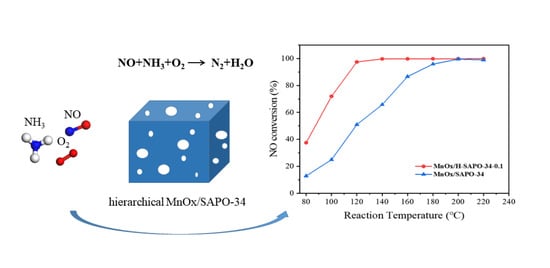
MnOx Supported on Hierarchical SAPO-34 for the Low-Temperature Selective Catalytic Reduction of NO with NH3: Catalytic Activity and SO2 Resistance
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
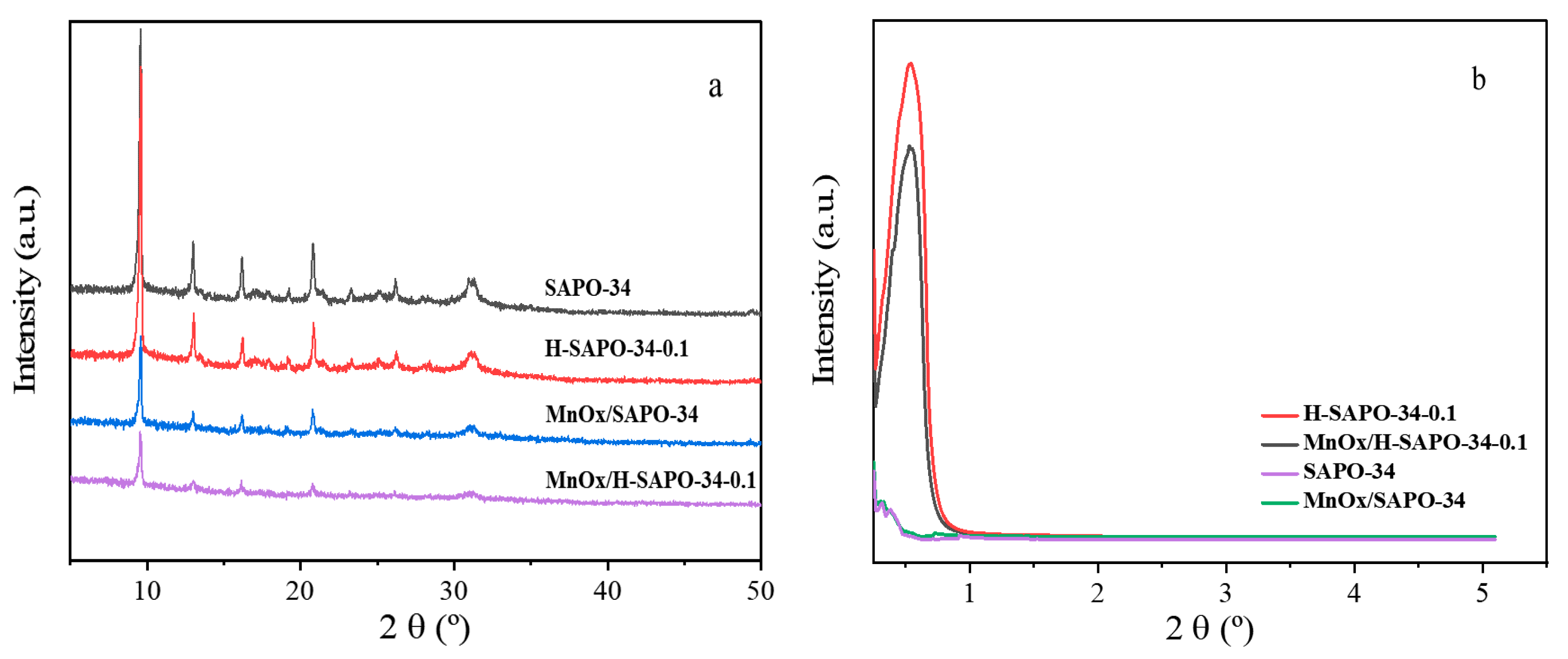
2.1.1. XRD
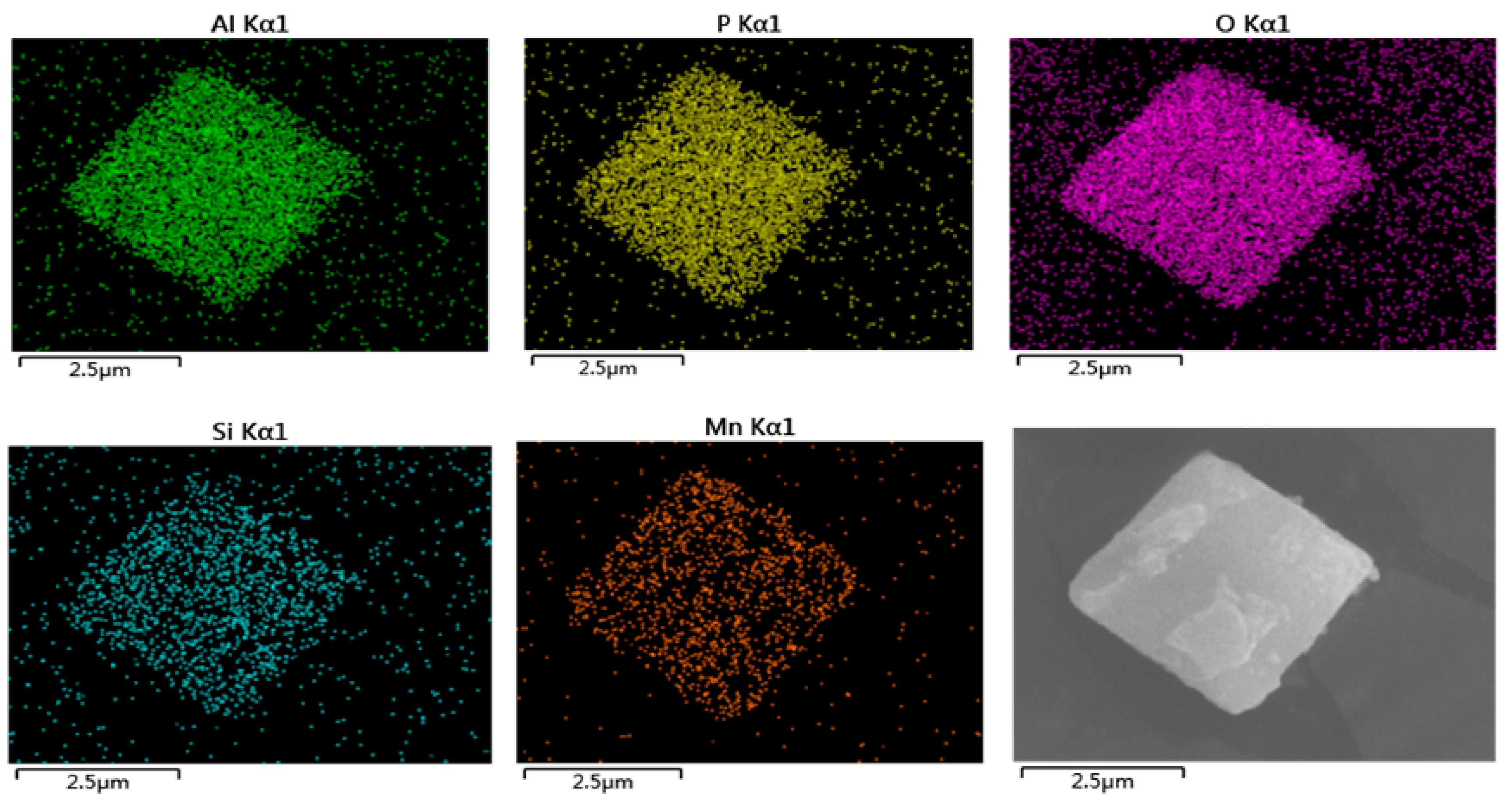
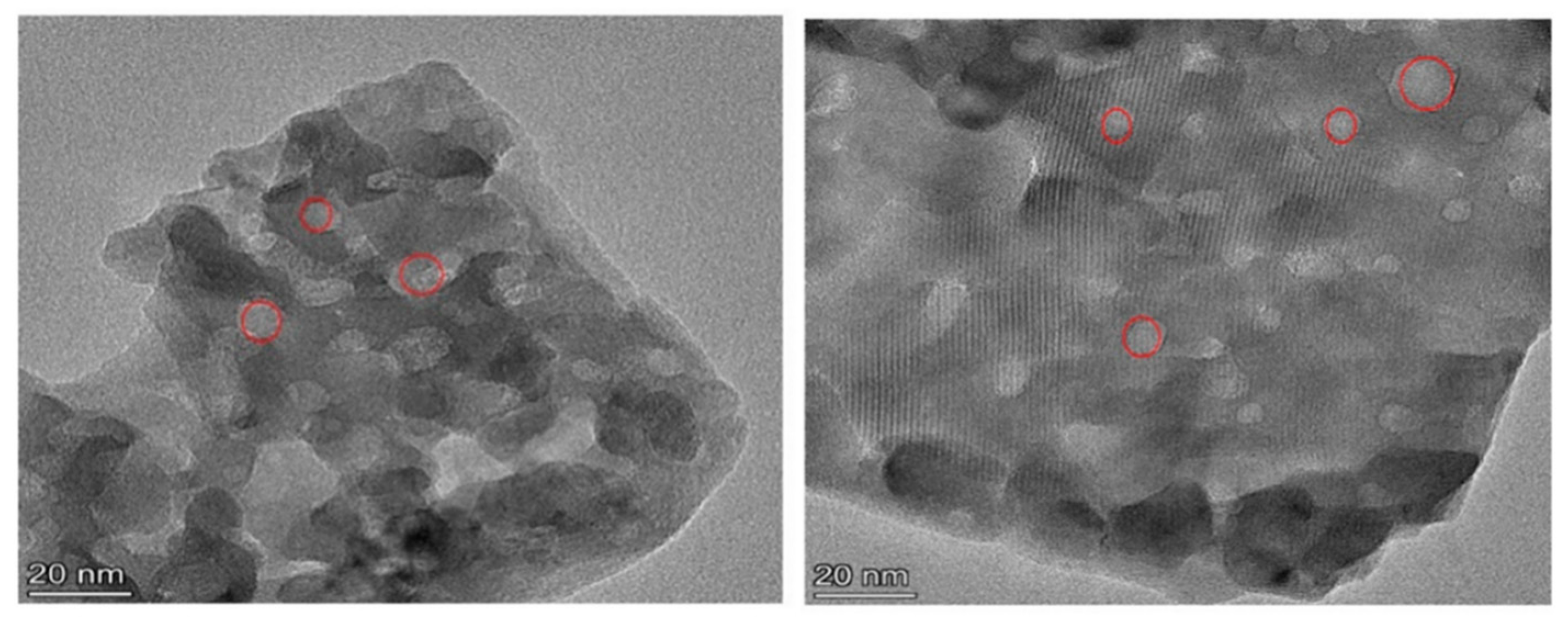
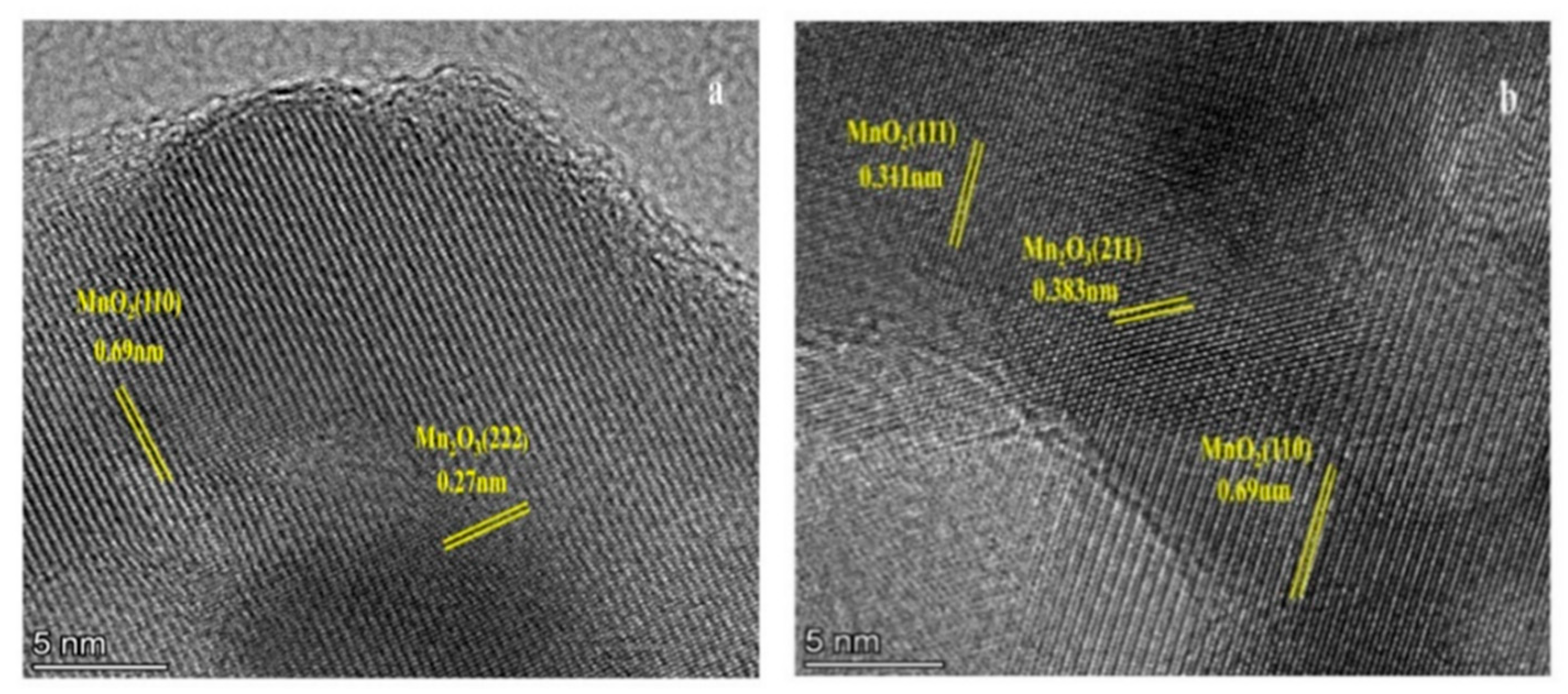
2.1.2. SEM and TEM
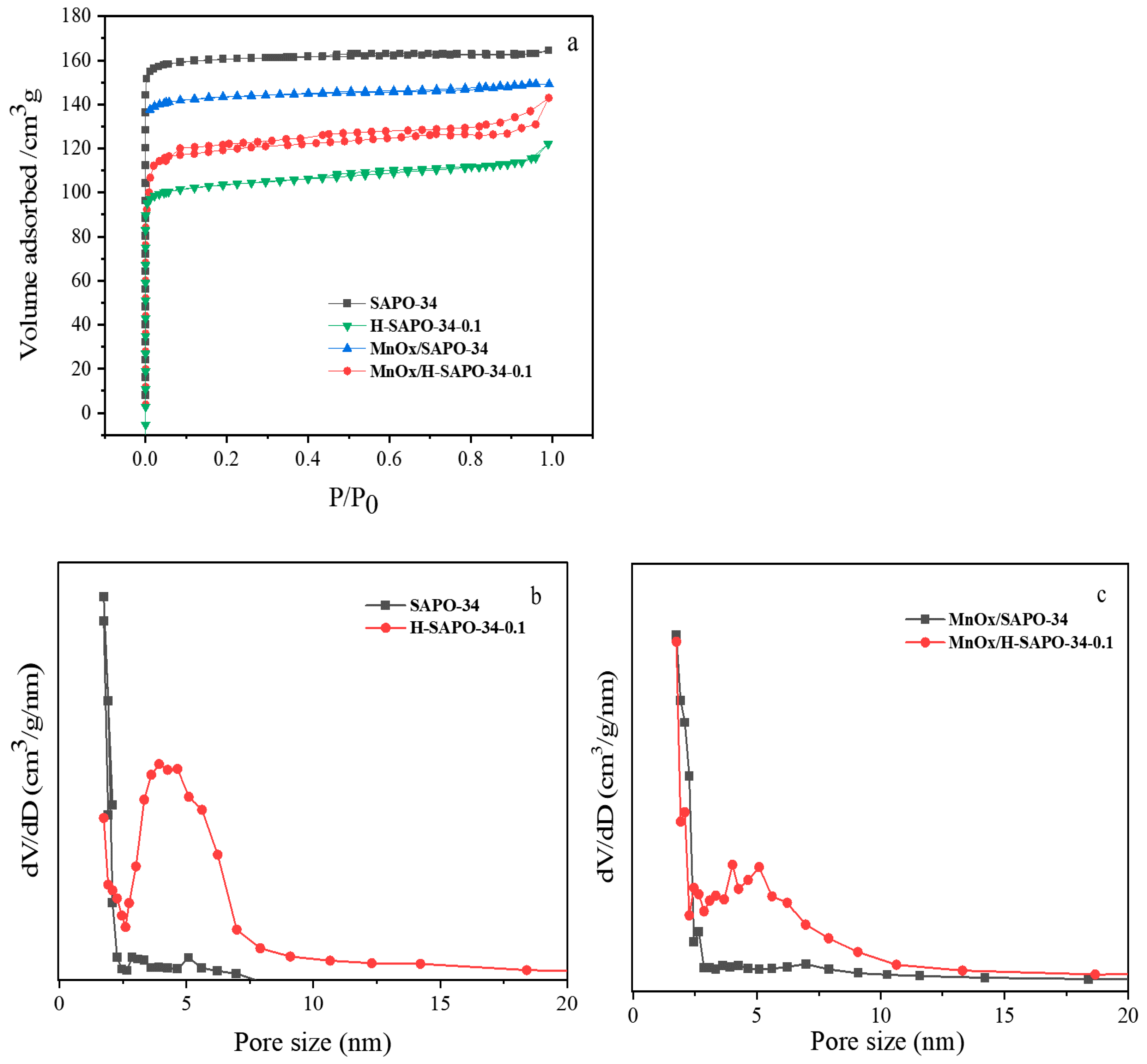
2.1.3. BET
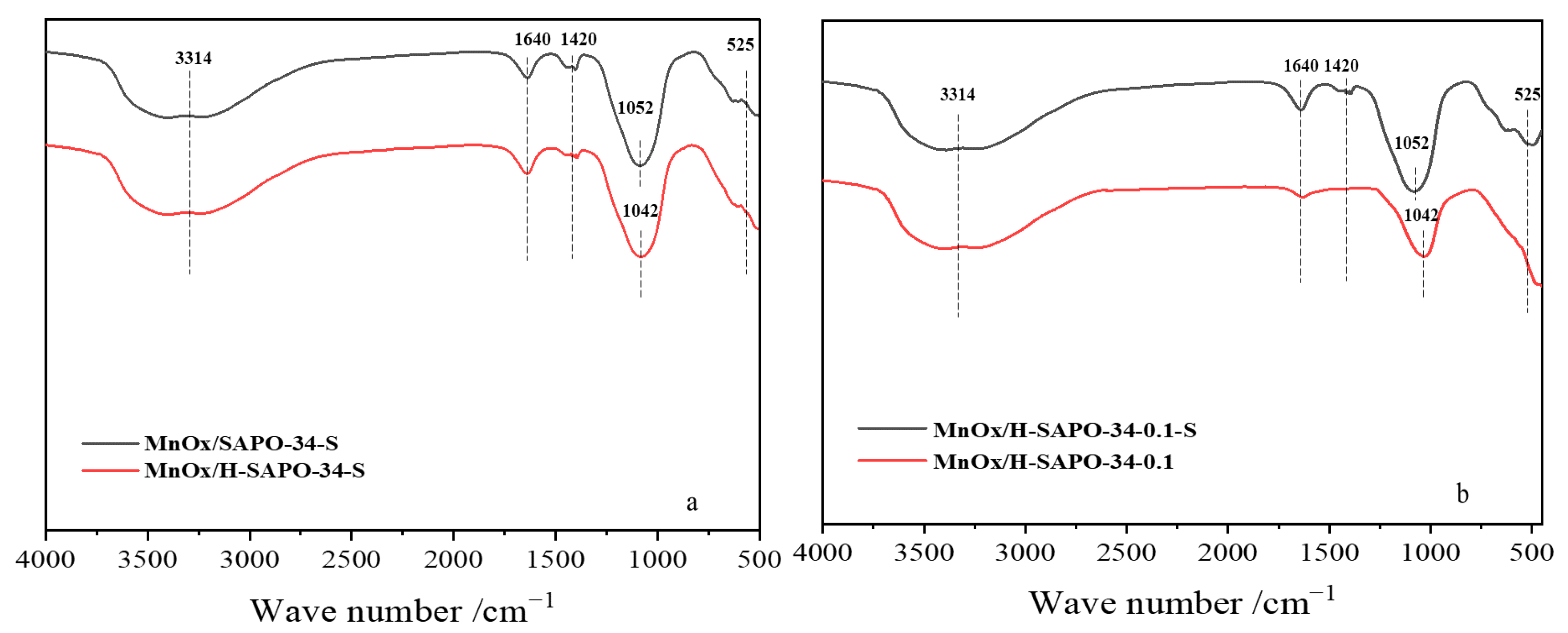
2.1.4. FT-IR
2.1.5. NH3-TPD and Py-IR
2.1.6. H2-TPR
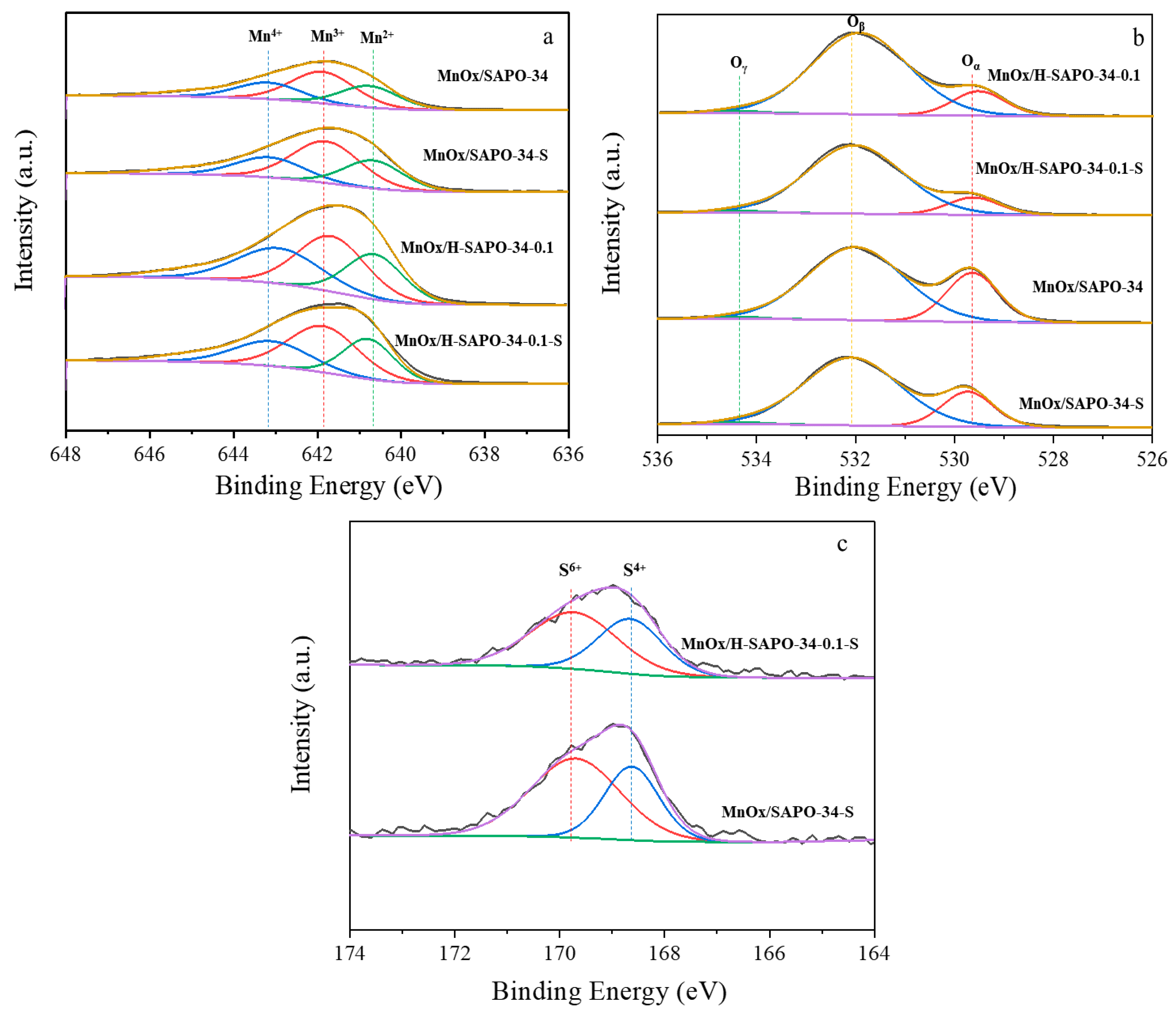
2.1.7. XPS
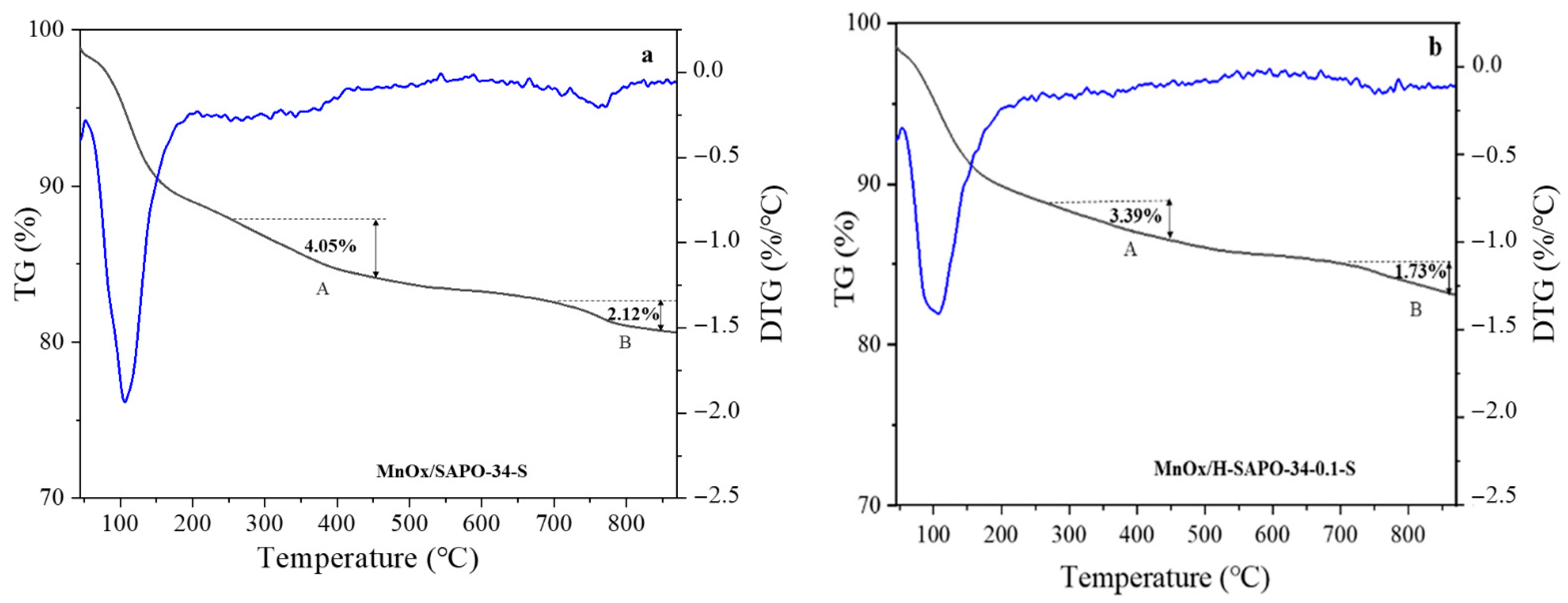
2.1.8. TG/DTG
2.2. Catalytic Performance of the Low-Temperature NH3-SCR
2.2.1. Catalytic Activity Tests
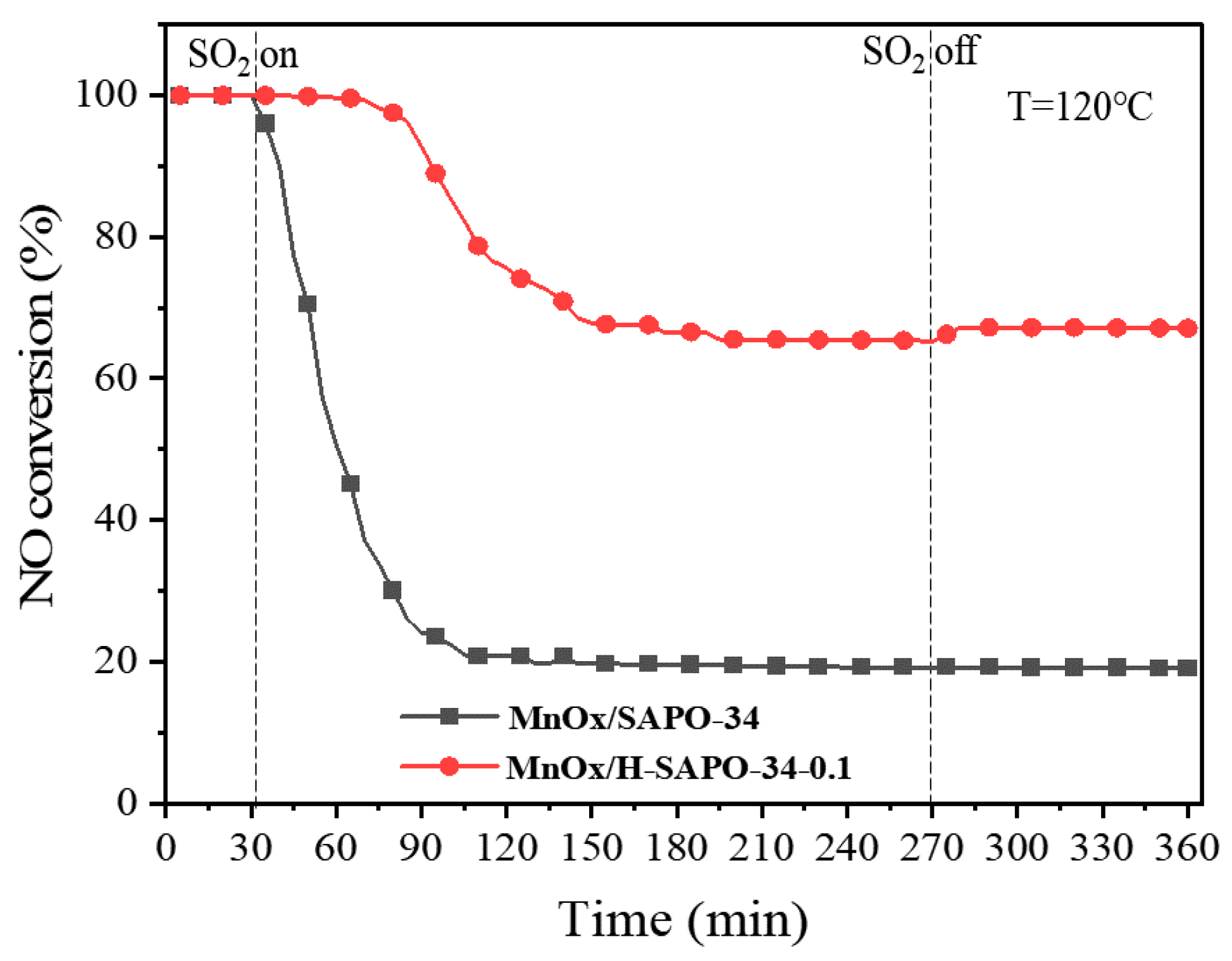
2.2.2. Impact of SO2 on Catalytic Activity
3. Materials and Methods
3.1. Catalysts Preparation
3.1.1. Preparation of Hierarchical SAPO-34
3.1.2. Preparation of the Catalysts
3.2. Catalysts Characterization
3.3. Catalysts Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Zhang, T.; Chang, H.; Li, K.; Peng, Y.; Li, X.; Li, J. Different exposed facets VOx/CeO2 catalysts for the selective catalytic reduction of NO with NH3. Chem. Eng. J. 2018, 349, 184–191. [Google Scholar] [CrossRef]
- Xie, L.; Liu, F.; Ren, L.; Shi, X.; Xiao, F.-S.; He, H. Excellent Performance of One-Pot Synthesized Cu-SSZ-13 Catalyst for the Selective Catalytic Reduction of NOx with NH3. Environ. Sci. Technol. 2014, 48, 566–572. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Hammershoi, P.S.; Vennestrom, P.N.R.; Falsig, H.; Jensen, A.D.; Janssens, T.V.W. Importance of the Cu oxidation state for the SO2-poisoning of a Cu-SAPO-34 catalyst in the NH3-SCR reaction. Appl. Catal. B Environ. 2018, 236, 377–383. [Google Scholar] [CrossRef]
- Jangjou, Y.; Do, Q.; Gu, Y.; Lim, L.-G.; Sun, H.; Wang, D.; Kumar, A.; Li, J.; Grabow, L.C.; Epling, W.S. Nature of Cu Active Centers in Cu-SSZ-13 and Their Responses to SO2 Exposure. ACS Catal. 2018, 8, 1325–1337. [Google Scholar] [CrossRef]
