Abstract
In the current study, we analysed the influence of metallic underlayers on carbon-doped TiO2 films for RhB decomposition and Salmonella typhimurium inactivation under visible-light irradiation. All the experiments were divided into two parts. First, layered M/C-doped-TiO2 film structures (M = Ni, Nb, Cu) were prepared by magnetron sputtering technique on borosilicate glass substrates in the two-step deposition process. The influence of metal underlayer on the formation of the carbon-doped TiO2 films was characterised by X-ray diffractometer, scanning electron microscope, and atomic force microscope. The comparison between the visible-light assisted photocatalytic activity of M/C-doped TiO2 structures was performed by the photocatalytic bleaching tests of Rhodamine B dye aqueous solution. The best photocatalytic performance was observed for Ni/C-doped-TiO2 film combination. During the second part of the study, the Ni/C-doped-TiO2 film combination was deposited on high-density polyethylene beads which were selected as a floating substrate. The morphology and surface chemical analyses of the floating photocatalyst were performed. The viability and membrane permeability of Salmonella typhimurium were tested in cycling experiments under UV-B and visible-light irradiation. Three consecutive photocatalytic treatments of fresh bacteria suspensions with the same set of floating photocatalyst showed promising results, as after the third 1 h-long treatment bacteria viability was still reduced by 90% and 50% for UV-B and visible-light irradiation, respectively. The membrane permeability and ethidium fluorescence results suggest that Ni underlayer might have direct and indirect effect on the bacteria inactivation process. Additionally, relatively low loss of the photocatalyst efficiency suggests that floating C-doped TiO2 photocatalyst with the Ni underlayer might be seen as the possible solution for the used photocatalyst recovery issue.
1. Introduction
In recent years, global society has expressed their concern regarding freshwater availability for drinking, food growing and sustaining life. One of the problems is that clean freshwater makes up only a small fraction of all water resources and they are unevenly distributed across the globe. Following the data presented by the World Health Organization, more than 2 billion people lack sanitation services and a huge part of them live in an area plagued by freshwater shortages [1]. The increasing scarcity of clean freshwater is the result of industrial growth, advanced manufacturing, increased water contamination and climate change as well as poor water treatment and insufficient disinfection [2]. The quality of freshwater can be jeopardised by many types of harmful pollutants, including toxic pesticides, industrial by-products, effluents, pharmaceuticals, human pathogens and so forth [3].
Conventional water treatment technologies, such as biological treatment, chemical precipitation, membrane filtration, and chlorination have been proposed in order to solve this issue [1,2,4]. However, in some cases these techniques lack effectiveness, particularly in the decomposition of microorganisms (bacteria, viruses, fungi, and etc.) which can pose serious health issues [3,5]. For example, Gram-negative pathogens such as Salmonella bacteria can cause food-borne infections (gastroenteritis, fever, abdominal discomfort, nausea and diarrhoea) [6]. Concerns surrounding these bacteria are fairly high, because several studies have revealed that they are usually present in wastewater treatment plants [7,8]. Therefore, the development of alternative water disinfection methods such as advanced oxidation process (AOP) has a huge interest [9,10]. AOPs are characterised by the in-situ generation of reactive oxygen species (ROS), especially superoxide anion (•O2−) and hydroxyl radicals (•OH) which act as powerful oxidising agents capable of decomposing various organic compounds [2]. The oxidation power of hydroxyl radicals (oxidation potential 2.8 V) is higher than chlorine (oxidation potential 1.36 V) and even ozone (oxidation potential 2.07 V) [1]. There are various types of AOPs including the Fenton process [11], electrochemical oxidation [12], photocatalysis [13], electrical discharge [14], catalytic ozonation [15,16], sonolysis [17], or wet air oxidation [18]. All of the mentioned AOPs have their own advantages as well as disadvantages, e.g., the Fenton process is known as an efficient, non-toxic and cost-effective process. However, some of disadvantages are chemical instability, low optimum reaction pH (2–4), subsequent separation and post-treatment of a large amount of sludge [19]. Meanwhile, photocatalysis is recognized as one of the most promising environmental purification process due to its wide application areas, uses earth abundant materials, reusability, possible complete degradation, eco-friendly, does not involve sludge formation, etc. [2,20,21,22]. Moreover, versatility of the photocatalysis can be emphasized by huge amount of publications, where scientists trying to adopt this technique using different catalysts (e.g., TiO2, ZnO, Ag2O, GO, etc. [23,24,25]), dyes (RhB, PhB, MB, MY, etc. [26,27,28]) or light irradiation (UV-C, UV-B, visible-light, etc. [29,30,31]). Therefore, photocatalytic water disinfection is one of the mostly researched AOPs methods. It utilises combinations of light and specific photocatalysts (usually metal oxide semiconductors). Photocatalytic water treatment is considered to be a promising and sustainable process. During photocatalyst irradiation, photoinduced electrons in the conduction band and holes in the valence band move to the surface of the semiconductor and react with oxygen and water molecules which leads to the production superoxide and hydroxyl radicals, respectively (generation of other ROS is also possible) [2,3]. Among many bacteria, Salmonella typhimurium as waterborne pathogen is frequently chosen as an indicator for performance of photocatalytic material [32,33,34]. Garvey et al. achieved optimal inactivation of Salmonella after 2 h exposure to UV light with photocatalyst [35]. Pino-Sandoval et al. showed promising results of the photocatalyst efficiency under solar radiation in the inactivation experiments of Escherichia coli and Salmonella typhimurium as strains found in wastewater [36]. Meanwhile, Gayan et al. showed that some strains of S. typhimurium may be resistant to simultaneous treatment of UV-C light and mild temperatures [37].
Among a variety of metal oxides (ZnO, WO3, Fe2O3, Bi2WO6, Nb2O5, etc.), TiO2 in the anatase crystalline structure is by far the most extensively investigated photocatalyst [2,38,39]. TiO2 is an attractive material due to its low toxicity, high physical and chemical stability and low cost [39]. However, due to its wide band gap (3.2 eV for anatase), ultraviolet light is necessary for the excitation of electron-hole pairs [40]. Furthermore, fast electron-hole pairs recombination results in suppressed photocatalytic efficiency [40]. To overcome these drawbacks and improve photocatalytic performance under visible-light a number of physical and chemical techniques have been researched. Up to now, most of the researcher was focused on the investigation of metal/nonmetal doping, TiO2 combining with other metal oxides [41,42,43,44], modifying particle size [45] or the influence of different supports (glass, ceramics, polymers, thin films, activated carbon, sand, paper, wood, etc.) [10,46,47,48]. Various synthesis methods, including sol-gel [49], precipitation [50], hydrothermal [51], wet impregnation [52], thermal spraying [53], magnetron sputtering [54], and electrochemical methods [55] have been applied. In comparison to various films and coatings, due to the huge specific surface area, TiO2 powder demonstrate a superior photocatalytic performance [56]. Nevertheless, the aggregation of particles and the difficulties involved in extracting them from the water limit practical application of powders [4].
