Abstract
The concept of very strong metal–support interactions (VSMSI) was defined in regard to the interactions that influence the catalytic properties of catalysts due to the creation of a new phase as a result of a solid-state chemical reaction between the metal and support. In this context, the high catalytic activity of the 1%Pt/Al2O3 catalyst in the CO oxidation reaction at room temperature was explained. The catalyst samples were reduced at different temperatures ranging from 500 °C to 800 °C and characterized using TPR, O2/H2 titration, CO chemisorption, TPD-CO, FTIR-CO, XRD, and TOF-SIMS methods. Based on the obtained results, it was claimed that with very high temperature reduction (800 °C), nonstoichiometric platinum species [Pt(Cl)Ox] strongly anchored to Al2O3 surface are formed. These species act as the oxygen adsorption sites.
1. Introduction
Supported metal catalysts comprising small metal particles deposited on a support surface are considered some of the most important components in heterogeneous catalysis. The primary goal of depositing metal onto a support material is to obtain a system with a highly dispersed form of metal, which is well-stabilized on the support surface, ensuring high catalytic efficiency. These catalysts are of considerable importance for industry because they allow more effective and economic usage of the metals, especially very expensive noble metals.
Initially, the support was treated as an inert material, the main role of which was to provide a large surface area for metal deposition. In the late 1970s, for the first time it was realized that interactions occur between the metal and support, whose strength depends on the nature and surface morphology of the support material, the type of metal, and the catalyst preparation conditions. Such interactions may influence both the activity and selectivity of the catalyst. Thus, it became clear that the role of the support goes far beyond physically increasing the surface area. The first well-described example of a catalyst showing these interactions, which were called strong metal–support interactions (SMSI), was Pt/TiO2 [1].
SMSI are usually induced under high-temperature treatment in hydrogen atmosphere and primarily involve reducible supports such as TiO2, CeO2, and Fe3O4 [1,2,3,4,5]. The most typical feature of the catalyst in SMSI state is a drastic decrease in H2 or CO adsorption capacity after reduction at temperatures usually higher than 400 or 500 °C [1,6,7]. Due to SMSI, in addition to the chemisorptive properties, the catalytic activity of the catalyst in various chemical reactions can also be altered. Different theories have been formulated to explain the SMSI phenomenon. The most respected SMSI explanations involve encapsulation by partly a reduced support material, which blocks active sites at the metal surface (decoration of metal sites) [8,9] and alloys formation [10] or electronic effects via charge transfer [11]. However, based on recent publications, the view that metal encapsulation is preceded by a partial support reduction as the main source of the SMSI state seems to dominate [12,13,14,15].
Nearly 40 years ago, Bond [16] proposed the classification of metal–support interactions into three groups—strong (SMSI), medium (MMSI), and weak (WMSI), depending on their strength. In contrast to SMSI (as described above), WMSI are ascribed to noble metals supported on nonreducible oxides, such as Al2O3, MgO, and SiO2. Small metal particles dispersed on zeolites can be considered examples of MMSI.
Nowadays, the definition of SMSI is often expanded to more widely understood support-induced changes in the catalytic activity of metal nanoparticles. In this regard, the interactions between irreducible support materials and noble metals are also often considered, although they do not show the most characteristic attributes of the classic SMSI state. In the literature, examples can be found indicating that the catalysts that should reveal only WMSI after a sufficiently high-temperature reduction, show unexpectedly high catalytic activity [17,18]. It is worth noting that for irreducible supports, the SMSI state is generally identified by an increase in the catalytic effectiveness of the catalyst after its high-temperature reduction. On the other hand, it can be assumed that in addition to the metal encapsulation mentioned earlier, high-temperature reduction (HTR) of the supported catalysts may also cause sintering of both the metal particles and support, diffusion of metal atoms or ions into the support, defects and dehydroxylation of support surface, the formation of Lewis acids centers, the creation of different interfacial chemical species that arise as a result of the reaction between the metal and support, and in extreme conditions the formation of alloys. These phenomena can cause decreased or increased catalytic activity after HTR. The authors of [19] proved that Pd–silicide (PdxSi) formed during the high-temperature reduction of Pd/SiO2 catalyst sharply increases activity in 2,2-dimethylpropane hydroconversion. In [20], it was stated that upon heating the Pt/Al2O3 system in reduced atmosphere at high temperature, tetragonal Pt3Al is formed, which can be further transformed into a cubic structure. Pt3Al and other platinum–aluminum intermetallic compounds show enhanced catalytic activity in oxygen reduction reactions.
The above examples prove that the changes in the catalytical performance of noble metals supported on irreducible oxides subjected to a high-temperature reduction are determined by a nuanced combination of the different effects listed earlier, although mainly involving a new phase formation as an effect of a very strong metal–support interaction. This new phase is responsible for changes in the catalytic performance. Bond’s classification does not include such systems, although they are relatively often reported in the literature. Therefore, we propose that the name very strong metal–support interactions (VSMSI) be assigned to such interactions, which influence the catalytic properties of catalysts due to the creation of a new phase as a result of a solid-state chemical reaction between the metal and support. On the other hand, we believe that the term SMSI, due to its history and widespread use in the literature, should be reserved for the interactions between metals and reducible supports and understood in accordance with its existing classic definition. The temperature conditions at which a given VSMSI system are induced led us to the term very high temperature reductions (VHTRs).
The results of a very high temperature reduction of Pt/Al2O3 catalyst are considered in the present work. We found that after reduction at 800 °C, this catalyst shows unexpectedly high activity during the CO oxidation reaction at room temperature (RT). It is worth mentioning that platinum is usually deposited on hard-to-reduce oxides such as Al2O3 or SiO2 and shows poor catalytic activity during CO oxidation at room temperature. These oxides do not participate in CO oxidation and serve as inert supports that maintain platinum dispersion. However, carbon monoxide adsorbed on platinum strongly inhibits oxygen adsorption and also hinders CO oxidation at room temperature. Therefore, to explain the high activity of the Pt/Al2O3 catalyst, it was assumed that additional O2 adsorption sites are the source of its activity, which are formed by the very strong platinum–Al2O3 interaction induced during reduction at 800 °C. The aim of this work was to confirm the above hypothesis
2. Results
2.1. Catalytic Activity
The results for carbon monoxide oxidation at room temperature (RT) are presented in Figure 1.
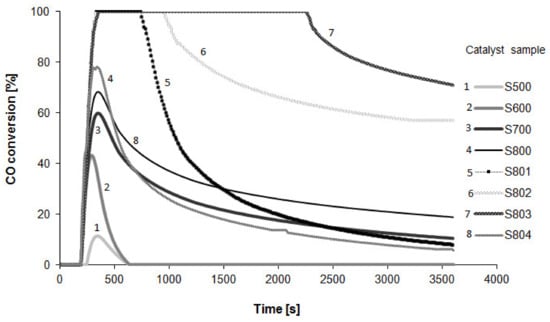
Figure 1.
CO conversion at room temperature vs. time on the Pt/Al2O3 catalyst sample reduced under different conditions.
The profiles reveal that the carbon monoxide oxidation over the Pt/Al2O3 catalyst at RT occurred significantly only after its very high temperature reduction. The highest activity was recorded for the sample reduced four times at 800 °C (sample S803). According to the above considerations, the areas under profiles are proportional to the CO2 volume arising for 3600 s of carbon monoxide oxidation.
Table 1 (column 6) shows yields of CO conversion at RT for all samples (a detailed description of the CO conversion calculation is given in Section 4.7). Carbon monoxide conversion for samples reduced at 500 and 600 °C equaled only a few percent and increased significantly with the increase in reduction temperature, with sample S803 reaching values as high as 85.3%.

Table 1.
Adsorption properties, dispersion rates, and platinum crystallites sizes of the Pt/Al2O3 catalysts.
These results were rather unexpected, since the reaction of CO oxidation over Pt/Al2O3 catalyst usually occurs at elevated temperature. However, it should be noted that the high catalytic activity of Pt/Al2O3 samples reduced at 800 °C was not stable and gradually decreased over time. The most active catalyst (S803) allowed total CO conversion at room temperature over 2400 s, although the conversion decreased significantly after 3600 s (to ca. 70%) and total deactivation was observed after 21,600 s. The catalyst can be regenerated by treatment in hydrogen atmosphere. One can observe that the first three regeneration cycles led to a gradual increase in catalytic activity. Rows 6–9 in Table 1 depict the yields of CO conversion changes with the number of regeneration cycles. These results are for sample S800 and reveal that the highest catalytic activity was achieved after 3 regeneration cycles. The next successive cycle caused a clear decrease in the catalytic activity (sample S804). The reasons for this decrease will be discussed in Section 3.
