Abstract
A wide series of copolymer materials with various contents of 4-vinyl-diisopropyl-phtalate ester (10–90 mol%), divinylbenzene (1–11 mol%) and styrene, as monomers, were obtained by radical copolymerization. In the last steps of the synthesis, diisopropyl ester functionalities were converted into the form of N-hydroxyphthalimide (NHPI) rings. The obtained materials with the NHPI groups immobilized in the copolymer structure were studied by various physicochemical techniques, including FT-IR, UV-Vis-DR, XPS, elemental analysis, and tested as catalysts in aerobic oxidation of p-methoxytoluene in the presence of Co(II) acetate co-catalyst. Conversion of the aromatic substrate was correlated with the NHPI content and cross-linking degree. The best catalytic performance (conversions higher than 23%) was achieved for the copolymer catalysts containing 60% and 30% of 4-vinyl-diisopropyl-phtalate ester. At too high concentrations of NHPI and DVB, some of the NHPI groups were transformed into inactive (C=O)-N=O species or not available due to embedding inside the copolymer structure. The mechanism of the process involving both NHPI centers, forming phthalimide N-oxyl (PINO) radicals, and Co(II) cations was discussed. Stability of the developed catalysts was also tested. The opening of imide rings took place during the catalytic process, resulting in the formation of carboxyl groups and the release of hydroxylamine molecules. The deactivated catalyst could be easily regenerated by repeating two last steps of closing imide ring.
1. Introduction
The processes of oxidation of organic compounds, consisting of an introduction of covalently bonded oxygen atom(s) into a molecule, are widespread both in nature (e.g., biochemical reactions, biomass combustion) and many fields of human activity (e.g., industrial chemical synthesis, combustion of fossil fuels). Of particular technological importance are the reactions of selective oxidation, which allow to obtain many products (including alcohols, aldehydes, ketones, carboxylic acids, and epoxides [1,2,3,4,5,6]) important in various applications, such as production of plastics, synthetic fibers, cleaning agents, dyes, pharmaceuticals, flavors, or fragrances. Nowadays, the industrial process of oxidation of cyclohexane to cyclohexanol and finally to adipic acid (annual production of ca. 3 mln tonnes), is carried out using cobalt(II) or manganese(II) catalysts in the presence of nitric acid at 150–170 °C under pressure of 1–2 MPa O2 [7]. The by-product of this reaction is N2O (recognized as a greenhouse gas), which is formed in amount of about 300 kg per 1 ton of the desired product. An advantage of this process is its relatively high selectivity (~83%), in view of low substrate conversion of 3–5%. In the Amoco process, terephthalic acid is produced (ca. 24 million tons per year) by the oxidation of p-xylene in the presence of cobalt(II) and manganese(II) salts, bromine compounds, acetic acid at 175–225 °C under pressure of 1.5–3 MPa O2 [8,9]. This reaction proceeds with a fairly high conversion of 98% and selectivity of 97%. The presence of aggressive reagents, i.e., bromine compounds, results in the use of anti-corrosion materials (e.g., titanium-containing alloys) in installations, which significantly increases operating costs. Moreover, acetic acid oxidizes in parallel to COx, causing significant economic losses. Phenol is mainly obtained in an industrial scale by the Hock’s process (approximately 12 million tons per year constituting about 95% of world production [10]) involving alkylation of benzene with propylene to cumene, which is then oxidized in the presence of air at 80–120 °C under pressure of 0.7 MPa O2, forming cumene hydroperoxide [7,11]. The last compound is subsequently decomposed with sulfuric acid to phenol and acetone. The end products of this three-step synthesis are obtained with only 30% yield.
As shown above, the most important technological processes of oxidation of hydrocarbons and their derivatives have serious limitations related mainly to the formation of harmful by-products and low yields of expected products. Therefore, alternative solutions focused on, among others, development of more active and selective catalysts have been still developed. One of the more promising approaches is the use of N-hydroxyphthalimide (NHPI) as a catalyst. In the presence of this compound (10 mol%) with the addition of Mn(II) salt and acetic acid, the oxidation of cyclohexane to adipic acid with the yield of 73% was carried out at 100 °C and 0.1 MPa O2 [12,13,14]. Such a significant increase in conversion under milder conditions should be noted as a milestone in the development of oxidation technologies, which has been confirmed by launching pilot plant by Daicel Chemical Industry Ltd. [15,16]. Similarly, NHPI was successfully used in the Amoco process, performed without the addition of bromine compounds [17]. In the presence of NHPI (20 mol%), cobalt(II) and manganese(II) salts, acetic acid, at 100 °C and 0.1 MPa O2, terephthalic acid was achieved at the yield of 99%. Furthermore, in the Hock process, the application of NHPI allowed the reaction conditions to moderate, increasing the yield from 30% to 80% (10 mol% NHPI, acetonitrile, 75 °C, ambient pressure) [18]. Moreover, NHPI was also used successfully in the oxidation of cellulose [19].
Taking into account the promising results for the implementation of NHPI, it is worth developing this type of catalysts further, trying to immobilize NHPI functionalities on solid supports, thus moving from a homogeneous system to a technologically more favorable heterogeneous one. In such a case, a used catalyst would not have to be recovered by controlled evaporation of a solvent combined with precipitation, which significantly increases operating costs. Immobilization of NHPI on a surface of poly(2-hydroxyethylacrylate) [20], cross-linked aminoethylstyrene, chloroethylstyrene [21,22], copolymer of styrene, and maleic anhydride [21], as well as copolymer of glycidyl methacrylate, diethyl glycol dimethacrylate, and methyl methacrylate [23] was described. It was shown that the deposition did not significantly affect the activity of NHPI in the process of aerobic oxidation of p-methoxytoluene, α-methylstyrene, cyclohexane, or acetophenone, respectively. Gao et al. [24] developed a catalyst by immobilizing an NHPI precursor on commercial cross-linked polystyrene using the Friedel–Crafts reaction. In the oxidation of 1-phenylethanol with the use of VO(acac)2 as a cocatalyst, acetophenone was obtained as the only product with the yield of ~40%. Another approach to the synthesis of NHPI-based catalysts for oxidizing alcohols to corresponding ketones was to obtain a derivative that could be attached to ionic liquids, as described by Koguchi et al. [25]. Su et al. [26] used immobilized NHPI for the synthesis of complex O-alkyl hydroxyamines. The method was based on the reaction of trimellitic chloride and NHPI precursor with aminomethyl polystyrene resin and the subsequent reaction with hydroxylamine hydrochloride. In turn, Maillard et al. [27] reacted trimellitic anhydride with O-para-methoxybenzylhydroxylamine hydrochloride, and then deposited the obtained product on aminomethyl polystyrene resin. In the last stage of the synthesis, catalytically active NHPI moieties were obtained in the reaction with hydroxylamine hydrochloride. A NHPI precursor was similarly deposited on commercial polystyrene resins (i.e., aminomethyl and chloromethyl), as well as chloromethylated copolymer of styrene and divinylbenzene [28].
NHPI species were immobilized on various solid supports, including silicas [29,30,31,32], zeolites [33], MOFs [34], diamond particles [35] or carbon nanotubes [36]. An interesting approach to anchoring NHPI groups on the surface of mesoporous silica was described by Li et al. [37]. The N,N-dihydroxypyromellitimide (NDHPI) was attached to 3-glycidyloxypropyltrimethoxysilane (GPTMS), and the resulting organosilane precursor was grafted on the surface of SBA-15. The synthesized catalyst gave toluene conversion of 14.8% at temperature of 90 °C under 1.6 MPa of O2. GPTMS was used as the linking molecule to graft NHPI onto the surface of commercial SiO2 [38,39]. In addition, NHPI was studied in photocatalytic [40,41] and electrocatalytic selective oxidation [42,43], aerobic dehydrogenation of N-heterocycles [44], and intermolecular anti-Markovnikov hydroamination of alkenes [45].
The developed strategies of grafting of NHPI precursors onto available polymeric substrates are based on using a variety of linkers that provide amide or ester bondings. Unfortunately, the formed connections are unstable since the immobilized groups exposed to catalytic reaction conditions are relatively easily hydrolyzed. As a result, detachment of functional groups and migration of NHPI molecules into the reaction environment may occur. Conducting the oxidation processes in the presence of such prepared NHPI-deposited catalysts is therefore associated with strict limitations.
In our previous paper [46], permanently anchoring NHPI groups in the copolymer structure was demonstrated. The binding of NHPI-containing monomer with styrene (S) and divinylbenzene (DVB) leads to stabilization of the functional groups, making them resistant to possible hydrolysis and withdrawal from the copolymer network. The proposed strategy consists of multi-stage transformations described in Scheme 1. The key starting step in this approach is the synthesis of a specially developed monomer—4-vinyl-diisopropyl-phtalate ester (VDPE) from phthalic anhydride (PA). After its embedding in the copolymer structure, diisopropyl ester functionalities are hydrolyzed. Afterwards, the reaction with acetic anhydride and activation to the NHPI groups occur. In the presented work, following this concept of synthesis, we have formed nine series of the copolymer catalysts differing in the content of VDPE-derived monomer (from 10 to 90 mol% at a step of 10 mol%) and the degree of cross-linking (content of DVB = 1, 3, 5, 7, 9, or 11 mol%). Such a wide range of the obtained catalysts allowed us to identify main factors determining a catalytic activity of the NHPI-containing copolymer catalysts in the model process of aerobic oxidation of p-methoxytoluene (p-methylanisole, PMT), as well as to recognize the mechanism of this process.
