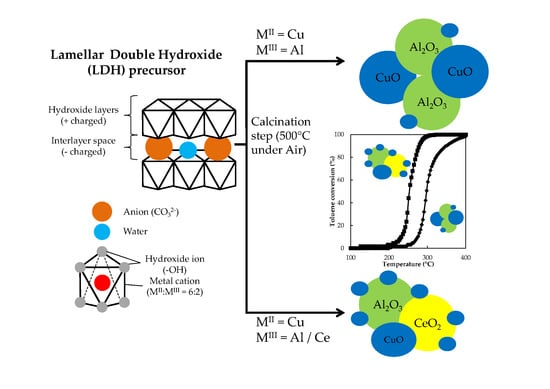
CuAlCe Oxides Issued from Layered Double Hydroxide Precursors for Ethanol and Toluene Total Oxidation
Abstract
1. Introduction
2. Results
2.1. Structural Properties
2.2. Textural Properties
2.3. H2-Temperature Programmed Reduction
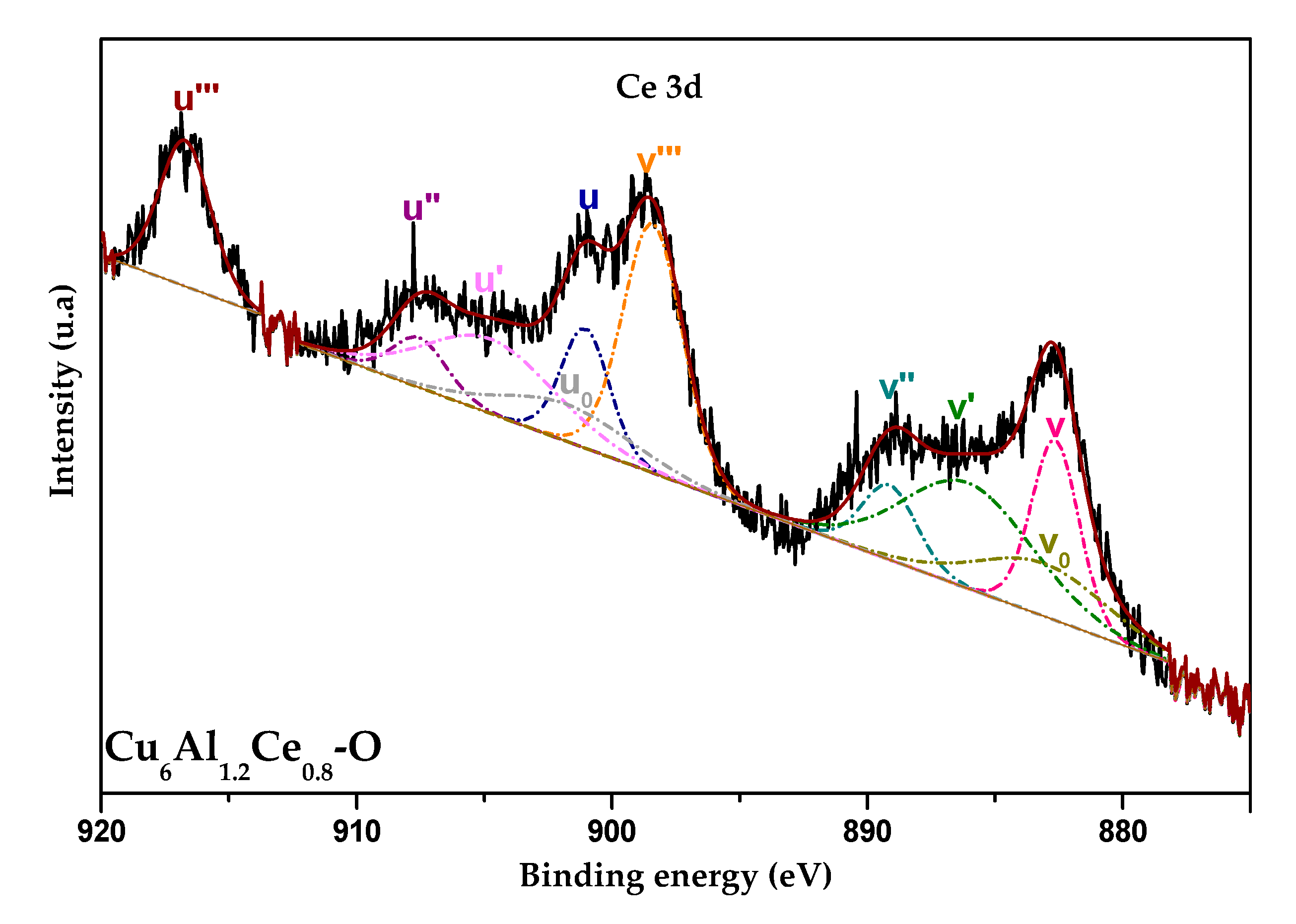
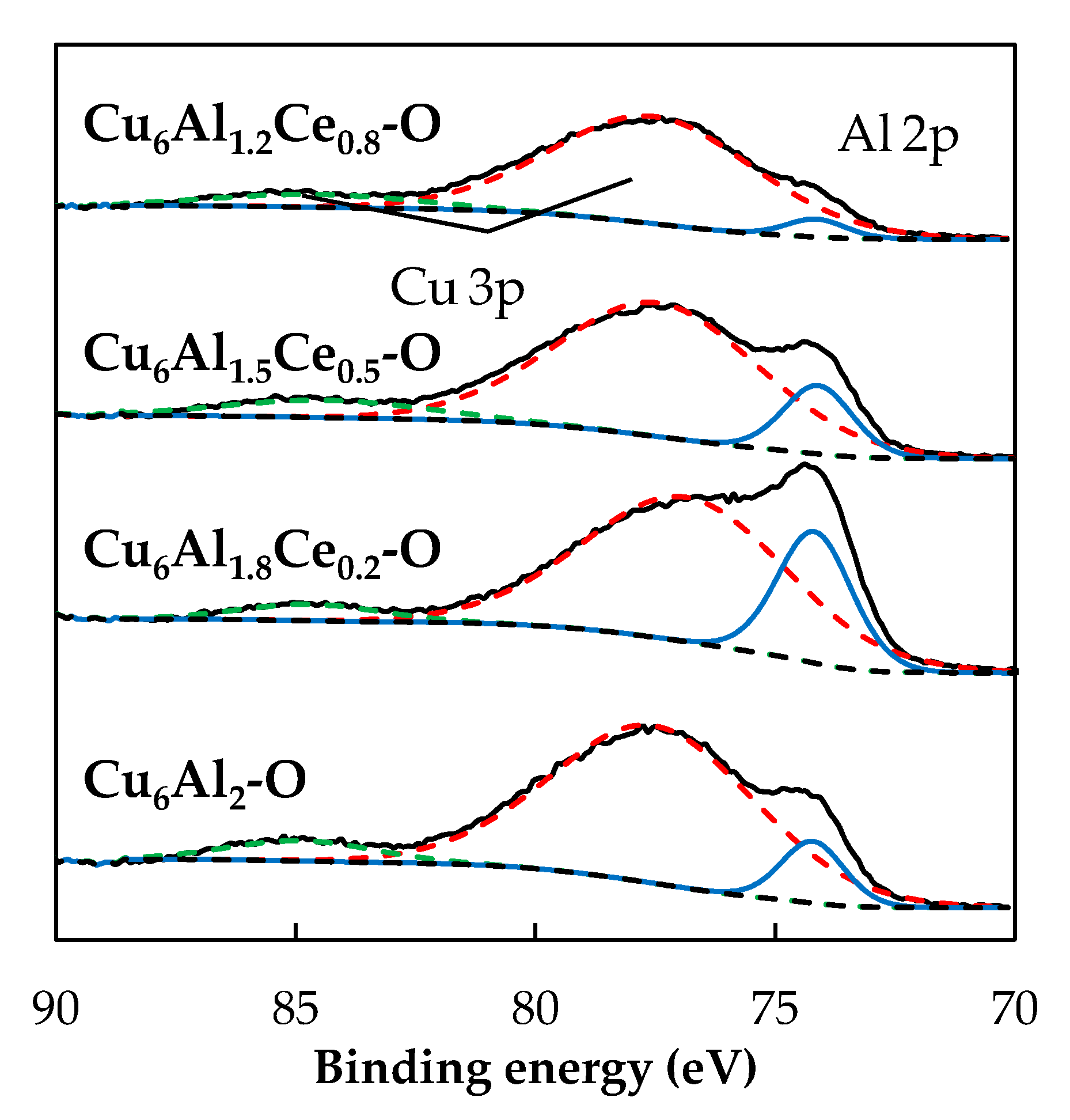
2.4. X-ray Photoelectron Spectroscopy
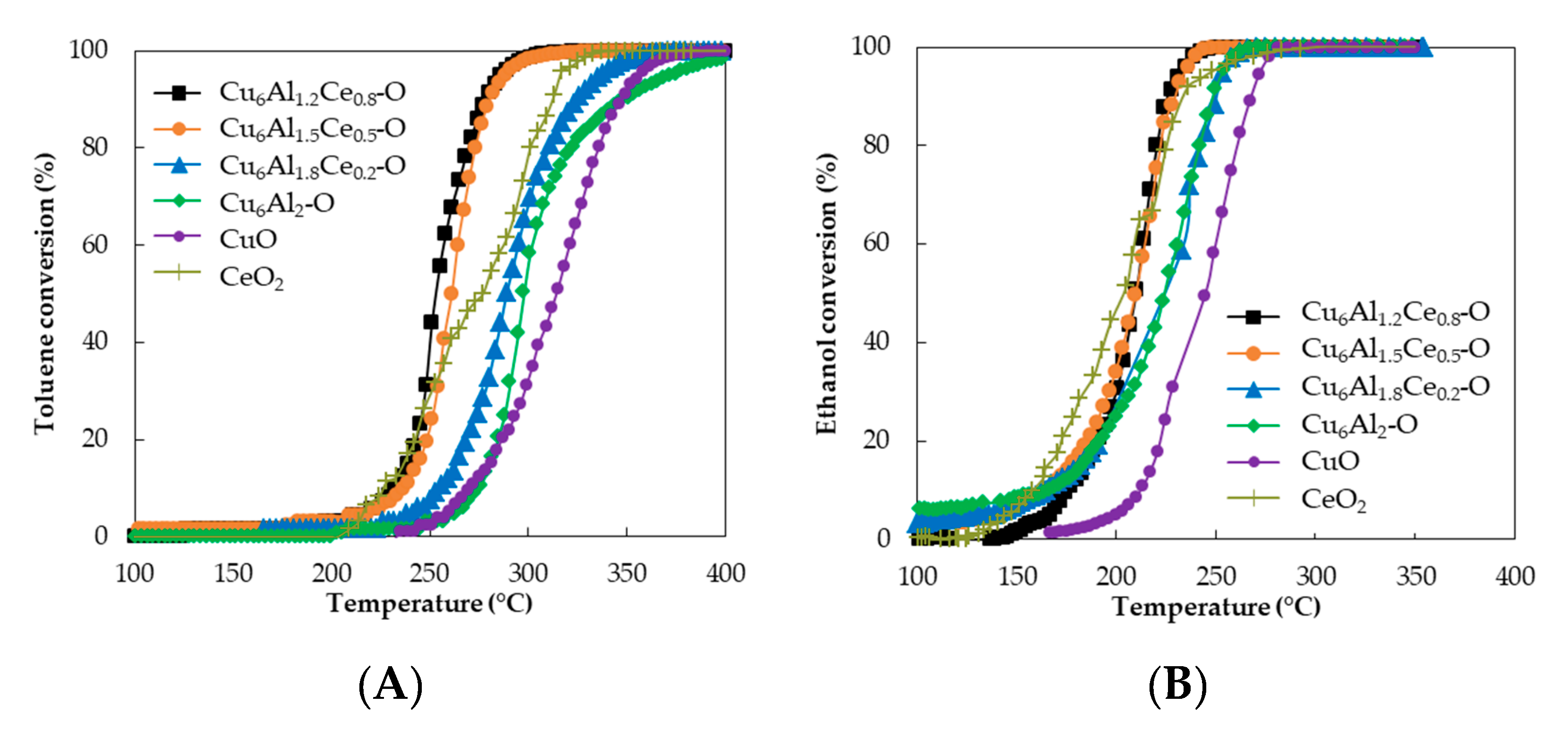
2.5. Catalytic Performance
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Catalyst Characterization
3.3. Catalytic Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y.C. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Torres, J.Q.; Royer, S.; Bellat, J.P.; Giraudon, J.M.; Lamonier, J.F. Formaldehyde: Catalytic oxidation as a promising soft way of elimination. ChemSusChem 2013, 6, 578–592. [Google Scholar] [CrossRef]
- Brunet, J.; Genty, E.; Landkocz, Y.; Zallouha, M.A.; Billet, S.; Courcot, D.; Siffert, S.; Thomas, D.; De Weireld, G.; Cousin, R. Identification of by-products issued from the catalytic oxidation of toluene by chemical and biological methods. C. R. Chim. 2015, 18, 1084–1093. [Google Scholar] [CrossRef]
- Santos, V.P.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. Mixture effects during the oxidation of toluene, ethyl acetate and ethanol over a cryptomelane catalyst. J. Hazard. Mater. 2011, 185, 1236–1240. [Google Scholar] [CrossRef]
- Wu, H.; Wang, L.; Zhang, J.; Shen, Z.; Zhao, J. Catalytic oxidation of benzene, toluene and p-xylene over colloidal gold supported on zinc oxide catalyst. Catal. Commun. 2011, 12, 859–865. [Google Scholar] [CrossRef]
- Burgos, N.; Paulis, M.; Mirari Antxustegi, M.; Montes, M. Deep oxidation of VOC mixtures with platinum supported on Al2O3/Al monoliths. Appl. Catal. B Environ. 2002, 38, 251–258. [Google Scholar] [CrossRef]
