Photodegradation of Carbol Fuchsin Dye Using an Fe2−xCuxZr2−xWxO7 Photocatalyst under Visible-Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photocatalysis Characterizations
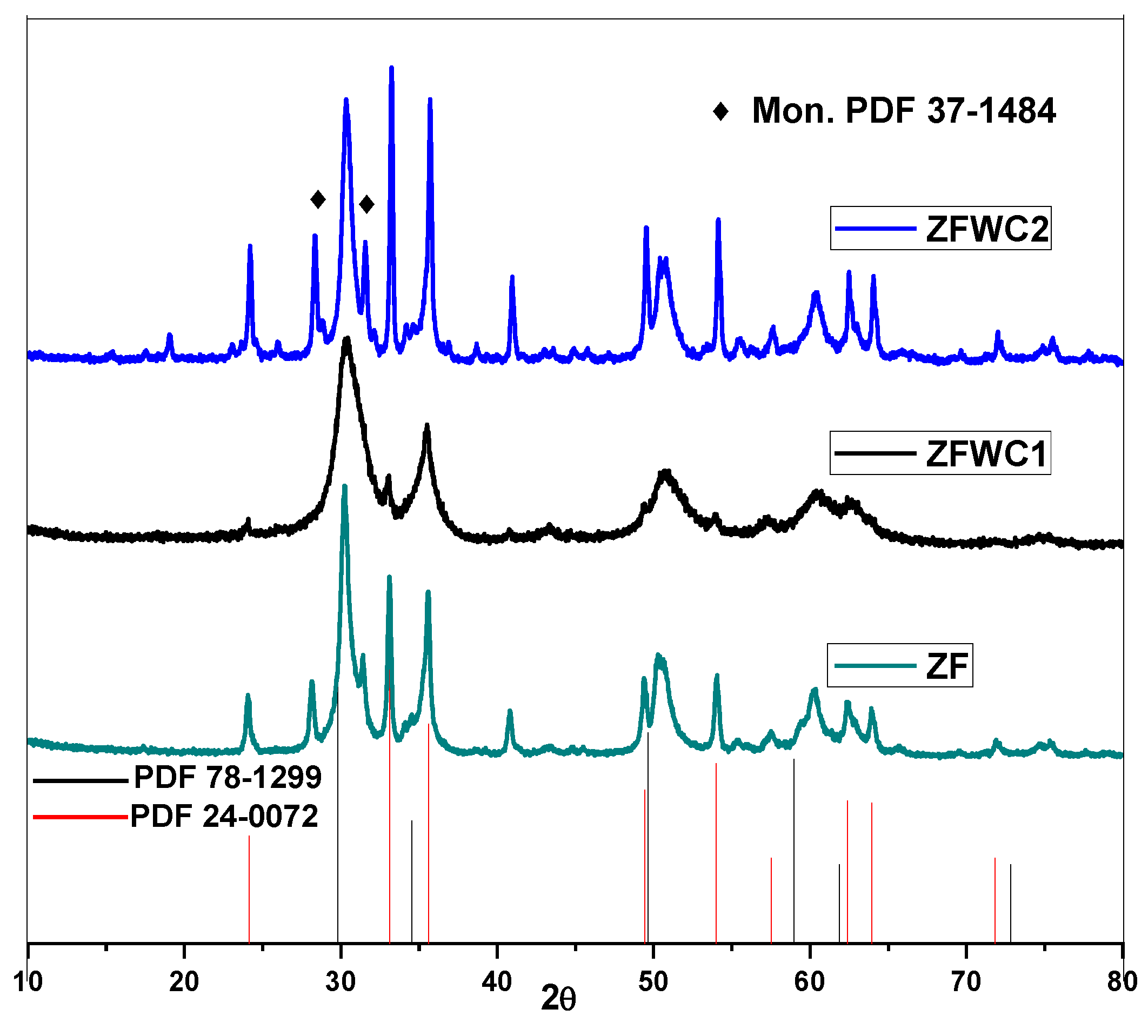
2.1.1. X-ray Diffraction
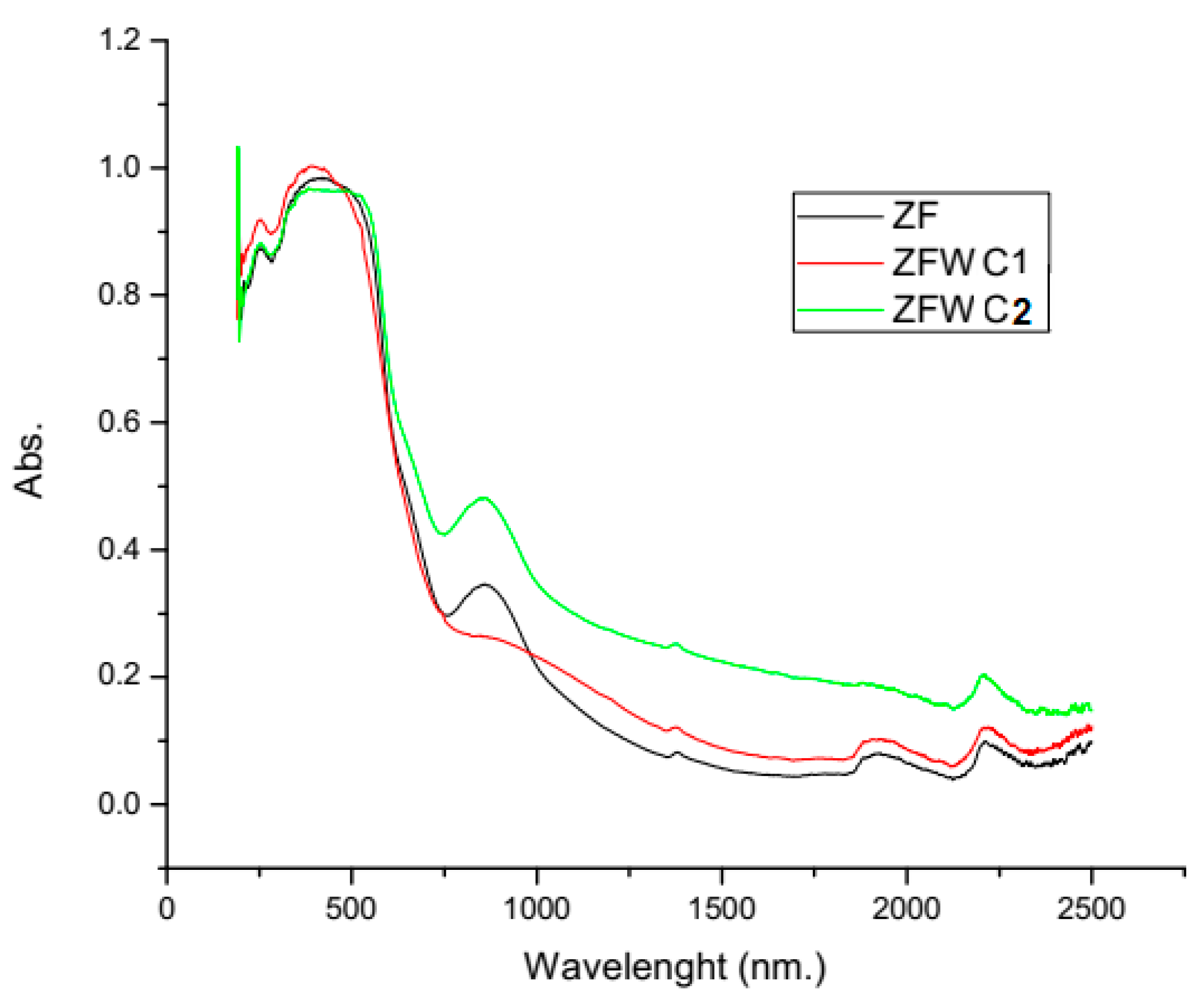
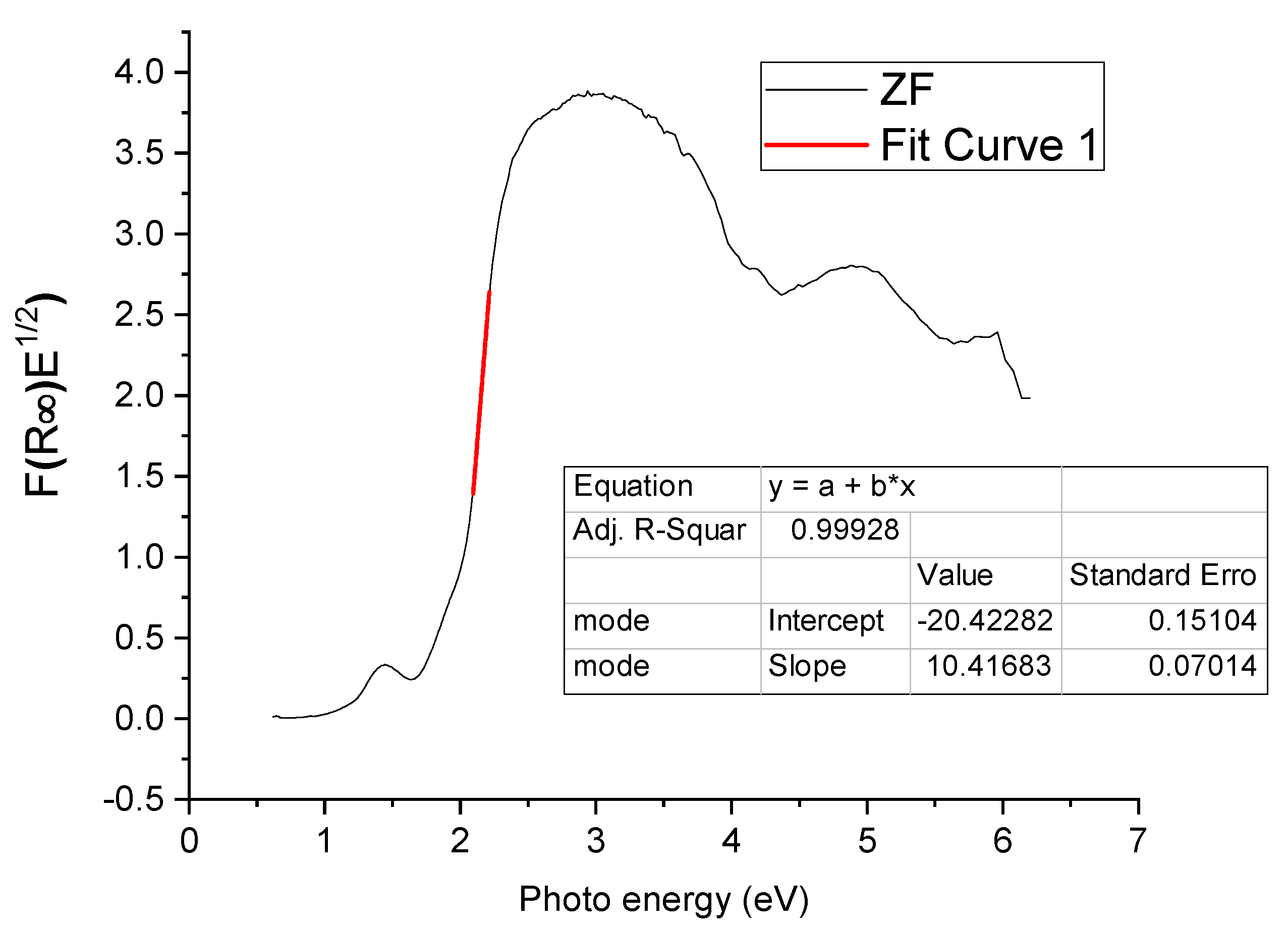
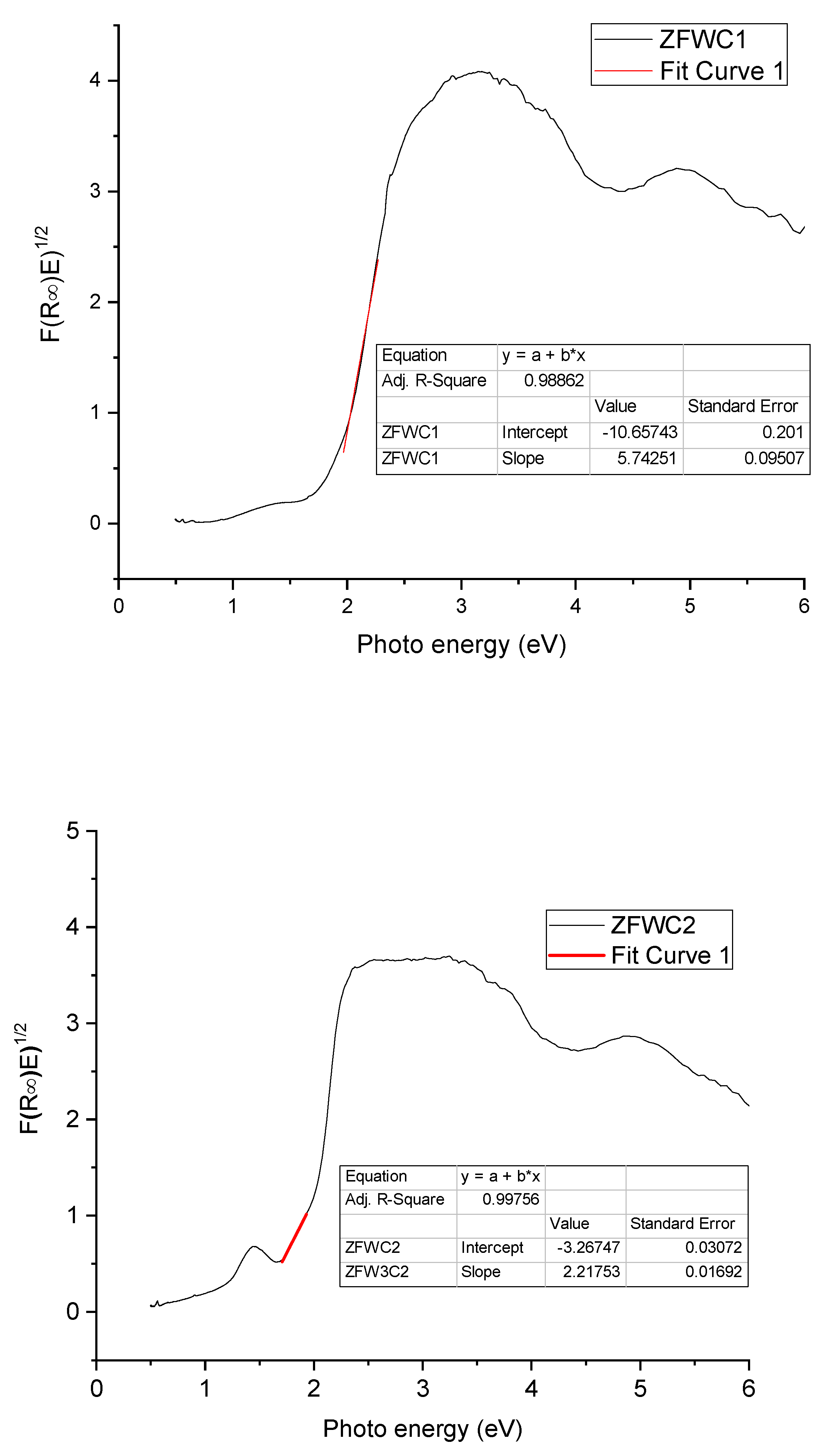
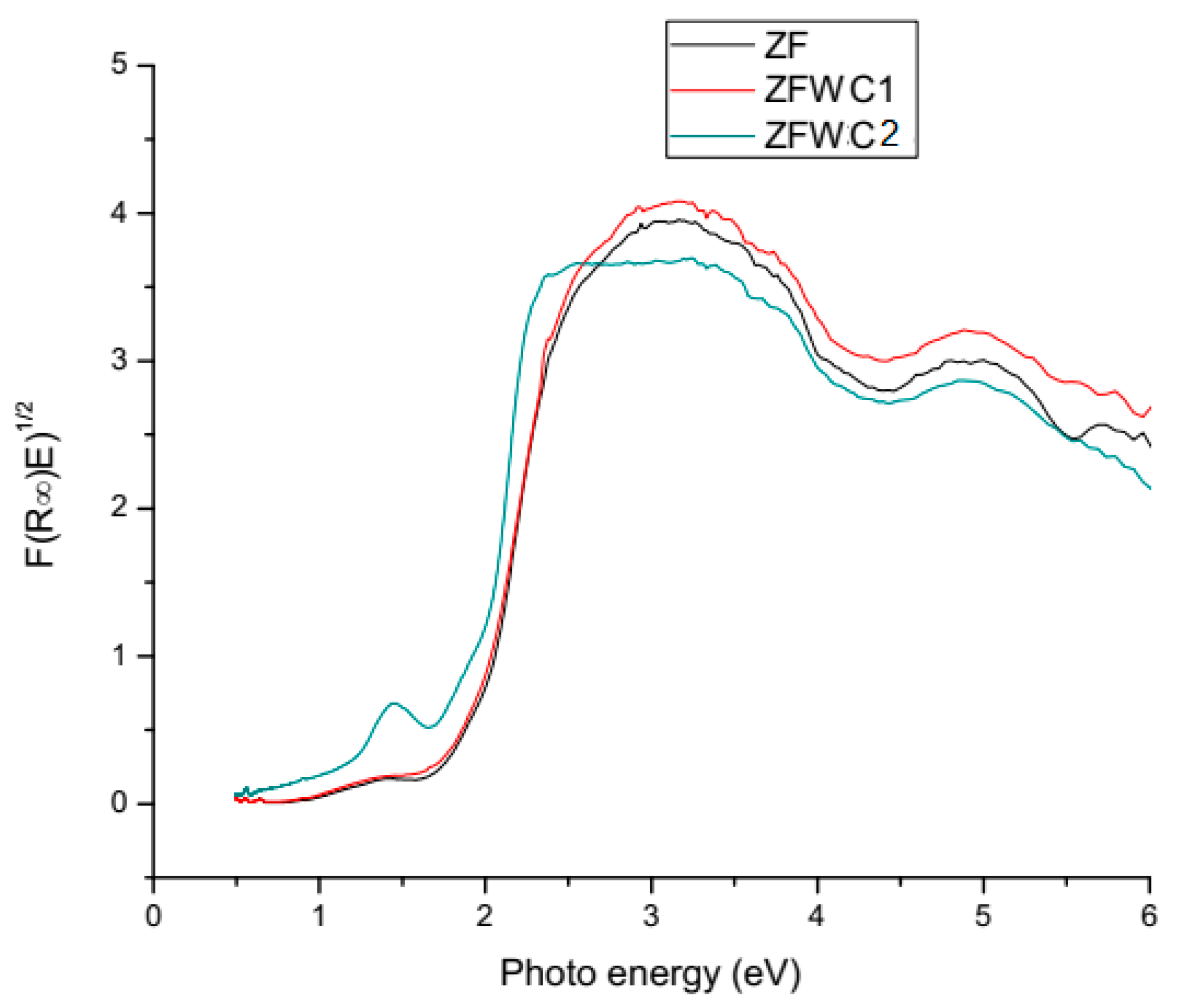
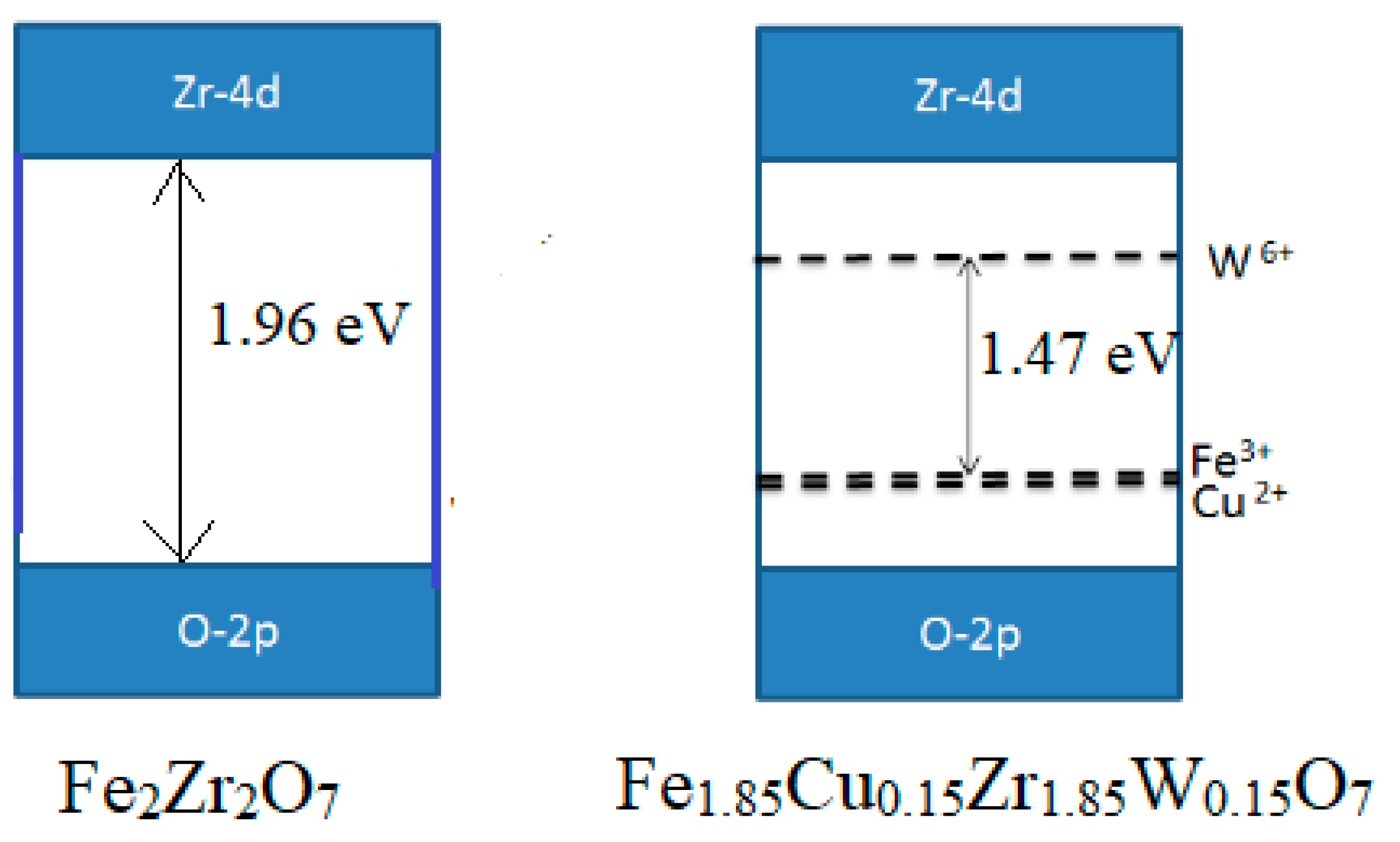
2.1.2. Diffuse Reflectance Spectroscopy (DRS)
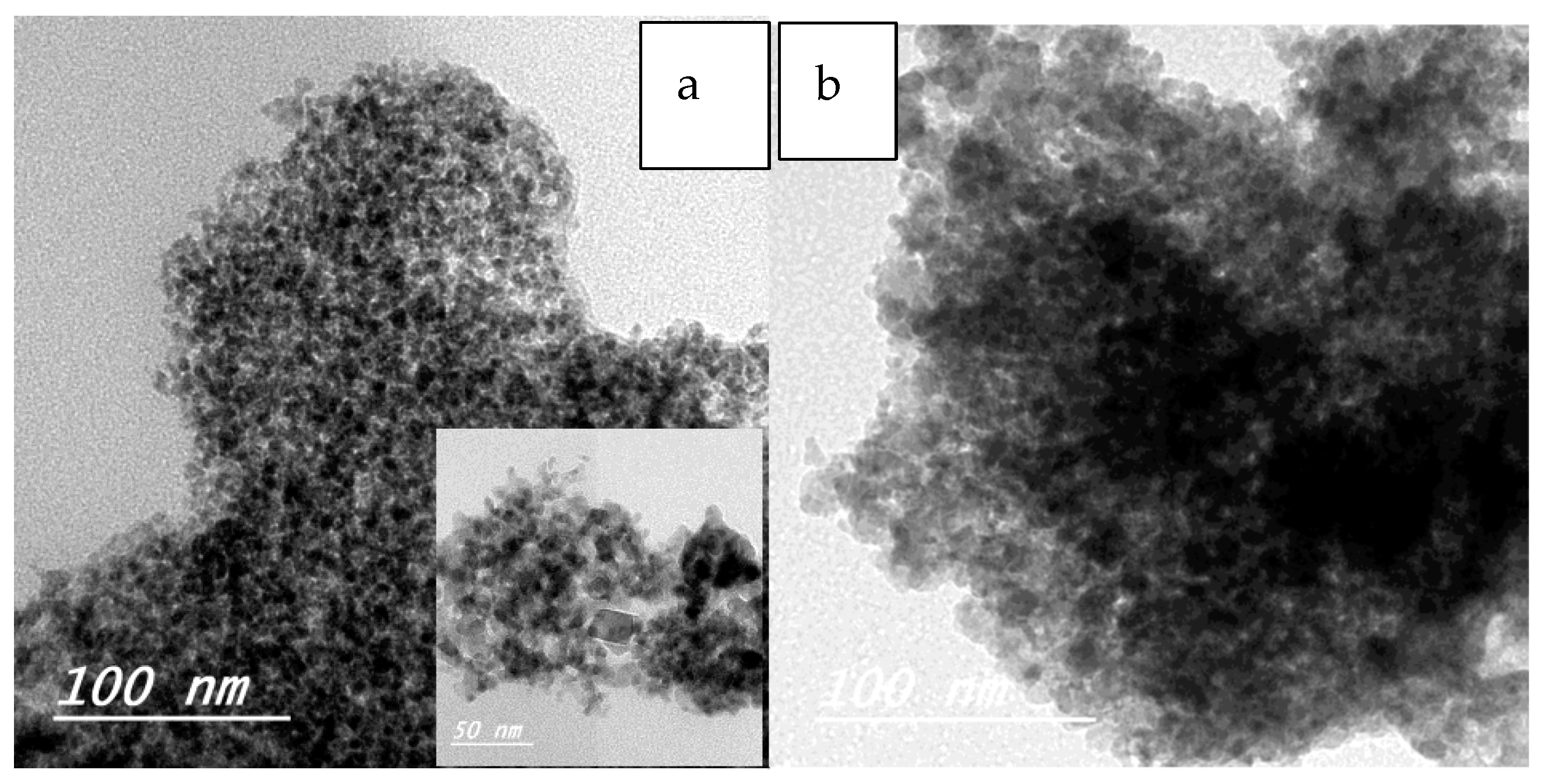
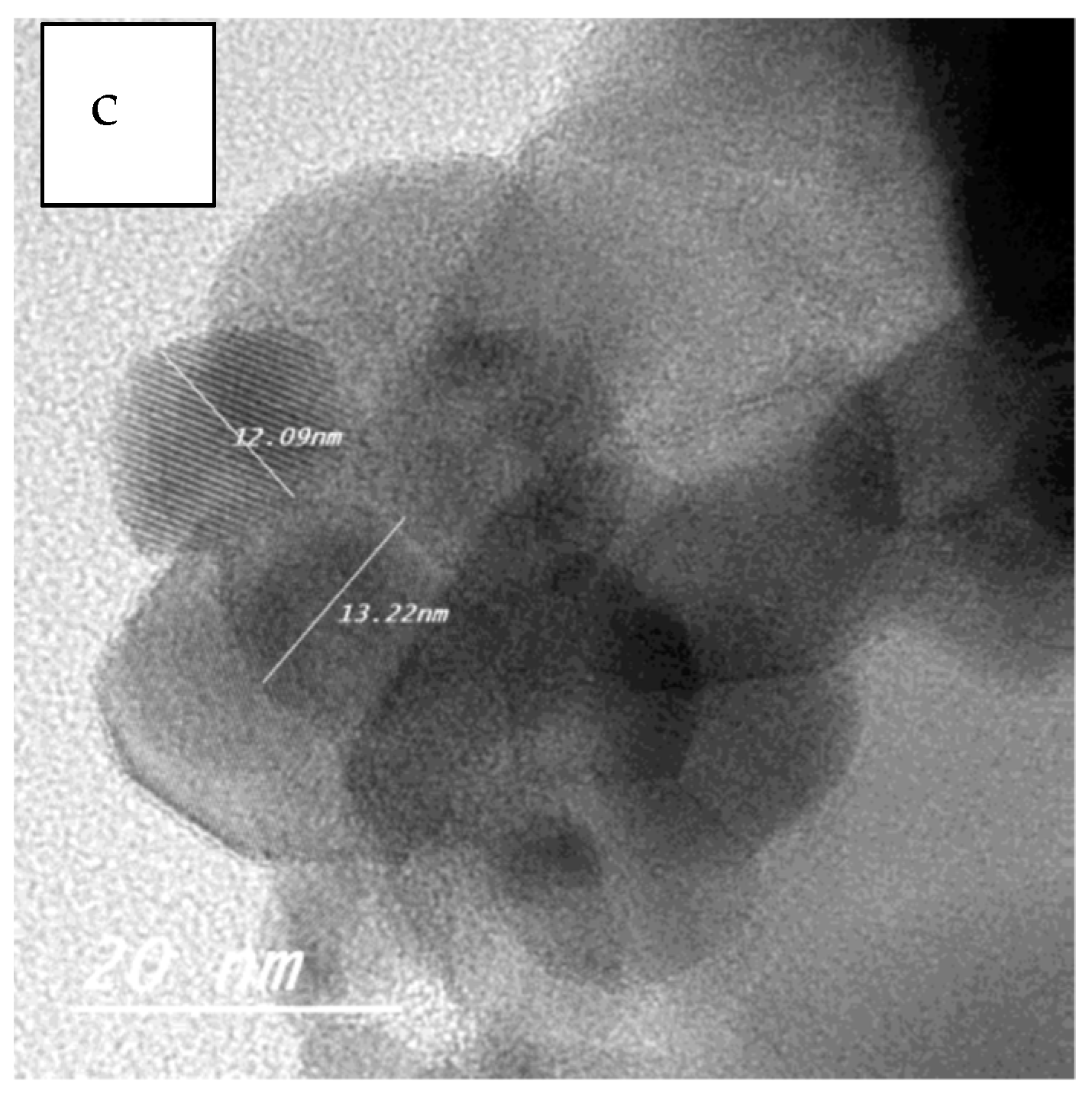
2.1.3. Transmission Electron Microscopy (TEM)
2.2. Photocatalytic Degradation of Dye
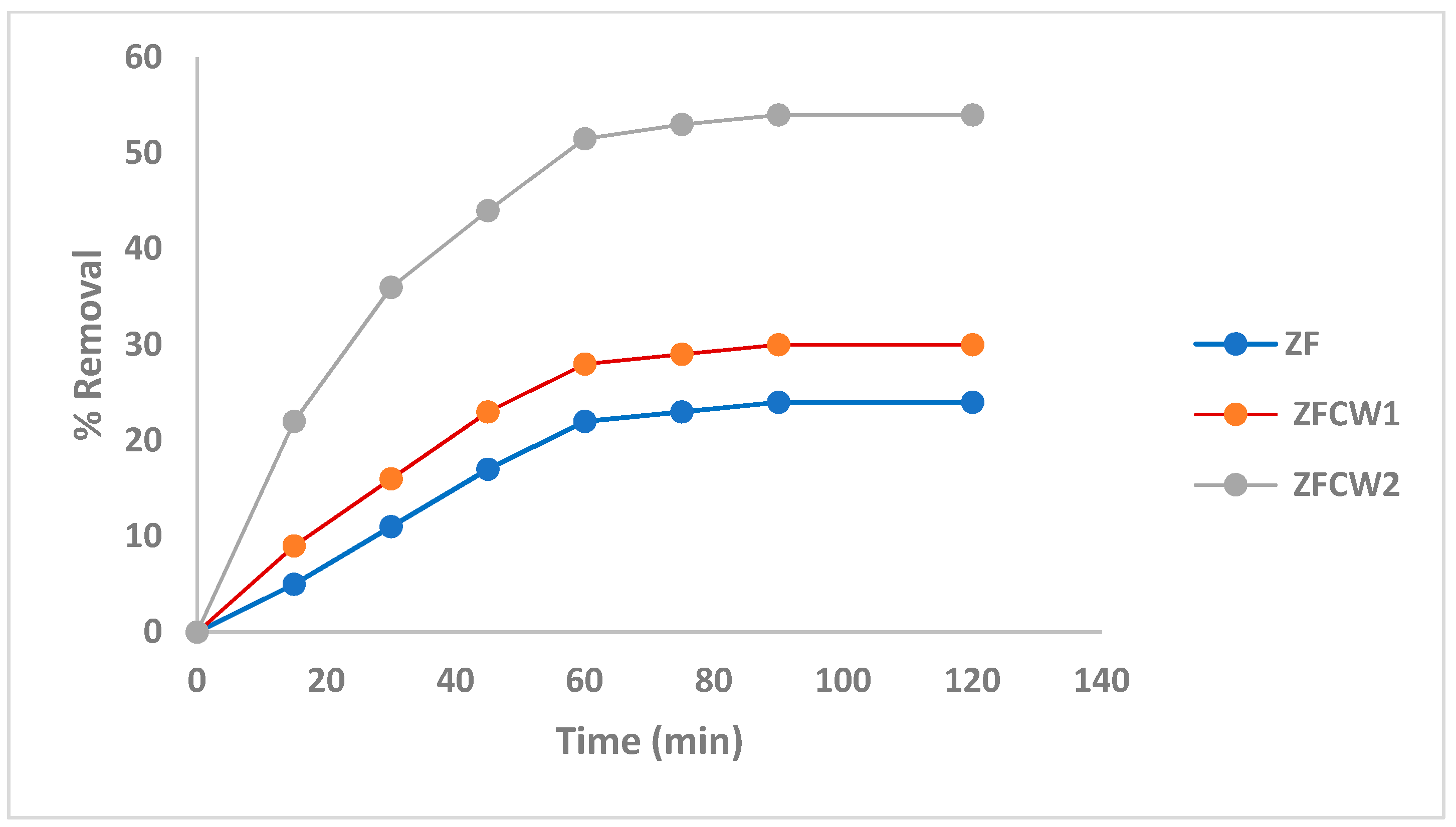
2.2.1. The Irradiation Time Effect
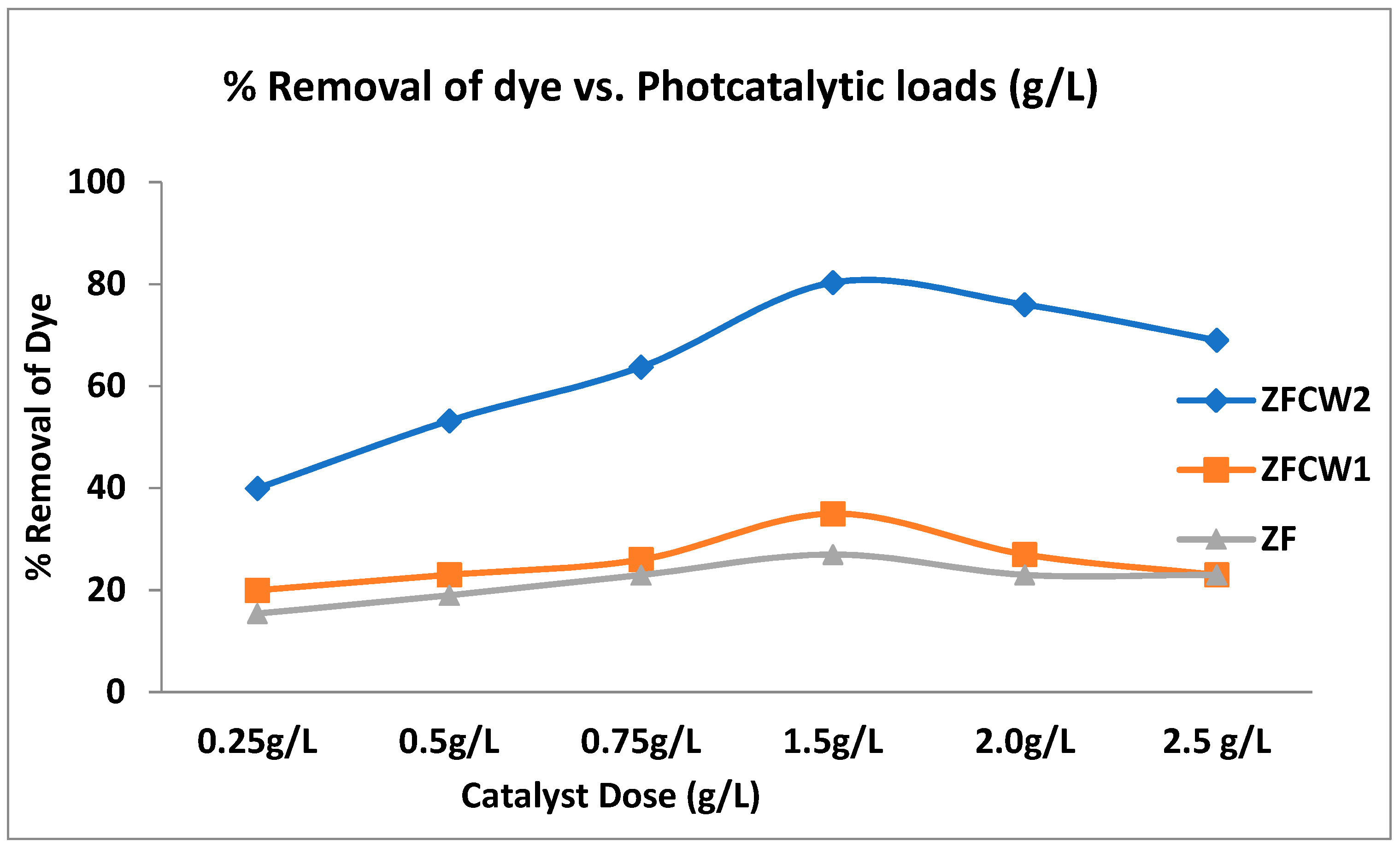
2.2.2. The Effect of Catalyst Dose
2.2.3. Effect of pH
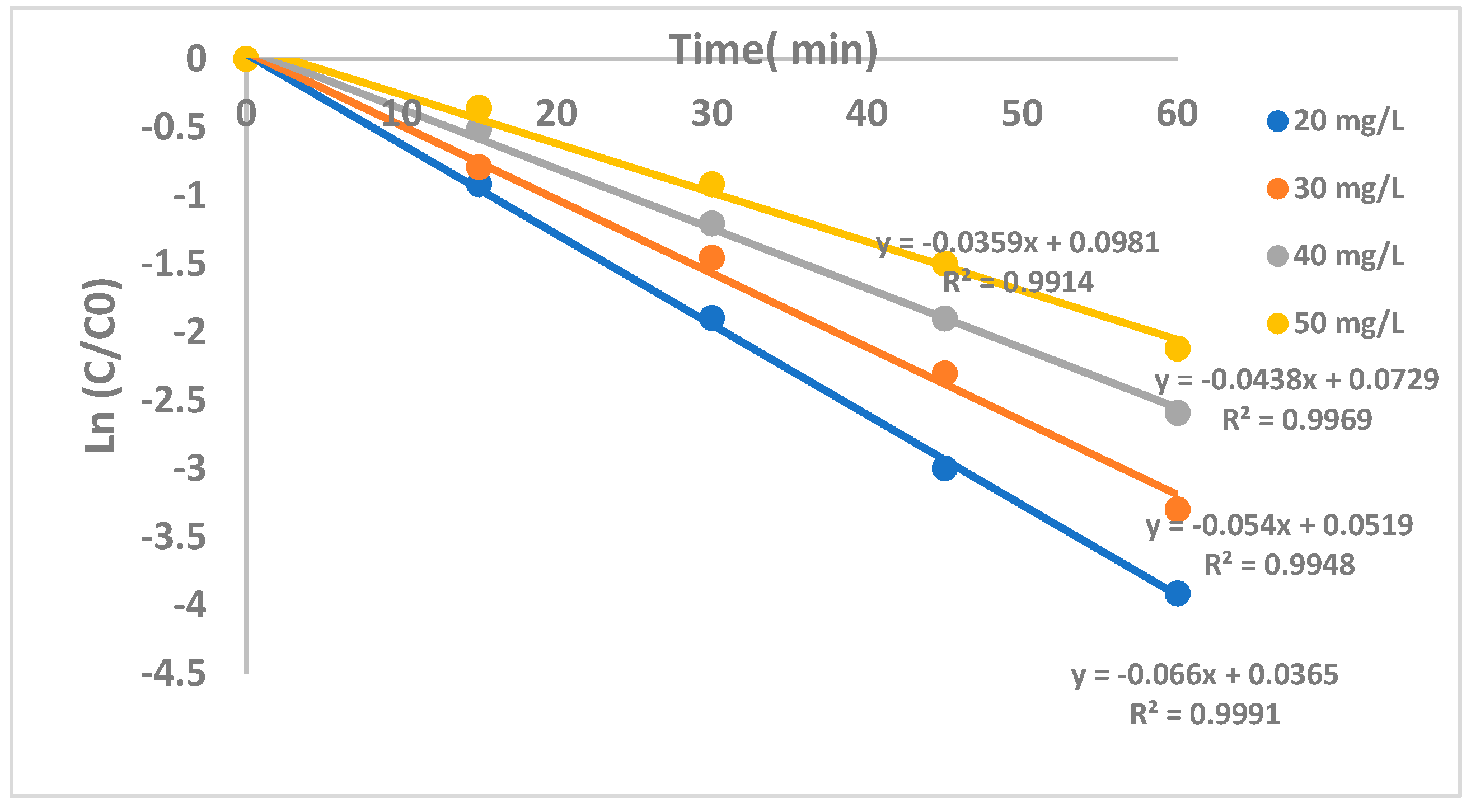
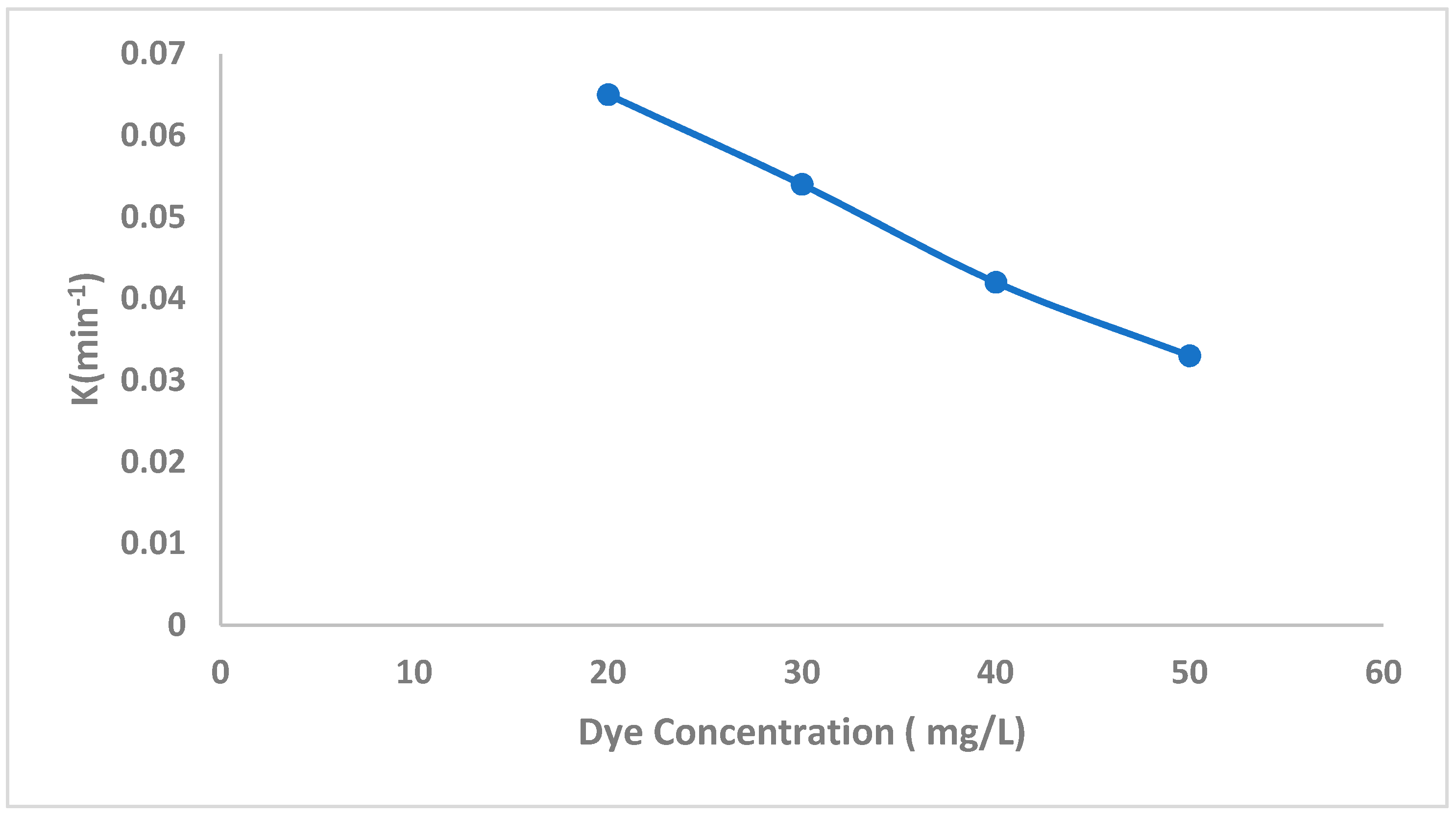
2.2.4. The Effect of CF Concentration
2.2.5. The Effect of Visible Light
2.2.6. Active Radical Trapping Experiments
2.2.7. Toxicity
2.2.8. The Proposed Mechanism of Photocatalytic Degradation of CF
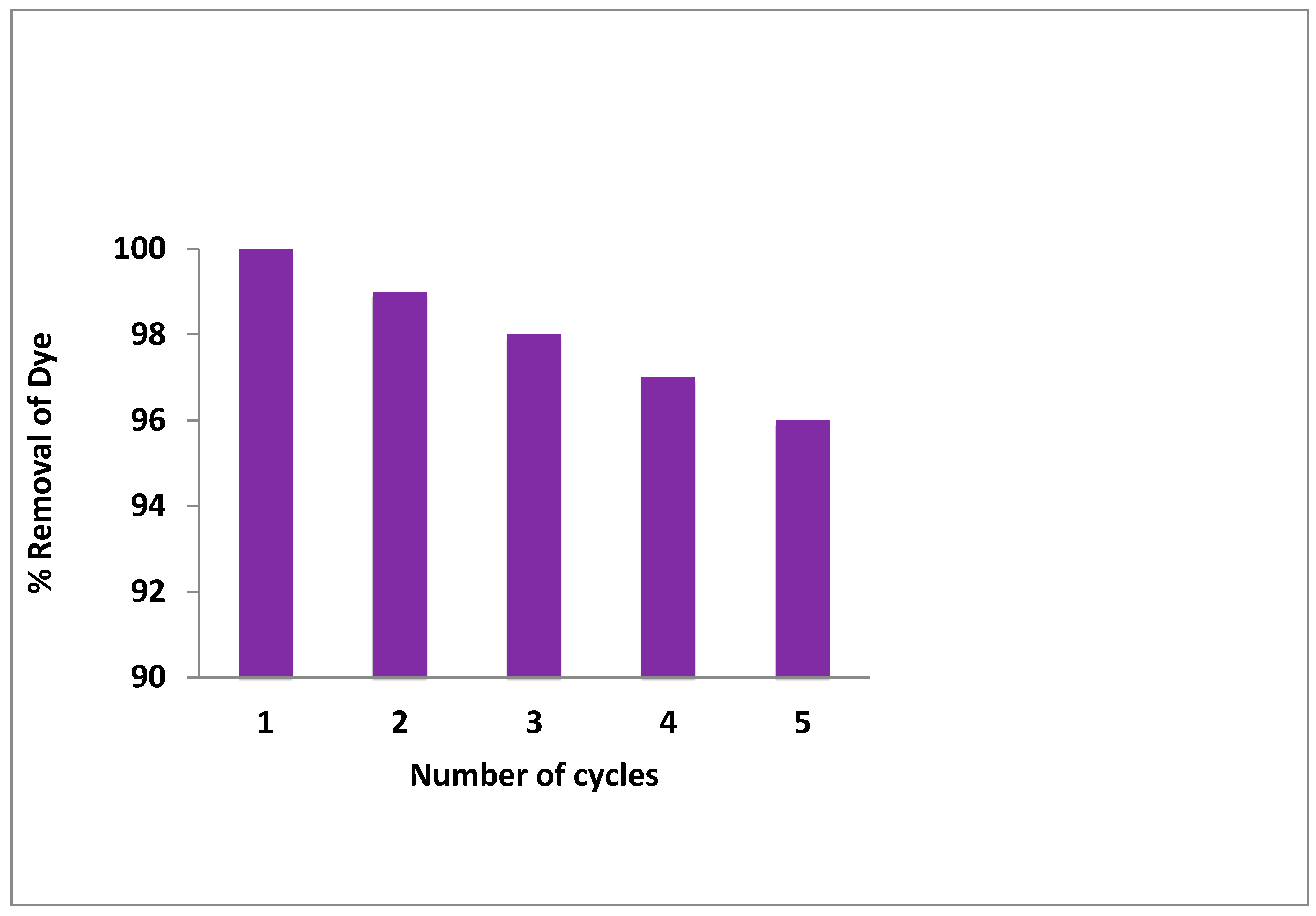
2.2.9. The Reusability of ZFWC2
3. Materials and Methods
3.1. Materials
3.2. Preparation of Photocatalysts
3.3. Characterization Photocatalysts
3.4. Carbol Fuchsin Degradation Experiments
3.5. Active Radical Trapping Experiments
3.6. Toxicity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pavithra, K.G.; Jaikumar, V. Removal of colorants from wastewater: A review on sources and treatment strategies. J. Ind. Eng. Chem. 2019, 75, 1–19. [Google Scholar] [CrossRef]