- Wang, L.; Huang, B.; Su, Y.; Zhou, G.; Wang, K.; Luo, H.; Ye, D. Manganese oxides supported on multi-walled carbon nanotubes for selective catalytic reduction of NO with NH3: Catalytic activity and characterization. Chem. Eng. J. 2012, 192, 232–241. [Google Scholar] [CrossRef]
- Liu, C.; Shi, J.-W.; Gao, C.; Niu, C. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A review. Appl. Catal. A Gen. 2016, 522, 54–69. [Google Scholar] [CrossRef]
- Wang, C.; Sun, L.; Cao, Q.; Hu, B.; Huang, Z.; Tang, X. Surface structure sensitivity of manganese oxides for low-temperature selective catalytic reduction of NO with NH3. Appl. Catal. B Environ. 2011, 101, 598–605. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, Y.; Wu, Z. Low-temperature selective catalytic reduction of NO on MnOx/TiO2 prepared by different methods. J. Hazard. Mater. 2009, 162, 1249–1254. [Google Scholar] [CrossRef]
- Chang, H.; Chen, X.; Li, J.; Ma, L.; Wang, C.; Liu, C.; Schwank, J.W.; Hao, J. Improvement of Activity and SO2 Tolerance of Sn-Modified MnOx-CeO2 Catalysts for NH3-SCR at Low Temperatures. Environ. Sci. Technol. 2013, 47, 5294–5301. [Google Scholar] [CrossRef]
- Marban, G.; Fuertes, A.B. Kinetics of the low-temperature selective catalytic reduction of NO with NH3 over activated carbon fiber composite-supported iron oxides. Catal. Lett. 2002, 84, 13–19. [Google Scholar] [CrossRef]
- Li, L.; Sun, B.; Sun, J.; Yu, S.; Ge, C.; Tang, C.; Dong, L. Novel MnOx-CeO2 nanosphere catalyst for low-temperature NH3-SCR. Catal. Commun. 2017, 100, 98–102. [Google Scholar] [CrossRef]
- Yu, C.; Hou, D.; Huang, B.; Lu, M.; Peng, R.; Zhong, Z. A MnOx@Eu-CeOx nanorod catalyst with multiple protective effects: Strong SO2-tolerance for low temperature DeNOx processes. J. Hazard. Mater. 2020, 399, 123011. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Lee, J.; Kwak, S.-Y. Manganese oxides with hierarchical structures derived from coordination polymers and their enhanced catalytic activity at low temperature for selective catalytic reduction of NOx. Dalton Trans. 2019, 48, 16395–16401. [Google Scholar] [CrossRef] [PubMed]
