Characterization of a Novel Thermophilic Mannanase and Synergistic Hydrolysis of Galactomannan Combined with Swollenin
Abstract
1. Introduction
2. Results
2.1. Sequence Analysis of AfMan5A
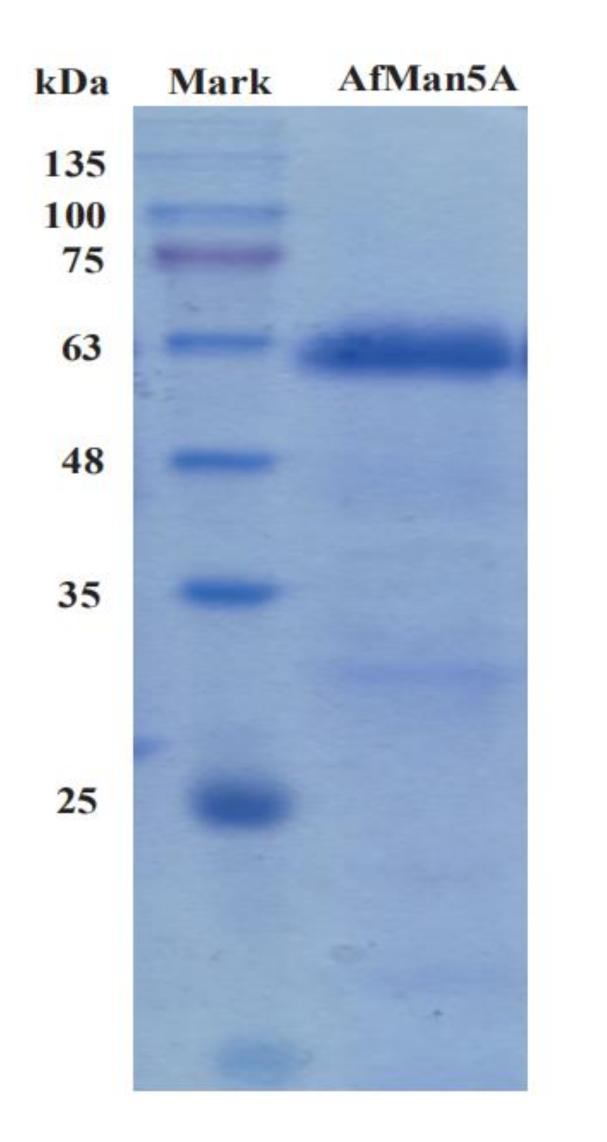
2.2. Expression and Purification of AfMan5A
2.3. Characteristics of Recombinant AfMan5A
2.4. Synergistic Action of AfMan5A and AfSowl on Galactomannan Degradation
3. Discussion
4. Materials and Methods
4.1. Strains, Vector and Medium
4.2. Genes Cloning and Bioinformatic Analysis
4.3. Heterologous Expression of AfMan5A
4.4. Purification and SDS-PAGE Analysis of Recombinant Protein
4.5. Enzyme Activity Assay
4.6. Biochemical Characterization
4.7. Synergistic Hydrolysis of Galactomannan by AfMan5A and AfSwo1
4.8. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malgas, S.; Dykan, S.J.V.; Pletschke, B.I. β-Mannanase (Man26A) and α-galactosidase (Aga27A) synergism—A key factor for the hydrolysis of galactomannan substrates. Enzym. Microb. Technol. 2017, 70, 1–8. [Google Scholar] [CrossRef]
- Terrett, O.M.; Dupree, P. Covalent interactions between lignin and hemicelluloses in plant secondary cell walls. Curr. Opin. Biotechnol. 2019, 56, 97–104. [Google Scholar] [CrossRef]
- Berglund, J.; Mikkelsen, D.; Flanagan, B.M.; Dhital, S.; Gaunitz, S.; Henriksson, G.; Lindstrom, M.E.; Yakubov, G.E.; Gidley, M.J.; Vilaplana, F. Wood hemicelluloses exert Distinct biomechanical contributions to cellulose fibrillar networks. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Jana, U.K.; Kango, N. Characteristics and bioactive properties of mannooligo saccharides derived from agro-waste manans. Int. J. Biol. Macromol. 2020, 149, 931–940. [Google Scholar] [CrossRef]
- Singh, S.; Singh, G.; Arya, S.K. Mannans: An overview of properties and application in food products. Int. J. Biol. Macromol. 2018, 119, 79–95. [Google Scholar] [CrossRef]
- Rashid, F.; Hussain, S.; Ahmed, Z. Extraction purification and characterization of galactomannan from fenugreek forindustrial utilization. Carbohyd. Polym. 2018, 180, 88–95. [Google Scholar] [CrossRef]
- Sharma, M.; Soni, R.; Nazir, A.; Oberoi, H.S.; Chadha, B.S. Evaluation of glycosyl hydrolases inthe secretome of Aspergillus fumigatus and saccharification of alkali-treated rice straw. Appl. Biochem. Biotechnol. 2011, 163, 577–591. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Kapoor, M. Production, properties, and applications of endo-beta-mannanases. Biotechnol. Adv. 2017, 35, 1–19. [Google Scholar] [CrossRef]
- Kaira, G.S.; Kapoor, M. Molecular advancements on over-expression, stability and catalytic aspects of endo-β-mannanases. Crit. Rev. Biotechnol. 2020, 1825320. [Google Scholar] [CrossRef]
- Malgas, S.; van Dyk, J.S.; Pletschke, B.I. A review of the enzymatic hydrolysis of mannans and synergistic interactions between β-mannanase, β-mannosidase and α-galactosidase. World J. Microb. Biotechnol. 2015, 31, 1167–1175. [Google Scholar] [CrossRef]
