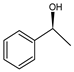
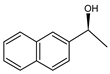
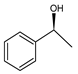
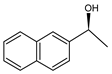
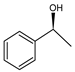
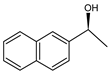
Abstract
Dynamic kinetic resolution (DKR) is one of the most attractive routes to enantioselective synthesis, and ruthenium complexes are often applied as racemization catalysts. Two substituted cyclopentadienyl ruthenium complexes were immobilized covalently and non-covalently on mesoporous silica of mesocellular foam (MCF) and Santa Barbara Amorphous (SBA)-15 type functionalized with a 3 carbon spacer and 4-(chloromethyl)-N-amidobenzoate moiety. The catalysts were studied in a model reaction of secondary alcohol racemization. The immobilization decreased catalyst activity, considerably more for SBA-15 than for MCFs, and complete racemization of 1-phenylethanol was achieved within 24 h with the MCF-supported catalyst. The catalyst could be recovered and reused, thus paving the way for further development of the DKR process. The synthesized materials were fully characterized by Fourier-transform infrared spectroscopy analysis, thermogravimetry analysis, inductively cou-pled plasma optical emission spectrometry, and nitrogen adsorption at 77 K.
1. Introduction
To date, over 80% of to date medicines contain chiral building blocks. Thus, the production of enantiopure materials is vital for the pharmaceutical and related industries [1]. The synthesis of pure enantiomers is very elegant, but costs can be prohibitively high, and conventional separation methods of racemates often remain a practical solution [2,3,4]. During the past three decades, however, a kinetic resolution (KR) and, more recently, its dynamic-kinetic mode (DKR) emerged as their viable alternatives [5,6]; these approaches are already applied in the production of chiral amines and alcohols with the use of ruthenium complexes [7,8]. In DKR, the slowly reacting enantiomer of the racemic mixture is catalytically transformed (racemized) into that preferentially converted isomer, whereas in KR, in principle, it is left unused [2,9,10]. In effect, the maximum product yield in DKR can reach 100%, whilst in KR it is 50% (Figure 1).
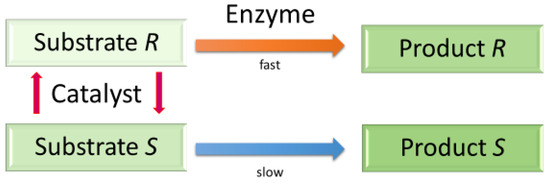
Figure 1.
Dynamic kinetic resolution.
Although various enzymes, due to excellent enantioselectivity, effectively catalyze the resolution, the racemization poses a problem because it escapes from the mainstream topics of organic synthesis. Fortunately enough, several transition metal complexes, and substituted cyclopentadienyl ruthenium complexes, in particular, appear to show significant potential as racemization catalysts and could be applied in DKR [11,12,13]. However, typically they are homogeneous catalysts, and hence separation and reuse are not possible and reports on their heterogeneous analogs are scarce [6,14]. Thus, we deemed it important to focus this study on the development of a heterogeneous racemization catalyst suitable for a chemo-enzymatic mode of the DKR process, i.e. with the supported-metal racemization catalyst compatible with the enzyme used in a resolution reaction.
Among various mesoporous siliceous materials (MPSs) applied as supports for both enzymes and metal complexes, mesocellular foams (MCFs) and SBA-15 (Santa Barbara Amorphous) have attracted the most attention [6,14,15,16]. They offer a large surface area that is easily accessible for reactants, which is considerably greater in MCF than SBA-15 due to larger pores and total pore volume. Hence, overall, MCFs are expected to offer higher reaction rates. However, more importantly for the cooperative bicatalytic tandem performance, the pores in MCFs are large enough to host comfortably host both the enzyme and the metal complex, and allow their co-immobilization in the same cavity to afford a bifunctional catalyst [6,15,17,18]. This feature is hardly possible for SBA-15 due to much smaller pores. However, the smaller and more finely tuned pore sizes of SBA-15 were found to improve the stereoselectivity of DKR due to the nanocage effect [14]. Thus, we face a dilemma about which of the two porous silicates is more suitable for DKR, to what extent, and when. Equally importantly, powdered catalysts obtained using MPSs as supports for the enzymes and/or the metal complexes can also serve as a benchmark in comparative studies of the batch slurry system with the continuous flow high-yield synthesis or resolution of enantiomers, which remains the ultimate practical goal [19].
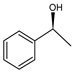
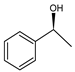
Given this background, we report on the preparation and performance of the racemization catalysts made of substituted cyclopentadienyl ruthenium complexes covalently and non-covalently immobilized on MCFs and SBA-15 materials. The complexes first proposed by Kim, Park, and Bäckvall [20] groups were shown to racemize secondary alcohols within a few hours. Those complexes were air-stable, compatible with the enzyme, and could also be recycled and reused [20,21,22,23]. Therefore, they are excellent candidates for application as racemization catalysts for (S)-phenylethanol, considered here as a model reaction, yet of major practical importance (Scheme 1).
