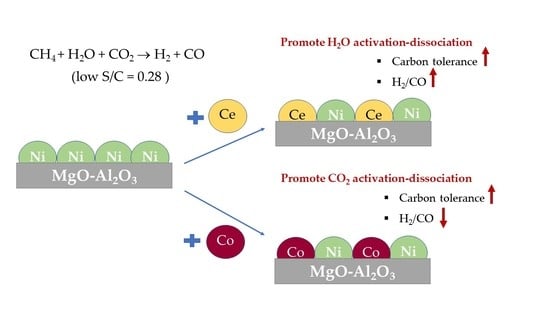
The Effects of CeO2 and Co Doping on the Properties and the Performance of the Ni/Al2O3-MgO Catalyst for the Combined Steam and CO2 Reforming of Methane Using Ultra-Low Steam to Carbon Ratio
Abstract
:1. Introduction
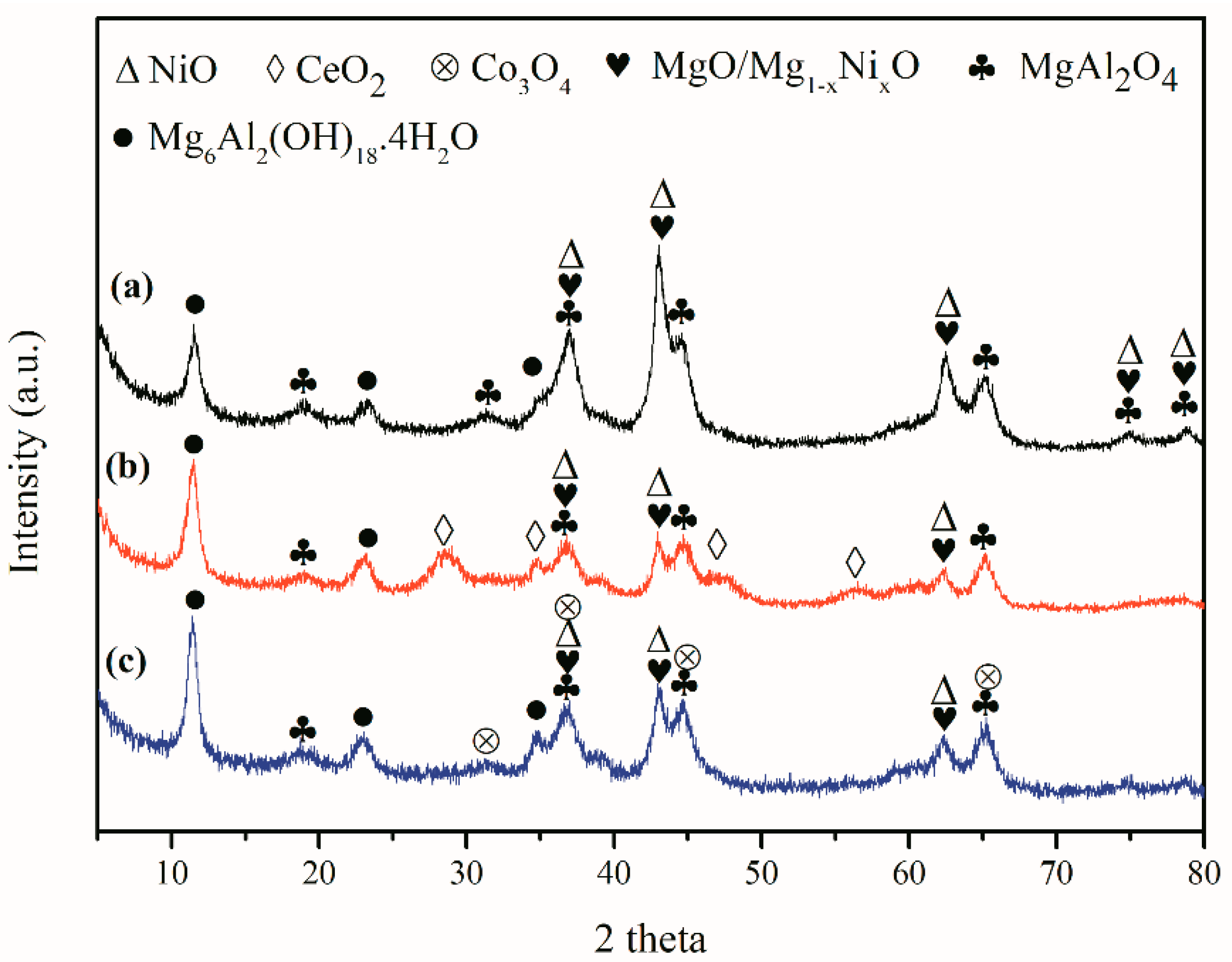
2. Results and Discussion
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Activity Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| BET | Brunauer–Emmett–Teller |
| BJH | Barrett–Joyner–Halenda |
| CSCRM | Combined steam and CO2 reforming of methane |
| CO2-TPD | CO2temperature programmed desorption |
| H2-TPR | H2 temperature programmed reduction |
| IUPAC | International Union of Pure and Applied Chemistry |
| RWGS | Reverse water gas shift |
| S/C ratio | Steam-to-carbon (H2O/(CH4 + CO2)) ratio |
| TCD | Thermal conductivity detector |
| TEM | Transmission electron microscope |
| TGA | Thermogravimetric analysis |
| XRD | X-ray diffraction |
References
- Jabbour, K. Tuning combined steam and dry reforming of methane for “metgas” production: A thermodynamic approach and state-of-the-art catalysts. J. Energy Chem. 2020, 48, 54–91. [Google Scholar] [CrossRef]
- Lee, S.M.; Hwang, I.H.; Kim, S.S. Enhancement of catalytic performance of porous membrane reactor with Ni catalyst for combined steam and carbon dioxide reforming of methane reaction. Fuel Process. Technol. 2019, 188, 197–202. [Google Scholar] [CrossRef]
- Jang, W.J.; Jeong, D.W.; Shim, J.O.; Kim, H.M.; Roh, H.S.; Son, I.H.; Lee, S.J. Combined steam and carbon dioxide reforming of methane and side reactions: Thermodynamic equilibrium analysis and experimental application. Appl. Energy 2016, 173, 80–91. [Google Scholar] [CrossRef]
- Ryi, S.K.; Lee, S.W.; Park, J.W.; Oh, D.K.; Park, J.S.; Kim, S.S. Combined steam and CO2 reforming of methane using catalytic nickel membrane for gas to liquid (GTL) process. Catal. Today 2014, 236, 49–56. [Google Scholar] [CrossRef]
- Jabbour, K.; Massiani, P.; Davidson, A.; Casale, S.; El Hassan, N. Ordered mesoporous “one-pot” synthesized Ni-Mg(Ca)-Al2O3 as effective and remarkably stable catalysts for combined steam and dry reforming of methane (CSDRM). Appl. Catal. B Environ. 2017, 201, 527–542. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yang, Z.; Chen, J.; Qin, X.; Jiang, X.; Wang, F.; Song, Z.; Ma, C. Performance of bio-char and energy analysis on CH4 combined reforming by CO2 and H2O into syngas production with assistance of microwave. Fuel 2018, 215, 655–664. [Google Scholar] [CrossRef]
