1. Introduction
The Association for Automotive Internal Combustion Engine Technology, so-called AICE, was once an organization of eight Japanese passenger car manufacturers (Original Equipment Manufacturers: OEMs) and the Japan Automobile Research Institute (JARI), and has since grown to include two organizations and nine OEMs. At the time of its launch, joint research was conducted with subsidies from the Ministry of Economy, Trade, and Industry (METI) and investments from OEMs. The first phase of the collaboration was a three-year scheme for 2014–2016. The second phase was the following two years 2017–2018, which was an opportunity for autonomy from government subsidies. It has been more than five years since this industries–academia–academia joint research started. This paper will introduce this section, which involves the culmination of five years of our work [
1].
The AICE mission originally started with the goal of increasing the combustion efficiency of internal combustion engines and reducing CO2 emissions in the name of the national project. The AICE, which is mainly in charge of after treatment, has been launched with the following six themes at the time of its inception, targeting diesel vehicles with high combustion efficiency among internal combustion engines.
- (1)
Basic research on the advancement of diesel particulate filter (DPF) functions.
- (2)
Basic research on the advancement of the regeneration function of DPF.
- (3)
DPF simulation application research.
- (4)
Basic research on white smoke control technology.
- (5)
Basic research on exhaust gas recirculation (EGR) deposit suppression technology.
- (6)
Basic research on innovative NOx reduction catalysts.
Themes (1) to (3) are related to the diesel particulate removal filter, as in diesel aftertreatment. Theme (4) deals with the suppression of relatively high boiling point fuel-derived components that precipitate on the catalytic converter during cold start, and subsequently become visible vapor (i.e., white smoke) when they are expelled by heating. Theme (5) is also related to relatively high boiling point compound-derived components that occur in diesel EGR systems. Our first mission was to develop a unique deNOx catalyst in the theme (6).
In Japan, diesel passenger cars are very rare. The research theme of AICE, which advocates the systemization of after treatment technologies for clean diesel, reminds us that there are urgent problems to be solved in the exhaust of clean diesel, although the exhaust is cleaner than it was in the past.
In response to the aforementioned theme (6), what is required to us “chemists”, perhaps, is a detailed clarification of the reaction mechanism and the derivation of kinetic equations. First, the following proposition was put forward.
- (I)
Development of a unique zeolite catalyst.
- (II)
Bridging chemistry and mechanical engineering.
The first proposition is, in essence, a screening work on zeolite catalysts (worldwide termed SCR, selective catalytic reduction of NOx, using ammonia as a reductant).
The NH
3-SCR (more precisely, using urea aqueous solution, AdBlue
®) is actually conducted as follows: urea aqueous solution is loaded on-board in a tank and sprayed into the exhaust gas pipe, which undergoes hydrolysis to form two molecules of ammonia on-site from a single molecule of urea, as the combustion exhaust naturally contains a large quantity of water. In the 1930s, NH
3-SCR, in which ammonia aqueous solution was sprayed, was applied to purify thermal NOx emitted from high-temperature steel melting furnaces. Due to the high concentration of water vapor in combustion flue gas, this water usually becomes a catalyst poisoning for oxide catalysts where Lewis acidity, the redox property of metal, and oxygen deficiency sites are important [
2]. However, the NH
3 molecule overcame the competitive adsorption of water molecules and reacted selectively with NOx at lower temperatures even in the exhaust gas with a high concentration of oxygen remaining. As an aside, the influence of the NO
2 concentration in NOx on the overall reaction rate was already clarified at that time [
3], at least the repetition of definitions such as “fast SCR” and “slow SCR” in the present day is seen.
At that time, an investigation of so-called first generation NH
3-SCR was considered, which was not suitable for on-board use due to the toxicity of ammonia, and the NH
3-SCR catalytic process was considered to be a countermeasure for stationary, fixed sources of NOx. Here, we would like to stress that Japanese researchers were the leaders in this generation, especially in the reaction mechanism studies [
4].
Subsequently, the rise of urea-SCR systems in Europe and the United States has become more pronounced in the 2000s. This can be regarded as the second generation investigation. First, iron ion-exchanged zeolite, and later copper ion-exchanged zeolite were found to be the most suitable catalysts.
In Japan, the selective reduction reaction HC-SCR using hydrocarbons HC for removal of NOx from the exhaust gas, was discovered in the 1990s. Screening of copper zeolites and the other catalytic systems and examining the reaction mechanisms were the main subject. Thus, many researchers have joined the field and conducted evaluation tests as if they were filling out a candidate from the Periodic Table [
5]. In the first-generation era, zeolite was not received that could survive under the severe conditions essential for automotive applications.
Zeolite was generally considered weak against heat, and did not reach practical use. There existed another question: is it possible for molecules in the exhaust gas to enter the molecular-sized pores of zeolite under such a massive stream of exhaust? Looking back at that time, the 1:1 cooperation between industry (government) and academia was not so intimate, and the exchange of information was insufficient. Finally, zeolite was not much put to use in a practical after treatment application.