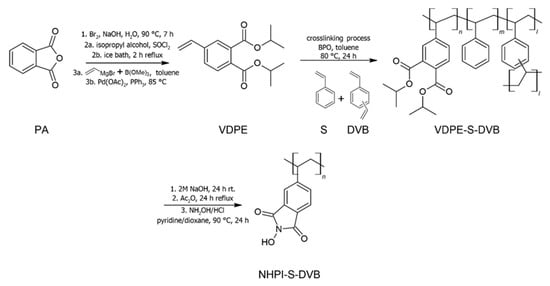
Scheme 1.
Synthetic route for novel NHPI-containing copolymer catalysts.
2. Results and Discussion
2.1. Effect of Composition of Copolymer Catalysts on Activity in Aerobic Oxidation of PMT
The aerobic oxidation of PMT is often used as the model reaction for testing various compounds and materials for their catalytic activity in the selective oxidation of organic substrates [20,46,47]. As shown in Scheme 2, the reaction proceeds with the formation of three major products, i.e., 4-methoxybenzyl alcohol (p-anisyl alcohol), 4-methoxybenzaldehyde (p-anisaldehyde), and 4-methoxybenzoic acid (p-anisic acid). In the catalytic tests, we used conditions that allow relatively easy comparison of the results obtained in the presence of NHPI-containing catalysts with the literature data, e.g., [20,47,48]. Furthermore, the temperature of 80 °C was selected to achieve the optimal reaction rate. It should be kept in mind that at higher temperatures decomposition of NHPI catalysts through self-decay of PINO radicals is favored [49,50]. We used fixed amounts of Co(II) acetate as a co-catalyst and AIBN as a radical initiator as well. It is worth emphasizing that no additional solvent was added.
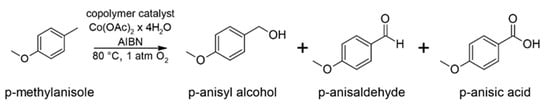
Scheme 2.
Model reaction of aerobic oxidation of p-methoxytoluene.
Changes in the PMT conversion observed under such reaction conditions during six-hour catalytic runs carried out in the presence of the developed NHPI-containing copolymer catalysts are presented in the form of two-dimensional maps in Figure 1. The maps collected at 1 h steps show the effect of the amount of the monomer with NHPI groups and the cross-linking degree (the DVB content) on the catalytic activity, while for the sake of simplicity six zones of the PMT conversions are distinguished with different colors—< 5% (blue, zone I), 5–9% (dark green, zone II), 9–13% (light green, zone III), 13–17% (yellow, zone IV), 17–21% (orange, zone V), and > 21% (red, zone VI).

Figure 1.
Conversion of PMT in the presence of the copolymer catalysts with different NHPI loadings and cross–linking degrees measured during 6 h tests at 80 °C (0.1 g copolymer catalyst, 5.0 mg AIBN, 5.0 mg Co(OAc)2∙4H2O, 2.0 mL PMT, 1 atm O2).
The presence of NHPI functionalities makes all the synthesized copolymer catalysts active in the aerobic PMT oxidation. The achieved conversion of substrate, however, strongly depends on the reaction time, as well as the composition of the catalyst, which determines the content of active functional groups (provided by the NHPI monomer) and the cross-linking degree (depended on the amount of DVB monomer). After 1 h, three catalysts, i.e., 30%NHPI_5%DVB, 40%NHPI_3%DVB, and 60%NHPI_5%DVB, reach the activity at zone II. Two of them (30%NHPI_5%DVB and 60%NHPI_5%DVB) come to zone IV after 2 h, and to zone V after 3 h of the test. Achieving zone VI, where the PMT conversion exceeds 21%, is much more difficult. Among the tested materials, only 60%NHPI_5%DVB (after 4 h), 30%NHPI_7%DVB (5 h), 30%NHPI_9%DVB (6 h), 40%NHPI_7%DVB (6 h) and 50%NHPI_7%DVB (6 h) show so high activity. It can be noticed that some catalysts, despite the high initial reaction rate, are not able to bring the reaction system to a sufficiently high degree of PMT conversion. On the other hand, a very interesting picture of the possibility of using the developed catalysts is drawn on the basis of the analysis of the results collected after 6 h (Table 1).

Table 1.
Conversion of PMT (%) and consumption of O2 (mmol, values given in brackets) in the presence of the copolymer catalysts with different NHPI loadings and cross-linking degrees measured after 6 h tests at 80 °C (0.1 g copolymer catalyst, 5.0 mg AIBN, 5.0 mg Co(OAc)2∙4H2O, 2.0 mL PMT, 1 atm O2).
At the end of the reaction, the most efficient catalysts form a specific diagonal in the matrix extending between 30%NHPI_9%DVB and 60%NHPI_5%DVB. This feature evidently indicates the mutual influence of at least two parameters (including active sites concentration and cross-linking degree) on the catalytic activity. If we compare the catalytic behavior of the materials on the left side of the diagonal with those on the right side, we notice a clear difference in the final PMT conversions. The catalysts with lower NHPI loadings and cross-linking degrees show much better catalytic performance than the materials with higher numbers of NHPI groups, which are additionally more strongly cross-linked. It can be concluded that after exceeding the critical NHPI and DVB contents, defined by the observed diagonal, the activity of the developed copolymer catalysts drops sharply.
Certainly, the observed effect is not only related to the NHPI content as confirmed by the results of the CHN analyzes. Table 2 exhibits the N content in the synthesized materials expressed in mmol in relation to 1 g of a copolymer.

Table 2.
Content of N (mmol/gcatalyst) in the synthesized copolymer catalysts determined by elemental analysis.
It can be clearly seen that, as predicted, the N content rises within the series with the increase in the amount of the NHPI monomer used in the synthesis. The experimental values confirm quite good agreement with the assumed ones. The highest amount of nitrogen incorporated into the copolymer structure (achieved for the 80%NHPI_1%DVB catalyst) is 6.46 mmol/g, which corresponds to 9.1 wt%.
A key parameter determining the activity of the studied catalysts must therefore be the availability of N-OH functionalities which are partially closed within the copolymer structure. These sites can be activated during the reaction, when the material swells in the presence of organic substrate. The pseudoporous structure created in this way enables relatively easy diffusion of reagent molecules to active centers. To determine the susceptibility of the studied copolymers to the formation of such structures, we studied the swelling capacity of the samples using chloroform as the solvent. Figure 2 demonstrates the determined values of swelling degree (Se) for two selected series of catalysts—30%NHPI_xDVB and xNHPI_9%DVB.
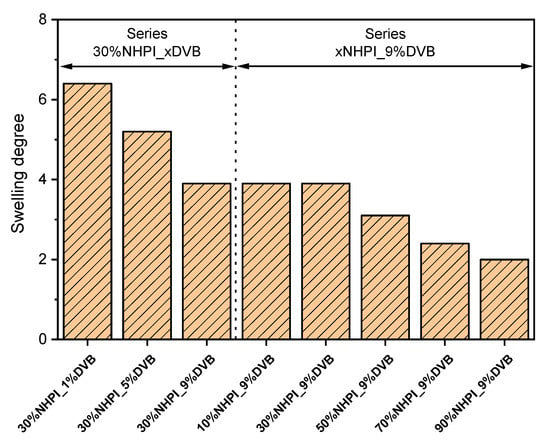
Figure 2.
Dependence of equilibrium swelling degree in chloroform on the content of NHPI and DVB monomers in the studied copolymer catalysts.
It can be seen that fairly small, non-polar chloroform molecules easily penetrate the copolymer structure, the swelling capacity of which is strongly depended on the cross-linking degree. For the 30%NHPI_xDVB series this expected effect results in a change in the value of Se from 6.4 (lower cross-linking degree) to 3.9 (higher cross-linking degree). However, the collected data reveal one more correlation, namely the decrease in the Se value with raising the NHPI content. The observed tendencies can certainly elucidate the catalytic behavior of the materials, but the deterioration of the swelling and catalytic properties for the copolymer catalysts with higher NHPI loadings is still unexplained.