To sum up, the very high reduction temperature (800 °C) resulted in high catalytic activity of the Pt/Al2O3 catalyst. It was found that the prepared catalyst was able to oxidize carbon monoxide at room temperature with very high efficiency. In order to explain this phenomenon, the processes of chemisorption, TPR, and TPD and the composition of the Pt/Al2O3 catalyst as a function of the reduction temperature were carried out and analyzed.
2.2. CO and Oxygen Chemisorption
On the basis of O2/H2 titration and CO chemisorption, the dispersion (D) and crystallites size (S) of platinum in the studied catalysts were calculated. The dispersion marked as DCO or DO2 was defined as the ratio of surface metal atoms to the total number of metal atoms [21]. It was assumed that only surface platinum atoms are the adsorption sites for CO. The mean platinum particle size was calculated from the equations given in [22], which describes the correlation between D and S.
The results of these calculations are presented in Table 1. They clearly show that dispersion of platinum strongly depends on the temperature of the catalyst reduction. The increase in the reduction temperature leads to the gradual decrease in platinum dispersion. These results are in accordance with results of different studies [23,24,25]. It is worth noting that the dispersion calculated based on the chemisorption of CO was significantly lower than that calculated from the oxygen adsorption calculated from O2/H2 titration. Such results suggest that the catalyst samples adsorb carbon monoxide not only in the linear form (Pt-CO) but also in the bridge [Pt-(2CO)] form. It may seem astonishing that successive reductions in S800 sample results in a decrease in carbon monoxide adsorption, while the volume of hydrogen adsorbed as an effect of oxygen–hydrogen titration practically remains constant. Comparing the values of DCO and Y (Table 1, columns 5 and 7, respectively), one can conclude that they change in a nonparallel way. For the samples with clearly lower dispersion, the CO conversion yields are significantly higher. Usually, high dispersion is essential to ensure high catalytic activity of metal-supported catalysts. The results presented above show an opposite tendency. In other words, the low dispersion and large platinum crystallites size promote high activity of the Pt/Al2O3 catalyst in carbon monoxide oxidation at room temperature. This conclusion suggests that the very high temperature reduction (VHRT) process leads to the creation of a new catalytic active phase, which might be responsible for CO oxidation at room temperature.
2.3. TPR, XRD, and TOF-SIMS Analysis
According to the conclusion from the previous section, the consequence of the very high temperature reduction of Pt/Al2O3 catalyst may be the formation of a new chemical species. To shed light on this assumption, TPR profiling of the calcined precursor of the Pt/Al2O3 catalyst was carried out. Usually, TPR profiles of the Pt/Al2O3 catalysts prepared from chloroplatinic acid include two peaks. They are ascribed to the different platinum species weakly and strongly interacting with the alumina.
The authors of [26,27,28,29] assumed that the first peak can be attributed to the reduction of oxy- or hydroxychloride platinum species, while the high temperature peak can be attributed to the two-dimensional dispersive oxychlorinated platinum species, which strongly interact with the alumina support. It should be noted that the above conclusions are generally accepted. Figure 2 shows the TPR-H2 profile of the Pt/Al2O3 sample investigated in the present work, directly after its calcination.
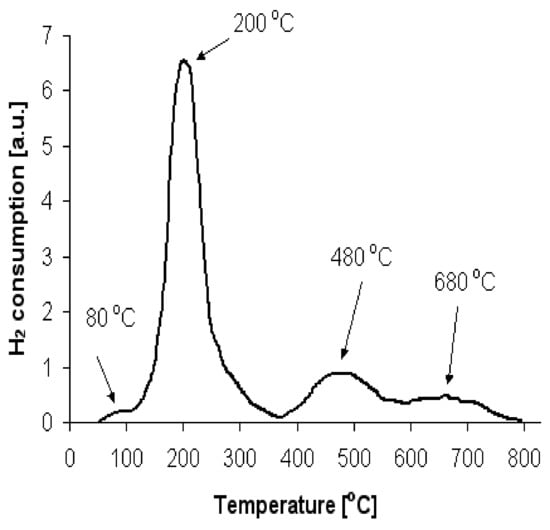
Figure 2.
TPR profile of the Pt/Al2O3 catalyst sample.
Four reduction peaks can be observed with maximum H2 consumption at around 80, 200, 480, and 680 °C, respectively. They correspond to the reductions in different platinum species, which may be formed both during the impregnation and calcination processes. For correct interpretation of TPR profiles, knowledge of the chemical composition before reduction is of fundamental importance. An attempt to use XRD for this purpose failed due to the low content of platinum in the sample and high dispersion of platinum species. There were neither metallic platinum nor any other platinum species lines on XRD diffractograms of the catalyst under study.
Thus, a more sensitive technique for surface analysis, namely TOF-SIMS, was used to analyze the chemical compositions of Pt/Al2O3 samples surface both before and after their reduction. The results are presented in Table 2.

Table 2.
The TOF-SIMS intensity levels of selected ions for 1%Pt/Al2O3 catalyst.
Regardless of the sample treatment, the different ionic species containing platinum (PtO−, PtOCl−, PtCl−) and intermetallic species such as PtAlO− or PtAlO2− were observed. Their concentrations changed with the increase in reduction temperature. Moreover, the results provided direct evidence that both Pt oxides and oxy-chloric-platinum or chloric-platinum species were still present on the catalyst surface, even after high-temperature reduction. The most significant observation was the confirmation that Al2O3 interacts with platinum-forming intermetallic compounds. It seems that they can be formed during the impregnation process.
The authors of [30] examined the compositions of Pt complexes adsorbed on alumina from H2(PtCl6) solutions with the used 195Pt-NMR and UV–Vis methods. They stated that two different types of adsorbed hexachloroplatinate ions can exist on the Al2O3 surface. The first is PtCl6]−2, while the second one is [PtCl−5(OH)]−2. The [PtCl6]−2/[PtCl5(OH)]−2 ratio of these ions depends on the pH of the impregnation solution, impregnation time, and acidity of the alumina support. The decomposition of [PtCl6]−2 and [PtCl5(OH)]−2 ions leads to the creation of oxy-chloride-platinum species. However, intermetallic species such as PtAlO− and PtAlO2−, which are characteristic of PtAlO3, can be formed as a result of the following reaction:
To enable Reaction (3), Al3+ cations must appear in the impregnation solution. It can be assumed that the reaction leading to their formation involves at least minor digestion of Al2O3 according to the reaction:
To confirm this assumption, we used the atomic absorption spectroscopy method to measure the aluminium concentration in the impregnation solution (8 wt.% H2[PtCl6] in H2O) after contact with Al2O3 for 12 h (impregnation time used in the catalyst’s preparation). It turned out that about 0.08% of alumina had dissolved during impregnation. This fact confirmed that Reaction (2) may proceed during impregnation of Al2O3 via the solution of chloroplatinic acid. Therefore, the reaction between Al3+ and [PtCl6]2− can lead to aluminium hexachloroplatinate, which after calcination creates PtAlO3, as identified by TOF-SIMS. Considering the above results, the process of oxy-chloride-platinum species formation can be described by a reaction comprising the presence of the hydroxyl group adsorbed on Al2O3:
Thus, the peaks observed on the TPR profile correspond to the reduction of these platinum compounds. The first and smallest peak, which starts at 50 °C and reaches its maximum at around 80 °C, may relate to the reduction of platinum clusters whose surface layers were passivated by oxygen. The second peak beginning at 110 °C with a maximum at 200 °C may be attributed to the reduction of all platinum oxides that weakly interact with the alumina support. The third peak with the maximum at 480 °C can be identified by the reduction of PtClx and all oxy-chloride connections (Pt-O-Cly) that strongly interact with the alumina support. A more ambiguous issue is the origin of the fourth peak with the maximum at 680 °C. Considering the fact that high catalytic activity is observed after VHRT, it could by that only the fourth reduction peak should is the source of that activity. Let us preliminarily assume that this peak relates to the reduction of Al2O3 and formation of alloys, such as PtAl:
Pt/Al2O3 + 3H2 = 2PtAl + 3H2O
On the other hand, it is well known that alumina possesses a very high chemical stability and is not prone to reduction even under harsh conditions. It is worth noting that the high-temperature interactions in the Pt-Al2O3 system are a subject of great research interest because of the significance for hermetic metal–ceramic joining [31,32]. At temperatures above 1500 °C, alumina creates a very dense structure with platinum as result of platinum encapsulation by Al2O3 [33]. However, reduction of Al2O3 in the presence of supported, well-dispersed platinum begins at significantly lower temperatures. In the literature, one can find reports showing that the process of PtAl alloy formation occurs at 800 °C [34] or even 600 °C as a result of Al2O3 reduction [35].
