Effects of Hydrothermal Aging on CO and NO Oxidation Activity over Monometallic and Bimetallic Pt-Pd Catalysts
Abstract
1. Introduction
2. Results
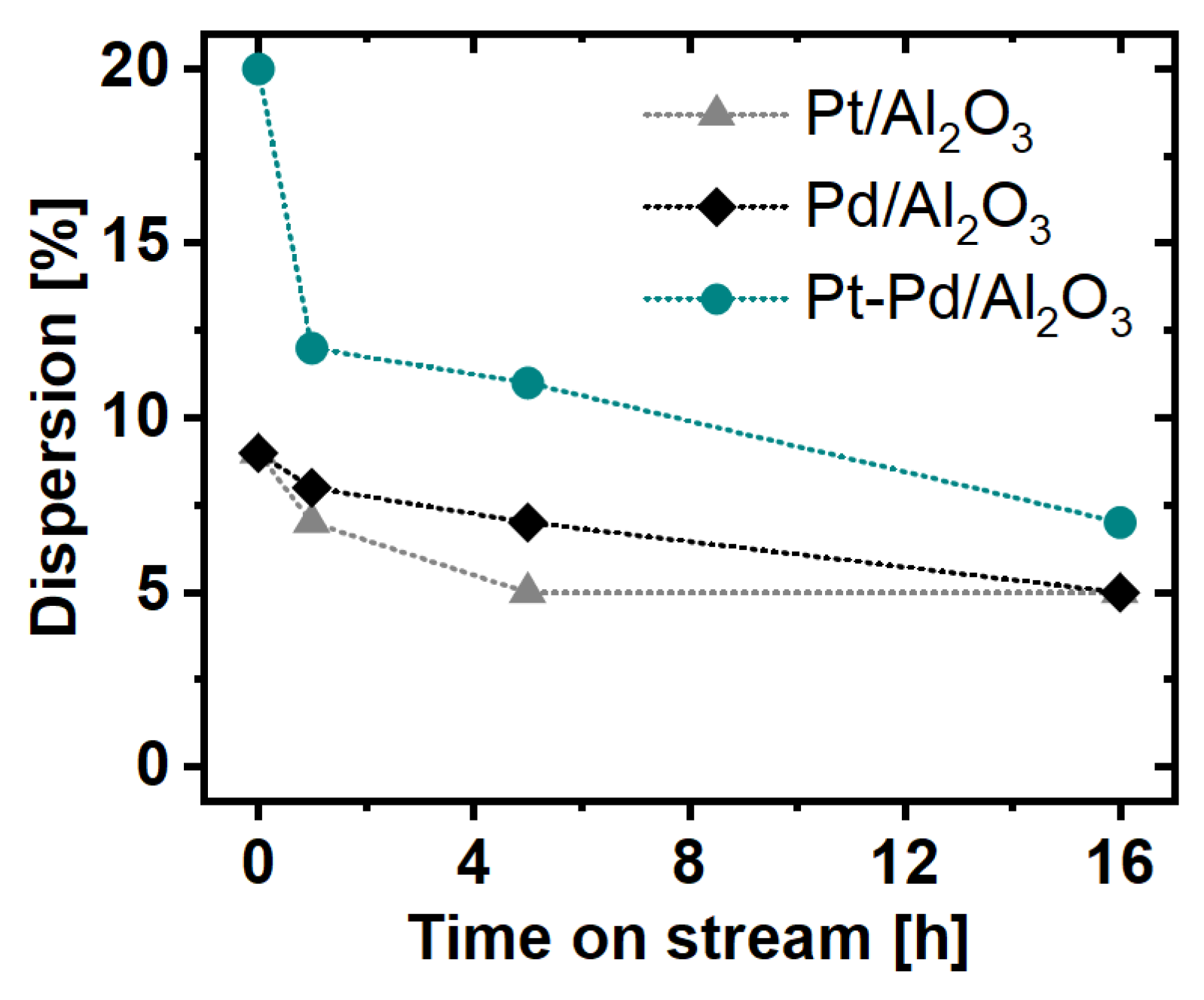
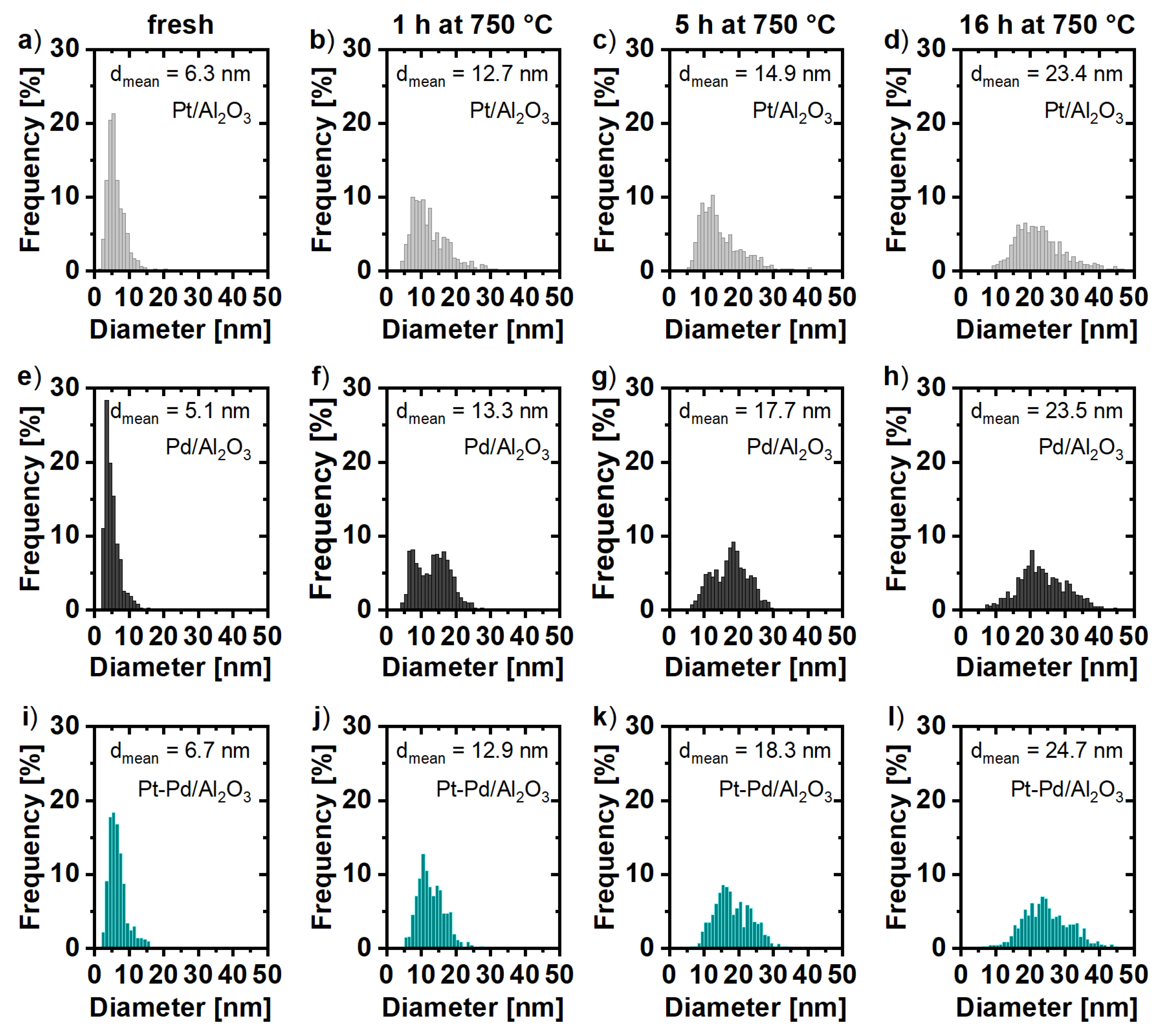
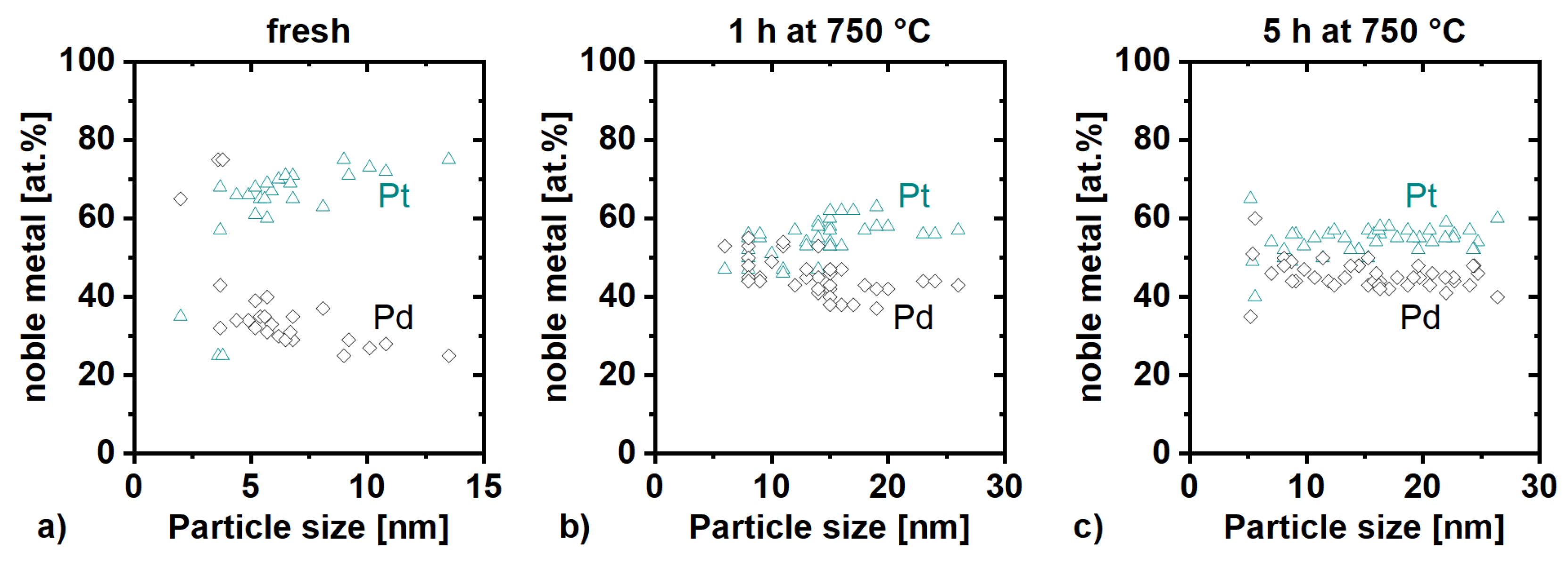
2.1. Particle Size Distribution and Dispersion
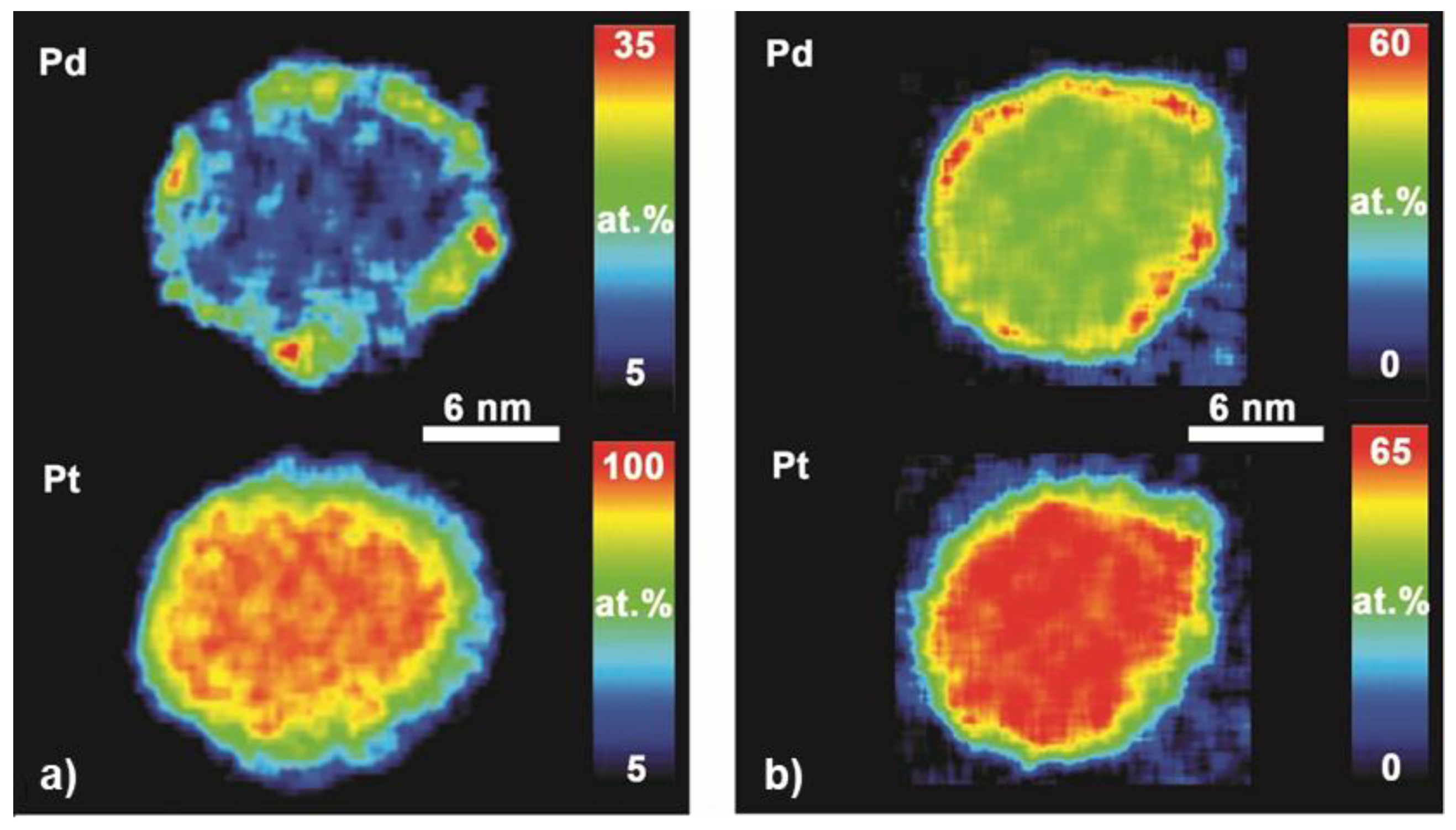
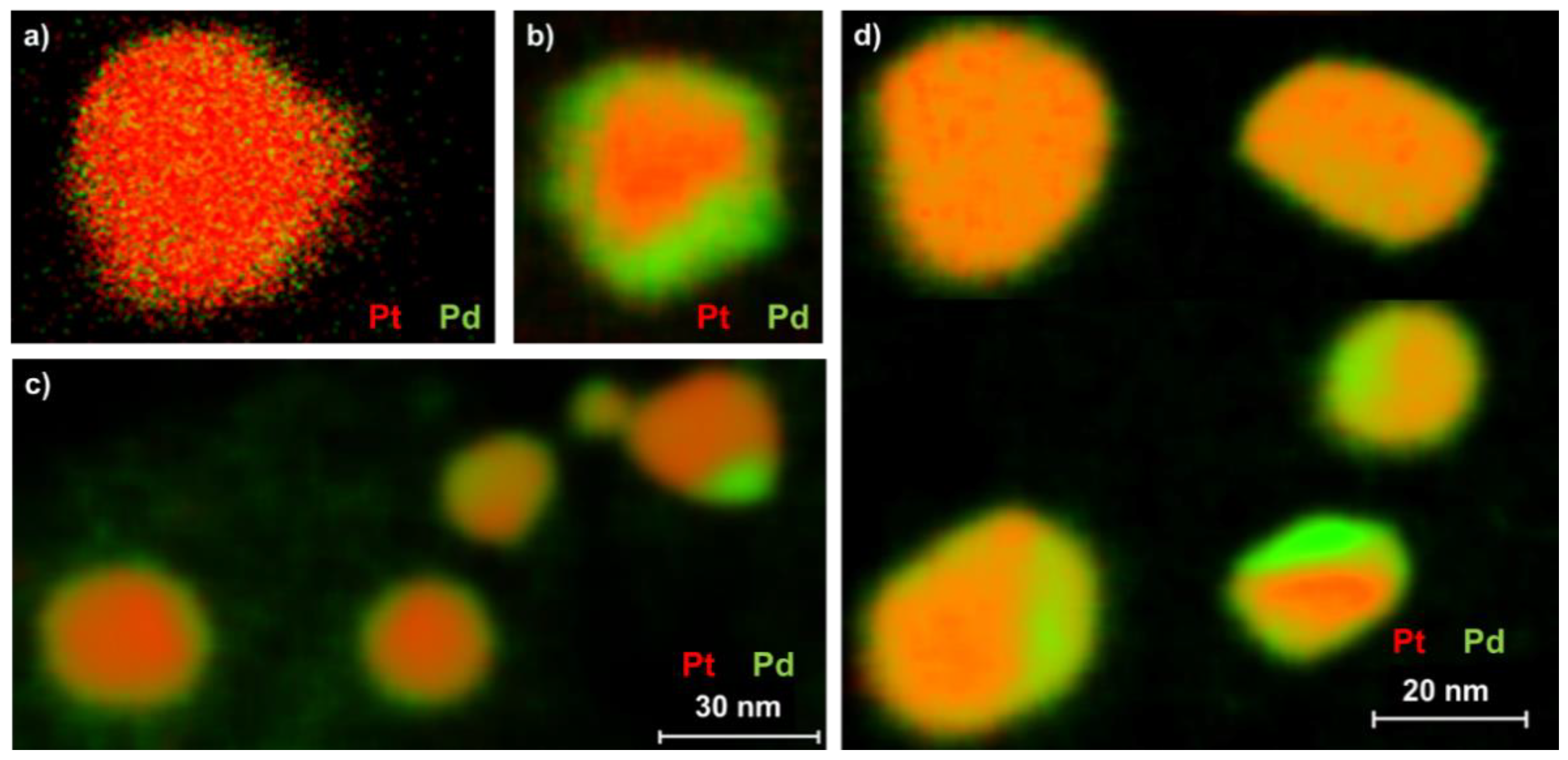
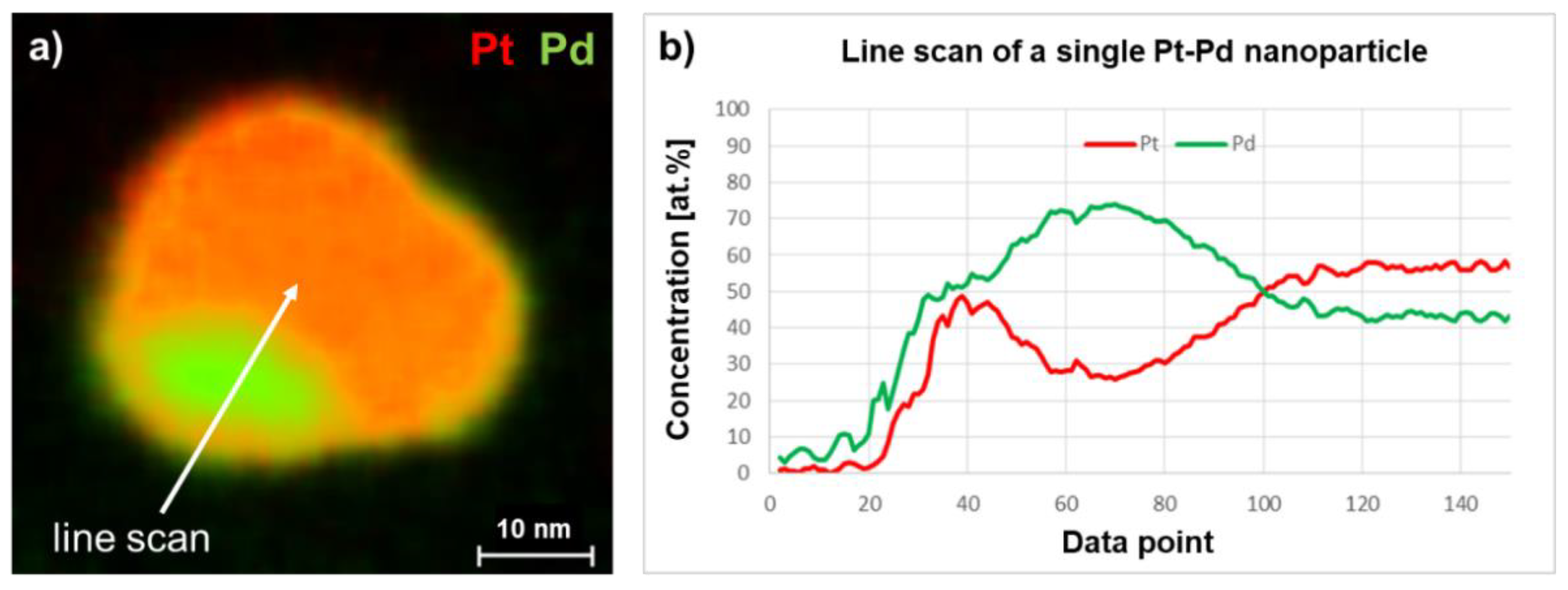
2.2. Particle Morphology
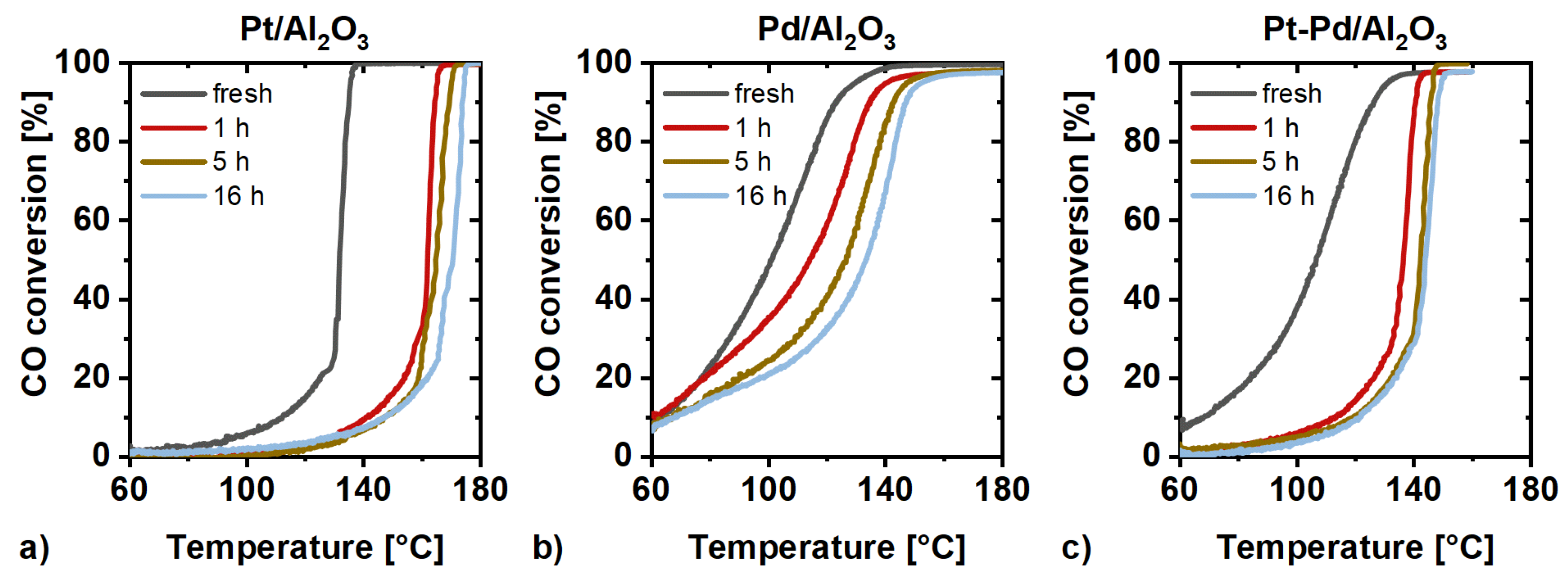
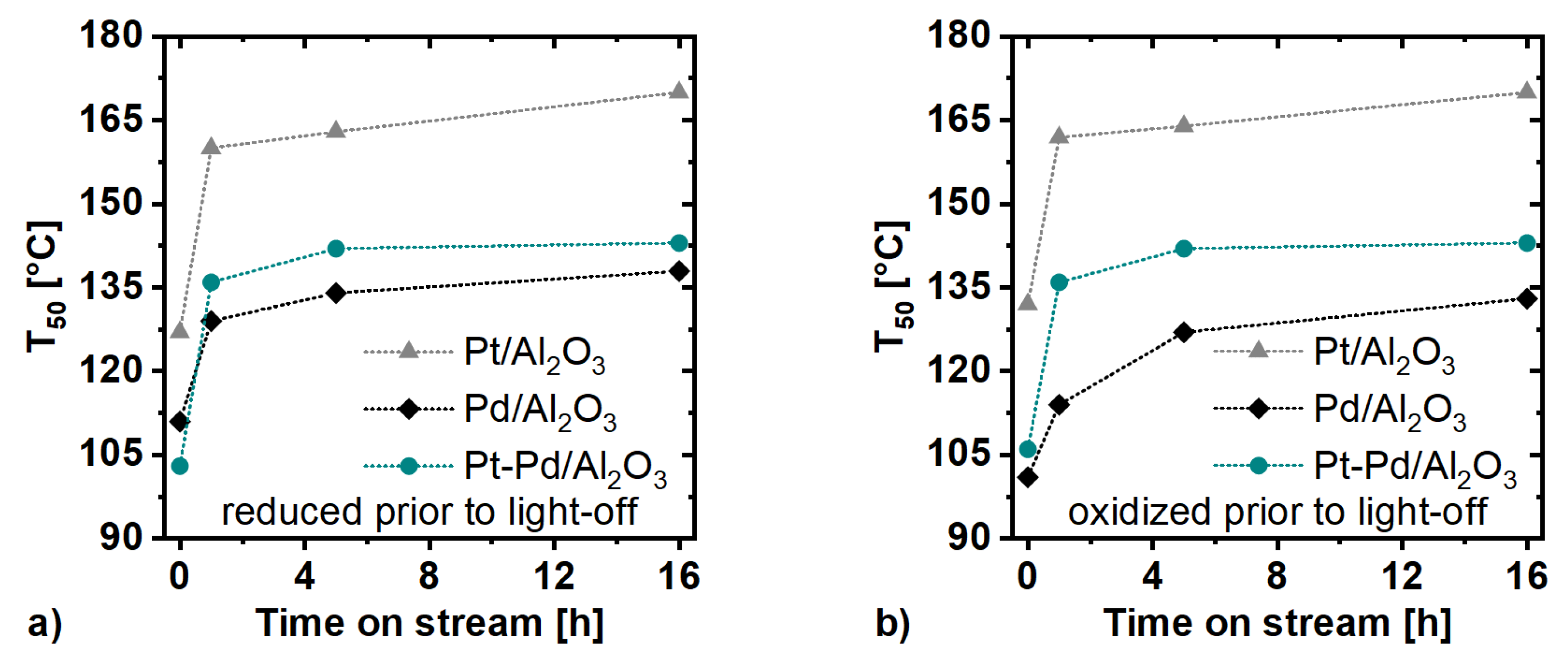
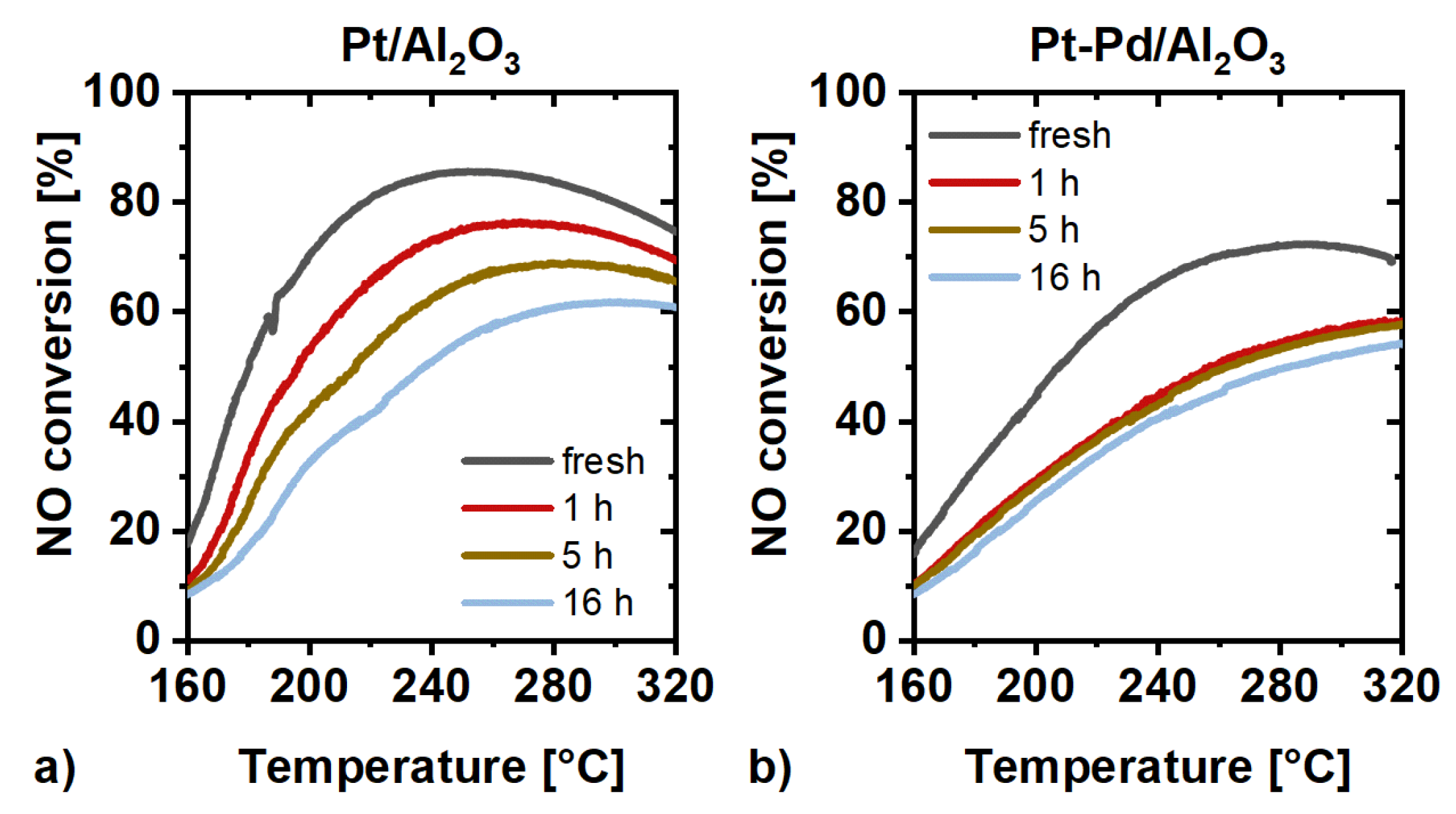
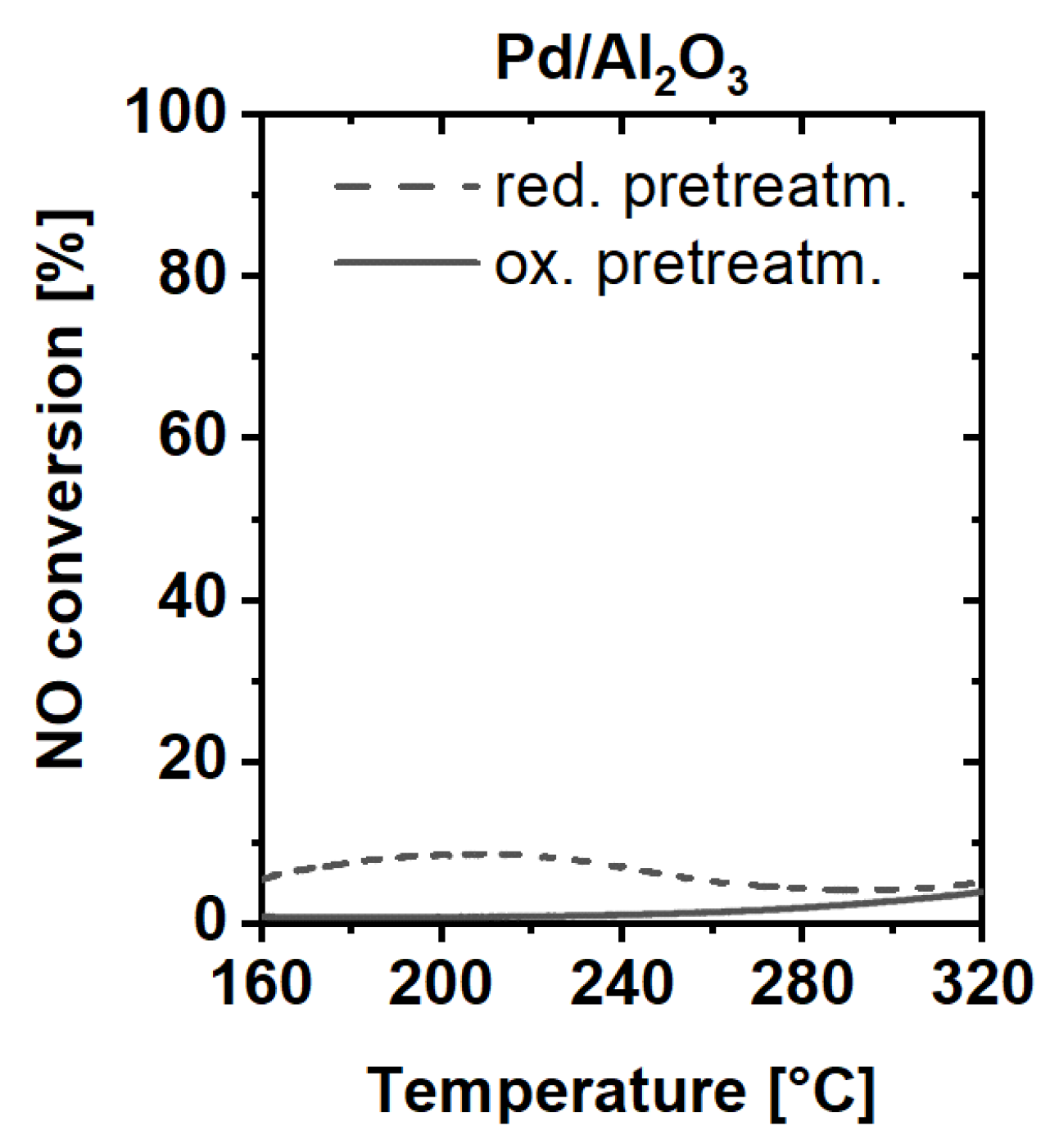
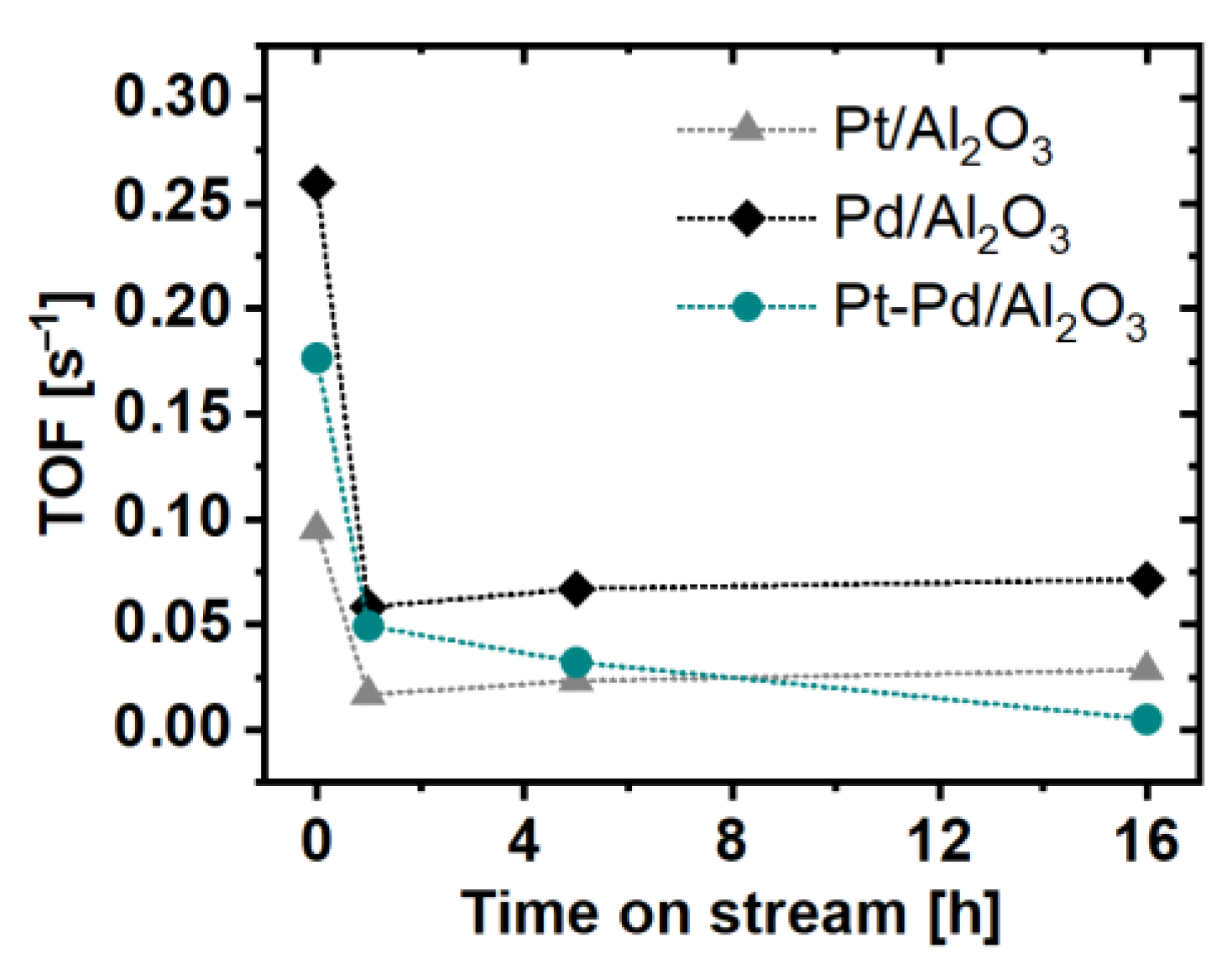
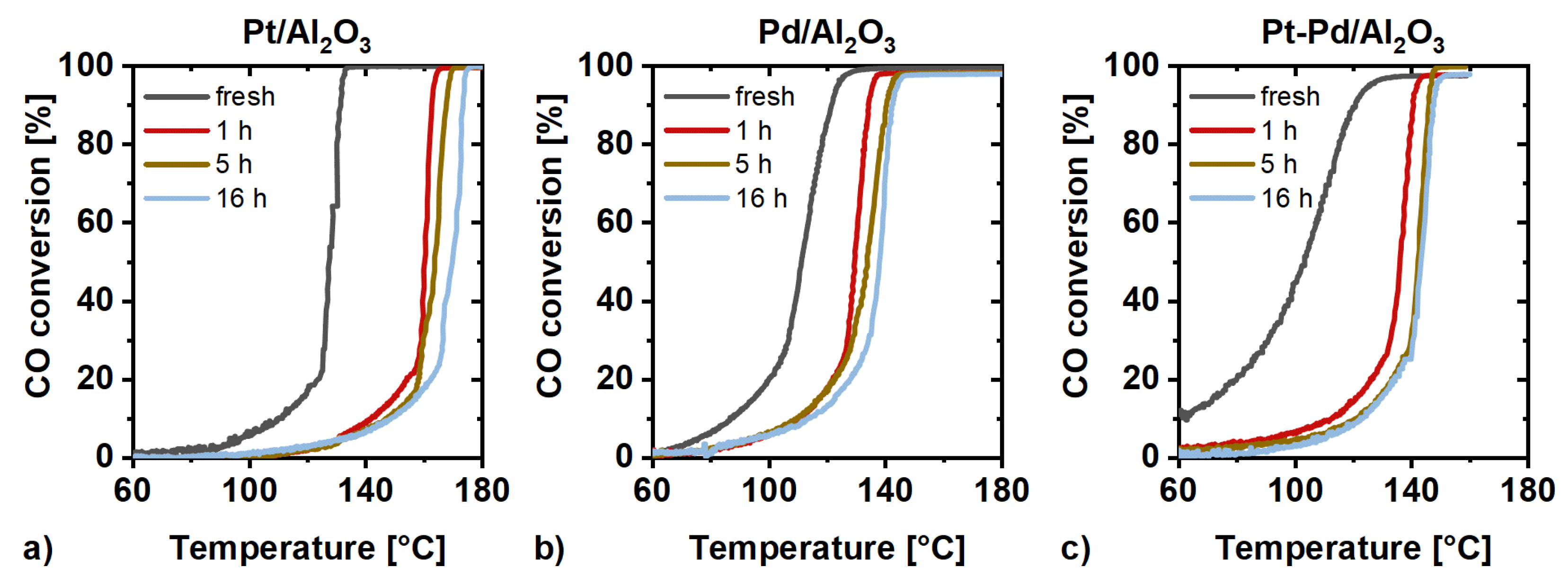
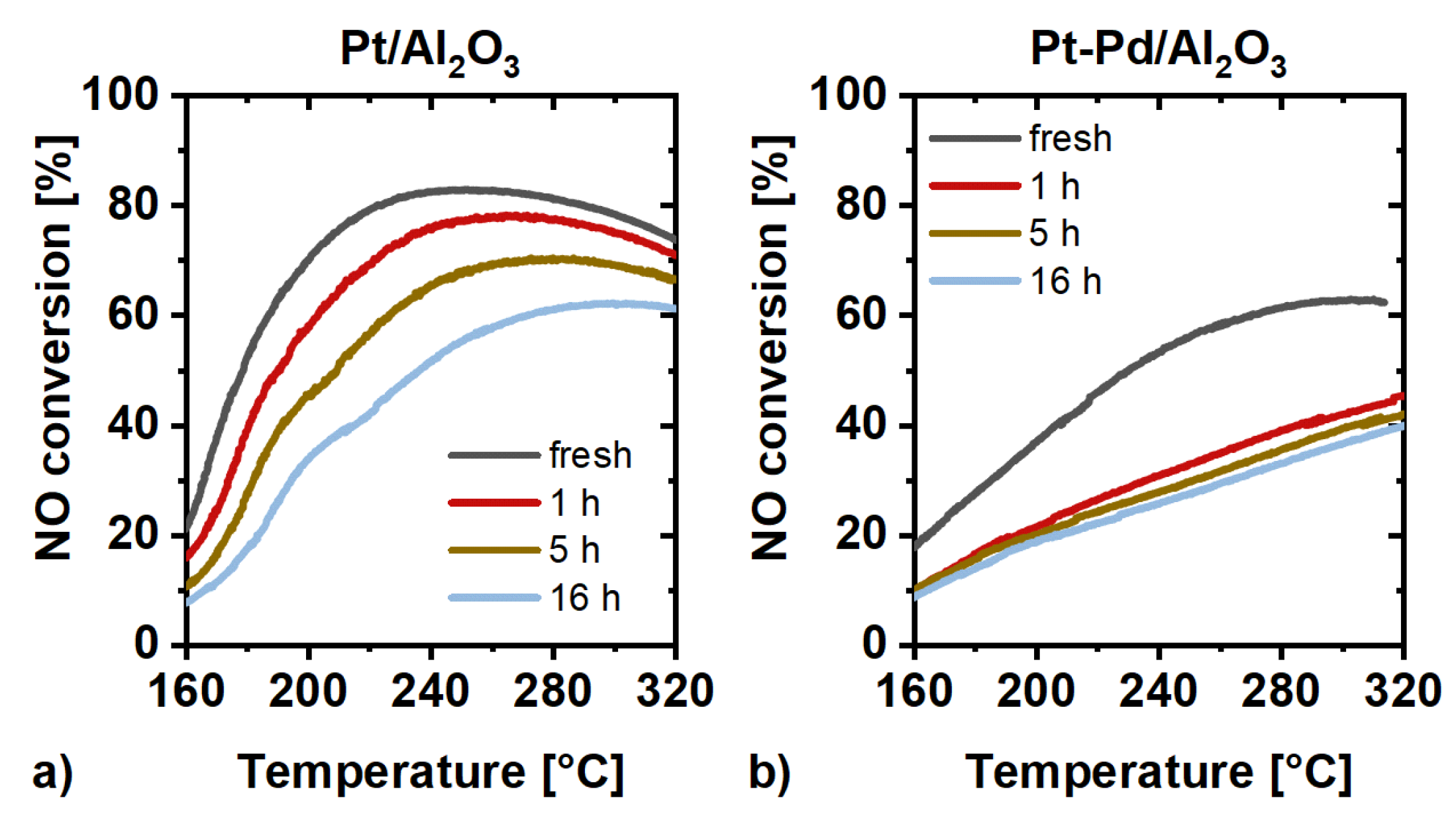
2.3. Catalytic Activity Measurements
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Catalyst Samples
5.2. Catalyst Characterization
5.3. Catalytic Tests
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Deutschmann, O.; Grunwaldt, J.-D. Exhaust Gas Aftertreatment in Mobile Systems: Status, Challenges, and Perspectives. Chem. Eng. Tech. 2013, 85, 595–617. [Google Scholar] [CrossRef]
- Russell, A.; Epling, W.S. Diesel Oxidation Catalysts. Catal. Rev. 2011, 53, 337–423. [Google Scholar] [CrossRef]
- Johnson, T.V. Review of diesel emissions and control. Int. J. Eng. Res. 2009, 10, 275–285. [Google Scholar] [CrossRef]
- Johnson, T.V. Diesel Emission Control in Review. SAE Int. J. Fuels Lubr. 2009, 1, 68–81. [Google Scholar] [CrossRef]
- Mulla, S.; Chen, N.; Cumaranatunge, L.; Blau, G.; Zemlyanov, D.; Delgass, W.; Epling, W.S.; Ribeiro, F. Reaction of NO and O2 to NO2 on Pt: Kinetics and catalyst deactivation. J. Catal. 2006, 241, 389–399. [Google Scholar] [CrossRef]
- Boubnov, A.; Dahl, S.; Johnson, E.; Molina, A.P.; Simonsen, S.B.; Cano, F.M.; Helveg, S.; Lemus-Yegres, L.J.; Grunwaldt, J.-D. Structure–activity relationships of Pt/Al2O3 catalysts for CO and NO oxidation at diesel exhaust conditions. Appl. Catal. B 2012, 126, 315–325. [Google Scholar] [CrossRef]