Recently floating photocatalytic materials have become a highly discussed topic. Lightweight materials (such as polymer, perlite, vermiculite, and glass beads) can be exploited as floating supports for photocatalysts [20]. Such systems have several advantages and most importantly, they enable the easy recovery of photocatalyst from the treated water.
From the other point of view, TiO2 immobilisation on metal substrates also remains a current method. The literature suggests that transition metals and even noble metals can reduce the charge carriers’ recombination rate [57,58,59]. A review by Lee and Park has summarised that noble metals can inhibit electron-hole recombination due to the low Fermi level of metals [2]. The other research reported that zinc-doped TiO2 has a red shift of absorption edge towards the visible-light region [60]. The same results were obtained with Ni doping by Niishiro et al. [61]. A few studies have demonstrated the improved photocatalytic properties of TiO2 immobilised on the porous nickel [47,62], stainless steel [46] and aluminium alloy [63]. However, investigations of TiO2 films immobilised onto metal supports are still not fully covered. There is a lack of information on photocatalyst deposited on nanocrystalline metallic films. In particular, floating substrates covered by subsidiary metal layers could be an interesting subject for further studies.
In the current study, the metal underlayer effect upon the photocatalytic activity and bactericidal effect of carbon-doped TiO2 was analysed. Our previous studies disclosed that C-doped TiO2 as well as HDPE beads is a suitable combination for S. Typhimurium inactivation under UV-B light irradiation [64,65]. Here, we step further by improving our previous approach by adopting metallic underlayers for better dye decomposition as well as bacteria inactivation under visible-light irradiation. To the extent of our knowledge, this is the first time that the floating photocatalyst covered by C-doped TiO2 with metallic underlayer has been applied to S. Typhimurium bacteria inactivation under visible-light irradiation.
Here, the magnetron sputtering technique was used for the deposition of both the metal underlayer and carbon-doped TiO2. Doping with carbon is straightforward to synthesise a photocatalyst with improved visible light photocatalytic activity [65]. Magnetron sputtering was chosen as a technique suitable for temperature-sensitive materials. The first part of this work reveals the photocatalytic activity, structure and morphology of carbon-doped TiO2 films deposited on borosilicate glass substrates which were pre-covered by metal underlayer. Three different metals (copper, nickel and niobium) were compared. In the second part of this study, carbon-doped anatase TiO2 was deposited onto high-density polyethylene (HDPE) beads pre-covered with a nickel underlayer. HDPE beads were selected as the floating support due to good durability, easy extraction from water, and recycling. The produced floating photocatalyst was cyclically tested in the inactivation of bacteria (Salmonella typhimurium) under UV-B and visible-light irradiation.
2. Results
The results of this article are divided into two parts. The first part presents the analysis of C-doped TiO2 films deposited on glass substrates with Cu, Nb or Ni underlayers (or without underlayer). This part covers the analysis of films’ structure, morphology, cross-section and roughness, as well as the photocatalytic decomposition of RhB using VL irradiation. Based on the obtained results, the most promising C-doped TiO2 film and Ni metal underlayer combination was selected and applied for the formation of floating photocatalyst. Accordingly, the second part of the results involves analysis of C-doped TiO2 films with Ni underlayer deposited on HDPE beads by analysing surface morphology, elemental mapping, surface elemental composition and chemical bond structure. Additionally, experiments with a practical value regarding Salmonella typhimurium inactivation under UV-B and VL irradiation were performed. These experiments involve general bacteria inactivation, cycled inactivation, membrane permeability and ethidium fluorescence measurement.
2.1. Results of C-Doped TiO2 Films Deposited on Glass Substrates with Cu, Nb or Ni Underlayers
2.1.1. Structural Analysis
The structural analysis of C-doped TiO2 films deposited on glass substrates with Cu, Nb or Ni underlayers (or without underlayer) was performed by the XRD technique. Figure 1 shows XRD results, where patterns represent C-doped TiO2 films: (a) without underlayer, (b) with Cu underlayer, (c) with Nb underlayer and (d) with Ni underlayer. XRD analysis demonstrated that all samples had two TiO2 phases: anatase (tetragonal, I41/amd, JCPDS No. 21-1272), which is the dominant phase, and rutile phase (tetragonal, P42/mnm, JCPDS No. 086-0148) with weak peaks. As it was presented by other authors, heterojunction between anatase and rutile TiO2 phases, when anatase covers the dominant part of whole TiO2 film, has synergistic effects and might improve its photocatalytic activity. This can be explained by a combination of different anatase and rutile band gaps, where electrons can jump from anatase to rutile conduction bands and electron-hole recombination process might be slowed down [20,66].
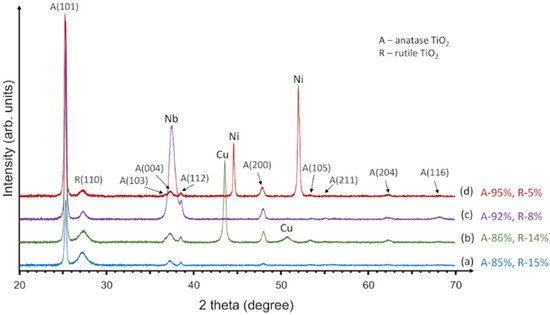
Figure 1.
XRD patterns of (a) the as deposited carbon-doped TiO2 film and carbon-doped TiO2 films deposited on (b) the Cu underlayer, (c) Nb underlayer and (d) Ni underlayer.
The percentage amount of anatase and rutile phases was evaluated and presented in Figure 1 (right side). The highest amount of anatase (95%) was found in C-doped TiO2 films with Ni underlayer while C-doped TiO2 films without underlayer showed the smallest amount of anatase (86%). The rutile phase varied from 5 to 15% which mainly depended on the area of crystallographic orientation (1 1 0). Kleiman et al. used cathodic arc for synthesis of TiO2 film which showed the crystalline anatase from 80% to 100% and rutile from 7% to 20% [67].
