Abstract
Biomass could be a source of the redox shuttles that have shown promise for operation as high potential, organic electrolytes for redox flow batteries. There is a sufficient quantity of biomass to satisfy the growing demand to buffer the episodic nature of renewably produced electricity. However, despite a century of effort, it is still not evident how to use existing information from organic electrochemistry to design the electrocatalysts or supporting electrolytes that will confer the required activity, selectivity and longevity. In this research, the use of a fiducial reaction to normalize reaction rates is shown to fail.
1. Introduction
Catalysis is a key component of technologies that promise to satisfy the growing demand for clean energy. However, we do not yet have ready access to correlations for selecting or interpreting the performance of electrocatalysts for storing the quantities of electricity that will permit grid-scale use of renewable resources (e.g., wind power and solar power). This article proposes the use of an infrequently employed approach to normalize electrocatalytic reaction rates, namely, a fiducial reaction. Here, fiducial means “faithful”, in the sense that the fiducial reaction faithfully tracks the number and rate of the active sites. While the discussion below was motivated by our study of electrocatalysts for redox flow batteries, this article is not intended to be a review of that technology, nor to bear on the choice of the electrolyte. Rather, it introduces the idea of a fiducial reaction as a way to systematize the rates of electrocatalytic reactions. The examples shown and discussed below illustrate an instance in which a fiducial reaction works and one in which it does not, for the particular cases of electrolytes derived from biomass.
Projections from the International Energy Agency (IEA) [1] indicate that the installed capacity of renewable energy may soon surpass the installed capacity of coal- and natural gas-fired powerplants. However, because the availability of the renewable resources fluctuates seasonally, diurnally and on even shorter times scales [2] (e.g., momentarily becalmed turbines, clouds passing overhead), the installed availability averages only about 40–70% of the world’s installed capacity [3]. In the U.S., after nearly two decades of steady growth [4], there is sufficient battery capacity to cover about 0.1% of the power demand but only about 3 × 10−5% of the energy demand (Figure 1). Therefore, supplying smooth, always-on power that the modern world demands will require technologies that can buffer multiple tranches of both power and energy. Consider that the characteristic power of a modern wind turbine is on the order of 2 MW per generator [5]. The amount of energy to be buffered for a short interruption in its operation is on the order of 10–100 MJ (= 2 MW × 5–50 s).
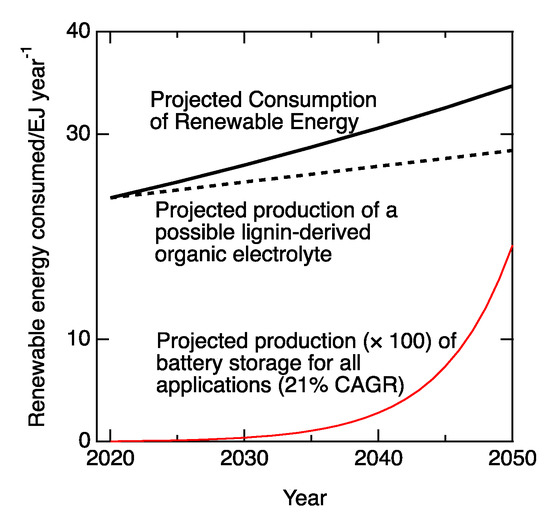
Figure 1.
Consumption or storage of renewable electricity. The solid black line shows projected global consumption of renewable electricity [18]. The dashed line shows how much electricity might be stored in a redox flow battery whose electrolytes were derived from the 160 Mt of lignin produced globally each year by the pulp and paper industry [11] if lignin were the source of a redox shuttle, had a molecular weight of 150 g/mol, and could store and release 2e− and an aspirational [12,13,14,15] cell voltage of 3 V. The lowest curve is a recent projection for the production of storage batteries for all applications, increasing at a compound annual growth rate (CAGR) of 21%.
Despite recent acceleration in the rate of growth of both capacities, projected developments in rechargeable batteries may satisfy power demands but not energy demands across days or seasons.