In order to clarify the above effects, spectroscopic studies of the fresh copolymer catalysts were carried out using FT-IR and XPS techniques. In Figure 3, the FT-IR spectra measured for the xNHPI_5%DVB series are presented. Many bands characteristic of C-H, C-C, as well as aromatic ring vibrations, can be easily identified, corresponding to all three monomers used to form the final copolymers. Nevertheless, there are also bands typical for NHPI groups, which confirm the presence of these functionalities [51]. Among them, first of all, we can point to the strong bands at 1713 and 1779 cm−1 (the positions found for 90%NHPI_5%DVB) assigned to the C=O stretching vibrations. Furthermore, less intense bands, designated to the C-N stretching vibrations, appear at 1333 and 1378 cm−1. The N-O vibrations occur in the region of lower wavenumbers and are manifested by the distinct band at 1146 cm−1 (for 90%NHPI_5%DVB). As the NHPI monomer content decreases, the intensities of the individual bands decrease, as expected, but also some of them shift. This effect is especially distinct in the case of the bands corresponding to the vibrations of C=O (for the 20%NHPI_5%DVB sample they are found at 1720 and 1782 cm−1), as well as N-O (the band at 1143 cm−1, respectively). Apparently, the chemical environment around these bonds changed. The explanation of this phenomenon may be the appearance of the band at 1534 cm−1, observed for the catalyst with the highest content of NHPI monomer, which is attributed to the N=O stretching vibrations in (C=O)-N=O groups. Thus, these types of moieties had to be formed within the copolymer structure at the higher NHPI amounts. Taking into account the high concentration of NHPI groups in the copolymers with the high N loadings and high cross-linking degrees, it can be assumed that during the last stage of synthesis, carried out at a relatively high temperature, the formation of adjacent PINO radicals could have occurred. The self-decomposition reaction, described in Scheme 3, took place transforming a part of NHPI groups into catalytically inactive sites [50,52,53].

Figure 3.
FT–IR spectra collected for the fresh xNHPI_5%DVB copolymer catalysts. In the shaded areas, the main vibrations of the NHPI groups are distinguished with the bands marked with asterisks (expected) and diamond (unexpected).
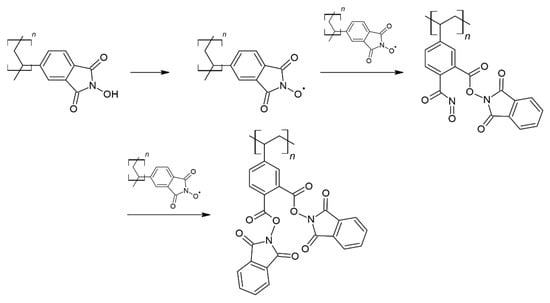
Scheme 3.
Possible transformations of NHPI groups in the copolymer structure with high N loading and high cross-linking degree.
XPS is another technique which confirms the formation of postulated NHPI dimers with the (C=O)-N=O groups. Figure 4 presents the XPS spectra collected in the N 1s and O 1s regions for three catalysts containing 60 mol% of NHPI monomer and differing in the cross-linking degree (the DVB content of 3, 5, or 7 mol%). For the copolymer catalysts with lower cross-linking degrees, where the distance between the NHPI moieties is relatively long, the measured spectra show features typical of N-hydroxyphthalimide.
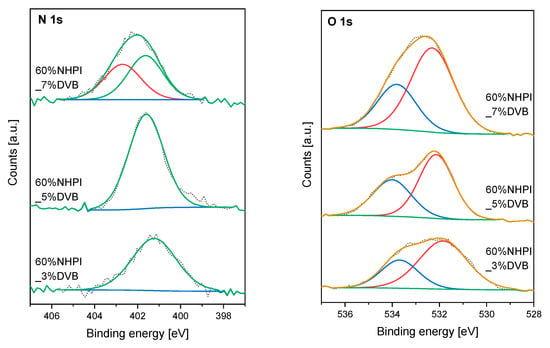
Figure 4.
XPS N 1s and O 1s spectra collected for the fresh 60%NHPI_3%DVB, 60%NHPI_5%DVB and 60%NHPI_7%DVB copolymer catalysts.
In the XPS N 1s spectrum the peak at binding energy (Eb) of 401.6 eV corresponds to the photoelectron emission from N atoms in the N-OH species [47]. On the other hand, the oxygen atoms present in the NHPI functionalities give two characteristic peaks in the O 1s spectrum. The photoemission at Eb = 532.1 eV comes from O atoms in the C=O groups, while at Eb = 533.9 eV—in the N-OH groups. The quantitative proportion of individual oxygen species is roughly maintained in relation to that resulting from the NHPI structure. The anomaly is found for the catalyst with the higher cross-linking degree (60%NHPI_7%DVB), in which self-condensation of adjacent NHPI groups occurred. In this case, the additional component appears in the N 1s spectrum at Eb = 402.7 eV that can be assigned to N atoms in a more oxidized environment (e.g., in N=O). Such clear features of this groups cannot be identified in the O 1s spectrum, because the photoemission of electrons from O atoms in N=O overlaps with the photoemission from O atoms in two other types of oxygen species present in the copolymer structure.
In conclusion, in order to achieve the highest activity of the developed copolymer catalysts, the contents of functional monomer and cross-linking monomer should be properly chosen so as to ensure the optimal concentration of active groups and their exposure during the contact of the catalyst with the solvent, while protecting them against the undesirable process of self-decomposition. The most active catalysts containing NHPI in a fairly wide range, from 1.66 (30%NHPI_9%DVB) to 3.78 mmol/gcatalyst (60%NHPI_5%DVB), were able to convert 3.47–3.81 mmol O2 within 6 h of the reaction at 80 °C in the presence of Co2+ and AIBN. Under the same reaction conditions, NHPI anchored to the surface of copolymer of 2-hydroxyethylacrylate (HEA) and divinylbenzene (NHPI loading = 2.06 mmol/gcatalyst), (aminomethyl)polystyrene (NHPI loading = 1.12 mmol/gcatalyst), and (chloromethyl)polystyrene (NHPI loading = 1.65 mmol/gcatalyst) exhibited significantly lower O2 consumption of 0.17 mmol, 0.41 mmol, and 0.38 mmol, respectively [20,47]. The developed catalysts are, therefore, considerably more active compared to those described previously in the literature.
2.2. Mechanism of Aerobic Oxidation of PMT over Copolymer Catalysts
As noted in Scheme 2, three major products were formed by the aerobic oxidation of PMT over the developed copolymer catalysts. The profiles of their yields are shown in the same way as the PMT conversion in the 2D maps in Figure 5. Six zones of yield values are indicated in the maps with different colors—< 1% (blue, zone I), 1–3% (dark green, zone II), 3–5% (light green, zone III), 5–7% (yellow, zone IV), 7–9% (orange, zone V), and > 9% (red, zone VI). In addition, the exact values of yields and selectivities of the individual products after 6 h of the reaction are summarized in Tables S1–S3 in the Supplementary Materials.

Figure 5.
Yields of p–anisyl alcohol, p–anisaldehyde, and p–anisic acid in the presence of the copolymer catalysts with different NHPI loadings and cross–linking degrees measured during 6 h tests at 80 °C (0.1 g copolymer catalyst, 5.0 mg AIBN, 5.0 mg Co(OAc)2∙4H2O, 2.0 mL PMT, 1 atm O2).
After 1 h the yields of all products are relatively low and do not exceed 5% (for p-anisyl alcohol < 3%, p-anisaldehyde < 5 %, and p-anisic acid < 3%). During the second hour, the yields of alcohol and aldehyde increase markedly. The best catalysts reach zone III for the alcohol production, and even zone V for the aldehyde formation. The acid is still below zone III. During the third hour, the amounts of all products are significantly higher. The alcohol yields are <7% and maintain this level until the catalytic test is complete. For comparison, in the case of aldehyde, a further increase in its amount is observed from 3 to 5 h of the reaction, in which the p-anisaldehyde yields enter zone VI for the most active catalysts. Interestingly, after 5 h, a slight decrease in the p-anisaldehyde yields (to values below 9%) is found. Furthermore, prolonged reaction time has a very positive effect on the p-anisic acid formation. After the third hour, the best catalysts show the acid yields in zone III, after the fourth and fifth hour in zone V, and after the six hours in zone VI. It should be emphasized that the yields above 9% at the end of the reaction are only noted for p-anisic acid—both p-anisyl alcohol and p-anisaldehyde remain in smaller quantities. Such changes in the products profile clearly suggest the occurrence of subsequent reactions in which initially produced p-anisyl alcohol is converted into p-anisaldehyde, and this, in turn, into p-anisic acid. These observations lead directly to the formulation of possible reaction mechanism (Scheme 4) that links the activity of NHPI and Co(OAc)2 as complementary components of the catalytic system for the aerobic oxidation of PMT. The lack of any of these components causes the system to stop working effectively. We tested the reaction in the presence of the best copolymer catalyst without the addition of Co(II) salt, as well as using only Co(II) acetate without the participation of NHPI, and in both cases after 6 h of the reaction, the PMT conversion ranged from 1.0% to 1.2%.
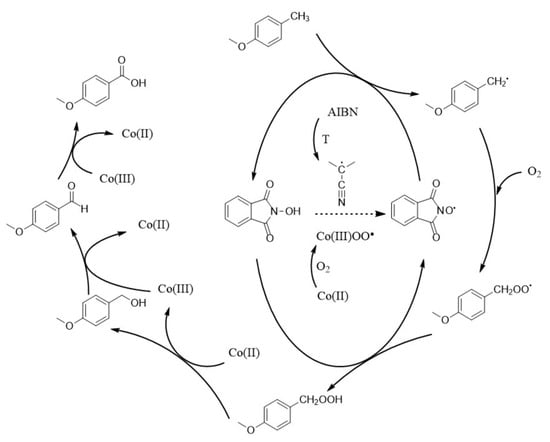
Scheme 4.
