Low Temperature Water-Gas Shift: Enhancing Stability through Optimizing Rb Loading on Pt/ZrO2
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
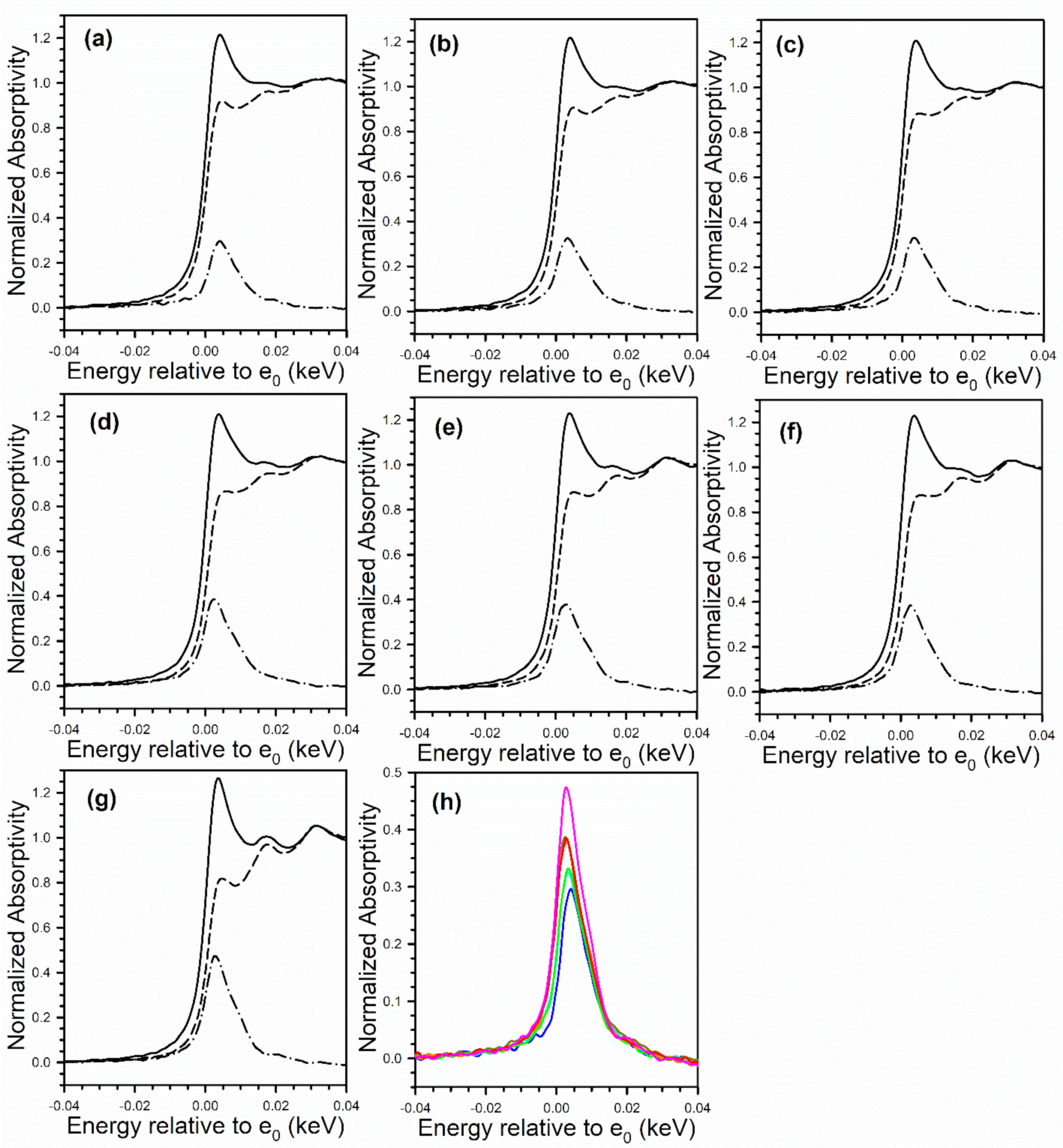
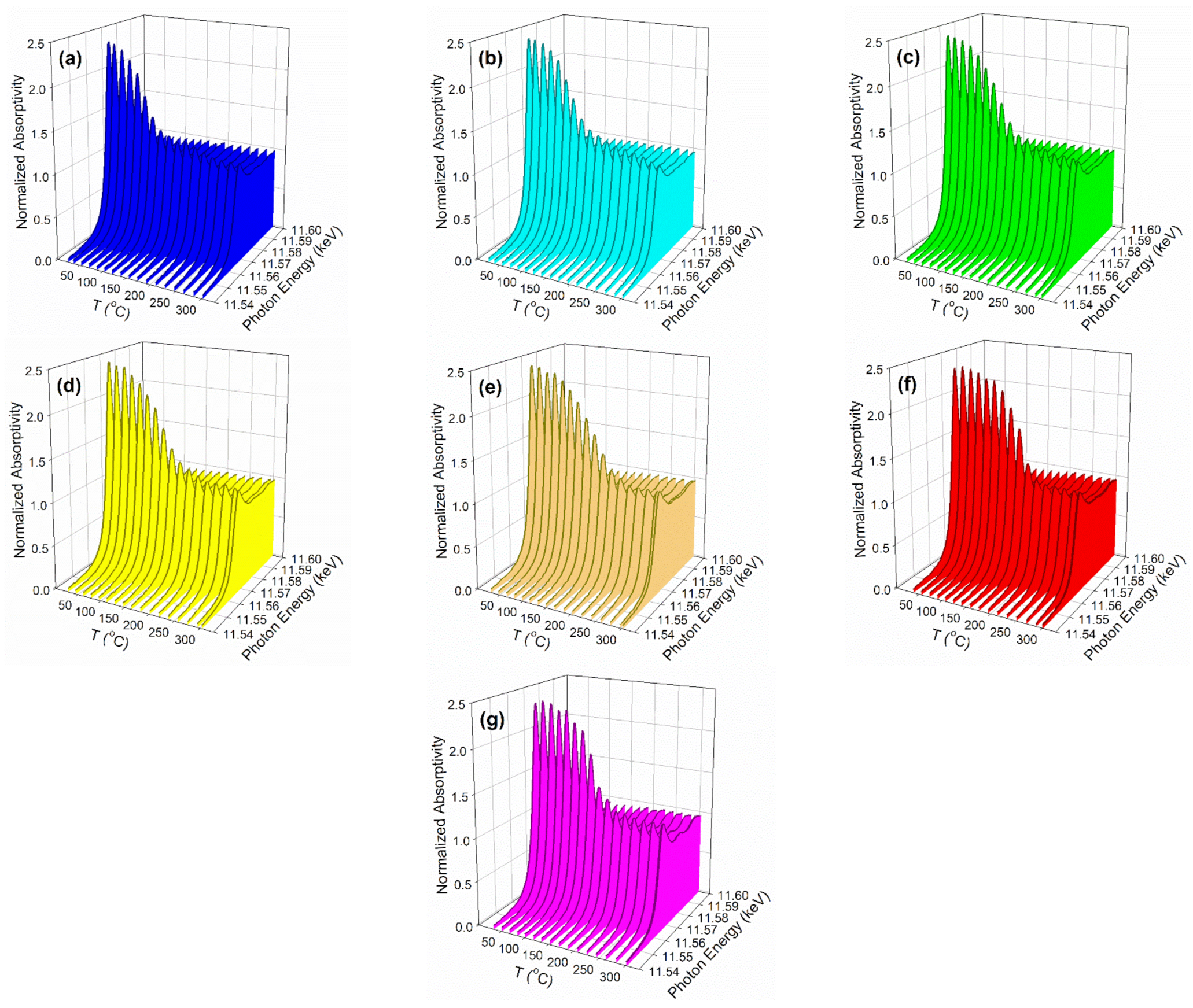
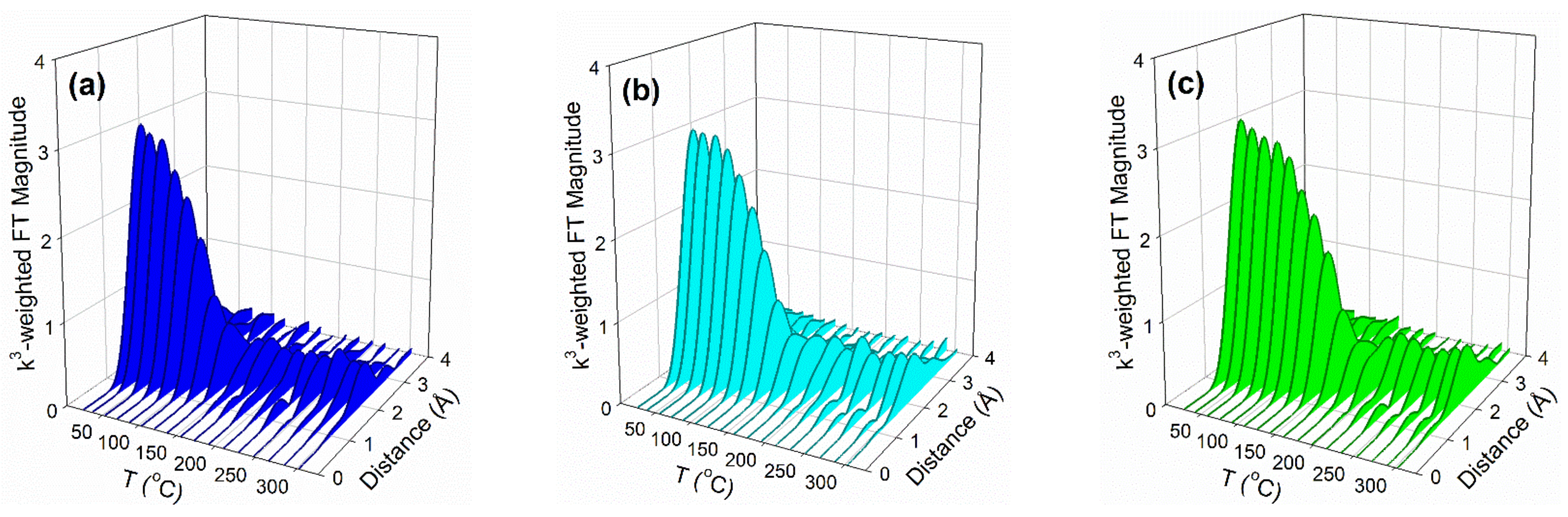
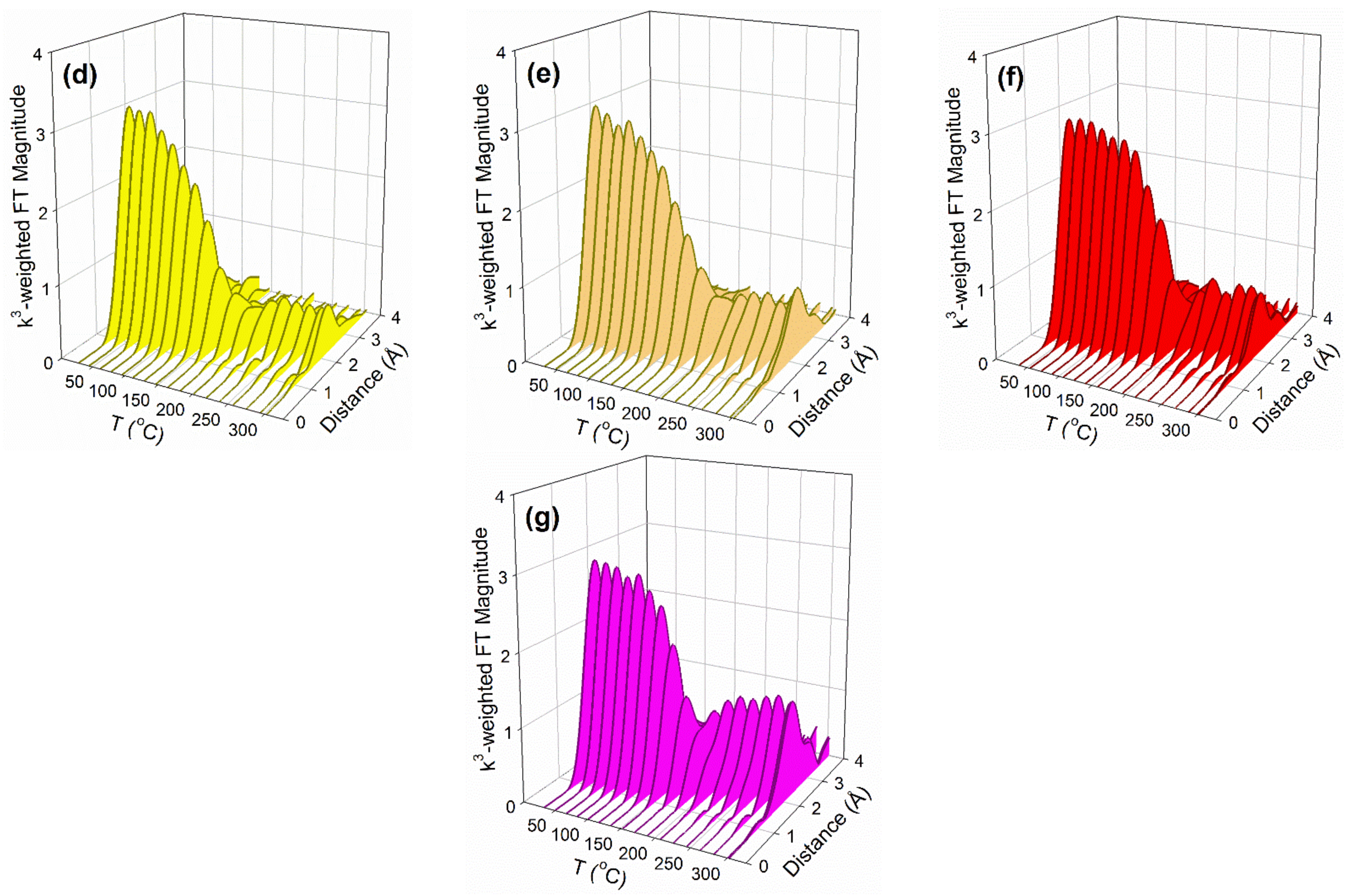
2.2. X-ray Absorption Spectroscopy
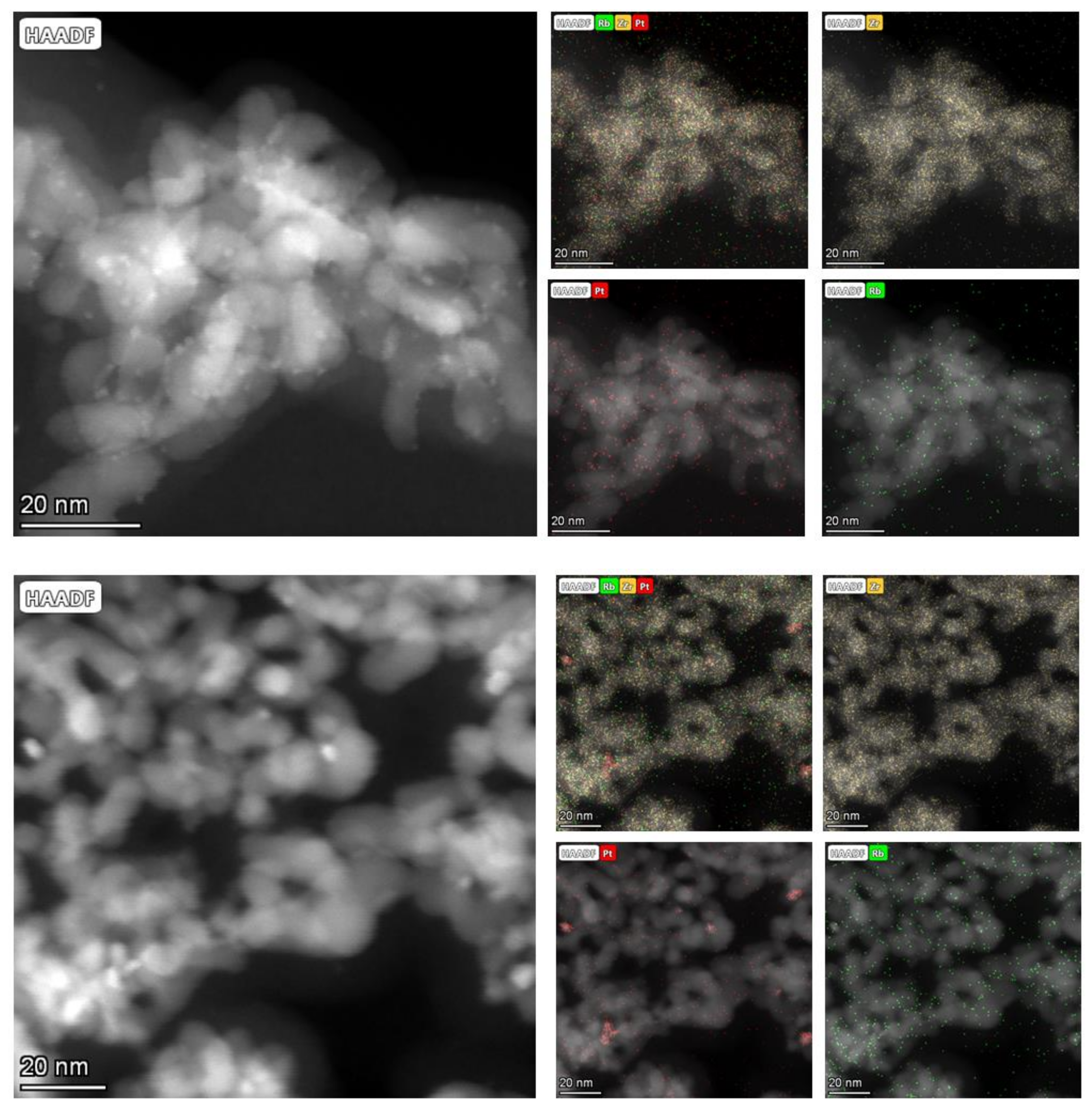
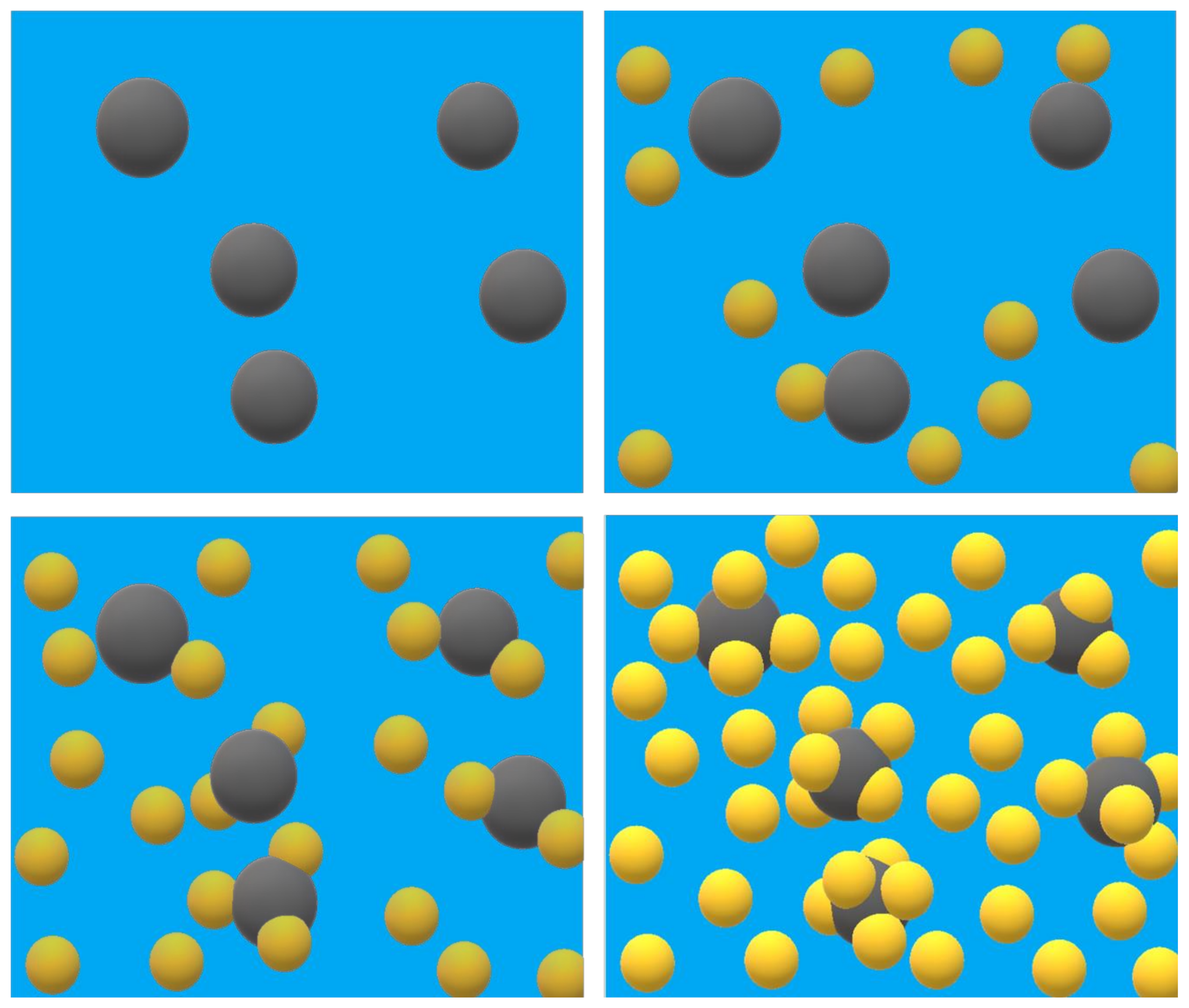
2.3. Transmission Electron Microscopy
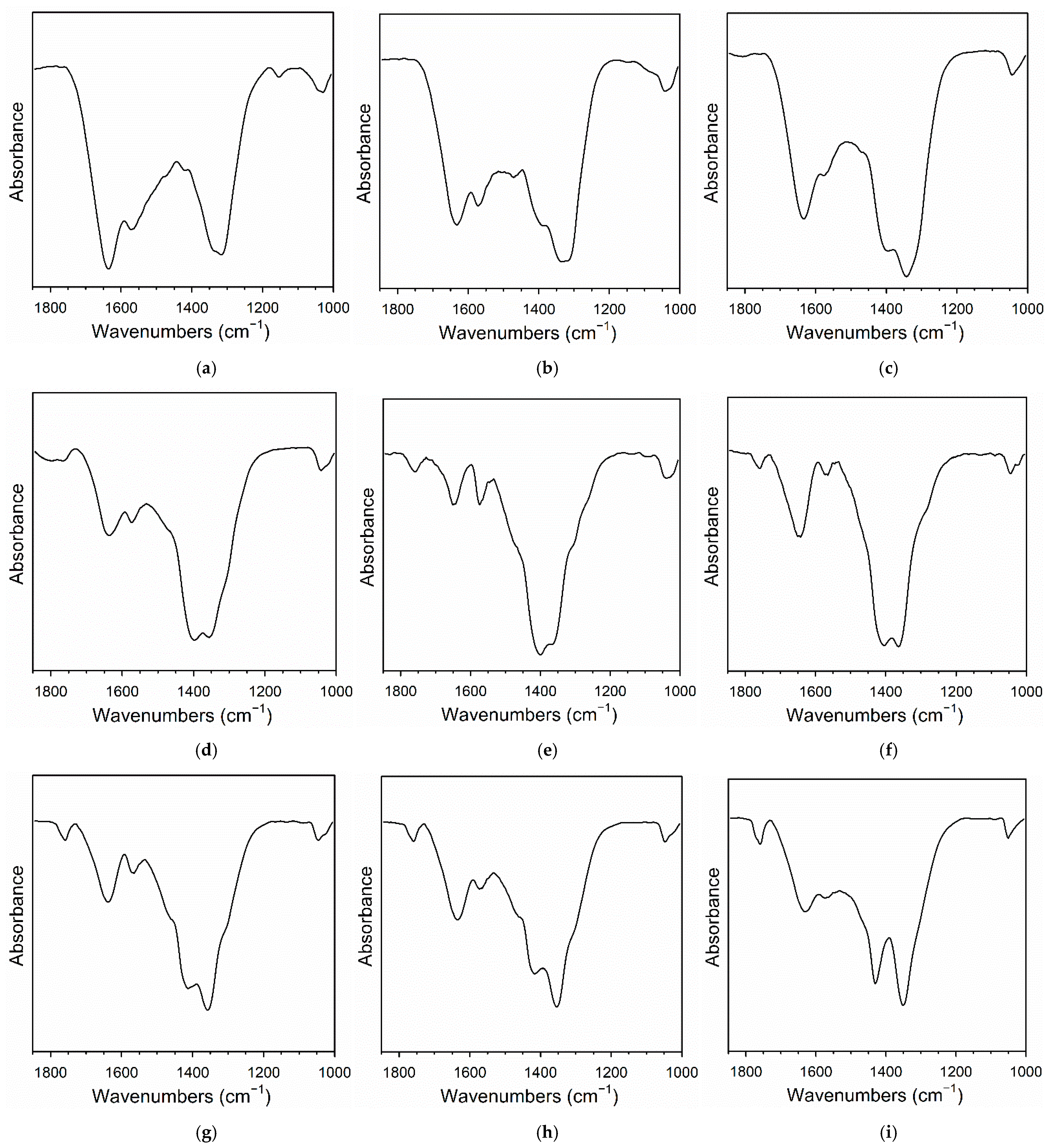
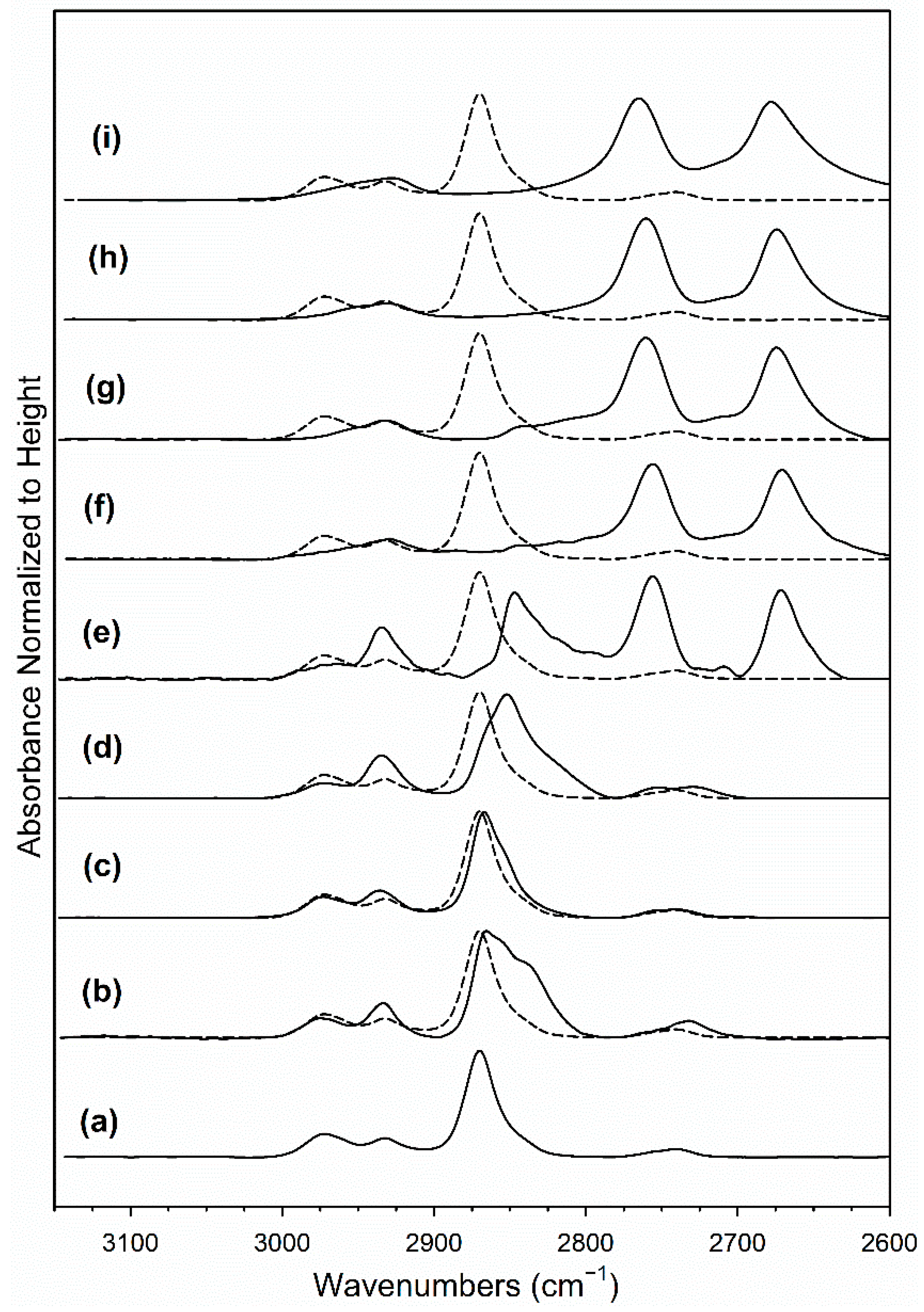
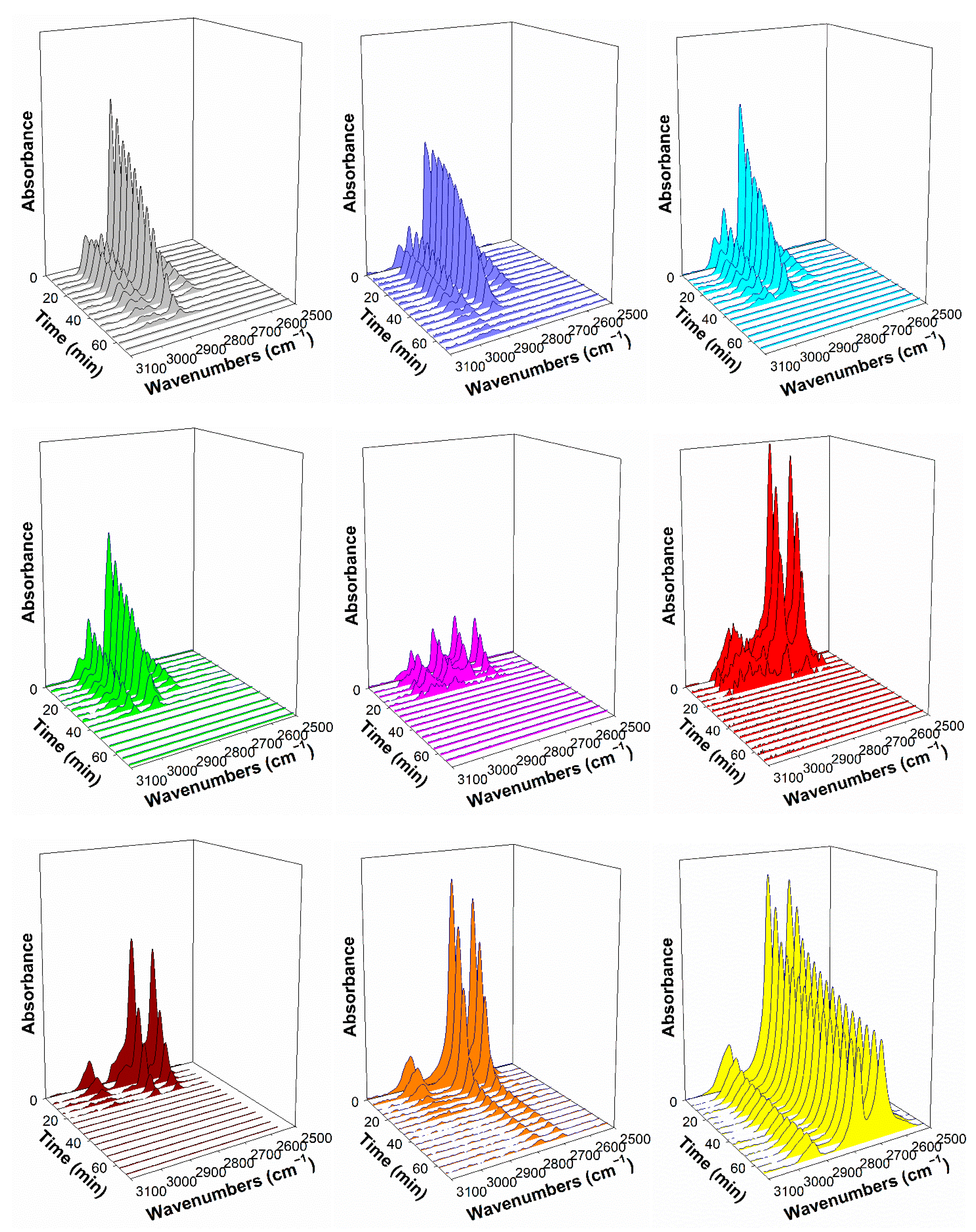
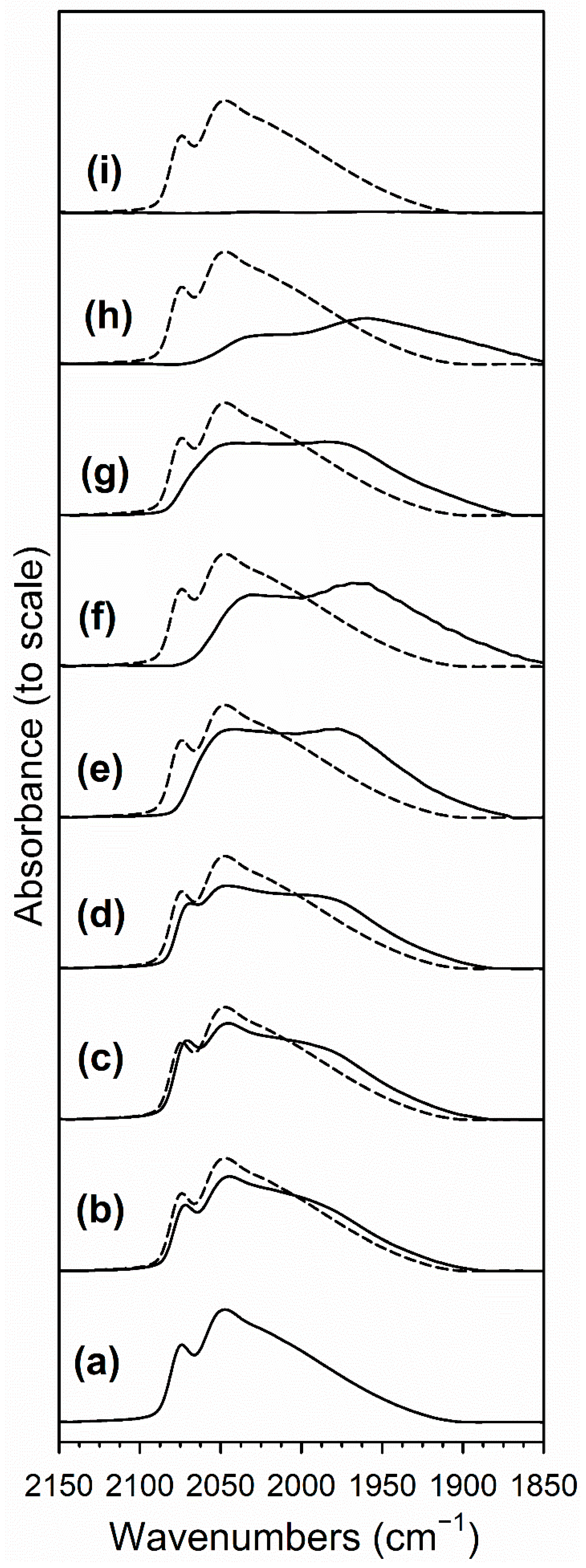
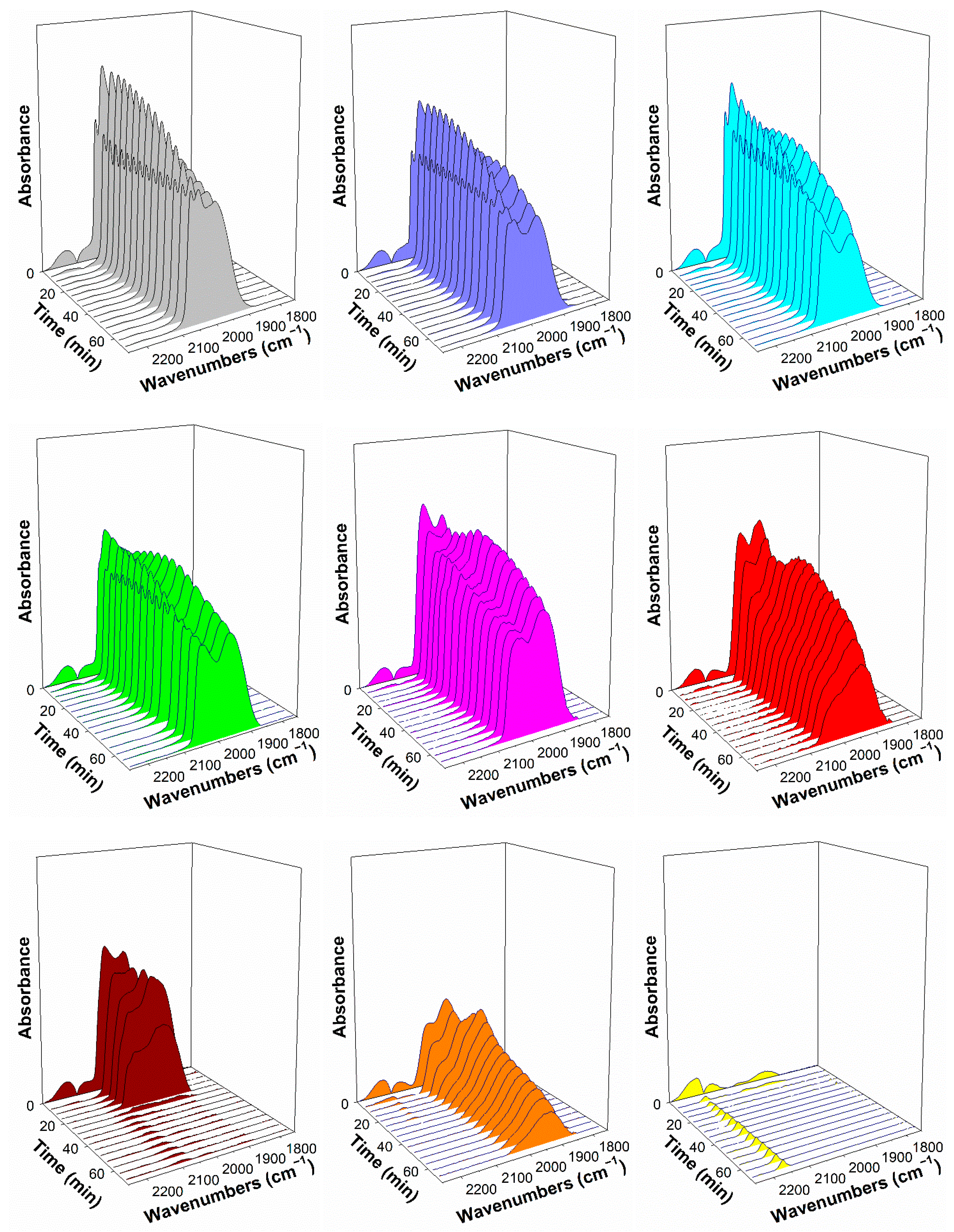
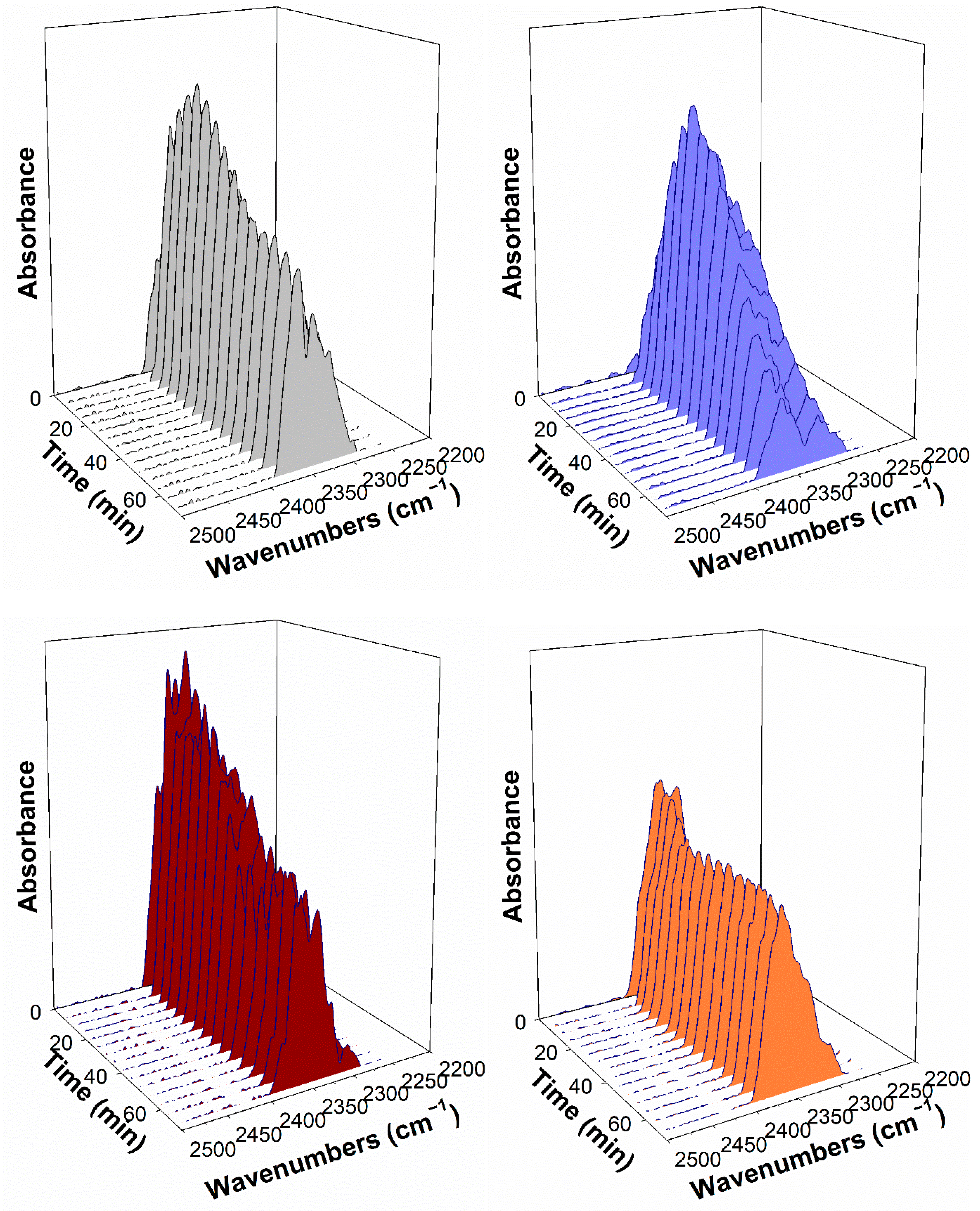
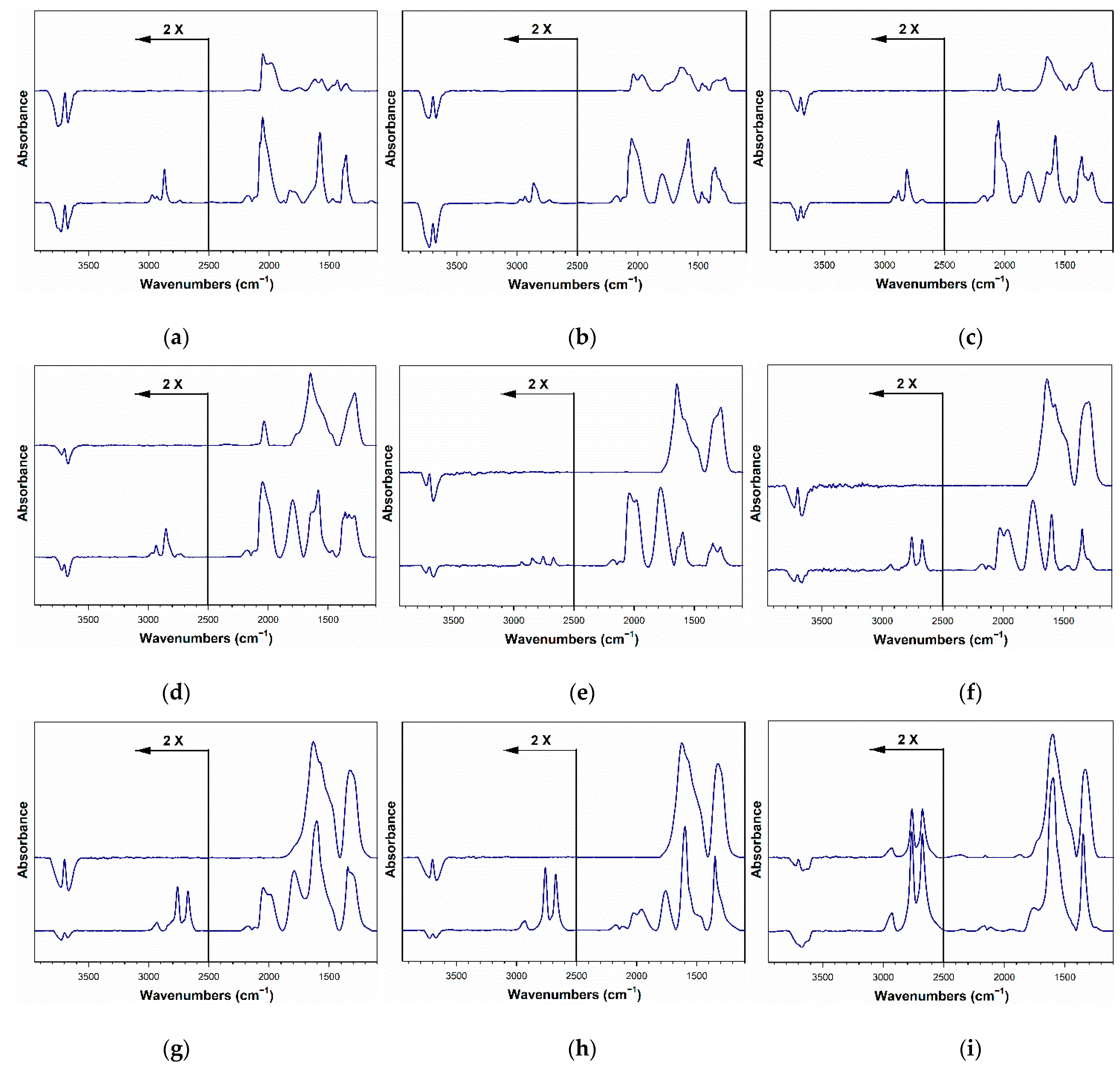
2.4. DRIFTS Studies
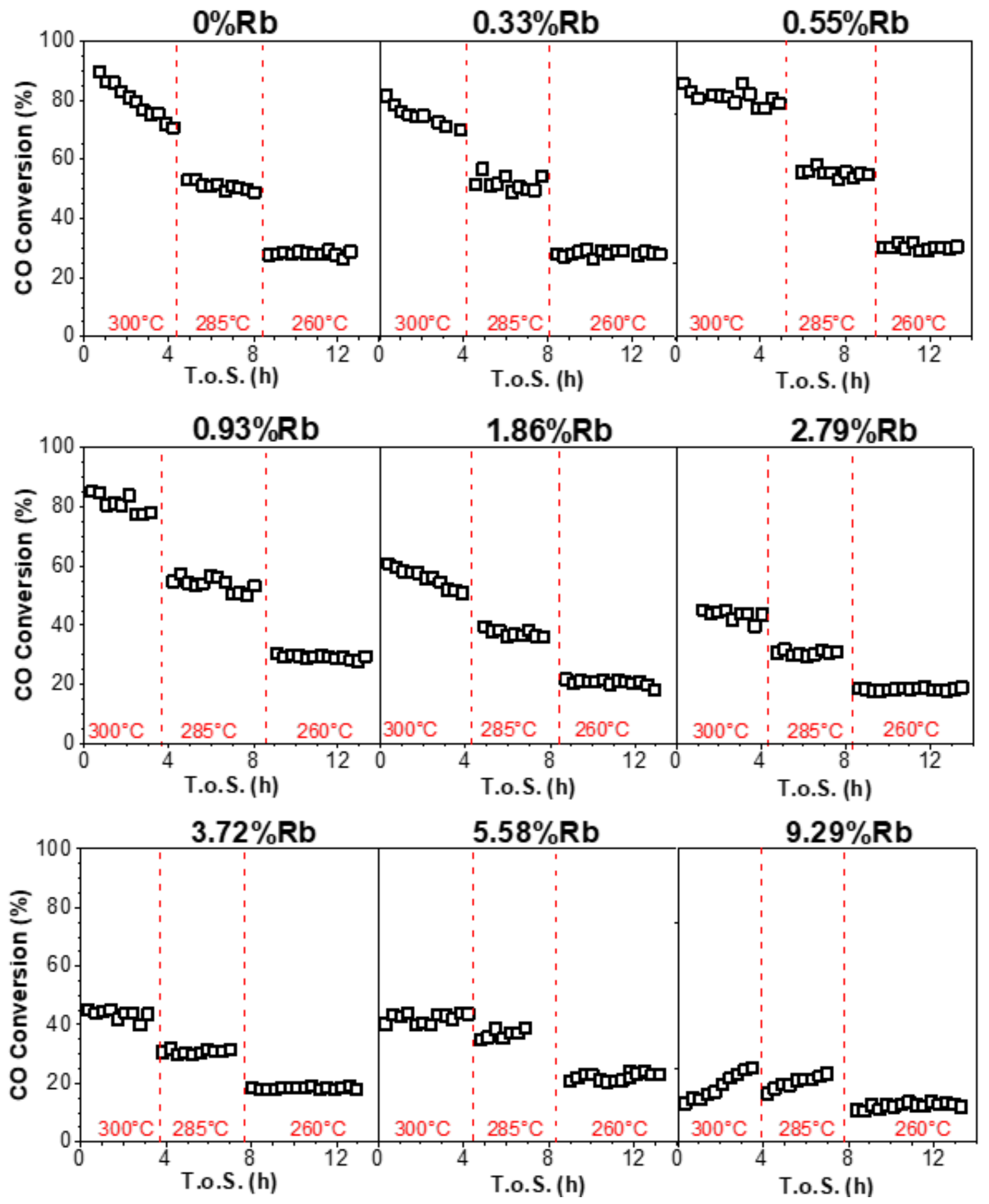
2.5. Reactor Testing
2.6. Alkali Comparison
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization
3.2.1. BET Surface Area
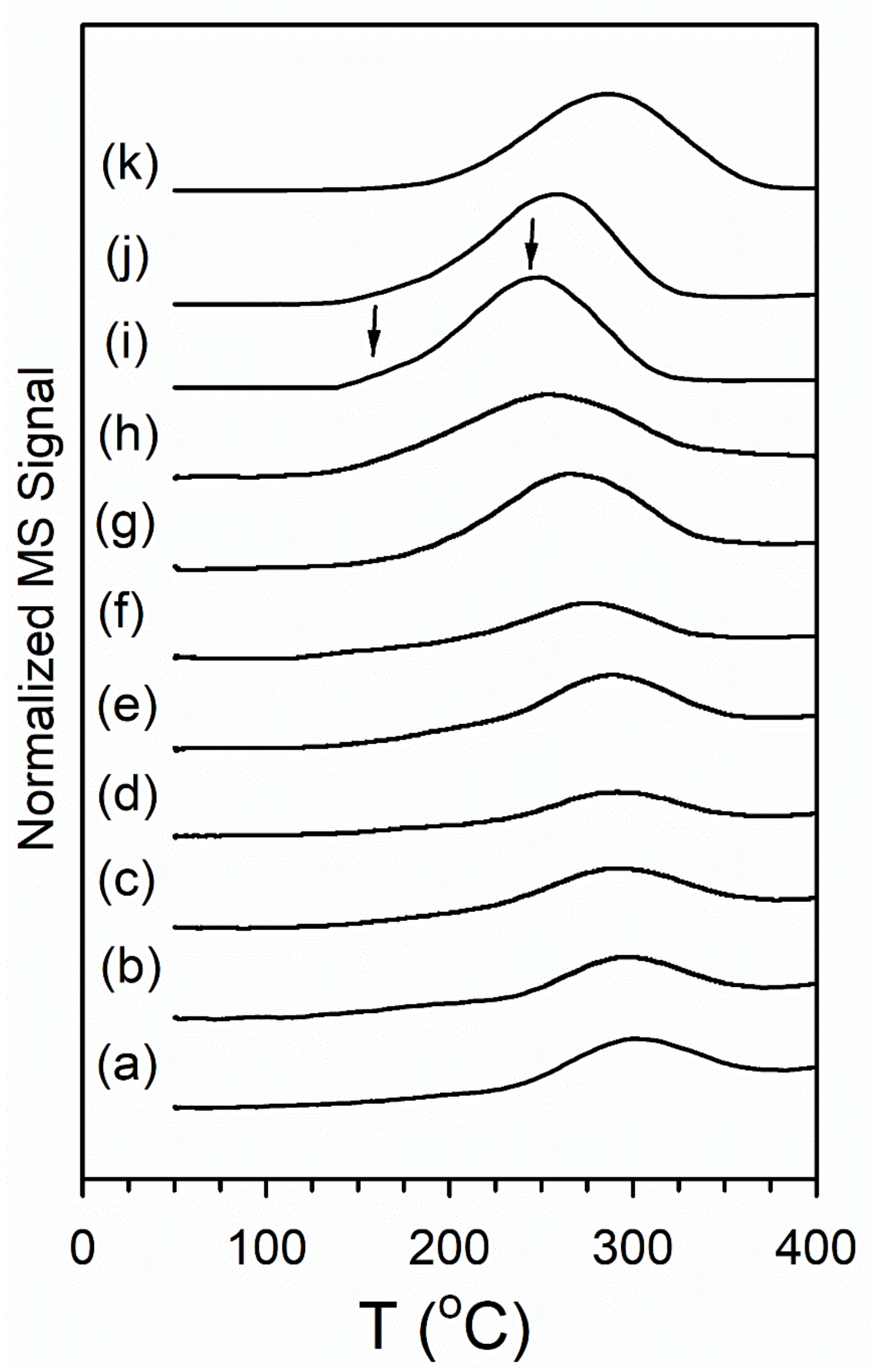
3.2.2. Temperature Programmed Reduction/Mass Spectrometry
3.2.3. Temperature Programmed Desorption
3.2.4. EXAFS
3.2.5. DRIFTS
3.2.6. Transmission Electron Microscopy
3.2.7. Reaction Testing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, D.; Liu, Y.; Liu, M.; Liu, J.; Liang, C.; Li, C.; Xu, J. Correlation between electrochemical performance degradation and catalyst structural parameters on polymer electrolyte membrane fuel cell. Nanotechnol. Rev. 2019, 8, 493–502. [Google Scholar] [CrossRef]
- Dias, F.G.D.A.; Veiga, A.G.; Andreopoulou, A.K.; Kallitsis, J.K.; Rocco, M.L.M. Spectroscopic Study of Reinforced Cross-Linked Polymeric Membranes for Fuel Cell Application. ACS Omega 2020, 5, 15901–15910. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Prasad, R. A Review on Preferential Oxidation of Carbon Monoxide in Hydrogen Rich Gases. Bull. Chem. React. Eng. Catal. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- Garbis, P.; Kern, C.; Jess, A. Kinetics and Reactor Design Aspects of Selective Methanation of CO over a Ru/γ-Al2O3 Catalyst in CO2/H2 Rich Gases. Energies 2019, 12, 469. [Google Scholar] [CrossRef]
- Aliaga, F.; Iglesias, I.; Tejeda, R.; Laborde, M. Hydrogen Production from Bioethanol: Behavior of a Carbon Oxide Preferential Oxidation Catalyst. Chem. Eng. Technol. 2017, 40, 1702–1712. [Google Scholar] [CrossRef]