2. Flow Batteries
In flow batteries, an electrochemically reversibly oxidizable and reducible species shuttles between the anode and cathode. Because the volume of the storage vessel can be independent of the size of the electrolysis cell, a flow battery, in principle, can satisfy the need for both large amounts of stored energy and large rates of energy delivery (power). Their features and limitations have already been reviewed elsewhere [2,6,7,8,9]. To store energy, the electrolyte in its oxidized form is reduced to a lower oxidation state. Then, when the energy is needed, the reduced electrolyte is directed to the anode of the electrolysis cell, where it is reoxidized. However, the species (or mixtures of them) that offer the right balance of rate and thermodynamics (potential difference) have proved to be costly and environmentally burdensome [6]. Here, we consider the implications for catalysis of substituting inorganic shuttles (e.g., V3+/5+, Br) with renewable energy carriers (e.g., liquids or solutes derived from biomass).
Biomass-derived fuels are abundant enough to fulfill only about 6% of the global demand for fuel [10]. However, as redox looping agents, their annual production could nearly buffer the increase in the global demand for renewable power. About 160 Mt of lignin is produced each year for making pulp and paper [11]. Mostly, it is now burned for process heat. Instead, the lignin could be a feedstock for an organic electrolyte. Suppose, as an example, that a redox shuttle had a molecular weight in the range of 150 g/mol and that it could shuttle 2e− at 3 V cell potential. That high a voltage is in the range of recent developments in organic electrolytes [12,13,14,15]. Lignin comprises about 25% of the mass of the wood that is used for making pulp [16]. Therefore, its annual production could source an amount of electrolyte sufficient to store about half of the energy (Figure 1) that is projected to be produced from renewable, episodic sources like wind and solar [17]. That is enough energy to approximately buffer diurnal cycling.
2.1. Renewable Organic Redox Shuttles
Molecules that have been considered and tested [6,13,15,19] as organic electrolytes consist of heteronuclear or substituted aromatics. Some of them occur naturally as fragments of lignin (Table 1).

Table 1.
Examples of organic electrolytes that operate at high potential that could be derived from lignin.
The characteristics of an electrocatalyst suitable for use with an organic electrolyte are: (1) high electrochemical reaction rates (i.e., at a small overpotential), (2) selectivity (to avoid undesired side reactions) and (3) long times on stream. These three requirements are equivalent to the usual triad of characteristics of any industrial catalyst: activity, selectivity and longevity.
In the case of electrocatalysis, it is impossible to ignore the effect of the electrolyte, which serves to transport the redox species and which, unlike a gaseous reaction medium, likely exhibits strong gradients in composition and structure over nanometer length scales in the double layer adjacent to the catalytic site. Therefore, in addition to first architecting the electron transfer site, the designer of an electrocatalyst must also consider what could jocularly be called the “Mr. Rogers’ Support Effect”. Such a support effect facilitates the approach to the neighborhood of an active site of the reaction intermediates and solution phase species that stabilizes the transition state. The structure of an organic electrolyte near the surface of the solid catalyst has been probed through modeling [21,22], spectroscopically [23,24], and empirically, through the use of surfactants [25,26,27,28].
The kinetics of the electrocatalytic reactions may be complex functions of the composition of the electrolyte, even for substrates that are infinitely miscible with the supporting electrolyte. For example, we found [29] that the kinetics of the oxidation of methanol and water followed Hill–Langmuir kinetics, which prevented high extents of conversion because water displaced the methanol from the vicinity of the electroactive site when the methanol concentration fell below about 0.5 M. Organics such as those in Table 1 could be expected to segregate from the polar media [30] needed to stabilize the polarized, likely charged, current carriers, leading to a complex dependence of the redox reaction rates on the concentration of the substrates.
The rates of electrochemical oxidations and reductions catalyzed by supported electrocatalysts are also sensitive to the composition and domain size of the metal [31], possibly because of associated changes in surface site densities. Moreover, the scarcely controlled, and possibly potential-dependent, interaction of the metal particles with the surface moieties of the supporting electrode and electrolyte further complicates efforts to identify correlations between the structure of electrocatalysts and their electrocatalytic activity. Despite decades of research [32,33], along with many review articles (for example, [31,33,34]), monographs [35,36,37,38] and handbooks on organic electrochemistry (for example, [39]), these complications continue to contribute to a lack of evident correlations to guide the choice of catalyst and to transform any particular functional group.