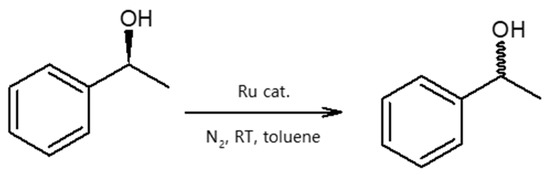
Scheme 1.
Racemization of (S)-phenylethanol.
2. Results and Discussion
Three substituted cyclopentadienyl ruthenium complexes, presented in Figure 2, were obtained as described in [21].
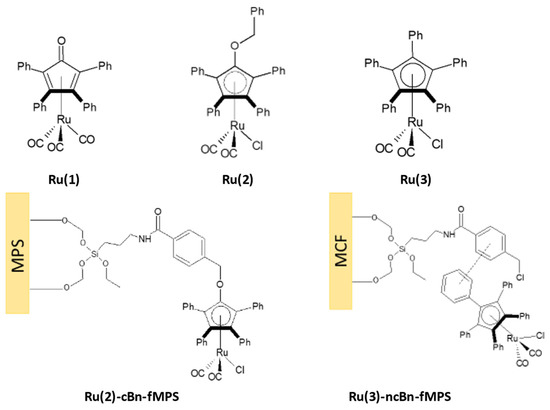
Figure 2.
Ruthenium Ru(1)-Ru(3), and covalent Ru(2)-cBn-fMPS and non-covalent Ru(3)-ncBn-fMCF immobilization of the complexes; MPS stands for both mesocellular foam (MCF) or Santa Barbara Amorphous (SBA)-15 support for Ru(2) complex.
The substituted cyclopentadienyl is one of the most important ligands because it can strongly bind via π-interactions and is relatively inert to nucleophilic or electrophilic reagents. This makes it a reliable stabilizing ligand for the whole series of CpMLn complexes (n = 2,3,4) [24]. Moreover, phenyl groups can be used for immobilization of the ruthenium complex employing π-stacking interaction to the functionalized MPS (Figure 2), whereas, the carbonyl group on the cyclopentadienone ligand in complex Ru(1) allows the transformation of complex Ru(1) into Ru(2), and this strategy can be applied to form the covalently immobilized complex Ru(2)-cBn-fMPS (Figure 2). Complex Ru(1) was obtained in the reaction of ruthenium carbonyl with pentaphenylcyclopentadienone. The reaction of Ru(1) with benzyl chloride led to Ru(2). A similar approach was used to obtain the immobilized Ru(2)-cBn-fMPS. Alternatively, the reaction of Ru(1) with phenyl magnesium bromide followed by reduction yielded Ru(3). The performed experiments showed very good catalytic activity of the complexes Ru(2) and Ru(3) (Table 1).

Table 1.
Conversion of secondary alcohols with the studied Ru complexes.
2.1. Immobilization of Ru complexes on MCFs and SBA-15
Encouraged by the high yields of racemization featuring Ru(2) and Ru(3) (Table 1), we decided to immobilize them on MCFs. To anchor the complex Ru(2) we used carbonyl group from the cyclopentadienyl fragment of complex Ru(1), which can react with 4-(chloromethyl)benzamidyl present on the previously functionalized siliceous support, thus leading to Ru(2)-cBn-fMPS. Due to the extended aromatic system consisting of several phenyl groups of cyclopentadienyl ligand and benzoyl fragments introduced to the silica, we could attach non-covalently attach the ruthenium complex Ru(3) by π-stacking interactions forming the immobilized catalyst Ru(3)-ncBn-fMCF.
The initial racemization reaction results utilizing Ru(2)-cBn-fMCF were disappointing, and the catalyst proved to be inactive. We hypothesized that this was caused by hydroxyl groups present on the silica surface, and possibly the distance between the complex and the surface was too short [25,26]. To reduce both of these factors, protection of hydroxyl groups of silane compounds was used but, in addition, prior to this the calcination temperature was increased from 550 to 700 °C, to leave only very strong single silanols on the silica surface [27,28]. Then, 3-(triethoxysilyl)-1-propanamine fragments (3 mmol for 1 g MCF) were attached to the MCF surface together with 4-(chloromethyl)benzoyl chloride (CMBC) (3 mmol for 1 g MCF), to pave the way for both covalent and non-covalent immobilization of complexes shown in Figure 2. Therefore, mesoporous silica materials (MCF and SBA-15) functionalized in this way were used as supports for the ruthenium complexes attached either covalently (complex Ru(2)) or via non-covalent interactions (complex Ru(3)) as shown in Scheme 2.
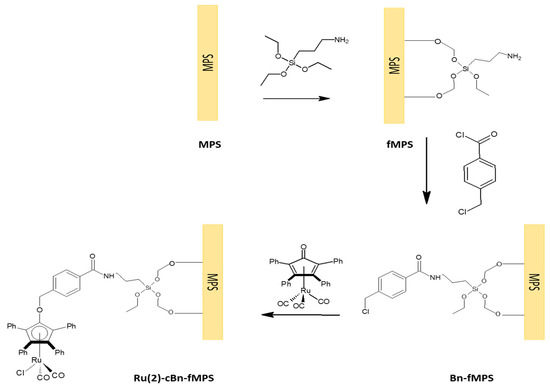
Scheme 2.