- Jacobs, G.; Davis, B.H. Low temperature water-gas shift catalysts. In Catalysis; Royal Society of Chemistry: Cambridge, UK, 2007; pp. 122–285. [Google Scholar]
- Ratnasamy, C.; Wagner, J.P. Water Gas Shift Catalysis. Catal. Rev. 2009, 51, 325–440. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Jacobs, G.; Linganiso, L.; Azzam, K.G.; Graham, U.M.; Davis, B.H. Low Temperature Water Gas Shift: Evaluation of Pt/HfO2 and Correlation between Reaction Mechanism and Periodic Trends in Tetravalent (Ti, Zr, Hf, Ce, Th) Metal Oxides. ACS Catal. 2011, 1, 1375–1383. [Google Scholar] [CrossRef]
- Jacobs, G.; Davis, B.H. Surface interfaces in low temperature water-gas shift: The metal oxide synergy, the assistance of co-adsorbed water, and alkali doping. Int. J. Hydrog. Energy 2010, 35, 3522–3536. [Google Scholar] [CrossRef]
- Vignatti, C.I.; Avila, M.S.; Apesteguía, C.R.; Garetto, T.F. Study of the water-gas shift reaction over Pt supported on CeO2–ZrO2 mixed oxides. Catal. Today 2011, 171, 297–303. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Dionysiou, D.D.; Efstathiou, A.M. Mechanistic Studies of the Water–Gas Shift Reaction over Pt/CexZr1–xO2 Catalysts: The Effect of Pt Particle Size and Zr Dopant. ACS Catal. 2012, 2, 2729–2742. [Google Scholar] [CrossRef]
- Palma, V.; Ruocco, C.; Cortese, M.; Renda, S.; Meloni, E.; Festa, G.; Martino, M. Platinum Based Catalysts in the Water Gas Shift Reaction: Recent Advances. Metals 2020, 10, 866. [Google Scholar] [CrossRef]
- Shido, T.; Iwasawa, Y. Reactant-Promoted Reaction Mechanism for Water-Gas Shift Reaction on Rh-Doped CeO2. J. Catal. 1993, 141, 71–81. [Google Scholar] [CrossRef]
- Jacobs, G.; Graham, U.M.; Chenu, E.; Patterson, P.M.; Dozier, A.; Davis, B.H. Low-temperature water–gas shift: Impact of Pt promoter loading on the partial reduction of ceria and consequences for catalyst design. J. Catal. 2005, 229, 499–512. [Google Scholar] [CrossRef]
- Jacobs, G.; Ricote, S.; Graham, U.M.; Patterson, P.M.; Davis, B.H. Low temperature water gas shift: Type and loading of metal impacts forward decomposition of pseudo-stabilized formate over metal/ceria catalysts. Catal. Today 2005, 106, 259–264. [Google Scholar] [CrossRef]
- Kauppinen, M.M.; Melander, M.M.; Bazhenov, A.S.; Honkala, K. Unraveling the Role of the Rh–ZrO2 Interface in the Water–Gas-Shift Reaction via a First-Principles Microkinetic Study. ACS Catal. 2018, 8, 11633–11647. [Google Scholar] [CrossRef]