- Fickel, D.W.; D’Addio, E.; Lauterbach, J.A.; Lobo, R.F. The ammonia selective catalytic reduction activity of copper-exchanged small-pore zeolites. Appl. Catal. B Environ. 2011, 102, 441–448. [Google Scholar] [CrossRef]
- Kwak, J.H.; Tonkyn, R.G.; Kim, D.H.; Szanyi, J.; Peden, C.H.F. Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J. Catal. 2010, 275, 187–190. [Google Scholar] [CrossRef]
- Korhonen, S.T.; Fickel, D.W.; Lobo, R.F.; Weckhuysen, B.M.; Beale, A.M. Isolated Cu2+ ions: Active sites for selective catalytic reduction of NO. Chem. Commun. 2011, 47, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, A.; Wang, W.; Xu, M.; Arnold, A.; Hunger, M. Thermal stability and dehydroxylation of Bronsted acid sites in silicoaluminophosphates H-SAPO-11, H-SAPO-81 H-SAPO-31, and H-SAPO-34 investigated by multi-nuclear solid-state NMR spectroscopy. Microporous Mesoporous Mater. 2002, 56, 267–278. [Google Scholar] [CrossRef]
- Li, R.; Wang, P.; Ma, S.; Yuan, F.; Li, Z.; Zhu, Y. Excellent selective catalytic reduction of NOx by NH3 over Cu/SAPO-34 with hierarchical pore structure. Chem. Eng. J. 2020, 379, 122376. [Google Scholar] [CrossRef]
- Liu, J.; Yu, F.; Liu, J.; Cui, L.; Zhao, Z.; Wei, Y.; Sun, Q. Synthesis and kinetics investigation of meso-microporous Cu-SAPO-34 catalysts for the selective catalytic reduction of NO with ammonia. J. Environ. Sci. 2016, 48, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Fan, G.; Gu, D.; Yu, S.; Ma, K.; Liu, A.; Tan, W.; Wang, J.; Du, X.; Zou, W.; et al. Pore Size Expansion Accelerates Ammonium Bisulfate Decomposition for Improved Sulfur Resistance in Low-Temperature NH3-SCR. ACS Appl. Mater. Interfaces 2019, 11, 4900–4907. [Google Scholar] [CrossRef]
