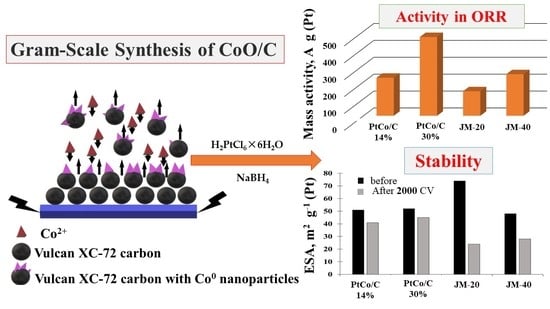
Gram-Scale Synthesis of CoO/C as Base for PtCo/C High-Performance Catalysts for the Oxygen Reduction Reaction
Abstract
:1. Introduction
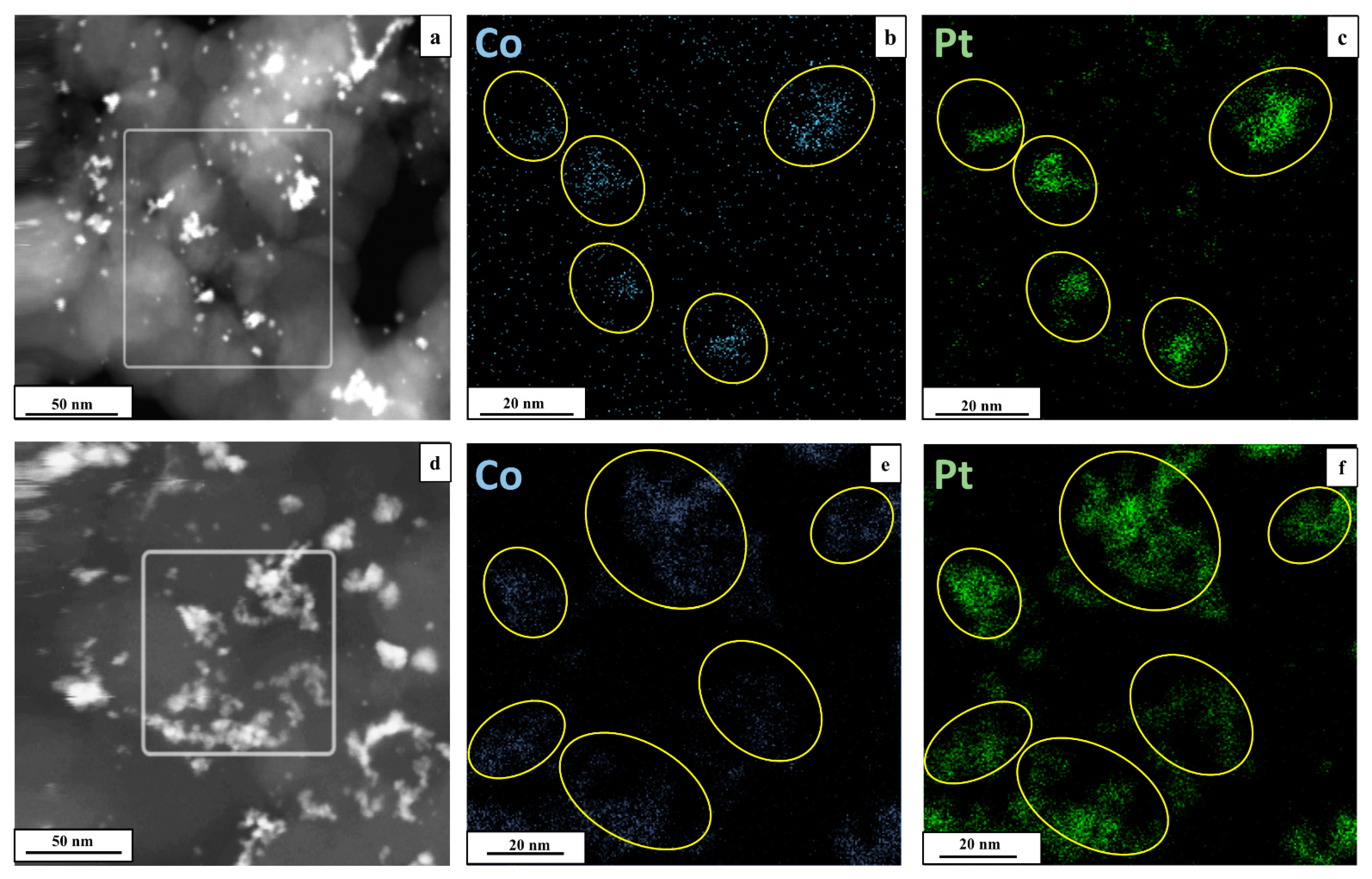
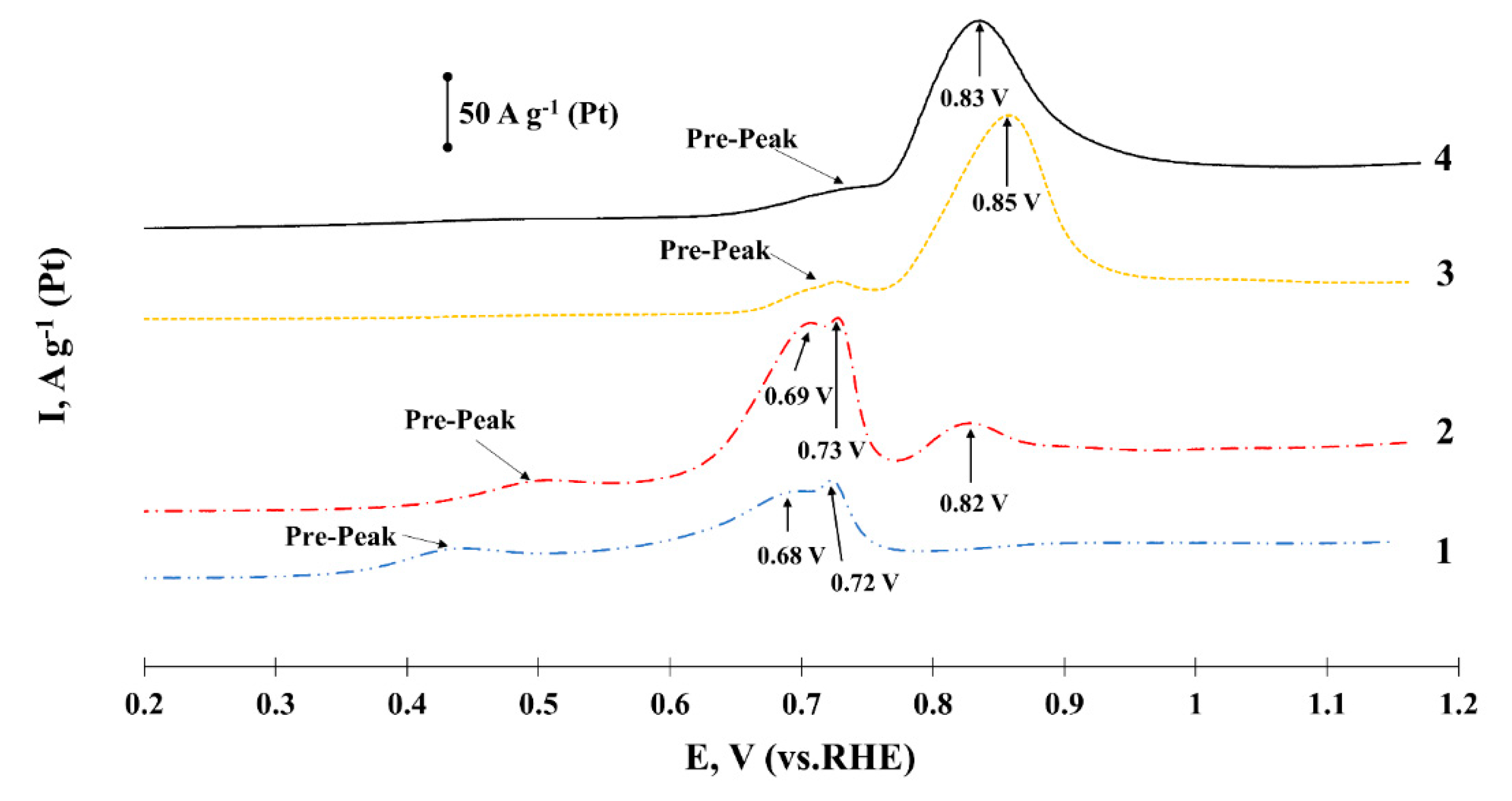
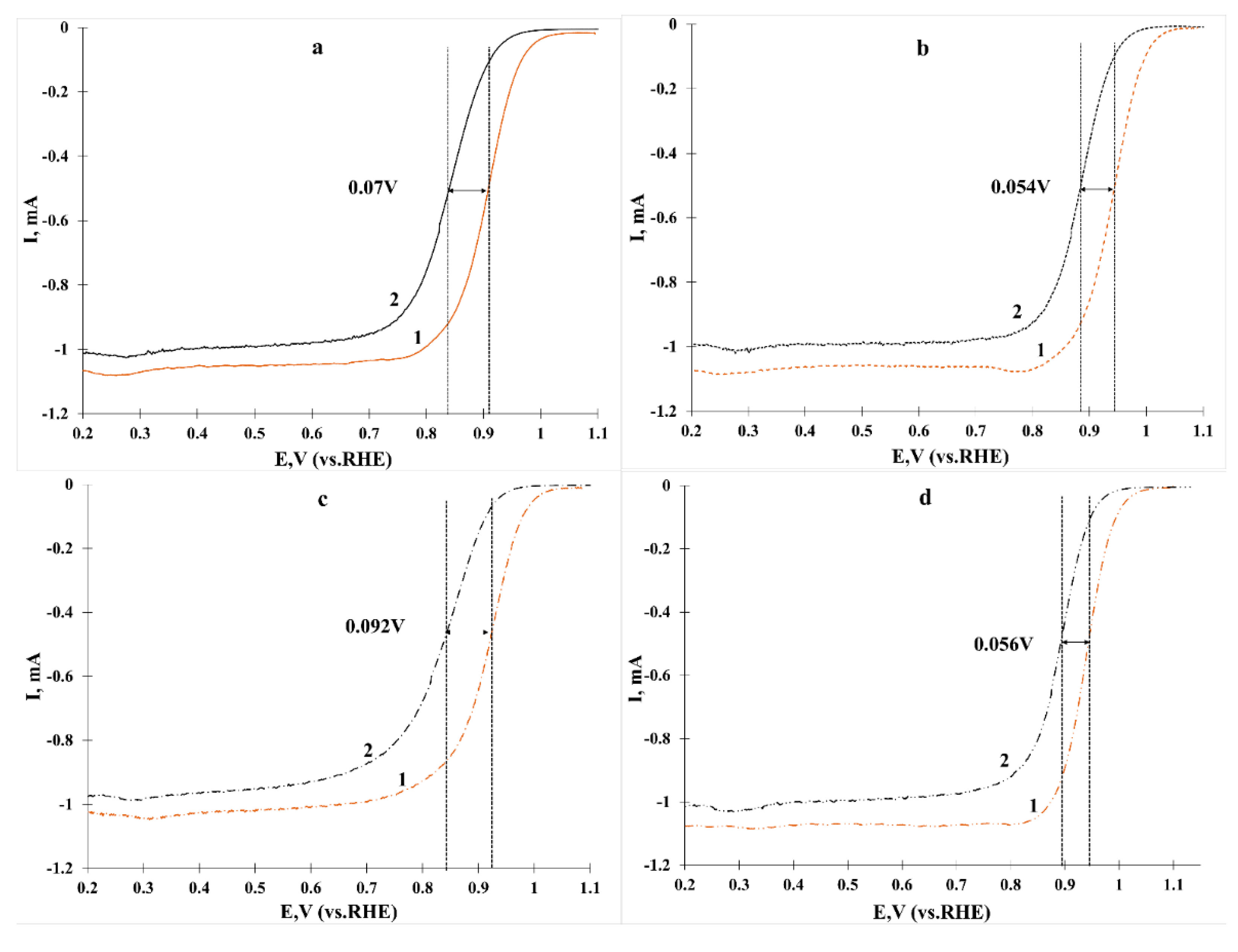
2. Results and Discussion
Characteristics of Bimetallic Catalysts
3. Experimental
3.1. Electrodeposition Technique
3.2. Measurement Methods and Techniques
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CV | Cyclic voltammogram |
| ESA | Electrometrically active surface area |
| LSV | Linear sweep voltammetry |
| ORR | Oxygen reduction reaction |
| RDE | Rotating disk electrode |
| RHE | Reversible hydrogen electrode |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence analysis |
| XPS | X-ray photoelectron spectroscopy |
| TEM | Transmission electron microscopy |
References
- Pettersson, J.; Ramsey, B.; Harrison, D. A review of the latest developments in electrodes for unitised regenerative polymer electrolyte fuel cells. J. Power Sources 2006, 157, 28–34. [Google Scholar] [CrossRef]
- Alaswad, A.; Baroutaji, A.; Achour, H.; Carton, J.; Al Makky, A.; Olabi, A.G. Developments in fuel cell technologies in the transport sector. Int. J. Hydrogen Energy 2016, 41, 16499–16508. [Google Scholar] [CrossRef] [Green Version]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Filippov, S.P.; Yaroslavtsev, A.B. Hydrogen energy: Development prospects and materials. Russ. Chem. Rev. 2021, 90, 627–643. [Google Scholar] [CrossRef]
- Gomez-Marin, A.M.; Rizo, R.; Feliu, J.M. Oxygen reduction reaction at Pt single crystals: A critical overview. Catal. Sci. Technol. 2014, 4, 1685–1698. [Google Scholar] [CrossRef]
- Sorsa, O.; Romar, H.; Lassi, U.; Kallio, T. Co-electrodeposited mesoporous PtM (M=Co, Ni, Cu) as an active catalyst for oxygen reduction reaction in a polymer electrolyte membrane fuel cell. Electrochim. Acta. 2017, 230, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Mohl, M.; Dobo, D.; Kukovecz, A.; Konya, Z.; Kordas, K.; Wei, J.; Vajtai, R.; Ajayan, P.M. Formation of CuPd and CuPt Bimetallic Nanotubes by Galvanic Replacement Reaction. J. Phys. Chem. C 2011, 115, 9403–9409. [Google Scholar] [CrossRef]
- Asset, T.; Chattot, R.; Fontana, M.; Mercier-Guyon, B.; Job, N.; Dubau, L.; Maillard, F. A Review on Recent Developments and Prospects for the Oxygen Reduction Reaction on Hollow Pt-alloy Nanoparticles. ChemPhysChem 2018, 19, 1552–1567. [Google Scholar] [CrossRef]
- Antolini, E. Formation of carbon-supported PtM alloys for low temperature fuel cells: A review. Mater. Chem. Phys. 2003, 78, 563–573. [Google Scholar] [CrossRef]