- Aranifard, S.; Ammal, S.C.; Heyden, A. On the Importance of the Associative Carboxyl Mechanism for the Water-Gas Shift Reaction at Pt/CeO2 Interface Sites. J. Phys. Chem. C 2014, 118, 6314–6323. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Wang, G.-C. A systematic theoretical study of the water gas shift reaction on the Pt/ZrO2 interface and Pt(111) face: Key role of a potassium additive. Catal. Sci. Technol. 2020, 10, 876–892. [Google Scholar] [CrossRef]
- Song, W.; Hensen, E.J.M. Mechanistic Aspects of the Water–Gas Shift Reaction on Isolated and Clustered Au Atoms on CeO2(110): A Density Functional Theory Study. ACS Catal. 2014, 4, 1885–1892. [Google Scholar] [CrossRef]
- Sun, K.; Kohyama, M.; Tanaka, S.; Takeda, S. Reaction Mechanism of the Low-Temperature Water–Gas Shift Reaction on Au/TiO2 Catalysts. J. Phys. Chem. C 2017, 121, 12178–12187. [Google Scholar] [CrossRef]
- Ziemba, M.; Ganduglia-Pirovano, V.; Hess, C. Insight into the mechanism of the water-gas shift reaction over Au/CeO2 catalysts using combined operando spectroscopies. Faraday Discuss. 2020. [Google Scholar] [CrossRef]
- Schilling, C.; Hess, C. Elucidating the Role of Support Oxygen in the Water–Gas Shift Reaction over Ceria-Supported Gold Catalysts Using Operando Spectroscopy. ACS Catal. 2019, 9, 1159–1171. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Panagiotopoulou, P.; Kondarides, D.I.; Efstathiou, A.M. Kinetic and mechanistic studies of the water–gas shift reaction on Pt/TiO2 catalyst. J. Catal. 2009, 264, 117–129. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Americanou, S.; Efstathiou, A.M. “Redox” vs. “associative formate with –OH group regeneration” WGS reaction mechanism on Pt/CeO2: Effect of platinum particle size. J. Catal. 2011, 279, 287–300. [Google Scholar] [CrossRef]
- Salcedo, A.; Irigoyen, B. Unraveling the Origin of Ceria Activity in Water–Gas Shift by First-Principles Microkinetic Modeling. J. Phys. Chem. C 2020, 124, 7823–7834. [Google Scholar] [CrossRef]
- Petallidou, K.C.; Polychronopoulou, K.; Boghosian, S.; Garcia-Rodriguez, S.; Efstathiou, A.M. Water–Gas Shift Reaction on Pt/Ce1–xTixO2−δ: The Effect of Ce/Ti Ratio. J. Phys. Chem. C 2013, 117, 25467–25477. [Google Scholar] [CrossRef]