Covalent immobilization of Ru(2) on functionalized mesoporous silicas to give Ru(2)-cBn-fMPS (MPS=MCF or SBA).
The racemization reaction experiments showed (cf. Table 2) that both of the ruthenium complexes immobilized on MCFs appeared to be highly active in the conversion of secondary alcohols, but the one attached covalently Ru(2)-cBn-fMCF was notably more active than that bonded by weaker forces, i.e. Ru(3)-ncBn-fMCF.

Table 2.
Racemization tests on secondary alcohols catalyzed by the Ru complexes attached either covalently, Ru(2)-cBn-fMCF, or non-covalently, Ru(3)-ncBn-fMCF.
Regardless of the immobilization method, the racemization time increased, most likely due to hindered access of the substrate to the immobilized complex. The amount of base needed to activate the complex also increased [21]. The studies of Ru(2)-cBn-fMCF catalyst in three successive reaction runs (Table 3) clearly demonstrate that it could be used twice, with no appreciable loss of activity, and that the latter decreased significantly during the third cycle.

Table 3.
Recycling tests of catalyst Ru(2)-cBn-fMCF.
Studies have shown that the catalyst can be reused after the racemization reaction without a significant reduction of its activity. Only in the third subsequent run, there is a decrease in the catalytical activity.
To addressthe dilemma of the support impact, the tests were also performed for the ruthenium complex deposited on SBA-15 (Ru(2)-cBn-fSBA catalyst). Clearly, the reaction conditions and reactant values were the same as for the MCF complexes.
As shown in Table 4 and Table 5, the catalysts attached to SBA-15 silica appeared to be much less active in comparison to their MCF-supported analogs. This is expected due to the catalysts’s activity resulting from the pore structure, i.e. active sites’ accessibility, and perspective.

Table 4.
Racemization tests on secondary alcohols catalyzed by the covalently bounded Ru(2)-cBn-fSBA.

Table 5.
Comparison of the activity of immobilized catalysts on MCF and SBA-15.
2.2. Characteristics of MCF, SBA, and the Immobilized Complexes
2.2.1. Fourier-Transform Infrared Spectroscopy Analysis
Pure MCF, functionalized MCF, and the immobilized catalyst were analyzed by FTIR (Figure 3). The samples were analyzed in reference to pure KBr. In the functionalized MCF the wide peak assigned to asymmetric stretching vibrations of the Si–O bond ranging from 1250 to 1000 cm−1 was reduced by 16% in the case of Ru(2)-cBn-fMCF and only 1% in the case of Ru(3)-ncBn-fMCF. Reduction of intensity by 19% and 6% respectively was also observed for the band of O-H stretching vibration at 3800-2500 cm−1 [29,30]. After immobilization, bands at 2048 cm−1 and 1980 cm−1 assigned to C=O stretching vibrations were observed. The peak at 750 cm−1 may be assigned to symmetric stretching vibrations of the Si-O bond. Peaks at 1643 cm−1 and 1549 cm−1 were assigned to C=C stretching vibrations of phenyl rings from cyclopenthadienyl ligands.
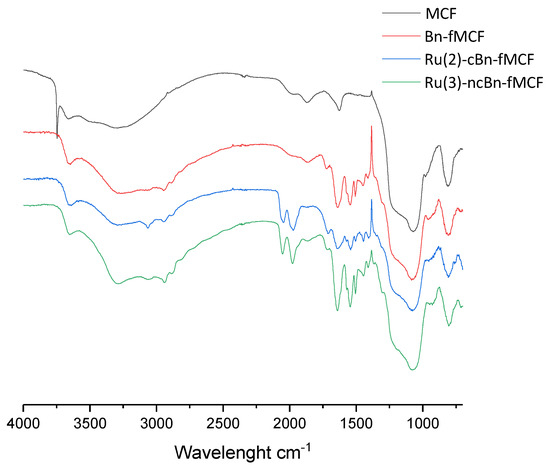
Figure 3.
IR spectra of native MCF and its functionalization products.
IR spectra of MCF- and SBA-based materials are very similar to the SBA’s (Figure 4). The signal from the O–H stretching vibration at 3800–2500 cm−1 is wider than that in MCS compounds which may indicate a higher number of hydroxyl groups in the carrier and, consequently, weaker catalytic properties of the complexes deposited on this carrier.