2.2. Choosing/Designing Electrocatalysts for Organic Redox Flow Batteries
Cathode catalysts are usually chosen from platinum group metals, as these elements are all active as hydrogenation catalysts [6,13,14,15,21,40,41,42,43,44,45,46,47]. Their utility is limited when the supporting electrolyte is aqueous because they are also active for reducing water (to make H2). This limitation can be obviated by using metals with a high overpotential against reducing water while still maintaining the ability to transfer hydrogen [48]. Alternately, it appears that some metals can bind the organic strongly enough to lower the surface concentration of H, such that the H–H combination reaction is suppressed [41].
Anode catalysts are also usually chosen from carbon-supported platinum group metals or metal oxides [44,49,50] because such materials do not readily oxidize. Boron-doped diamond offers a high overpotential for the oxygen evolution reaction [51,52,53,54,55], and is therefore useful for oxidizing organics at voltages above the decomposition voltage of water.
Beyond those rules of thumb, there is little guidance available for the choice of either the cathode or anode. There are structure/activity correlations in electrochemistry, for example, volcano plots [40,56,57], but they are more interpolative than predictive towards the detailed aspect of the metal (alloy composition, particle size, particle shape, etc.) or towards the substrate.
For example, volcano curves have been compiled for the reductions of three organic oxygenates, benzaldehyde, furfural, and heptanal [40,57]. The original correlations plotted the adsorption energies of the substrates on the indicated metals on the x axis. Here, for the sake of comparison, we renormalized the y values by the turnover rate of the most active catalyst for each reaction and the x axis to span, in each case, from the weakest bonding (0.0) to the strongest (1.0). While the peaked shape that gives the correlation its name is evident (Figure 2), in this case, the trend lines are heavily influenced only by the most active catalyst. The activity of all the other catalysts lies in a band approximately half as high. The volcano-like pattern exhibits two more characteristics that attenuate its value. The trend for a particular substrate or across substrates does not vary systematically with the position of the catalytic metal in the periodic chart; cobalt is much more active than its neighbor rhodium for the electrochemical hydrogenation of heptanal, but the two elements exhibit about equal activity for the hydrogenation of benzaldehyde.
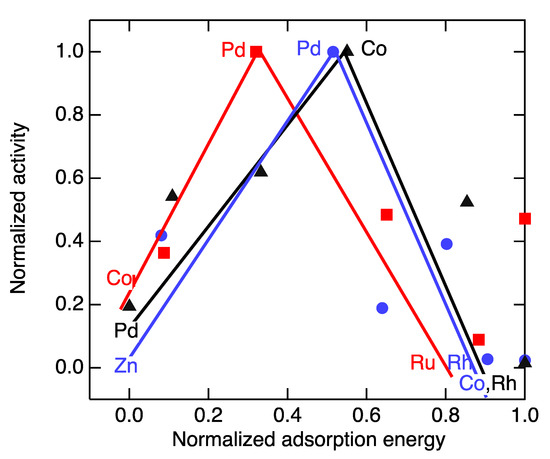
Figure 2.
Superposed volcano graphs for the electrochemical hydrogenation of benzaldehyde (●), furfural (▲) and heptanal (■). Data from [40,57].
2.3. Electrochemical Hydrogenation of Benzaldehyde as a Fiducial Reaction
Plotting the rates of hydrogenations against each other (Figure 3) provides a compelling depiction of the lack of predictive power of the volcano correlation: the rate of hydrogenation of heptanal tracks with the rate of hydrogenation of benzaldehyde (positive slope), but the rate of hydrogenation of furfural tracks inversely with the rate of hydrogenation of benzaldehyde (negative slope).