- Burch, R. Gold catalysts for pure hydrogen production in the water–gas shift reaction: Activity, structure and reaction mechanism. Phys. Chem. Chem. Phys. 2006, 8, 5483–5500. [Google Scholar] [CrossRef]
- Vovchok, D.; Guild, C.J.; Llorca, J.; Palomino, R.M.; Waluyo, I.; Rodriguez, J.A.; Suib, S.L.; Senanayake, S.D. Structural and chemical state of doped and impregnated mesoporous Ni/CeO2 catalysts for the water-gas shift. Appl. Catal. Gen. 2018, 567, 1–11. [Google Scholar] [CrossRef]
- Vecchietti, J.; Bonivardi, A.; Xu, W.; Stacchiola, D.; Delgado, J.J.; Calatayud, M.; Collins, S.E. Understanding the Role of Oxygen Vacancies in the Water Gas Shift Reaction on Ceria-Supported Platinum Catalysts. ACS Catal. 2014, 4, 2088–2096. [Google Scholar] [CrossRef]
- Fonseca, A.A.; Fisher, J.M.; Ozkaya, D.; Shannon, M.D.; Thompsett, D. Ceria-zirconia supported Au as highly active low temperature Water-gas shift catalysts. Top. Catal. 2007, 44, 223–235. [Google Scholar] [CrossRef]
- Pigos, J.M.; Brooks, C.J.; Jacobs, G.; Davis, B.H. Low temperature water-gas shift: Characterization of Pt-based ZrO2 catalyst promoted with Na discovered by combinatorial methods. Appl. Catal. Gen. 2007, 319, 47–57. [Google Scholar] [CrossRef]
- Pigos, J.M.; Brooks, C.J.; Jacobs, G.; Davis, B.H. Low temperature water–gas shift: The effect of alkali doping on the CH bond of formate over Pt/ZrO2 catalysts. Appl. Catal. Gen. 2007, 328, 14–26. [Google Scholar] [CrossRef]
- Watson, C.D.; Martinelli, M.; Cronauer, D.C.; Kropf, A.J.; Marshall, C.L.; Jacobs, G. Low temperature water-gas shift: Optimization of K loading on Pt/m-ZrO2 for enhancing CO conversion. Appl. Catal. Gen. 2020, 598, 117572. [Google Scholar] [CrossRef]
- Jacobs, G.; Davis, B.; Pigos, J.; Brooks, C. Low-temperature water-gas shift: Assessing formates as potential intermediates over Pt/ZrO2 and na-doped Pt/ZrO2 catalysts employing the SSITKA-DRIFTS technique. In Advances in Fischer-Tropsch Synthesis, Catalysts and Catalysis; Davis, B.H., Occelli, M.L., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 365–394. [Google Scholar]
- Evin, H.N.; Jacobs, G.; Ruiz-Martinez, J.; Graham, U.M.; Dozier, A.; Thomas, G.; Davis, B.H. Low Temperature Water–Gas Shift/Methanol Steam Reforming: Alkali Doping to Facilitate the Scission of Formate and Methoxy C–H Bonds over Pt/ceria Catalyst. Catal. Lett. 2008, 122, 9–19. [Google Scholar] [CrossRef]
