TBO Degradation by Heterogeneous Fenton-like Reaction Using Fe Supported over Activated Carbon
Abstract
:1. Introduction
2. Results and Discussion
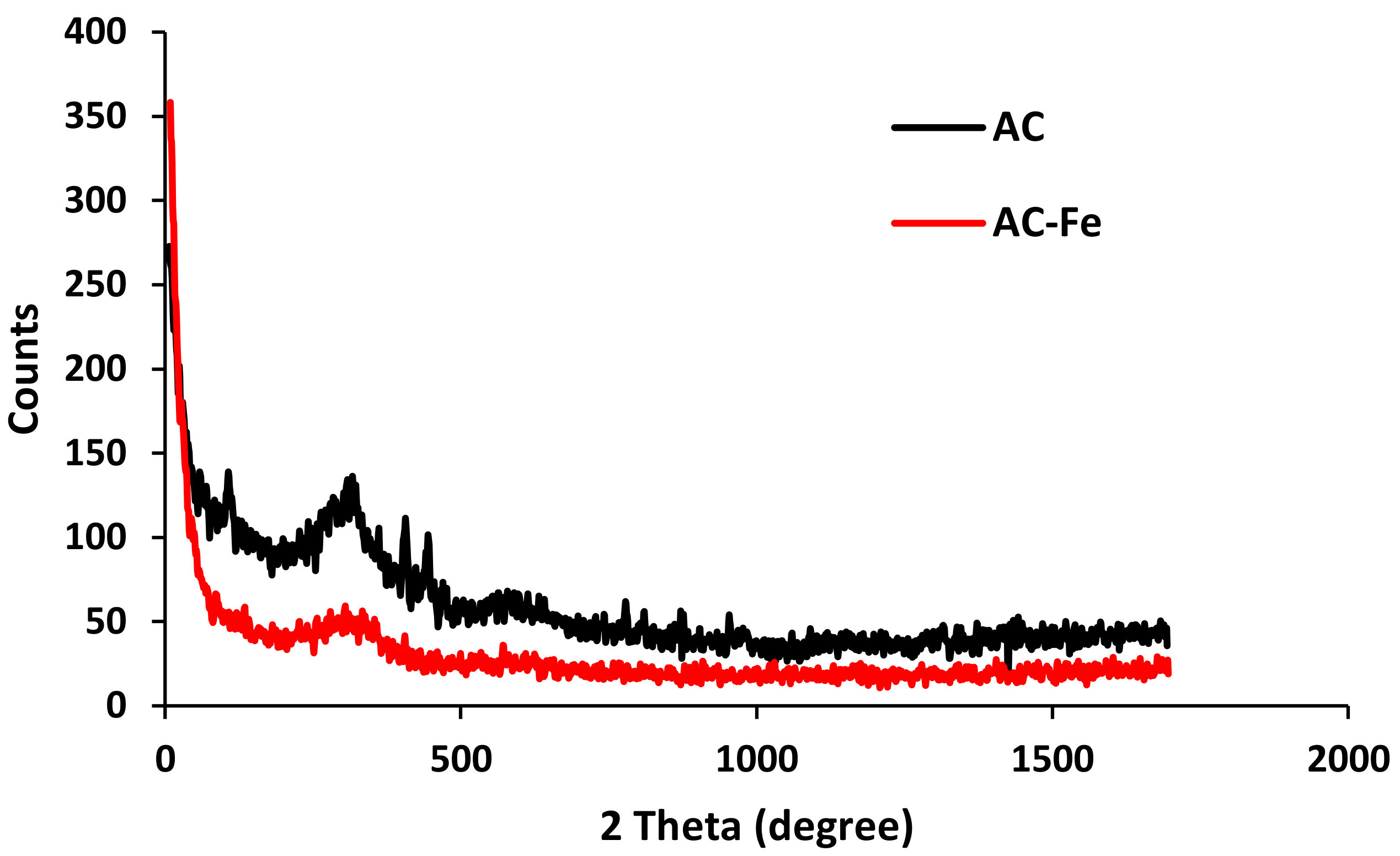
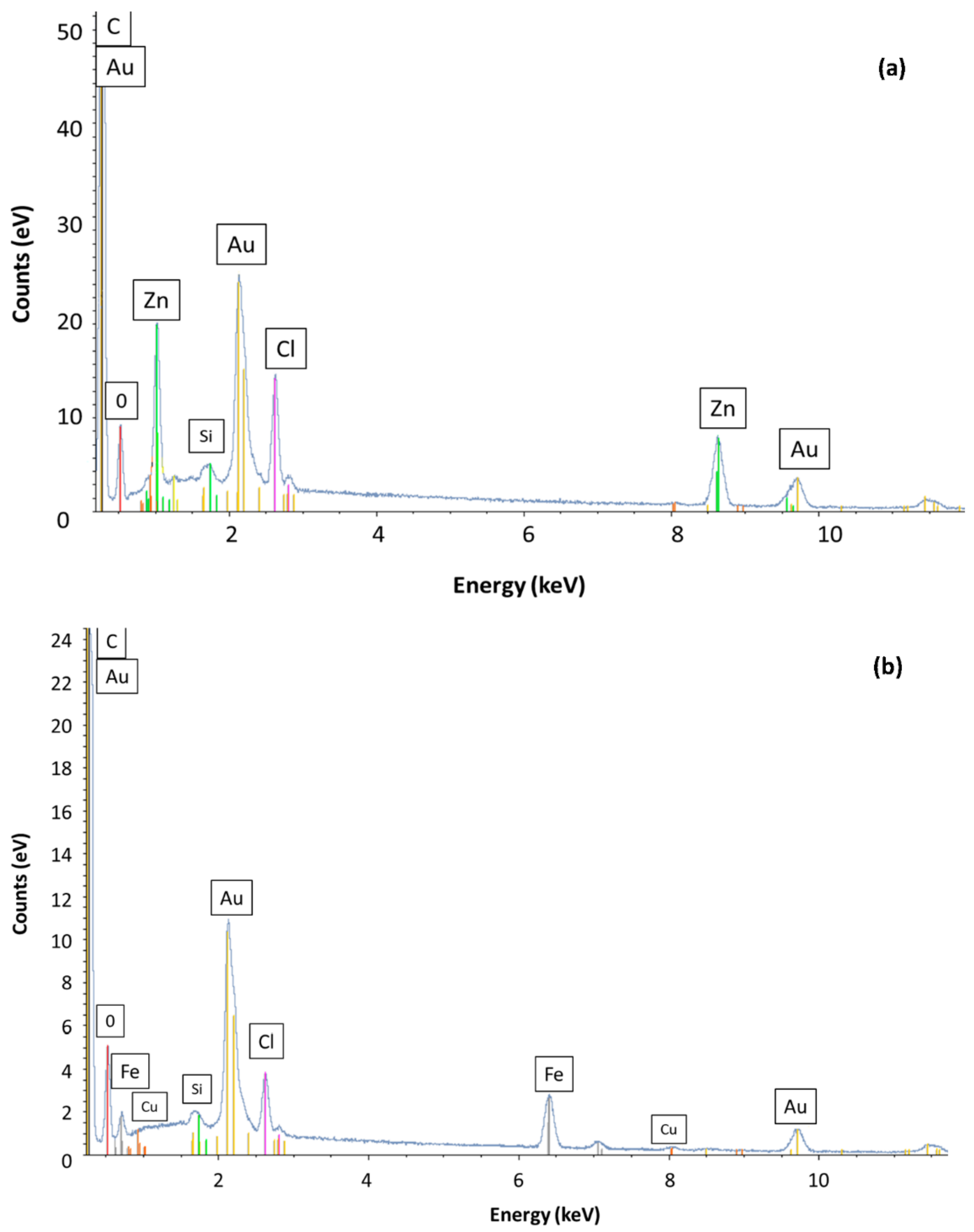
2.1. Structural and Textural Properties
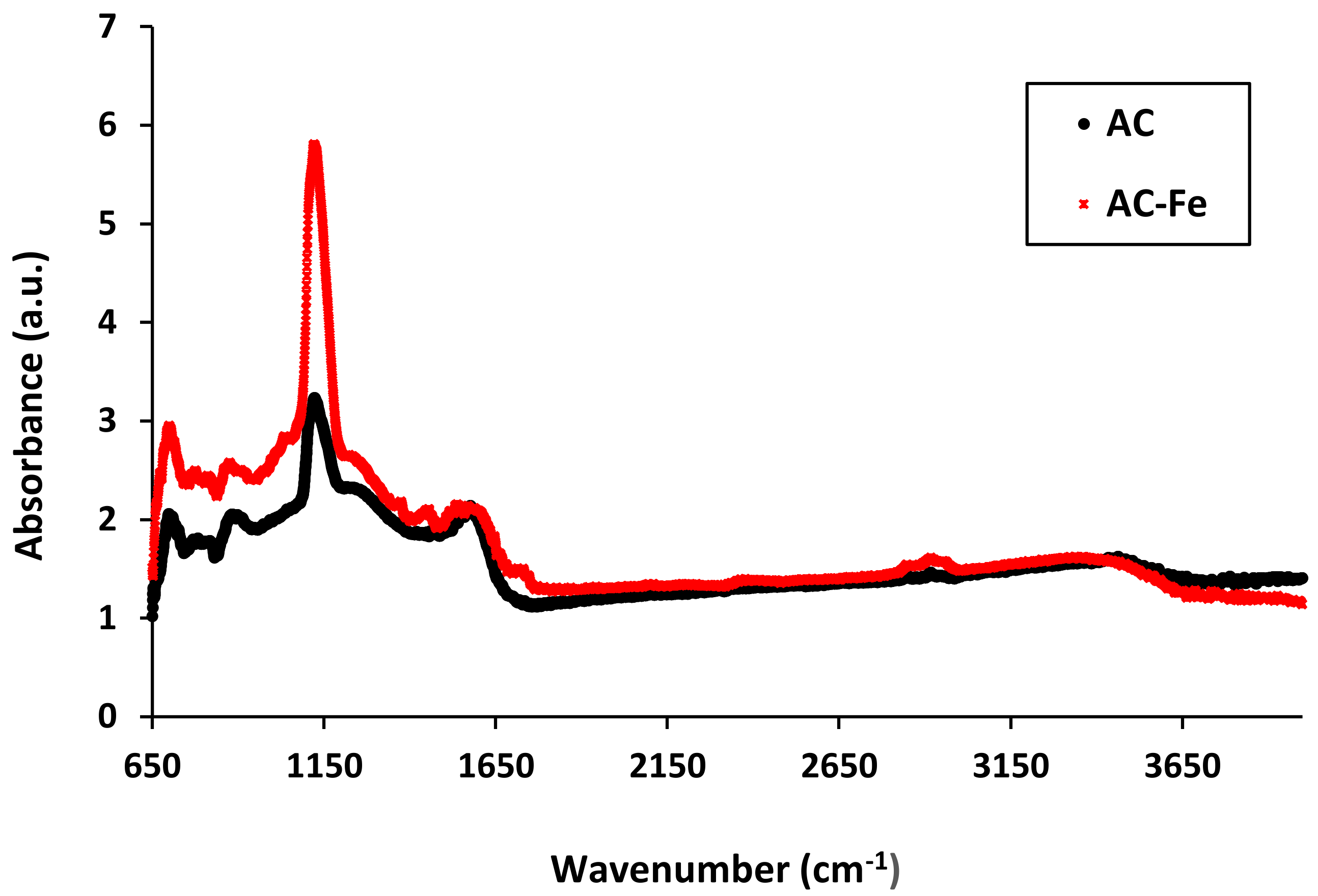
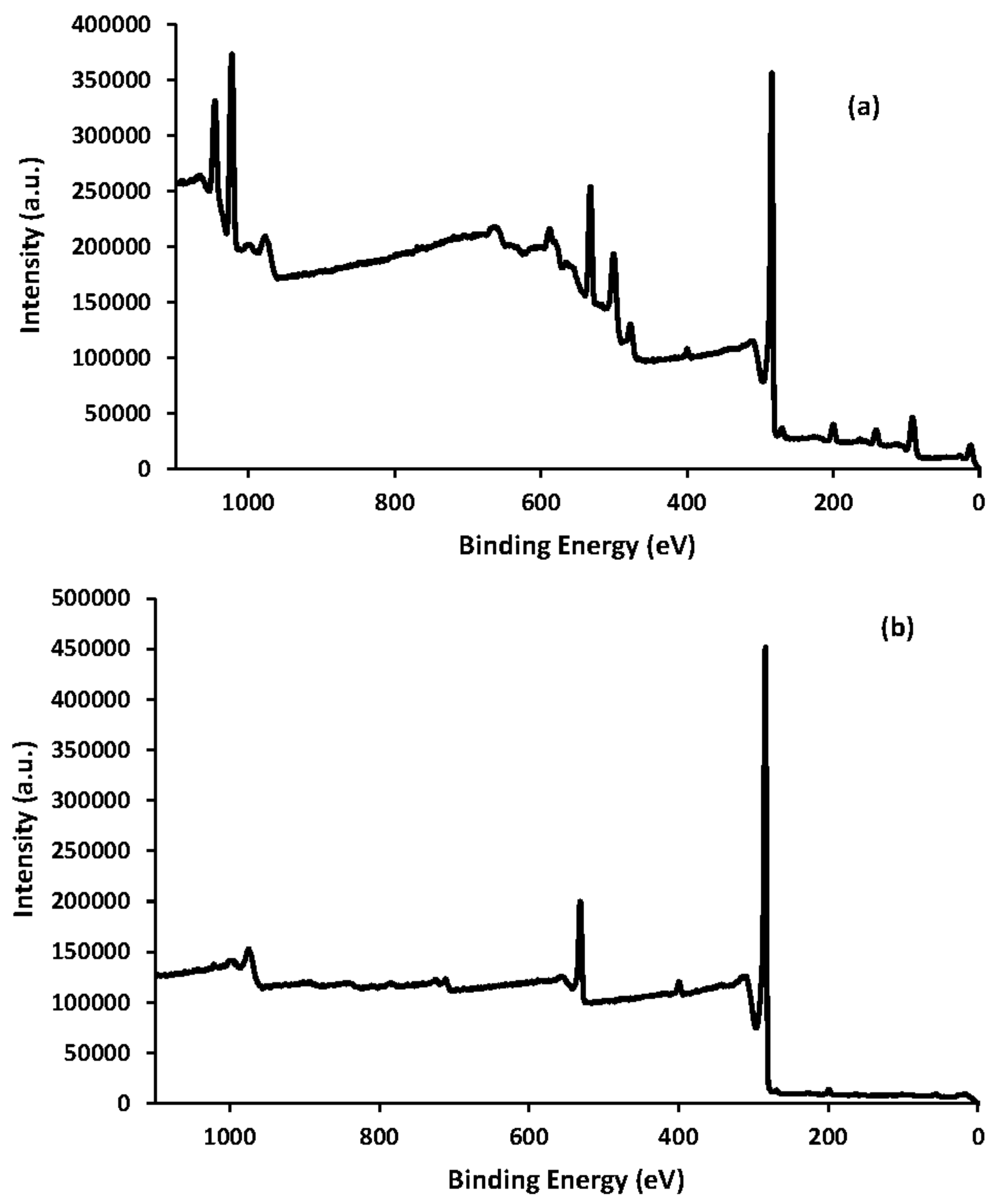
2.2. Changes in Surface Chemistry Features
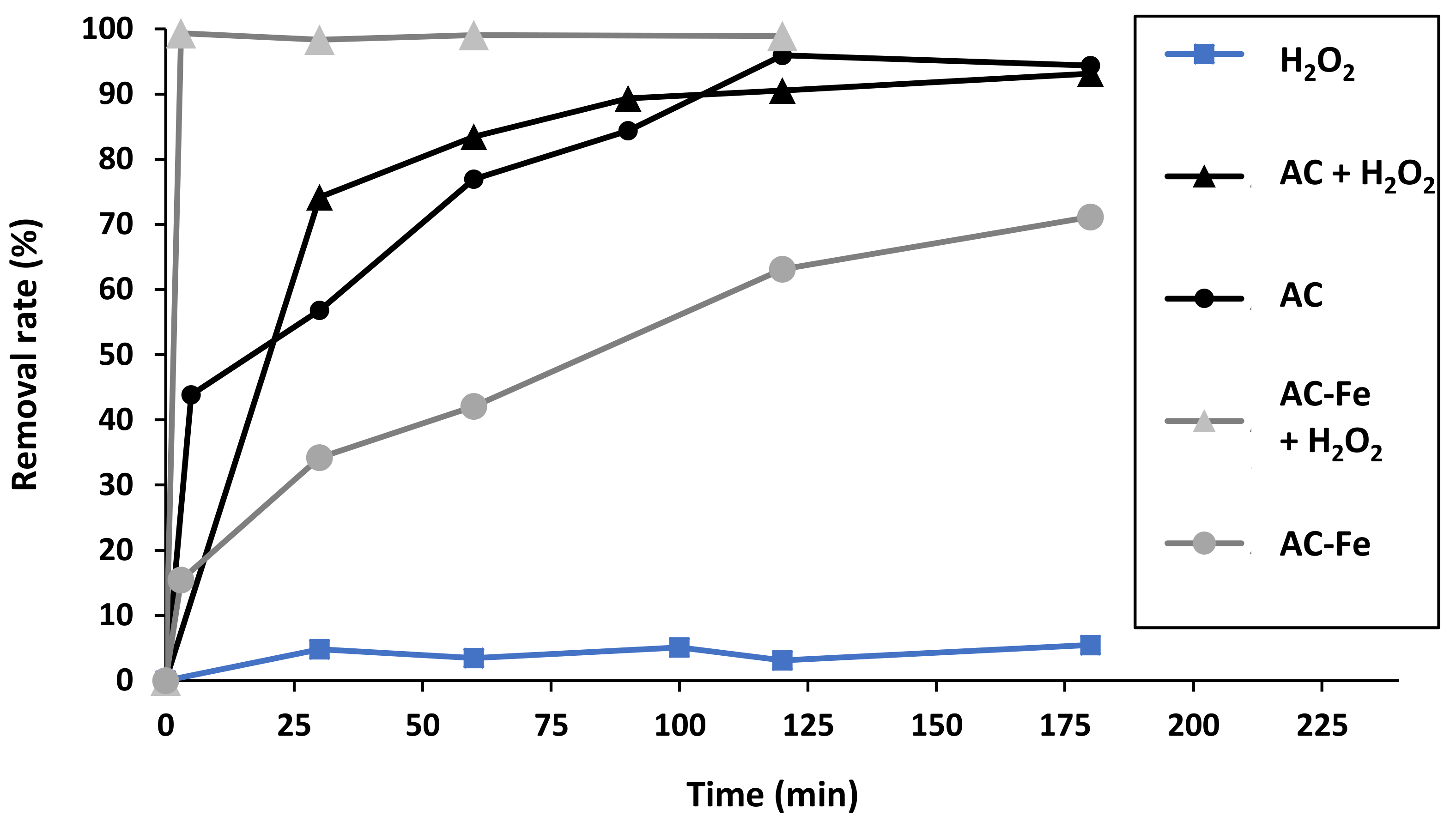
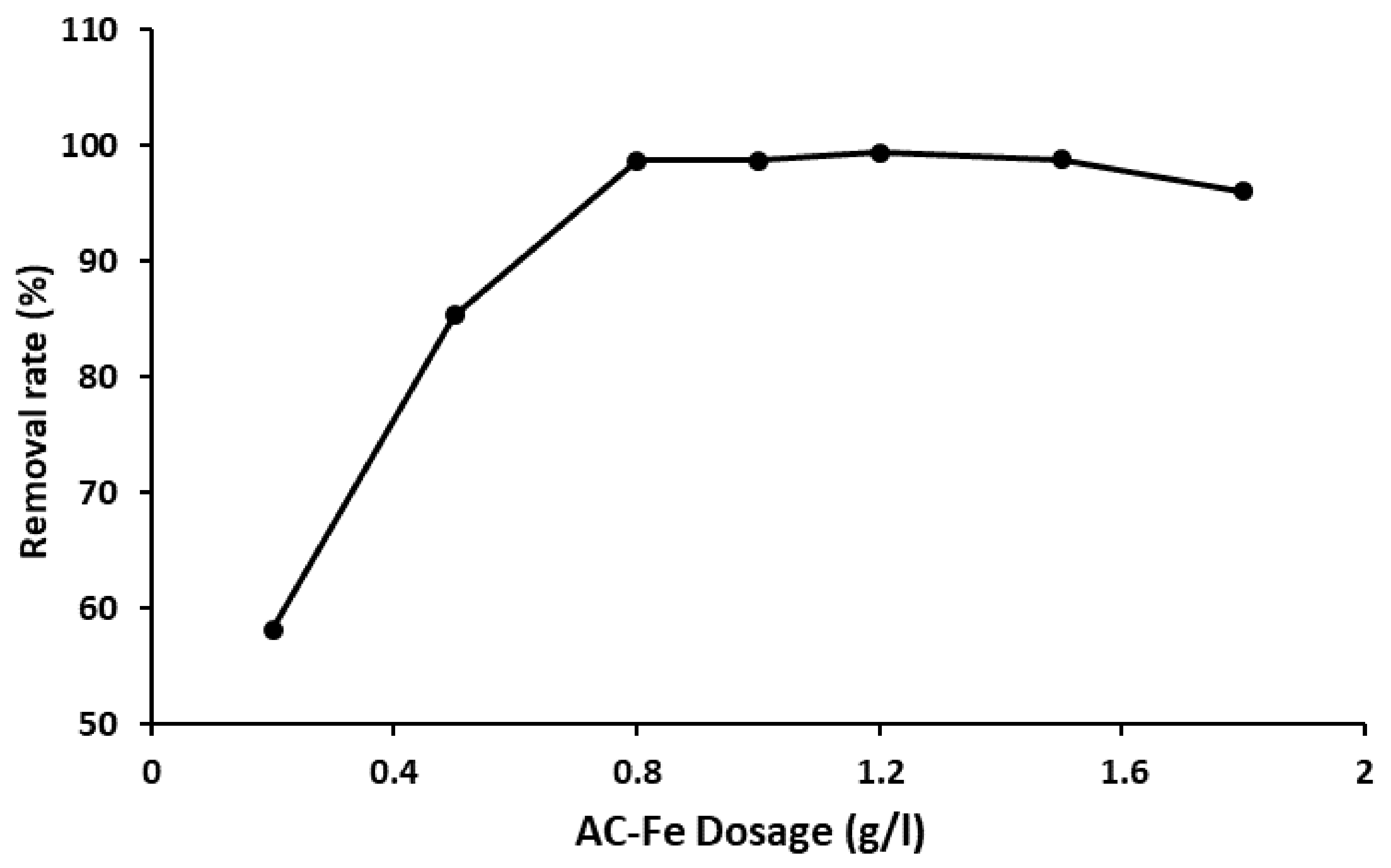
2.3. TBO Adsorption and Fenton Oxidation
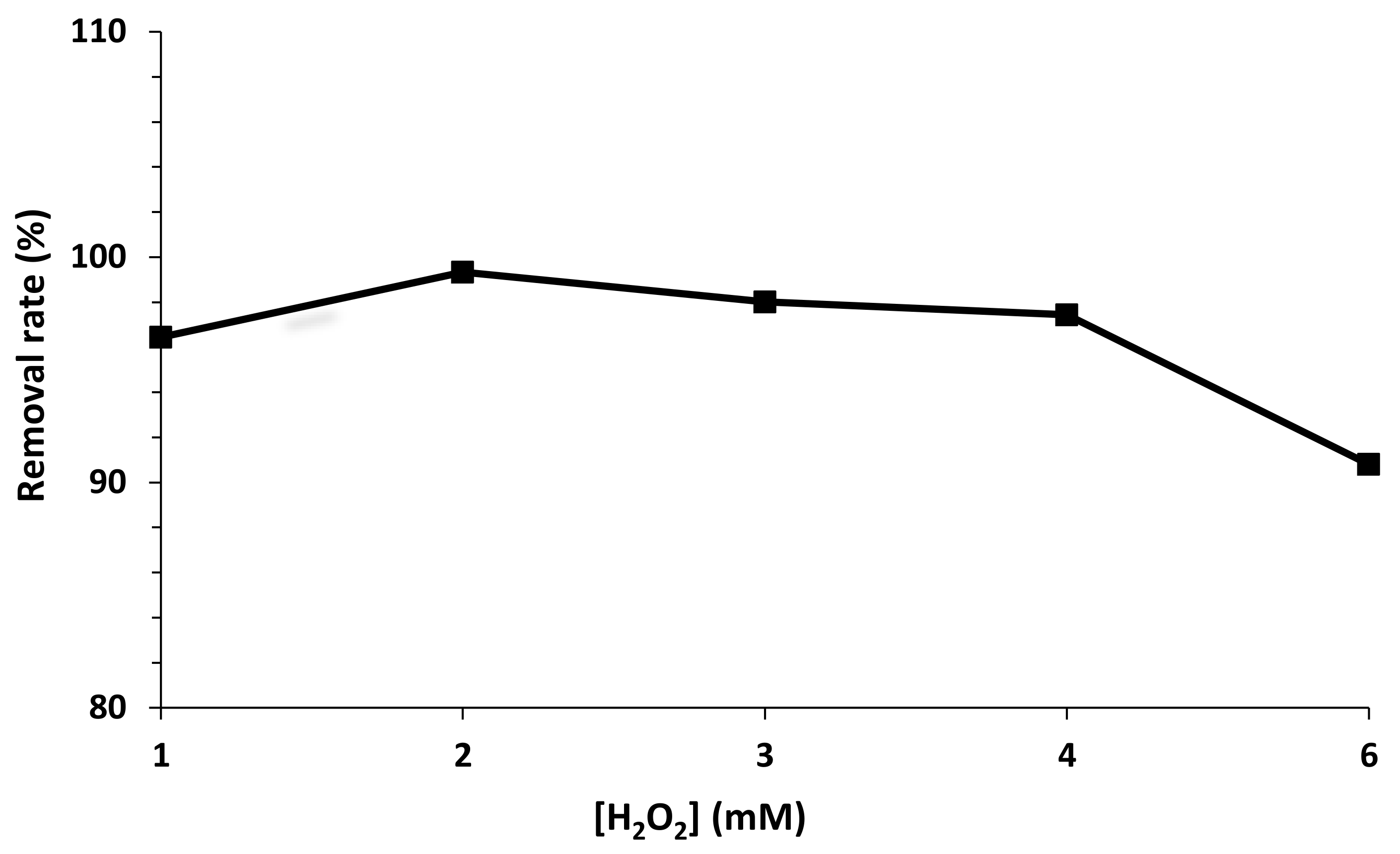
2.3.1. Effect of H2O2
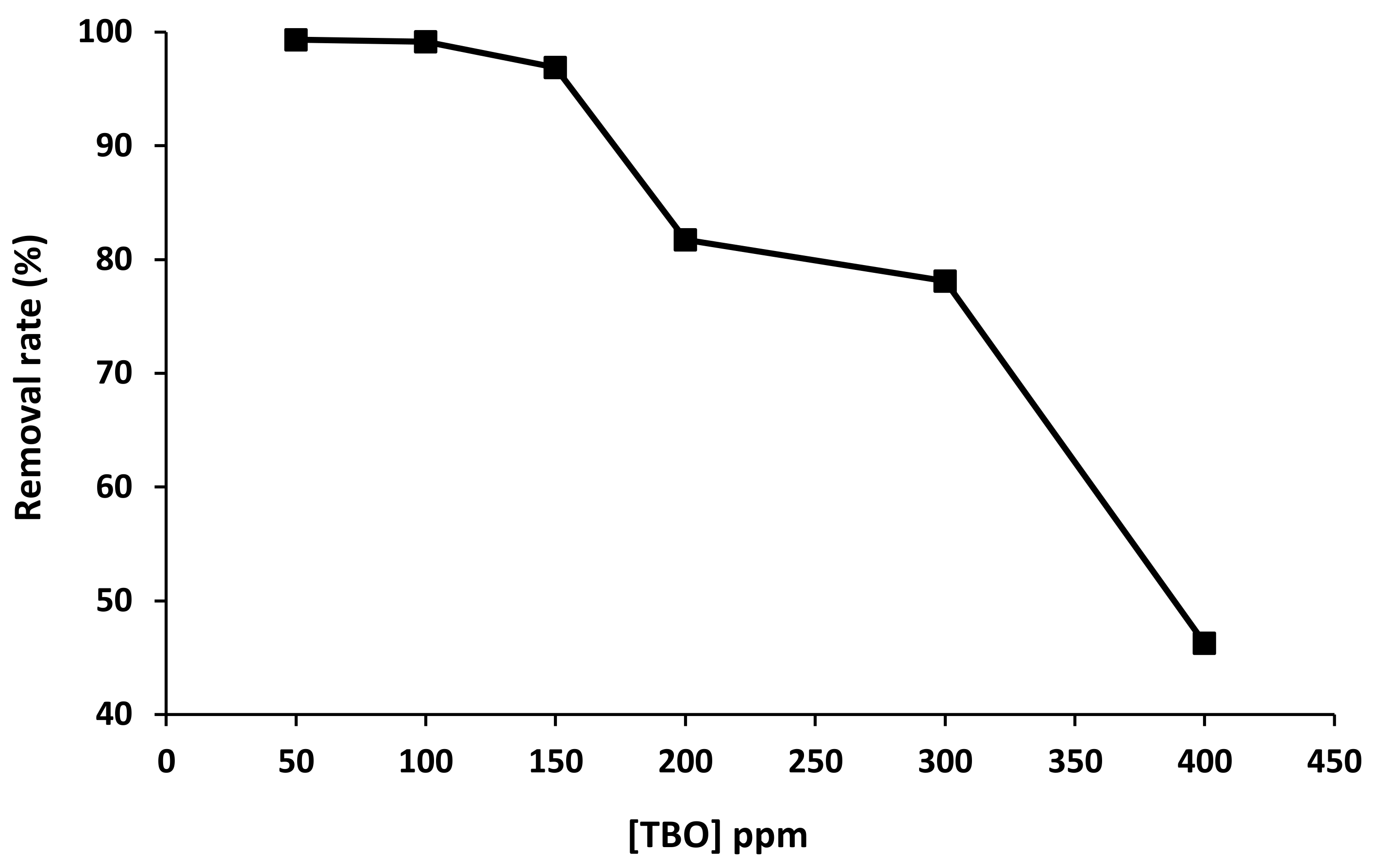
2.3.2. Effect of the Initial TBO Concentration
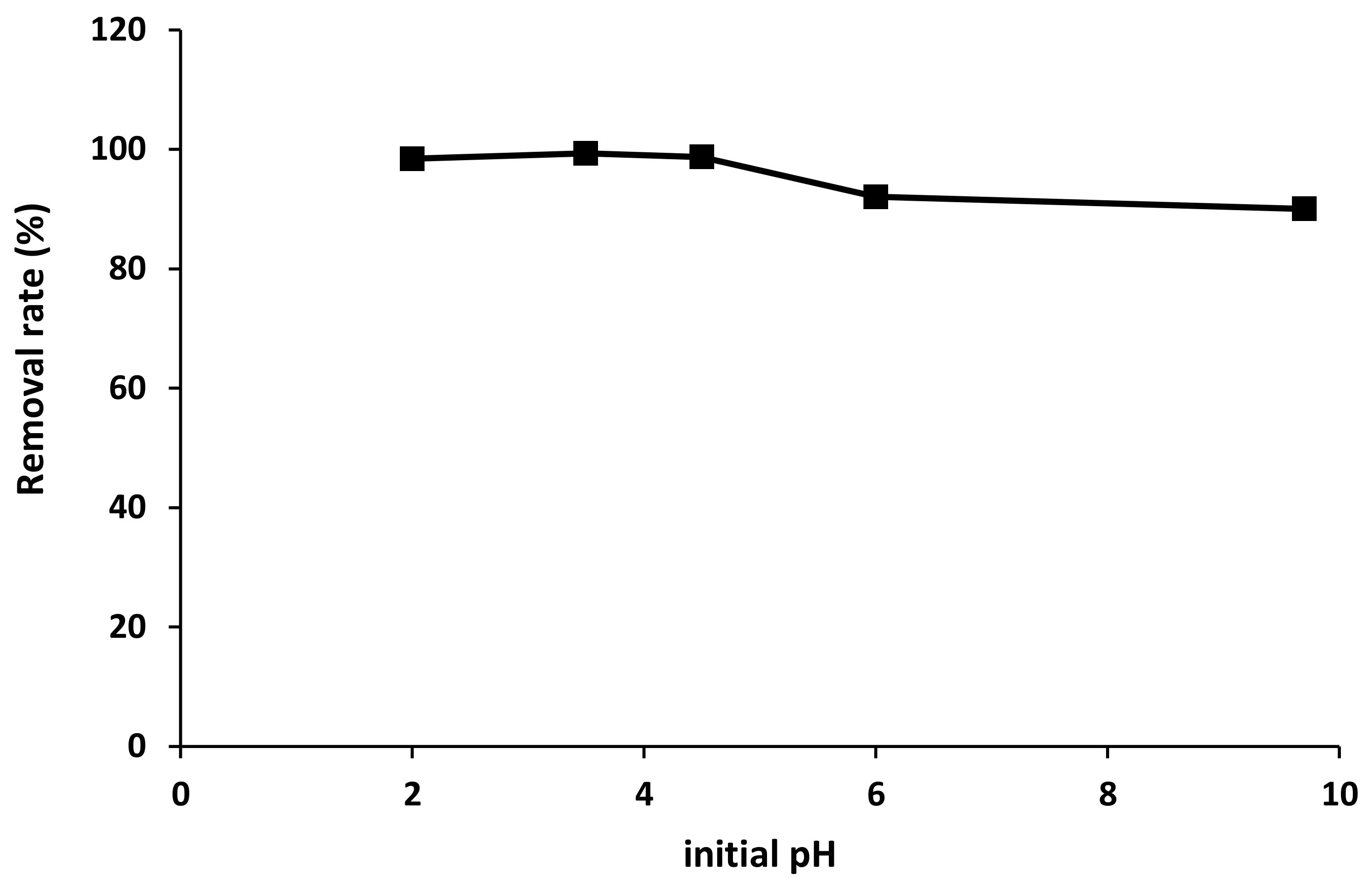
2.3.3. Effect of Initial pH
2.4. Mineralization Study
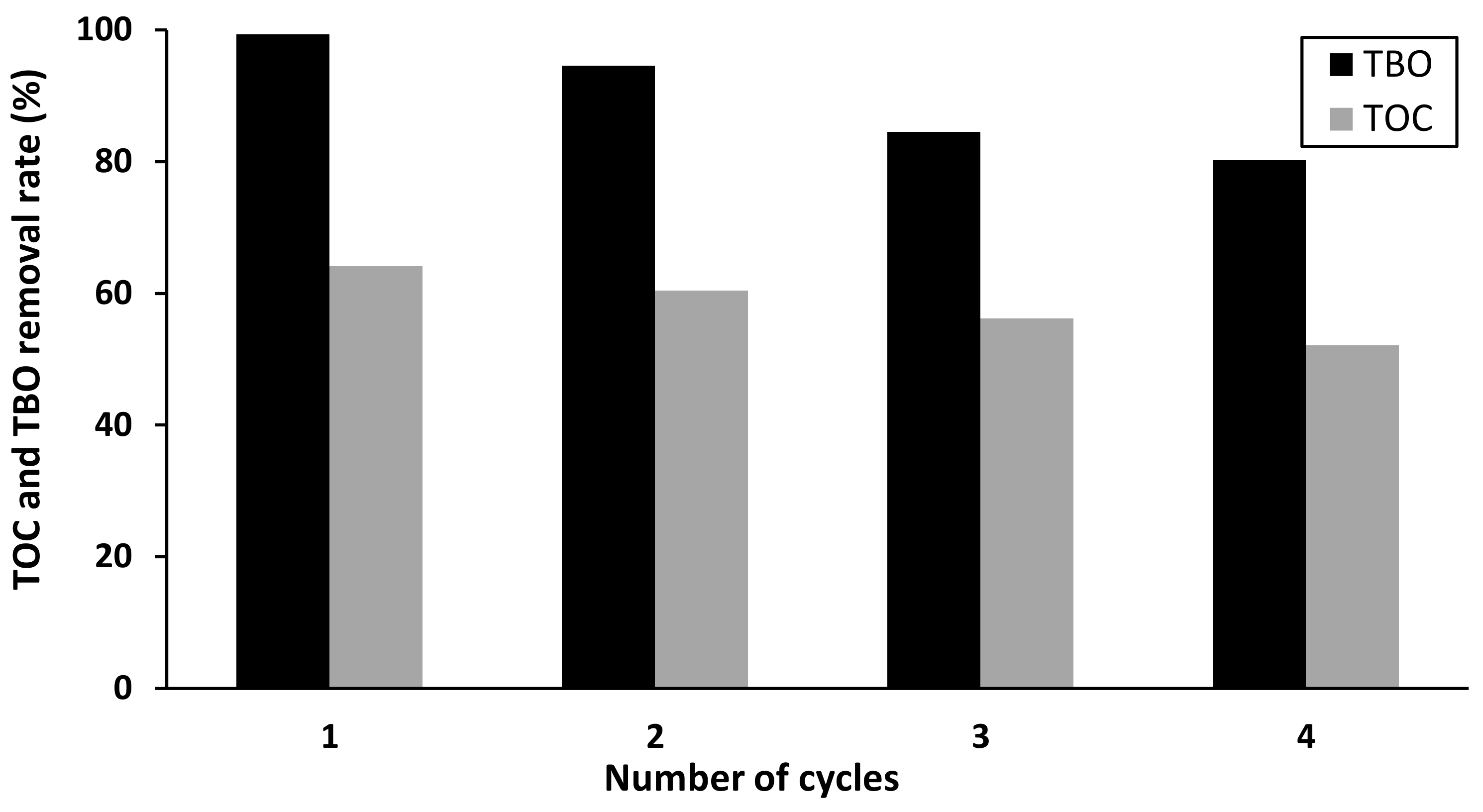
2.5. Stability and Reuse of the Catalyst
3. Materials and Methods
3.1. Chemicals
3.2. Catalyst Preparation and Characterization
3.3. Catalyst Characterization
3.4. Catalytic Experimental Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yao, T.; Qi, Y.; Mei, Y.; Yang, Y.; Aleisa, R.; Tong, X.; Wu, J. One-step preparation of reduced graphene oxide aerogel loaded with mesoporous copper ferrite nanocubes: A highly efficient catalyst in microwave-assisted Fenton reaction. J. Hazard. Mater. 2019, 378, 120712. [Google Scholar] [CrossRef]
- Alpat, S.K.; Özbayrak, Ö.; Alpat, Ş.; Akçay, H. The adsorption kinetics and removal of cationic dye, Toluidine Blue O, from aqueous solution with Turkish zeolite. J. Hazard. Mater. 2008, 151, 213–220. [Google Scholar] [CrossRef]
- Zheng, H.; Pan, Y.; Xiang, X. Oxidation of acidic dye Eosin Y by the solar photo-Fenton processes. J. Hazard. Mater. 2007, 141, 457–464. [Google Scholar] [CrossRef]