Plausible mechanism of aerobic oxidation of PMT in the presence of NHPI-containing copolymer and Co(OAc)2.
A key step in the free-radical reaction of aerobic oxidation of PMT is the generation of phthalimide N-oxyl radicals (PINO) from the NHPI species. PINO are initially formed with the involvement of azobisisobutyronitrile (AIBN), which at an elevated temperature decomposes into two 2-cyanoprop-2-yl radicals and a N2 molecule evolved to the gas phase. The presence of PINO enables the entire cycle of transformations based on the activation of the C-H bond through hydrogen atom transfer [54,55]. Namely, the CH2 radicals are generated in the methyl group of PMT, which subsequently react with molecular oxygen giving corresponding peroxy radicals. Finally, the hydroperoxide compound is produced by abstraction of hydrogen from another NHPI functionality. Despite the unlikely termination of PINO [5], the described transformation chain may be interrupted and the reconstruction of PINO would become impossible. However, the presence of 2-cyanoprop-2-yl radicals in the initial period of the reaction, and then Co(III)OO radicals (formed by binding O2 on Co(II)), make possible to keep the concentration of PINO in the system at a constant level. The further stages of the reaction are catalyzed exclusively by the Co(II)/Co(III) redox system, which facilitates the formation of p-anisyl alcohol, p-anisaldehyde and finally p-anisic acid.
Taking into account the above-described mechanism, it is very important to ensure the optimal molar ratio of Co(II)/NHPI in the catalytic system. It was examined on an example of 30%NHPI_5%DVB, and the results in the form of PMT conversion obtained after 6 h of the aerobic oxidation reaction are presented in Figure 6. Keeping the Co(II)/NHPI molar ratio between 1:8 and 1:12 was found to give the best efficiency. If this ratio is changed beyond the determined optimum, a clear decrease in the activity, associated with the imbalance between the subsequent reaction steps and extinction of PINO radicals, is observed.
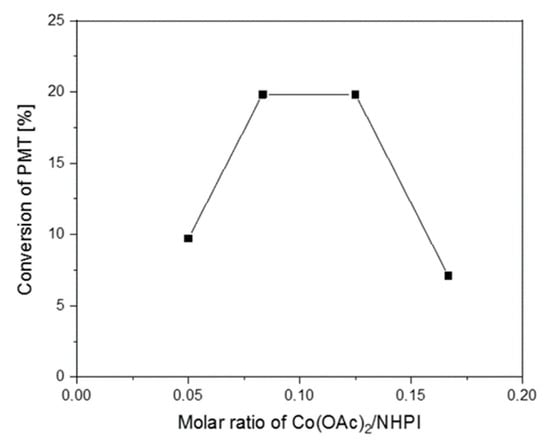
Figure 6.
Influence of Co(OAc)2/NHPI molar ratio on conversion of PMT achieved in the presence of 30%NHPI_5%DVB after 6h at 80 °C (0.1 g copolymer catalyst, 5.0 mg AIBN, 2.0–6.5 mg Co(OAc)2∙4H2O, 2.0 mL PMT, 1 atm O2).
2.3. Stability of Copolymer Catalysts during Aerobic Oxidation of PMT
Stability of copolymer catalysts in the aerobic PMT oxidation was analyzed using 30% NHPI_5% DVB material. The catalytic test was interrupted after 1, 2, or 6 h, respectively, and then the catalyst was separated from the reaction mixture, washed with a solvent and dried. During the studied period, the PMT conversion increased from 6.1% (after 1 h) by 13.2% (2 h) finally to 18.5% (6 h). With the increase in the PMT conversion, a decrease in the N content in the catalyst was observed. The fresh 30%NHPI_5%DVB catalyst contained 4.0 wt% of nitrogen corresponding to 2.84 mmol of N-OH groups in 1 g of the catalyst. After 1 h of the reaction the number of N-OH groups dropped to 2.29 mmol/g (3.2 wt% of N). The prolonged reaction time resulted in a further decrease in the N-OH amount to 1.79 mmol/g (2.5 wt% of N) and 0.93 mmol/g (1.3 wt% of N), after 2 and 6 h of the catalytic test, respectively.
The changes in the catalyst structure were studied by FT-IR (Figure 7). After the catalytic reaction, a new intense IR band appears at 1675 cm−1 corresponding to the vibrations of carboxylic groups that may be derived from p-anisic acid (one of the PMT oxidation products) deposited on the catalyst surface. On the other hand, NHPI decomposition may occur during the reaction according to the mechanism proposed by Hamdah et al. [56], who described the opening of imide ring leading to the formation of carboxyl groups and free hydroxylamine. For a proper diagnosis of this effect, the catalyst after 6 h of the reaction was rinsed copiously with MeCN, which effectively removed p-anisic acid. The FT-IR spectrum of 30%NHPI_5%DVB_6h_MeCN reveals an IR band at 1686 cm−1 confirming the presence of carboxyl groups formed by the NHPI decomposition. Furthermore, the FT-IR spectra of the 30%NHPI_5%DVB catalyst after 6 h of the catalytic test show bands at 3444 cm−1 and 3393 cm−1 attributed to asymmetric and symmetrical NH stretching vibrations, as well as at 1633 cm−1 assigned to the deformation vibrations of primary NH2 groups present in released NH2OH molecules deposited inside the copolymer structure.
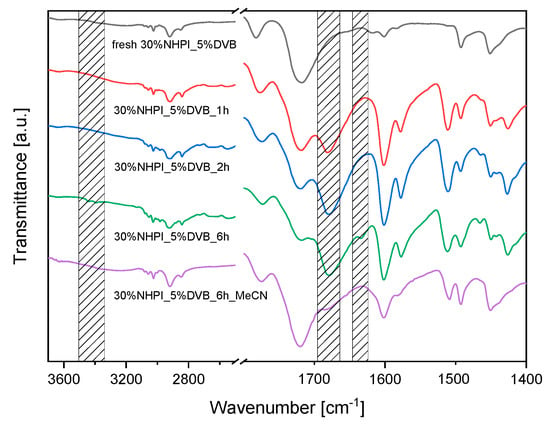
Figure 7.
FT–IR spectra collected for the 30%NHPI_5%DVB copolymer—fresh and after various times (1, 2, and 6 h) of the aerobic PMT oxidation at 80 °C (0.1 g copolymer catalyst, 5.0 mg AIBN, 5.0 mg Co(OAc)2∙4H2O, 2.0 mL PMT, 1 atm O2), as well as 30%NHPI_5%DVB after 6 h of the catalytic reaction, separated, and washed with MeCN. In the shaded areas, the new bands are indicated.
Additionally, UV-VIS measurements were performed for the fresh and used 30%NHPI_5%DVB copolymer material (Figure 8).
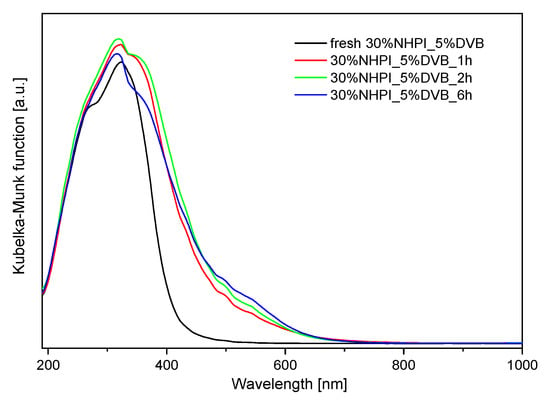
Figure 8.
UV-Vis spectra collected for the 30%NHPI_5%DVB copolymer—fresh and after various times (1, 2, and 6 h) of the aerobic PMT oxidation at 80 °C (0.1 g copolymer catalyst, 5.0 mg AIBN, 5.0 mg Co(OAc)2∙4H2O, 2.0 mL PMT, 1 atm O2).
The UV-Vis spectra of the spent catalysts exhibit an increase in intensity of absorption bands in the wavelength range of 400 to 600 nm compared to the fresh sample. The observed effect can be attributed to the electronic transitions characteristic of Co(II) and Co(III) ion complexes with carboxyl and amine ligands, such as Co(II) with COO− which presents an absorption band at 415 nm [57]. The previously published papers reported absorption bands at 520 nm for Co(II) complexes with NH2 amine ligands [58], at 550 nm for Co(III) with ethylenediamine ligands [59] and at 470 nm for Co(III) in Co(NH3)63+ complex [60]. Thus, the presence of primary NH2 groups and carboxyl groups formed in the copolymer structure as a result of the decomposition of NHPI groups results in the adsorption of Co(II) or Co(III) cations.