- Arstad, B.; Lind, A.; Cavka, J.H.; Thorshaug, K.; Akporiaye, D.; Wragg, D.; Fjellvag, H.; Gronvold, A.; Fuglerud, T. Structural changes in SAPO-34 due to hydrothermal treatment. A NMR, XRD, and DRIFTS study. Microporous Mesoporous Mater. 2016, 225, 421–431. [Google Scholar] [CrossRef]
- Jin, H.X.; Gu, X.J.; Hong, B.; Lin, L.S.; Wang, C.Y.; Jin, D.F.; Peng, X.L.; Wang, X.Q.; Ge, H.L. Fabrication of Mesoporous Co3O4 from LP-FDU-12 via Nanocasting Route and Effect of Wall/Pore Size on Their Magnetic Properties. J. Phys. Chem. C 2012, 116, 13374–13381. [Google Scholar] [CrossRef]
- Shao, J.; Cheng, S.; Li, Z.; Huang, B. Enhanced Catalytic Performance of Hierarchical MnOx/ZSM-5 Catalyst for the Low-Temperature NH3-SCR. Catalysts 2020, 10, 311. [Google Scholar] [CrossRef]
- Tian, J.; Peng, H.; Xu, X.; Liu, W.; Ma, Y.; Wang, X.; Yang, X. High surface area La2Sn2O7 pyrochlore as a novel, active and stable support for Pd for CO oxidation. Catal. Sci. Technol. 2015, 5, 2270–2281. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Li, R.M.; Wang, M.J.; Li, Y.S.; Tong, Y.M.; Yang, P.P.; Zhu, Y.J. Two steps synthesis of CeTiOx oxides nanotube catalyst: Enhanced activity, resistance of SO2 and H2O for low temperature NH3-SCR of NOx. Appl. Catal. B Environ. 2021, 282, 119542. [Google Scholar] [CrossRef]
- Gao, J.; Han, Y.; Mu, J.; Wu, S.; Tan, F.; Shi, Y.; Li, X. 2D, 3D mesostructured silicas templated mesoporous manganese dioxide for selective catalytic reduction of NOx with NH3. J. Colloid Interface Sci. 2018, 516, 254–262. [Google Scholar] [CrossRef]
- Yu, J.; Guo, F.; Wang, Y.; Zhu, J.; Liu, Y.; Su, F.; Gao, S.; Xu, G. Sulfur poisoning resistant mesoporous Mn-base catalyst for low-temperature SCR of NO with NH3. Appl. Catal. B Environ. 2010, 95, 160–168. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Shi, L.; Fang, C.; Li, H.; Gao, R.; Huang, L.; Zhang, J. In situ supported MnOx-CeOx on carbon nanotubes for the low-temperature selective catalytic reduction of NO with NH3. Nanoscale 2013, 5, 1127–1136. [Google Scholar] [CrossRef]