The main investigation of the urea (NH
3)-SCR was carried out on copper zeolites, a group of zeolites defined as small pore zeolites with a CHA structure, SSZ-13 [
6]. For iron zeolites, a large pore zeolite beta with *BEA structure was mainly used. Other complex oxides such as V
2O
5 and CeO
2, which have been well studied by Chinese researchers, are also known [
7], and their catalytic properties are summarized in a review by a certain catalyst manufacturer [
8]. We do not need to compete with the catalyst technologies in the same field as the other researchers in the world [
9], because we in Japan produce the world’s leading researchers in zeolite synthesis. The expertise in zeolite synthesis in Japan has provided us with “durable” zeolite, where probably the first hurdle exists to be overcome. The “robustness” above-mentioned is based on the assumption that the zeolite crystal structure is not collapsed and dealumination from the zeolite framework does not occur even after exposure to 800 °C for several hours in a water vapor atmosphere, when considering the application as a catalyst for automotive exhaust purification. The original screening target was not to find a zeolite with a novel structure, but the same type of zeolite as that crystallized by the conventional hydrothermal method would be acceptable. Furthermore, we invited to test some zeolites that had been used by professors in other fields working on other reactions, but had never been used for automotive emission control before.
In this way, a journey in search of “good” zeolite was about to begin. We received a zeolite sample from a Japanese researcher who provided us with a potential new SCR catalyst candidate, and we decided to evaluate the NH3-SCR characteristics and hydrothermal resistance of the zeolite under unified conditions by ion exchange of Cu. It is important to note that these characterization conditions as well as catalyst evaluation have been made as the AICE standard agreed by all OEMs. Our site will become a base for comparison on the same level of evaluation, and we will form a center to aggregate the conditions, samples for testing, and the results of each analysis.
3. Method
With the aforementioned strategy, we began, in essence, to work on zeolite screening. It was decided to investigate whether there were any common “descriptors” among the various zeolites provided, without preconceptions such as that a small pore 8-membered ring zeolite is better, or that a large pore zeolite is affected by the adsorption of coexisting HCs, as is generally believed. About 30 structures of zeolites of various sizes and synthetic methods have been investigated [
1] and their results are summarized by the following three items:
As the reaction was under the standard SCR condition, and the molar ratio of NO to NH
3 supplied to the catalyst was 1:1, only this 1:1 reaction proceeded at a low temperature of about 200 °C. Based on the previous studies, this reaction was known to be catalyzed by Cu cationic species located at the ion exchange site [
14]. However, at high temperatures such as 600 °C, the excess oxygen causes the ammonia to undergo oxidation [
15]. Therefore, the term “ammonia selectivity toward SCR” was defined as follows: a value close to 1 indicates the presence of Cu cations and the progress of only selective reduction reaction, while a value away from 1 indicates the progress of non-selective reaction by copper oxide species located outside the zeolite framework.
- (2)
Si/Al ratio, Cu loading
The Si/Al molar ratio of the zeolite framework applied is one of the major basic properties of the zeolite, and it can be a parameter that controls not only the surface properties of the zeolite, but also the structural properties. A zeolite with a high Si/Al ratio is generally stable against high hydrothermal conditions, while a zeolite with a low Si/Al ratio possesses many metal ion exchange sites such as Cu ions and have a large ion exchange capacity. A zeolite with both high ion exchange capacity and high hydrothermal stability is highly desired.
- (3)
Large micropore
In the absence of HC, a large pore zeolite such as *BEA and EMT showed a high SCR performance. Some of them such as OSDA-free zeolite beta maintained a high NO conversion after hydrothermal treatment [
1]. In general, inhibition of adsorption by coexisting HCs and structural disintegration due to the heat of combustion of HC are often pointed out as a disadvantage of large-pore to extra-large-pore zeolites. However, the size of the pore does not seem to affect the essence of the “intrinsic” activity of Cu such as the ability to maintain active copper species and surface properties.
More unfortunate is that by-product selectivity to N
2O is unusually high in large pore zeolites, in particular EMT, accounting for more than half of the nitrogen-containing products. This can be explained by the presence of a large cage that can easily form the intermediate NH
4NO
3 for N
2O formation, as discussed in the reaction mechanism [
16,
17].