The most striking feature of the TOF-SIMS analysis of reduced samples is the presence of chlorine ions and the oxy-chloride platinum species on the catalyst surface, despite the very high reduction temperatures. This fact indicates that Al2O3 bonds chloride and oxy-chloride platinum species very strongly. However, ions that might have arisen due to the presence of PtAl alloys were not observed. Thus, these results confirm that the reducibility of Al2O3 is very low and that the reaction under TPR conditions does not occur. Concurrently, the results of TOF-SIMS measurements clearly proved that the concentration of oxy-chloric-platinum species contained in S800 sample is clearly smaller than in the S500 sample. This means that parts of these species such as PtAlO3 are reduced during the fourth step of the TPR process, with maximal hydrogen consumption at about 680 °C. This can lead to an increase in the number of adsorption sites. Simultaneously, because the platinum dispersion DCO values decrease with temperature more rapidly than DO2, it can be assumed that high reduction temperatures promote CO chemisorption in bridge form. In order to verify this preliminary conclusion, FTIR spectroscopy measurements were carried out.
2.4. FTIR Analysis
Figure 3 shows FTIR spectra of CO adsorption on the samples reduced at 500 and 800 °C.
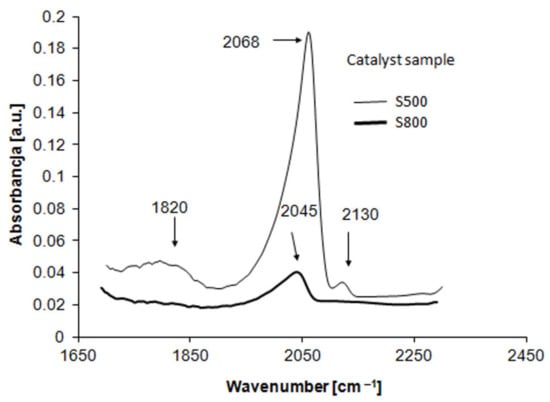
Figure 3.
FTIR spectra of CO adsorption on Pt/Al2O3 catalysts reduced at 500 and 800 °C.
For the S500 sample, both linearly [Pt-CO (2068 cm−1)] and bridge [Pt-(2CO) (1820 cm−1)]-bonded carbon monoxide was detected. In addition, a very small peak at 2130 cm−1 was also recorded. This peak is usually assigned to CO adsorption on the oxidized form of platinum atoms [36]. Comparing the peaks areas, it can be stated that the percent concentration of the bridge-bonded CO is ca. 11%. It is worth emplacing that the difference between the DCO and DO2 dispersion values is equal to 11.5% (Table 1). Thus, one can conclude that FTIR and CO chemisorption results for S500 sample are in line.
However, for the S800 sample, there is only one peak at 2045 cm−1 corresponding to the linear form of adsorbed CO. Contrary to expectations, the peak characteristic for [Pt-(2CO)] is not observed. This means that the Pt/Al2O3 catalyst after the VHTR creates adsorption sites that adsorb CO only in the linear form. Thus, these results do not confirm the conclusion presented in the previous section. Furthermore, the FTIR Pt-CO peak is strongly shifted towards a lower wavenumber. With respect to the S500 sample, this shift is equal to 23 cm−1. The direction of this shift is also rather unexpected. According to the literature [37,38], the high-temperature reduction should shift the CO peak towards higher wavenumber due to the increase in CO-CO interactions resulting from the increase in platinum particle size (the effect of dipole–dipole coupling). This phenomenon corresponds to the adsorption sites located mainly in the adjacent position and for high CO coverage. Only in such situations can CO molecules interact with each other. A shift of the CO peak towards a lower wavenumber is observed when adsorbed CO molecules are better spread over the platinum surface, causing a decrease in the dipole–dipole coupling. For instance, the coadsorption process can minimize or eliminate the dipole–dipole coupling as a consequence of carbon monoxide dilution. In this regard, the CO peak’s shift towards a lower wavenumber was interpreted by the authors of [39]. Dilution of adsorbed CO can also occur as a result of the presence of different adatoms on the platinum surface. This leads to a decrease in the number of exposed Pt sites for CO adsorption, and consequently prevents CO adsorption on the Pt sites situated in adjacent positions. In this regard, the shift of the CO peak to lower values for the bimetallic catalysts containing platinum as a primary metal is also considered. For instance, it was stated that the presence of Cu or Ge [40,41] on the platinum surface decreases the dipole–dipole coupling due to changes in the morphology of the platinum surface.
Therefore, considering the above aspects, one can conclude that the VHTR process creates the conditions under which platinum atoms can be insulated from each other, reducing the effects of CO-CO interactions (dipole–dipole coupling). On the other hand, the observed strong shift towards lower wavenumbers can also be attributed to the change in platinum electronic properties due to the VHTR process. According to the model given by Blyholder [42], which considers molecular orbitals, the chemisorption between CO and Pt is the result of electron transfer from the filled CO 5σ orbital to platinum (donation) and electron transfer from platinum into the empty CO 2π* orbital (back donation). The increase in electron density of the 2π* orbital leads to weakening of the carbon–oxygen bond in the CO molecule and strengthening of the Pt–CO bond. Since the vibrational frequency of the C–O bond is proportional to the strength of the C–O bond, one can observe a frequency shift towards a lower wavenumber when the strength of this bond decreases in comparison to the free CO molecule. The value of this shift depends on the extent to which the 2π* orbital is filled. However, it is dependent on the electronic properties of the supported platinum. Thus, all factors that increase the electron concentration in the platinum particles can also be responsible for the increase in the bond strength between platinum and carbon monoxide due to the increase in back donation.
In light of above considerations, it can be regarded that the shift of the FTIR CO peak is due to the increase in electron density of the platinum particles that occurs after the VHTR process. However, the mechanism responsible for this increase is an open issue. One can preliminarily assume that the strong electronic interaction between platinum and Al2O3 results in electron transfer from Al2O3 to platinum. Consequently, both back donation and the Pt–CO bond strength increase.
On the other hand, it is well known that Al2O3 is a very good electrical insulator with a forbidden band width higher than 7 eV [43]. When Al2O3 is in close contact with platinum an electrical junction is created. Therefore, the platinum–alumina interface in the Pt/Al2O3 catalyst can be treated as a metal–insulator electronic junction. In this junction, the Fermi levels of platinum and alumina are aligned to the same level by the charge transfer between them. The direction of the electron flow depends on whether the work function of platinum is above or below the work function of alumina. At room temperature, polycrystalline platinum has a relatively high work function equal to 5.7 eV [44]. However, because the width of the forbidden band of Al2O3 is ca. 8 eV and assuming that its conduction band is about 1 eV, the work function of Al2O3 can be estimated as ca. 4.5 eV. These values mean that when Pt and Al2O3 are brought into contact, the electrons should move from alumina to platinum. The consequence of this phenomenon should be the growth of the positive charge on the alumina side and a negative one on the platinum side. On the other hand, this electronic junction probably does not play a significant role in electron transfer if Al2O3 is the insulator. Due to the very high value of the width of the forbidden band of alumina at room temperature, its conduction band is empty and the flow of electrons across the junction is negligible. Thus, proper conditions for the electron transfer across the Pt-A2O3 junction are not provided.