- Jalan, V.M.; Taylor, E.J. Importance of Interatomic Spacing in Catalytic Reduction of Oxygen in Phosphoric Acid. J. Electrochem. Soc. 1983, 130, 2299–2302. [Google Scholar] [CrossRef]
- Toda, T.; Igarashi, H.; Uchida, H.; Watanabe, M. Enhancement of the Electroreduction of Oxygen on Pt Alloys with Fe, Ni, and Co. J. Electrochem. Soc. 1999, 146, 3750–3756. [Google Scholar] [CrossRef]
- Munoz, M.; Ponce, S.; Zhang, G.R.; Etzold, B.J.M. Size-controlled PtNi nanoparticles as highly efficient catalyst for hydrodechlorination reactions. Appl. Catal. B Environ. 2016, 192, 1–7. [Google Scholar] [CrossRef]
- Beard, B.C.; Ross, P.N. The Structure and Activity of Pt-Co Alloys as Oxygen Reduction Electrocatalysts. J. Electrochem. Soc. 1990, 137, 3368–3374. [Google Scholar] [CrossRef]
- He, C.; Zhang, S.; Tao, J.; Shen, P.K. One-step solid state synthesis of PtCo nanocubes graphene nanocomposites as advanced oxygen reduction reaction electrocatalysts. J. Catal. 2018, 362, 85–93. [Google Scholar] [CrossRef]
- Stamenkovic, V.; Schmidt, T.J.; Ross, P.N.; Markovic, N.M. Surface Composition Effects in Electrocatalysis: Kinetics of Oxygen Reduction on Well-Defined Pt3Ni and Pt3Co Alloy Surfaces. J. Phys. Chem. 2002, 106, 11970–11979. [Google Scholar] [CrossRef] [Green Version]
- Konno, N.; Mizuno, S.; Nakaji, H.; Ishikawa, Y. Development of compact and high-performance fuel cell stack. SAE Int. J. Altern. Powertains 2015, 4, 123–129. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhu, X.Y.; Feng, J.J.; Wang, A.J. Solvothermal synthesis of N-doped graphene supported PtCo nanodendrites with highly catalytic activity for 4-nitrophenol reduction. Appl. Surf. Sci. 2018, 428, 798–808. [Google Scholar] [CrossRef]
- Meng, H.B.; Zhang, X.F.; Pu, Y.L.; Chen, X.L.; Feng, J.J.; Han, D.M.; Wang, A.J. One-pot solvothermal synthesis of reduced graphene oxide-supported uniform PtCo nanocrystals for efficient and robust electrocatalysis. J. Colloid Interface Sci. 2019, 543, 17–24. [Google Scholar] [CrossRef]
- Hu, S.; Wang, Z.; Chen, H.; Wang, S.; Li, X.; Zhang, X.; Shen, P.K. Ultrathin PtCo nanorod assemblies with self-optimized surface for oxygen reduction reaction. J. Electroanal. Chem. 2020, 870, 114194–114201. [Google Scholar] [CrossRef]
- Stamenkovic, V.; Mun, B.S.; Mayrhofer, K.J.J.; Ross, P.N.; Markovic, N.M.; Rossmeisl, J.; Greeley, J.; Nørskov, J.K. Changing the activity of electrocatalysts for oxygen reduction by tuning the surface electronic structure. Angew. Chem. Int. Ed. 2006, 45, 2897–2901. [Google Scholar] [CrossRef]