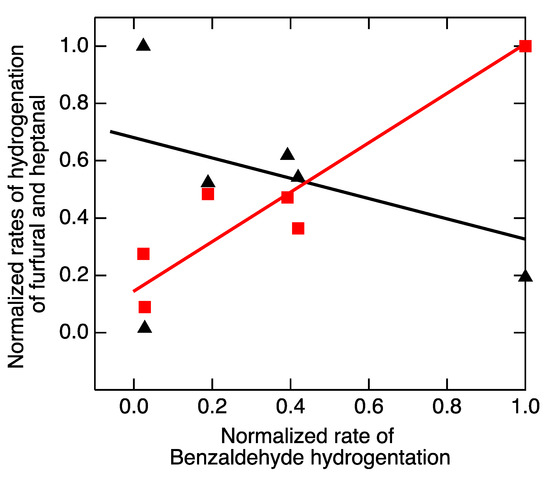
Figure 3.
Comparison of rates of hydrogenation of furfural (▲) and heptanal (■) with the rate of hydrogenation of benzaldehyde. The lines serve merely to illustrate that the two test reactions trend oppositely when compared with the rate of the index reaction.
The issue is not the accuracy of the calculations of heats of adsorption that produced the x values in the original version of Figure 2, nor is it the accuracy of the experiments that measured the reaction rates. Rather, it points to the inadequacy of the rationale that underlies the Balandin concept, which has been noted previously [58], and the complex interactions that contribute to electrochemical kinetics [59].
3. Discussion
An example of a biomass-derived electrolyte is 2-methoxyhydroquinone [45], which can be synthesized from vanillin, which can, in turn, be derived from lignin [60,61,62,63]. That material has been successfully employed as a redox shuttle. However, those experiments involved an aqueous electrolyte, so the corresponding cell voltage was closer to 1 V than to the 3 V assumed in constructing Figure 1.
Regardless, deconvoluting the complexities noted above for the oxidation and reductions of substrates, such as those in Table 1, will require access to intrinsic kinetics, preferably differential kinetics over wide ranges of substrate concentration [29]. It will also require access to well normalized reaction rates, preferably using a site-counting method that can be used under conditions close to those of the relevant reactions. Titration of the sites by a selective poison is one classical method [31]. Other selective site-counting methods have been described [64,65], but they are used too infrequently in the development and description of electrocatalysts for organic reactions [66]. Finally, we need more frequent use of in situ and in operando probes of the organic surrounding of the electrocatalytic site [23,24]. Normalizing electrocatalytic rates by the rate of a fiducial reaction is an approach that uses in operando information. Its partial success here suggests that the reported reaction rates may not reflect the intrinsic kinetics, but rather, are confounded with finite rates of transport.
4. Conclusions
The annual production of biomass should be capable of keeping up with the materials demand for organic electrolytes, provided that it could be converted into, and employed as, a high voltage redox shuttle. While the prospects for this are plausible, regrettably, it is not yet evident how to use the corpus of organic electrochemistry to design catalysts to promote the intrinsic kinetics of the redox couples. Therefore, at this point, organic shuttles, supporting electrolytes and catalysts will need to be developed in parallel, and likely empirically. The advantage of doing so would be the production of a sustainable, and potentially environmentally friendly, electrical grid.
Funding
The work was supported by Pacific Northwest National Laboratory’s Laboratory Directed Research and Development (LDRD) program through the Chemical Transformation Initiative. PNNL is operated by Battelle for the U.S. Department of Energy under Contract DE-AC05-76RL01830.
Conflicts of Interest
The author declares no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- International Energy Agency. IEA Renewables 2020. 2020. Available online: https://www.iea.org/reports/renewables-2020 (accessed on 10 November 2020).
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- International Renewable Energy Agency. Renewable Energy Statistics 2020. Available online: https://irena.org/publications/2020/Mar/Renewable-Capacity-Statistics-2020 (accessed on 28 February 2021).
- US Energy Information Agency. Battery Storage in the United States: An Update on Market Trends. 2020. Available online: https://www.eia.gov/analysis/studies/electricity/batterystorage/pdf/battery_storage.pdf (accessed on 29 November 2020).
- American Wind Energy Association. US Wind Industry Quarterly Market Report, Fourth Quarter 2019. 2019. Available online: https://cleanpower.org/resources/american-clean-power-market-report-q4-2020/ (accessed on 20 April 2020).