Further analysis revealed that all applied underlayers (Cu, Nb and Ni) had crystalline structure. The XRD pattern of the photocatalyst without an underlayer (Figure 1a) had less intensive anatase (1 0 1) peak compared to any other sample with an underlayer. This result is in agreement with other authors’ works, which showed that metallic substrate or underlayer might have positive influence on crystalline TiO2 structure formation [62,63,68]. Quantitative analysis confirmed this suggestion by indicating the anatase phase crystallite sizes of 49 nm, 54 nm, 57 nm, and 55 nm for the sample without an underlayer and with the Cu, Nb, and Ni underlayers, respectively.
However, the majority of other authors generally calculated smaller crystallites of anatase. For example, the crystallite size of TiO2 deposited on various floating substrates varied from 12.2 nm to 21 nm [69,70,71,72,73]. Davidsdottir et al. deposited the TiO2 films on metallic substrate by DC magnetron sputtering which had a size of approximately 26 nm of crystallite size [63], as shown by XRD, while Gualdrón-Reyes et al. calculated approximately 17 nm after the TiO2 deposition on the stainless steel [74]. Similar crystallite sizes (11–35 nm) were obtained preparing TiO2 by sol gel method [36,75] or other chemically based methods (10–19) [39,76].
As it was presented in the methodology part, TiO2 deposition was implemented in conjunction with the carbon doping process. XRD analysis revealed that no peaks of any carbon containing phase (neither separate carbon) were observed and this allows to presume that carbon was fully integrated into TiO2 structure.
2.1.2. Morphology, Cross-Section and Roughness Analysis
The SEM views of the samples’ surfaces and cross-sections are presented in Figure 2. Image analysis revealed that C-doped TiO2 films with and without metal underlayers had slightly different surface morphology. The Sample without underlayer had relatively flat surface base with nano-scaled holes/defects, which can be seen in Figure 2a. The formation of underlayers prior to C-doped TiO2 films deposition invoked noticeable TiO2 morphology changes. Namely, the surfaces became noticeably grainier in comparison to the sample without underlayer. The estimated grain sizes for samples with Cu, Nb and Ni underlayer were approximately 180 nm, 120 nm and 100 nm, respectively. These results coincide with the AFM surface roughness results (Figure 3), which showed the lowest Ra value for the sample without underlayer (~2.1 nm), the highest for the Cu underlayer (~26.7 nm) and mediocre roughness values for the samples with Nb and Ni underlayers (5.0 nm and 6.1 nm, respectively). The cross-section images revealed that for all samples, the TiO2 film thickness was approximately 1500 nm. All C-doped TiO2 samples had columnar structure, which is inherent to crystalline TiO2 films [77,78].
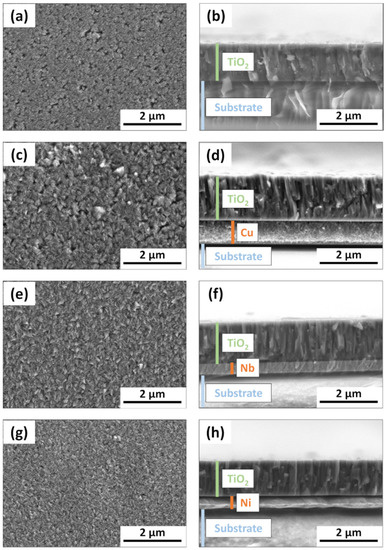
Figure 2.
SEM surface and cross-section views of C-doped TiO2 films deposited on various underlayers: (a,b) no underlayer; (c,d) Cu underlayer; (e,f) Nb underlayer; (g,h) Ni underlayer.
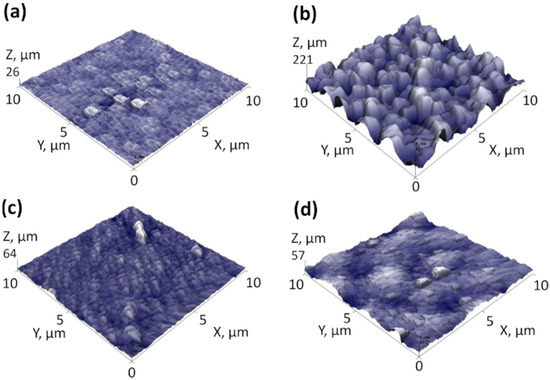
Figure 3.
AFM surface measurements of C-doped TiO2 films deposited on various underlayers: (a) C-doped TiO2 film without underlayer; (b) Cu underlayer; (c) Nb underlayer; (d) Ni underlayer.
2.1.3. Transmittance and Optical Band Gap
Pure TiO2 thin film was deposited on borosilicate glass substrate and compared to C-doped TiO2 film using transmittance and corresponding Tauc plots in order to understand their differences in optical parameters. Figure 4a showed that both of analysed samples optical transmittance is near 80–90% in visible light spectra, which is typical for TiO2 films [79,80]. Meanwhile, the slight shift to higher wavelength of C-doped TiO2 film can be seen in a range of about 350–400 nm. The Tauc plot calculations revealed the reduction of band gap comparing C-doped TiO2 and pure TiO2 films with the values of 3.16 eV and 3.22 eV, respectively (Figure 4b). These results suggest the possibility to adsorb higher amount of visible light spectra for C-doped TiO2 films. Since all the C-doped TiO2 films were formed using the same deposition conditions despite the type of underlayers (i.e., Cu, Nb or Ni), we presume that similar band gap reduction characteristics should be observed for the all samples as well. On the other hand, since all the samples with metallic underlayers showed zero-level transmittance characteristics, the band gap measurements weren’t performed.
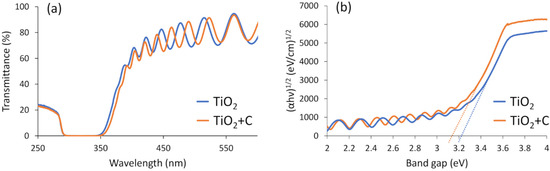
Figure 4.
Transmittance (a) and band gap (b) spectra of pure TiO2 and C-doped TiO2 films deposited on borosilicate glass substrates.
