Photoelectrocatalytic Degradation of Congo Red Dye with Activated Hydrotalcites and Copper Anode
Abstract
1. Introduction
2. Results and Discussion
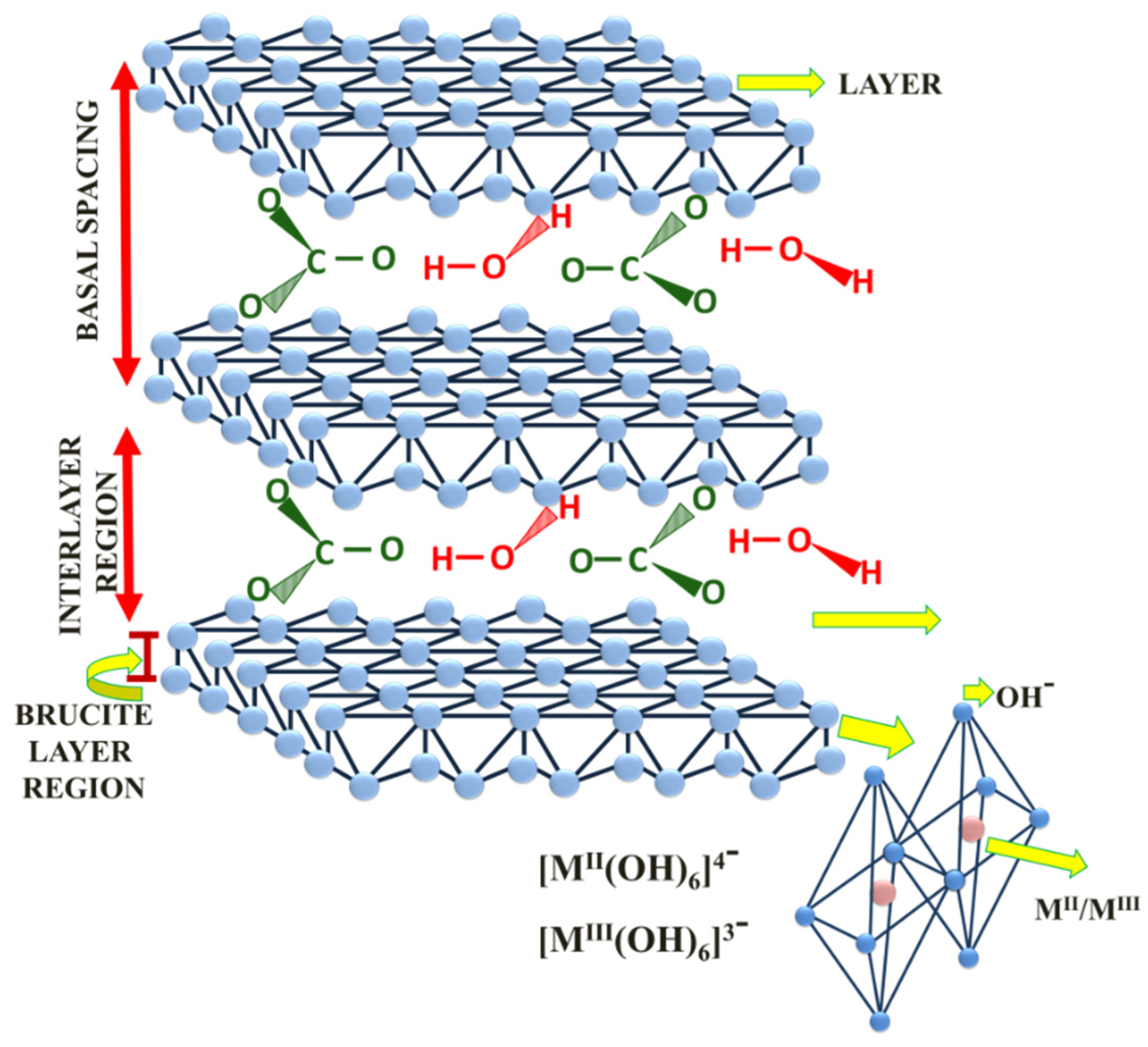
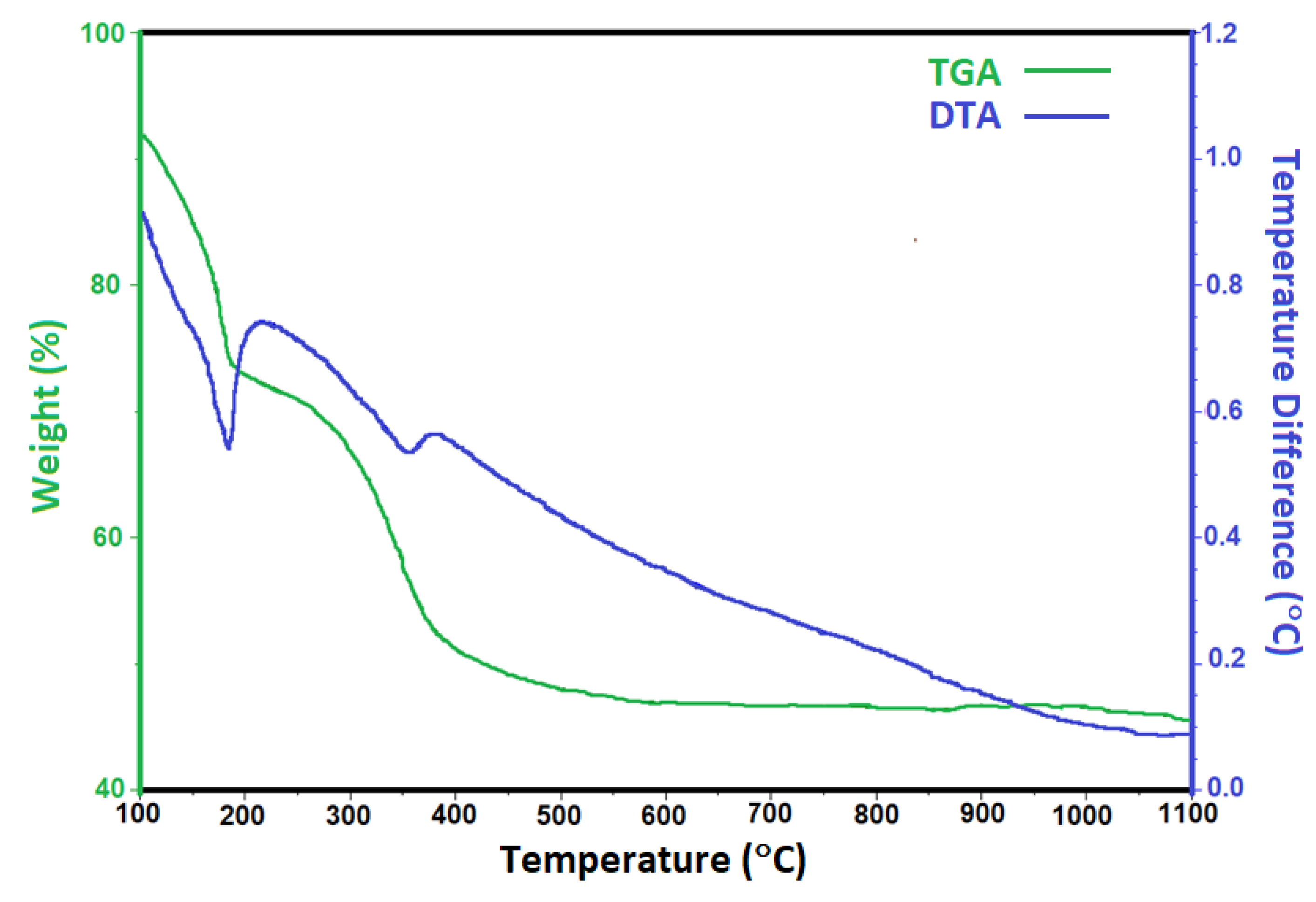
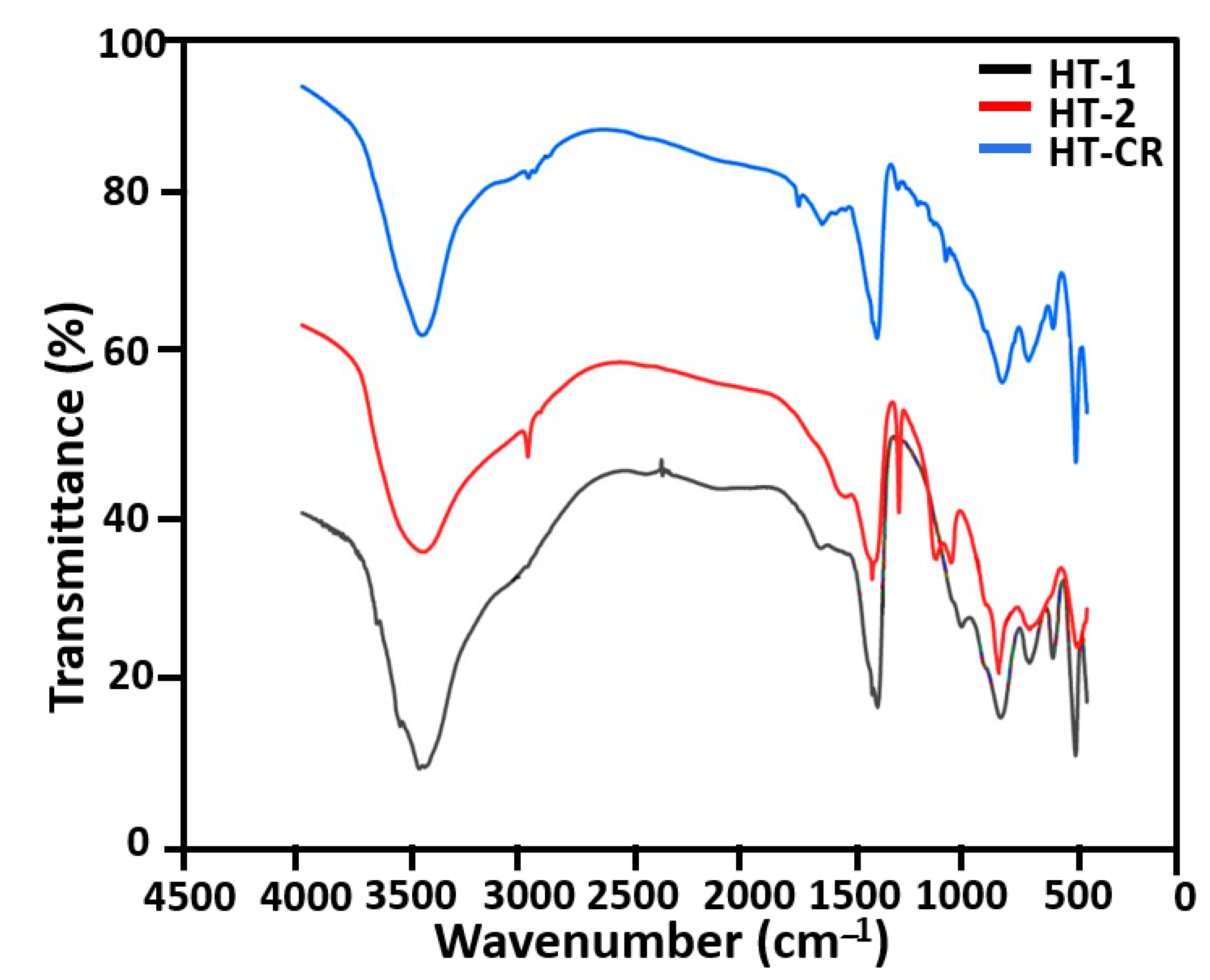
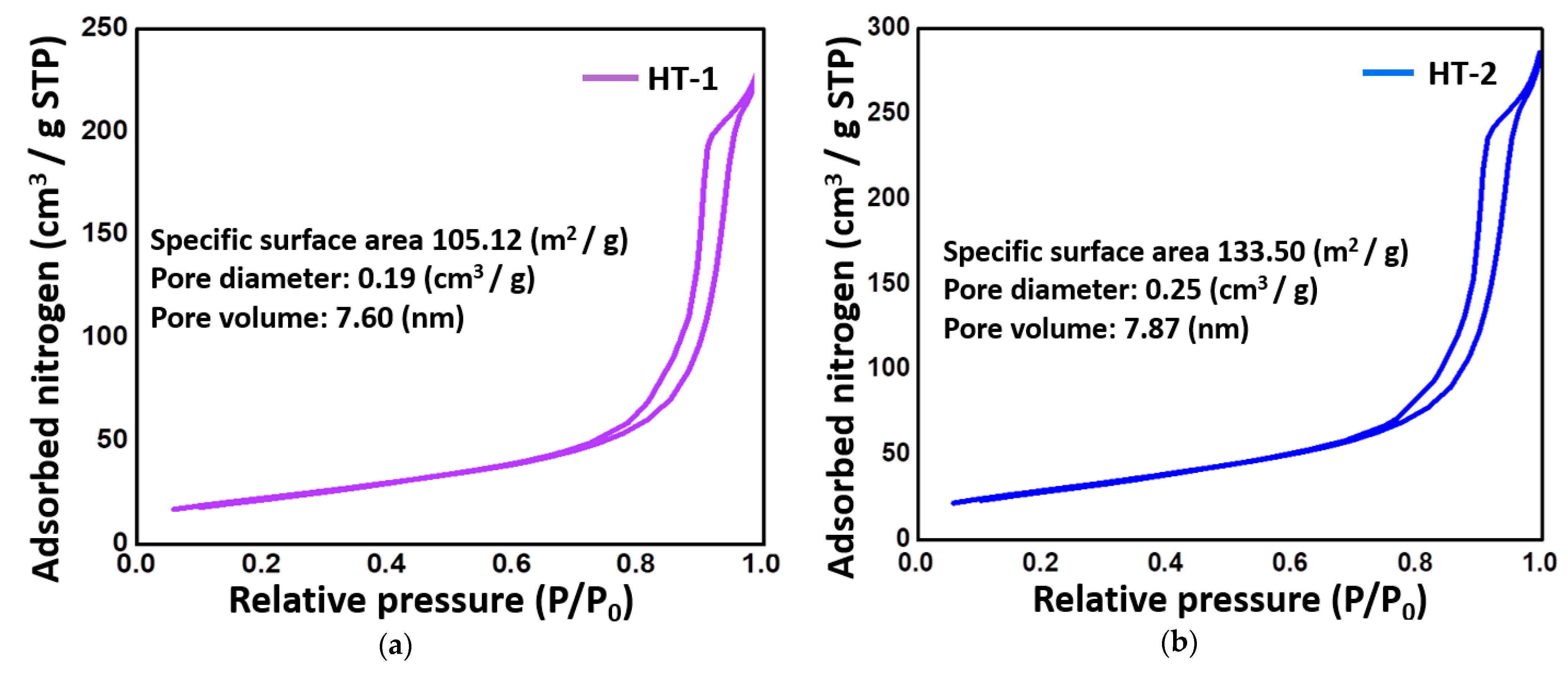
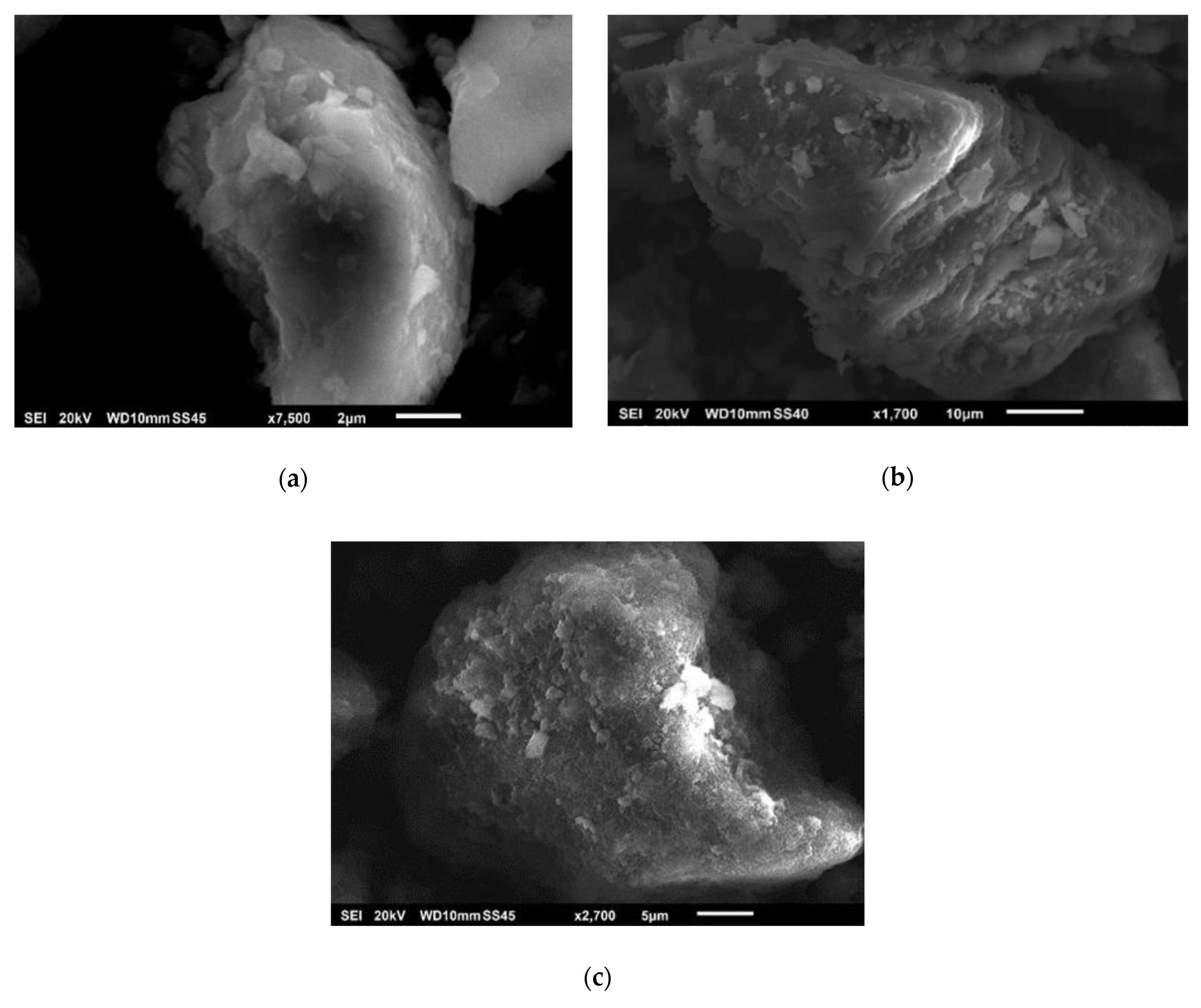
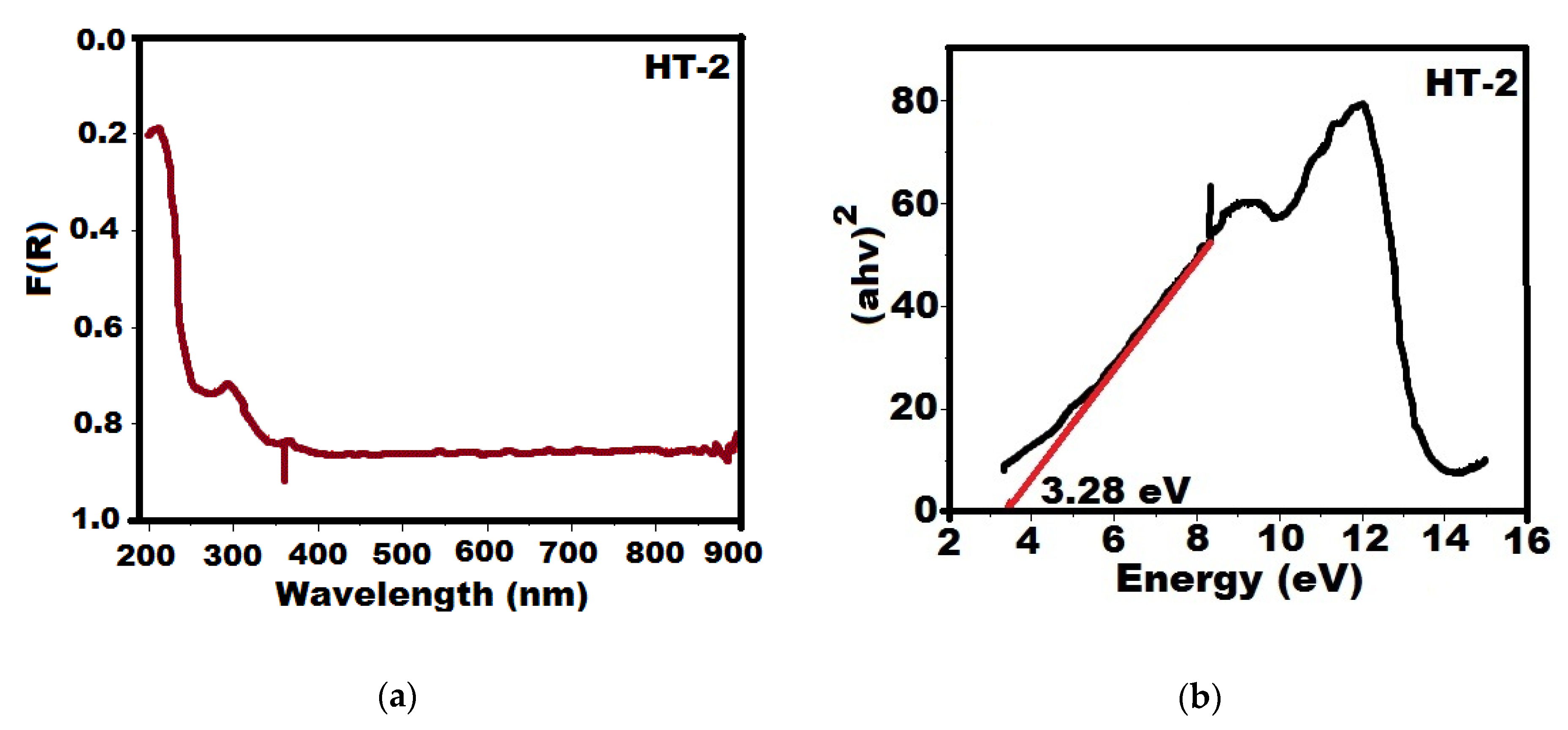
2.1. Characterization of Catalytic Precursors of the Hydrotalcites
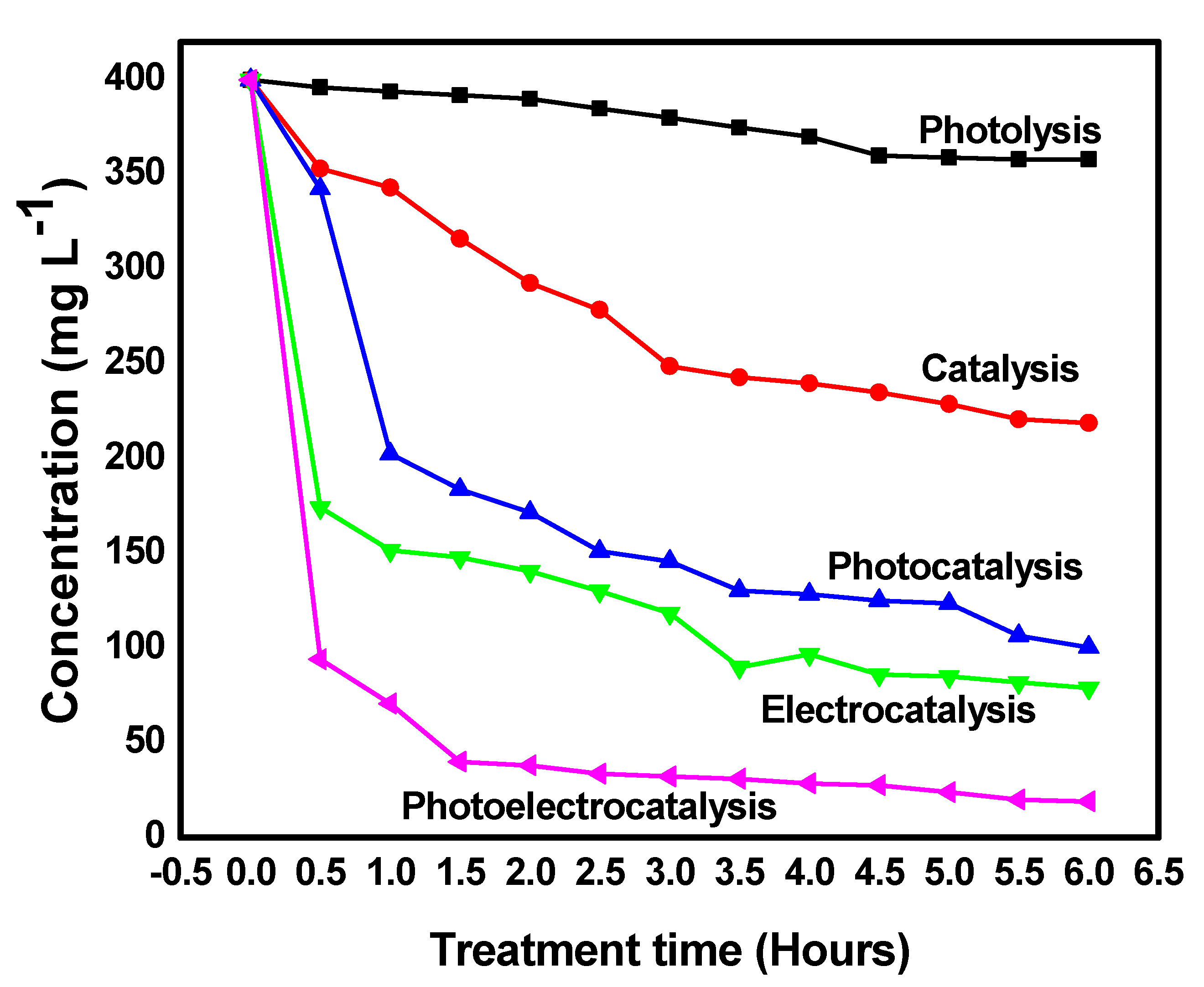
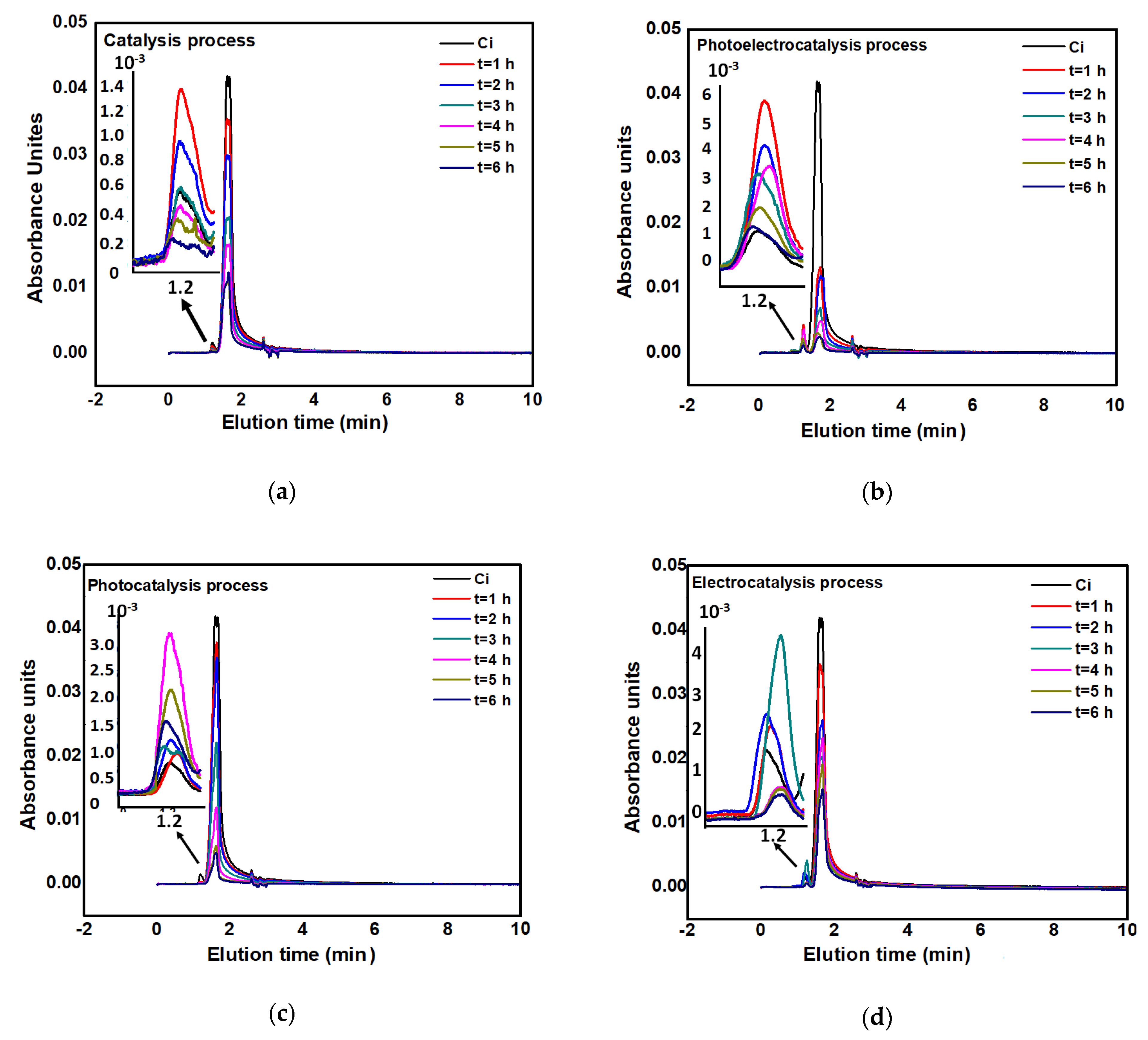
2.2. Evaluation of the Degradation Capacity of the Congo Red Dye by Photolysis, Catalysis, Photocatalysis, Electrocatalysis, and Photoelectrocatalysis
3. Materials and Methods
3.1. Obtaining the Catalytic Precursors of the Hydrotalcites
3.2. Structural, Thermal, and Textural Characterization of Hydrotalcite-type Catalytic Precursors
3.3. Optimization of the Analytical Method for the Quantification of the Degradation of the Congo Red Dye
3.4. Evaluation of the Degradation Capacity of Congo Red Dye by Photolysis, Catalysis, Photocatalysis, Electrocatalysis, and Photoelectrocatalysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- El Gaini, L.; Lakraimi, M.; Sebbar, E.; Meghea, A.; Bakasse, M. Removal of indigo carmine dye from water to Mg–Al–CO3-calcined layered double hydroxides. J. Hazard. Mater. 2009, 161, 627–632. [Google Scholar] [CrossRef]
- Chen, F.; Wu, X.; Bu, R.; Yang, F. Co–Fe hydrotalcites for efficient removal of dye pollutants via synergistic adsorption and degradation. RSC Adv. 2017, 7, 41945–41954. [Google Scholar] [CrossRef]
- Van Der Zee, F.P.; Font, J.; Fortuny, A.; Fabregat, A. Towards advanced aqueous dye removal processes: A short review on the versatile role of activated carbon. J. Environ. Manag. 2012, 102, 148–160. [Google Scholar]
- Guzmán-Vargas, A.; Limb, E.; Uriostegui-Ortega, G.A.; Oliver-Tolentino, M.A.; Rodríguez, E.E. Adsorption and subsequent partial photodegradation of methyl violet 2B on Cu/Al layered double hydroxides. Appl. Surf. Sci. 2016, 363, 372–380. [Google Scholar] [CrossRef]