- Wang, L.; Fu, X.; Han, Y.; Chang, E.; Wu, H.; Wang, H.; Li, K.; Qi, X. Preparation, Characterization, and Photocatalytic Activity of TiO2/ZnO Nanocomposites. J. Nanomater. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Koli, P.B.; Kapadnis, K.H.; Deshpande, U.G. Transition metal decorated Ferrosoferric oxide (Fe3O4): An expeditious catalyst for photodegradation of Carbol Fuchsin in environmental remediation. J. Environ. Chem. Eng. 2019, 7, 103373. [Google Scholar] [CrossRef]
- Qin, J.; Qiu, F.; Rong, X.; Yan, J.; Zhao, H.; Yang, D. Removal of basic fuchsin dye from aqueous solutions using graphite oxide modified aromatic polyurethane foam material. Toxicol. Environ. Chem. 2014, 96, 849–860. [Google Scholar] [CrossRef]
- Huong, D.T.M.; Liu, B.-L.; Chai, W.S.; Show, P.L.; Tsai, S.-L.; Chang, Y.-K. Highly efficient dye removal and lysozyme purification using strong and weak cation-exchange nanofiber membranes. Int. J. Biol. Macromol. 2020, 165, 1410–1421. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.N.; Ferreira, M.B.; de Moura Santos, E.C.M.; Léon, J.J.L.; Ganiyu, S.O.; Martínez-Huitle, C.A. Electrochemical degradation of Azo-dye Acid Violet 7 using BDD anode: Effect of flow reactor configuration on cell hydrodynamics and dye removal efficiency. J. Appl. Electrochem. 2018, 48, 1321–1330. [Google Scholar] [CrossRef]
- Droguett, T.; Mora-Gómez, J.; García-Gabaldón, M.; Ortega, E.; Mestre, S.; Cifuentes, G.; Pérez-Herranz, V. Electrochemical Degradation of Reactive Black 5 using two-different reactor configuration. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Cerboneschi, M.; Corsi, M.; Bianchini, R.; Bonanni, M.; Tegli, S. Decolorization of acid and basic dyes: Understanding the metabolic degradation and cell-induced adsorption/precipitation by Escherichia coli. Appl. Microbiol. Biotechnol. 2015, 99, 8235–8245. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.; El-Sayed, R.; Osman, T.; Toprak, M.; Muhammed, M.; Uheida, A. Composite nanofibers for highly efficient photocatalytic degradation of organic dyes from contaminated water. Environ. Res. 2016, 145, 18–25. