0.6 | 4.08 | 60.97 | 5.12 | 29.83 | 31.9 | 36.8 | 31.3 | 69.8 | 30.2 |
| Catalysts | Weak Acid (mmol·g−1) | Medium-Strong Acid (mmol·g−1) | Total Acid Amount (mmol·g−1) | H2-TPR Curve Area |
|---|---|---|---|---|
| Mn/Z | 0.605 | 0.304 | 0.909 | 0.31435 |
| GdMn/Z-0.1 | 0.789 | 0.465 | 1.254 | 0.25803 |
| GdMn/Z-0.3 | 0.702 | 0.542 | 1.244 | 0.69167 |
| GdMn/Z-0.6 | 0.638 | 0.588 | 1.226 | 0.40507 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, J.; Zhou, L.; Li, W.; Hu, D.; Wen, J.; Huang, B. Improving the Performance of Gd Addition on Catalytic Activity and SO2 Resistance over MnOx/ZSM-5 Catalysts for Low-Temperature NH3-SCR. Catalysts 2021, 11, 324. https://doi.org/10.3390/catal11030324
Guan J, Zhou L, Li W, Hu D, Wen J, Huang B. Improving the Performance of Gd Addition on Catalytic Activity and SO2 Resistance over MnOx/ZSM-5 Catalysts for Low-Temperature NH3-SCR. Catalysts. 2021; 11(3):324. https://doi.org/10.3390/catal11030324
Chicago/Turabian StyleGuan, Jinkun, Lusha Zhou, Weiquan Li, Die Hu, Jie Wen, and Bichun Huang. 2021. "Improving the Performance of Gd Addition on Catalytic Activity and SO2 Resistance over MnOx/ZSM-5 Catalysts for Low-Temperature NH3-SCR" Catalysts 11, no. 3: 324. https://doi.org/10.3390/catal11030324
APA StyleGuan, J., Zhou, L., Li, W., Hu, D., Wen, J., & Huang, B. (2021). Improving the Performance of Gd Addition on Catalytic Activity and SO2 Resistance over MnOx/ZSM-5 Catalysts for Low-Temperature NH3-SCR. Catalysts, 11(3), 324. https://doi.org/10.3390/catal11030324