- Bilińska, L.; Gmurek, M.; Ledakowicz, S. Comparison between industrial and simulated textile wastewater treatment by AOPs—Biodegradability, toxicity and cost assessment. Chem. Eng. J. 2016, 306, 550–559. [Google Scholar] [CrossRef]
- Ersoz, G.; Napoleoni, A.; Atalay, S. Comparative study using chemical wetoxidation for removal of reactive black 5 in the presence of activated carbon. J. Environ. Eng. 2013, 139, 1462–1469. [Google Scholar] [CrossRef]
- Li, X.L.; Zhang, J.; Jiang, Y.C.; Hu, M.C.; Li, S.N.; Zhai, Q.G. Highly efficient biodecolorization/degradation of Congo red and alizarin yellow R bychloroperoxidase from Caldariomyces fumago: Catalytic mechanism anddegradation pathway. Ind. Eng. Chem. Res. 2013, 52, 13572–13579. [Google Scholar] [CrossRef]
- Wang, Y.S.; Shen, J.H.; Horng, J.J. Chromate enhanced visible light driven TiO2 photocatalytic mechanism on acid orange 7 photodegradation. J. Hazard. Mater. 2014, 274, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Erdemoglu, S.; Aksu, S.K.; Sayilkan, F.; Izgi, B.; Asilturk, M.; Sayılkan, H.; Frimmel, F.; Gucer, S. Photocatalytic degradation of Congo red by hydrothermally synthesized nanocrystalline TiO2 and identification of degradation products by LC–MS. J. Hazard. Mater. 2008, 155, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Starukh, G. Photocatalytically Enhanced Cationic Dye Removal with Zn-Al Layered Double Hydroxides. Nanoscale Res. Lett. 2017, 12, 391. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E. On the Origin of Enhanced Photocatalytic Activity of Copper-Modified Titania in the Oxidative Reaction Systems. Catalysts 2017, 7, 317. [Google Scholar] [CrossRef]
- Dorian, A.H.H.; Sorrell, C.C. Sand supported mixed-phase TiO2 photocatalysts for water decontamination applications. Adv. Eng. Mater. 2014, 16, 248–254. [Google Scholar]
- Pattnaik, S.P.; Behera, A.; Acharya, R.; Parida, K. Green exfoliation of graphitic carbon nitride towards decolourization of Congo-Red under solar irradiation. J. Environ. Chem. Eng. 2019, 7, 103456. [Google Scholar] [CrossRef]