- Jin, W.; Wang, B.; Tuo, P.; Li, C.; Li, L.; Zhao, H.; Gao, X.; Shen, B. Selective Desilication, Mesopores Formation, and MTO Reaction Enhancement via Citric Acid Treatment of Zeolite SAPO-34. Ind. Eng. Chem. Res. 2018, 57, 4231–4236. [Google Scholar] [CrossRef]
- Li, J.; Guo, J.; Shi, X.; Wen, X.; Chu, Y.; Yuan, S. Effect of aluminum on the catalytic performance and reaction mechanism of Mn/MCM-41 for NH3-SCR reaction. Appl. Surf. Sci. 2020, 534, 147592. [Google Scholar] [CrossRef]
- Qiu, M.; Zhan, S.; Yu, H.; Zhu, D.; Wang, S. Facile preparation of ordered mesoporous MnCo2O4 for low-temperature selective catalytic reduction of NO with NH3. Nanoscale 2015, 7, 2568–2577. [Google Scholar] [CrossRef]
- Sun, X.; Guo, R.-T.; Liu, J.; Fu, Z.-G.; Liu, S.-W.; Pan, W.-G.; Shi, X.; Qin, H.; Wang, Z.-Y.; Liu, X.-Y. The enhanced SCR performance of Mn/TiO2 catalyst by Mo modification: Identification of the promotion mechanism. Int. J. Hydrog. Energy 2018, 43, 16038–16048. [Google Scholar] [CrossRef]
- Chen, J.; Shen, M.; Wang, X.; Qi, G.; Wang, J.; Li, W. The influence of nonstoichiometry on LaMnO3 perovskite for catalytic NO oxidation. Appl. Catal. B Environ. 2013, 134, 251–257. [Google Scholar] [CrossRef]
- Yang, S.; Wang, C.; Li, J.; Yan, N.; Ma, L.; Chang, H. Low temperature selective catalytic reduction of NO with NH3 over Mn-Fe spinel: Performance, mechanism and kinetic study. Appl. Catal. B Environ. 2011, 110, 71–80. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, L.; Ning, P.; Gu, J.; Guan, Q. Surface characterization studies of CuO-CeO2-ZrO2 catalysts for selective catalytic reduction of NO with NH3. Appl. Surf. Sci. 2014, 317, 955–961. [Google Scholar] [CrossRef]
- Ren, Q.; Mo, S.; Peng, R.; Feng, Z.; Zhang, M.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Controllable synthesis of 3D hierarchical Co3O4 nanocatalysts with various morphologies for the catalytic oxidation of toluene. J. Mater. Chem. A 2018, 6, 498–509. [Google Scholar] [CrossRef]