- Liotta, L.F.; Ousmane, M.; Di Carlo, G.; Pantaleo, G.; Deganello, G.; Boreave, A.; Giroir-Fendler, A. Catalytic removal of toluene over Co3O4-CeO 2 mixed oxide catalysts: Comparison with Pt/Al2O 3. Catal. Lett. 2009, 127, 270–276. [Google Scholar] [CrossRef]
- Genty, E.; Cousin, R.; Capelle, S.; Gennequin, C.; Siffert, S. Catalytic oxidation of toluene and CO over nanocatalysts derived from hydrotalcite-like compounds (X 62+Al 23+): Effect of the bivalent cation. Eur. J. Inorg. Chem. 2012, 2802–2811. [Google Scholar] [CrossRef]
- Busca, G.; Daturi, M.; Finocchio, E.; Lorenzelli, V.; Ramis, G.; Willey, R.J. Transition metal mixed oxides as combustion catalysts: Preparation, characterization and activity mechanisms. Catal. Today 1997, 33, 239–249. [Google Scholar] [CrossRef]
- Sekine, Y.; Nishimura, A. Removal of formaldehyde from indoor air by passive type air-cleaning materials. Atmos. Environ. 2007, 35, 2001–2007. [Google Scholar] [CrossRef]
- Trigueiro, F.E.; Ferreira, C.M.; Volta, J.C.; Gonzalez, W.A.; de Oliveria, P.G.P. Effect of niobium addition to Co/γ-Al2O3 catalyst on methane combustion. Catal. Today 2006, 118, 425–432. [Google Scholar] [CrossRef]
- Garcia, T.; Agouram, S.; Sánchez-Royo, J.F.; Murillo, R.; Mastral, A.M.; Aranda, A.; Vázquez, I.; Dejoz, A.; Solsona, B. Deep oxidation of volatile organic compounds using ordered cobalt oxides prepared by a nanocasting route. Appl. Catal. A Gen. 2010, 386, 16–27. [Google Scholar] [CrossRef]
- Papageorgiou, I.; Brown, C.; Schins, R.; Singh, S.; Newson, R.; Davis, S.; Fisher, J.; Ingham, E.; Case, C.P. The effect of nano- and micron-sized particles of cobalt-chromium alloy on human fibroblasts in vitro. Biomaterials 2007, 28, 2946–2958. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Dash, S.K.; Tripathy, S.; Das, B.; Mandal, D.; Pramanik, P.; Roy, S. Toxicity of cobalt oxide nanoparticles to normal cells; An in vitro and in vivo study. Chem. Biol. Interact. 2015, 226, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; He, J.; Zhang, J.; He, Z.; Hu, Y.; Zhang, C.; He, H. Facile in-situ synthesis of manganese dioxide nanosheets on cellulose fibers and their application in oxidative decomposition of formaldehyde. J. Phys. Chem. C 2011, 115, 16873–16878. [Google Scholar] [CrossRef]