- Kumru, B.; Antonietti, M. Colloidal properties of the metal-free semiconductor graphitic carbon nitride. Adv. Colloid Interfac. Sci. 2020, 283, 102229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic carbon nitridebased nanocomposites: A review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef]
- Hieu-Nguyen, C.; Chun-Chieh, F.; Ruey-Shin, J. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Kushwaha, H.S.; Vaish, R. Enhanced visible light photocatalytic activity of curcumin-sensitized perovskite Bi0.5Na0.5TiO3 for rhodamine 6G degradation. Int. J. Appl. Ceram. Technol. 2016, 13, 333–339. [Google Scholar] [CrossRef]
- Subhan, M.A.; Saha, P.C.; Uddin, N.; Sarker, P. Synthesis, structure, spectroscopy and photocatalytic studies of nano multimetal oxide MgO·Al2O3 ·ZnO and MgO·Al2O3 ·ZnO curcumin composite. Int. J. NanoSci. NanoTechnol. 2017, 13, 69–82. [Google Scholar]
- Li, Y.; Cui, W.; Liu, L.; Zong, R.; Yao, W.; Liang, Y.; Zhu, Y. Removal of Cr(VI) by 3D TiO2 -graphene hydrogel via adsorption enriched with photocatalytic reduction. Appl. Catal. B Environ. 2016, 199, 412–423. [Google Scholar] [CrossRef]
- Arotiba, O.A.; Orimolade, B.O.; Koiki, B.A. Visible light–driven photoelectrocatalytic semiconductor heterojunction anodes for water treatment applications. Curr. Opin. Electrochem. 2020, 22, 25–34. [Google Scholar] [CrossRef]
- Turolla, A.; Bestetti, M.; Antonelli, M. Optimization of heterogeneous photoelectrocatalysis on nanotubular TiO2 electrodes: Reactor configuration and kinetic modelling. Chem. Eng. Sci. 2018, 182, 171–179. [Google Scholar] [CrossRef]
- Figueras, F. Base Catalysis in the Synthesis of Fine Chemicals. Top. Catal. 2004, 29, 189–196. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–302. [Google Scholar] [CrossRef]
- Rives, V. Layered Double Hydroxides: Present and Future, 1st ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2001; pp. 9–321. [Google Scholar]
- Timar, Z.; Varga, G.; Murath, S. Synthesis, characterization and photocatalytic activity of crystalline Mn(II) Cr(III)-layered double hydroxide. Catal. Today 2017, 284, 195–201. [Google Scholar] [CrossRef]