- Grolleau, C.; Coutanceau, C.; Pierre, F.; Leger, J.M. Optimization of a surfactant free polyol method for the synthesis of platinum–cobalt electrocatalysts using Taguchi design of experiments. J. Power Sources 2010, 195, 1569–1576. [Google Scholar] [CrossRef]
- Miyatake, K.; Shimizu, Y. Pt/Co Alloy Nanoparticles Prepared by Nanocapsule Method Exhibit a High Oxygen Reduction Reaction Activity in the Alkaline Media. ACS Omega 2017, 2, 2085–2089. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.Z.; Gao, P.; Wang, F.H.; Zhu, H. Carbon supported chemically ordered nanoparicles with stable Pt shell and their superior catalysis toward the oxygen reduction reaction. Electrochim. Acta 2017, 245, 924–933. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, B.; Jiang, K.; Cai, W.B. Surfactant-Free Synthesis of Carbon-Supported Palladium Nanoparticles and Size-Dependent Hydrogen Production from Formic Acid–Formate Solution. ACS Appl. Mater. Interfaces 2017, 9, 24678–24687. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ding, C.; Yang, Y.; Liu, G.; Cai, W.B. An alternate aqueous phase synthesis of the Pt3Co/C catalyst towards efficient oxygen reduction reaction. Chin. J. Catal. 2019, 40, 1895–1903. [Google Scholar] [CrossRef]
- Antolini, E.; Salgado, J.; Giz, M.; Gonzalez, E. Effects of geometric and electronic factors on ORR activity of carbon supported Pt–Co electrocatalysts in PEM fuel cells. Int. J. Hydrogen Energy 2005, 30, 1213–1220. [Google Scholar] [CrossRef]
- Jiang, R.; Rong, C.; Chu, D. Surface coverage of Pt atoms on PtCo nanoparticles and catalytic kinetics for oxygen reduction. Electrochim. Acta 2011, 56, 2532–2540. [Google Scholar] [CrossRef]
- Hernández-Fernández, P.; Rojas, S.; Ocón, P.; de la Fuente, J.L.G.; Terreros, P.; Peña, M.A.; García-Fierro, J.L. An opening route to the design of cathode materials for fuel cells based on PtCo nanoparticles. Appl. Catal. B Environ. 2007, 77, 19–28. [Google Scholar] [CrossRef]
- Koh, S.; Toney, M.F.; Strasser, P. Activity–stability relationships of ordered and disordered alloy phases of Pt3Co electrocatalysts for the oxygen reduction reaction (ORR). Electrochim. Acta 2007, 52, 2765–2774. [Google Scholar] [CrossRef]
- Hou, F.; Zhao, H.; Song, H.; Chou, L.; Zhao, J.; Yang, J.; Yan, L. Effect of impregnation strategy on catalytic hydrogenation behavior of PtCo catalysts supported on La2O2CO3 nanorods. J. Rare Earths 2018, 36, 965–973. [Google Scholar] [CrossRef]
- Mondal, A.; De, A.; Datta, J. Selective methodology for developing PtCo NPs and performance screening for energy efficient electro-catalysis in direct ethanol fuel cell. J. Int. J. Hydrogen Energy 2019, 44, 10996–11011. [Google Scholar] [CrossRef]