- Guibal, E.; Roussy, J. Coagulation and flocculation of dye-containing solutions using a biopolymer (Chitosan). React. Funct. Polym. 2007, 67, 33–42. [Google Scholar] [CrossRef]
- Kornaros, M.; Lyberatos, G. Biological treatment of wastewaters from a dye manufacturing company using a trickling filter. J. Hazard. Mater. 2006, 136, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Dutta, K.; Mukhopadhyay, S.; Bhattacharjee, S.; Chaudhuri, B. Chemical oxidation of methylene blue using a Fenton-like reaction. J. Hazard. Mater. 2001, 84, 57–71. [Google Scholar] [CrossRef]
- Rodríguez, R.; Espada, J.J.; Pariente, M.I.; Melero, J.A.; Martínez, F.; Molina, R. Comparative life cycle assessment (LCA) study of heterogeneous and homogenous Fenton processes for the treatment of pharmaceutical wastewater. J. Clean. Prod. 2016, 124, 21–29. [Google Scholar] [CrossRef]
- Barbusiński, K.; Majewski, J. Discoloration of azo dye Acid Red 18 by Fenton reagent in the presence of iron powder. Pol. J. Environ. Stud. 2003, 12, 151–155. [Google Scholar]
- Huang, Q.; Song, S.; Chen, Z.; Hu, B.; Chen, J.; Wang, X. Biochar-based materials and their applications in removal of organic contaminants from wastewater: State-of-the-art review. Biochar 2019, 1, 45–73. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Xiang, J.; Lin, J.; Xiang, L.; Chen, S.; Yan, B.; Fan, H.; Zhang, S.; Shi, X. Sustainable Advanced Fenton-like Catalysts Based on Mussel-Inspired Magnetic Cellulose Nanocomposites to Effectively Remove Organic Dyes and Antibiotics. ACS Appl. Mater. Interfaces 2020, 12, 51952–51959. [Google Scholar] [CrossRef]