- Sekine, Y. Oxidative decomposition of formaldehyde by metal oxides at room temperature. Atmos. Environ. 2002, 36, 5543–5547. [Google Scholar] [CrossRef]
- Kim, S.C. The catalytic oxidation of aromatic hydrocarbons over supported metal oxide. J. Hazard. Mater. 2002, 91, 285–299. [Google Scholar] [CrossRef]
- Zedan, A.F.; Polychronopoulou, K.; Asif, A.; AlQaradawi, S.Y.; AlJaber, A.S. Cu-Ce-O catalyst revisited for exceptional activity at low temperature CO oxidation reaction. Surf. Coat. Technol. 2018, 354, 313–323. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Song, L.; Hou, F.; Yang, Y.; Wang, Y.; Liu, N. Enhanced catalytic performance for CO oxidation and preferential CO oxidation over CuO/CeO2 catalysts synthesized from metal organic framework: Effects of preparation methods. Int. J. Hydrogen Energy 2018, 43, 18279–18288. [Google Scholar] [CrossRef]
- Alejandre, A.; Medina, F.; Salagre, P.; Correig, X.; Sueiras, J.E. Preparation and study of Cu-Al mixed oxides via hydrotalcite-like precursors. Chem. Mater. 1999, 11, 939–948. [Google Scholar] [CrossRef]
- Kannan, S.; Rives, V.; Knözinger, H. High-temperature transformations of Cu-rich hydrotalcites. J. Solid State Chem. 2004, 177, 319–331. [Google Scholar] [CrossRef]
- Teixeira, C.D.O.P.; Montani, S.D.S.; Palacio, L.A.; Zotin, F.M.Z. The effect of preparation methods on the thermal and chemical reducibility of Cu in Cu-Al oxides. Dalton Trans. 2018, 47, 10989–11001. [Google Scholar] [CrossRef] [PubMed]
- Delimaris, D.; Ioannides, T. VOC oxidation over CuO-CeO2 catalysts prepared by a combustion method. Appl. Catal. B Environ. 2009, 89, 295–302. [Google Scholar] [CrossRef]