- Choi, J.; Jang, J.H.; Roh, C.W.; Yang, S.; Kim, J.; Lim, J.; Lee, H. Gram-scale synthesis of highly active and durable octahedral PtNi nanoparticle catalysts for proton exchange membrane fuel cell. Appl. Catal. B Environ. 2018, 225, 530–537. [Google Scholar] [CrossRef] [Green Version]
- Lim, I.; Lee, E.; Park, H.U.; Jang, J.; Jung, N.; Yang, T.H.; Park, G.G. Sonochemical gram-scale synthesis of core–shell PdCo@Pt nanoparticle and investigation of post heat-treatment effect for various gas atmospheres. J. Alloys Compd. 2021, 879, 160441. [Google Scholar] [CrossRef]
- Poly, S.S.; Hashiguchi, Y.; Sultana, A.; Nakamura, I.; Shimizu, K.; Yasumura, S.; Fujitani, T. Flow reactor approach for the facile and continuous synthesis of efficient Pd@Pt core-shell nanoparticles for acceptorless dehydrogenative synthesis of pyrimidines from alcohols and amidines. Appl. Catal. A Gen. 2021, 619, 118158. [Google Scholar] [CrossRef]
- Lin, R.; Cai, X.; Hao, Z.; Pu, H.; Yan, H. Rapid microwave-assisted solvothermal synthesis of shape-controlled Pt-Ni alloy nanoparticles for PEMFC. Electrochim. Acta 2018, 283, 764–771. [Google Scholar] [CrossRef]
- Domynguez-Domynguez, S.; Arias-Pardilla, J.; Berenguer-Murcia, Á.; Morallyn, E.; Cazorla-Amorys, D. Electrochemical deposition of platinum nanoparticles on different carbon supports and conducting polymers. J. Appl. Electrochem. 2008, 38, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.J.; Li, A.Q.; Wang, A.J.; Lei, Z.; Chen, J.R. Electrodeposition of monodispersed platinum nanoparticles on a glassy carbon electrode for sensing methanol. Microchim. Acta 2011, 173, 383–389. [Google Scholar] [CrossRef]
- Tang, H.; Chen, J.H.; Huang, Z.P.; Wang, D.Z.; Ren, Z.F.; Nie, L.H.; Kuang, Y.F.; Yao, S.Z. High dispersion and electrocatalytic properties of platinum on well-aligned carbon nanotube arrays. Carbon 2004, 42, 191–197. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, Z.; Zhao, G.C.; Wei, X.W. Electrodeposited platinum nanoparticles on the multi-walled carbon nanotubes and its electrocatalytic for nitric oxide. Int. J. Electrochem. Sci. 2008, 3, 746–754. [Google Scholar]
- Wei, Z.D.; Chan, S.H.; Li, L.L.; Cai, H.F.; Xia, Z.T.; Sun, C.X. Electrodepositing Pt on a Nafion-bonded carbon electrode as a catalyzed electrode for oxygen reduction reaction. Electrochim. Acta 2005, 50, 2279–2287. [Google Scholar] [CrossRef]
- Santiago, D.; Rodryguez-Calero, G.G.; Rivera, H.; Tryk, D.A.; Scibioh, M.A. Cabrera CR Platinum electrodeposition at high surface area carbon Vulcan XC-72 material using a rotating disk -slurry electrode technique. J. Electrochem. Soc. 2010, 157, F189–F195. [Google Scholar] [CrossRef]