- González-Rodríguez, J.; Gamallo, M.; Conde, J.J.; Vargas-Osorio, Z.; Vázquez-Vázquez, C.; Piñeiro, Y.; Rivas, J.; Feijoo, G.; Moreira, M.T. Exploiting the Potential of Supported Magnetic Nanomaterials as Fenton-like Catalysts for Environmental Applications. Nanomaterials 2021, 11, 2902. [Google Scholar] [CrossRef]
- Hajjaji, W.; Pullar, R.C.; Labrincha, J.A.; Rocha, F. Aqueous Acid Orange 7 dye removal by clay and red mud mixes. Appl. Clay Sci. 2016, 126, 197–206. [Google Scholar] [CrossRef]
- Ünnü, B.A.; Gündüz, G.; Dükkancı, M. Heterogeneous Fenton-like oxidation of crystal violet using an iron loaded ZSM-5 zeolite. Desalin. Water Treat. 2016, 57, 11835–11849. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, P.; Gong, B.; Yang, S.; Li, H.; Zhu, Z.; Cui, L. Preservation of glutamic acid-iron chelate into montmorillonite to efficiently degrade Reactive Blue 19 in a Fenton system under sunlight irradiation at neutral pH. Appl. Surf. Sci. 2016, 370, 209–217. [Google Scholar] [CrossRef]
- Bello, M.M.; Raman, A.A.A.; Asghar, A. Activated carbon as carrier in fluidized bed reactor for Fenton oxidation of recalcitrant dye: Oxidation-adsorption synergy and surface interaction. J. Water Process Eng. 2020, 33, 101001. [Google Scholar] [CrossRef]
- Bounab, L.; Iglesias, O.; Pazos, M.; Sanromán, M.Á.; Gonzalez-Romero, E. Effective monitoring of the electro-Fenton degradation of phenolic derivatives by differential pulse voltammetry on multi-walled-carbon nanotubes modified screen-printed carbon electrodes. Appl. Catal. B Environ. 2016, 180, 544–550. [Google Scholar] [CrossRef]