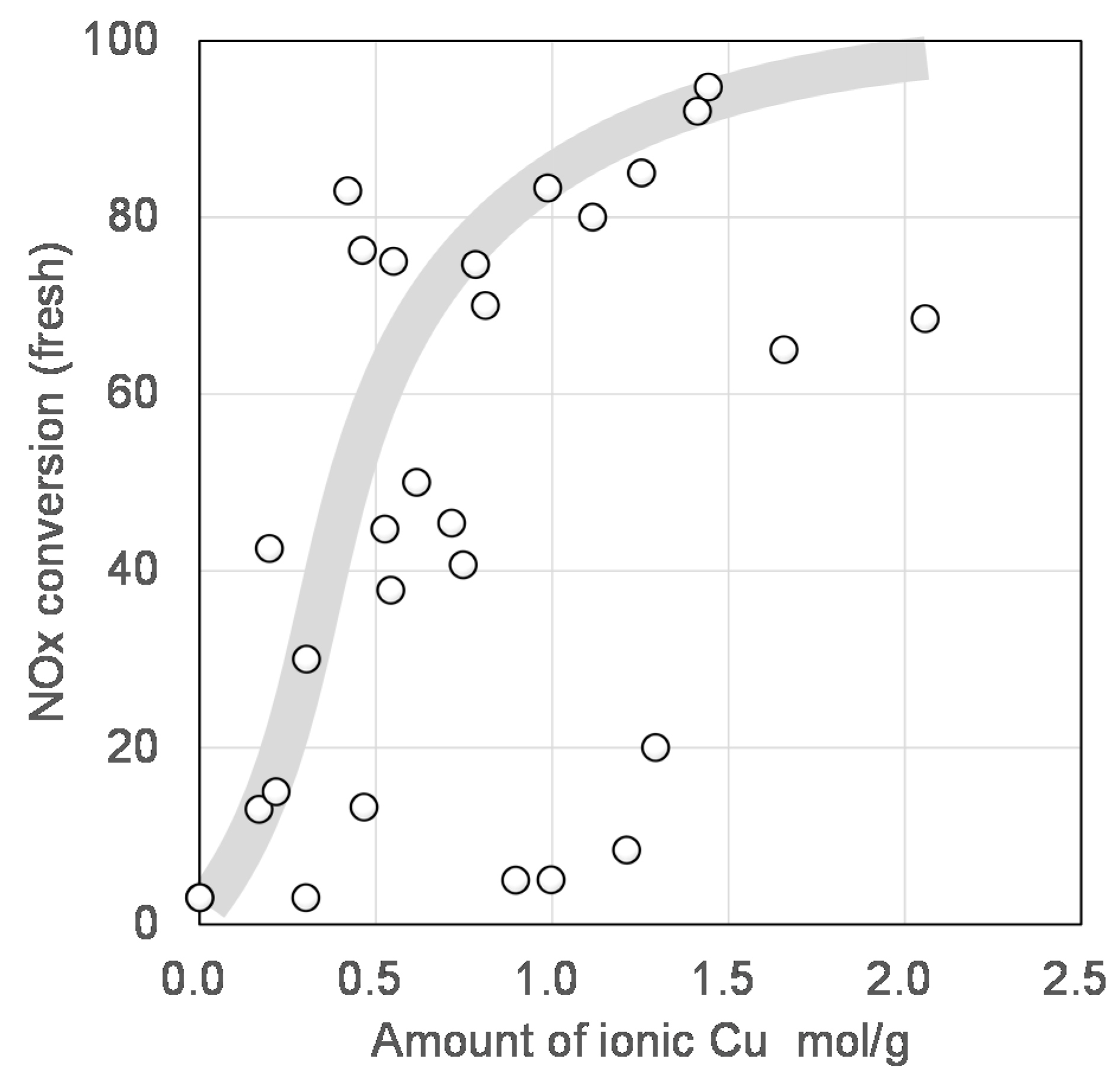
Figure 9 shows the preliminary summary of zeolite mining for use as the support of the NH
3-SCR catalyst [
1]. It is clearly demonstrated that NO conversion at 200 °C was increased with an increase in the amount of ionic Cu loaded on each zeolite, where the ionic Cu was detected as another desorption peak in the NH
3-TPD profile between 150 and 300 °C, apparently different from the original NH