XPS measurements were performed for the spent catalysts containing 60 mol% of the functional monomer with different cross-linker contents (3, 5, or 7 mol% of DVB), which has been previously analyzed in the fresh form (cf. Figure 4). The results collected for the materials after 6 h of the catalytic run, isolation, washing with PMT and effective drying are shown in Figure 9. In the XPS N 1s spectrum of the 60%NHPI_7% DVB material with the highest cross-linking degree, beside the peak characteristic for N-OH functionalities, an additional peak appears at 399.2 eV. This can be attributed to the presence of amine groups in hydroxylamine trapped in the copolymer structure [61]. Interestingly, in the case of this sample, significant amounts of Co are also found on the surface. The chemical nature of these species can be determined on the basis of the XPS Co 2p spectrum (Figure 9). The parent Co 2p3/2 and 2p1/2 peaks at 781.9 eV and 797.5 eV (spin-orbit splitting Δ = 15.6 eV), respectively, with very intense satellite structure (at 786.0 eV and 802.6 eV), suggest that high-spin Co(II) complexes were formed with NH2OH species [62,63]. The intensities of the peaks attributed to photoemission from Co atoms in the less cross-linked samples are significantly lower. It is also worth noting that the content of NH2OH and, thus, the formed Co(II) complexes is strongly correlated with the catalytic activity in the aerobic PMT oxidation. The high cross-linking most likely causes that the hydroxylamine released as a result of the decomposition of NHPI groups remains bound inside the polymer network, while attaching Co(II) cations. Consequently, this leads to a fast loss of activity of the material.
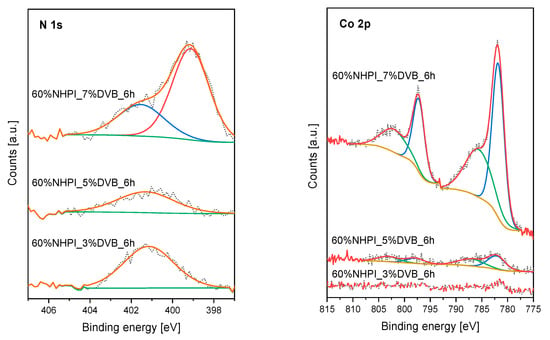
Figure 9.
XPS N 1s and Co 2p spectra collected for the 60NHPI_3%DVB, 60NHPI_5%DVB and 60NHPI_7%DVB copolymer catalysts after the aerobic PMT oxidation at 80 °C (0.1 g copolymer catalyst, 5.0 mg AIBN, 5.0 mg Co(OAc)2∙4H2O, 2.0 mL PMT, 1 atm O2, 6 h).
Fortunately, there is a relatively simple way to regenerate the spent catalyst, which was confirmed for one of the most active materials—60%NHPI_5%DVB. After the first catalytic cycle, in which after 6 h the PMT conversion of 23.3% was achieved, the catalyst was separated, washed with PMT and introduced into the fresh reaction system. After the repeated 6 h test, the obtained PMT conversion significantly decreased 6.6% due to the limited number of available active sites. The initial nitrogen content of 5.3 wt% corresponding to 3.78 mmol N-OH/g reduced after the first test to 2.4 wt% (1.71 mmol N-OH/g). The used catalyst was activated by repeating the last two steps of NHPI group formation, i.e., the reaction leading to the generation of phthalic anhydride structure, and then its transformation into the active forms of NHPI. In this way, the N content was restored to the level of 4.8 wt% (3.43 mmol N-OH/g), which allowed to restore high catalytic activity of 21.3% PMT conversion.
3. Materials and Methods
3.1. Synthesis
3.1.1. Synthesis of Functional Monomer (VDPE)
The VDPE monomer was synthesized by dissolving phthalic anhydride (100.0 g, 0.676 mol) in water (448 mL) containing NaOH (54.0 g, 1.352 mol) under vigorous stirring. Subsequently, ice cooled bromine mixture (36.5 mL, 0.712 mol) was added to the prepared solution and heated to 90 °C for 7 h. Afterwards, the mixture was cooled to room temperature and kept overnight. The obtained product—4-bromophtalic acid monosodium and disodium salts (86.624 g)—was filtered and recrystallized in water to clean the precipitate, and then dried in a vacuum oven. The obtained salt was suspended in isopropanol (600 mL) and cooled in an ice bath for 1 h. Subsequently, thionyl chloride (170.5 g, 1.434 mol) was added dropwise, and refluxed for 2 h. The resulting product was isolated from the cooled mixture using a rotary evaporator and mixed with water (1300 mL). The mixture was extracted three times with ethyl acetate (150 mL). The organic phases were combined and washed with a saturated aqueous solution of NaHCO3 (100 mL), dried overnight with MgSO4, filtered, and concentrated in vacuo. The produced oil (i.e., 4-bromo-diisopropy-phthalate) was purified using a silica gel chromatography with cyclohexane/ethyl acetate (v:v = 3:1).
According to the procedure described by Braslau et al. [64], vinyl magnesium bromide solution (12.6 mL 1 M in THF, 12.6 mmol) was added to trimethyl borate (1.311 g, 12.6 mmol) in anhydrous toluene (44 mL) at −78 °C under a nitrogen atmosphere. Afterwards, the mixture was slowly heated to 20 °C, and water (10 mL) containing 4-bromo-diisopropy-phthalate (1.197 g, 4.2 mmol) and K2CO3 (0.841 g, 8.4 mmol) was introduced. After degassing by bubbling Ar for 30 min, palladium(II) acetate (0.019 g, 0.085 mmol) and triphenylphosphine (0.134 g, 0.51 mmol) were added to the reaction mixture and heated at 85 °C for 18 h and then at 90 °C for 24 h. Upon cooling, the organic and aqueous phases were separated. The organic phase was cleaned with brine, dried overnight with MgSO4, filtered, and concentrated in vacuo. The resulting oil was purified by a silica gel chromatography with ethyl acetate/cyclohexane (v:v = 15:85) giving the desired product of VDPE (1.010 g).
3.1.2. Copolymerization of VDPE, DVB and Styrene
The synthesized VDPE monomer was subjected to the bulk copolymerization with DVB (used as a cross-linking monomer) and styrene (another hydrophobic vinyl monomer). In each synthesis, 1 g of VDPE was used, to which an appropriate amount of styrene and DVB were introduced to obtain an assumed molar ratio of VDPE:S:DVB. The content of VDPE varied in the range from 10 to 90 mol% (at 10 mol% step), while the amount of DVB changed in the range from 1 to 11 mol% (at 2 mol% step). Styrene was always the balance to 100%. The monomers were mixed with toluene (0.5 mL) and benzoyl peroxide (BPO, 2 mol% in relation to other monomers) as an initiator. Before the copolymerization reaction, the mixture was degassed by bubbling with Ar for 5 min. The vial was sealed and placed in an oil bath kept at 80 °C for 24 h. The obtained copolymer was crushed, washed with toluene and CH2Cl2, and dried in a vacuum oven at 50 °C. Subsequently, the material was suspended in the mixture of NaOH (3.2 g, 80 mmol), water (20 mL), and dioxane (20 mL), and refluxed for 48 h. After cooling, it was washed with 1M HCl, dioxane, CH2Cl2 and Et2O, followed by drying in a vacuum oven. Next, the copolymer was dispersed in acetic anhydride (20 mL) and mixed with dioxane (20 mL), refluxed for 24 h, again washed with dioxane, CH2Cl2 and Et2O, and dried in a vacuum oven. Finally, the product was dispersed in a mixture of pyridine (30 mL) and dioxane (10 mL). To the prepared solution, hydroxylammonium chloride (0.514 g, 7.4 mmol) was added, and the obtained suspension was refluxed for 24 h. The resulting solid was washed with dioxane, H2O/DMF, DMF, toluene, EtOAc, CH2Cl2, and Et2O, and dried in a vacuum oven.
The finally obtained copolymer catalysts were denoted as aNHPI_bDVB, where a and b mean the content of the functional monomer and DVB, respectively, used in the copolymerization process, in relation to styrene. For example, the symbol 10%NHPI_1%DVB refers to the copolymer synthesized in the mixture containing 10 mol% VDPE, 1 mol% DVB, and 89 mol% styrene.
3.2. Characterization
Elemental analysis of C, H, and N was carried out in a FLASH 2000 elemental analyzer (ThermoFisher Scientific, Waltham, MA, USA).
Fourier transform infrared spectra were recorded for powder samples with a Nicolet 6700 spectrometer (Thermo Scientific, Waltham, MA, USA) using an attenuated total reflectance (ATR) mode (200 scans, resolution = 4 cm−1, wavenumber range = 650 to 4000 cm−1).
A swelling behavior was determined using a standard procedure, in which a sample dried to constant weight was immersed in chloroform for 24 h at room temperature. The isolated swollen catalyst was dried with a Sartorius MA37-1 analyzer (Sartorius, Getynga, Germany) at 80 °C to constant weight. The swelling degree (Se) was calculated applying the formula:
where me means the weight of swollen copolymer at equilibrium, and m0—the weight of dried copolymer.