- Cleveland, V.; Bingham, J.P.; Kan, E. Heterogeneous Fenton degradation of bisphenol A by carbon nanotube-supported Fe3O4. Sep. Purif. Technol. 2014, 133, 388–395. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, Y.; Lu, F.; Wei, F.; Wang, X.; Wang, S. Magnetic recoverable MnFe2O4 and MnFe2O4-graphene hybrid as heterogeneous catalysts of peroxymonosulfate activation for efficient degradation of aqueous organic pollutants. J. Hazard. Mater. 2014, 270, 61–70. [Google Scholar] [CrossRef]
- Jain, A.K.; Gupta, V.K.; Bhatnagar, A.; Suhas, A. A comparative study of adsorbents prepared from industrial wastes for removal of dyes. Sep. Sci. Technol. 2003, 38, 463–481. [Google Scholar] [CrossRef]
- Gupta, V.K.; Saleh, T.A. Sorption of pollutants by porous carbon, carbon nanotubes and fullerene-an overview. Environ. Sci. Pollut. Res. 2013, 20, 2828–2843. [Google Scholar] [CrossRef]
- Gu, Z.; Fang, J.; Deng, B. Preparation and evaluation of GAC-based iron-containing adsorbents for arsenic removal. Environ. Sci. Technol. 2005, 39, 3833–3843. [Google Scholar] [CrossRef]
- Deng, H.; Yang, L.; Tao, G.; Dai, J. Preparation and characterization of activated carbon from cotton stalk by microwave assisted chemical activation—Application in methylene blue adsorption from aqueous solution. J. Hazard. Mater. 2009, 166, 1514–1521. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, L.; Ma, A.; Xia, H.; Peng, J.; Li, C.; Shu, J. Comparison of activated carbon and iron/cerium modified activated carbon to remove methylene blue from wastewater. J. Environ. Sci. 2018, 65, 92–102. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Mileva, A.; Tsyntsarski, B.; Paneva, D.; Spassova, I.; Kovacheva, D.; Petrov, N. Activated carbon from Bulgarian peach stones as a support of catalysts for methanol decomposition. Biomass Bioenergy 2018, 109, 135–146. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Kang, X.; Zhang, C. Rapid and efficient recovery of silver with nanoscale zerovalent iron supported on high performance activated carbon derived from straw biomass. Environ. Pollut. 2019, 255, 113043. [Google Scholar] [CrossRef] [PubMed]