- Lira, E.; Merte, L.R.; Behafarid, F.; Ono, L.K.; Zhang, L.; Roldan Cuenya, B. Role and Evolution of Nanoparticle Structure and Chemical State during the Oxidation of NO over Size- and Shape-Controlled Pt/γ-Al2O3 Catalysts under Operando Conditions. ACS Catal. 2014, 4, 1875–1884. [Google Scholar] [CrossRef]
- Hansen, T.K.; Høj, M.; Hansen, B.B.; Janssens, T.V.W.; Jensen, A.D. The Effect of Pt Particle Size on the Oxidation of CO, C3H6, and NO Over Pt/Al2O3 for Diesel Exhaust Aftertreatment. Top. Catal. 2017, 60, 1333–1344. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kung, H.H. Effect of Pt dispersion on the reduction of NO by propene over alumina-supported Pt catalysts under lean-burn conditions. Catal. Lett. 1998, 51, 1–4. [Google Scholar] [CrossRef]
- Auvray, X.; Pingel, T.; Olsson, E.; Olsson, L. The effect gas composition during thermal aging on the dispersion and NO oxidation activity over Pt/Al2O3 catalysts. Appl. Catal. B 2013, 129, 517–527. [Google Scholar] [CrossRef]
- Johns, T.R.; Gaudet, J.R.; Peterson, E.J.; Miller, J.T.; Stach, E.A.; Kim, C.H.; Balogh, M.P.; Datye, A.K. Microstructure of Bimetallic Pt-Pd Catalysts under Oxidizing Conditions. ChemCatChem 2013, 5, 2636–2645. [Google Scholar] [CrossRef]
- Ogel, E.; Casapu, M.; Doronkin, D.E.; Popescu, R.; Störmer, H.; Mechler, C.; Marzun, G.; Barcikowski, S.; Türk, M.; Grunwaldt, J.-D. Impact of Preparation Method and Hydrothermal Aging on Particle Size Distribution of Pt/γ-Al2O3 and Its Performance in CO and NO Oxidation. J. Phys. Chem. C 2019, 123, 5433–5446. [Google Scholar] [CrossRef]
- Casapu, M.; Fischer, A.; Gänzler, A.M.; Popescu, R.; Crone, M.; Gerthsen, D.; Türk, M.; Grunwaldt, J.-D. Origin of the Normal and Inverse Hysteresis Behavior during CO Oxidation over Pt/Al2O3. ACS Catal. 2016, 7, 343–355. [Google Scholar] [CrossRef]
- Hauff, K.; Tuttlies, U.; Eigenberger, G.; Nieken, U. Platinum oxide formation and reduction during NO oxidation on a diesel oxidation catalyst—Experimental results. Appl. Catal. B 2012, 123–124, 107–116. [Google Scholar] [CrossRef]