2.1.4. Photocatalysis: Decomposition of RhB Solution
The decomposition of the RhB solution was used as an indicator to identify the combination with the highest photocatalytic activity. Figure 5 displays graphs of photolysis and photocatalytic degradation of RhB solution versus the exposure time for each sample. Prior to the experiments, RhB photodegradation (photolysis) under VL without photocatalyst was evaluated. For photolysis, the value of the first-order reaction constant (k value) was approximately 4 × 10−4 min−1 (Figure 5a), while the adsorption measurements (without light) disclosed very negligible influence on the photocatalysis results (k values < 1 × 10−4 min−1) with all the samples. The results using a combination of VL and photocatalysts showed significantly larger k values. They were calculated at approximately 22 × 10−4 min−1, 15 × 10−4 min−1, 22 × 10−4 min−1, and 27 × 10−4 min−1 for C-doped TiO2 films without underlayer and with Cu, Nb and Ni underlayers, respectively. As it can be seen, samples with Cu and Nb underlayers have the same or even lower photocatalytic activity than C-doped TiO2 film without an underlayer. It is known, that some metal dopants can act as recombination centres of photogenerated electrons and holes. For example, despite the fact that Cu is recognised as one of the most promising dopants, an excessively high Cu concentration may even reduce the photocatalytic performance [38,74,81]. Still, the highest photoactivity was observed for the sample with Ni underlayer. As other authors showed, nickel might alter the TiO2 growth mode by inducing more anatase phase compared to rutile [82,83]. Moreover, as it was concluded by I. Ganesh et. al, if Ni is doped into TiO2 film, it can stimulate a redshift to the light-absorbing nature of TiO2. This improves the amount of absorbed energy from visible-light [84]. In our case, some of Ni atoms might diffuse to C-doped TiO2 film from an underlayer by affecting photocatalysts characteristics. Although for these samples elemental analysis in bulk was not performed, potential Ni diffusion through C-doped TiO2 film was observed by analysing floating photocatalyst (more details are provided in the following paragraphs).
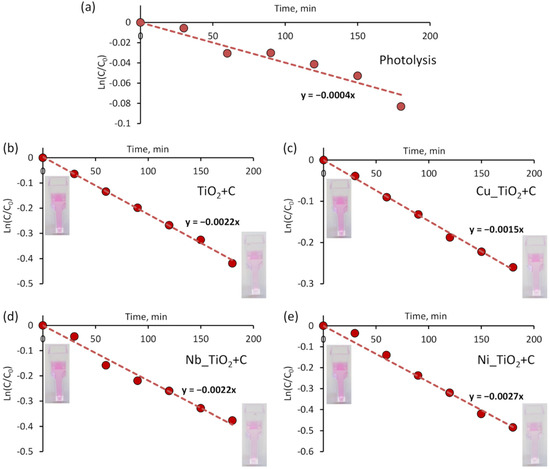
Figure 5.
Photocatalytic bleaching of Rhodamine B under VL light irradiation (a) without sample and using various C-doped TiO2 film underlayers; (b) no underlayer; (c) Cu underlayer; (d) Nb underlayer; and (e) Ni underlayer.
2.2. Results of C-Doped TiO2 Films Deposited on HDPE Beads with Ni Underlayer
2.2.1. Morphology and Elemental Mapping Analysis
SEM images and elemental mapping of floating photocatalyst granules (HDPE beads with Ni underlayer and C-doped TiO2 film) are presented in Figure 6. Surface morphology of such floating photocatalyst granules can be characterised by the presence of various surface defects, cracks and high roughness (Figure 6a and its insert). Such surface morphology is similar to the native HDPE bead structure. Therefore, it can be assumed that in general deposited Ni underlayer together with C-doped TiO2 film reasonably good repeat HDPE surface texture. These insights were confirmed by elemental mapping analysis, which revealed that all elements (i.e., Ni, O, Ti and C) were distributed relatively uniformly all over the surface of HDPE beads (Figure 6b–f). It is important to mention, that in this case, carbon is not only the dopant of TiO2 film, but it is the main element of HDPE bead (–(CH2-CH2)n–) as well as carbon-based sticker, which was used for the immobilization of HDPE beads during SEM/EDS analysis.
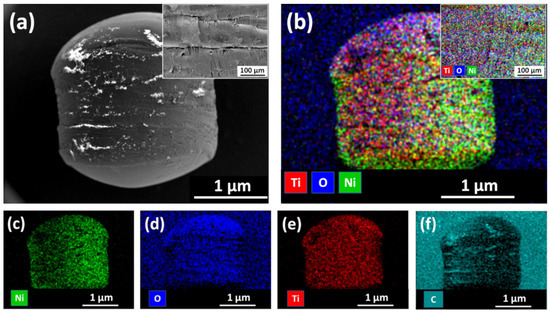
Figure 6.
SEM image and EDS elemental mapping results of C-doped TiO2 films with Ni underlayer deposited on HDPE beads: (a) surface morphology (insert—the central zone of the sample at higher magnification); (b) combined mapping of Ti, O, and Ni elements (insert—sample mapping at higher magnification); (c) Ni mapping results; (d) O mapping results; (e) Ti mapping results; (f) C mapping results.
2.2.2. Chemical Bond and Elemental Composition Analysis
XPS analysis was performed to understand the elemental composition, its distribution in bulk and the chemical bonds of C-doped TiO2 films with an Ni underlayer deposited on HDPE beads. Figure 7a presents XPS survey spectra, while 7b shows elemental distribution after a different sputtering time. The results disclosed that the top layer of the analysed structure consists of three main elements (C—44.5 at. %, O—41.2 at. % and Ti—12.2 at. %) and a small amount of Ni (2.1 at. %) without any additional elements or impurities. Further analysis revealed clear changes of C and Ni concentrations through the bulk: concentration of Ni increased till approximately 11 at. %, while C concentration decreased till 33 at. %. Concentrations of O and Ti remained relatively stable with approximately ±2 at. % variation. These values remained relatively stable through the C-doped TiO2 film bulk. The obtained results suggest several assumptions: (i) our suggested deposition method is suitable for complex photocatalyst structure formation without unwanted impurities; (ii) Ni from the underlayer penetrated through the film and mixed up with C-doped TiO2; (iii) the observed concentration of carbon was much higher than expected. A similar C-doped TiO2 film technique was applied in our previous study, where 5–7 at. % of carbon was incorporated [65]. We presume that due to native HDPE beads surface morphology, deposited Ni might be penetrated or mixed with beads surface layers (i.e., polyethylene). A similar process might occur when the C-doped TiO2 film was deposited. Therefore, we suggest that a double saturation process with carbon (from deposition process and surface layers of HDPE beads) was observed.