- Winsberg, J.; Hagemann, T.; Janoschka, T.; Hager, M.D.; Schubert, U.S. Redox-Flow Batteries: From Metals to Organic Redox-Active Materials. Angew. Chem. Int. Ed. Engl. 2017, 56, 686–711. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Wei, X.; Pan, W.; Duan, W.; Hollas, A.; Yang, Z.; Li, B.; Nie, Z.; Liu, J.; Reed, D.; Wang, W.; et al. Materials and Systems for Organic Redox Flow Batteries: Status and Challenges. ACS Energy Lett. 2017, 2, 2187–2204. [Google Scholar] [CrossRef]
- Leung, P.; Li, X.; De León, C.P.; Berlouis, L.; Low, C.T.J.; Walsh, F.C. Progress in redox flow batteries, remaining challenges and their applications in energy storage. RSC Adv. 2012, 2, 10125–10156. [Google Scholar] [CrossRef]
- Weber, R.S.; Holladay, J.E.; Jenks, C.; Panisko, E.A.; Snowden-Swan, L.J.; Ramirez-Corredores, M.; Baynes, B.; Angenent, L.T.; Boysen, D. Modularized production of fuels and other value-added products from distributed, wasted, or stranded feedstocks. Wiley Interdiscip. Rev. Energy Environ. 2018, 7, e308. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Pulp and Paper Capacity Survey 2017–2022. 2018. Available online: http://www.fao.org/3/CA1791T/ca1791t.pdf (accessed on 19 May 2020).
- Tabor, D.P.; Gómez-Bombarelli, R.; Tong, L.; Gordon, R.G.; Aziz, M.J.; Aspuru-Guzik, A. Mapping the frontiers of quinone stability in aqueous media: Implications for organic aqueous redox flow batteries. J. Mater. Chem. A 2019, 7, 12833–12841. [Google Scholar] [CrossRef]
- Chen, R. Redox flow batteries for energy storage: Recent advances in using organic active materials. Curr. Opin. Electrochem. 2020, 21, 40–45. [Google Scholar] [CrossRef]
- Xing, X.; Huo, Y.; Wang, X.; Zhao, Y.; Li, Y. A benzophenone-based anolyte for high energy density all-organic redox flow battery. Int. J. Hydrog. Energy 2017, 42, 17488–17494. [Google Scholar] [CrossRef]
- Huo, Y.; Xing, X.; Zhang, C.; Wang, X.; Li, Y. An all organic redox flow battery with high cell voltage. RSC Adv. 2019, 9, 13128–13132. [Google Scholar] [CrossRef]
- Sjöström, E. Wood Chemistry, Fundamentals and Applications, 2nd ed.; Academic Press: New York, NY, USA, 1993. [Google Scholar]
- International Renewable Energy Agency. Renewable Capacity Statisitics 2020; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2020. [Google Scholar]
- US Energy Information Agency. International Energy Outlook 2019. 2019. Available online: https://www.eia.gov/outlooks/aeo/data/browser/#/?id=24-IEO2019&cases=Reference&sourcekey=0, (accessed on 10 April 2020).