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Z.; Liu, D.; Gao, Z. Preparation of ZnO Photocatalyst for the Efficient and Rapid Photocatalytic Degradation of Azo Dyes. Nanoscale Res. Lett. 2017, 12, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwer, H.; Mahmood, A.; Lee, J.; Kim, K.-H.; Park, J.-W.; Yip, A. Photocatalysts for degradation of dyes in industrial effluents: Opportunities and challenges. Nano Res. 2019, 12, 955–972. [Google Scholar] [CrossRef]
- Tichapondwa, S.; Newman, J.; Kubheka, O. Effect of TiO2 phase on the photocatalytic degradation of methylene blue dye. Phys. Chem. Earth Parts A/B/C 2020, 118-119, 102900. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Un-derstanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Badvi, K.; Javanbakht, V. Enhanced photocatalytic degradation of dye contaminants with TiO2 immobilized on ZSM-5 zeolite modified with nickel nanoparticles. J. Clean. Prod. 2021, 280, 124518. [Google Scholar] [CrossRef]
- Oyelude, E.; Frimpong, F.; Dawson, D. Studies on the Removal of Basic Fuchsin Dye from Aqueous Solution by HCl Treated Malted Sorghum Mash. J. Mater. Environ. Sci. 2015, 6, 1126–1136. [Google Scholar]
- Shinde, S.; Shrivastava, V.S. Ni and Zn modified acid activated montmorillonite clay for effective removal of carbol fuchsin dye from aqueous solution. SN Appl. Sci. 2020, 2, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Li, X.; Ma, W.; Zhao, J.; Hidaka, H.; Serpone, N. Effect of Transition Metal Ions on the TiO2-Assisted Photodegradation of Dyes under Visible Irradiation: A Probe for the Interfacial Electron Transfer Process and Reaction Mechanism. J. Phys. Chem. B 2002, 106, 318–324. [Google Scholar] [CrossRef]