UV-Vis diffuse reflectance spectra were recorded using a Thermo Scientific Evolution 220 spectrometer (ThermoFisher Scientific, Waltham, MA, USA). (resolution = 1 nm, 60 scans per minute, wavelength range = 200 to 1000 nm).
X-ray photoelectron spectra were collected using a photoelectron spectrometer (PREVAC, Rogów, Poland) equipped with a hemispherical VG SCIENTA R3000 analyzer (Scienta Omicron Uppsala, Sweden). The spectra were taken using a monochromatized aluminum source Al Kα (E = 1486.6 eV) and a low-energy electron flood gun (FS40A-PS) to compensate for a charge accumulation on a surface of nonconductive samples. Binding energy scale was calibrated by referring to a position of C 1s (Eb = 284.8 eV). The Shirley background and fitting with the mixed function of Gauss and Lorentz (GL = 30) were used during interpretation of the spectra in the CasaXPS software.
3.3. Catalytic Tests
Catalytic tests were performed in a dedicated system based on a flask tightly connected to a gasometric apparatus containing a gas burette filled with oxygen under atmospheric pressure. Prior to the catalytic run, a synthesized NHPI-S-DVB catalyst (0.10 g) was activated by soaking in PMT (2.0 mL, 15.8 mmol) for 24 h at room temperature. Subsequently, the reaction mixture was heated to a reaction temperature (80 °C) under stirring (1100 rpm), and then AIBN (5.0 mg, 0.03 mmol) as an initiator and Co(OAc)2∙4H2O (typically 5.0 mg, 0.02 mmol, but in some cases its amount varied within the range of 2.0 to 6.5 mg) as a co-catalyst were added. Typically, the reaction was run for 6 h, but in some cases it was stopped after 1 or 2 h. During the whole catalytic test an oxygen uptake was measured and recalculated to the standard conditions of 25 °C and 1 atm. The products of PMT oxidation were additionally analyzed in a gas chromatograph (GC) equipped with a DB-WAX capillary column (30 m × 0.32 mm × 0.5 µm) and a flame ionization detector (FID). The withdrawn samples of the reaction mixture (10 µL) were mixed with cyclohexane (10 µL), used as a standard, and the prepared solutions (0.3 µL) were injected into the GC.
4. Conclusions
Various amounts of VDPE monomer were incorporated with styrene and DVB into the copolymer structure using the developed copolymerization strategy. Moreover, the content of DVB was changed giving different cross-linking degrees of the produced copolymer materials. Finally, they were converted into a catalytically active form by hydrolysis of diisopropyl ester functionalities and ring closure towards the formation of NHPI groups. A wide series of the synthesized materials allowed the analysis of the influence of the copolymer composition on its catalytic activity in the aerobic PMT oxidation. Obviously, the observed PMT conversions were correlated with the NHPI species concentration. It was however found that too high NHPI and DVB contents resulted in a clear decrease in the catalytic activity due to limited availability of N-OH functionalities embedded inside the copolymer structure. Furthermore, at the high NHPI loadings the formation of noticeable number of inactive (C=O)-N=O functionalities was confirmed by FT-IR and XPS.
The presence of Co(II) cations in the reaction mixture was necessary for the functioning of NHPI centers anchored in the copolymer structure. At a properly selected Co(II)/NHPI molar ratio, the optimal concentration of PINO radicals was maintained, which activated C-H bonds in PMT through hydrogen atom transfer. The formed CH2• radicals reacted with molecular O2 giving corresponding hydroperoxide compound. The Co(II)/Co(III) redox system facilitated the formation of p-anisyl alcohol, p-anisaldehyde, and p-anisic acid, being the main products of the studied reaction.
It was observed that along with the reaction time, the copolymer catalysts were deactivated gradually due to decomposition of NHPI groups. The FT-IR results revealed that the main cause of this negative effect was the opening of imide ring leading to the formation of carboxyl groups and hydroxylamine. The latter compound remained in the catalyst structure, especially with high cross-linking degree, additionally complexing Co(II) ions, which was confirmed by XPS and UV-Vis-DR techniques. The spent copolymer catalyst could, however, be relatively easily regenerated by repeating the last two steps of NHPI group formation.
Taking into account high catalytic activity of the developed copolymer catalysts compared to other systems described in the literature (Table 3), as well as the efficient regeneration procedure, they can be candidates for commercial use. A very significant advantage of their application is the lack of necessity to use a solvent, which reduces the costs of separation of the reaction mixture.

Table 3.
Comparison of activity in the aerobic oxidation of aromatic compounds for selected catalysts described in the literature and the NHPI-containing copolymer studied in this work.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal11121474/s1, Table S1. Yield (%) and selectivity to p-anisyl alcohol (%, values given in brackets) in the presence of the copolymer catalysts with different NHPI loadings and cross-linking degrees measured after 6 h tests at 80 °C (0.1 g copolymer catalyst, 5.0 mg AIBN, 5.0 mg Co(OAc)2∙4H2O, 2.0 mL PMT, = 1 atm); Table S2. Yield (%) and selectivity to p-anisaldehyde (%, values given in brackets) in the presence of the copolymer catalysts with different NHPI loadings and cross-linking degrees measured after 6 h tests at 80 °C (0.1 g copolymer catalyst, 5.0 mg AIBN, 5.0 mg Co(OAc)2∙4H2O, 2.0 mL PMT, = 1 atm); Table S3. Yield (%) and selectivity to p-anisic acid (%, values given in brackets) in the presence of the copolymer catalysts with different NHPI loadings and cross-linking degrees measured after 6 h tests at 80 °C (0.1 g copolymer catalyst, 5.0 mg AIBN, 5.0 mg Co(OAc)2∙4H2O, 2.0 mL PMT, = 1 atm).
Author Contributions
Conceptualization, T.B., P.Ł. and P.K.; methodology, T.B., P.Ł. and P.K.; investigation, T.B., P.Ł., A.R. and K.S.; writing—original draft preparation, T.B., P.Ł. and P.K.; writing—review and editing, T.B. and P.K.; visualization, T.B.; supervision, P.K. All authors have read and agreed to the published version of the manuscript.
Funding
T.B. has been partly supported by the EU Project POWR.03.02.00-00-I004/16. Some measurements were carried out with the equipment purchased thanks to the financial support of the European Regional Development Fund in the framework of the Polish Innovation Economy Operational Program (contract no. POIG.02.01.00-12-023/08).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Wang, L.; Zhang, Y.; Du, R.; Yuan, H.; Wang, Y.; Yao, J.; Li, H. Selective One-Step Aerobic Oxidation of Cyclohexane to ϵ-Caprolactone Mediated by N-Hydroxyphthalimide (NHPI). ChemCatChem 2019, 11, 423–427. [Google Scholar] [CrossRef] [Green Version]
- Melone, L.; Franchi, P.; Lucarini, M.; Punta, C. Sunlight Induced Oxidative Photoactivation of N-Hydroxyphthalimide Mediated by Naphthalene Imides. Adv. Synth. Catal. 2013, 355, 3210–3220. [Google Scholar] [CrossRef]
- Tong, X.; Xu, J.; Miao, H. Highly Efficient and Metal-Free Aerobic Hydrocarbons Oxidation Process by an o-Phenanthroline-Mediated Organocatalytic System. Adv. Synth. Catal. 2005, 347, 1953–1957. [Google Scholar] [CrossRef]
- Kuznetsova, N.I.; Kuznetsova, L.I.; Yakovina, O.A.; Karmadonova, I.E.; Balzhinimaev, B.S. An Improved Catalytic Performance of Fe(III)-promoted NHPI in the Oxidation of Hydrocarbons to Hydroperoxides. Catal. Lett. 2020, 150, 1020–1027. [Google Scholar] [CrossRef]
- Figiel, P.J.; Sobczak, J.M. Mechanistic Investigation of the Catalytic System Based on N-Hydroxy-Phthalimide with Vanadium co-Catalysts for Aerobic Oxidation of Alcohols with Dioxygen. J. Catal. 2009, 263, 167–172. [Google Scholar] [CrossRef]
- Zheng, G.; Liu, C.; Wang, Q.; Wang, M.; Yang, G. Metal-Free: An Efficient and Selective Catalytic Aerobic Oxidation of Hydrocarbons with Oxime and N-Hydroxyphthalimide. Adv. Synth. Catal. 2009, 351, 2638–2642. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Methods and tools of sustainable industry chemistry: Catalysis. In Sustainable Industrial Chemistry; Cavani, F., Centi, G., Perathoner, S., Trifiro, F., Eds.; Wiley-VCH: Weinheim, Germany, 2009; pp. 73–188. [Google Scholar]
- Weissermel, K.; Arpe, H.J. Oxidation Products of Xylene and Naphthalene. In Industrial Organic Chemistry, 3rd ed.; Wiley-VCH: Weinheim, Germany, 1997; pp. 385–404. [Google Scholar]
- Sheehan, R.J. Terephthalic acid, dimethyl terephthalate, and isophthalic acid. In Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed.; Wiley-VCH: Weinheim, Germany, 2012; Volume 36. [Google Scholar]
- Melone, L.; Gambarotii, C.; Prosperini, S.; Pastori, N.; Recupero, F.; Punta, C. Hydroperoxidation of Tertiary Alkylaromatics Catalyzed By N-Hydroxyphthalimide and Aldehydes under Mild Condition. Adv. Synth. Catal. 2011, 353, 147–154. [Google Scholar] [CrossRef]
- Hock, H.; Lang, S. Autoxydation von Kohlenwasserstoffen, IX. Mitteil.: Über Peroxyde von Benzol-Derivaten. Ber. Dtsch. Chem. Ges. 1944, 77, 257–264. [Google Scholar] [CrossRef]
- Ishii, Y.; Sakaguchi, S.; Obora, Y. Aerobic oxidations and related reactions catalyzed by N-hydroxyphthalimide. In Modern Oxidation Methods; Bäckvall, J.-E., Ed.; Willey-VCH: Weinheim, Germany, 2010; pp. 187–240. [Google Scholar]
- Ishii, Y.; Sakaguchi, S. Recent progress in aerobic oxidation of hydrocarbons by N-hydroxyimide. Catal. Today 2006, 117, 105–113. [Google Scholar] [CrossRef]
- Ishii, Y.; Iwahama, T.; Sakaguchi, S.; Nakayama, K.; Nishiyama, Y. Alkane Oxidation with Molecular Oxygen Using a New Efficient Catalytic System: N-Hydroxyphthalimide (NHPI) Combined with Co(acac)n (n = 2 or 3). J. Org. Chem. 1996, 61, 4520–4526. [Google Scholar] [CrossRef] [PubMed]
- Daicel Chemical Industries, LTD. Environment and Safety Report, Responsible Care Activities/Social Activities. 2006. Available online: https://www.daicel.com/en/sustainability/pdf/library/ar2006e.pdf (accessed on 17 November 2021).