A different situation occurs when surface platinum atoms (Pts0) are surrounded by semiconductive materials. As has been shown in Table 2, despite the very high reduction temperature, the surface of the Pt/A2O3 catalyst still contains the oxide, chloric, and oxy-chloric platinum ions. The sources of these ions are platinum oxides, chlorides, or oxy-chlorides. Their presence in the S800 sample means that they are very strongly anchored to the Al2O3 surface, which makes their total reduction to Pt0 very difficult. Thus, the sample of S800 catalyst contains irreducible platinum species surrounding reduced surface platinum atoms (Pts0). It can be presumed that these species after the VHTR process are strongly nonstoichiometric due to the following reduction reactions:
PtO2-(Al2O3) + xH2 = PtO2−x − (Al2O3) + xH2O
PtCl4-(Al2O3) + xH2 = PtCl4−x − (Al2O3) + xHCl
Pt OxCly-(Al2O3) + (x1 + y1)H2 = PtOx−x1Cly−y1 (Al2O3) + y1HCl + x1H2O
These nonstoichiometric platinum species strongly anchored to the Al2O3, labeled by us generally as [Pt(Cl)Ox]Al2O3, could be treated as a type n semiconductor, although the high anion vacancy concentration means that they also can be regarded as a degenerate semiconductor. In such materials, at room temperature the Fermi level is in the conduction band [45]. Consequently, these materials can show quasi-metallic properties with lower work function values in comparison to platinum (5.7 eV).
Therefore, the [Pt(Cl)Ox]Al2O3 species can be treated as an electron-donating compound in the vicinity of the platinum (Pt0) atoms. The interaction of the Pt0 with an electron-donating compound can cause an increase in the electron density of the platinum. This creates conditions for enhancing the back donation towards the 2π* CO orbital and leads to a weakening of the C–O bond and to a decrease in the FTIR CO frequency. Simultaneously, this phenomenon creates conditions for increased bond strength between Pt and CO.
Thus, it can be concluded that [Pt(Cl)Ox]Al2O3 species induced by the VHTR process act as promoters for carbon monoxide adsorption over platinum surfaces. This conclusion is, however, contrary to expectations. It is expected that the VHTR process will cause an opposite effect, i.e., weakening of the Pt–CO bond. A weak Pt–CO bond should facilitate coadsorption of CO and O2 over the platinum surface and increase the catalytic activity in CO oxidation. The effect of CO and O2 coadsorption can be observed for platinum catalysts promoted with alkali metals [46] because of Pt–CO bond weakening. According to the results presented above, the VHTR process strengthens the Pt–CO bond. In order to confirm this conclusion, the TPD-CO process was assessed.
2.5. TPD of Carbon Monoxide
The TPD-CO profile for the S500 catalyst sample is shown in Figure 4 (curve labeled as CO). Only two small and broad peaks with maxima at 110 °C and 380 °C, respectively, can be observed. The volume of desorbed CO (0.005 cm3/gcat) does not correspond to the result presented in Table 1 for adsorbed CO (0.814 cm3/gcat). To explain this phenomenon, it is assumed that the process of CO desorption is also connected with the formation of CO2. Carbon dioxide can arise as a product of the Boudouard reaction:
2CO = CO2 + C
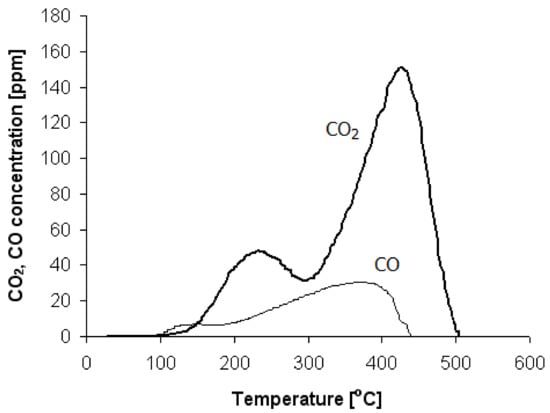
Figure 4.
The CO2 and CO concentration profile changes obtained during the TPD-CO process over the S500 sample recorded simultaneously during the same experiment.
Additionally, it can arise a product of CO oxidation by hydroxyl groups:
and between water and carbon monoxide:
CO + 2OH = CO2 + H2O
CO + H2O = CO2 + H2
The last reaction, called the water–gas shift reaction, is well known and often used as a source of hydrogen [47] or in the process of gas purification from CO [48].
The hydroxyl groups present on the catalyst surface can be weakly or strongly bonded by Al2O3. These groups can relatively easily react with adsorbed carbon monoxide, oxidizing it to carbon dioxide in accordance with Reaction (9) or (10).
The curve labeled as CO2 in Figure 4 shows the profile of CO2 formed during the TPD-CO process. Two CO2 peaks can be clearly distinguished. One can assume that the first peak arises as a product of a reaction between the adsorbed carbon monoxide and hydroxyl groups, which are weakly bonded by Al2O3. This process is described by Reactions (9) and (10) and starts at relatively low temperatures (around 110 °C). In the second stage, adsorbed CO reacts with hydroxyl groups, which are strongly bonded by alumina. This stage starts at temperatures higher than 300 °C and reaches its maximum at 450 °C. To sum up, during TPD-CO, the dehydroxylation of alumina resulting in the release of CO2 occurs. Therefore, after the first run, the catalyst sample was again saturated with CO and the process of TPD-CO was repeated. The details of the whole procedure are described in Section 4.5.
The TPD-CO profiles after the second CO adsorption are presented in Figure 5. The amount of recorded CO2 (not shown here) was negligible. The profiles of the samples reduced at temperatures lower than 800 °C clearly show two poorly separated CO desorption stages. In the first one, CO starts to desorb at 90 °C, reaching a blurry maximum at about 125 °C, whereas the second stage starts at 160 °C and shows a maximum in the range of 360–400 °C, depending on the sample’s reduction temperature. This course of TPD-CO, including two CO desorption stages, is in general accordance with the FTIR results, which also indicate two predominating CO adsorption sites attributed to linearly and bridge-bonded carbon monoxide. The lack of a third adsorption site assigned to CO adsorption on oxidized platinum, which is hardly observed in FTIR spectra, may relate to the heterogeneous character of the catalyst sample, as reflected by broad desorption peaks.
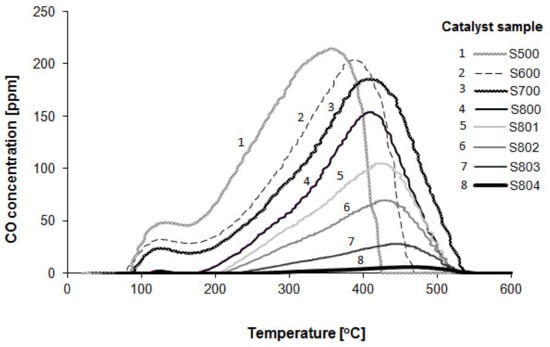
Figure 5.
TPD-CO profiles for the 1%Pt/Al2O3 catalysts depending on their reduction temperature.
All samples reduced at 800 °C show single-stage CO desorption, starting at about 200 °C and reaching a maximum in the range of 400–460 °C, depending on the sample’s pretreatment. The absence of the low-temperature desorption stage seems to indicate that on the sample reduced at VHRT, the adsorption of CO in bridge form does not take place. CO desorption for samples reduced at temperatures lower than 800 °C begins at a temperature of around 100 °C, corresponding to that in which the process of CO oxidation over the Pt/Al2O3 catalyst operating in oxygen excess at atmospheric pressure starts (the results are not shown here). As was previously stated, carbon monoxide behaves as a poison for platinum catalysts. The desorption of CO activates the platinum surface by creating sites for oxygen adsorption, allowing coadsorption of CO and oxygen. Therefore, one can expect that for samples showing very high catalytic activity at room temperature (S801, S802, or S803), the process of CO desorption should start practically at RT. However, as is shown in Figure 5, CO desorption begins at around 200 °C. Moreover, the increase in the TPD-CO maximum can be clearly observed for the sample subjected to each successive reduction cycle (S800 → S804). The shift of the maximum towards higher temperatures means that the strength of the interaction between carbon monoxide and platinum surfaces gradually increases.
Thus, the results of TPD-CO measurements confirmed that the VHTR process strengthens the bond between Pt and CO, which means that coadsorption of CO and O2 over platinum surfaces at room temperature can be excluded. This last statement raises questions regarding the source of high catalytic activity of the Pt/Al2O3 catalyst.
3. Discussion—The Source of High Catalytic Activity of Pt/Al2O3 Catalyst
The results presented in Section 2.1 show that the very high reduction temperature for Pt/Al2O3 catalysts strongly promotes their activity in the reaction of CO oxidation at room temperature. Simultaneously, platinum particles become larger during the high-temperature reduction due to their agglomeration and sintering. This conclusion suggests that the catalytic activity of Pt/Al2O3 catalysts in CO oxidation is facilitated by the large platinum crystallites. On the other hand, the platinum black powder with relatively large Pt crystallites is not highly active in the CO oxidation reaction at room temperature [49,50].