- Geigle, P.; Hartwig, J.; Larionov, E.; Baal, E. Redox-Active Compounds and Uses Thereof; CMBlu Projekt AG: Alzenau, Germany, 2006. [Google Scholar]
- Lee, J.; Park, M.J. Tattooing Dye as a Green Electrode Material for Lithium Batteries. Adv. Energy Mater. 2017, 7. [Google Scholar] [CrossRef]
- Cantu, D.C.; Padmaperuma, A.B.; Nguyen, M.-T.; Akhade, S.A.; Yoon, Y.; Wang, Y.-G.; Lee, M.-S.; Glezakou, V.-A.; Rousseau, R.; Lilga, M.A. A Combined Experimental and Theoretical Study on the Activity and Selectivity of the Electrocatalytic Hydro-genation of Aldehydes. ACS Catal. 2018, 8, 7645–7658. [Google Scholar] [CrossRef]
- Baskin, A.; Prendergast, D. Exploring chemical speciation at electrified interfaces using detailed continuum models. J. Chem. Phys. 2019, 150, 041725. [Google Scholar] [CrossRef]
- Tong, Y.J. In situ electrochemical nuclear magnetic resonance spectroscopy for electrocatalysis: Challenges and prospects. Curr. Opin. Electrochem. 2017, 4, 60–68. [Google Scholar] [CrossRef]
- Singh, N.; Song, Y.; Gutiérrez, O.Y.; Camaioni, D.M.; Campbell, C.T.; Lercher, J.A. Electrocatalytic Hydrogenation of Phenol over Platinum and Rhodium: Unexpected Temperature Effects Resolved. ACS Catal. 2016, 6, 7466–7470. [Google Scholar] [CrossRef]
- Chambrion, P.; Roger, L.; Lessard, J.; Béraud, V.; Mailhot, J.; Thomalla, M. The influence of surfactants on the electrocatalytic hydrogenation of organic compounds in micellar, emulsified, and hydroorganic solutions at Raney nickel electrodes. Can. J. Chem. 1995, 73, 804–815. [Google Scholar] [CrossRef]
- Ilikti, H.; Rekik, N.; Thomalla, M. Electrocatalytic hydrogenation of alkyl-substituted phenols in aqueous solutions at a Raney nickel electrode in the presence of a non-micelle-forming cationic surfactant. J. Appl. Electrochem. 2004, 34, 127–136. [Google Scholar] [CrossRef]
- Jaeger, D.A.; Bolikal, D.; Nath, B. Surfactant Effects on the Electrochemical Reduction of an α,β-Unsaturated Ketone. In Surfactants in Solution; Mittal, K.L., Ed.; Springer: Boston, MA, USA, 1989; pp. 149–152. [Google Scholar]
- Moorthy, P.N.; Kishore, K. Electrochemical Studies in Surfactant Solutions. In Surfactants in Solution; Mittal, K.L., Ed.; Springer: Boston, MA, USA, 1989; pp. 135–147. [Google Scholar]
- Andrews, E.M.; Egbert, J.D.; Sanyal, U.; Holladay, J.D.; Weber, R.S. Anode-Boosted Electrolysis in Electrochemical Upgrading of Bio-oils and in the Production of H2. Energy Fuels 2020, 34, 1162–1165. [Google Scholar] [CrossRef]
- Nguyen, M.-T.; Akhade, S.A.; Cantu, D.C.; Lee, M.-S.; Glezakou, V.-A.; Rousseau, R. Electro-reduction of organics on metal cathodes: A multiscale-modeling study of benzaldehyde on Au (111). Catal. Today 2020, 350, 39–46. [Google Scholar] [CrossRef]
- Stonehart, P.; Ross, P.N. The Commonality of Surface Processes in Electrocatalysis and Gas-Phase Heterogeneous Catalysis. Catal. Rev. 1975, 12, 1–35. [Google Scholar] [CrossRef]
- Lund, H. A Century of Organic Electrochemistry. J. Electrochem. Soc. 2002, 149, S21–S33. [Google Scholar] [CrossRef]
- Baizer, M.M. Recent developments in organic synthesis by electrolysis. Tetrahedron 1984, 40, 935–969. [Google Scholar] [CrossRef]
- Weinberg, N.L.; Weinberg, H.R. Electrochemical oxidation of organic compounds. Chem. Rev. 1968, 68, 449–523. [Google Scholar] [CrossRef]
- Lob, W. Electrochemistry of Organic Compounds; John Wiley & Sons: New York, NY, USA, 1906. [Google Scholar]