- Evin, H.N.; Jacobs, G.; Ruiz-Martinez, J.; Thomas, G.A.; Davis, B.H. Low Temperature Water–Gas Shift: Alkali Doping to Facilitate Formate C–H Bond Cleaving over Pt/Ceria Catalysts—An Optimization Problem. Catal. Lett. 2008, 120, 166–178. [Google Scholar] [CrossRef]
- Brooks, C.; Cypes, S.; Grasselli, R.K.; Hagemeyer, A.; Hogan, Z.; Lesik, A.; Streukens, G.; Volpe, A.F.; Turner, H.W.; Weinberg, W.H.; et al. High throughput discovery of CO oxidation/VOC combustion and water–gas shift catalysts for industrial multi-component streams. Top. Catal. 2006, 38, 195–209. [Google Scholar] [CrossRef]
- Figueiredo, R.T.; Santos, M.S.; Andrade, H.M.C.; Fierro, J.L.G. Effect of alkali cations on the CuZnOAl2O3 low temperature water gas-shift catalyst. Catal. Today 2011, 172, 166–170. [Google Scholar] [CrossRef]
- Gao, P.; Graham, U.M.; Shafer, W.D.; Linganiso, L.Z.; Jacobs, G.; Davis, B.H. Nanostructure and kinetic isotope effect of alkali-doped Pt/silica catalysts for water-gas shift and steam-assisted formic acid decomposition. Catal. Today 2016, 272, 42–48. [Google Scholar] [CrossRef]
- Komarov, Y.M.; Il’in, A.A.; Smirnov, N.N.; Il’in, A.P.; Babaikin, D.B. Effect of alkali metal oxides on the selectivity of carbon monoxide conversion to give hydrogen on copper-containing catalysts. Russ. J. Appl. Chem. 2013, 86, 27–31. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Jacobs, G.; Shafer, W.D.; Sparks, D.E.; Hopps, S.; Thomas, G.A.; Davis, B.H. Low Temperature Water–Gas Shift Reaction Over Alkali Metal Promoted Cobalt Carbide Catalysts. Top. Catal. 2014, 57, 612–618. [Google Scholar] [CrossRef]
- Kusche, M.; Bustillo, K.; Agel, F.; Wasserscheid, P. Highly Effective Pt-Based Water–Gas Shift Catalysts by Surface Modification with Alkali Hydroxide Salts. ChemCatChem 2015, 7, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Li, Y.; Zou, X.; Zhuo, H.; Yao, Y.; Suo, Z. Effect of Alkali Metal Promoters on Water-Gas Shift Activity over Au-Pt/CeO2 Catalyst: Effect of Alkali Metal Promoters on Water-Gas Shift Activity over Au-Pt/CeO2 Catalyst. Chin. J. Catal. Chin. Version 2010, 31, 671–676. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Kondarides, D.I. Effects of alkali promotion of TiO2 on the chemisorptive properties and water–gas shift activity of supported noble metal catalysts. J. Catal. 2009, 267, 57–66. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, M.; Lobban, L.L.; Mallinson, R.G. Structural effects of Na promotion for high water gas shift activity on Pt–Na/TiO2. J. Catal. 2011, 278, 123–132. [Google Scholar] [CrossRef]