- Daicel Chemical Industries, LTD. Environment and Safety Report. 2008. Available online: https://www.daicel.com/en/sustainability/pdf/library/ar2008e.pdf (accessed on 17 November 2021).
- Yoshino, Y.; Hayashi, Y.; Iwahama, T.; Sakaguchi, S.; Ishii, Y. Catalytic Oxidation of Alkylbenzenes with Molecular Oxygen under Normal Pressure and Temperature by N-Hydroxyphthalimide Combined with Co(OAc)2. J. Org. Chem. 1997, 62, 6810–6813. [Google Scholar] [CrossRef]
- Yoshino, Y.; Iwahama, T.; Sakaguchi, S.; Ishii, Y. A New Strategy for the Preparation of Terephthalic Acid by the Aerobic Oxidation of p-Xylene using N-Hydroxyphthalimide as a Catalyst. Adv. Synth. Catal. 2001, 343, 220–225. [Google Scholar]
- Coseri, S.; Biliuta, G.; Simionescu, B.C. Selective oxidation of cellulose, mediated by N-hydroxyphthalimide, under metal-free environment. Polym. Chem. 2018, 9, 961–967. [Google Scholar] [CrossRef]
- Kasperczyk, K.; Orlinska, B.; Witek, E.; Łątka, P.; Zawadiak, J.; Proniewicz, L. Polymer-Supported N-Hydroxyphthalimide as Catalyst for Toluene and p-Methoxytoluene Aerobic Oxidation. Catal. Lett. 2015, 145, 1856–1867. [Google Scholar] [CrossRef]
- Huang, J.-L.; Gao, B.-J.; Yang, X.-L. Catalytic Performance of N-Hydroxyphthalimide-immobilized Cross-linked Polystyrene Microspheres in Oxidation of Toluene and Cyclohexanol. Chin. J. Process Eng. 2014, 4, 683–688. [Google Scholar]
- Yang, D.-H.; Zhao, W.-J.; Gao, L. Study of Catalyzed Oxidation of Cyclohexane in a Solvent-free System using a unique Combination of two Heterogeneous Catalysts. J. Mol. Catal. 2008, 6, 513–518. [Google Scholar]
- Yang, X.-L.; Gao, B.-J.; Huang, J.-L. Preparation of N-Hydroxyphthalimide Catalyst Supported on Polymer Microspheres and Preliminary Study on Its Catalytic Oxidation Performance. J. Funct. Polym. 2014, 27, 278–284. [Google Scholar]
- Gao, B.; Bi, C. Some Catalytic Characteristics of Compositional Catalysts of Immobilized N-Hydroxyphthalimide and Metal Salts in Aerobic Oxidation of 1-Phenylethanol. Catal. Commun. 2018, 115, 6–11. [Google Scholar] [CrossRef]
- Koguchi, S.; Kitazume, T. Synthetic Utilities of Ionic Liquid-Supported NHPI Complex. Tetrahedron Lett. 2006, 47, 2797–2801. [Google Scholar] [CrossRef]
- Su, S.; Giguere, J.R.; Schaus, S.E.; Parco, J.A. Synthesis of Complex Alkoxyamines Using a Polymer-Supported N-Hydroxyphthalimide. Tetrahedron 2004, 60, 8645–8657. [Google Scholar] [CrossRef]
- Maillard, L.T.; Benohound, M.; Durand, P.; Badet, B. A New Supported Reagent for the Parallel Synthesis of Primary and Secondary O-Alkyl Hydroxylamines through a Base-Catalyzed Mitsunobu Reaction. J. Org. Chem. 2005, 70, 6303–6321. [Google Scholar] [CrossRef]
- Culica, M.E.; Kasperczyk, K.; Baron, R.I.; Biliuta, G.; Macsim, A.M.; Lazea-Stoyanova, A.; Orlińska, B.; Coseri, S. Recyclable Polymer-Supported N-Hydroxyphthalimide Catalysts for Selective Oxidation of Pullulan. Materials 2019, 12, 3585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Guo, L.; He, P.; Yuan, X.; Jiao, F. Co-SBA-15-Immobilized NDHPI as a New Composite Catalyst for Toluene Aerobic Oxidation. Catal. Lett. 2017, 147, 856–864. [Google Scholar] [CrossRef]
- Hermans, H.; Deun, J.V.; Houthoofd, K.; Peeters, J.; Jacobs, P.A. Silica-immobilized N-hydroxyphthalimide: An efficient heterogeneous autoxidation catalyst. J. Catal. 2007, 251, 204–212. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Mavvaji, M.; Tajbakhsh, M.; Lasemi, Z. Synthesis and characterization of N-hydroxyphthalimide immobilized on SiO2-coated Fe3O4 nanoparticles as magnetic catalyst for oxidation of benzyl alcohols and hydrocarbons. J. Iran. Chem. Soc. 2018, 15, 893–904. [Google Scholar] [CrossRef]
- Dobras, G.; Kasperczyk, K.; Jurczyk, S.; Orlińska, B. N-Hydroxyphthalimide Supported on Silica Coated with Ionic Liquids Containing CoCl2 (SCILLs) as New Catalytic System for Solvent-Free Ethylbenzene Oxidation. Catalysts 2020, 10, 252. [Google Scholar] [CrossRef] [Green Version]
- Hosseinzadeh, R.; Mavvaji, M.; Tajbakhsh, M.; Lasemi, Z. Synthesis and Characterization of N-Hydroxyphthalimide Immobilized on NaY Nano-Zeolite as a Novel and Efficient Catalyst for the Selective Oxidation of Hydrocarbons and Benzyl Alcohols. React. Kinet. Mech. Catal. 2018, 124, 839–855. [Google Scholar] [CrossRef]
- Wang, M.; Liang, G.; Wang, Y.; Fan, T.; Yuan, B.; Liu, M.; Yin, Y.; Li, L. Merging N-hydroxyphthalimide into Metal−Organic Frameworks for Highly Efficient and Environmentally Benign Aerobic Oxidation. Chem. A Eur. J. 2021, 2, 9674–9685. [Google Scholar] [CrossRef]
- Blandez, J.F.; Navalon, S.; Alvaro, M.; Garcia, H. N-hydroxyphthalimide anchored on diamond nanoparticles as selective heterogeneous metal-free oxidation catalyst of benzylic hydrocarbons and cyclic alkenes by molecular O2. ChemCatChem 2018, 10, 34–41. [Google Scholar] [CrossRef]
- Luo, J.; Peng, F.; Yu, H.; Wang, H.; Zheng, W. Aerobic Liquid-Phase Oxidation of Ethylbenzene to Acetophenone Catalyzed by Carbon Nanotubes. ChemCatChem 2013, 5, 1578–1586. [Google Scholar] [CrossRef]
- Zhou, M.; Li, X.X.; Bao, L.; Yuan, X.; Luo, H.A. A new method for immobilization of NDHPI on SBA-15 carrier used as catalyst for selective oxidation of toluene. Catal. Lett. 2016, 146, 383–390. [Google Scholar] [CrossRef]
- Xu, J.; Shi, G.; Liang, Y.; Lu, Q.; Ji, L. Selective aerobic oxidation of toluene to benzaldehyde catalyzed by covalently anchored N-hydroxyphthalimide and cobaltous ions. Mol. Catal. 2020, 503, 111440–111450. [Google Scholar] [CrossRef]
- Shi, G.; Lu, Q.; Xu, J.; Wang, J.; Ji, L. Co-immobilization of N-hydroxyphthalimide and cobaltous ions as a recyclable catalyst for selective aerobic oxidation of toluene to benzaldehyde. J. Environ. Chem. Eng. 2021, 9, 106234–106244. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, K.; Li, X.; Hai, G.; Huang, X.; Wang, G. Conjugated polymer coated MIL-125(Ti) as an efficient photocatalyst for selective oxidation of benzylic C–H bond under visible light. Appl. Surf. Sci. 2021, 555, 149732. [Google Scholar] [CrossRef]