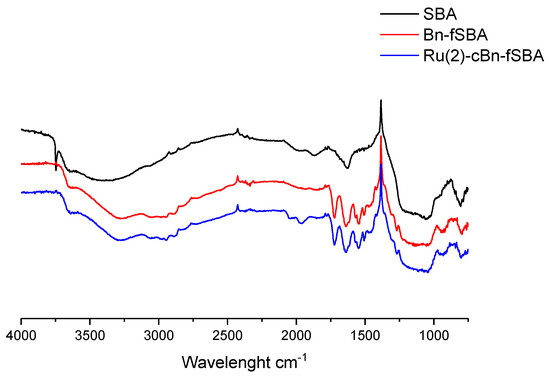
Figure 4.
IR spectra of native SBA and its functionalization products.
2.2.2. Atmospheric Solid Analysis Probe
Table 6 lists the properties of the pore structure of pristine and functionalized materials of the MCF- and SBA family, and the corresponding Ru catalysts obtained from nitrogen adsorption experiments. Adsorption-desorption isotherms showed Type IV isotherms characteristic of mesoporous materials It is worth noting that after surface modification with organic moiety the mesoporous nature was preserved. As expected, after modification the surface area, pore size, and pore volume were decreased. It appeared that the specific surface area and mesopore volume of MCF modified with amino and benzyl groups (Bn-fMCF) was about half the value of the pristine MCF. Further modification with the Ru complex had less impact on porous texture parameters. Small differences in surface area and pore volume, observed for catalysts Ru(2)-cBn-fMCF and Ru(3)-ncBn-fMCF, tend to suggest that almost the same amount of Ru-complex was immobilized using the covalent and non-covalent methods. Unlike in the case of MCFs, the modification of SBA-15 and further immobilization of the complex almost completely blocked the mesopores present in the native material. Clearly, this must have had a major influence on the racemization properties of the immobilized catalyst, reflecting a drastic reduction in catalytic activity, as seen in Table 4 and Table 5.

Table 6.
Pore structure properties of MCF- and SBA-15-based materials before and after modification.
2.2.3. Thermogravimetry Analysis
Pure MCF, Bn-fMCF, Ru(2)-cBn-fMCF and Ru(3)-ncBn-fMCF were analyzed by TGA (Figure 5). For the native MCF a slight weight loss at 100 °C was ascribed to desorption of water. However, no further appreciable weight loss proves the high thermostability of native MCFs. For the Bn-fMCF a significant mass reduction, from 98% to ca. 75%, was observed in the temperature range of 230–650 °C, and was ascribed to calcinations of organic fragments present in the modified support [31]. For MCFs loaded with ruthenium complexes, in the temperature range of 250-400 °C about 30% for Ru(3)-ncBn-fMCF and 35% for Ru(2)-cBn-fMCF mass loss was observed. It is noteworthy that the slope for Ru(2)-cBn-fMCF was greater than that for Ru(3)-ncBn-fMCF, which indicates that Ru-complex 1 is more closely associated with MCF than Ru- complex 3 [32].
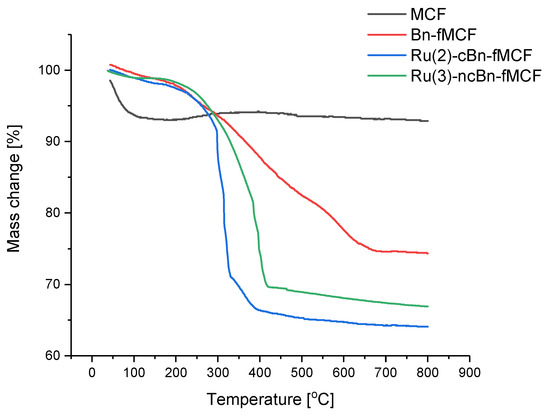
Figure 5.
Thermogravimetry analysis (TGA) of MCF’s compounds.
For SBA materials, much greater weight losses were observed than for the corresponding MCFs, which on the whole, indicates larger content of the attached organic compounds (Figure 6). This is in line with a more significant decrease in the pore volume of SBA catalysts detected by pore size analyses (cf. Table 6). Both the complex itself and the molecules used for functionalization are less bounded to the carrier than in the corresponding MCF material. Surprisingly, the course of the Ru(2)-cBn-fSBA curve resembles that of the TGA curve for the non-covalently immobilized complex, Ru(3)-ncBn-fMCF. This may indicate that narrower pores present in SBA-15 facilitate phenyl group π-stacking interaction. It may also be responsible for a larger content of organic compounds in functionalized SBA and hinder the access of substrates to active sites, ultimately reducing the catalytic activity.
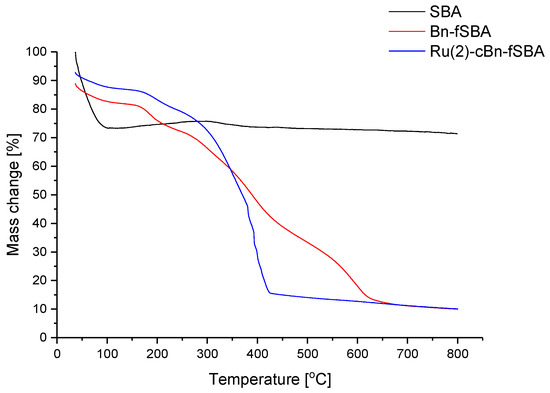
Figure 6.