- Anjum, H.; Johari, K.; Gnanasundaram, N.; Appusamy, A.; Thanabalan, M. Impact of surface modification on adsorptive removal of BTX onto activated carbon. J. Mol. Liq. 2019, 280, 238–251. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. Int. J. Devoted Dev. Appl. Tech. Anal. Surf. Interfaces Thin Film. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Guimarães, J.R.; Maniero, M.G.; de Araújo, R.N. A comparative study on the degradation of RB-19 dye in an aqueous medium by advanced oxidation processes. J. Environ. Manag. 2012, 110, 33–39. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When I’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Dou, X.; Deng, D.; Guan, Y.; Zhang, L.; He, G. Decolourization of the azo dye Orange G in aqueous solution via a heterogeneous Fenton-like reaction catalysed by goethite. Environ. Technol. 2012, 33, 1545–1552. [Google Scholar] [CrossRef]
- Carvalho, S.S.; Carvalho, N.M. Dye degradation by green heterogeneous Fenton catalysts prepared in presence of Camellia sinensis. J. Environ. Manag. 2017, 187, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Park, J.A.; Yang, B.; Park, C.; Choi, J.W.; van Genuchten, C.M.; Lee, S.H. Oxidation of microcystin-LR by the Fenton process: Kinetics, degradation intermediates, water quality and toxicity assessment. Chem. Eng. J. 2017, 309, 339–348. [Google Scholar] [CrossRef]
- Navalon, S.; Alvaro, M.; Garcia, H. Heterogeneous Fenton catalysts based on clays, silicas and zeolites. Appl. Catal. B Environ. 2010, 99, 1–26. [Google Scholar] [CrossRef]