- Gänzler, A.M.; Casapu, M.; Boubnov, A.; Müller, O.; Conrad, S.; Lichtenberg, H.; Frahm, R.; Grunwaldt, J.-D. Operando spatially and time-resolved X-ray absorption spectroscopy and infrared thermography during oscillatory CO oxidation. J. Catal. 2015, 328, 216–224. [Google Scholar] [CrossRef]
- Boubnov, A.; Gänzler, A.M.; Conrad, S.; Casapu, M.; Grunwaldt, J.-D. Oscillatory CO Oxidation Over Pt/Al2O3 Catalysts Studied by In situ XAS and DRIFTS. Top. Catal. 2013, 56, 333–338. [Google Scholar] [CrossRef]
- Dubbe, H.; Eigenberger, G.; Nieken, U. Modeling of Conversion Hysteresis Phenomena for Pt/Pd-based Diesel Oxidation Catalysts. Chem. Eng. Tech. 2018, 90, 625–633. [Google Scholar] [CrossRef]
- Hauff, K.; Dubbe, H.; Tuttlies, U.; Eigenberger, G.; Nieken, U. Platinum oxide formation and reduction during NO oxidation on a diesel oxidation catalyst—Macrokinetic simulation. Appl. Catal. B 2013, 129, 273–281. [Google Scholar] [CrossRef]
- Olsson, L.; Fridell, E. The Influence of Pt Oxide Formation and Pt Dispersion on the Reactions NO2⇔NO+1/2 O2 over Pt/Al2O3 and Pt/BaO/Al2O3. J. Catal. 2002, 210, 340–353. [Google Scholar] [CrossRef]
- Gänzler, A.M.; Casapu, M.; Doronkin, D.E.; Maurer, F.; Lott, P.; Glatzel, P.; Votsmeier, M.; Deutschmann, O.; Grunwaldt, J.-D. Unravelling the Different Reaction Pathways for Low Temperature CO Oxidation on Pt/CeO2 and Pt/Al2O3 by Spatially Resolved Structure-Activity Correlations. J. Phys. Chem. Lett. 2019, 10, 7698–7705. [Google Scholar] [CrossRef]
- Cargnello, M.; Doan-Nguyen, V.V.T.; Gordon, T.R.; Diaz, R.E.; Stach, E.A.; Gorte, R.J.; Fornasiero, P.; Murray, C.B. Control of metal nanocrystal size reveals metal-support interface role for ceria catalysts. Science 2013, 341, 771–773. [Google Scholar] [CrossRef]
- Gänzler, A.M.; Casapu, M.; Vernoux, P.; Loridant, S.; Cadete Santos Aires, F.J.; Epicier, T.; Betz, B.; Hoyer, R.; Grunwaldt, J.-D. Tuning the Structure of Platinum Particles on Ceria In Situ for Enhancing the Catalytic Performance of Exhaust Gas Catalysts. Angew. Chem. Int. Ed. 2017, 56, 13078–13082. [Google Scholar] [CrossRef] [PubMed]
- Datye, A.K.; Votsmeier, M. Opportunities and challenges in the development of advanced materials for emission control catalysts. Nat. Mater. 2020. [Google Scholar] [CrossRef]