- Heynderickx, P.M.; Thybaut, J.W.; Poelman, H.; Poelman, D.; Marin, G.B. The total oxidation of propane over supported Cu and Ce oxides: A comparison of single and binary metal oxides. J. Catal. 2010, 272, 109–120. [Google Scholar] [CrossRef]
- Qian, J.; Hou, X.; Wang, F.; Hu, Q.; Yuan, H.; Teng, L.; Li, R.; Tong, Z.; Dong, L.; Li, B. Catalytic reduction of NO by CO over promoted Cu3Ce0.2Al0.8 composite oxides derived from hydrotalcite-like Compounds. J. Phys. Chem. C 2018, 122, 2097–2106. [Google Scholar] [CrossRef]
- Chen, C.; Wang, R.; Shen, P.; Zhao, D.; Zhang, N. Inverse CeO2/CuO catalysts prepared from heterobimetallic metal-organic framework precursor for preferential CO oxidation in H2-rich stream. Int. J. Hydrogen Energy 2015, 40, 4830–4839. [Google Scholar] [CrossRef]
- Genty, E.; Dib, H.; Brunet, J.; Poupin, C.; Siffert, S.; Cousin, R. Effect of Ce Addition on mgal mixed oxides for the total oxidation of CO and toluene. Top. Catal. 2019, 62, 397–402. [Google Scholar] [CrossRef]
- Sharma, M.K.; Melesse, S.F. Optimal block designs for CDC experiments method (2). Metron 2011, 69, 297–307. [Google Scholar] [CrossRef]
- Alejandre, A.; Medina, F.; Rodriguez, X.; Salagre, P.; Sueiras, J.E. Preparation and activity of copper, nickel and copper-nickel-al mixed oxides via hydrotalcite-like precursors for the oxidation of phenol aqueous solutions. Stud. Surf. Sci. Catal. 2000, 130 B, 1763–1768. [Google Scholar] [CrossRef]
- Dow, W.P.; Wang, Y.P.; Huang, T.J. TPR and XRD studies of yttria-doped ceria/γ-alumina-supported copper oxide catalyst. Appl. Catal. A Gen. 2000, 190, 25–34. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, R. A new insight into the morphology effect of ceria on CuO/CeO2 catalysts for CO selective oxidation in hydrogen-rich gas. Catal. Sci. Technol. 2016, 6, 3862–3871. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Arango-Díaz, A.; Marrero-Jerez, J.; Núñez, P.; Moretti, E.; Storaro, L.; Rodríguez-Castellón, E. Catalytic behaviour of CuO-CeO2 systems prepared by different synthetic methodologies in the CO-PROX reaction under CO2-H2O feed stream. Catalysts 2017, 7, 160. [Google Scholar] [CrossRef]