- Shrestha, S.; Fonoll, X.; Khanal, S.K.; Raskin, L. Biological strategies for enhanced hydrolysis of lignocellulosic biomass during anaerobic digestion: Current status and future perspectives. Bioresour. Technol. 2017, 245, 1245–1257. [Google Scholar] [CrossRef]
- Andberg, M.; Penttilä, M.; Saloheimo, M. Swollenin from Trichoderma reesei exhibits hydrolytic activity against cellulosic substrates with features of both endoglucanases and cellobiohydrolases. Bioresour. Technol. 2015, 181, 105–113. [Google Scholar] [CrossRef]
- Nakatani, Y.; Yamada, R.; Ogino, C.; Kondo, A. Synergetic effect of yeast cell-surface expression of cellulase and expansin-like protein on direct ethanol production from cellulose. Microb. Cell Fact. 2013, 12, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, C.; Qu, M.; Pan, K.; OuYang, K.; Song, X.; Zhao, X. Construction ancharacterization of a chimeric enzyme of swollenin and xylanase to improve soybean straw hydrolysis. Int. J. Biol. Macromol. 2020, 156, 558–564. [Google Scholar] [CrossRef]
- Meng, X.; Ma, L.; Li, T.; Zhu, H.; Guo, K.; Liu, D.; Ran, W.; Shen, Q. The functioning of a novel protein, Swollenin, in promoting the lignocellulose degradation capacity of Trichoderma guizhouense NJAU 4742 from a proteomic perspective. Bioresour. Technol. 2020, 317, 123992. [Google Scholar] [CrossRef]
- Sharma, K.; Dhillon, A.; Goyal, A. Insights into structure and reaction mechanism of β-mannanases. Curr. Protein Pept. Sci. 2018, 19, 34–47. [Google Scholar] [CrossRef]
- Bien-Cuong, D.; Thi-Thu, D.; Berrin, J.G.; Haltrich, D.; Kim-Anh, T.; Sigoillot, J.C.; Yamabhai, M. Cloning, expression in Pichia pastoris, and characterization of a thermostable GH5 mann an endo-1, 4-β-mannosidase from Aspergillus niger BK01. Microb. Cell Fact. 2009, 8, 59–70. [Google Scholar] [CrossRef]
- Luo, H.; Wang, Y.; Wang, H.; Yang, J.; Yang, Y.; Huang, H.; Yang, P.; Bai, Y.; Shi, P.; Yao, B. A novel highly acidic β-mannanase from the acidophilic fungus Bispora sp. MEY-1: Gene cloning and overexpression in Pichia pastoris. Appl. Microbiol. Biotechnol. 2009, 82, 453–461. [Google Scholar] [CrossRef]
- Rosengren, A.; Hägglund, P.; Anderson, L.; Pavon-Orozco, P.; Peterson-Wulff, R.; Nerinckx, W.; Stålbrand, H. The role of subsite +2 of the Trichodermareesei β-mannanase TrMan5A in hydrolysis and transglycosylation. Biocatal. Biotransform. 2012, 30, 338–352. [Google Scholar] [CrossRef]
- Liu, W.; Tu, T.; Gu, Y.; Wang, Y.; Zheng, F.; Zheng, J.; Wang, Y.; Su, X.; Yao, B.; Luo, H. Insight into the thermophilic mechanism of a glycoside hydrolase family 5 β-mannanase. J. Agric. Food Chem. 2018, 67, 473–483. [Google Scholar] [CrossRef]
- Karahalil, E.; Germec, M.; Karaoglan, M.; Yatmaz, E.; Coban, H.B.; Inan, M.; Turhan, I. Partial purification and characterization of a recombinant β-mannanase from Aspergillus fumigatus expressed in Aspergillus sojae grown on carob extract. Biomass Convers. Biorefin. 2019, 10, 1189–1205. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, H.; Shi, P.; Luo, H.; Wang, Y.; Yang, P.; Yao, B. A family 5 β-mannanase from the thermophilic fungus Thielavia arenaria XZ7 with typical thermophilic enzyme features. Appl. Microbiol. Biotechnol. 2013, 97, 8121–8128. [Google Scholar] [CrossRef]