- Cao, A.; Lu, R.; Veser, G. Stabilizing metal nanoparticles for heterogeneous catalysis. Phys. Chem. Chem. Phys. 2010, 12, 13499–13510. [Google Scholar] [CrossRef]
- Wong, A.P.; Kyriakidou, E.A.; Toops, T.J.; Regalbuto, J.R. The catalytic behavior of precisely synthesized Pt–Pd bimetallic catalysts for use as diesel oxidation catalysts. Catal. Today 2016, 267, 145–156. [Google Scholar] [CrossRef]
- Etheridge, J.E.; Watling, T.C.; Izzard, A.J.; Paterson, M.A.J. The Effect of Pt:Pd Ratio on Light-Duty Diesel Oxidation Catalyst Performance: An Experimental and Modelling Study. SAE Int. J. Engines 2015, 8, 1283–1299. [Google Scholar] [CrossRef]
- Kim, C.H.; Schmid, M.; Schmieg, S.J.; Tan, J.; Li, W. The Effect of Pt-Pd Ratio on Oxidation Catalysts Under Simulated Diesel Exhaust. SAE Tech. Pap. 2011. [Google Scholar] [CrossRef]
- Kaneeda, M.; Iizuka, H.; Hiratsuka, T.; Shinotsuka, N.; Arai, M. Improvement of thermal stability of NO oxidation Pt/Al2O3 catalyst by addition of Pd. Appl. Catal. B 2009, 90, 564–569. [Google Scholar] [CrossRef]
- Morlang, A.; Neuhausen, U.; Klementiev, K.V.; Schütze, F.-W.; Miehe, G.; Fuess, H.; Lox, E.S. Bimetallic Pt/Pd diesel oxidation catalysts: Structural characterisation and catalytic behaviour. Appl. Catal. B 2005, 60, 191–199. [Google Scholar] [CrossRef]
- Johns, T.R.; Goeke, R.S.; Ashbacher, V.; Thüne, P.C.; Niemantsverdriet, J.W.; Kiefer, B.; Kim, C.H.; Balogh, M.P.; Datye, A.K. Relating adatom emission to improved durability of Pt–Pd diesel oxidation catalysts. J. Catal. 2015, 328, 151–164. [Google Scholar] [CrossRef]
- Plessow, P.N.; Abild-Pedersen, F. Sintering of Pt Nanoparticles via Volatile PtO2. ACS Catal. 2016, 6, 7098–7108. [Google Scholar] [CrossRef]
- Alcock, C.B.; Hooper, G.W. Thermodynamics of the gaseous oxides of the platinum-group metals. Proc. R. Soc. Lond. A 1960, 254, 551–561. [Google Scholar] [CrossRef]
- Peuckert, M. XPS study on surface and bulk palladium oxide, its thermal stability, and a comparison with other noble metal oxides. J. Phys. Chem. 1985, 89, 2481–2486. [Google Scholar] [CrossRef]