- Saeed, K.; Khan, I.; Gul, T.; Sadiq, M. Efficient photodegradation of methyl violet dye using TiO2/Pt and TiO2/Pd photocatalysts. Appl. Water Sci. 2017, 7, 3841–3848. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Likodimos, V.; Figueiredo, J.; Faria, J.; Falaras, P.; Silva, A. Advanced nanostructured photocatalysts based on reduced graphene oxide–TiO2 composites for degradation of diphenhydramine pharmaceutical and methyl orange dye. Appl. Catal. B Environ. 2012, 123-124, 241–256. [Google Scholar] [CrossRef]
- Sriramoju, J.B.; Muniyappa, M.; Marilingaiah, N.R.; Sabbanahalli, C.; Shetty, M.; Mudike, R.; Chitrabanu, C.P.; Shivaramu, P.D.; Nagaraju, G.; Rangappa, K.S.; et al. Carbon-based TiO2-x heterostructure nanocomposites for enhanced photocatalytic degradation of dye molecules. Ceram. Int. 2021, 47, 10314–10321. [Google Scholar] [CrossRef]
- Gouvêa, C.A.; Wypych, F.; Moraes, S.G.; Durán, N.; Peralta-Zamora, P. Semiconductor-assisted photodegradation of lignin, dye, and kraft effluent by Ag-doped ZnO. Chemosphere 2000, 40, 427–432. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Dutta, B.K. Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst. J. Hazard. Mater. 2004, 112, 269–278. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, Q.; Xing, H.; Chen, D.; Wang, H. Construction of Pillared-Layer MOF as Efficient Visible-Light Photocatalysts for Aqueous Cr(VI) Reduction and Dye Degradation. ACS Sustain. Chem. Eng. 2017, 5, 4449–4456. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Zeng, T.; Shang, Q.; Zhou, H.; Pan, Z.; Cheng, Q. Two pure MOF-photocatalysts readily prepared for the degradation of methylene blue dye under visible light. Dalton Trans. 2018, 47, 4251–4258. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhai, B.; Liang, Y.; Li, Y.; Li, J. Preparation of CdS/ g-C3N4/ MOF composite with enhanced visible-light photocatalytic activity for dye degradation. J. Solid State Chem. 2019, 274, 32–39. [Google Scholar] [CrossRef]