- Qi, L.; Yu, Q.; Dai, Y.; Tang, C.; Liu, L.; Zhang, H.; Gao, F.; Dong, L.; Chen, Y. Influence of cerium precursors on the structure and reducibility of mesoporous CuO-CeO2 catalysts for CO oxidation. Appl. Catal. B Environ. 2012, 119–120, 308–320. [Google Scholar] [CrossRef]
- Sun, S.; Mao, D.; Yu, J.; Yang, Z.; Lu, G.; Ma, Z. Lowerature CO oxidation on CuO/CeO2 catalysts: The significant effect of copper precursor and calcination temperature. Catal. Sci. Technol. 2015, 5, 3166–3181. [Google Scholar] [CrossRef]
- Sumrunronnasak, S.; Chanlek, N.; Pimpha, N. Improved CeCuOx catalysts for toluene oxidation prepared by aqueous cationic surfactant precipitation method. Mater. Chem. Phys. 2018, 216, 143–152. [Google Scholar] [CrossRef]
- Deng, C.; Li, B.; Dong, L.; Zhang, F.; Fan, M.; Jin, G.; Gao, J.; Gao, L.; Zhang, F.; Zhou, X. NO reduction by CO over CuO supported on CeO2-doped TiO2: The effect of the amount of a few CeO2. Phys. Chem. Chem. Phys. 2015, 17, 16092–16109. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Du, L.; Wang, W.; Yan, H.; Wang, X.; Jin, Z.; Song, Q.; Si, R.; Jia, C. Copper-ceria sheets catalysts: Effect of copper species on catalytic activity in CO oxidation reaction. J. Rare Earths 2017, 35, 1186–1196. [Google Scholar] [CrossRef]
- Khassin, A.A.; Yurieva, T.M.; Kaichev, V.V.; Bukhtiyarov, V.I.; Budneva, A.A.; Paukshtis, E.A.; Parmon, V.N. Metal-support interactions in cobalt-aluminum co-precipitated catalysts: XPS and CO adsorption studies. J. Mol. Catal. A Chem. 2001, 175, 189–204. [Google Scholar] [CrossRef]
- Cheng, J.; Yu, J.; Wang, X.; Li, L.; Li, J.; Hao, Z. Novel CH4 combustion catalysts derived from Cu-Co/X-Al (X = Fe, Mn, La, Ce) hydrotalcite-like compounds. Energy Fuels 2008, 22, 2131–2137. [Google Scholar] [CrossRef]