- Chum, H.L.; Baizer, M.M. The Electrochemistry of Biomass and Derived Materials; American Chemical Society: Washington, DC, USA, 1985. [Google Scholar]
- Fry, A.J. Synthetic Organic Electrochemistry; Harper & Row: New York, NY, USA, 1972. [Google Scholar]
- Frumkin, A.N.; Érshler, A.B. Progress in Electrochemistry of Organic Compounds; Plenum Press: London, UK, 1971. [Google Scholar]
- Meites, L.; Zuman, P.; Rupp, E.B.; Fenner, T.L.; Spritzer, L. Handbook Series in Organic Electrochemistry; CRC Press: Cleveland, OH, USA, 1976. [Google Scholar]
- Akhade, S.A.; Singh, N.; Gutierrez, O.Y.; Lopez-Ruiz, J.; Wang, H.; Holladay, J.D.; Liu, Y.; Karkamkar, A.; Weber, R.S.; Padmaperuma, A.B.; et al. Electrocatalytic Hydrogenation of Biomass-Derived Organics: A Review. Chem. Rev. 2020, 120, 11370–11419. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, U.; Lopez-Ruiz, J.A.; Padmaperuma, A.B.; Holladay, J.; Gutiérrez, O.Y. Electrocatalytic Hydrogenation of Oxygenated Compounds in Aqueous Phase. Org. Process. Res. Dev. 2018, 22, 1590–1598. [Google Scholar] [CrossRef]
- Sanyal, U.; Song, Y.; Singh, N.; Fulton, J.L.; Herranz, J.; Jentys, A.; Gutiérrez, O.Y.; Lercher, J.A. Structure Sensitivity in Hydrogenation Reactions on Pt/C in Aqueous-phase. ChemCatChem 2018, 11, 575–582. [Google Scholar] [CrossRef]
- Song, Y.; Chia, S.H.; Sanyal, U.; Gutiérrez, O.Y.; Lercher, J.A. Integrated catalytic and electrocatalytic conversion of substituted phenols and diaryl ethers. J. Catal. 2016, 344, 263–272. [Google Scholar] [CrossRef]
- Amini, K.; Gostick, J.; Pritzker, M.D. Metal and Metal Oxide Electrocatalysts for Redox Flow Batteries. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Schlemmer, W.; Nothdurft, P.; Petzold, A.; Riess, G.; Fruhwirt, P.; Schmallegger, M.; Gescheidt-Demner, G.; Fischer, R.; Freunberger, S.A.; Kern, W.; et al. 2-Methoxyhydroquinone from Vanillin for Aqueous Redox-Flow Batteries. Angew. Chem. Int. Ed. Engl. 2020, 59, 22943–22946. [Google Scholar] [CrossRef]
- Wang, H.; Sayed, S.Y.; Luber, E.J.; Olsen, B.C.; Shirurkar, S.M.; Venkatakrishnan, S.; Tefashe, U.M.; Farquhar, A.K.; Smotkin, E.S.; McCreery, R.L.; et al. Redox Flow Batteries: How to Determine Electrochemical Kinetic Parameters. ACS Nano 2020, 14, 2575–2584. [Google Scholar] [CrossRef]
- Lister, T.E.; Diaz, L.A.; Lilga, M.A.; Padmaperuma, P.A.; Lin, Y.J.; Palakkal, V.M.; Arges, C.G. Low-Temperature Electrochemical Upgrading of Bio-oils Using Polymer Electrolyte Membranes. Energy Fuels 2018, 32, 5944–5950. [Google Scholar] [CrossRef]
- Carroll, K.J.; Burger, T.; Langenegger, L.; Chavez, S.; Hunt, S.T.; Román-Leshkov, Y.; Brushett, F.R. Electrocatalytic Hydrogenation of Oxygenates using Earth-Abundant Transition-Metal Nanoparticles under Mild Conditions. ChemSusChem 2016, 9, 1904–1910. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-S.; Huang, S.-L.; Chen, M.-L.; Tsai, T.-J.; Lin, Y.-S. Improving Electrochemical Activity in a Semi-V-I Redox Flow Battery by Using a C–TiO2–Pd Composite Electrode. J. Nanomater. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Chu, Y. Bismuth Trioxide Modified Carbon Nanotubes as Negative Electrode Catalysts for all Vanadium Redox Flow Batteries. Int. J. Electrochem. Sci. 2020, 7733–7743. [Google Scholar] [CrossRef]
- Cañizares, P.; Saez, C.; Lobato, J.; Rodrigo, M.A. Electrochemical Oxidation of Polyhydroxybenzenes on Boron-Doped Diamond Anodes. Ind. Eng. Chem. Res. 2004, 43, 6629–6637. [Google Scholar] [CrossRef]