- Graham, G.W.; Jen, H.-W.; Ezekoye, O.; Kudla, R.J.; Chun, W.; Pan, X.Q.; McCabe, R.W. Effect of alloy composition on dispersion stability and catalytic activity for NO oxidation over alumina-supported Pt–Pd catalysts. Catal. Lett. 2007, 116, 1–8. [Google Scholar] [CrossRef]
- Neyestanaki, A.K.; Klingstedt, F.; Salmi, T.; Murzin, D.Y. Deactivation of postcombustion catalysts, a review. Fuel 2004, 83, 395–408. [Google Scholar] [CrossRef]
- Ciuparu, D.; Pfefferle, L. Methane combustion activity of supported palladium catalysts after partial reduction. Appl. Catal. A 2001, 218, 197–209. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Chen, J.J. Spreading and surface tension gradient driven phenomena during heating of alumina-supported palladium crystallites in oxygen. J. Catal. 1981, 70, 233–236. [Google Scholar] [CrossRef]
- Carrillo, C.; Johns, T.R.; Xiong, H.; DeLaRiva, A.; Challa, S.R.; Goeke, R.S.; Artyushkova, K.; Li, W.; Kim, C.H.; Datye, A.K. Trapping of Mobile Pt Species by PdO Nanoparticles under Oxidizing Conditions. J. Phys. Chem. Lett. 2014, 5, 2089–2093. [Google Scholar] [CrossRef] [PubMed]
- Arnal, P.M.; Comotti, M.; Schüth, F. High-temperature-stable catalysts by hollow sphere encapsulation. Angew. Chem., Int. Ed. 2006, 45, 8224–8227. [Google Scholar] [CrossRef]
- Hill, A.J.; Seo, C.Y.; Chen, X.; Bhat, A.; Fisher, G.B.; Lenert, A.; Schwank, J.W. Thermally Induced Restructuring of Pd@CeO2 and Pd@SiO2 Nanoparticles as a Strategy for Enhancing Low-Temperature Catalytic Activity. ACS Catal. 2020, 10, 1731–1741. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, J.; Guo, Y.; Liu, J. Ultrathin, Polycrystalline, Two-Dimensional Co3O4 for Low-Temperature CO Oxidation. ACS Catal. 2019, 9, 2558–2567. [Google Scholar] [CrossRef]
- Yang, X.; Li, Q.; Lu, E.; Wang, Z.; Gong, X.; Yu, Z.; Guo, Y.; Wang, L.; Guo, Y.; Zhan, W.; et al. Taming the stability of Pd active phases through a compartmentalizing strategy toward nanostructured catalyst supports. Nat. Commun. 2019, 10, 1611. [Google Scholar] [CrossRef] [PubMed]