- Puchart, V.; Vršanská, M.; Svoboda, P.; Pohl, J.; Ögel, Z.B.; Biely, P. Purification and characterization of two forms of endo-β-1, 4-mannanase from a thermotolerant fungus, Aspergillus fumigatus IMI 385708 (formerly Thermomyces lanuginosus IMI 158749). BBA-Gen. Subj. 2004, 1674, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Duruksu, G.; Ozturk, B.; Biely, P.; Bakir, U.; Ogel, Z.B. Cloning, expression and characterization of endo-β-1, 4-mannanase from Aspergillus fumigatus in Aspergillus sojae and Pichia pastoris. Biotechnol. Progr. 2009, 25, 271–276. [Google Scholar] [CrossRef]
- Fischer, J.E.; Glieder, A. Current advances in engineering tools for Pichia pastoris. Curr. Opin. Biotechnol. 2019, 59, 175–181. [Google Scholar] [CrossRef]
- Ismail, S.A.; Hassan, A.A.; Emran, M.A. Economic production of thermo-active endo β-mannanase for the removal of food stain and production of antioxidant manno-oligosaccharides. Biocatal. Agr. Biotechnol. 2019, 22, 101387. [Google Scholar] [CrossRef]
- Karnaouri, A.; Antonopoulou, I.; Zerva, A.; Dimarogona, M.; Topakas, E.; Rova, U.; Christakopoulos, P. Thermophilic enzyme systems for efficient conversion of lignocellulose to valuable products: Structural insights and future perspectives for esterases and oxidative catalysts. Bioresour. Technol. 2019, 279, 362–372. [Google Scholar] [CrossRef]
- Salem, R.B.; Abbassi, M.S.; Cayol, J.L.; Amel, B.; Belhadj, O. Thermophilic bacillus licheniformis rbs 5 isolated from hot tunisian spring co-producing alkaline and thermostable α-amylase and protease enzymes. J. Microbiol. Biotechnol. Food Sci. 2016, 5. [Google Scholar] [CrossRef][Green Version]
- Guo, H.; Lin, C.; Wang, S.; Jiang, D.; Zheng, B.; Liu, Y.; Qin, W. Characterization of a novel laccase-producing Bacillus sp. A4 and its application in Miscanthus degradation. BioResources 2017, 12, 4776–4794. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Y.; Liu, X.; Li, C.; Lin, Y.; Liang, S. High-Level Expression and Biochemical Properties of A Thermo-Alkaline Pectate Lyase From Bacillus sp. RN1 in Pichia pastoris With Potential in Ramie Degumming. Front. Bioeng. Biotechnol. 2020, 8, 850–860. [Google Scholar] [CrossRef]
- Malgas, S.; Pletschke, B.I. Combination of CTec2 and GH5 or GH26 En do-Mannanases for Effective Lignocellulosic Biomass Degradation. Catalysts 2020, 10, 1193. [Google Scholar] [CrossRef]
- Yan, Z.; Ming-Xiong, H.; Bo, W.; Qi-Chun, H.; Qing, L.; Jian, Z. Recombinant EXLX1 from Bacillus subtilis for enhancing enzymatic hydrolysis of corn stover with low cellulase loadings. Afr. J. Biotechnol. 2012, 11, 11126–11131. [Google Scholar]
- Yang, X.; Shi, P.; Huang, H.; Luo, H.; Wang, Y.; Zhang, W.; Yao, B. Two xylose-tolerant GH43 bifunctional β-xylosidase/α-arabinosidases and one GH11 xylanase from Humicola insolens and their synergy in the degradation of xylan. Food Chem. 2014, 148, 381–387. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Kim, M. An overview of techniques in enzyme immobilization. Appl. Sci. Converg. Technol. 2017, 26, 157–163. [Google Scholar] [CrossRef]