- Sisi, A.J.; Khataee, A.; Fathinia, M.; Vahid, B. Ultrasonic-assisted degradation of a triarylmethane dye using combined peroxydisulfate and MOF-2 catalyst: Synergistic effect and role of oxidative species. J. Mol. Liq. 2020, 297, 111838. [Google Scholar] [CrossRef]
- Khairnar, S.D.; Kulkarni, A.N.; Shinde, S.G.; Marathe, S.D.; Marathe, Y.V.; Dhole, S.D.; Shrivastava, V.S. Synthesis and characterization of 2-D La-doped Bi2O3 for photocatalytic degradation of organic dye and pesticide. J. Photochem. Photobiol. 2021, 6, 100030. [Google Scholar] [CrossRef]
- Abbas, H.; Nasr, R.A.; Khalaf, A.; Al Bawab, A.; Jamil, T.S. Photocatalytic degradation of methylene blue dye by fluorite type Fe2Zr2−xWxO7 system under visible light irradiation. Ecotoxicol. Environ. Saf. 2020, 196, 110518. [Google Scholar] [CrossRef]
- Malathi, M.; Sreenu, K.; Ravi, G.; Kumar, P.; Sudhakar Reddy, C.; Guje, R.; Velchuri, R.; Vithal, M. Low temperature synthesis of fluorite-type Ce-based oxides of composition Ln2Ce2O7 (Ln = Pr, Nd and Eu): Photodegradation and Luminescence studies. J. Chem. Sci. 2017, 129, 1193–1203. [Google Scholar] [CrossRef]
- Devi, V.R.; Ravi, G.; Velchuri, R.; Muniratnam, N.R.; Prasad, G.; Vithal, M. Preparation, Characterization, Photocatalytic Activity and Conductivity Studies of YLnTi2O7(Ln = Nd, Sm, Eu and Gd). Trans. Indian Ceram. Soc. 2013, 72, 241–251. [Google Scholar] [CrossRef]
- Ma, T.T.; Zhou, R.S.; Li, X. Synthesis of Pyrochlore-Type K2Ta2O6 with High Photocatalytic Activity for Dye Degradation. Key Eng. Mater. 2016, 680, 203–207. [Google Scholar] [CrossRef]
- Ravi, G.; Mansouri, S.; Palla, S.; Vithal, M. Synthesis of Y2Ti2O7-XNywith Visible Light Responsive Photocatalytic Activity. Indian J. Chem. Sect. Inorg. Phys. Theor. Anal. Chem. 2015, 54, 20–26. [Google Scholar]