- Malamis, S.A.; Harold, M.P.; Epling, W.S. Coupled NO and C3H6 Trapping, Release and Conversion on Pd/BEA: Evaluation of the Lean Hydrocarbon NOx Trap. Ind. Eng. Chem. Res. 2019, 58, 22912–22923. [Google Scholar] [CrossRef]
- Zheng, Y.; Kovarik, L.; Engelhard, M.H.; Wang, Y.; Wang, Y.; Gao, F.; Szanyi, J. Low-Temperature Pd/Zeolite Passive NOx Adsorbers: Structure, Performance, and Adsorption Chemistry. J. Phys. Chem. C 2017, 121, 15793–15803. [Google Scholar] [CrossRef]
- Theis, J.R. An assessment of Pt and Pd model catalysts for low temperature NO adsorption. Catal. Today 2016, 267, 93–109. [Google Scholar] [CrossRef]
- Günter, T.; Pesek, J.; Schäfer, K.; Bertótiné Abai, A.; Casapu, M.; Deutschmann, O.; Grunwaldt, J.-D. Cu-SSZ-13 as pre-turbine NOx-removal-catalyst: Impact of pressure and catalyst poisons. Appl. Catal. B 2016, 198, 548–557. [Google Scholar] [CrossRef]
- Xu, Q.; Kharas, K.C.; Croley, B.J.; Datye, A.K. The Sintering of Supported Pd Automotive Catalysts. ChemCatChem 2011, 3, 1004–1014. [Google Scholar] [CrossRef]
- McCarty, J.G.; Malukhin, G.; Poojary, D.M.; Datye, A.K.; Xu, Q. Thermal coarsening of supported palladium combustion catalysts. J. Phys. Chem. B 2005, 109, 2387–2391. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, G.A.; Salinas-Rodríguez, E. Realistic Particle Size Distributions during Sintering by Ostwald Ripening. Stud. Surf. Sci. Catal. 2001, 139, 503–510. [Google Scholar] [CrossRef]
- Hansen, T.W.; Delariva, A.T.; Challa, S.R.; Datye, A.K. Sintering of catalytic nanoparticles: Particle migration or Ostwald ripening? Acc. Chem. Res. 2013, 46, 1720–1730. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Pulvermacher, B. Kinetics of crystallite sintering during heat treatment of supported metal catalysts. AIChE J. 1973, 19, 356–364. [Google Scholar] [CrossRef]
- Bergeret, G.; Gallezot, P. Particle Size and Dispersion Measurements. In Handbook of Heterogeneous Catalysis, 2nd ed.; Ertl, G., Ed.; Wiley-VCH: Weinheim, Germany; Chichester, UK, 2008; ISBN 9783527610044. [Google Scholar]