- Pokutsa, A.; Ohkubo, K.; Zaborovski, A.; Bloniarz, P. UV-induced oxygenation of toluene enhanced by Co (acac)2/9-mesityl-10-methylacridinium ion/N-hydroxyphthalimide tandem. Asia-Pac. J. Chem. Eng. 2021, e2714. [Google Scholar] [CrossRef]
- Rafiee, M.; Karimi, B.; Alizadeh, S. Mechanistic Study of the Electrocatalytic Oxidation of Alcohols by TEMPO and NHPI. ChemElectroChem 2014, 1, 455–462. [Google Scholar] [CrossRef]
- Mo, Y.; Jensen, K.F. Continuous N-Hydroxyphthalimide (NHPI)-Mediated Electrochemical Aerobic Oxidation of Benzylic C@H Bonds. Chem. Eur. J. 2018, 24, 10260–10265. [Google Scholar] [CrossRef]
- Chen, W.; Tang, H.; Wang, W.; Fu, Q.; Luo, J. Catalytic Aerobic Dehydrogenation of N-Heterocycles by N-Hydoxyphthalimide. Adv. Synth. Catal. 2020, 362, 3905–3911. [Google Scholar] [CrossRef]
- Lardy, S.W.; Schmidt, V.A. Intermolecular Radical Mediated Anti-Markovnikov Alkene Hydroamination Using N-Hydroxyphthalimide. J. Am. Chem. Soc. 2018, 140, 12318–12322. [Google Scholar] [CrossRef]
- Łątka, P.; Berniak, T.; Drozdek, M.; Witek, E.; Kuśtrowski, P. Formation of N-Hydroxyphthalimide Species in Poly(Vinyl-Diisopropyl-Phtalate Ester-co-Styrene-co-Divinylbenzene) and its Application in Aerobic Oxidation of p-Methoxytoluene. Catal. Comm. 2018, 115, 73–77. [Google Scholar] [CrossRef]
- Łątka, P.; Kasperczyk, K.; Orlińska, B.; Drozdek, M.; Skorupska, B.; Witek, E. N-Hydroxyphthalimide Immobilized on Poly (HEA-co-DVB) as Catalyst for Aerobic Oxidation Processes. Catal. Lett. 2016, 146, 1991–2000. [Google Scholar] [CrossRef] [Green Version]
- Jian, M.; Jianlan, C.; Dongmei, L.; Meina, X. Catalytic Properties of N-hydroxyphthalimide Immobilized on a Novel Porous Organic Polymer in the Oxidation of Toluene by Molecular Oxygen. RSC Adv. 2016, 6, 68170–68177. [Google Scholar] [CrossRef]
- Minisci, F.; Recupero, F.; Cecchetto, A.; Gambarotti, C.; Punta, C.; Paganelli, R.; Pedulli, G.F.; Fontana, F. Solvent and Temperature Effects in the Free Radical Aerobic Oxidation of Alkyl and Acyl Aromatics Catalysed by Transition Metal Salts and N-Hydroxyphthalimide: New Processes for the Synthesis of p-Hydroxybenzoic Acid, Diphenols, and Dienes for Liquid Crystals and Cross-Linked Polymers. Org. Process Res. Dev. 2004, 8, 163–168. [Google Scholar]
- Melone, L.; Punta, C. N-Hydroxyphthalimide (NHPI)—Organocatalyzed aerobic oxidations: Advantages, limits, and industrial perspectives. In Liquid Phase Aerobic Oxidation Catalysis: Industrial Applications and Academic Perspectives: Industrial Applications and Academic Perspectives; Stahl, S.S., Alsters, P.L., Eds.; Wiley-VCH: Weinheim, Germany, 2016; pp. 253–265. [Google Scholar]
- Krishnakumar, V.; Sivasubramanian, M.; Muthunatesan, S. Density Functional Theory Study and Vibrational Analysis of FT-IR and FT-Raman Spectra of N-Hydroxyphthalimide. J. Raman. Spectrosc. 2009, 40, 987–991. [Google Scholar] [CrossRef]
- Amorati, R.; Lucarini, M.; Mugnaini, V.; Pedulli, G.F.; Minisci, F.; Recupero, F.; Fontana, F.; Astolfi, P.; Greci, L. Hydroxylamines as Oxidation Catalysts: Thermochemical and Kinetic Studies. J. Org. Chem. 2003, 68, 1747–1754. [Google Scholar] [CrossRef]
- Ueda, C.; Noyama, M.; Ohmori, H.; Masui, M. Reactivity of Phthalimide-N-oxyl: A Kinetic Study. Chem. Pharm. Bull. 1986, 35, 1372–1377. [Google Scholar] [CrossRef] [Green Version]
- Petroselli, M.; Melone, L.; Cametti, M.; Punta, C. Lipophilic N-Hydroxyphthalimide Catalysts for the Aerobic Oxidation of Cumene: Towards Solvent-Free Conditions and Back. Chem. Eur. J. 2017, 23, 10616–10625. [Google Scholar] [CrossRef]
- Liang, F.; Zhong, W.; Xiang, L.; Mao, L.; Xu, W.; Kirk, S.R.; Yin, D. Synergistic Hydrogen Atom Transfer with the Active Role of Solvent: Preferred One-Step Aerobic Oxidation of Cyclohexane to Adipic Acid by N-Hydroxyphthalimide. J. Catal. 2019, 378, 256–269. [Google Scholar] [CrossRef]
- Hamdah, W.; Ahmad, W.; Sim, Y.-L.; Khan, M.N. Kinetics and mechanism of the general base-catalyzed hydrolysis of N-hydroxyphthalimide. Mon. Chem. Chem. Mon. 2013, 144, 1299. [Google Scholar]
- Rokicińka, A.; Drozdek, M.; Dudek, B.; Gil, B.; Michorczyk, P.; Brouri, D.; Dzwigaj, S.; Kuśtrowski, P. Cobalt-containing BEA zeolite for catalytic combustion of toluene. Appl. Catal. B-Environ. 2017, 212, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Diaz, J.F.; Balkus, K.J., Jr. Synthesis and Characterization of Cobalt-Complex Functionalized MCM-42. Chem. Mater. 1997, 9, 61–67. [Google Scholar] [CrossRef]
- Massoud, S.S. Spectroscopic Characterization for the Geometrical Isomers of Azido-Amide-Cobalt(III) Complexes. Polyhedron 1994, 13, 3127–3134. [Google Scholar] [CrossRef]
- Deng, H.; Bloomfield, V.A. Structural Effects of Cobalts-Amine Compounds on DNA Condensation. Biophys. J. 1999, 77, 1556–1561. [Google Scholar] [CrossRef] [Green Version]
- Graf, N.; Yegen, E.; Gross, T.; Lippitz, A.; Weigel, W.; Krakert, S.; Terfort, A.; Unger, W.E.S. XPS and NEXAFS studies of aliphatic and aromatic amine species on functionalized surfaces. Surf. Sci. 2009, 603, 2849–2860. [Google Scholar] [CrossRef]
- Tufts, B.J.; Abraham, I.L.; Caley, C.E.; Lunt, S.R.; Miskelly, G.M.; Sailor, M.J.; Santangelo, G.S.; Lewis, N.S.; Roe, A.L.; Hodgson, K.O. XPS and EXAFS Studies of the Reactions of Co(III) Ammine Complexes with GaAs Surfaces. J. Am. Chem. Soc. 1990, 112, 5123–5136. [Google Scholar] [CrossRef]
- Ivanova, T.; Naumkin, A.; Sidorov, A.; Eremenko, I.; Kiskin, M. X-ray photoelectron spectra and electron structure of polynuclear cobalt complexes. J. Electron Spectrosc. Relat. Phenom. 2007, 156–158, 200–203. [Google Scholar] [CrossRef]
- Braslau, R.; Schaffner, F.; Earla, A. Polymeric Phthalates: Potential Nonmigratory Macromolecular Plasticizer. J. Polym. Sci. A 2012, 51, 1175–1184. [Google Scholar] [CrossRef]
- Gao, B.; Bi, C. Synchronously Synthesizing and Immobilizing N-Hydroxyphthalimide on Polymer Microspheres and Catalytic Performance of Solid Catalyst in Oxidation of Ethylbenzene by Molecular Oxygen. Org. Process Res. Dev. 2015, 19, 1374–1382. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).