- Chaisubanan, N.; Tantavichet, N. Pulse reverse electrodeposition of Pt–Co alloys onto carbon cloth electrodes. J. Alloys Compd. 2013, 559, 69–75. [Google Scholar] [CrossRef]
- Burk, J.J.; Buratto, S.K. Electrodeposition of Pt Nanoparticle Catalysts from H2Pt(OH)6 and Their Application in PEM Fuel Cells. J. Phys. Chem. C 2013, 117, 18957–18966. [Google Scholar] [CrossRef]
- Sieben, J.M.; Morallón, E.; Cazorla-Amorós, D. Flexible ruthenium oxide-activated carbon cloth composites prepared by simple electrodeposition methods. Energy 2013, 58, 519–526. [Google Scholar] [CrossRef] [Green Version]
- Novomlinski, I.N.; Tabachkova, N.Y.; Safronenko, O.I.; Guterman, V.E. A novel electrochemical method for the preparation of Pt/C nanostructured materials. Mon. Für Chem. Chem. Mon. 2019, 150, 631–637. [Google Scholar] [CrossRef]
- Kuriganova, A.B.; Leontyeva, D.V.; Ivanov, S.; Bund, A.; Smirnova, N.V. Electrochemical dispersion technique for preparation of hybrid MOx–C supports and Pt/MOx–C electrocatalysts for low temperature fuel cells. J. Appl. Electrochem. 2016, 46, 1245–1260. [Google Scholar] [CrossRef]
- Guterman, V.E.; Novomlinskij, I.N.; Skibina, L.M.; Mauer, D.K. Method for Obtaining Nanostructural Material of Tin Oxide on Basis of Carbon. Invention Patent RUS 2656914, 7 June 2018. [Google Scholar]
- Mauer, D.K.; Belenov, S.V.; Skibina, L.M.; Guterman, V.E. Composite Pt/(SnO2/C) and PtSnNi/C Catalysts for Oxygen Reduction and Alcohol Electrooxidation Reaction. Russ. J. Electrochem. 2021, 57, 898–910. [Google Scholar] [CrossRef]
- Novomlinskiy, I.N.; Danilenko, M.V.; Safronenko, O.I.; Tabachkova, N.Y.; Guterman, V.E. Influence of the Sn-Oxide-Carbon Carrier Composition on the Functional Characteristics of Deposited Platinum Electrocatalysts. Electrocatalysis 2021, 12, 489–498. [Google Scholar] [CrossRef]
- Menshchikov, V.S.; Belenov, S.V.; Guterman, V.E.; Novomlinskiy, I.N.; Nevel’skaya, A.K.; Nikulin, A.Y. Methanol Electrooxidation on PtM/C (M = Ni, Co) and Pt/(SnO2/C) Catalysts. Russ. J. Electrochem. 2018, 54, 937–948. [Google Scholar] [CrossRef]
- Travitsky, N.; Ripenbein, T.; Golodnitsky, D.; Rosenberg, Y.; Burshtein, L.; Peled, E. Pt-, PtNi- and PtCo-supported catalysts for oxygen reduction in PEM fuel cells. J. Power Sources 2006, 161, 782–789. [Google Scholar] [CrossRef]
- Denton, A.R.; Ashcroft, N.W. Vegard’s law. Phys. Rev. A 1991, 43, 3161–3164. [Google Scholar] [CrossRef]