- González-Cobos, J.; Valverde, J.L.; De Lucas-Consuegra, A. Electrochemical vs. chemical promotion in the H2 production catalytic reactions. Int. J. Hydrog. Energy 2017, 42, 13712–13723. [Google Scholar] [CrossRef]
- Menacherry, P.V.; Haller, G.L. The effect of water on the infrared spectra of CO adsorbed on Pt/K L-zeolite. Catal. Lett. 1997, 44, 135–144. [Google Scholar] [CrossRef]
- Jentys, A. Estimation of mean size and shape of small metal particles by EXAFS. Phys. Chem. Chem. Phys. 1999, 1, 4059–4063. [Google Scholar] [CrossRef]
- Marinković, N.S.; Sasaki, K.; Adžić, R.R. Nanoparticle size evaluation of catalysts by EXAFS: Advantages and limitations. Zaštita Materijala 2016, 57, 101–109. [Google Scholar] [CrossRef]
- Binet, C.; Daturi, M.; Lavalley, J.-C. IR study of polycrystalline ceria properties in oxidised and reduced states. Catal. Today 1999, 50, 207–225. [Google Scholar] [CrossRef]
- Martinelli, M.; Watson, C.D.; Jacobs, G. Sodium doping of Pt/m-ZrO2 promotes C–C scission and decarboxylation during ethanol steam reforming. Int. J. Hydrog. Energy 2020, 45, 18490–18501. [Google Scholar] [CrossRef]
- Martinelli, M.; Alhraki, N.; Castro, J.D.; Matamoros, M.E.; Jacobs, G. 6-Water-gas shift: Effect of Na loading on Pt/m-zirconia catalysts for low-temperature shift for the production and purification of hydrogen. In New Dimensions in Production and Utilization of Hydrogen; Nanda, S., Vo, D.-V.N., Nguyen-Tri, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–160. ISBN 978-0-12-819553-6. [Google Scholar]
- Allen, L.C. Electronegativity is the average one-electron energy of the valence-shell electrons in ground-state free atoms. J. Am. Chem. Soc. 1989, 111, 9003–9014. [Google Scholar] [CrossRef]
- Jacoby, M. X-ray Absorption Spectroscopy. Chem. Eng. News Arch. 2001, 79, 33–38. [Google Scholar] [CrossRef]
- Ressler, T. WinXAS: A Program for X-ray Absorption Spectroscopy Data Analysis under MS-Windows. J. Synchrotron Radiat. 1998, 5, 118–122. [Google Scholar] [CrossRef]
- Ravel, B. IUCr ATOMS: Crystallography for the X-ray Absorption Spectroscopist. Available online: https://scripts.iucr.org/cgi-bin/paper?hf5152 (accessed on 22 November 2020).