- Taheri-Kafrani, A.; Kharazmi, S.; Nasrollahzadeh, M.; Soozanipour, A.; Ejeian, F.; Etedali, P.; Mansouri-Tehrani, H.A.; Razmjou, A.; Yek, S.M.G.; Varma, R.S. Recent developments in immobilization technology for high-throughput processing in food industries. Crit. Rev. Food Sci. 2020, 1–37. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, M.C.; Liu, R.L. Recent Developments in Carriers and Non-Aqueous Solvents for Enzyme Immobilization. Catalysts 2019, 9, 647–661. [Google Scholar] [CrossRef]
- Dal Magro, L.; Kornecki, J.F.; Klein, M.P.; Rodrigues, R.C.; Fernandez-Lafuente, R. Pectin lyase immobilization using the glutaraldehyde chemistry increases the enzyme operation range. Enzym. Microb. Technol. 2020, 132, 109397. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]


| Metal Ions and Chemicals | Relative Activity (%) a | |
|---|---|---|
| 1 mM | 5 mM | |
| None | 100.0 ± 2.4 | 100.0 ± 1.9 |
| Fe3+ | 83.8 ± 4.2 | 97.9 ± 3.7 |
| Cu2+ | 119.7 ± 1.5 | 137.6 ± 4.8 |
| K+ | 41.2 ± 2.0 | 109.6 ± 3.1 |
| Li+ | 99.7 ± 3.1 | 90.0 ± 4.9 |
| Na+ | 96.8 ± 3.2 | 106.5 ± 0.3 |
| Zn2+ | 108.5 ± 7.1 | 109.1 ± 2.0 |
| Mn2+ | 79.3 ± 4.7 | 13.0 ± 5.0 |
| Mg2+ | 75.5 ± 0.8 | 112.1 ± 1.2 |
| Ca2+ | 35.8 ± 7.9 | 82.6 ± 0.7 |
| EDTA | 49.4 ± 5.8 | 106.7 ± 0.4 |
| SDS | 0 ± 7.4 | 63.6 ± 5.8 |
| Enzyme Added | Reducing Sugar Content (µ mol) | Synergistic Effect (Degree of Synergy (DS)) | |
|---|---|---|---|
| First Enzyme | Second Enzyme | ||
| AfMan5A | None | 0.458 | None |
| AfSwol | None | None | None |
| AfMan5A | AfSwol | 0.600 | 1.31 |
| AfSwol | AfMan5A | 0.581 | 1.26 |
| AfMan5A + AfSwol | None | 0.516 | 1.13 |
| Primers | Sequences (5′→3′) a | Size (bp) | Tm |
|---|---|---|---|
| AfMan5A-F | ATGAAGTTCTCCTGGCTCAC | 20 | 56 |
| AfMan5A-R | CTAAACCCACCCCTGCTTC | 19 | 56 |
| AfMan5A-PF | CGGAATTCGCCCCCAGCGCAAAG | 23 | 62 |
| AfMan5A-PR | AGAATGCGGCCGCAACCCACCCCTGCTTC | 29 | 56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, X.; Lu, H.; Chen, W.; Meng, X. Characterization of a Novel Thermophilic Mannanase and Synergistic Hydrolysis of Galactomannan Combined with Swollenin. Catalysts 2021, 11, 254. https://doi.org/10.3390/catal11020254
Gu X, Lu H, Chen W, Meng X. Characterization of a Novel Thermophilic Mannanase and Synergistic Hydrolysis of Galactomannan Combined with Swollenin. Catalysts. 2021; 11(2):254. https://doi.org/10.3390/catal11020254
Chicago/Turabian StyleGu, Xinxi, Haiqiang Lu, Wei Chen, and Xiangchen Meng. 2021. "Characterization of a Novel Thermophilic Mannanase and Synergistic Hydrolysis of Galactomannan Combined with Swollenin" Catalysts 11, no. 2: 254. https://doi.org/10.3390/catal11020254
APA StyleGu, X., Lu, H., Chen, W., & Meng, X. (2021). Characterization of a Novel Thermophilic Mannanase and Synergistic Hydrolysis of Galactomannan Combined with Swollenin. Catalysts, 11(2), 254. https://doi.org/10.3390/catal11020254