TGA analysis of SBA compounds.
2.2.4. ICP-OES Analysis
The ICP analysis of the obtained catalytic systems was performed (Table 7). The measurement was preceded by four-fold mineralization of the samples.

Table 7.
Ruthenium content on silica supports measured by ICP-OES method.
For samples with a non-covalently deposited catalyst, the result was very surprising. The percentage of ruthenium was much lower than in the other samples. The difference between covalent and noncovalent ruthenium deposition is fivefold, while the decrease in an activity does not reflect this ratio. Both carriers allow the deposition of ruthenium in an amount up to 4%. Although there is a slight difference between the amount of ruthenium for MCF and SBA, the latter has much lower catalytic activity, which suggests that the structure of the carrier is of great importance. On the other hand, in the case of ruthenium catalysts, their deposition method is not as important as the structure of the support itself.
3. Experimental
Chemicals were purchased in the highest available purity from Acros Organics, Sigma-Aldrich, or Avantor Poland, and used without purification unless reported otherwise. Solvents were distilled at no earlier than two weeks before synthesis. All syntheses were carried out under nitrogen. atmosphere spectroscopy—instrumentation details: 1HNMR and 13CNMR spectra were recorded by Varian Unity Inova 300 MHz and Agilent Technologies 400 MHz. FTIR spectroscopy was carried out with a NicoletTM iS50 FT-IR Spectrometer from Thermo Electron Corporation using the potassium bromide (KBr) method. Before analysis, KBr and the immobilized complex were dried for at least 24 hours at 110 °C. Characterization of silica materials was determined from the nitrogen adsorption at −196 °C utilizing a Micromeritics ASAP 2020 instrument. GC analysis was performed on a chiral GC system (Agilent Technology 6890N) equipped with a capillary column MEGA-DEX DMP Beta (25 m × 0.25 mm × 0.25 μm). Injection was performed in split mode (100:1), injector and FID detector temperatures were 250 °C. The analysis was carried out at constant pressure mode (19.91 psi). Oven program: initial temperature 100 °C, then 10 °C min−1 to 150 °C and hold for 1.5 min. TGA curves were recorded using a LINSEIS STA PT1600 thermobalance at a heating rate of 10 °C/min under air atmosphere. The temperature range was between 30 and 1000 °C. ICP-OES procedure: for the microwave digestion 0.3 ± 0.0005 g of sample was weighed into a digestion vessel. The samples were spiked with 8 mL of concentrated nitric acid and 2 mL of hydrogen peroxide. Subsequently, the vessels were closed.
Digestion was performed in the Ethos Up microwave. After each mineralization, the vessels were cooled to room temperature to reach pressure that allowed them to be opened safely. The contents of the vessels were gravity filtered in 25 mL volumetric flasks, the filters had been weighed before the process. The solutions were filled up to the mark with demineralized water. For the determination of ruthenium ICP-OES (iCap 7400, Thermo Scientific) was used in combination with an autosampler (Teledyne Cetac ASX 520). The sediments were dried, weighed, and used in further mineralization (first sediments were used in second mineralization, and then consecutively to the fourth mineralization). Due to the undissolved sediment after the first mineralization and to obtain quantitative digestion of the analyzed element into the liquid phase, the mineralization was repeated four times. Calibration standards were prepared from ruthenium standard (Agilent Technologies Ruthenium Standard, 1000 μg/ml, Ru in 20 % HCl ).
3.1. General Procedure of MCF and SBA Functionalization
MCF synthesis was performed according to our previously the published methods [29] but without contact with water vapor. IR spectra: 1250–1000cm−1 and 750 cm−1 Si–O stretching vibrations, 3800–2500 cm−1 O–H stretching vibrations.
SBA-15 synthesis was performed according to the published methods [33] but without contact with water vapor. IR spectra: 1250–1000cm−1 and 750 cm−1 Si–O stretching vibrations, 3800–2200 cm−1 O–H stretching vibrations.
Pure MCF or SBA was calcinated by heating to 550 °C within 8 h followed by 8 h of dwell time and then heating to 700 °C within 2 h. Then, MCF (1 g) was refluxed under nitrogen with 3-(triethoxysilyl)-1-propanamine (0.54 mL, 3 mmol) in toluene (20 mL) over 24 h. The functionalized carrier was filtered off, washed with acetone and dichloromethane, and then dried under vacuum over 4 days. The solid was transferred to a flask and dichloromethane (30 mL) was added followed by cooling to 0 °C. Then 4-(chloromethyl)benzoyl chloride (400 mg; 2 mmol) in dichloromethane (4 mL) was added dropwise. The mixture was stirred under nitrogen for 24 h at room temperature and then filtered. The received precipitate was washed with acetone and dichloromethane and dried under vacuum for 4 days. IR spectra: 1643 cm−1 and 1549 cm−1 C=C stretching vibrations.