- Ballarin, B.; Mignani, A.; Scavetta, E.; Giorgetti, M.; Tonelli, D.; Boanini, E.; Mousty, C. Ruta de síntesis para hidróxidos dobles en capas de nanopartículas de oro soportadas como catalizadores eficaces en la electro oxidación del metanol. Langmuir 2012, 28, 15065–15074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, H.; Azat, S.; Mansurov, Z.; Liu, X.; Wang, J.; Su, X.; Wu, R. Super adsorption capability of rhombic dodecahedral Ca-Al layered double oxides for Congo red removal. J. Alloy. Comp. 2018, 768, 572–581. [Google Scholar] [CrossRef]
- Ramos-Ramírez, E.; Tzompantzi-Morales, F.; Gutiérrez-Ortega, N.; Mojica-Calvillo, H.G.; Castillo-Rodríguez, J. Photocatalytic Degradation of 2,4,6-Trichlorophenol by MgO–MgFe2O4 Derived from Layered Double Hydroxide Structures. Catalysts 2019, 9, 454. [Google Scholar] [CrossRef]
- Ramos-Ramírez, E.; Gutiérrez-Ortega, N.L.; Tzompantzi-Morales, F.; Barrera-Rodríguez, A.; Castillo-Rodríguez, J.C.; Tzompantzi-Flores, C.; Guevara-Hornedo, M.P. Photocatalytic Degradation of 2,4-Dichlorophenol on NiAl-Mixed Oxides Derivatives of Activated Layered Double Hydroxides. Top. Catal. 2020, 63, 546–563. [Google Scholar] [CrossRef]
- Jawad, A.; Peng, L.; Liao, Z.; Zhou, Z.; Shahzad, A.; Ifthikar, J.; Chen, Z. Selective removal of heavy metals by hydrotalcites as adsorbents in diverse wastewater: Different intercalated anions with different mechanisms. J. Clean. Prod. 2018, 211, 1112–1126. [Google Scholar] [CrossRef]
- Setshedi, K.; Ren, J.; Aoyi, O.; Onyango, M.S. Removal of Pb(II) from aqueous solution using hydrotalcite-like nanostructured material. Int. J. Phys. Sci. 2012, 7, 63–72. [Google Scholar]
- Dias, A.C.; Fontes, M.P.F. Arsenic (V) removal from water using hydrotalcites as adsorbents: A critical review. Appl. Clay. Sci. 2020, 191, 105615. [Google Scholar] [CrossRef]
- Nong, L.; Xiao, C.; Jiang, W. Azo dye removal from aqueous solution by organic-inorganic hybrid dodecanoic acid modified layered Mg-Al hydrotalcite. Korean J. Chem. Eng. 2011, 28, 933–938. [Google Scholar] [CrossRef]