- Bai, Y.; Bian, X.; Wu, W. Catalytic properties of CuO/CeO2-Al2O3 catalysts for low concentration NO reduction with CO. Appl. Surf. Sci. 2019, 463, 435–444. [Google Scholar] [CrossRef]
- Chang, Z.; Zhao, N.; Liu, J.; Li, F.; Evans, D.G.; Duan, X.; Forano, C.; De Roy, M. CuCeO mixed oxides from Ce-containing layered double hydroxide precursors: Controllable preparation and catalytic performance. J. Solid State Chem. 2011, 184, 3232–3239. [Google Scholar] [CrossRef]
- Brunet, J.; Genty, E.; Barroo, C.; Cazier, F.; Poupin, C.; Siffert, S.; Thomas, D.; De Weireld, G.; de Bocarmé, T.V.; Cousin, R. The CoAlCeO mixed oxide: An alternative to palladium-based catalysts for total oxidation of industrial VOCs. Catalysts 2018, 8. [Google Scholar] [CrossRef]
- He, C.; Yu, Y.; Yue, L.; Qiao, N.; Li, J.; Shen, Q.; Yu, W.; Chen, J.; Hao, Z. Low-temperature removal of toluene and propanal over highly active mesoporous CuCeOx catalysts synthesized via a simple self-precipitation protocol. Appl. Catal. B Environ. 2014, 147, 156–166. [Google Scholar] [CrossRef]
- Pérez, A.; Montes, M.; Molina, R.; Moreno, S. Cooperative effect of Ce and Pr in the catalytic combustion of ethanol in mixed Cu/CoMgAl oxides obtained from hydrotalcites. Appl. Catal. A Gen. 2011, 408, 96–104. [Google Scholar] [CrossRef]








| Samples | CuO Crystallite Size (nm) * | Experimental Atomic Ratio (Cu/Al/Ce) ** | BET Surface Area (m2/g) | Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|---|---|
| Cu6Al1.2Ce0.8-O | 15.2 | 5.5/1.2/0.8 | 47 | 17.3 | 0.19 |
| Cu6Al1.5Ce0.5-O | 20.7 | 5.9/1.5/0.5 | 37 | 22.7 | 0.22 |
| Cu6Al1.8Ce0.2-O | 17.7 | 5.6/1.8/0.2 | 33 | 23.9 | 0.19 |
| Cu6Al2-O | 17.5 | 5.6/2.0/- | 17 | 35.4 | 0.14 |
| Samples | Temperature (°C) | H2 Consumption (μmol/g) (T ˂ 350 °C) | ||||
|---|---|---|---|---|---|---|
| T α | T β | T γ | Peak α | Peak β | Peak γ | |
| CuO | - | - | 289 | n.d * | n.d * | n.d * |