- Koo, K.Y.; Lee, S.; Jung, U.H.; Roh, H.-S.; Yoon, W.L. Syngas production via combined steam and carbon dioxide reforming of methane over Ni–Ce/MgAl2O4 catalysts with enhanced coke resistance. Fuel Process. Technol. 2014, 119, 151–157. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, H.Y.; Cho, J.M.; Choi, J.-H.; Bae, J.W. Ni/M-Al2O3 (M=Sm, Ce or Mg) for combined steam and CO2 reforming of CH4 from coke oven gas. J. CO2 Util. 2017, 21, 211–218. [Google Scholar] [CrossRef]
- Banisharifdehkordi, F.; Baghalha, M. Catalyst deactivation in industrial combined steam and dry reforming of natural gas. Fuel Process. Technol. 2014, 120, 96–105. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Gangadharan, P.; Kanchi, K.C.; Lou, H.H. Evaluation of the economic and environmental impact of combining dry reforming with steam reforming of methane. Chem. Eng. Res. Des. 2012, 90, 1956–1968. [Google Scholar] [CrossRef]
- Álvarez, M.A.; Centeno, M.Á.; Odriozola, J.A. Ru-Ni Catalyst in the Combined Dry-Steam Reforming of Methane: The Importance in the Metal Order Addition. Top. Catal. 2016, 59, 303–313. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Chen, X.; Liang, X.; Zou, X.; Lu, X. Combined steam and CO2 reforming of methane over one-pot prepared Ni/La-Si catalysts. Int. J. Hydrog. Energy 2019, 44, 4780–4793. [Google Scholar] [CrossRef]
- Al-Fatesh, A.; Singh, S.K.; Kanade, G.S.; Atia, H.; Fakeeha, A.H.; Ibrahim, A.A.; El-Toni, A.M.; Labhasetwar, N.K. Rh promoted and ZrO2/Al2O3 supported Ni/Co based catalysts: High activity for CO2 reforming, steam–CO2 reforming and oxy–CO2 reforming of CH4. Int. J. Hydrogen Energy 2018, 43, 12069–12080. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An overview on dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Abdel, N.; Aramouni, K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018. [Google Scholar] [CrossRef]
- Ay, H.; Üner, D. Dry reforming of methane over CeO2 supported Ni, Co and Ni–Co catalysts. Appl. Catal. B Environ. 2015, 179, 128–138. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Palcheva, R.; Karakirova, Y.; Sanchez, M.C.C.; Tyuliev, G.; Gaigneaux, E.; Fierro, J.L.G. Structure and surface properties of ceria-modified Ni-based catalysts for hydrogen production. Appl. Catal. B Environ. 2018, 225, 340–353. [Google Scholar] [CrossRef]
- Wang, L.; Meng, F.; Li, K.; Lu, F. Characterization and optical properties of pole-like nano-CeO2 synthesized by a facile hydrothermal method. Appl. Surf. Sci. 2013, 286, 269–274. [Google Scholar] [CrossRef]
- Setiabudi, H.D.; Chong, C.C.; Abed, S.M.; Teh, L.P.; Chin, S.Y. Comparative study of Ni-Ce loading method: Beneficial effect of ultrasonic-assisted impregnation method in CO2 reforming of CH4 over Ni-Ce/SBA-15. J. Environ. Chem. Eng. 2018, 6, 745–753. [Google Scholar] [CrossRef]
- Li, L.; Tang, D.; Song, Y.; Jiang, B.; Zhang, Q. Hydrogen production from ethanol steam reforming on Ni-Ce/MMT catalysts. Energy 2018, 149, 937–943. [Google Scholar] [CrossRef]