3 desorption derived from the Broensted acid site in each zeolite. Apart from the discussion on the active Cu species, it must be a natural trend of the activity increment. In another word, the zeolitic framework structure seems to have little influence on the “intrinsic” activity of the Cu ion. This strongly indicates that the main and important role of zeolite support is, first of all, to hold such an active Cu ionic species while maintaining its activity not to be deactivated. Additionally, it is quite natural that a large number of Cu species result in high catalytic performance for NH
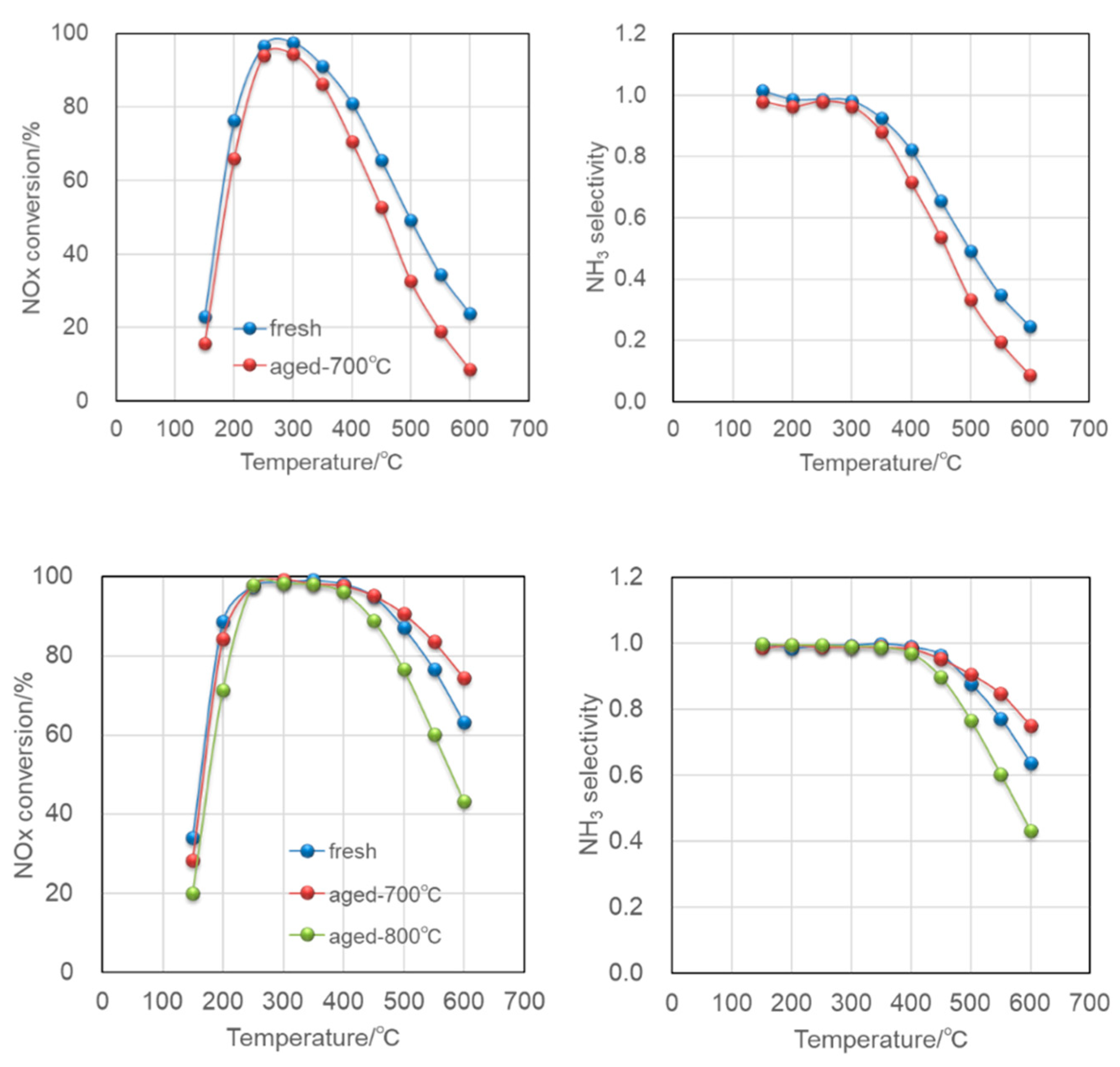
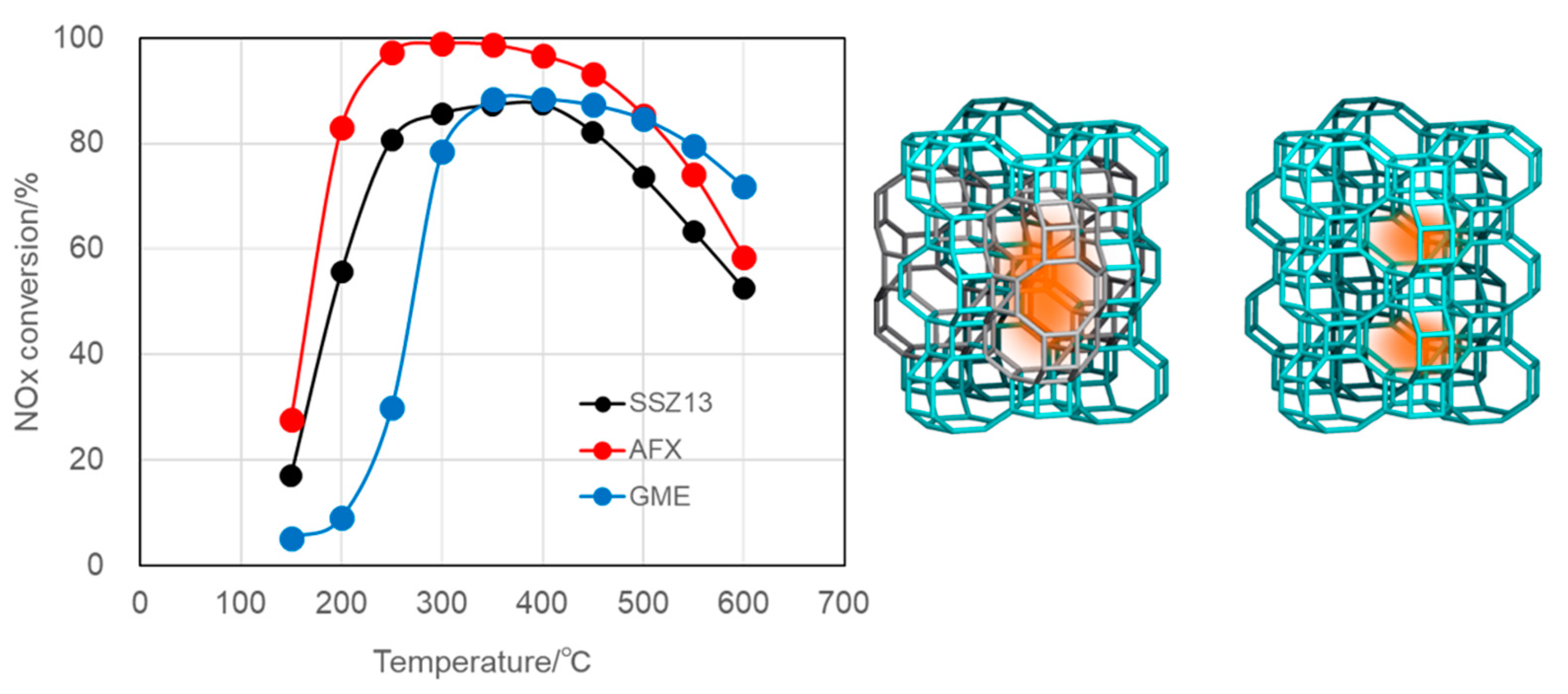
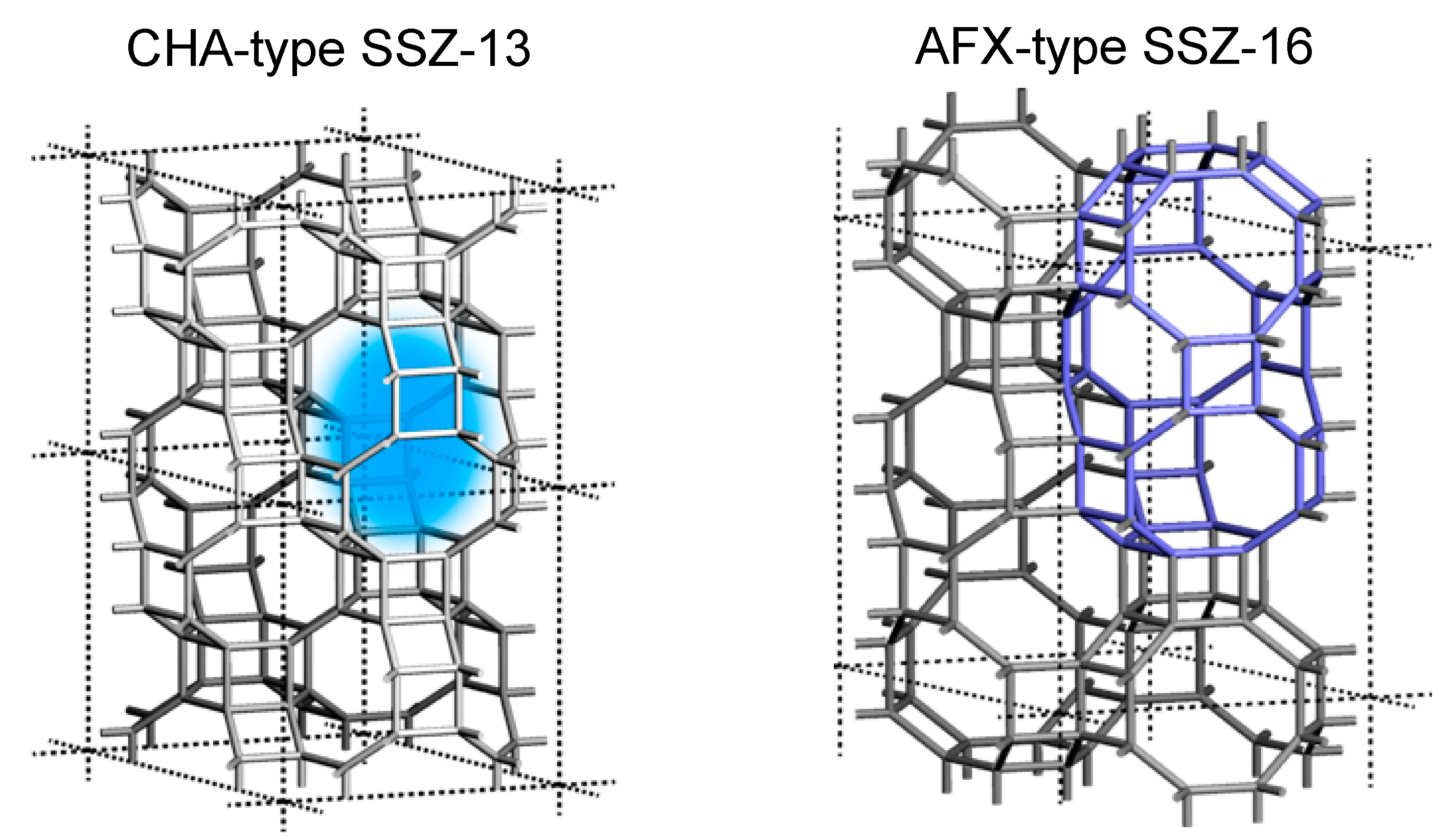
3-SCR; therefore, a large number of ion-exchange sites, that is, Al in the framework of aluminosilicate zeolites, should be kept inside the framework even under severe conditions exposed by automobile exhaust. The plots showing above 70% NO conversion were derived from Al-rich SSZ-13 (Si/Al = 9), beta (Si/Al = 5), and SSZ-16 (Si/Al = 3.3). We focused on SSZ-16 in this study.