3.2. General Procedure of Immobilization Ru(2)-cBn-fMCF
Functionalized MCF (630 mg) was refluxed under nitrogen with complex 1 (470 mg; 0.83 mmol) in dry toluene (30 mL) for 46 h. Then the mixture was filtered and washed with acetone and dichloromethane and dried under vacuum for 4 days. IR spectra: 2048 cm−1 and 1980 cm−1 C=O stretching vibrations, 1643 cm−1 and 1549 cm−1 C=C stretching vibrations.
3.3. General Procedure of Immobilization Ru(3)-ncBn-fMCF
Functionalized MCF (1 g) was refluxed under nitrogen with ruthenium complex 3 (200 mg; 0.31 mmol) in dry toluene (25 mL) for 5 h. Then the mixture was filtered and washed with acetone and dichloromethane and dried under vacuum for few days. IR spectra: 2048 cm−1 and 1980 cm−1 C=O stretching vibrations, 1643 cm−1 and 1549 cm−1 C=C stretching vibrations
3.4. General Procedure of Immobilization Ru(2)-cBn-fSBA
Functionalized SBA-117 (630 mg) was refluxed under nitrogen with complex 1 (470 mg; 0.83 mmol) in dry toluene (30 mL) for 46 h. Then the mixture was filtered and washed with acetone and dichloromethane and dried under vacuum for 4 days. IR spectra: 2048 cm−1 and 1980 cm−1 C=O stretching vibrations, 1643 cm−1 and 1549 cm−1 C=C stretching vibrations.
3.5. General Procedure of Synthesis Ru(1)
The procedure described by E. Haak [34] was modified as follows: Dodecacarbonyltriruthen(0) (561 mg, 0.877 mmol) was refluxed with 2,3,4,5-tetraphenylocyclopentadien-1-on (0.972 mg; 2.528 mmol) and dry toluene (20 mL). After 3 h the solvent was evaporated. Ruthenium complex was crystalized from THF. 1HNMR (CD3COCD3) ẟ: 7.15–7.28 (m, 15 H); 7.55–7.58 (m, 5 H) ppm. 13CNMR (CD3COCD3) ẟ: 195.7 (CO); 174.7; 133.1; 132.8; 131.1; 130.9; 128.9; 128.2; 128.0; 117.4; 108.9; 81.6 (CH2) ppm.
3.6. General Synthesis of Ru(2)
Ruthenium complex 2 was synthesized according to the literature [21] but time of complex synthesis has been shortened to 24 h. 1HNMR (CD3COCD3) ẟ: 7.59–7.64 (m, 4H); 7.00–7.28 (m, 21 H), 4.81 (s, 2H, CH2) ppm. 13CNMR (CD3COCD3) ẟ: 197.5 (CO); 135.9; 132.9; 132.2; 129.9; 129.9; 128.6; 128.5; 128.3; 128.3; 127.8; 102.6; 88.8; 75.3 (CH2) ppm
3.7. General Synthesis of Ru(3)
Ruthenium complex 3 was synthesize according to the literature [23] 1HNMR (CDCl3) ẟ: 7.17–7.20 (m, 5H); 7.08–7.12 (dd, J = 7.10 Hz, 10 H); 7.02–7.06 (m, 10 H) ppm. 13CNMR (CDCl3) ẟ: 196.9; 132.2; 128.4; 127.8; 127.5; 106.5 ppm.
4. Conclusions
In summary, three cyclopentadienyl ruthenium homogeneous complexes were studied as racemization catalysts of secondary 1-arylethanols. Their immobilization of mesoporous cellular foams was investigated. The support was functionalized with a 3-carbon spacer and 4-(chloromethyl)-N-amidobenzoate moiety. Approaches with covalently and non-covalently immobilized Ru catalysts were studied in a model reaction of secondary alcohol racemization. Although the immobilization resulted in the decrease of the activity of the catalyst, complete racemization of 1-phenylethanol could be achieved within 24 h. The activity of the MCF immobilized ruthenium catalyst was maintained for the recycled catalyst. These results Provide a promising vision for the application of MCF-supported racemization catalysts.
These catalysts combined with the enzymes may be used in the dynamic kinetic resolution. Deposition of the metal catalyst and enzyme on the same support allows to use them in the chemical processes carried out in “one reactor”, especially in the cascade reaction systems.
Author Contributions
M.H. Methodology, Validation, Formal analysis, Investigation, Resources, Writing—Original Draft, Visualization; D.S. Validation, Formal analysis, Investigation, Resources; K.S. Conceptualization, Methodology, Formal analysis, Investigation, Resources, Supervision, Project administration, Visualization; A.K. Formal analysis, Data Curation, Visualization; K.A. Funding acquisition, Writing—Review & Editing; M.M. Formal analysis, Data Curation, Visualization; W.P. Data Curation; A.J. Conceptualization, Investigation, Writing—Review & Editing, Supervision, Funding acquisition; N.K. Conceptualization, Methodology, Investigation, Writing—Review & Editing, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Science Centre of Poland, grant number 2016/23/B/ST8/00627.