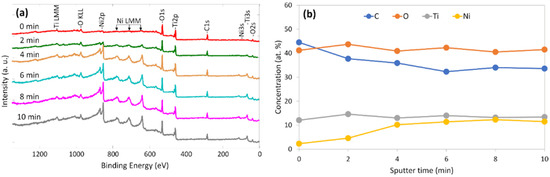
Figure 7.
XPS analysis of C-doped TiO2 film with the Ni underlayer deposited on HDPE beads: (a) survey spectra and (b) distribution of elemental composition after different sputtering time.
The chemical bond analysis of the C-doped TiO2 film with the Ni underlayer deposited on HDPE beads was performed by the fitting of four different areas of the XPS spectrum (Figure 8) including C 1 s, O 1 s, Ti 2p and Ni 2p. It is important to note that chemical bond investigation was implemented after 10 min of sputtering by 2 kV Ar+ ions. Prior to the analysis, all spectra were aligned by placing C 1 s peak of C–C bond at 284.8 eV. The deconvolution of Ti 2p3/2 core level spectra revealed the existence of three components observed at 456.2 eV, 458.0 eV and 458.9 eV, which can be attributed to Ti2+, Ti3+, Ti4+ respectively (Figure 8a). As emphasised in other articles, the appearance of Ti2+ and Ti3+ is generally attributed to the sputtering of Ar ions prior the XPS measurement [85,86]. Meanwhile, the existence of TiO2 compound was confirmed by Ti4+ binding energy (458.9 eV) and the separation between this value and its doublet (Δ = 5.7 eV), while both values correspond to the theoretical TiO2 binding energies [87].
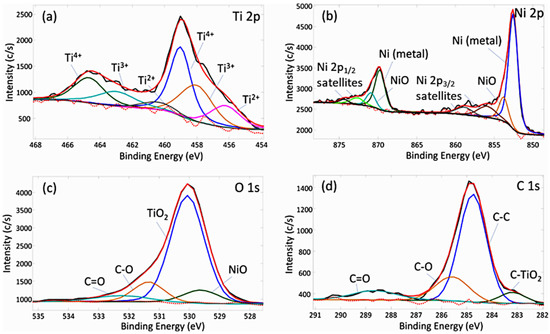
Figure 8.
XPS chemical bond analysis: (a) Ti 2p, (b) Ni 2p, (c) O 1 s and (d) C 1 s spectra.
Further analysis of the Ni 2p core level spectra disclosed two types of chemical structures: metallic Ni (852.6 eV for Ni 2p3/2 and 869.7 for Ni 2p1/2) and oxidised Ni–O (853.8 eV for Ni 2p3/2 and 870.9 for Ni 2p1/2) bonds (Figure 7b). Other observed peaks were ascribed to satellites, which are inherent to Ni 2p spectra [88,89]. The analysis of surface area ratio revealed that metallic Ni peaks make up approximately 78%, while NiO compose 22% of Ni 2p spectra area.
The existence of both oxygen-based chemical bonds (i.e. NiO and TiO2) was confirmed by analysing O 1 s spectra at binding energies of 529.5 eV and 530.0 eV, which fully agreed with theoretical values. Additional two compounds were fitted at 531.4 eV and 532.3 eV suggesting the presence of C–O and C=O chemical bonds as well as adsorption of moisture (Figure 8c). The same carbon-oxygen compounds were observed by the fitting of C 1 s spectra, which in total consists of four components: C–TiO2 (283.2 eV), C–C (284.8 eV), C–O (285.6 eV) and C=O (288.6 eV), with area contributions of approximately 6.4%, 65.5%, 18.2% and 9.9%, respectively (Figure 8d). These results suggest that only a small part of observed carbon (6.4%) has a direct influence on TiO2 states. The existence of C-doped TiO2 chemical bonds can be confirmed by other authors as well, which showed that the occurrence of a peak at 282–283 eV in C 1 s spectra can be attributed to doping process of anionic carbon species into TiO2 lattice [85,90].
2.2.3. Salmonella typhimurium Bacteria Inactivation: Viability, Membrane Permeability and Ethidium Fluorescence Measurement
Our previous study [54] showed that ROS generated in media cause additional intracellular oxidative stress to bacteria. Due to this, we first carried out control measurements with C-doped TiO2 with Ni underlayer deposited on HDPE beads, in order to investigate whether bacteria cells generate intracellular reactive oxygen species (ROS) after the treatment by these beads (Figure 9). Three types of ROS were fixed: •OH, •ROO, H2O2. After 1 h of treatment, the control as well as UV-B irradiation alone showed a relatively low intensity of DCFH-DA fluorescence with the growth tendencies from 3000 to 6000 or from 10,000 to 17,500 units, respectively during the 15 min of measurement. The fluorescence measurement using the floating photocatalyst (C-doped TiO2 with Ni underlayer deposited on HDPE beads) together with UV-B irradiation showed highly increased fluorescence in the range between 43,280 and 62,250 units, which is approximately four times higher compared to UV-B alone. This result suggests that C-doped TiO2 with Ni underlayer deposited on HDPE beads formed more ROS in the medium which caused high oxidative stress to bacteria and as a result, a high amount of intracellular ROS (•OH, •ROO, H2O2) were formed.
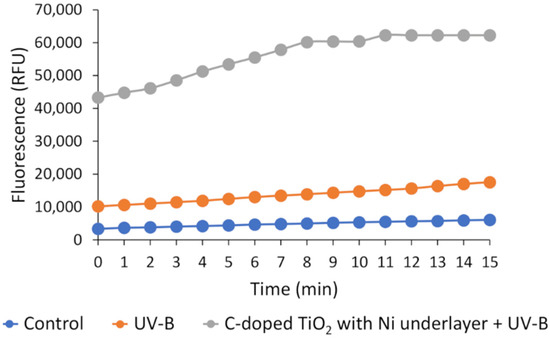
Figure 9.
Intracellular ROS formation in S. typhimurium bacteria after the treatment of floating photocatalyst.