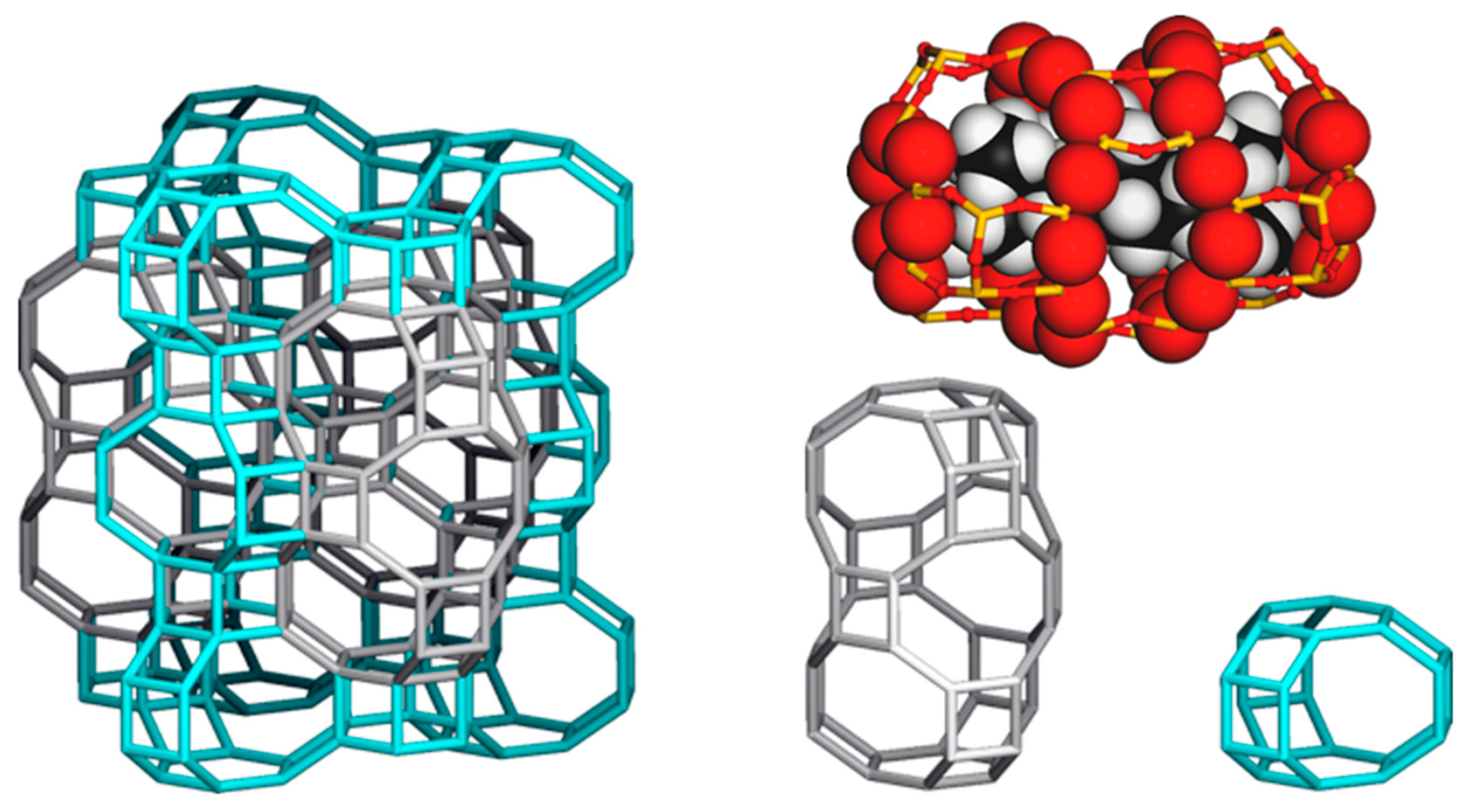
The framework type AFX, the three letter code of the structure of SSZ-16 [
18] and SAPO-56 [
19] assigned by the IZA synthesis committee in 1994, has a large cavity of 0.55 × 1.35 nm in size, named
aft as the composite building unit, and a small
gme cage of 0.33 × 0.74 nm, each of which is connected by a
d6r unit [
10]. Compared to the SSZ-13 CHA structure (see
Figure 10), which holds only one type of medium
cha cage of 0.70 × 0.83 nm in size, a large reaction field is expected even though the cages are connected through an 8-membered ring [
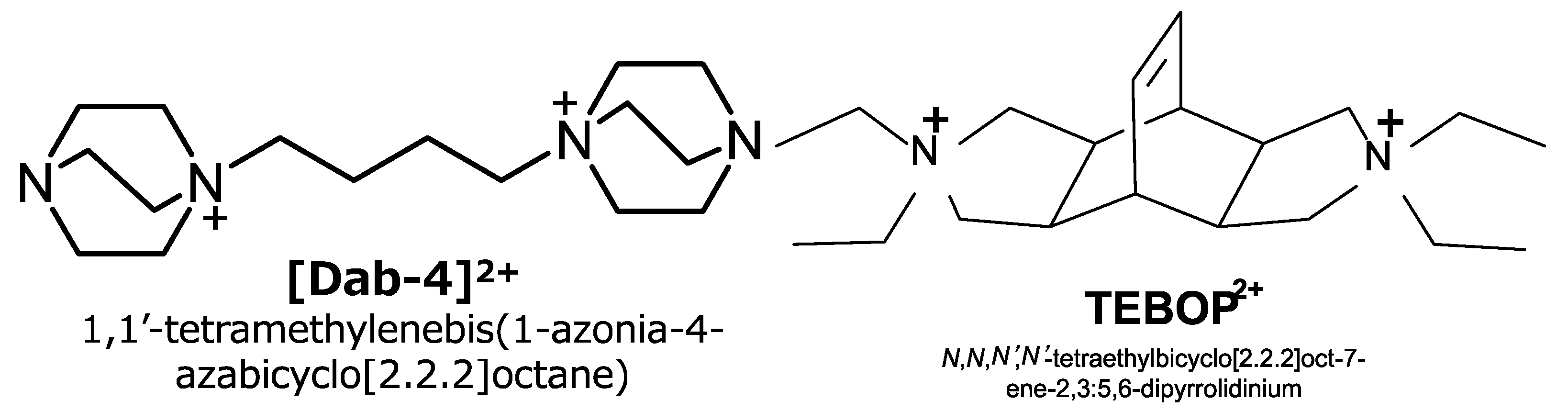
10]. In this sense, the AFX zeolite is classified in the group of small pore zeolites, the same class of CHA. SSZ-16 was first reported to synthesize hydrothermally by use of 1,1′-tetramethylenebis(1-azonia-4-azabicylco[2,2,2]octane, [Dab-4]
2+, a cationic compound, as an organic structure-directing agent [
10,
20], and the product has a well-crystalline rugby-ball-like particle of 2–3 μm in size with Si/Al = 6 or so. High silica SSZ-16 was also synthesized by use of 1,3-bis(1-adamantyl)imidazolium cation to obtain Si/Al = 16 with a larger crystalline size of 5 μm [
21].