- Mustapha, M.; Derriche, Z.; Denoyel, R.; Prevot, V.; Forano, C. Thermodynamical and structural insights of orange II adsorption by MgRAlNO3 layered double hydroxides. J. Solid State Chem. 2011, 184, 1016–1024. [Google Scholar] [CrossRef]
- Sels, B.F.; de Vos, D.E.; Jacobs, P.A. Hydrotalcite-like anionic clays in catalytic organic reactions. Catal. Rev. 2001, 43, 443–488. [Google Scholar] [CrossRef]
- Shakeel, M.; Arif, M.; Yasin, G.; Li, B.; Khan, H.D. Layered by Layered Ni-Mn-LDH/g-C3N4 Nanohybrid for Multi-Purpose Photo/electrocatalysis: Morphology Controlled Strategy for Effective Charge Carriers Separation. Appl. Catal. B 2018, 242, 485–498. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Z.; Wang, L.; Qin, H.; Tong, M. Synthesis of Hollow Spherical Zinc-Aluminum Hydrotalcite and Its Application as Zinc Anode Material. J. Electrochem. Soc. 2019, 166, A2589–A2596. [Google Scholar] [CrossRef]
- Pan, L.; Huang, H.; Niederberger, M. Layered cobalt hydrotalcite as an advanced lithium-ion anode material with high capacity and rate capability. J. Mater. Chem. A 2019, 7, 21264–21269. [Google Scholar] [CrossRef]
- Zhou, F.; Pan, N.; Chen, H.; Xu, X.; Wang, C.; Du, Y.; Li, L. Hydrogen production through steam reforming of toluene over Ce, Zr or Fe promoted Ni-Mg-Al hydrotalcite-derived catalysts at low temperature. Energy Convers. Manag. 2019, 196, 677–687. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Brillas, E. Applied photoelectrocatalysis on the degradation of organicpollutants in wastewaters. J. PhotoChem. Photobiol. C 2017, 31, 1–35. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, W.; An, W.; Liu, L.; Liang, Y.; Zhu, Y. Combination of photoelectrocatalysis and adsorption for removal of bisphenol A over TiO2-graphene hydrogel with 3D network structure. Appl. Catal. B 2018, 221, 36–46. [Google Scholar] [CrossRef]
- Ghenaatgar, A.; Tehrani, R.M.A.; Khadir, A. Photocatalytic degradation and mineralization of dexamethasone using WO3 and ZrO2 nanoparticles: Optimization of operational parameters and kinetic studies. J. Water Process Eng. 2019, 32, 100969. [Google Scholar] [CrossRef]