- Azmi, N.H.M.; Ayodele, O.B.; Vadivelu, V.M.; Asif, M.; Hameed, B.H. Fe-modified local clay as effective and reusable heterogeneous photo-Fenton catalyst for the decolorization of Acid Green 25. J. Taiwan Inst. Chem. Eng. 2014, 45, 1459–1467. [Google Scholar] [CrossRef]
- Jafari, A.J.; Kakavandi, B.; Jaafarzadeh, N.; Kalantary, R.R.; Ahmadi, M.; Babaei, A.A. Fenton-like catalytic oxidation of tetracycline by AC@ Fe3O4 as a heterogeneous persulfate activator: Adsorption and degradation studies. J. Ind. Eng. Chem. 2017, 45, 323–333. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q.; Tang, B. Effective degradation of C.I. Acid Red 73 by advanced Fenton process. J. Hazard. Mater. 2010, 174, 17–22. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, L.; Sun, L.; Zhu, S.; Huang, Z.; Mao, Y.; Lu, W.; Chen, W. Efficient removal of dyes using heterogeneous Fenton catalysts based on activated carbon fibers with enhanced activity. Chem. Eng. Sci. 2013, 101, 424–431. [Google Scholar] [CrossRef]
- Tao, H.C.; Wei, X.Y.; Zhang, L.J.; Lei, T.; Xu, N. Degradation of p-nitrophenol in a BES-Fenton system based on limonite. J. Hazard. Mater. 2013, 254, 236–241. [Google Scholar] [CrossRef]
- Gomes, H.T.; Miranda, S.M.; Sampaio, M.J.; Figueiredo, J.L.; Silva, A.M.; Faria, J.L. The role of activated carbons functionalized with thiol and sulfonic acid groups in catalytic wet peroxide oxidation. Appl. Catal. B Environ. 2011, 106, 390–397. [Google Scholar] [CrossRef]
- Zazo, J.A.; Casas, J.A.; Mohedano, A.F.; Rodríguez, J.J. Catalytic wet peroxide oxidation of phenol with a Fe/active carbon catalyst. Appl. Catal. B Environ. 2006, 65, 261–268. [Google Scholar] [CrossRef]