- Abbas, H.A.; Nasr, R.A.; Abu-Zurayk, R.; Al Bawab, A.; Jamil, T.S. Decolourization of crystal violet using nano-sized novel fluorite structure Ga2Zr2−xWxO7 photocatalyst under visible light irradiation. R. Soc. Open Sci. 2020, 7, 191632. [Google Scholar] [CrossRef] [Green Version]
- Holland, T.J.B.; Redfern, S. Unit cell refinement from powder diffraction data: The use of regression diagnostics. Miner. Mag. 1997, 61, 65–77. [Google Scholar] [CrossRef]
- Nasr, R.A.; Abbas, H.; Khalaf, A.; Bozeya, A.; Jamil, T.S. Nano-sized Ga2-xCuxZr2-xWxO7 for Malachite green decolorization under visible light. DESALINATION Water Treat. 2020, 183, 389–403. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Jamil, T.; Mansor, E.; Nasr, R. Degradation of Lindane using two nanosized BiOXs and their heterojunction under visible light. DESALINATION Water Treat. 2015, 57, 14750–14761. [Google Scholar] [CrossRef]
- Kiriakidou, F.; Kondarides, D.; Verykios, X.E. The effect of operational parameters and TiO2-doping on the photocatalytic degradation of azo-dyes. Catal. Today 1999, 54, 119–130. [Google Scholar] [CrossRef]
- Arul, N.S.; Mangalaraj, D.; Han, J.I. Enhanced photocatalytic property of self-assembled Fe-doped CeO2 hierarchical nanostructures. Mater. Lett. 2015, 145, 189–192. [Google Scholar] [CrossRef]
- Sayed, M.; Arooj, A.; Shah, N.S.; Khan, J.A.; Shah, L.A.; Rehman, F.; Arandiyan, H.; Khan, A.M.; Khan, A.R. Narrowing the band gap of TiO2 by co-doping with Mn2+ and Co2+ for efficient photocatalytic degradation of enoxacin and its additional peroxidase like activity: A mechanistic approach. J. Mol. Liq. 2018, 272, 403–412. [Google Scholar] [CrossRef]
- Mohagheghian, A.; Karimi, S.-A.; Yang, J.-K.; Shirzad-Siboni, M. Photocatalytic Degradation of a Textile Dye by Illuminated Tungsten Oxide Nanopowder. J. Adv. Oxid. Technol. 2015, 18, 18. [Google Scholar] [CrossRef]