- Tavakoli-Azar, T.; Reza-Mahjoub, A.; Seyed-Sadjadi, M.; Farhadyar, N.; Hossaini-Sadr, M. Improving the photocatalytic performance of a perovskite ZnTiO3 through ZnTiO3@S nanocomposites for degradation of Crystal violet and Rhodamine B pollutants under sunlight. Inorg. Chem. Commun. 2020, 119, 108091. [Google Scholar] [CrossRef]
- Kamarudin, N.S.; Jusoh, R.; Jalil, A.A.; Setiabudi, H.D.; Sukora, N.F. Synthesis of silver nanoparticles in green binarysolvent for degradation of 2,4-D herbicide:Optimization and kinetic studies Faculty. Chem. Eng. Res. Des. 2020, 159, 300–314. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, T.; Chen, W.; Xu, H.; Tao, H. Degradation kinetics, byproducts formation and estimated toxicity of metronidazole (MNZ) during chlor(am)ination. Chemosphere 2019, 235, 21–31. [Google Scholar] [CrossRef]
- Tantawi, O.; Baalbaki, A.; El Asmar, R.; Ghauch, A. A rapid and economical method for the quantification of hydrogen peroxide (H2O2) using a modified HPLC apparatus. Sci. Total Environ. 2019, 654, 107–117. [Google Scholar] [CrossRef]
- Pinkernell, U.; Effkemann, S.; Karst, U. Simultaneous HPLC Determination of Peroxyacetic Acid and Hydrogen Peroxide. Anal. Chem. 1997, 69, 3623–3627. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, L.; Chen, B.; Zhang, Q.; Zhu, R. Detecting hydrogen peroxide reliably in water by ion chromatography: A method evaluation update and comparison in the presence of interfering components. Environ. Sci. Water Res. Technol. 2020, 6, 2396–2404. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Li, F.; Zhao, Y.; Liu, Y.; Hao, Y.; Liu, R.; Zhao, D. Solution combustion synthesis and visible light-induced photocatalytic activity of mixed amorphous and crystalline MgAl2O4 nanopowders. Chem. Eng. J. 2011, 173, 750–759. [Google Scholar] [CrossRef]
- Sánchez-Cantú, M.; Galicia-Aguilar, J.A.; Santamaría-Juárez, D.; Hernández-Moreno, L.E. Evaluation of the mixed oxides produced from hydrotalcite-like compound’s thermal treatment in arsenic uptake. Appl. Clay. Sci. 2016, 121–122, 146–153. [Google Scholar]