- Shan, W.; Luo, M.; Ying, P.; Shen, W.; Li, C. Reduction property and catalytic activity of Ce1−XNiXO2 mixed oxide catalysts for CH4 oxidation. Appl. Catal. A Gen. 2003, 246, 1–9. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Papageridis, K.N.; Baklavaridis, A.; Tzounis, L.; Avraam, D.G.; Goula, M.A. Syngas production via the biogas dry reforming reaction over nickel supported on modified with CeO2 and/or La2O3 alumina catalysts. J. Nat. Gas Sci. Eng. 2016, 31, 164–183. [Google Scholar] [CrossRef]
- Han, J.; Zhan, Y.; Street, J.; To, F.; Yu, F. Natural gas reforming of carbon dioxide for syngas over Ni–Ce–Al catalysts. Int. J. Hydrogen Energy 2017, 42, 18364–18374. [Google Scholar] [CrossRef]
- Boaro, M.; Colussi, S.; Trovarelli, A. Ceria-Based Materials in Hydrogenation and Reforming Reactions for CO2 Valorization. Front. Chem. 2019, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Nikoo, M.K.; Amin, N.A.S. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef] [Green Version]
- Siang, T.J.; Pham, T.L.M.; Van Cuong, N.; Phuong, P.T.T.; Phuc, N.H.H.; Truong, Q.D.; Vo, D.-V.N. Combined steam and CO2 reforming of methane for syngas production over carbon-resistant boron-promoted Ni/SBA-15 catalysts. Microporous Mesoporous Mater. 2018, 262, 122–132. [Google Scholar] [CrossRef]
- Cao, P.; Adegbite, S.; Wu, T. Thermodynamic Equilibrium Analysis of CO2 Reforming of Methane: Elimination of Carbon Deposition and Adjustment of H2/CO Ratio. Energy Procedia 2017, 105, 1864–1869. [Google Scholar] [CrossRef]
- Lim, Y.; Lee, C.-J.; Jeong, Y.S.; Song, I.H.; Lee, C.J.; Han, C. Optimal Design and Decision for Combined Steam Reforming Process with Dry Methane Reforming to Reuse CO2 as a Raw Material. Ind. Eng. Chem. Res. 2012. [Google Scholar] [CrossRef]
- Oyama, S.T.; Hacarlioglu, P.; Gu, Y.; Lee, D. Dry reforming of methane has no future for hydrogen production: Comparison with steam reforming at high pressure in standard and membrane reactors. Int. J. Hydrog. Energy 2012, 37, 10444–10450. [Google Scholar] [CrossRef]
- Tongamp, W.; Zhang, Q.; Saito, F. Preparation of meixnerite (Mg–Al–OH) type layered double hydroxide by a mechanochemical route. J. Mater. Sci. 2007, 42, 9210–9215. [Google Scholar] [CrossRef]
- Hassani Rad, S.J.; Haghighi, M.; Alizadeh Eslami, A.; Rahmani, F.; Rahemi, N. Sol–gel vs. impregnation preparation of MgO and CeO2 doped Ni/Al2O3 nanocatalysts used in dry reforming of methane: Effect of process conditions, synthesis method and support composition. Int. J. Hydrog. Energy 2016, 41, 5335–5350. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, X.; Pan, M.; Shi, Y. Nano-scale pore structure and its multi-fractal characteristics of tight sandstone by n2 adsorption/desorption analyses: A case study of shihezi formation from the sulige gas filed, ordos basin, china. Minerals 2020, 10, 377. [Google Scholar] [CrossRef]