- Du, W.; Xu, Y.; Wang, Y. Photoinduced degradation of orange II on different iron (hydr) oxides in aqueous suspension: Rate enhancement on addition of hydrogen peroxide, silver nitrate, and sodium fluoride. Langmuir 2008, 24, 175–181. [Google Scholar] [CrossRef]
- Liang, C.; Liang, C.P.; Chen, C.C. pH dependence of persulfate activation by EDTA/Fe (III) for degradation of trichloroethylene. J. Contam. Hydrol. 2009, 106, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.W.; Hwang, K.Y. Effects of reaction conditions on the oxidation efficiency in the Fenton process. Water Res. 2000, 34, 2786–2790. [Google Scholar] [CrossRef]
- Feng, J.; Hu, X.; Yue, P.L. Effect of initial solution pH on the degradation of Orange II using clay-based Fe nanocomposites as heterogeneous photo-fenton catalyst. Water Res. 2006, 40, 641–646. [Google Scholar] [CrossRef]
- Huang, H.H.; Lu, M.C.; Chen, J.N. Catalytic decomposition of hydrogen peroxide and 2-chlorophenol with iron oxides. Water Res. 2001, 35, 2291–2299. [Google Scholar] [CrossRef]
- Laiju, A.R.; Sivasankar, T.; Nidheesh, P.V. Iron-loaded mangosteen as a heterogeneous Fenton catalyst for the treatment of landfill leachate. Environ. Sci. Pollut. Res. 2014, 21, 10900–10907. [Google Scholar] [CrossRef]
- Bakhta, S.; Sadaoui, Z.; Lassi, U.; Romar, H.; Kupila, R.; Vieillard, J. Performances of metals modified activated carbons for fluoride removal from aqueous solutions. Chem. Phys. Lett. 2020, 754, 137705. [Google Scholar] [CrossRef]
- Allalou, O.; Miroud, D.; Belmedani, M.; Sadaoui, Z. Performance of surfactant-modified activated carbon prepared from dates wastes for nitrate removal from aqueous solutions. Environ. Prog. Sustain. Energy 2019, 38, S403–S411. [Google Scholar] [CrossRef]