- Favilla, P.C.; Acosta, J.J.; Schvezov, C.E.; Sercovich, D.J.; Collet-Lacos, J.R. Size control of carbon-supported platinum nanoparticles made using polyol method for low temperature fuel cells. Chem. Eng. Sci. 2013, 101, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Moriau, L.J.; Hrnjic, A.; Pavlisic, A.; Kamsek, A.R.; Petek, U.; Ruiz-Zepeda, F.; Sala, M.; Pavko, L.; Selih, V.S.; Bele, M.; et al. Resolving the nanoparticles’ structure-property relationships at the atomic level: A study of Pt-based electrocatalysts. iScience 2021, 24, 102102. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yan, Z.; Xiao, Q.; Dai, J.; Yang, D.; Zhang, C.; Cai, M.; Ma, J. Highly active carbon-supported Pt nanoparticles modified and dealloyed with Co for the oxygen reduction reaction. J. Power Sources 2014, 270, 201–207. [Google Scholar] [CrossRef]
- García-Contreras, M.A.; Fernández-Valverde, S.M.; Vargas-García, J.R.; Cortés-Jácome, M.A.; Toledo-Antonio, J.A.; Ángeles-Chavez, C. Pt, PtCo and PtNi electrocatalysts prepared by mechanical alloying for the oxygen reduction reaction in 0.5 M H2SO4. Int. J. Hydrogen Energy 2008, 33, 6672–6680. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Tian, X.C.; Zhang, B.W.; Huang, L.; Zhu, F.C.; Qu, X.-M.; Sun, S.G. Engineering phase and surface composition of Pt 3 Co nanocatalysts: A strategy for enhancing CO tolerance. Nano Energy 2017, 34, 224–232. [Google Scholar] [CrossRef]
- de la Fuente, J.L.G.; Rojas, S.; Martínez-Huerta, M.V.; Terreros, P.; Peña, M.A.; Fierro, J.L.G. Functionalization of carbon support and its influence on the electrocatalytic behaviour of Pt/C in H2 and CO electrooxidation. Carbon 2006, 44, 1919–1929. [Google Scholar] [CrossRef]
- Urchaga, P.; Baranton, S.; Coutanceau, C.; Jerkiewicz, G. Electro-oxidation of COchem on Pt Nanosurfaces: Solution of the Peak Multiplicity Puzzle. Langmuir 2011, 28, 3658–3663. [Google Scholar] [CrossRef]
- López-Cudero, A.; Cuesta, A.; Gutiérrez, C. Potential dependence of the saturation CO coverage of Pt electrodes: The origin of the pre-peak in CO-stripping voltammograms. Part 1: Pt (111). J. Electroanal. Chem. 2005, 579, 1–12. [Google Scholar] [CrossRef]
- Zamanzad Ghavidel, M.R.; Monteverde Videla, A.H.A.; Specchia, S.; Easton, E.B. The relationship between the structure and ethanol oxidation activity of Pt-Cu/C alloy catalysts. Electrochim. Acta 2017, 230, 58–72. [Google Scholar] [CrossRef]
- Rudi, S.; Cui, C.; Gan, L.; Strasser, P. Comparative Study of the Electrocatalytically Active Surface Areas (ECSAs) of Pt Alloy Nanoparticles Evaluated by Hupd and CO-stripping voltammetry. Electrocatalysis 2014, 5, 408–418. [Google Scholar] [CrossRef]