- Nassar, M.Y.; Ahmed, I.S.; Samir, I. A novel synthetic route for magnesium aluminate (MgAl2O4) nanoparticles using sol–gel auto combustion method and their photocatalytic properties. Spectrochim. Acta A 2014, 131, 329–334. [Google Scholar] [CrossRef]
- Bard, A.J.; Parsons, R.; Jordan, J. Standard Potentials in Aqueous Solutions; Marcel Dekker: New York, NY, USA, 1985. [Google Scholar]
- Araña, J.; Peña Alonso, A.; Doña Rodríguez, J.M.; Herrera Melián, J.A.; González Díaz, O.; Pérez Peña, J. Comparative study of MTBE photocatalytic degradation with TiO2 and Cu-TiO2. Appl. Catal. B 2008, 78, 355–363. [Google Scholar] [CrossRef]
- Irie, H.; Kamiya, K.; Shibanuma, T.; Miura, S.; Tryk, D.A.; Yokoyama, T.; Hashimoto, K. Visible light-sensitive Cu(II)-grafted TiO2 photocatalysts: Activities and X-ray absorption fine structure analyses. J. Phys. Chem. C 2009, 113, 10761–10766. [Google Scholar] [CrossRef]
- Jalife-Jacobo, H.; Feria-Reyes, R.; Serrano-Torres, O.; Gutiérrez-Granados, S.; Peralta-Hernández, J.M. Diazo dye Congo red degradation using a Boron-doped diamond anode: An experimental study on the effect of supporting electrolytes. J. Hazard. Mater. 2016, 319, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Naikoo, G.A.; Sheikh, M.U.D.; Bano, M.; Khan, F. Effective photocatalytic degradation of Congo red dye using alginate/carboxymethyl cellulose/TiO2 nanocomposite hydrogel under direct sunlight irradiation. J. Photochem. Photobiol. A 2016, 327, 33–43. [Google Scholar] [CrossRef]
- Bhagwat, U.O.; Wu, J.J.; Asiri, A.M.; Anandan, S. Sonochemical Synthesis of Mg-TiO2 nanoparticles for persistent Congo red dye degradation. J. PhotoChem. Photobiol. A 2017, 346, 559–569. [Google Scholar] [CrossRef]
- Ullah, I.; Haider, A.; Khalid, N.; Ali, S.; Ahmed, S.; Khan, Y.; Ahmed, N.; Zubair, M. Tuning the band gap of TiO2 by tungsten doping for efficient UV and visible photodegradation of Congo red dye. Spectrochim. Acta A 2018, 204, 150–157. [Google Scholar] [CrossRef]
- Curkovic, L.; Ljubas, D.; Juretic, H. Photocatalytic decolorization kinetics of diazo dye Congo red aqueous solution by UV/TiO2 nanoparticles. React. Kinet. Mech. Catal. 2010, 99, 201–208. [Google Scholar]