- Jiang, B.Q.; Wu, Z.B.; Liu, Y.; Lee, S.C.; Ho, W.K. DRIFT Study of the SO2 Effect on Low-Temperature SCR Reaction over Fe-Mn/TiO2. J. Phys. Chem. C 2010, 114, 4961–4965. [Google Scholar] [CrossRef]
- Wu, S.; Yao, X.; Zhang, L.; Cao, Y.; Zou, W.; Li, L.; Ma, K.; Tang, C.; Gao, F.; Dong, L. Improved low temperature NH3-SCR performance of FeMnTiOx mixed oxide with CTAB-assisted synthesis. Chem. Commun. 2015, 51, 3470–3473. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Y.; Yan, N.; Wu, D.; He, H.; Qu, Z.; Yang, C.; Zhou, Q.; Jia, J. Nanosized Cation-Deficient Fe-Ti Spinel: A Novel Magnetic Sorbent for Elemental Mercury Capture from Flue Gas. ACS Appl. Mater. Interfaces 2011, 3, 209–217. [Google Scholar] [CrossRef]
- Liu, H.; Fan, Z.X.; Sun, C.Z.; Yu, S.H.; Feng, S.; Chen, W.; Chen, D.Z.; Tang, C.J.; Gao, F.; Dong, L. Improved activity and significant SO2 tolerance of samarium modified CeO2-TiO2 catalyst for NO selective catalytic reduction with NH3. Appl. Catal. B Environ. 2019, 244, 671–683. [Google Scholar] [CrossRef]
- Lian, Z.; Liu, F.; Shan, W.; He, H. Improvement of Nb Doping on SO2 Resistance of VOx/CeO2 Catalyst for the Selective Catalytic Reduction of NOx with NH3. J. Phys. Chem. C 2017, 121, 7803–7809. [Google Scholar] [CrossRef]
- Li, B.; Ren, Z.; Ma, Z.; Huang, X.; Liu, F.; Zhang, X.; Yang, H. Selective catalytic reduction of NO by NH3 over CuO-CeO2 in the presence of SO2. Catal. Sci. Technol. 2016, 6, 1719–1725. [Google Scholar] [CrossRef]
- Bai, B.; Arandiyan, H.; Li, J. Comparison of the performance for oxidation of formaldehyde on nano-Co3O4, 2D-Co3O4, and 3D-Co3O4 catalysts. Appl. Catal. B Environ. 2013, 142, 677–683. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, R.; Liu, Y.; Wang, H. Ceria modified MnOx/TiO2 as a superior catalyst for NO reduction with NH3 at low-temperature. Catal. Commun. 2008, 9, 2217–2220. [Google Scholar] [CrossRef]