- Newville, M.; Ravel, B.; Haskel, D.; Rehr, J.J.; Stern, E.A.; Yacoby, Y. Analysis of multiple-scattering XAFS data using theoretical standards. Phys. B Condens. Matter 1995, 208–209, 154–156. [Google Scholar] [CrossRef]

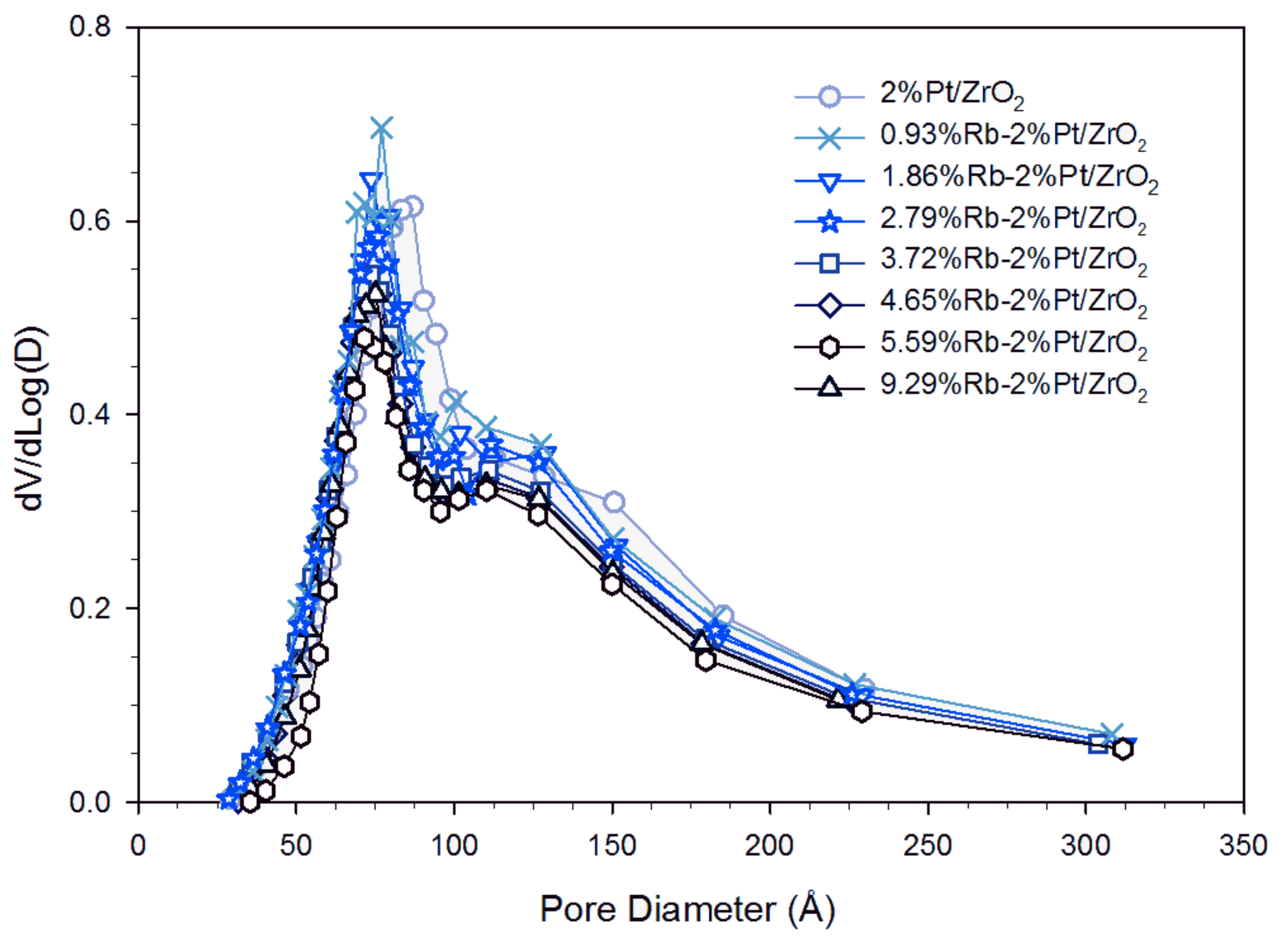
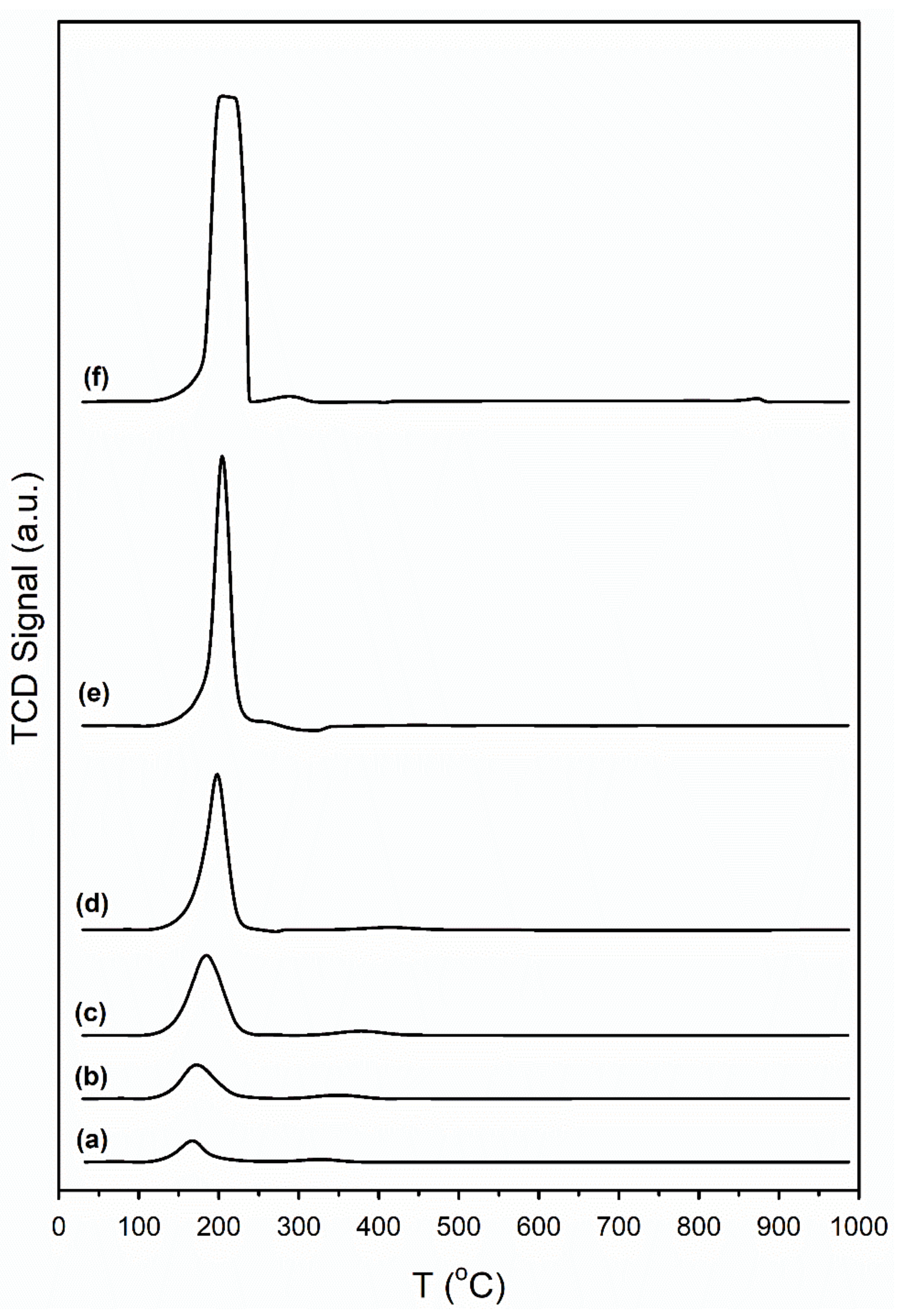
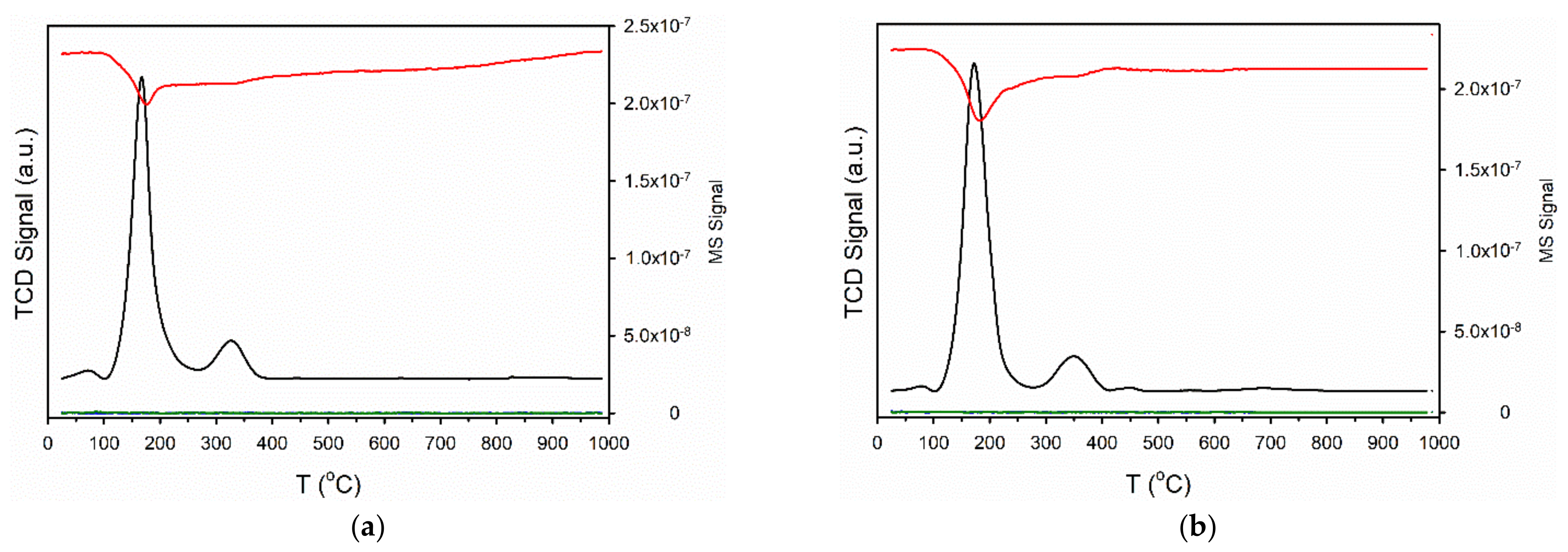
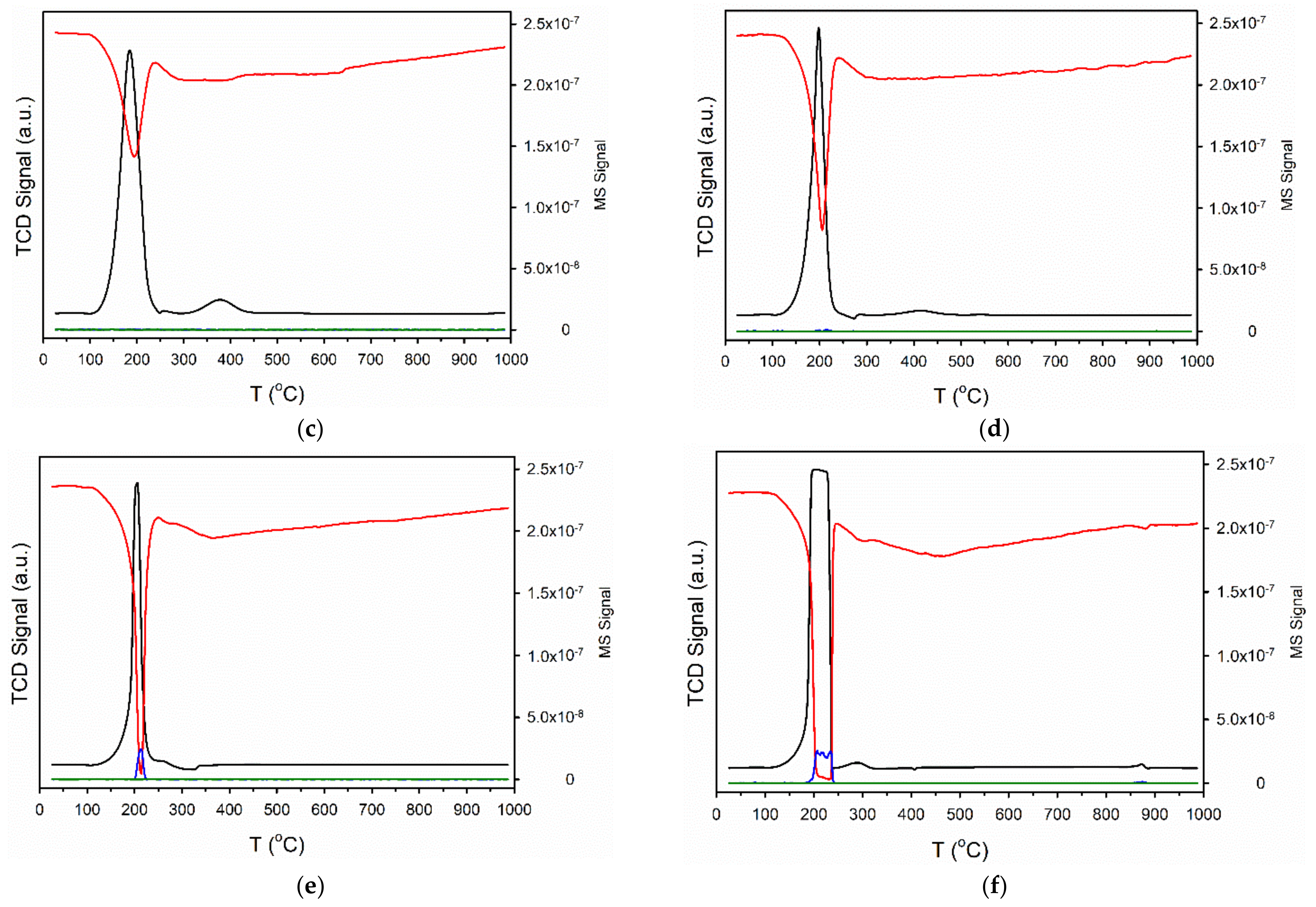


| Sample ID | As (BET) [m2/g] | Vp (BJH Des) [cm3/g] | Dp (BJH Des) [Å] |
|---|---|---|---|
| ZrO2 | 95.4 | 0.289 | 95 |
| 2%Pt/ZrO2 | 89.7 | 0.260 | 95 |
| 0.33%Rb-2%Pt/ZrO2 | 88.8 | 0.271 | 95 |
| 0.55%Rb-2%Pt/ZrO2 | 87.9 | 0.268 | 96 |
| 0.93%Rb-2%Pt/ZrO2 | 91.6 | 0.275 | 93 |
| 1.86%Rb-2%Pt/ZrO2 | 88.7 | 0.262 | 94 |
| 2.79%Rb-2%Pt/ZrO2 | 86.7 | 0.260 | 93 |
| 3.72%Rb-2%Pt/ZrO2 | 78.2 | 0.244 | 93 |
| 4.65%Rb-2%Pt/ZrO2 | 72.3 | 0.235 | 95 |
| 5.58%Rb-2%Pt/ZrO2 | 69.1 | 0.227 | 96 |
| 9.29%Rb-2%Pt/ZrO2 | 58.2 | 0.202 | 102 |
| Sample Description | N Pt-Pt Metal | R Pt-Pt (Å) Metal | e0 (eV) | σ2 (Å2) | r-Factor | Est. # Atoms * | Est. Diam. (nm) */** | Est. % Disp. (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Pt0 foil | 12 (fixed) | 2.760 (0.0058) | 8.75 (0.632) | 0.00523 (0.00043) | 0.0084 | - | - | - | |
| 2%Pt/m-ZrO2 | 4.7 (0.70) | 2.669 (0.0157) | 4.22 (1.08) | 0.0100 (0.00249) | 0.0184 | 10 | 0.80 | 94 | |
| 0.72 | |||||||||
| 0.93%Rb-2%Pt/m-ZrO2 | 5.2 (0.42) | 2.690 (0.0084) | 4.30 (0.580) | 0.00971 (0.00133) | 0.0055 | 13 | 0.86 | 92 | |
| 0.78 | |||||||||
| 1.86%Rb-2%Pt/m-ZrO2 | 6.3 (0.41) | 2.705 (0.0072) | 4.89 (0.467) | 0.0112 (0.00116) | 0.0034 | 22 | 1.0 | 87 | |
| 0.93 | |||||||||
| 2.79%Rb-2%Pt/m-ZrO2 | 6.7 (0.39) | 2.720 (0.0064) | 6.04 (0.415) | 0.0103 (0.00102) | 0.0030 | 27 | 1.1 | 85 | |
| 0.99 | |||||||||
| 4.65%Rb-2%Pt/m-ZrO2 | 7.7 (0.62) | 2.736 (0.0081) | 6.51 (0.557) | 0.00834 (0.00128) | 0.0060 | 53 | 1.3 | 77 | |
| 1.2 | |||||||||
| 5.58%Rb-2%Pt/m-ZrO2 | 7.7 (0.55) | 2.745 (0.0068) | 7.15 (0.490) | 0.00665 (0.00107) | 0.0049 | 53 | 1.3 | 77 | |
| 1.2 | |||||||||
| 9.29%Rb-2%Pt/m-ZrO2 | 8.9 (0.47) | 2.750 (0.0047) | 7.96 (0.352) | 0.00505 (0.000723) | 0.0028 | 191 | 2.0 | 56 | |
| 1.9 | |||||||||
| Catalyst | Band Position (cm−1) | |||||
|---|---|---|---|---|---|---|
| ν(CH) | δ(CH) + νs(OCO) | 2δ(CH) | ν(OCO) Formate | ν(OCO) Carbonate | ν(OH) | |
| 2%Pt/ZrO2 (reference) | 2870 | 2973, 2932 | (2752) 2741 | 1386, 1361 sy 1578 asy | 1617, 1560, (1472) 1434, 1362 | 3762, 3675 |
| 0.55%Rb-2%Pt/ZrO2 | 2866 2838 | 2975 (2970), 2933 | (2741) 2732 | (1385) 1375, 1357 sy 1581 asy | (1641) 1619, 1566, 1467, 1377, 1348 (1280) | 3729, 3674 |