| Process | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|
| K (h−1) | t1/2 (h) | R2 | K (L·mg−1·h−1) | t1/2 (h) | R2 | |
| Photolysis | 0.0212 | 32.69 | 0.9638 | 0.00006 | 41.67 | 0.9612 |
| Catalysis | 0.0990 | 7.00 | 0.9343 | 0.0004 | 6.25 | 0.9594 |
| Photocatalysis | 0.1975 | 3.51 | 0.8421 | 0.0011 | 2.27 | 0.9448 |
| Electrocatalysis | 0.2027 | 3.42 | 0.7876 | 0.0015 | 1.67 | 0.9226 |
| Photoelectrocatalysis | 0.3515 | 1.97 | 0.7016 | 0.0072 | 0.35 | 0.9457 |
| Photocatalyst | Congo Red Concentration (mg/L) | Catalyst Concentration (g/L) | Time (h) | Removal (%) | Irradiation Source | Reference |
|---|---|---|---|---|---|---|
| Nanocrystalline TiO2 Degussa P-25 | 20 | 0.25 | 0.5 | 98 | Suntest 675 Wm−2 | [10] |
| Mg-TiO2-P25 NPs | 7 | 0.5 | 7 | 80 | 400 nm vis lamp | [60] |
| TiO-W | 30 | 0.15 | 0.83 | 96 | 365 nm vis lamp | [61] |
| TiO2 | 55 | 1.0 | 8 | 20 | 254 nm UV lamp | [62] |
| Photoelectrocatalysis | 400 | 0.66 | 1 | 91 | 254 nm UV lamp | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argote-Fuentes, S.; Feria-Reyes, R.; Ramos-Ramírez, E.; Gutiérrez-Ortega, N.; Cruz-Jiménez, G. Photoelectrocatalytic Degradation of Congo Red Dye with Activated Hydrotalcites and Copper Anode. Catalysts 2021, 11, 211. https://doi.org/10.3390/catal11020211
Argote-Fuentes S, Feria-Reyes R, Ramos-Ramírez E, Gutiérrez-Ortega N, Cruz-Jiménez G. Photoelectrocatalytic Degradation of Congo Red Dye with Activated Hydrotalcites and Copper Anode. Catalysts. 2021; 11(2):211. https://doi.org/10.3390/catal11020211
Chicago/Turabian StyleArgote-Fuentes, Sara, Rossy Feria-Reyes, Esthela Ramos-Ramírez, Norma Gutiérrez-Ortega, and Gustavo Cruz-Jiménez. 2021. "Photoelectrocatalytic Degradation of Congo Red Dye with Activated Hydrotalcites and Copper Anode" Catalysts 11, no. 2: 211. https://doi.org/10.3390/catal11020211
APA StyleArgote-Fuentes, S., Feria-Reyes, R., Ramos-Ramírez, E., Gutiérrez-Ortega, N., & Cruz-Jiménez, G. (2021). Photoelectrocatalytic Degradation of Congo Red Dye with Activated Hydrotalcites and Copper Anode. Catalysts, 11(2), 211. https://doi.org/10.3390/catal11020211