The effect on viability and membrane integrity of Salmonella typhimurium cells by photocatalyst granules (C-doped TiO2 with an Ni underlayer deposited on HDPE beads) activated by UV-B (Figure 10) and VL (Figure 11) was investigated. Three consecutive photocatalytic treatment experiments with the same set of photocatalyst granules (fresh bacteria suspensions were used for each treatment run) were performed to evaluate the changes in efficiency. UV-B light acting in isolation decreased the viability of S. typhimurium cells by approximately 60%. The highest reduction of bacteria viability (approximately 95%) was observed during the first run using photocatalyst granules and UV-B light. After this test, only 5% of bacteria were able to form colonies in comparison to the control group. After the second run, the impact of treatment with photocatalyst granules and UV-B light was weaker and the viability of the cells was just slightly lower than the UV-B light treated group. The viability was 10% after the third run. Thus, the effect of UV-B activated photocatalyst granules on the viability of S. typhimurium cells was not constant. We assume that one of the factors which could significantly influence the results (namely the second run) was how neatly we manage to wash photocatalyst granules by water between the consecutive runs. The second factor, which could have higher effect for higher number of the tests, is partial tearing of the film and consecutive fractional loss of photocatalytic material.
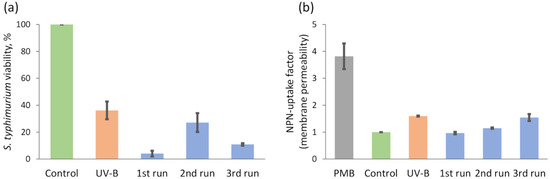
Figure 10.
S. typhimurium bacteria photocatalytic inactivation experiments with UV-B light irradiation: (a) viability and (b) membrane permeability.
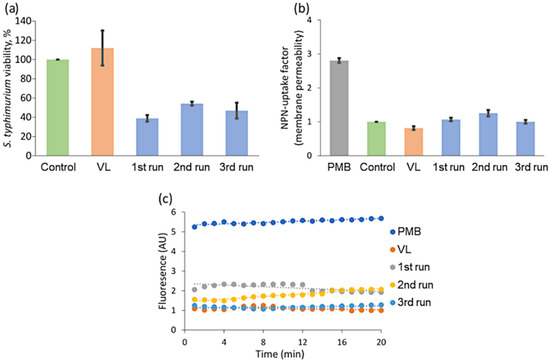
Figure 11.
S. typhimurium bacteria photocatalytic inactivation experiments with visible-light irradiation: (a) viability, (b) membrane permeability, and (c) ethidium fluorescence.
On the other hand, despite the fluctuations in bacteria viability, the effect of photocatalytic treatment on membrane permeability was more coherent. For these tests in addition to two standard control groups (untreated bacteria and UV-B light treated bacteria), one additional control group was measured. More specifically, Polymixin B (PMB) was used as control of dead cells with a fully permeabilized membrane. The permeability of the bacteria membrane was decreased after the first and the second run in comparison with UV-B treated group because granules partially shield the bacteria from UV-B light. But after the third run, membrane permeability factor was 1.5 times higher in comparison with a control group and almost did not differ from the UV-B group. It should be noted, that all these experiments were conducted using the same sets with the same number of photocatalyst granules, therefore shielding effect should be the same.
Summing up, the obtained results showed that membrane permeability was not as high as possible. The viability of cell was mostly reduced after the first run, whereas membrane permeability was affected stronger with each of the consecutive treatment run. This means that the outer membrane of bacteria is not the main target of photocatalytic treatment by C-doped TiO2 with Ni underlayer deposited on HDPE beads. Still the results with UV-B light showed that outer membrane integrity was slightly affected. According to this, we hypothesized that the granules could affect the efficiency of the efflux pumps in bacteria. Therefore, the efficiency of ethidium fluorescence was investigated after the treatment with granules activated by VL (Figure 11c) as well.
Photocatalytic treatment experiments with visible light source showed that VL alone had no effect on viability and membrane permeability of S. typhimurium cells (Figure 11a,b, respectively). The first run using a combination of VL and photocatalyst granules showed that viability of S. typhimurium cells decreases by 60%, which is fairly good result for 1 h VL assisted treatment. Interestingly, despite the decreased viability, the permeability of the outer membrane was the same as in a control group. We also observed, that during the first run ethidium accumulation was increased two times, but efflux pumps were still active because after 12 min efflux of ethidium was determined and fluorescence decreased. After second and third runs the viability of bacteria was approximately the same (≈50%), although after the second run NPN and ethidium accumulations were slightly higher—by 0.2 and 0.5 units respectively.
Among many different studies, various modifications and synthesis methods of TiO2 irradiated under solar or visible light can be found for complete S. typhimurium inactivation within 60–360 min [49,91,92]. E. Coli as other gram-negative bacteria are also investigated in experiments with TiO2 when in best scenarios reaching complete inactivation within 20–240 min using either UV or visible light [93,94,95]. However, it is difficult to compare the bacteria inactivation results with each other due to pretty different synthesis and measurement conditions (e.g., various chemical or physical photocatalyst synthesis route; films or powders; different light sources with their inherent parameters; different irradiation power; and bacteria cultivation or estimation).
The obtained results suggest, that during the first VL photocatalytic treatment run, we had the largest area of active centres and generated the highest amounts of reactive oxygen species. Strong photocatalytic activity eventually resulted in damages to the cells and the large reduction of bacteria viability. Similarly, during UV-B assisted photocatalysis the largest viability drop was also observed during the 1st run with “new” photocatalyst granules. On the other hand, the first VL photocatalytic treatment run did not show the high membrane damage, whereas decreased activity of efflux pumps showed inhibition of energy depended processes. The second run showed increased membrane permeability as well as efflux pumps activity and viability compared with the previous run. The third run showed generally similar result: the low amount of accumulated ethidium and intact membrane of bacteria, but viability only about 50%. These disparities suggest that we indicate different effects from photocatalytic treatment and photocatalyst granules themselves which cause different processes in bacteria cells. It is hard to determine the exact mechanisms, but we suppose that for the “new” photocatalyst granules the most important factor could be the generation of ROS. Whereas as C-doped TiO2 film and Ni underlayer material partially tears off and submicrometric particles enter into solution they can start to interact with bacteria cells. Accordingly, the decreased viability of bacteria could be observed due to the metal caused indirect oxidative stress [96]. For example, it is possible that during the third run we registered increased efflux pumps activity due to the partial tear off of nickel, because it causes the overexpression of these pumps. Due to the pumps characteristic to extrude metal ions actively, higher accretion of toxic by-product inside the bacterial cells can happen [96,97]. This process could have an influence on the decrement of bacteria viability too.