- Buvaneswari, K.; Karthiga, R.; Kavitha, B.; Rajarajan, M.; Suganthi, A. Effect of FeWO 4 doping on the photocatalytic activity of ZnO under visible light irradiation. Appl. Surf. Sci. 2015, 356, 333–340. [Google Scholar] [CrossRef]
- Coleman, H.M.; Eggins, B.R.; Byrne, J.; Palmer, F.L.; King, E. Photocatalytic degradation of 17-β-oestradiol on immobilised TiO2. Appl. Catal. B Environ. 2000, 24, L1–L5. [Google Scholar] [CrossRef]
- Chanu, L.A.; Singh, W.J.; Singh, K.J.; Devi, K.N. Effect of operational parameters on the photocatalytic degradation of Methylene blue dye solution using manganese doped ZnO nanoparticles. Results Phys. 2019, 12, 1230–1237. [Google Scholar] [CrossRef]
- Barakat, M.; Schaeffer, H.; Hayes, G.; Ismat-Shah, S. Photocatalytic degradation of 2-chlorophenol by Co-doped TiO2 nanoparticles. Appl. Catal. B Environ. 2005, 57, 23–30. [Google Scholar] [CrossRef]
- Chiou, C.-H.; Juang, R.-S. Photocatalytic degradation of phenol in aqueous solutions by Pr-doped TiO2 nanoparticles. J. Hazard. Mater. 2007, 149, 1–7. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Method for Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- ISO 11348-3. Water Quality-Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio Fischeri (Luminescent Bacteria Test)-Part 3: Method Using Freeze-Dried Bacteria; International Organization for Standardization: London, UK, 2007. [Google Scholar]

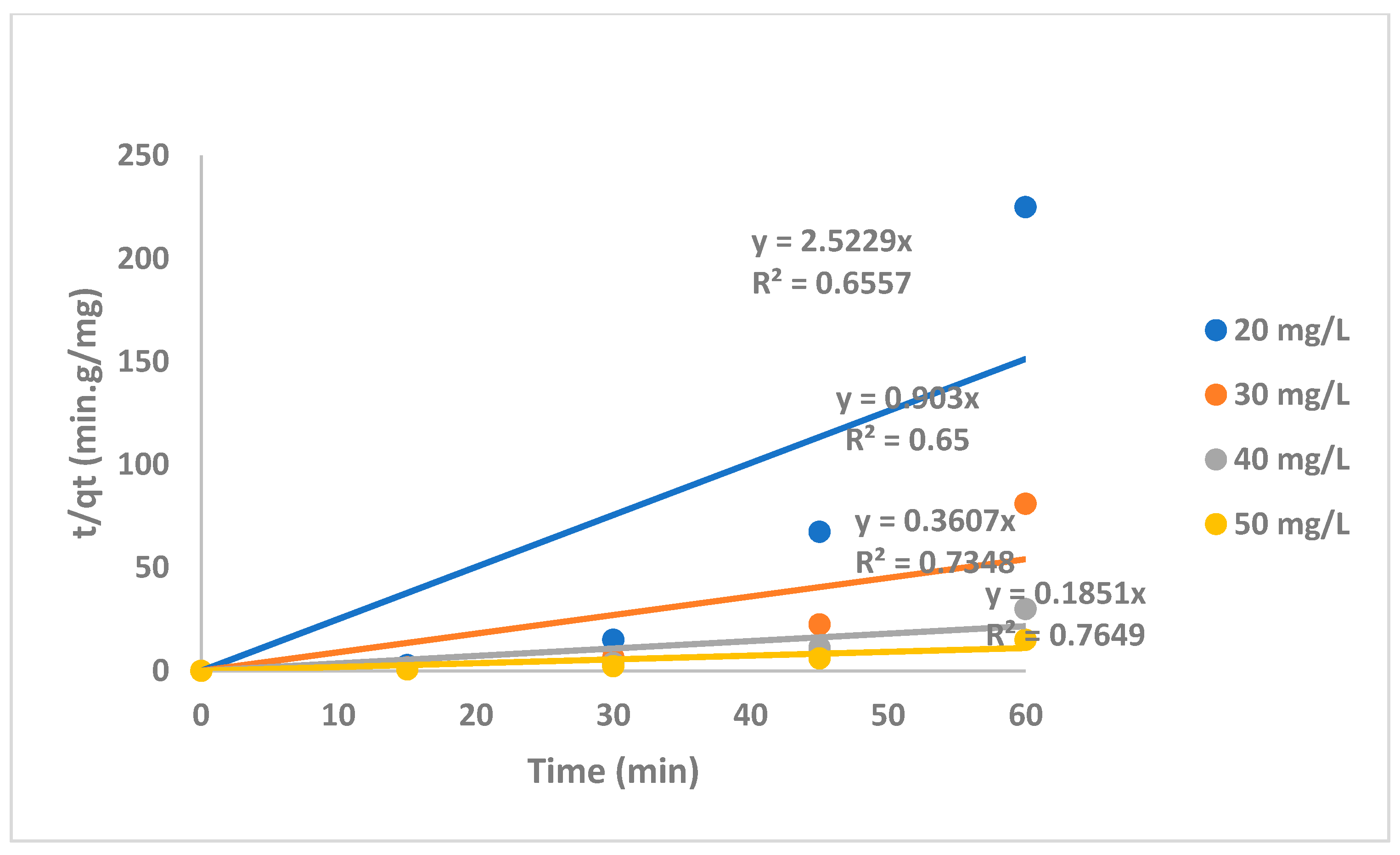
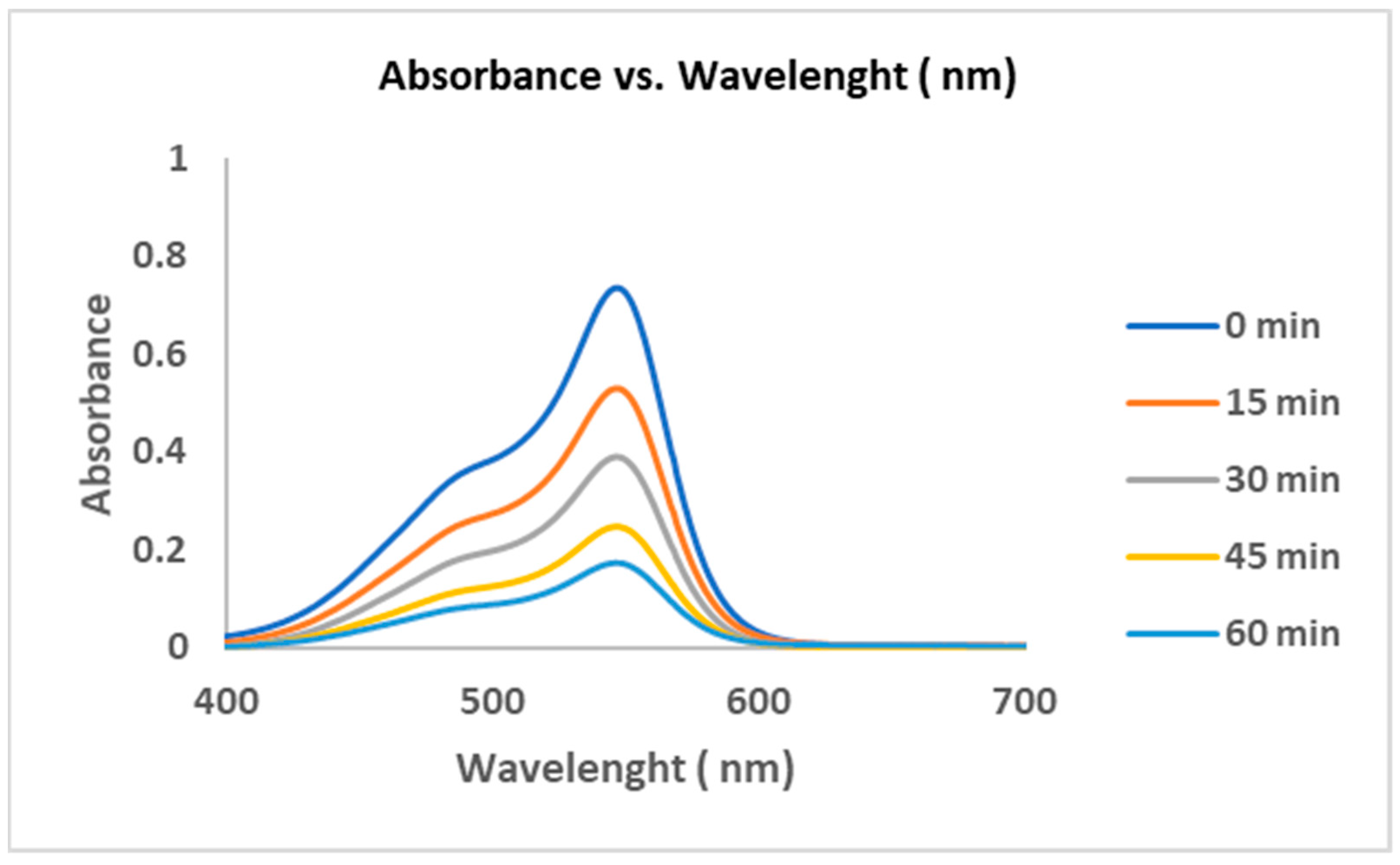
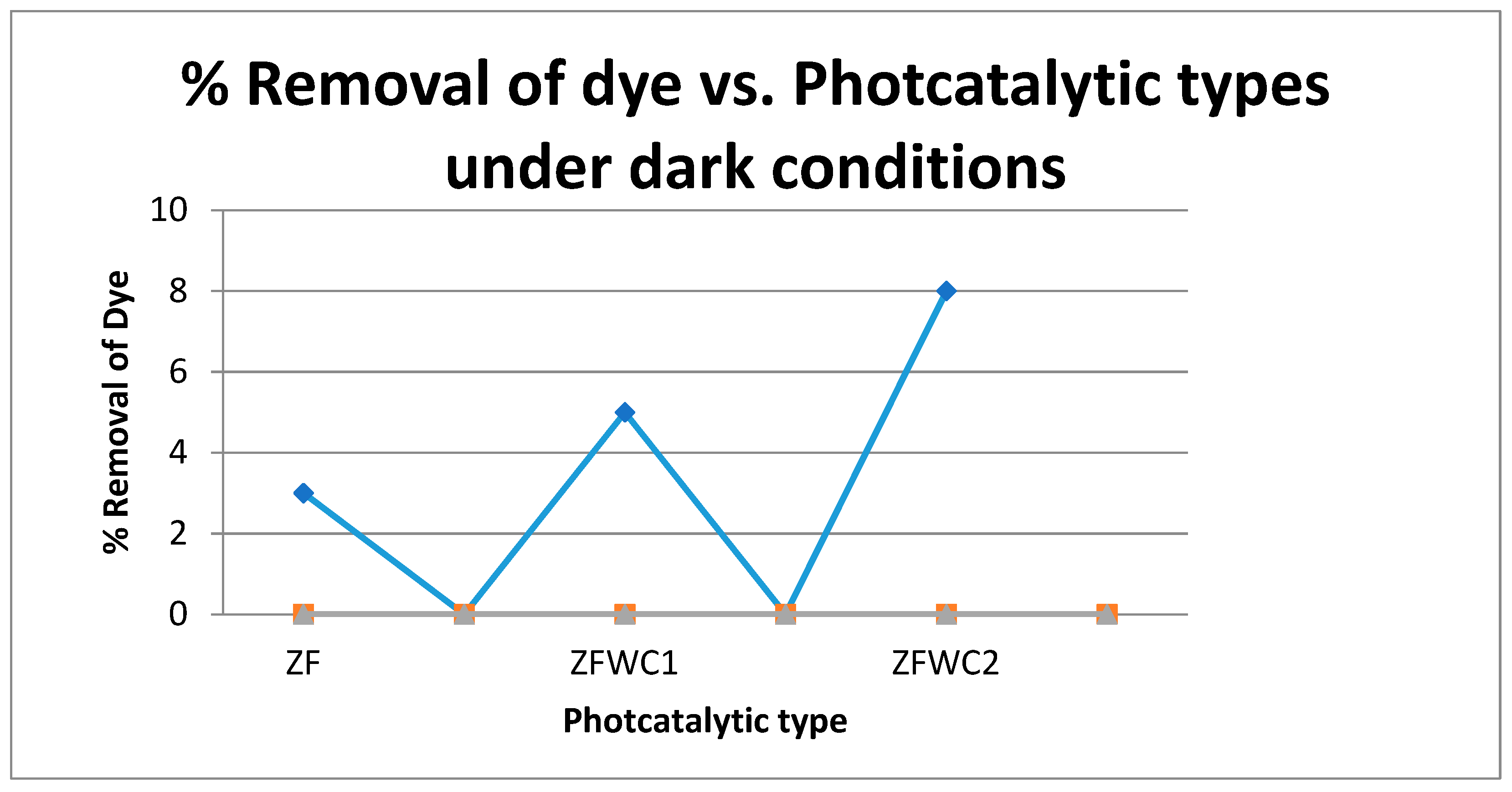
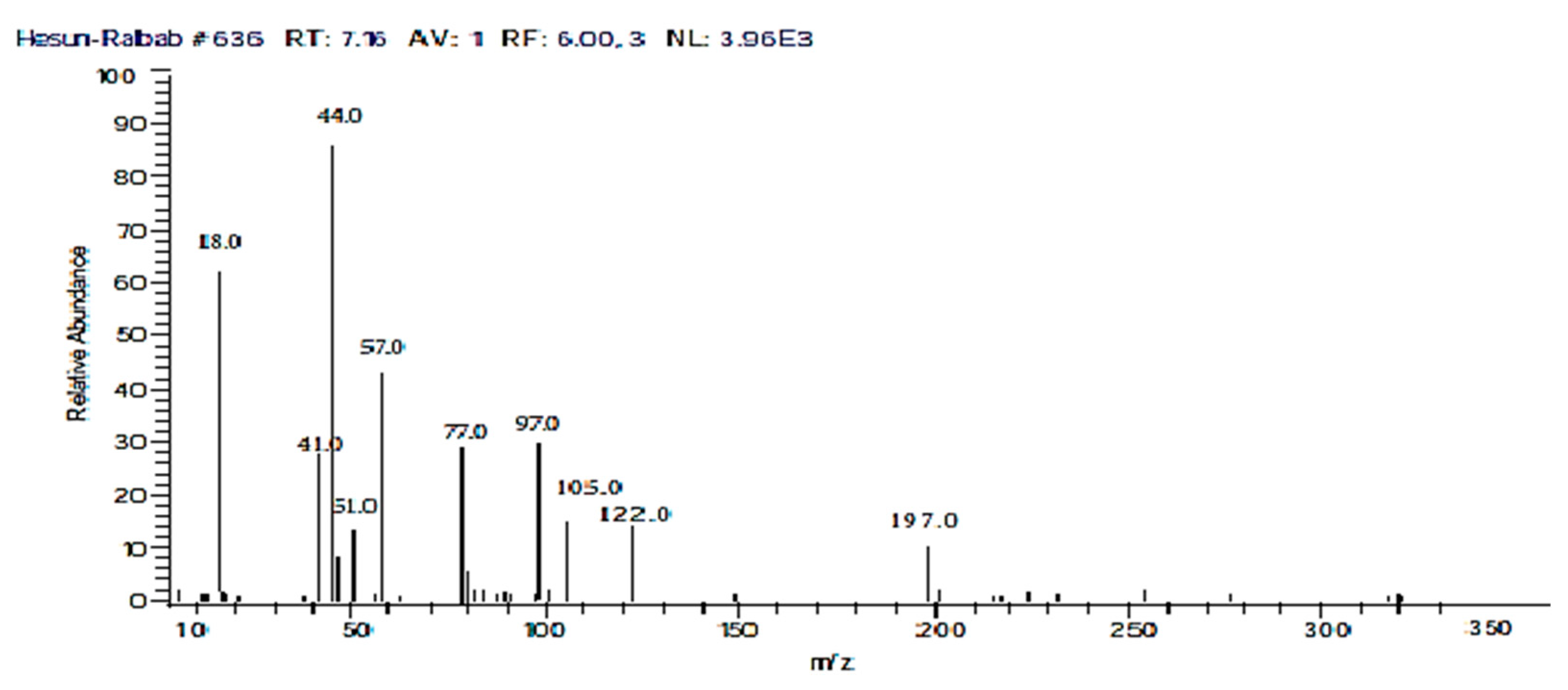

| Name of Catalyst | Composition of Catalyst |
|---|---|
| ZF | Fe2Zr2O7 |
| ZFWC1 | Fe1.95Cu0.05Zr1.95W0.05O7 |
| ZFWC2 | Fe1.85Cu0.15Zr1.85W0.15O7 |
| Sample | a (Å) | V (Å3) |