- Macías-Pérez, M.C.; Bueno-López, A.; Lillo-Rodenas, M.A.; de Lecea, C.S.M.; Linares-Solano, A. SO2 retention on CaO/activated carbon sorbents. Part I: Importance of calcium loading and dispersion. Fuel 2007, 86, 677–683. [Google Scholar] [CrossRef]


| BET | |||
|---|---|---|---|
| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
| AC | 1308.3 | 1.05 | 3.21 |
| AC-Fe | 1069.3 | 0.89 | 3.33 |
| Sample | C (wt%) | O (wt%) | Si (wt%) | Fe (wt%) | Cl (wt%) | Cu (wt%) |
|---|---|---|---|---|---|---|
| AC | 74.89 | 5.31 | 0.44 | - | 2.77 | 0.20 |
| AC-Fe | 85.74 | 2.86 | 0.22 | 9.09 | 1.50 | 0.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samir, B.; Bakhta, S.; Bouazizi, N.; Sadaoui, Z.; Allalou, O.; Le Derf, F.; Vieillard, J. TBO Degradation by Heterogeneous Fenton-like Reaction Using Fe Supported over Activated Carbon. Catalysts 2021, 11, 1456. https://doi.org/10.3390/catal11121456
Samir B, Bakhta S, Bouazizi N, Sadaoui Z, Allalou O, Le Derf F, Vieillard J. TBO Degradation by Heterogeneous Fenton-like Reaction Using Fe Supported over Activated Carbon. Catalysts. 2021; 11(12):1456. https://doi.org/10.3390/catal11121456
Chicago/Turabian StyleSamir, Brahim, Soumia Bakhta, Nabil Bouazizi, Zahra Sadaoui, Ouiza Allalou, Franck Le Derf, and Julien Vieillard. 2021. "TBO Degradation by Heterogeneous Fenton-like Reaction Using Fe Supported over Activated Carbon" Catalysts 11, no. 12: 1456. https://doi.org/10.3390/catal11121456
APA StyleSamir, B., Bakhta, S., Bouazizi, N., Sadaoui, Z., Allalou, O., Le Derf, F., & Vieillard, J. (2021). TBO Degradation by Heterogeneous Fenton-like Reaction Using Fe Supported over Activated Carbon. Catalysts, 11(12), 1456. https://doi.org/10.3390/catal11121456