| Cu6Al1.2Ce0.8-O | 168 | 206 | 239 | 942 | 6081 | 1542 |
| Cu6Al1.5Ce0.5-O | 187 | 221 | 249 | 590 | 4974 | 2866 |
| Cu6Al1.8Ce0.2-O | 210 | 230 | 277 | 263 | 1666 | 6838 |
| Cu6Al2-O | 215 | 259 | 302 | 181 | 5235 | 3610 |
| Samples | B.E. * Ce 3d u’’’/eV | CeIII/CeIV | B.E. * O 1s/eV (%) | nCu/nM ** | nO/nTot *** | |
|---|---|---|---|---|---|---|
| O-I | O-II | |||||
| Cu6Al1.2Ce0.8-O | 917.1 | 0.11 | 530.2 (26.1%) | 532.6 (73.9%) | 0.48 | 0.81 |
| Cu6Al1.5Ce0.5-O | 916.8 | 0.13 | 530.0 (23.0%) | 532.0 (77.0%) | 0.39 | 0.47 |
| Cu6Al1.8Ce0.2-O | 916.4 | 0.36 | 529.8 (17.9%) | 531.8 (82.1%) | 0.33 | 0.57 |
| Cu6Al2-O | - | - | 530.3 (24.0%) | 532.2 (76.0%) | 0.65 | 0.56 |
| Samples | T50 (°C) | |
|---|---|---|
| Toluene | Ethanol | |
| Cu6Al1.2Ce0.8-O | 254 | 210 |
| Cu6Al1.5Ce0.5-O | 261 | 210 |
| Cu6Al1.8Ce0.2-O | 286 | 230 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dib, H.; El Khawaja, R.; Rochard, G.; Poupin, C.; Siffert, S.; Cousin, R. CuAlCe Oxides Issued from Layered Double Hydroxide Precursors for Ethanol and Toluene Total Oxidation. Catalysts 2020, 10, 870. https://doi.org/10.3390/catal10080870
Dib H, El Khawaja R, Rochard G, Poupin C, Siffert S, Cousin R. CuAlCe Oxides Issued from Layered Double Hydroxide Precursors for Ethanol and Toluene Total Oxidation. Catalysts. 2020; 10(8):870. https://doi.org/10.3390/catal10080870
Chicago/Turabian StyleDib, Hadi, Rebecca El Khawaja, Guillaume Rochard, Christophe Poupin, Stéphane Siffert, and Renaud Cousin. 2020. "CuAlCe Oxides Issued from Layered Double Hydroxide Precursors for Ethanol and Toluene Total Oxidation" Catalysts 10, no. 8: 870. https://doi.org/10.3390/catal10080870
APA StyleDib, H., El Khawaja, R., Rochard, G., Poupin, C., Siffert, S., & Cousin, R. (2020). CuAlCe Oxides Issued from Layered Double Hydroxide Precursors for Ethanol and Toluene Total Oxidation. Catalysts, 10(8), 870. https://doi.org/10.3390/catal10080870