|---|---|---|
| ZF | 5.10 | 132.29 |
| ZFWC1 | 5.08 | 130.80 |
| ZFWC2 | 5.09 | 131.17 |
| Sample | BG |
|---|---|
| ZF | 1.96 |
| ZFWC1 | 1.86 |
| ZFWC2 | 1.47 |
| Sample | Particle Size Range (nm) |
|---|---|
| ZF | 3–7 |
| ZFWC1 | 5–7 |
| ZFWC2 | 3–6 |
| Photocatalyst | Reaction Time (min) | Catalyst Dose (g/L) | CF Concentration | % Degradation | Reference |
|---|---|---|---|---|---|
| Ni- and Zn-modified montmorillonite clay | 90 | 3 | 15 | 94 | [16] |
| Undoped Fe3O4 | 90 | 8 | 20 | 90 | [3] |
| Co2+- and Ni2+-doped Fe3O4 nanoparticles | 90 | 8 | 20 | 95 | [3] |
| Undoped Fe2Zr2−xWxO7 | 60 | 1.5 | 30 | 77 | Present work |
| Fe1.85Cu0.15Zr1.85W0.15O7 | 60 | 1.5 | 30 | 100 | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Zurayk, R.; Khalaf, A.; Abbas, H.A.; Nasr, R.A.; Jamil, T.S.; Al Bawab, A. Photodegradation of Carbol Fuchsin Dye Using an Fe2−xCuxZr2−xWxO7 Photocatalyst under Visible-Light Irradiation. Catalysts 2021, 11, 1473. https://doi.org/10.3390/catal11121473
Abu-Zurayk R, Khalaf A, Abbas HA, Nasr RA, Jamil TS, Al Bawab A. Photodegradation of Carbol Fuchsin Dye Using an Fe2−xCuxZr2−xWxO7 Photocatalyst under Visible-Light Irradiation. Catalysts. 2021; 11(12):1473. https://doi.org/10.3390/catal11121473
Chicago/Turabian StyleAbu-Zurayk, Rund, Aya Khalaf, Hussien A. Abbas, Rabab A. Nasr, Tarek S. Jamil, and Abeer Al Bawab. 2021. "Photodegradation of Carbol Fuchsin Dye Using an Fe2−xCuxZr2−xWxO7 Photocatalyst under Visible-Light Irradiation" Catalysts 11, no. 12: 1473. https://doi.org/10.3390/catal11121473
APA StyleAbu-Zurayk, R., Khalaf, A., Abbas, H. A., Nasr, R. A., Jamil, T. S., & Al Bawab, A. (2021). Photodegradation of Carbol Fuchsin Dye Using an Fe2−xCuxZr2−xWxO7 Photocatalyst under Visible-Light Irradiation. Catalysts, 11(12), 1473. https://doi.org/10.3390/catal11121473