One of the coauthors of this research has already reported that the same zeolite could be crystallized by
N,
N,
N′,
N′-tetraethylbicyclo[2.2.2]oct-7-ene-2,3:5,6-dipyrrolidinium cation, TEBOP
2+, and they successfully obtained the zeolite with less than 500 nm tetragonal particles having Si/Al = 6~9 [
22]. Such unique OSDA could be used to synthesize MSE or other types of zeolite by the same group. Professor Corma and his co-workers reported simultaneously that AFX was crystallized by use of TEBOP
2+ in a similar manner (see
Figure 11) [
23]. This zeolite seems to deliver an ideal structure and composition in the name of automobile purification; thus we decided to call it the “AFX” zeolite in this paper to differentiate and categorize a different case with SSZ-16.
Experimental
All zeolite samples were supplied from industries or academia [
1,
22]. SSZ-16 and AFX zeolites were home-made according to the procedures reported elsewhere [
22]. The copper ion exchange was carried out at room temperature for 24 h using an aqueous solution of copper acetate. Typically, the chemical compositions of Cu-SSZ-16 and Cu-AFX used in this study were Si/Al = 3.3 and 5.3, and Cu/Al = 0.30 and 0.32, respectively, which corresponds to 5.5 and 4.1 wt% of Cu loading. Characterization of the product with Cu ion exchange was conducted by an inductively coupled plasma-atomic emission spectrometry (ICP-AES, SPS4000, Seiko Instruments Inc., Chiba, Japan), a X-ray diffractometer (XRD, Rint2100, Rigaku, Tokyo, Japan), a N
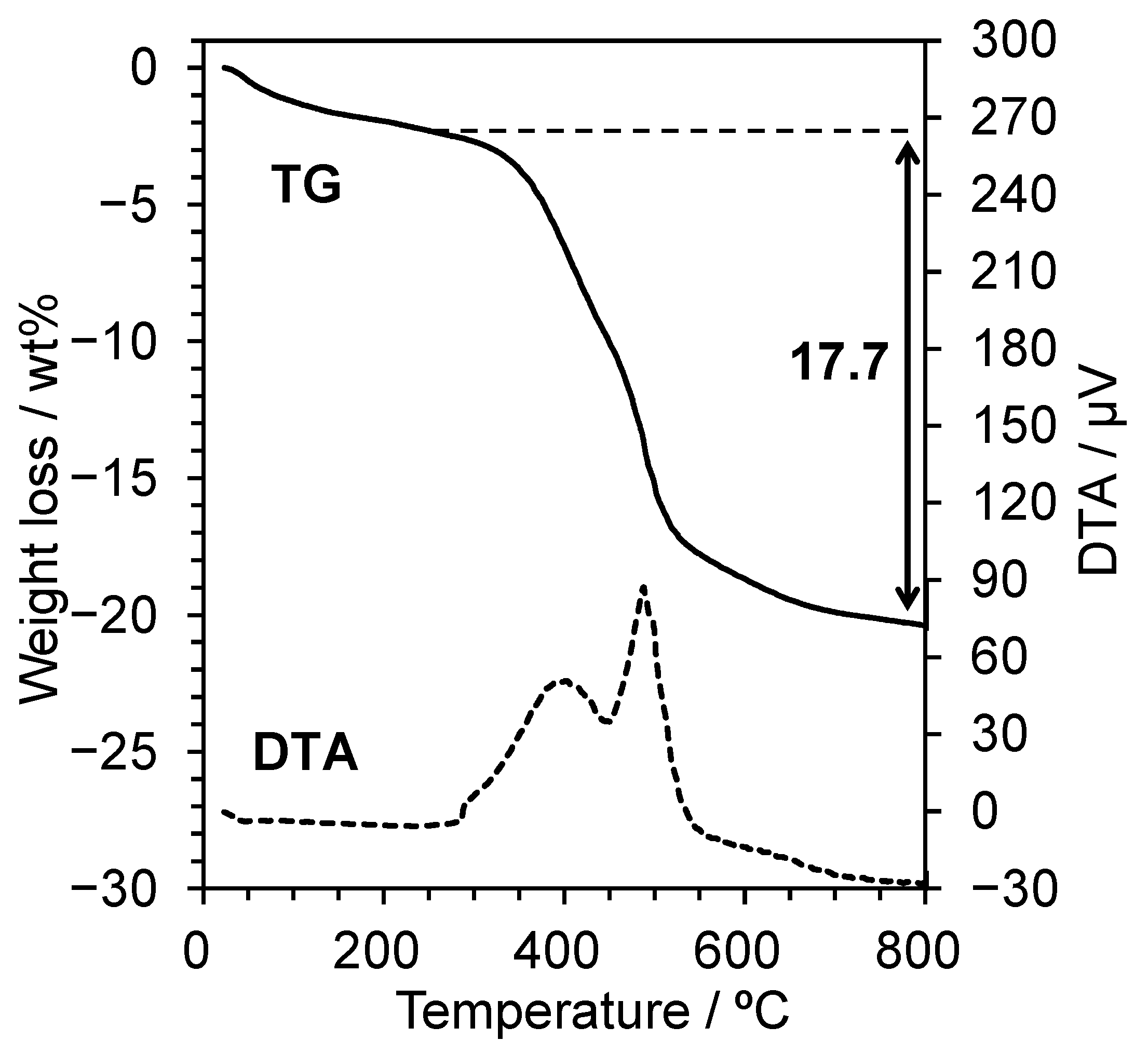
2 sorption equipment (Quadrosorb, Quantachrome, Boynton Beach, FL, USA), a thermogravimetry-differential thermal analysis (TG-DTA, Thermoplus EVO II TG8120, Rigaku, Tokyo, Japan; TGDTA2000SA, Bruker AXS, Billerica, MA, USA), and
29Si magic angle spinning-nuclear magnetic resonance (MASNMR, ECA-600, JEOL, Tokyo, Japan).
The SCR test reaction was conducted by a plug-flow, fix-bed reactor attached with a 10 mm diameter glass tube and an electric furnace, which attained an increase in the catalyst bed temperature up to 900 °C. The mixed gas composed of NO, 300 ppm; NH3, 300 ppm; O2, 5%; H2O, 3% was balanced by N2 to be 200 cm3/min, then flown onto a catalyst 30 mg (SV = 144,000 h−1). After degreening the catalyst at 600 °C, the catalytic performance was measured from 150 °C to 600 °C. The conditions for hydrothermal aging was as follows: temperature, 700–900 °C; duration, 16 h; composition of the feed, 5%H2O balanced by 10% oxygen, and the remaining nitrogen. Infrared detectors and a chemiluminescence NOx analyzer (VA-5000 series, Horiba Ltd., Kyoto, Japan) were used to detect those components, quantify, and calculate the NO and NH3 conversions. A small amount of N2O below 10 ppm was detected in almost all of our experiments; nevertheless, the experimental conditions or catalytic nature was altered.