- Shao, J.; Lin, F.; Huang, Y.; Wang, Z.; Li, Y.; Chen, G.; Cen, K. MnOx fabrication with rational design of morphology for enhanced activity in NO oxidation and SO2 resistance. Appl. Surf. Sci. 2020, 503, 144064. [Google Scholar] [CrossRef]
- Li, C.; Tang, X.; Yi, H.; Wang, L.; Cui, X.; Chu, C.; Li, J.; Zhang, R.; Yu, Q. Rational design of template-free MnOx-CeO2 hollow nanotube as de-NOx catalyst at low temperature. Appl. Surf. Sci. 2018, 428, 924–932. [Google Scholar] [CrossRef]
- Ma, Z.; Sheng, L.; Wang, X.; Yuan, W.; Chen, S.; Xue, W.; Han, G.; Zhang, Z.; Yang, H.; Lu, Y.; et al. Oxide Catalysts with Ultrastrong Resistance to SO2 Deactivation for Removing Nitric Oxide at Low Temperature. Adv. Mater. 2019, 31, 1903719. [Google Scholar] [CrossRef]
- Pan, S.; Luo, H.; Li, L.; Wei, Z.; Huang, B. H2O and SO2 deactivation mechanism of MnOx/MWCNTs for low-temperature SCR of NOx with NH3. J. Mol. Catal. A Chem. 2013, 377, 154–161. [Google Scholar] [CrossRef]




| Samples | SBET a/ (m2·g−1) | Smic a/ (m2·g−1) | Sext a/ (m2·g−1) | V total b/ (cm3·g−1) | Vmicro b/ (cm3·g−1) | Vmeso/ (cm3·g−1) | Ave. Dmeso c (nm) |
|---|---|---|---|---|---|---|---|
| SAPO-34 | 489.90 | 444.04 | 25.86 | 0.212 | 0.212 | - | - |
| H-SAPO-34-0.1 | 548.80 | 510.24 | 38.56 | 0.253 | 0.211 | 0.042 | 5.19 |
| MnOx/SAPO-34 | 248.91 | 222.29 | 26.61 | 0.131 | 0.131 | - | - |
| MnOx/H-SAPO-34-0.1 | 428.26 | 390.37 | 37.89 | 0.222 | 0.177 | 0.045 | 5.22 |
| Sample | Amount of Acid Sites (mmol/g) a | Amount of Acid Sites (umol/g) b | B/L | Total Acidity (mmol/g) | ||
|---|---|---|---|---|---|---|
| Weak | Strong | Lewis Acid Sites | Brønsted Acid Sites | |||
| MnOx/SAPO-34 | 0.771 | 0.316 | 70.22 | 29.78 | 0.42 | 1.098 |
| MnOx/H-SAPO-34-0.01 | 0.593 | 0.327 | 65.96 | 34.04 | 0.52 | 0.899 |
| MnOx/H-SAPO-34-0.1 | 0.537 | 0.377 | 60.75 | 39.25 | 0.65 | 0.914 |
| MnOx/H-SAPO-34-0.125 | 0.534 | 0.336 | 61.60 | 38.4 | 0.62 | 0.873 |
| Element | Binding Energy/eV | Assignment | FWHM/eV |
|---|---|---|---|
| O 1s | 529.6–530.0 | lattice oxygen species | 1.28 |
| 531.9–532.3 | chemisorbed oxygen species | 2.39 | |