The efficiency of the CO oxidation over platinum surfaces at room temperature mainly depends on the possibility of CO and O2 coadsorption. After adsorption on the Pt surface, oxygen dissociates into oxygen atoms and reacts with adsorbed carbon monoxide molecules to CO2. This reaction is an example of the Langmuir–Hinshelwood (LH) mechanism, which requires the adsorption of both CO and oxygen over the metallic platinum surface. It is generally accepted that the process of CO oxidation occurs at low temperatures according to this mechanism [51,52]. However, the rate of reaction depends on the fractional surface coverage of CO (θCO) and oxygen atoms (θO). On the other hand, it is well known that the affinity of carbon monoxide towards platinum surfaces is so great that it may be considered a poison for the platinum catalyst. The presence of CO on the surface of the platinum catalyst can totally block oxygen adsorption. Thus, oxygen must compete with carbon monoxide on the adsorption sites on the platinum surface. Moreover, in contrast to carbon monoxide, the adsorption of oxygen molecules requires a relatively large surface area of Pt due to its dissociative nature. According to [53], after dissociation, the O atoms are separated by two lattice constants on the Pt(111) due to the reduction of the lateral O–O interaction. These determinants clearly show that reaction of CO oxidation at room temperature over platinum surfaces is not easy. A good platinum catalyst for low-temperature CO oxidation should enable simultaneous adsorption of both CO and O2. Therefore, the platinum catalysts supported on irreducible oxides such as Al2O3 or SiO2 are usually not very active in this reaction, contrary to those supported on partly reducible oxides, which are able to adsorb oxygen, such as TiO2 [54], SnO2 [55], or Fe2O3 [56].
Therefore, the question arises regarding the source of high catalytic activity of the Pt/Al2O3 catalyst reduced at very high temperature. One can assume that two forms of adsorption sites on the Pt/Al2O3 catalyst exist. The first form is the surface metallic platinum atoms (Pts0) generated during the reduction of the Pt/Al2O3 catalyst at relatively low temperatures (lower than 500 °C), while the second is nonstoichiometric platinum species [Pt(Cl)Ox] strongly anchored to Al2O3, created at very high reduction temperatures. The adsorption properties of both these forms are quite different. Platinum (Pts0) sites show a clearly higher adsorption affinity to carbon monoxide than to oxygen, while reduced [Pt(Cl)Ox]Al2O3 sites show the opposite properties. They facilitate the adsorption and activation of oxygen molecules. The above findings mean that the Pt/Al2O3 catalyst after the VHRT process provides separate sites for oxygen and carbon monoxide adsorption. Thus, these gases do not have to compete for the same adsorption sites over the platinum surface. Carbon monoxide strongly adsorbed on Pt0 can easily react with oxygen adsorbed on [Pt(Cl)Ox]Al2O3 species, according to the LH mechanism:
Pt-CO(ads) + {[Pt(Cl)Ox]Al2O3}-O(ads) = Pt + [Pt(Cl)Ox]Al2O3 + CO2(gas)
This mechanism ensures effective CO oxidation at room temperature, if [Pt(Cl)Ox]Al2O3 sites are present on the catalyst surface in the reducible form. However, the oxygen adsorption over [Pt(Cl)Ox]Al2O3 sites is also the first step toward oxygen incorporation into the lattice of these nonstoichiometric oxy-chloride-platinum species. This progressive oxidation of [Pt(Cl)Ox]Al2O3 leads to the decrease in the efficiency of CO oxidation with time. For these reasons, the very high catalytic activity of Pt/Al2O3 catalyst is not stable. As it was shown in Section 2.1, the catalyst gradually lost its high activity during the reaction. When the [Pt(Cl)Ox]Al2O3 adsorption sites lose capacity to oxygen adsorption, the catalytic activity of the Pt/Al2O3 catalyst at RT practically disappears due to the reaction:
where [Pt(Cl)Ox]OLAl2O3 means that the oxidized oxygen adsorption sites also do not interact with carbon monoxide adsorbed on the platinum surface (Pt-CO(ads)). However, the repeated reduction (re-reduction) of the catalyst sample restores its high catalytic activity.
Pt + [Pt(Cl)Ox]Al2O3 + 2COgas + O2gas = [Pt(Cl)Ox]OLAl2O3 + Pt-COads + CO2gas
The above considerations indicate that the ability for oxygen adsorption by nonstoichiometric oxy-chloric-platinum species strongly anchored to Al2O3 is a crucial factor that determines whether the Pt/Al2O3 catalyst is active or inactive in CO oxidation at room temperature. They also shed new light on the oxygen–hydrogen titration results shown in Table 1. The comparison of the data presented in columns 3 and 5 shows that the DO2 values were higher than DCO values for all samples. For samples S500 and S600, this discrepancy could be attributed to CO adsorption in the bridge form. However, according to the FTIR results, this form is not observed for the samples reduced at a very high temperature. Thus, it can be concluded that for the S800 sample, differences between DO2 and DCO result from additional oxygen adsorption sites formed during the VHTR process.
Therefore, the whole process of oxygen–hydrogen titration can be described by the following reactions:
- Oxidation of the catalyst surface:
- Oxygen titration:{[Pt(Cl)Ox]OLAl2O3} + H2 = {[Pt(Cl)Ox]Al2O3} + H2OPts-Oads + 3/2H2 = Pt-Hads + H2O
As it results from Equations (14) and (15), the volume of consumed hydrogen corresponds to the total number of the adsorption sites (NPtT) present on the surface of the Pt/Al2O3 catalyst. However, the number of oxygen adsorption sites (NPtOx) is the difference between the total number of available adsorption sites (NPtT) and the number of surface metallic platinum atoms (NPts0):
NPtOx = NPtT − NPts0
NPts0 was determined during the measurements of CO adsorption, while the values of NPtT were calculated based on the O2-H2 titration. The ratios of NPtOx to NPtT show the concentrations of NPtOx oxygen adsorption sites in the investigated catalyst samples. Therefore, given that the concentrations of the bridge form of adsorbed CO are 10% for S500 and S600 samples, 5% for S700, and 0% for S800, the percentage content of oxygen adsorption sites in the investigated catalyst samples can be calculated. The results of these calculations are presented in Table 1 (column 8). They indicate that the concentration of oxygen adsorption sites significantly increases with the reduction temperature. For sample S804, nearly 90% of adsorption sites consist of oxygen adsorption sites.
However, Figure 6 shows the CO conversion yields (Y) as a function of the ratio of NPtOx to NPts0, labeled as NO2/CO (Table 1, column 9). The presented curve shows that the catalytic activity of the Pt/Al2O3 catalyst in the CO oxidation reaction at room temperature is determined by the presence of oxygen adsorption sites. For the Pt/Al2O3 samples reduced at 500 and 600 °C, the NO2/CO ratios are very low and the catalytic activity is also low, despite NPts0 value being high. Simultaneously, when the NO2/CO ratio is clearly higher than 1, the catalytic activity is low.
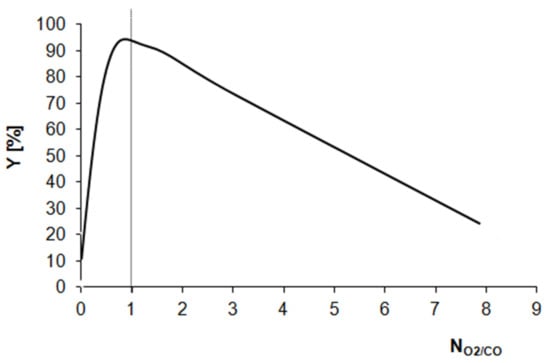
Figure 6.
The CO conversion yields (Y) as a function of the ratio of the concentration of O2 adsorption sites to the concentration of CO adsorption sites (NO2/CO).
Thus, the conclusion drawn from Figure 6 is unequivocal. The concentration of oxygen adsorption sites should be equal to the concentration of the adsorption sites for carbon monoxide. Only under such conditions can the process of CO oxidation occur at room temperature over the Pt/Al2O3 catalyst. In the investigated Pt/Al2O3 catalyst samples, this condition ensures the presence of nonstoichiometric oxy-chloride-platinum species strongly anchored to the Al2O3 surface as a result of very high temperature reduction.
4. Materials and Methods
Al2O3, (Fluka, type 507, Buchs, Switzerland, SBET = 125 m2/g), H2PtCl6 solution (8 wt.% in H2O), Sigma Aldrich, Poznan, Poland), were used as received. All gases used in this study were purchased from Linde Poland (ultra-high-purity grade 5.0) and used without any further purification.