| 0.93%Rb-2%Pt/ZrO2 | (2863) 2855 (2837) | 2967, 2930 | (2756) 2727 | (1386) 1359 sy (1648) 1578 asy | 1647 1466 1277 | 3722, 3674 |
| 1.86%Rb-2%Pt/ZrO2 | (2866) 2849 (2832) | 2970, 2931 | (2750) 2725 (2683) | (1374) 1358 (1326) sy (1637) 1582 asy | 1647 (1473) 1278 | 3724, 3673 |
| 2.79%Rb-2%Pt/ZrO2 | 2848, 2756 | 2934 | (2709) 2674 | (1377) 1347 sy (1639) 1597 asy | 1648 (1350) 1280 | 3734, 3666 |
| 3.72%Rb-2%Pt/ZrO2 | 2756 | 2932 | (2705) 2670 | 1346 sy, 1600 asy | 1633, (1573) 1304 | 3733, 3670 |
| 4.65%Rb-2%Pt/ZrO2 | 2761 | 2934 | (2707) 2675 | 1346 sy, 1600 asy | 1635 (1567) 1327 | 3723, 3673 |
| 5.58%Rb-2%Pt/ZrO2 | 2760 | 2934 | (2710) 2675 | 1348 sy, 1598 asy | 1624, 1328 | 3730, 3671 |
| 9.29%Rb-2%Pt/ZrO2 | 2766 | 2927 | (2713) 2678 | 1352 sy, 1594 asy | 1601, 1337 | 3730, 3651 |
| Sample ID | Dispersion Relative to 2%Pt/ZrO2 | Initial Pt-CO Magnitude Relative to 2%Pt/ZrO2 |
|---|---|---|
| 2%Pt/ZrO2 | 1 | 1 |
| 0.93%Rb-2%Pt/ZrO2 | 0.98 | 1.05 |
| 1.86%Rb-2%Pt/ZrO2 | 0.93 | 0.97 |
| 2.79%Rb-2%Pt/ZrO2 | 0.90 | 1.11 |
| 4.65%Rb-2%Pt/ZrO2 | 0.82 | 1.02 |
| 5.59%Rb-2%Pt/ZrO2 | 0.82 | 0.42 |
| 9.29%Rb-2%Pt/ZrO2 | 0.60 | 0.10 |
| Alkali Dopant | Optimal Weight% | Optimal Atom% | Maximum Formate ν(CH) Shift (cm−1) Relative to 2%Pt/ZrO2 | Allen Electronegativity of Alkali Metal [54] |
|---|---|---|---|---|
| Na | 1.8–2.5 | 3.15–4.38 | −64 | 0.869 |
| K | 2.55 | 2.69 | −94 | 0.734 |
| Rb | 0.55–0.93 | 0.27–0.46 | −109 | 0.706 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watson, C.D.; Martinelli, M.; Cronauer, D.C.; Kropf, A.J.; Jacobs, G. Low Temperature Water-Gas Shift: Enhancing Stability through Optimizing Rb Loading on Pt/ZrO2. Catalysts 2021, 11, 210. https://doi.org/10.3390/catal11020210
Watson CD, Martinelli M, Cronauer DC, Kropf AJ, Jacobs G. Low Temperature Water-Gas Shift: Enhancing Stability through Optimizing Rb Loading on Pt/ZrO2. Catalysts. 2021; 11(2):210. https://doi.org/10.3390/catal11020210
Chicago/Turabian StyleWatson, Caleb Daniel, Michela Martinelli, Donald Charles Cronauer, A. Jeremy Kropf, and Gary Jacobs. 2021. "Low Temperature Water-Gas Shift: Enhancing Stability through Optimizing Rb Loading on Pt/ZrO2" Catalysts 11, no. 2: 210. https://doi.org/10.3390/catal11020210
APA StyleWatson, C. D., Martinelli, M., Cronauer, D. C., Kropf, A. J., & Jacobs, G. (2021). Low Temperature Water-Gas Shift: Enhancing Stability through Optimizing Rb Loading on Pt/ZrO2. Catalysts, 11(2), 210. https://doi.org/10.3390/catal11020210