| 534.3–534.7 | surface hydroxyl species/adsorbed water molecules | 1.20 | |
| Mn 2p3/2 | 640.5–641.1 | Mn2+ | 1.98 |
| 641.6–642.0 | Mn3+ | 2.21 | |
| 642.6–643.6 | Mn4+ | 1.67 | |
| S 2p | 169.5–169.9 | S6+ | 2.01 |
| 168.4–168.8 | S4+ | 1.22 |
| Sample | Atomic Fraction (%) | XS (%) | XO (%) | XMn (%) | X(Mn3+ + Mn4+)/ X(Mn3+ + Mn2+ + Mn4+) (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mn | O | Others a | S | Oα | Oβ | Oγ | Mn2+ | Mn3+ | Mn4+ | ||
| MnOx/SAPO-34 | 7.87 | 59.12 | 40.88 | - | 26.26 | 72.69 | 1.05 | 28.88 | 46.46 | 24.66 | 71.12 |
| MnOx/SAPO-34-S | 6.07 | 62.86 | 28.92 | 2.15 | 21.26 | 77.44 | 1.30 | 30.87 | 43.70 | 25.43 | 69.13 |
| MnOx/H-SAPO-34-0.1 | 13.96 | 59.8 | 26.24 | - | 13.69 | 84.98 | 1.33 | 27.55 | 40.74 | 31.71 | 72.45 |
| MnOx/H-SAPO-34-0.1-S | 11.30 | 59.51 | 27.88 | 1.31 | 11.82 | 86.17 | 2.01 | 28.05 | 48.30 | 23.65 | 71.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Guan, J.; Yu, C.; Huang, B. MnOx Supported on Hierarchical SAPO-34 for the Low-Temperature Selective Catalytic Reduction of NO with NH3: Catalytic Activity and SO2 Resistance. Catalysts 2021, 11, 314. https://doi.org/10.3390/catal11030314
Zhou L, Guan J, Yu C, Huang B. MnOx Supported on Hierarchical SAPO-34 for the Low-Temperature Selective Catalytic Reduction of NO with NH3: Catalytic Activity and SO2 Resistance. Catalysts. 2021; 11(3):314. https://doi.org/10.3390/catal11030314
Chicago/Turabian StyleZhou, Lusha, Jinkun Guan, Chenglong Yu, and Bichun Huang. 2021. "MnOx Supported on Hierarchical SAPO-34 for the Low-Temperature Selective Catalytic Reduction of NO with NH3: Catalytic Activity and SO2 Resistance" Catalysts 11, no. 3: 314. https://doi.org/10.3390/catal11030314
APA StyleZhou, L., Guan, J., Yu, C., & Huang, B. (2021). MnOx Supported on Hierarchical SAPO-34 for the Low-Temperature Selective Catalytic Reduction of NO with NH3: Catalytic Activity and SO2 Resistance. Catalysts, 11(3), 314. https://doi.org/10.3390/catal11030314