- Galeev, T.K.; Bulgakov, N.N.; Savelieva, G.A.; Popova, N.M. Surface properties of platinum and palladium. React. Kinet. Catal. Lett. 1980, 14, 61–65. [Google Scholar] [CrossRef]
- Lott, P.; Dolcet, P.; Casapu, M.; Grunwaldt, J.-D.; Deutschmann, O. The Effect of Prereduction on the Performance of Pd/Al2O3 and Pd/CeO2 Catalysts during Methane Oxidation. Ind. Eng. Chem. Res. 2019, 58, 12561–12570. [Google Scholar] [CrossRef]
- Ho, Y.-S.; Wang, C.-B.; Yeh, C.-T. Calorimetric study on interaction of dioxygen with alumina supported palladium. J. Mol. Catal. A Chem. 1996, 112, 287–294. [Google Scholar] [CrossRef]
- Légaré, P.; Hilaire, L.; Maire, G.; Krill, G.; Amamou, A. Interaction of oxygen and hydrogen with palladium. Surf. Sci. 1981, 107, 533–546. [Google Scholar] [CrossRef]
- Wrobel, R.J.; Becker, S.; Weiss, H. Influence of Subsurface Oxygen in the Catalytic CO Oxidation on Pd(111). J. Phys. Chem. C 2015, 119, 5386–5394. [Google Scholar] [CrossRef]
- Phan, D.Q.; Kureti, S. CO Oxidation on Pd/Al2O3 Catalysts under Stoichiometric Conditions. Top. Catal. 2017, 60, 260–265. [Google Scholar] [CrossRef]
- Sheppard, T.L.; Price, S.W.T.; Benzi, F.; Baier, S.; Klumpp, M.; Dittmeyer, R.; Schwieger, W.; Grunwaldt, J.-D. In Situ Multimodal 3D Chemical Imaging of a Hierarchically Structured Core@Shell Catalyst. J. Am. Chem. Soc. 2017, 139, 7855–7863. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Xiong, H.; Delariva, A.T.; Peterson, E.J.; Pham, H.; Challa, S.R.; Qi, G.; Oh, S.; Wiebenga, M.H.; Pereira Hernández, X.I.; et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 2016, 353, 150–154. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalytic Properties of Ceria and CeO2-Containing Materials. Catal. Rev. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Suchorski, Y.; Wrobel, R.; Becker, S.; Weiss, H. CO Oxidation on a CeOx/Pt(111) Inverse Model Catalyst Surface: Catalytic Promotion and Tuning of Kinetic Phase Diagrams. J. Phys. Chem. C 2008, 112, 20012–20017. [Google Scholar] [CrossRef]