- Van der Vliet, D.F.; Wang, C.; Li, D.; Paulikas, A.P.; Greeley, J.; Rankin, R.B.; Strmcnik, D.; Tripkovic, D.; Markovic, N.M.; Stamenkovic, V.R. Unique Electrochemical Adsorption Properties of Pt-Skin Surfaces. Angew. Chem. Int. Ed. 2012, 51, 3139–3142. [Google Scholar] [CrossRef]
- Petrii, O.A. The progress in understanding the mechanisms of methanol and formic acid electrooxidation on platinum group metals. Russ. J. Electrochem. 2019, 55, 3–38. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Key, J.; Linkov, V.; Ji, S.; Wang, X.; Wang, R. Strain Effect of Core-Shell Co@Pt/C Nanoparticle Catalyst with Enhanced Electrocatalytic Activity for Methanol Oxidation. J. Electrochem. Soc. 2012, 159, B270–B276. [Google Scholar] [CrossRef]
- Lin, R.; Cao, C.; Zhao, T.; Huang, Z.; Li, B.; Wieckowski, A.; Ma, J. Synthesis and application of core–shell Co@Pt/C electrocatalysts for proton exchange membrane fuel cells. J. Power Sources 2013, 223, 190–198. [Google Scholar] [CrossRef]
- Jayasayee, K.; Veen, J.A.R.V.; Manivasagam, T.G.; Celebi, S.; Hensen, E.J.M.; de Bruijn, F.A. Oxygen reduction reaction (ORR) activity and durability of carbon supported PtM (Co, Ni, Cu) alloys: Influence of particle size and non-noble metals. Appl. Catal. B Environ. 2012, 111–112, 515–526. [Google Scholar] [CrossRef]
- Jeon, M.K.; Zhang, Y.; McGinn, P.J. A comparative study of PtCo, PtCr, and PtCoCr catalysts for oxygen electro-reduction reaction. Electrochim. Acta 2010, 55, 5318–5325. [Google Scholar] [CrossRef]
- Guterman, V.E.; Belenov, S.V.; Alekseenko, A.A.; Lin, R.; Tabachkova, N.Y.; Safronenko, O.I. Activity and Stability of Pt/C and Pt-Cu/C Electrocatalysts. Electrocatalysis 2018, 9, 550–562. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Norton, M.G. X-ray Diffraction: A Practical Approach; Springer: Boston, MA USA, 2013; p. 273. [Google Scholar] [CrossRef] [Green Version]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quirós, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; Le Bail, A. Crystallography Open Database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2012, 40, D420–D427. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures from 376 Polycrystalline and Amorphous Materials; John Wiley: New York, NY, USA, 1974. [Google Scholar]
- Shinozaki, K.; Zack, J.W.; Pylypenko, S.; Pivovar, B.S.; Kocha, S.S. Oxygen Reduction Reaction Measurements on Platinum Electrocatalysts Utilizing Rotating Disk Electrode Technique: II. Influence of Ink Formulation, Catalyst Layer Uniformity and Thickness. J. Electrochem. Soc. 2015, 162, 1144–1158. [Google Scholar] [CrossRef]