- Lips, S.; Waldvogel, S.R. Use of Boron-Doped Diamond Electrodes in Electro-Organic Synthesis. ChemElectroChem 2019, 6, 1649–1660. [Google Scholar] [CrossRef]
- Peralta-Hernández, J.M.; Méndez-Tovar, M.; Guerra-Sánchez, R.; Martínez-Huitle, C.A.; Nava, J.L. A Brief Review on Envi-ronmental Application of Boron Doped Diamond Electrodes as a New Way for Electrochemical Incineration of Synthetic Dyes. Int. J. Electrochem. 2012, 2012, 1–18. [Google Scholar] [CrossRef]
- Pleskov, Y.; Evstefeeva, Y.; Krotova, M.; Varnin, V.; Teremetskaya, I. Synthetic semiconductor diamond electrodes: Electrochemical behaviour of homoepitaxial boron-doped films orientated as (111), (110), and (100) faces. J. Electroanal. Chem. 2006, 595, 168–174. [Google Scholar] [CrossRef]
- Salazar-Banda, G.R.; Eguiluz, K.I.; Avaca, L.A. Boron-doped diamond powder as catalyst support for fuel cell applications. Electrochem. Commun. 2007, 9, 59–64. [Google Scholar] [CrossRef]
- Trasatti, S. Work Function, Electronegativity and Electrochemical Behaviour of Metals III. Electrolytic Hydrogen Evolution in Acid Solutions. J. Electroanal. Chem. Interfacial Electrochem. 1972, 39, 163–184. [Google Scholar] [CrossRef]
- Lopez-Ruiz, J.A.; Andrews, E.M.; Akhade, S.A.; Lee, M.-S.; Koh, K.; Sanyal, U.; Yuk, S.F.; Karkamkar, A.J.; Derewinski, M.A.; Holladay, J.; et al. Understanding the Role of Metal and Molecular Structure on the Electrocatalytic Hydrogenation of Oxygenated Organic Compounds. ACS Catal. 2019, 9, 9964–9972. [Google Scholar] [CrossRef]
- Barteau, M.A. Linear free energy relationships for C1-oxygenate decomposition on transition metal surfaces. Catal. Lett. 1991, 8, 175–183. [Google Scholar] [CrossRef]
- Quaino, P.; Juarez, F.; Santos, E.; Schmickler, W. Volcano plots in hydrogen electrocatalysis—Uses and abuses. Beilstein J. Nanotechnol. 2014, 5, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Calvillo, M.; Córdova, I.; Del Valle, M.; Oropeza, M.T. Electrochemical Oxidation of Vanillin and Capsaicin in Hartmann Solution. ECS Trans. 2010, 29, 339–347. [Google Scholar] [CrossRef]
- Parpot, P.; Bettencourt, A.; Carvalho, A.; Belgsir, E. Biomass conversion: Attempted electrooxidation of lignin for vanillin production. J. Appl. Electrochem. 2000, 30, 727–731. [Google Scholar] [CrossRef]
- Schmitt, D.; Regenbrecht, C.; Hartmer, M.; Stecker, F.; Waldvogel, S.R. Highly selective generation of vanillin by anodic degra-dation of lignin: A combined approach of electrochemistry and product isolation by adsorption. Beilstein J. Org. Chem. 2015, 11, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Stecker, F.; Malkowsky, I.M.; Fischer, A.; Waldvogel, S.R.; Regenbrecht, C. Method for Producing Vanillin by Electrochemical Oxidation of Aqueous Lignin Solutions or Suspensions; Rheinisch Friedrich Wilhems Universtät Bonn: Bonn, Germany, 2014. [Google Scholar]
- Moniri, S.; van Cleve, T.; Linic, S. Pitfalls and best practices in measurements of the electrochemical surface area of platinum-based nanostructured electro-catalysts. J. Catal. 2017, 345, 1–10. [Google Scholar] [CrossRef]
- Egbert, J.D.; Lopez-Ruiz, J.A.; Prodinger, S.; Holladay, J.D.; Mans, D.M.; Wade, C.E.; Weber, R.S. Counting surface redox sites in carbon-supported electrocatalysts by cathodic stripping of O deposited from N2O. J. Catal. 2018, 365, 405–410. [Google Scholar] [CrossRef]
- Weber, R.S. Normalizing Hetereogeneous Electrocatalytic and Photocatalytic Rates. ACS Omega 2019, 4, 4109–4112. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).