3. Methodology
3.1. Thin Films Deposition
3.1.1. C-Doped TiO2 Films Deposition on Glass Substrates with Various Underlayers
The borosilicate discs (D263 glass, SCHOTT) with a diameter of 30 mm were selected as substrates for various underlayer and C-doped TiO2 films deposition. A custom modified physical vapour deposition system (PVD-75, Kurt J. Lesker Company, Jefferson Hills, PA, USA) was used for a film deposition process. Prior to C-doped TiO2 films formation, Cu, Nb and Ni underlayers were deposited on borosilicate glass substrates using a 76 mm diameter unbalanced magnetron. The main deposition parameters were: (i) pressure—6 × 10−3 mbar; (ii) Ar gas purity—99.99%; (iii) Cu, Nb and Ni target purity—99.99%; (iv) magnetron operated by direct current (DC) power supply (170 W for Cu, 200 W for Nb, and 170 W for Ni). Due to lower Nb deposition rate, it was decided to increase DC power (200 W) in order to obtain similar deposition rates in all cases. After underlayers formation, C-doped TiO2 films were deposited using the same magnetron sputtering system. In this case, a 99.99% purity Ti target was used with four graphite pieces, which were attached to the most intensive sputtering region of Ti disc. The carbon-covered area on the Ti disc was approximately 1 cm2 for each piece. Pressure during C-doped TiO2 film formation was kept constant at 6 × 10−3 mbar using Ar-O2 gas mixture (mass flow controller (MFC) flow ratio 5:1). The deposition process was maintained by a pulsed DC power supply using 300 W power. The deposition time was 180 min, while distance the between Ti target and substrate was 4 cm.
3.1.2. C-Doped TiO2 Films Deposition on Floating Substrate with Ni Underlayer
As it is shown in the results part, films deposited onto borosilicate glass substrates with Ni underlayer showed the most promising photocatalytic performance compared to samples with Cu or Nb underlayers. Therefore, Ni was chosen for underlayer formation in the second step. High-density polyethylene beads (HDPE, obtained from GoodFellow, Huntingdon, England; nominal bead size 2–4 mm, density 0.95 g/cm3) were selected as substrate due to its proper characteristics for a floating photocatalyst application: high durability, buoyancy, cheapness, and size of beads is appropriate for their extraction from the water after photocatalysis process.
The formation of Ni underlayer was performed in the same way as it was explained in Section 3.1.1, except that HDPE beads were used as substrate instead of borosilicate discs. But the attempt to use the same C-doped TiO2 films deposition set-up with HDPE beads covered by Ni underlayer has dealt with an issue. Namely, during C-doped TiO2 films deposition process HDPE beads eventually started to melt, although substrate holder temperature implicitly (based on ex-situ assessment) did not reach the relatively low HDPE beads melting point, which is approximately 130 °C.
Throughout analysis confirmed that HDPE beads remain stable during Cu, Nb, and Ni formation, therefore the melting issue was only actual for the step of deposition of C-doped TiO2 films. The reduction of deposition power and/or increase of the distance between sample and cathode were capable to solve the melting issue. But these changes invoked the formation of amorphous TiO2 which is known for the lower photocatalytic activity than anatase phase. While looking for the solution, it was decided to apply slightly different experimental set-up, which was successfully used in our previous work [65] of anatase phase C-doped TiO2 film deposition on uncovered HDPE beads. The alternative set-up used slightly larger magnetron (95 mm diameter, 300 W pulsed DC power) that was placed at 10 cm distance from the HDPE beads. The more detailed description of this set-up is provided at [65].
3.2. Characterization
Samples with and without various underlayers were characterized by X-ray diffractometer (XRD, Bruker D8, Hamburg, Germany) operating with Cu Kα radiation (λ = 0.15406 nm) in the 2θ range between 20° and 70° to study phase structure of deposited C-doped TiO2 films. The crystallite sizes were calculated by Topas 6.0 software based on Scherrer equation [98] with Lorentzian convolution. Measurements of surface morphology, as well as cross-section imaging, were performed using a scanning electron microscope (SEM, Hitachi S-3400N, Tokyo, Japan). X-ray energy dispersive spectroscope (EDS, Bruker Quad 5040, Hamburg, Germany) was used for the elemental mapping analysis. Surface roughness was evaluated by Atomic Force Microscope (AFM, NT-206 Microtestmachine, Gomel, Belarus). Chemical bond analysis was evaluated by X-ray photoelectron spectroscope (XPS, PHI 5000 Versaprobe, Boston, MA, USA). The principal settings of XPS measurements were: monochromated 1486.6 eV Al radiation, 12.5 W beam power, 50 μm beam size and 45° measurement angle. Measurement of XPS depth profile was implemented using gentle etching process with 2 kV Ar+ ions rastered over 2 × 2 mm area. The optical transmittances were evaluated using ultraviolet-visible (UV-VIS) spectrophotometer (Jasco V-650, Tokyo, Japan). Calculations of the optical band gap were implemented using Tauc plot (the following procedure described elsewhere [99]).
3.3. Photocatalysis
The evaluation of the photocatalytic performance of C-doped TiO2 films with Cu, Nb or Ni underlayers (and without underlayer) was assessed by photocatalytic decomposition of Rhodamine B (RhB) in aqueous solution using visible-light (VL) irradiation (Solis-3C, 5700 K, Thorlabs, Dachau, Germany). The irradiation intensity at the surface of the sample was approximately 65 mW/cm2 (measured with Thorlabs PM16-401 Power meter, Dachau, Germany). 10 mL of 10 mg/L concentration aqueous RhB solution was used for the photocatalysis test. The distance between lamp and the top of the solution was 23 cm. 500 rpm magnetic stirrer was used to ensure RhB solution mixing process. Before the experiments, the prepared reactor with sample and RhB solution was kept for 1 h in the dark to ensure the adsorption equilibrium. The solution temperature was kept constant at 20 °C using the Peltier element during the photocatalysis experiment. No addition of air flux was used during the experiments. The RhB solution concentration changes were measured by UV-VIS spectrophotometer (Jasco V-650, Tokyo, Japan) for evaluation of samples photocatalytic activity.
3.4. Bacterial Inactivation
3.4.1. Bacteria Cultivation
Gram-negative Salmonella enterica ser. typhimurium SL1344 bacteria were cultivated as described in our previous studies [100] with one modification: diluted overnight culture was grown to OD600 of 0.7.