Data Availability Statement
Data is contained within the article.
Acknowledgments
Financial support from National Science Centre of Poland (grant number 2016/23/B/ST8/00627) is gratefully acknowledged.
Conflicts of Interest
There is no conflict to declare.
References
- Bhardwaj, K.K.; Gupta, R. Synthesis of Chirally Pure Enantiomers by Lipase. J. Oleo Sci. 2017, 66, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, H. Recent developments in dynamic kinetic resolution. Tetrahedron 2008, 64, 1563–1601. [Google Scholar] [CrossRef]
- Wu, D.; Pan, F.; Tan, W.; Gao, L.; Tao, Y.; Kong, Y. Recent progress of enantioseparation under scale production (2014–2019). J. Sep. Sci. 2020, 43, 337–347. [Google Scholar] [CrossRef]
- Xiouras, C.; Fytopoulos, A.; Jordens, J.; Boudouvis, A.G.; Van Gerven, T.; Stefanidis, G.D. Applications of ultrasound to chiral crystallization, resolution and deracemization. Ultrason. Sonochem. 2018, 43, 184–192. [Google Scholar] [CrossRef]
- Ozkan, S.A.; Chankvetadze, B. Analytical and Preparative Scale Separation of Enantiomers of Chiral Drugs by Chromatography and Related Methods. Curr. Med. Chem. 2018, 25, 4152–4188. [Google Scholar] [CrossRef]
- Engström, K.; Johnston, E.V.; Verho, O.; Gustafson, K.P.J.; Shakeri, M.; Tai, C.-W.; Bäckvall, J.-E. Co-immobilization of an Enzyme and a Metal into the Compartments of Mesoporous Silica for Cooperative Tandem Catalysis: An Artificial Metalloenzyme. Angew. Chem. Int. Ed. 2013, 52, 14006–14010. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, A.S.; Miranda, L.S.; De Souza, R.O. Lipases: Valuable catalysts for dynamic kinetic resolutions. Biotechnol. Adv. 2015, 33, 372–393. [Google Scholar] [CrossRef]
- Verho, O.; Bäckvall, J.-E. Chemoenzymatic Dynamic Kinetic Resolution: A Powerful Tool for the Preparation of Enantiomerically Pure Alcohols and Amines. J. Am. Chem. Soc. 2015, 137, 3996–4009. [Google Scholar] [CrossRef] [PubMed]
- Coldham, I.; Dufour, S.; Haxell, T.F.N.; Patel, J.J.; Sanchez-Jimenez, G. Dynamic Thermodynamic and Dynamic Kinetic Resolution of 2-Lithiopyrrolidines. J. Am. Chem. Soc. 2006, 128, 10943–10951. [Google Scholar] [CrossRef]
- Veum, L.; Hanefeld, U. Enantioselective formation of mandelonitrile acetate: Investigation of a dynamic kinetic resolution II. Tetrahedron Asymmetry 2004, 15, 3707–3709. [Google Scholar] [CrossRef]
- Huerta, F.F.; Minidis, A.B.E.; Bäckvall, J.E. Racemisation in asymmetric synthesis. Dynamic kinetic resolution and related processes in enzyme and metal catalysis. Chem. Soc. Rev. 2001, 30, 321–331. [Google Scholar] [CrossRef]
- Pàmies, O.; Bäckvall, J.-E. Combination of Enzymes and Metal Catalysts. A Powerful Approach in Asymmetric Catalysis. Chem. Rev. 2003, 103, 3247–3262. [Google Scholar] [CrossRef] [PubMed]
- Karvembu, R.; Prabhakaran, R.; Tamizh, M.M.; Natarajan, K. Ruthenium and enzyme-catalyzed dynamic kinetic resolution of alcohols. Comptes Rendus Chim. 2009, 12, 951–962. [Google Scholar] [CrossRef]
- Cao, H.; Zhu, X.-H.; Wang, N.; Sun, Z.; Deng, Y.; Hou, X.-F.; Zhao, D. Selectivity Enhancement in Dynamic Kinetic Resolution of Secondary Alcohols through Adjusting the Micro-Environment of Metal Complex Confined in Nanochannels: A Promising Strategy for Tandem Reactions. ACS Catal. 2014, 5, 27–33. [Google Scholar] [CrossRef]
- Magner, E. Immobilisation of enzymes on mesoporous silicate materials. Chem. Soc. Rev. 2013, 42, 6213–6222. [Google Scholar] [CrossRef] [PubMed]
- Jia, A.Q.; Shi, L.M.; Wu, F.L.; Xin, Z.F.; Zhang, Q.F. Syntheses, structures and immobilization of ruthenium complexes bearing N,O-Schiff-base or N,N′-diamine ligands functionalized with alkoxysilyl groups. J. Organomet. Chem. 2018, 855, 33–43. [Google Scholar] [CrossRef]
- Szymańska, K.; Bryjak, J.; Mrowiec-Białoń, J.; Jarzebski, A.B. Application and properties of siliceous mesostructured cellular foams as enzymes carriers to obtain efficient biocatalysts. Microporous Mesoporous Mater. 2007, 99, 167–175. [Google Scholar] [CrossRef]