- Lott, P.; Eck, M.; Doronkin, D.E.; Popescu, R.; Casapu, M.; Grunwaldt, J.-D.; Deutschmann, O. Regeneration of Sulfur Poisoned Pd–Pt/CeO2–ZrO2–Y2O3–La2O3 and Pd–Pt/Al2O3 Methane Oxidation Catalysts. Top. Catal. 2019, 62, 164–171. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.; Choung, S.-J. Comparison studies on sintering phenomenon of diesel oxidation catalyst depending upon aging conditions. Catal. Today 2012, 185, 296–301. [Google Scholar] [CrossRef]

| Mean Particle Size [nm] | ||||
|---|---|---|---|---|
| Catalyst | fresh | 1 h, 750 °C | 5 h, 750 °C | 16 h, 750 °C |
| Pt/Al2O3 | 6.3 ± 2.9 1 | 12.7 ± 5.7 1 | 14.9 ± 6.2 1 | 23.4 ± 8.1 1 |
| 12.3 2 | 15.9 2 | 22.2 2 | 22.2 2 | |
| Pd/Al2O3 | 5.1 ± 2.2 1 | 13.3 ± 4.9 1 | 17.7 ± 5.5 1 | 23.5 ± 7.0 1 |
| 12.3 2 | 13.9 2 | 15.9 2 | 22.2 2 | |
| Pt-Pd/Al2O3 | 6.7 ± 2.6 1 | 12.9 ± 3.9 1 | 18.3 ± 5.2 1 | 24.7 ± 6.9 1 |
| 5.55 2 | 9.25 2 | 10.1 2 | 15.9 2 | |
| CO | NO | C3H6 | CO2 | O2 | H2O | N2 | GHSV | |
|---|---|---|---|---|---|---|---|---|
| CO light-off | 0.08 | - | - | 7 | 5 | 5 | Bal. | 80,000 h−1 |
| NO light-off | - | 0.08 | - | 7 | 5 | 5 | Bal. | 40,000 h−1 |
| Hydrothermal aging | 0.04 | - | 0.01 | 7 | 12 | 10 | Bal. | 20,000 h−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schütz, J.; Störmer, H.; Lott, P.; Deutschmann, O. Effects of Hydrothermal Aging on CO and NO Oxidation Activity over Monometallic and Bimetallic Pt-Pd Catalysts. Catalysts 2021, 11, 300. https://doi.org/10.3390/catal11030300
Schütz J, Störmer H, Lott P, Deutschmann O. Effects of Hydrothermal Aging on CO and NO Oxidation Activity over Monometallic and Bimetallic Pt-Pd Catalysts. Catalysts. 2021; 11(3):300. https://doi.org/10.3390/catal11030300
Chicago/Turabian StyleSchütz, Jochen, Heike Störmer, Patrick Lott, and Olaf Deutschmann. 2021. "Effects of Hydrothermal Aging on CO and NO Oxidation Activity over Monometallic and Bimetallic Pt-Pd Catalysts" Catalysts 11, no. 3: 300. https://doi.org/10.3390/catal11030300
APA StyleSchütz, J., Störmer, H., Lott, P., & Deutschmann, O. (2021). Effects of Hydrothermal Aging on CO and NO Oxidation Activity over Monometallic and Bimetallic Pt-Pd Catalysts. Catalysts, 11(3), 300. https://doi.org/10.3390/catal11030300