4.1. Sample Preparation
The 1%Pt/Al2O3 catalyst was prepared via the wet impregnation method using an aqueous solution of H2PtCl6. The general procedure for wet impregnation was as follows: A support (Al2O3) was immersed in an appropriate volume of H2PtCl6 solution to achieve the loading of 1 wt.% Pt for 12 h at RT. The excess water was evaporated in a rotary evaporator. The impregnated catalyst was further dried at 120 °C overnight and finally calcined at 400 °C for 4 h. Next, the samples were reduced by heating in hydrogen at 500, 600, 700, and 800 °C for 2 h and denoted as S500, S600, S700, and S800, respectively. The S800 sample after chemisorption and catalytic measurements was again subjected to the reduction in the TPR regime (described below). After reaching 800 °C, the reduction at this temperature was continued for 1 h. This cycle was repeated four times and the samples after each of them were as S801, S802, S803, and S804, respectively. It should be underlined that the same sample S800 was used the whole time.
4.2. Structural and Morphological Characterization of Samples
XRD measurements were carried out in a PANalytical X’Pert Pro (Malvern PANalytical, Malvern, United Kingdom), diffractometer equipped with an Anton Paar XRK900 reactor chamber (Anton Paar, Graz, Austria). The X-ray source was a long fine-focus X-ray diffraction copper tube operating at 40 kV and 30 mA.
The surface species were examined by TOF-SIMS (time-of-flight secondary ion mass spectrometry). The TOF-SIMS measurements were taken in the static mode using an ION–TOF instrument ((TOF-SIMS IV, IONTOF GmbH, Münster, Germany) equipped with a 25-kV pulsed 69Ga+ primary ion gun.
4.3. Temperature Programmed Reduction (TPR)
TPR-H2 experiments were performed in the PEAK-4 apparatus, using the H2/Ar (5 vol.% H2, 95 vol.% Ar) gas mixture with a flow rate of 40 cm3 min−1 in the temperature range of 0–800 °C with a linear ramp rate of 20 °C min−1. The PEAK-4 was manufactured by us and its design and characteristics are described in the paper [57]. Prior to the TPR run, the 0.2 g sample directly after calcination was pretreated in situ by heating in O2 flow for 1 h at 400 °C, followed by cooling to RT. A thermal conductivity detector (TCD) was used to detect the changes in hydrogen concentration behind the reactor. The reduction of high-purity CuO was used to quantify the H2 consumption.
4.4. Chemisorption Measurements using the Dynamic Pulse Method
The O2/H2 titration was used to determine the chemisorption properties of the Pt/Al2O3 catalyst. The sorbed oxygen was titrated by hydrogen. It was assumed that the stoichiometry of chemisorption (H/Pt) is equal to 1, while the equation describing this process is as follows:
PtOads + 3/2H2 = PtH + H2O
The catalyst measuring 0.2 g was placed in a glass tube reactor with an internal diameter of 5 mm. The catalyst was activated at a given temperature of range of 500–800 °C for 2 h in a stream of hydrogen with a flow rate of 40 cm3 min−1. Then, the reactor was cooled to room temperature in a flow of N2 and finally nitrogen was replaced by air stream, flowing at a rate of 40 cm3 min−1 for 10 min. Next, air was replaced by nitrogen and the pulses of 0.05 cm3 hydrogen were introduced into the reactor by a six-way valve. The changes in hydrogen concentration were detected by a thermal conductivity detector (TCD). For comparison, the chemisorption properties of the Pt/Al2O3 catalyst were also determined by CO adsorption at room temperature. Prior to the adsorption, the sample (0.2 g) was reduced in the H2 stream under the same conditions as those preceding O2/H2 titration. Then, it was cooled to RT in a flow of nitrogen. Next, the pulses of 0.05 cm3 carbon monoxide were introduced into the carrier gas by the six-way valve. An infrared gas analyzer type ZRJ-4 (Fuji Electric System Co., Tokyo, Japan) detected the changes in CO concentration behind the reactor.
4.5. TPD-CO Measurements
Two consecutive TPD-CO measurements were conducted. The Pt/Al2O3 sample, after reduction at a given temperature, was cooled to RT in the flow of N2, then the flow of nitrogen was replaced by the gas mixture of 5% vol. CO balanced by nitrogen. The sample was saturated with CO for 15 min, then the flow of nitrogen was restored and the first (preliminary) TPD-CO run was recorded. The temperature was increased from 25 to 500 °C at a temperature ramp of 10 °C/min.
Next, after cooling to RT, nitrogen was again replaced by the gas mixture containing carbon monoxide and the described above TPD-CO procedure was repeated but in the temperature range 25–800 °C. The concentration of CO and CO2 was measured using Gas Analyzer type ZRJ-4. This device allows concurrent detection of CO and CO2, shows very high sensitivity (detection limit 1 ppm), and is highly selective to both CO and CO2.
4.6. CO-FTIR Spectroscopy
The nature of adsorbed CO species on Pt/Al2O3 surfaces was studied by FTIR spectroscopy using a Nicolet 6700 infrared spectrometer (Specac Ltd., Orpington, UK) equipped with a high-pressure and high-temperature transmission mode. IR spectra were recorded in the 4000–1000 cm−1 region of a wave number at RT. The spectral resolution was 4 cm−1. The reduced catalyst sample (0.3 g) in the form of a pressed pellet under 5 ton pressure for 10 min was mounted in the IR cell and pretreated in situ in 5 vol.% H2/Ar for 1 h at 500 °C at a 40 cm3 min−1 flow rate. After reduction, the sample was cooled to room temperature either in a flow of 5 vol.% H2/Ar or in a flow of Ar. Next, the flow of hydrogen or argon was replaced by 5 vol.% CO/Ar. The spectra of adsorbed species were collected after 60 min.
4.7. Catalytic Tests
The process of CO oxidation was conducted at room temperature (RT) in the flow bed reactor (ϕ = 5 mm). The reaction gas mixture consisted of 1.1 vol.% CO in air, the weight of the catalyst sample was 0.1 g, and the flow rate was 40 cm3 min−1. The gas hourly space velocity (GHSV) was equal to 20,000 h−1.
The outlet gas mixture was analyzed using a Fuji infrared gas analyzer. The samples after reduction in H2 at the given temperature were flushed with ultra-pure (99.9999%) nitrogen and then cooled to room temperature (RT). The aim of this process was to remove the adsorbed H2. After cooling and temperature stabilization at 23 °C (±3), nitrogen was changed for the reaction gas mixture and concentrations of CO and CO2 behind the reactor were recorded. Figure 7 shows an illustrative CO conversion profile, which was derived during CO oxidation over 360 min at room temperature over Pt/Al2O3 catalyst (S803 sample).
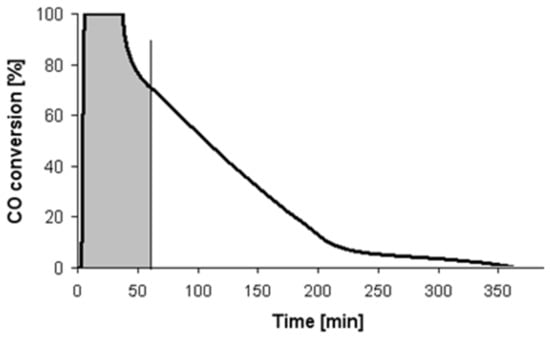
Figure 7.
The profiles of CO conversion changes vs. time at room temperature over Pt/Al2O3 catalyst (sample S803).
The CO oxidation reaction at room temperature was carried for 60 min. In order to compare the catalytic activities of the investigated samples, we introduced the value Y, i.e., the CO conversion yield.
Yields of CO conversion were defined according to the following equation:
where represents the maximum volume of CO2, which should increase for 60 min, assuming the total carbon monoxide conversion at the given flow rate and reaction gas mixture composition. One can easily calculate that with a flow of 40 cm3 min−1 and CO concentration of 1.1% vol., CO/air equals 26.4 cm3, which represents the volume of CO2 that really arises during 60 min of carbon monoxide oxidation. This value is calculated on the basis of the area under the curve of the CO2 concentration versus time. In Figure 7, the area in marked grey corresponds to the volume of CO2 that arises during CO oxidation.