| Sample | ω(Pt), %. | Metal Composition (According to XRF) | Metal Composition (According to XRD) | Lattice Parameter of the Metal Component a, Å | Average crystallite size (XRD) Pt, nm |
|---|---|---|---|---|---|
| PCC1 | 14 ± 0.28 | Pt1.56Co | Pt0.86Co | 3.875 | 2.6 ± 0.2 |
| PCC2 | 30 ± 0.6 | Pt1.12Co | Pt0.86Co | 3.876 | 3.3 ± 0.3 |
| JM20 | 20 ± 0.4 | - | - | 3.923 | 2.3 ± 0.2 |
| JM40 | 40 ± 0.8 | - | - | 3.923 | 3.0 ± 0.3 |
| Sample | Pt4f7/2 A | Pt4f7/2 B | Co2p3/2 A | Co2p3/2 B |
|---|---|---|---|---|
| JM-40 | 70.84 eV | 73.44 eV | - | - |
| 94.2% | 5.8% | 0.0% | 0.0% | |
| PCC2 | 70.89 eV | 73.42 eV | 777.8 eV | 779.6 eV |
| 94.9% | 5.1% | 5.7% | 94.4% | |
| PCC1 | 70.96 eV | 73.47 eV | 778.0 eV | 779.7 eV |
| 95.1% | 4.9% | 6.5% | 93.5% |
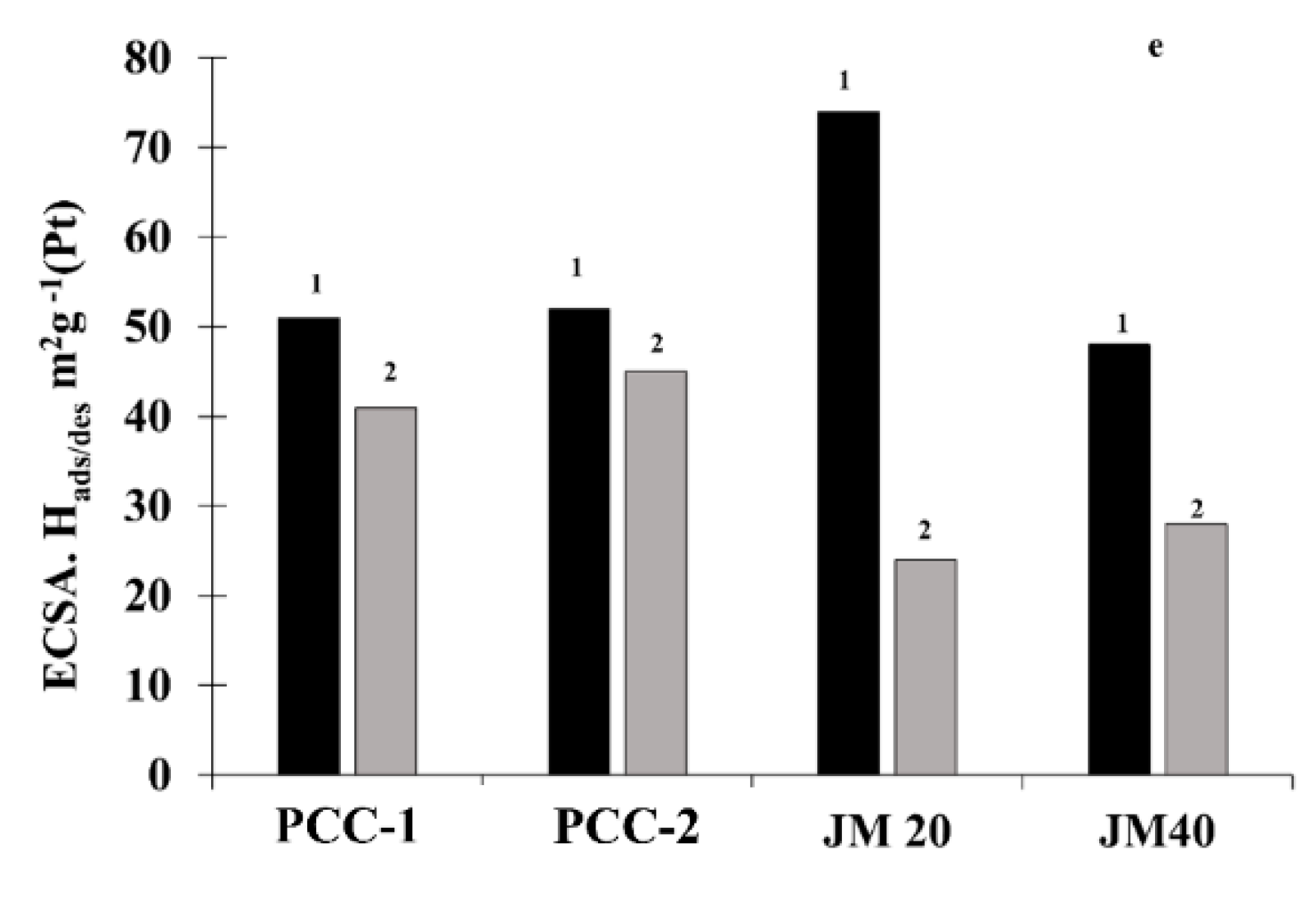
| Sample | ESA | Imass, Ag−1 (Pt) (E = 0.90 V) | Is, Am−2 (Pt) (E = 0.90 V) | E1/2 | Number of ē (E = 0.90 V) | |
|---|---|---|---|---|---|---|
| Hads/des m2g−1 (Pt) | CO m2g−1 (Pt) | |||||
| PCC1 | 51 ± 5 | 58 ± 5 | 407 ± 20 | 8.0 | 0.92 | 4.1 |
| PCC2 | 52 ± 5 | 45 ± 4 | 426 ± 21 | 8.9 | 0.94 | 4.0 |
| JM20 | 74 ± 7 | 76 ± 7 | 220 ± 11 | 2.9 | 0.92 | 3.9 |
| JM40 | 48 ± 5 | 51 ± 5 | 196 ± 10 | 4.1 | 0.92 | 3.8 |
| Sample | ESA Hads/des m2g−1 (Pt) | Imass, Ag−1 (Pt) (E = 0.85 V) | E1/2 |
|---|---|---|---|
| PCC1 | 41 ± 4 | 225 ± 11 | 0.85 |
| PCC2 | 45 ± 4 | 466 ± 23 | 0.88 |
| JM20 | 24 ± 2 | 145 ± 7 | 0.85 |
| JM40 | 28 ± 3 | 246 ± 12 | 0.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauer, D.; Belenov, S.; Guterman, V.; Nikolsky, A.; Kozakov, A.; Nikulin, A.; Alexeenko, D.; Safronenko, O. Gram-Scale Synthesis of CoO/C as Base for PtCo/C High-Performance Catalysts for the Oxygen Reduction Reaction. Catalysts 2021, 11, 1539. https://doi.org/10.3390/catal11121539
Mauer D, Belenov S, Guterman V, Nikolsky A, Kozakov A, Nikulin A, Alexeenko D, Safronenko O. Gram-Scale Synthesis of CoO/C as Base for PtCo/C High-Performance Catalysts for the Oxygen Reduction Reaction. Catalysts. 2021; 11(12):1539. https://doi.org/10.3390/catal11121539
Chicago/Turabian StyleMauer, Dmitry, Sergey Belenov, Vladimir Guterman, Anatoly Nikolsky, Alexey Kozakov, Alexey Nikulin, Danil Alexeenko, and Olga Safronenko. 2021. "Gram-Scale Synthesis of CoO/C as Base for PtCo/C High-Performance Catalysts for the Oxygen Reduction Reaction" Catalysts 11, no. 12: 1539. https://doi.org/10.3390/catal11121539
APA StyleMauer, D., Belenov, S., Guterman, V., Nikolsky, A., Kozakov, A., Nikulin, A., Alexeenko, D., & Safronenko, O. (2021). Gram-Scale Synthesis of CoO/C as Base for PtCo/C High-Performance Catalysts for the Oxygen Reduction Reaction. Catalysts, 11(12), 1539. https://doi.org/10.3390/catal11121539