- Rekuć, A.; Bryjak, J.; Szymańska, K.; Jarzębski, A.B. Laccase immobilization on mesostructured cellular foams affords preparations with ultra high activity. Process. Biochem. 2009, 44, 191–198. [Google Scholar] [CrossRef]
- Van Der Helm, M.P.; Bracco, P.; Busch, H.; Szymańska, K.; Jarzębski, A.B.; Hanefeld, U. Hydroxynitrile lyases covalently immobilized in continuous flow microreactors. Catal. Sci. Technol. 2019, 9, 1189–1200. [Google Scholar] [CrossRef]
- Choi, J.H.; Choi, Y.K.; Kim, Y.H.; Park, E.S.; Kim, E.J.; Kim, M.-J.; Park, J. Aminocyclopentadienyl Ruthenium Complexes as Racemization Catalysts for Dynamic Kinetic Resolution of Secondary Alcohols at Ambient Temperature. J. Org. Chem. 2004, 69, 1972–1977. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Ko, S.-B.; Kwon, M.S.; Kim, M.-J.; Park, J. Air-Stable Racemization Catalyst for Dynamic Kinetic Resolution of Secondary Alcohols at Room Temperature. Org. Lett. 2005, 7, 4523–4526. [Google Scholar] [CrossRef]
- Martín-Matute, B.; Edin, M.; Bogár, K.; Bäckvall, J.-E. Highly Compatible Metal and Enzyme Catalysts for Efficient Dynamic Kinetic Resolution of Alcohols at Ambient Temperature. Angew. Chem. Int. Ed. 2004, 43, 6535–6539. [Google Scholar] [CrossRef] [PubMed]
- Martín-Matute, B.; Edin, M.; Bogár, K.; Kaynak, F.B.; Bäckvall, J.-E. Combined Ruthenium(II) and Lipase Catalysis for Efficient Dynamic Kinetic Resolution of Secondary Alcohols. Insight into the Racemization Mechanism. J. Am. Chem. Soc. 2005, 127, 8817–8825. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, R.H. The Organometallic Chemistry of the Transition Metals, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1994; p. 487. [Google Scholar]
- Shylesh, S.; Hanna, D.; Gomes, J.; Canlas, C.G.; Head-Gordon, M.; Bell, A.T. The Role of Hydroxyl Group Acidity on the Activity of Silica-Supported Secondary Amines for the Self-Condensation ofn-Butanal. ChemSusChem 2014, 8, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ji, S.; Liu, Q.; Wu, P.; Zhu, J.; Li, C. Surface acidity and hydroxyl groups of SBA-15 mesoporous silica catalysts containing tungsten species. Chin. J. Catal. 2007, 11, 980–986. [Google Scholar] [CrossRef]
- Wouters, B.H.; Chen, T.; Dewilde, M.; Grobet, P.J. Reactivity of the surface hydroxyl groups of MCM-41 towards silylation with trimethylchlorosilane. Microporous Mesoporous Mater. 2001, 44–45, 453–457. [Google Scholar] [CrossRef]
- Mrowiec-Bialoń, J. Determination of hydroxyls density in the silica-mesostructured cellular foams by thermogravimetry. Thermochim. Acta 2006, 443, 49–52. [Google Scholar] [CrossRef]
- Gac, W.; Derylo-Marczewska, A.; Pasieczna-Patkowska, S.; Popivnyak, N.; Zukocinski, G. The influence of the preparation methods and pretreatment conditions on the properties of Ag-MCM-41 catalysts. J. Mol. Catal. A Chem. 2007, 268, 15–23. [Google Scholar] [CrossRef]
- Derylo-Marczewska, A.; Gac, W.; Popivnyak, N.; Zukocinski, G.; Pasieczna, S. The influence of preparation method on the structure and redox properties of mesoporous Mn-MCM-41 materials. Catal. Today 2006, 114, 293–306. [Google Scholar] [CrossRef]
- Zverev, S.A.; Andreev, S.; Zamilatskov, I.A.; Kurochkina, N.M.; Tyurin, V.S.; Senchikhin, I.N.; Ponomarev, G.V.; Erzina, D.R.; Chernyshev, V. Structure of ruthenium(II) complexes with coproporphyrin I tetraethyl ester. Russ. J. Phys. Chem. A 2017, 91, 1462–1467. [Google Scholar] [CrossRef]
- Mbese, J.Z.; Ajibade, P.A. Homonuclear tris-dithiocarbamato ruthenium(III) complexes as single-molecule precursors for the synthesis of ruthenium(III) sulfide nanoparticles. J. Sulfur Chem. 2016, 38, 173–187. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Haak, E. Ruthenium Complexes of Electronically Coupled Cyclopentadienone Ligands—Catalysts for Transformations of Propargyl Alcohols. Eur. J. Org. Chem. 2007, 2007, 2815–2824. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).