- Akbari, E.; Alavi, S.M.; Rezaei, M. Synthesis gas production over highly active and stable nanostructured NiMgOAl2O3 catalysts in dry reforming of methane: Effects of Ni contents. Fuel 2017, 194, 171–179. [Google Scholar] [CrossRef]
- Cao, Y.; Li, H.; Zhang, J.; Shi, L.; Zhang, D. Promotional effects of rare earth elements (Sc, Y, Ce, and Pr) on NiMgAl catalysts for dry reforming of methane. RSC Adv. 2016, 6, 112215–112225. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, S.; Johnston-Peck, A.; Xu, W.; Rodriguez, J.A.; Senanayake, S.D. Methanol steam reforming over Ni-CeO2 model and powder catalysts: Pathways to high stability and selectivity for H2/CO2 production. Catal. Today 2018, 311, 74–80. [Google Scholar] [CrossRef]
- Sangsong, S.; Ratana, T.; Tungkamani, S.; Sornchamni, T.; Phongaksorn, M. Effect of CeO2 loading of the Ce-Al mixed oxide on ultrahigh temperature water-gas shift performance over Ce-Al mixed oxide supported Ni catalysts. Fuel 2019, 252, 488–495. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Battocchio, C.; Lo Mastro, S.; Sodo, A. Ni/CeO2–Al2O3 catalysts for the dry reforming of methane: The effect of CeAlO3 content and nickel crystallite size on catalytic activity and coke resistance. Appl. Catal. A Gen. 2015, 500, 12–22. [Google Scholar] [CrossRef]
- Lim, T.H.; Cho, S.J.; Yang, H.S.; Engelhard, M.; Kim, D.H. Effect of Co/Ni ratios in cobalt nickel mixed oxide catalysts on methane combustion. Appl. Catal. A Gen. 2015, 505, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Lv, P.; Yuan, Z.; Li, H. The study of bimetallic Ni-Co/cordierite catalyst for cracking of tar from biomass pyrolysis. Renew. Energy 2013, 60, 522–528. [Google Scholar] [CrossRef]
- Zhao, L.; Mu, X.; Liu, T.; Fang, K. Bimetallic Ni-Co catalysts supported on Mn-Al oxide for selective catalytic CO hydrogenation to higher alcohols. Catal. Sci. Technol. 2018, 8, 2066–2076. [Google Scholar] [CrossRef]
- You, X.; Wang, X.; Ma, Y.; Liu, J.; Liu, W.; Xu, X.; Peng, H.; Li, C.; Zhou, W.; Yuan, P.; et al. Ni-Co/Al2O3 Bimetallic Catalysts for CH4 Steam Reforming: Elucidating the Role of Co for Improving Coke Resistance. ChemCatChem 2014, 6, 3377–3386. [Google Scholar] [CrossRef]
- Li, X.; Ai, J.; Li, W.; Li, D. Ni-Co bimetallic catalyst for CH4 reforming with CO2. Front. Chem. Eng. China 2010, 4, 476–480. [Google Scholar] [CrossRef]