5. Conclusions
In summary, it can be stated that the VHTR process creates the conditions under which platinum atoms can be insulated from each other and in this way reduce the effects of CO-CO interactions (dipole–dipole coupling). The lack of the bridge form of adsorbed CO confirms the presence of the isolated platinum atoms. Simultaneously, the VHTR process creates the conditions under which the metal–semiconductor junction have an impact on both the strength of the Pt-CO interaction and catalytic activity of the Pt/Al2O3 catalysts. Moreover, the VHTR process forms nonstoichiometric platinum species [Pt(Cl)Ox]Al2O3 that are strongly anchored to the Al2O3 surface. These species act as the oxygen adsorption sites. When the concentration of the oxygen adsorption sites is equal to the concentration of the adsorption sites for carbon monoxide, the process of CO oxidation can occur with very high effectiveness at room temperature.
Author Contributions
Conceptualization, I.K. and J.M.R.; writing—original draft preparation, I.K.; writing—review and editing, J.M.R.; investigations, I.Ś., M.J., I.K. and J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tauster, S.J.; Fung, S.C.; Garten, R.L. Strong metal-support interactions. Group 8 noble metals supported on titanium dioxide. J. Am. Chem. Soc. 1978, 100, 170–175. [Google Scholar] [CrossRef]
- Horsley, J.A. A molecular orbital study of strong metal-support interaction between platinum and titanium dioxide. J. Am. Chem. Soc. 1979, 101, 2870–2874. [Google Scholar] [CrossRef]
- Herrmann, J.M. Electronic effects in strong metal-support interactions on titania deposited metal catalysts. J. Catal. 1984, 89, 404–412. [Google Scholar] [CrossRef]
- Figueiredo, W.T.; Della Mea, G.B.; Segala, M.; Baptista, D.L.; Escudero, C.; Pérez-Dieste, V.; Bernardi, F. Understanding the strong metal−support interaction (SMSI) effect in CuxNi1−x/CeO2(0 < x < 1) nanoparticles for enhanced catalysis. ACS Appl. Nano Mater. 2019, 2, 2559–2573. [Google Scholar] [CrossRef]
- Zhang, K.; Shaikhutdinov, S.; Freund, H.J. Does the surface structure of oxide affect the strong metal–support interaction with platinum? Platinum on Fe3O4(001) versus Fe3O4(111). ChemCatChem 2015, 7, 73725–73730. [Google Scholar] [CrossRef]
- Alexeev, O.S.; Chin, S.Y.; Engelhard, M.H.; Ortiz-Soto, L.; Amiridis, M.D. Effects of reduction temperature and metal-support interactions on the catalytic activity of Pt/γ-Al2O3 and Pt/TiO2 for the oxidation of co in the presence and absence of H2. J. Phys. Chem. B 2005, 109, 23430–23443. [Google Scholar] [CrossRef]
- Tauster, S.J.; Fung, S.C.; Baker, R.T.K.; Horsley, J.A. Strong interactions in supported-metal catalysts. Science 1981, 211, 1121–1125. [Google Scholar] [CrossRef]
- Zhang, S.; Plessow, P.N.; Willis, J.J.; Dai, S.; Xu, M.; Graham, G.W.; Cargnello, M.; Abild-Pedersen, F.; Pan, X. Dynamical observation and detailed description of catalysts under strong metal–support interaction. Nano Lett. 2016, 16, 4528–4534. [Google Scholar] [CrossRef]
- Beck, A.; Huang, X.; Artiglia, L.; Zabilskiy, M.; Wang, X.; Rzepka, P.; Palagin, D.; Willinger, M.G.; van Bokhoven, J.A. The dynamics of overlayer formation on catalyst nanoparticles and strong metal-support interaction. Nat. Commun. 2020, 11, 3220–3228. [Google Scholar] [CrossRef]
- Beard, B.C.; Ross, P.N. Pt-Ti alloy formation from high-temperature reduction of a titania-impregnated Pt catalyst: Implications for strong metal-support interaction. J. Phys. Chem. 1986, 90, 6811–6817. [Google Scholar] [CrossRef]
- Resasco, D.E.; Weber, R.S.; Sakellson, S.; McMillan, M.; Haller, G.L. X-ray absorption near-edge structure evidence for direct metal-metal bonding and electron transfer in reduced rhodium/titania catalysts. J. Phys. Chem. 1988, 92, 189–193. [Google Scholar] [CrossRef]
- Wu, P.; Tan, S.; Moon, J.; Yan, Z.; Fung, V.; Li, N.; Yang, S.Z.; Cheng, Y.; Abney, C.W.; Wu, Z.; et al. Harnessing strong metal–support interactions via a reverse route. Nat. Commun. 2020, 11, 3042. [Google Scholar] [CrossRef]
- Chen, J.Z.; Gao, J.; Probus, P.R.; Liu, W.; Wu, X.; Wegener, E.C.; Kropf, A.J.; Zemlyanov, D.; Zhang, G.; Yang, X.; et al. The effect of strong metal-support interaction (SMSI) on Pt-Ti/SiO2 and Pt-Nb/SiO2 catalysts for propane dehydrogenation. Catal. Sci. Technol. 2020, 10, 5973–5982. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Okejiri, F.; Xu, C.; Dai, S. Deep understanding of strong metal interface confinement: A journey of Pd/FeOx catalysts. ACS Catal. 2020, 15, 8950–8959. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Wiese, K.; Parlinska-Wojtan, M.; Rabeah, J.; Brueckner, A.; Behm, R.J. Encapsulation of Ru nanoparticles: Modifying the reactivity toward CO and CO2 methanation on highly active Ru/TiO2 catalysts. Appl. Catal. B 2020, 270, 118846. [Google Scholar] [CrossRef]
- Imelik, B.; Naccache, C.; Coudurier, G.; Praliaud, H.; Vedrine, J.; Bond, G.C.; Huizinga, T.; Prins, R. (Eds.) Metal-Support and Metal-Additive Effects in Catalysis; Elsevier: Amsterdam, The Netherlands, 1982. [Google Scholar]
- Hu, Z.P.; Wang, Z.; Yuan, Z.Y. Cr/Al2O3 catalysts with strong metal-support interactions for stable catalytic dehydrogenation of propane to propylene. Mol. Catal. 2020, 493, 111052. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, Q.; Yu, L.; Chen, L.; Crocker, M.; Shi, C. New insights into the size and support effects of γ-Al2O3 supported Au catalysts for HCHO oxidation at room temperature. Catal. Sci. Technol. 2020, 10, 4571–4579. [Google Scholar] [CrossRef]
- Juszczyk, W.; Łomot, D.; Pielaszek, J.; Karpinski, Z. Transformation of Pd/SiO2 catalysts during high temperature reduction. Catal. Lett. 2002, 78, 95–98. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, M. Intermetallic compound catalysts: Synthetic scheme, structure characterization and catalytic application. J. Mater. Chem. A 2020, 8, 2207–2221. [Google Scholar] [CrossRef]
- Bergeret, G.; Gallezot, P. Handbook of Heterogeneous Catalysis, Vol. 2; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; p. 739. [Google Scholar]
- Borodziński, A.; Bonarowska, M. Relation between crystallite size and dispersion on supported metal catalysts. Langmuir 1997, 13, 5613–5620. [Google Scholar] [CrossRef]
- Lee, J.; Jang, E.J.; Kwak, J.H. Effect of number and properties of specific sites on alumina surfaces for Pt-Al2O3 catalysts. Appl. Catal. A 2019, 569, 8–19. [Google Scholar] [CrossRef]
- Hu, C.; Creaser, D.; Grönbeck, H.; Ojagh, H.; Skoglundh, M. Selectivity and kinetics of methyl crotonate hydrogenation over Pt/Al2O3. Catal. Sci. Technol. 2015, 5, 1716–1730. [Google Scholar] [CrossRef][Green Version]
- Chan, D.; Tischer, S.; Heck, J.; Diehm, C.; Deutschmann, O. Correlation between catalytic activity and catalytic surface area of a Pt/Al2O3 DOC: An experimental and microkinetic modeling study. Appl. Catal. B 2014, 156–157, 153–165. [Google Scholar] [CrossRef]