4. Conclusions
In summary, our zeolite mining project led us to discuss the role of zeolite as the support of the active Cu ion for SCR of NO by use of a NH3 reductant. We focused on the selectivity of NH3 for SCR as the key term to figure out the better candidate. From this sense, the capacity of Cu ion storage, which simply means the Al content in the zeolite framework, is quite important to at last obtain high NO conversion in a wider active temperature window.
We focused on the AFX zeolite, which we differentiated in this paper with SSZ-16 having the same topology, but with a different synthesis method using a different OSDA TEBOP2+ in the synthesis media. The rigid feature of the OSDA molecule led to adjusting the Al location in the topology, resulting in a large amount of highly isolated Cu ionic species on zeolites with high hydrothermal stability, even at 800~900 °C.
Based on the above characteristic samples, the next generation of zeolites will be possibly developed, and people may think that this is not going to be easy. After all, it is still the largest common SCR candidate zeolite (distinguished as a new zeolite in our opinion) within the nine Japanese automobile OEMs. From these findings, it seems that it is not so easy to find out the common descriptor of activity and durability. In this sense, we could figure out the common descriptor for high NH
3 selectivity against SCR and the wide temperature window of SCR. The zeolitic catalyst carrier should be capable of providing ion exchange sites (stable retention of active Cu ion species) and inhibiting Al removal from its framework. Finally, these functions result in high selectivity and wide temperature window. It is important to note that Cu ion capacity is a key obtained from the examination of our AFX zeolites and many zeolites, and Al retention is a key obtained from our P-modified zeolites [
24,
25]. Both researchers who are familiar with this field will submit maximal common descriptors and solutions that are considered to be “natural”. The knowledge provided by the zeolite synthesizer, which is the pride of Japan, is very important.
Not only that, what we should do is to make the greatest common descriptor into a least common multiple. Each OEM has developed their own engine philosophy, its own exhaust gas concepts, its actual exhaust composition, and its own measures. The proposal from the academic world would become common for OEMs to come up with each solution. In this AICE project, both parties could make the best performance in identical research.