- Sikander, U.; Sufian, S.; Salam, M.A. A review of hydrotalcite based catalysts for hydrogen production systems. Int. J. Hydrog. Energy 2017, 42, 19851–19868. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Galvez, M.E.; Grzybek, T.; Da Costa, P. Promotion effect of zirconia on Mg(Ni,Al)O mixed oxides derived from hydrotalcites in CO2 methane reforming. Appl. Catal. B Environ. 2018, 223, 36–46. [Google Scholar] [CrossRef]
- Challiwala, M.S.; Ghouri, M.M.; Linke, P.; El-Halwagi, M.M.; Elbashir, N.O. A combined thermo-kinetic analysis of various methane reforming technologies: Comparison with dry reforming. J. CO2 Util. 2017, 17, 99–111. [Google Scholar] [CrossRef]
- Özkara-Aydınoğlu, Ş. Thermodynamic equilibrium analysis of combined carbon dioxide reforming with steam reforming of methane to synthesis gas. Int. J. Hydrog. Energy 2010, 35, 12821–12828. [Google Scholar] [CrossRef]
- Carrasco, J.; López-Durán, D.; Liu, Z.; Duchoň, T.; Evans, J.; Senanayake, S.D.; Crumlin, E.J.; Matolín, V.; Rodríguez, J.A.; Ganduglia-Pirovano, M.V. In Situ and Theoretical Studies for the Dissociation of Water on an Active Ni/CeO2 Catalyst: Importance of Strong Metal-Support Interactions for the Cleavage of O-H Bonds. Angew. Chem. Int. Ed. 2015, 54, 3917–3921. [Google Scholar] [CrossRef]
- Sepehri, S.; Rezaei, M. Ce promoting effect on the activity and coke formation of Ni catalysts supported on mesoporous nanocrystalline γ-Al2O3 in autothermal reforming of methane. Int. J. Hydrog. Energy 2017, 42, 11130–11138. [Google Scholar] [CrossRef]
- Li, D.; Xu, S.; Song, K.; Chen, C.; Zhan, Y.; Jiang, L. Hydrotalcite-derived Co/Mg(Al)O as a stable and coke-resistant catalyst for low-temperature carbon dioxide reforming of methane. Appl. Catal. A Gen. 2018, 552, 21–29. [Google Scholar] [CrossRef]
- Liu, Z.; Lustemberg, P.; Gutiérrez, R.A.; Carey, J.J.; Palomino, R.M.; Vorokhta, M.; Grinter, D.C.; Ramírez, P.J.; Matolín, V.; Nolan, M.; et al. In Situ Investigation of Methane Dry Reforming on Metal/Ceria(111) Surfaces: Metal–Support Interactions and C−H Bond Activation at Low Temperature. Angew. Chem. Int. Ed. 2017, 56, 13041–13046. [Google Scholar] [CrossRef] [PubMed]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Shahul Hamid, M.Y. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Li, H.; Meng, F.; Gong, J.; Fan, Z.; Qin, R. Structural, morphological and optical properties of shuttle-like CeO2 synthesized by a facile hydrothermal method. J. Alloys Compd. 2017, 722, 489–498. [Google Scholar] [CrossRef]