- Jongpatiwut, S.; Rattanapuchapong, N.; Rirksomboon, T.; Osuwan, S.; Resasco, D.E. Enhanced sulfur tolerance of bimetallic PtPd/Al2O3 catalysts for hydrogenation of tetralin by addition of fluorine. Catal. Lett. 2008, 122, 214–222. [Google Scholar] [CrossRef]
- Borgna, A.; Garetto, T.; Apesteguia, C.; Le Normand, F.; Moraweck, B. Sintering of chlorinated Pt/-Al2O3 catalysts: An in situ study by X-Ray absorption spectroscopy. J. Catal. 1999, 186, 433–441. [Google Scholar] [CrossRef]
- Lieske, H.; Lietz, G.; Spindler, H.; Volteri, J. Reactions of platinum in oxygen- and hydrogen-treated Ptγ-A12O3 catalysts. J. Catal. 1983, 81, 8–16. [Google Scholar] [CrossRef]
- Contreras-Andrade, I.; Vázquez-Zavala, A.; Viveros, T. Influence of the synthesis method on the catalytic behavior of Pt and PtSn/Al2O3 reforming catalyst. Energy Fuels 2009, 23, 3835–3841. [Google Scholar] [CrossRef]
- Shelimov, B.; Lambert, J.F.; Che, M.; Didillon, B. Initial steps of the alumina-supported platinum catalyst preparation: A molecular study by 195Pt NMR, UV–Visible, EXAFS and Raman Spectroscopy. J. Catal. 1999, 185, 462–478. [Google Scholar] [CrossRef]
- Lu, H.; Svehla, M.J.; Skalsky, M.; Kong, C.; Sorrell, C. Pt-Al2O3 interfacial bonding in implantable hermetic feedthroughs: Morphology and orientation. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 817–824. [Google Scholar] [CrossRef]
- Guenther, T.; Kong, C.; Lu, H.; Svehla, M.J.; Lovell, N.H.; Ruys, A. Pt-Al2O3 interfaces in cofired ceramics for use in miniaturized neuroprosthetic implants. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 500–507. [Google Scholar] [CrossRef]
- Karbasi, A.; Hadjikhani, A.; Hrubiak, R.; Durygin, A. High temperature interactions in Platinum/Alumina system. Ceram. Trans. 2013, 240, 45. [Google Scholar] [CrossRef]
- Zhong, X.; Zhu, J.; Liu, J. Study of the interfacial structure of Pt/α-Al2O3 model catalyst under high-temperature hydrogen reduction. J. Catal. 2005, 236, 9–13. [Google Scholar] [CrossRef]
- Hayek, K.; Goller, H.; Penner, S.; Rupprechter, G.; Zimmerman, C. Regular alumina-supported nanoparticles of iridium, rhodium and platinum under hydrogen reduction: Structure, morphology and activity in the neopentane conversion. Catal. Lett. 2004, 92, 1–9. [Google Scholar] [CrossRef]
- Oh, S.; Ha, H.; Choi, H.; Jo, C.; Cho, J.; Choi, H.; Ryoo, R.; Kim, H.Y.; Park, J.Y. Oxygen activation on the interface between Pt nanoparticles and mesoporous defective TiO2 during CO oxidation. J. Chem. Phys. 2019, 151, 234716. [Google Scholar] [CrossRef] [PubMed]
- Severson, W.; Stuhlmann, C.; Villegas, I.; Weaver, M.J. Dipole–dipole coupling effects upon infrared spectroscopy of compressed electrochemical adlayers: Application to the Pt(111)/CO system. J. Chem. Phys. 1995, 103, 9832. [Google Scholar] [CrossRef]
- Deshlahra, P.; Conway, J.; Wolf, E.E.; Schneider, W.F. Influence of dipole–dipole interactions on coverage-dependent adsorption: CO and NO on Pt(111). Langmuir 2012, 28, 8408–8417. [Google Scholar] [CrossRef] [PubMed]
- Stoop, F.; Toolenaar, F.J.C.M.; Ponec, V. Geometric and ligand effects in the infrared spectra of adsorbed carbon monoxide. J. Catal. 1982, 73, 50–56. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Truong, C.M.; Goodman, D.W. Infrared vibrational studies of CO adsorption on Cu/Pt(111) and CuPt(111) surfaces. J. Chem. Phys. 1992, 96, 7814–7825. [Google Scholar] [CrossRef]
- de Menorval, L.C.; Chaqroune, A.; Coq, B.; Figueras, F. Characterization of mono- and bi-metallic platinum catalysts using CO FTIR spectroscopy. Size effects and topological segregation. J. Chem. Soc. Faraday Trans. 1997, 93, 3715–3720. [Google Scholar] [CrossRef]
- Blyholder, G. Molecular orbital view of chemisorbed carbon monoxide. J. Phys. Chem. 1964, 68, 2772. [Google Scholar] [CrossRef]
- Filatova, E.O.; Konashuk, A. Interpretation of the changing the band gap of Al2O3 depending on its crystalline form: Connection with different local symmetries. J. Phys. Chem. C 2015, 119, 20755–20761. [Google Scholar] [CrossRef]
- Gupta, R.K.; Singh, R.A. Junction properties of Schottky diode based on composite organic semiconductors. J. Mater. Sci. Mater. Electron. 2005, 16, 253–256. [Google Scholar] [CrossRef]
- Colinge, J.P.; Colinge, C.A. Physics of Semiconductor Devices; Springer: Berlin/Heidelberg, Germany, 2006; p. 39. [Google Scholar]
- Tanaka, H.; Kuriyama, M.; Ishida, Y.; Ito, S.I.; Kubota, T.; Miyao, T.; Naito, S.; Tomishige, K.; Kunimori, K. Preferential CO oxidation in hydrogen-rich stream over Pt catalysts modified with alkali metals Part II. Catalyst characterization and role of alkali metals. Appl. Catal. A Gen. 2008, 343, 125–133. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Kumar, A.; Khraisheh, M. A review of recent advances in water-gas shift catalysis for hydrogen production. Emerg. Mater. 2020, 3, 881–917. [Google Scholar] [CrossRef]
- LeValley, T.L.; Richard, A.R.; Fan, M. The progress in water gas shift and steam reforming hydrogen production technologies. A review. Int. J. Hydrogen Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Dekker, F.H.M.; Nazloomian, J.G.; Bliek, A.; Kapteijn, F.; Moulijn, J.A.; Coulson, D.R.; Mills, P.L.; Lerou, J.J. Carbon monoxide oxidation over platinum powder: A comparison of TAP and step-response experiments. Appl. Catal. A Gen. 1997, 151, 247–266. [Google Scholar] [CrossRef]
- Burnett, D.J.; Capitano, A.T.; Gabelnick, A.M.; Marsh, A.L.; Fischer, D.A.; Gland, J.L. In-situ soft X-ray studies of CO oxidation on the Pt(111) surface. Surf. Sci. 2004, 564, 29–37. [Google Scholar] [CrossRef]
- Campbell, C.T.; Ertl, G.; Kuipers, H.; Segner, J. A molecular beam study of the catalytic-oxidation of CO on a Pt(111) surface. J. Chem. Phys. 1980, 73, 5862–5873. [Google Scholar] [CrossRef]
- Allian, A.D.; Takanabe, K.; Fujdala, K.L.; Haoet, X.; Truex, T.J.; Cai, J.; Buda, C.; Neurock, M.; Iglesia, E. Chemisorption of CO and mechanism of CO oxidation on supported platinum nanoclusters. J. Am. Chem. Soc. 2011, 133, 4498–4517. [Google Scholar] [CrossRef]
- Van Spronsen, M.A.; Frenken, J.W.M.; Groot, I.M.N. Surface science under reaction conditions: CO oxidation on Pt and Pd model catalysts. Chem. Soc. Rev. 2017, 46, 4347–4374. [Google Scholar] [CrossRef]
- Zhao, K.F.; Tang, H.L.; Qiao, B.T.; Li, L.; Wang, J.H. High Activity of Au/γ-Fe2O3 for CO Oxidation: Effect of Support Crystal Phase in Catalyst Design. ACS Catal. 2015, 5, 3528–3539. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Naoto, K.; Hiroki, M.; Muroyama, T.; Toshiaki, M.; Koichi, E. Effect of reduction treatment on CO oxidation over Pt/SnO2 catalyst. Catal. Today 2011, 164, 169–175. [Google Scholar] [CrossRef]
- Liu, G.; Walsh, A.G.; Zhang, P. Synergism of iron and platinum species for low-temperature CO oxidation: From two-dimensional surface to nanoparticle and single-atom catalysts. J. Phys. Chem. Lett. 2020, 11, 2219–2229. [Google Scholar] [CrossRef]
- Kocemba, I. Apparatus for investigation of catalysts by temperature programmed methods—Peak4. Przem. Chem. 2003, 82, 142–148. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).