- Jafarbegloo, M.; Tarlani, A.; Mesbah, A.W.; Muzart, J.; Sahebdelfar, S. NiO-MgO Solid Solution Prepared by Sol-Gel Method as Precursor for Ni/MgO Methane Dry Reforming Catalyst: Effect of Calcination Temperature on Catalytic Performance. Catal. Lett. 2016, 146, 238–248. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Q.; Hao, Z.; Zhang, T.; Xie, Z. Development of a Co-Ni bimetallic aerogel catalyst for hydrogen production via methane oxidative CO2 reforming in a magnetic assisted fluidized bed. Int. J. Hydrog. Energy 2010, 35, 8494–8502. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Duraczyska, D.; Launay, F.; Galvez, M.E.; Grzybek, T.; Da Costa, P. Methane dry reforming over hydrotalcite-derived Ni–Mg–Al mixed oxides: The influence of Ni content on catalytic activity, selectivity and stability. Catal. Sci. Technol. 2016, 6, 6705–6715. [Google Scholar] [CrossRef]
- Ni, J.; Chen, L.; Lin, J.; Kawi, S. Carbon deposition on borated alumina supported nano-sized Ni catalysts for dry reforming of CH4. Nano Energy 2012, 1, 674–686. [Google Scholar] [CrossRef]






| N2 Adsorption-Desorption a | H2-TPR b | Deconvolution of the CO2-TPD b (mmol/gcat) | |||||
|---|---|---|---|---|---|---|---|
| Catalyst | Surface Area (m2/g) | Total Pore Volume (cm3/g) | H2 Uptake (mmol/gcat) | Weak | Medium | Strong | Total Basicity |
| 10Ni/MA | 97 | 0.20 | 11.9 | 0.04 | 0.02 | 0.13 | 0.19 |
| 5Ni5Ce/MA | 111 | 0.24 | 4.3 | 0.02 | 0.03 | 0.17 | 0.22 |
| 5Ni5Co/MA | 108 | 0.22 | 10.8 | 0.03 | 0.03 | 0.10 | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dharmasaroja, N.; Ratana, T.; Tungkamani, S.; Sornchamni, T.; Simakov, D.S.A.; Phongaksorn, M. The Effects of CeO2 and Co Doping on the Properties and the Performance of the Ni/Al2O3-MgO Catalyst for the Combined Steam and CO2 Reforming of Methane Using Ultra-Low Steam to Carbon Ratio. Catalysts 2020, 10, 1450. https://doi.org/10.3390/catal10121450
Dharmasaroja N, Ratana T, Tungkamani S, Sornchamni T, Simakov DSA, Phongaksorn M. The Effects of CeO2 and Co Doping on the Properties and the Performance of the Ni/Al2O3-MgO Catalyst for the Combined Steam and CO2 Reforming of Methane Using Ultra-Low Steam to Carbon Ratio. Catalysts. 2020; 10(12):1450. https://doi.org/10.3390/catal10121450
Chicago/Turabian StyleDharmasaroja, Nichthima, Tanakorn Ratana, Sabaithip Tungkamani, Thana Sornchamni, David S. A. Simakov, and Monrudee Phongaksorn. 2020. "The Effects of CeO2 and Co Doping on the Properties and the Performance of the Ni/Al2O3-MgO Catalyst for the Combined Steam and CO2 Reforming of Methane Using Ultra-Low Steam to Carbon Ratio" Catalysts 10, no. 12: 1450. https://doi.org/10.3390/catal10121450
APA StyleDharmasaroja, N., Ratana, T., Tungkamani, S., Sornchamni, T., Simakov, D. S. A., & Phongaksorn, M. (2020). The Effects of CeO2 and Co Doping on the Properties and the Performance of the Ni/Al2O3-MgO Catalyst for the Combined Steam and CO2 Reforming of Methane Using Ultra-Low Steam to Carbon Ratio. Catalysts, 10(12), 1450. https://doi.org/10.3390/catal10121450