3.4.2. Preparation of Bacteria Suspensions
The procedure of photocatalytic treatment in thermostated vessels was described in [65]. After 60 min of experiment, 100 μL of suspension were used for viability assay. The remainder parts of suspensions from the vessels were centrifuged at 3000× g for 5 min at 4 °C (Heraeus™ Megafuge™ 16R, Thermo Scientific, Bremen, Germany). The pellet was collected with NaPi buffer (100 mM, pH 8) to obtain 3.6 × 109 cfu/mL (OD600 of 12), divided in two parts and further used for NPN uptake factor assay for membrane permeability determination (as described in [64] and for ethidium accumulation assay. 1.2 µM of EtBr was added into cell suspension then mixed and poured into wells to obtain a concentration 0.6 OD600 in an each well. Fluorescence was measured immediately.
3.4.3. Fluorescence Measurements of DCFH-DA
Preparation of dichlorodihydrofluorescein diacetate (DCFH-DA, Sigma-Aldrich, Germany) solution for measurements was described in our previous study [54]. DCFH-DA is an indicator of intracellular hydroxyl radicals (•OH), peroxyl (•ROO) and hydrogen peroxide (H2O2) radicals. After 1 h of treatment, 100 μL of bacteria suspensions (as described in Section 3.4.2) were mixed and transferred into 96-well flat-bottom black plates. DCFH was added to obtain concentration of 10 µM. Relative intensity of the fluorescence was measured in “TECAN GENios Pro” (Männedorf, Switzeland) plate reader (excitation 492 nm, emission 535 nm) for 15 min.
3.4.4. Viability Assay
Viability of S. typhimurium cells was determined by spread-plate technique with sterile glass beads, named “Copacabana Method” [101]. Bacteria suspension was diluted 2500 times before plating then aliquots of 100 μL were spread on LB-Agar (Roth, Germany). The number of grown colonies was counted after 22–24 h of incubation at 37 °C. The treatment time of bacteria viability experiment was 60 min using UV-B (PL-S 9W/01/2p 1CT, Philips, Amsterdam, the Netherlands) and VL (Solis-3C, 5700 K, Thorlabs, Dachau, Germany) irradiation. The intensity was 5 mW/cm2 for UV-B and 65 mW/cm2 for VL irradiation, respectively.
3.4.5. Ethidium Accumulation Assay
Ethidium bromide is a lipophilic dye which has a very weak fluorescence in aqueous solution (or buffer). As concentration of this dye in the cytoplasm/periplasm of viable cells increases (mostly due to intercalating of the dye to cellular components, like DNA), intensity of fluorescence rises [102]. Ethidium is an indicator of efflux pumps. Higher accumulation of indicatory compound shows inhibition of active transport. In S. typhimurium RND-type efflux pumps are the most common. RND family pumps are proton motive force-dependent transporters which generally occur as tripartite efflux complexes that span the cytoplasmic and outer membranes, thus providing a mechanism to directly extrude antimicrobial substrates out of the cell [103]. The proton motive force (pmf) can be lost after the disruption of membrane integrity [104]. Due to this, measurements of ethidium fluorescence could be useful to determine the mechanisms of photocatalytic Ni-TiO2 effect on S. typhimurium bacteria.
Fluorescence measurements were done in 96-well flat-bottom black plates filled with TRIS buffer 100 mM with 0.1% of glucose pH 8. The samples of bacteria suspension (described in Section 3.4.1) were mixed and inoculated into plates. The volume of bacteria suspension with buffer was 200 µL per well at the time of measurement. Relative intensity of the fluorescence (excitation 535 nm, emission 590 nm) was monitored in “TECAN GENios ProTM” (Tecan, Männedorf, Switzeland) plate reader for 20 min, thermostatting the plate at 37 °C. The plate was shaken 5 s before each registration point. The ratio of fluorescence with control group is presented in a part of results.
4. Conclusions
In this study, the effect of different metal underlayers (Cu, Nb and Ni) on the photocatalytic activity and bactericidal effect of carbon-doped TiO2 films was investigated. First, borosilicate glass substrates pre-covered by corresponding thin metal layers were used for the deposition of carbon-doped TiO2. In all cases, the XRD analysis confirmed the formation of nanocrystalline anatase phase with predominant (101) orientation. A small peak of rutile phase was detected in all samples as well. In general, SEM analysis showed a relatively flat surface with the formation of columnar TiO2 structures. However, it was noticed that surface morphology of carbon-doped TiO2 films depended on the used metal underlayer.
In the photocatalytic test of RhB solution bleaching, the sample with Ni underlayer showed the highest efficiency (k value 27 × 10−4 min−1). Consequently, the production of floating photocatalyst started with the deposition of Ni underlayer on the surface of HDPE beads. Elemental mapping results showed that Ni and carbon-doped TiO2 distributed evenly over the surface of HDPE beads. XPS results revealed that Ni from the underlayer penetrated through the film and mixed up with C and TiO2. Moreover, the existence of C-TiO2 chemical bonds was confirmed by XPS fitting.
Inactivation cycling experiments of S. typhimurium treatment by floating photocatalyst under UV-B or VL irradiation, indicated that the largest inactivation efficiency of 95% and 60%, respectively, is obtained with the un-used photocatalyst granules. However, after the 3rd consecutive treatment run with the same set of photocatalyst granules under UV-B and VL irradiation bacteria viability still can be reduced by 90% and 50%, respectively. The loss of the photocatalyst efficiency is relatively low and this allows to suggest that such floating C-doped TiO2 photocatalyst with Ni underlayer at least partially can be seen as the possible solution for the used photocatalyst recovery issue.
Author Contributions
Conceptualization and methodology, S.V. and M.U.; investigation, S.S., S.T., S.V., M.U. and E.D.; writing—original draft preparation, S.V., M.U. and S.S.; writing—review and editing, S.V. and M.U.; visualization, S.T., M.U. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the European Social Fund according to the activity “Improvement of researchers” qualification by implementing world-class R&D projects of Measure No. 09.3.3-LMT-K-712, project “Investigation of the application of TiO2 and ZnO for the visible light assisted photocatalytical disinfection of the biologically contaminated water” (09.3.3-LMT-K-712-01-0175).
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors express gratitude for Darius Milcius and Albinas Svirskis for their input in material synthesis; Martynas Lelis and Rimantas Daugelavicius for their input in conceptualization and supervision; Mindaugas Aikas, Rolandas Uscila, Neringa Kuliesiene and Deimante Vasiliauske for their valuable input in preparation and characterization of the samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- García-Espinoza, J.D.; Robles, I.; Durán-Moreno, A.; Godínez, L.A. Photo-assisted electrochemical advanced oxidation processes for the disinfection of aqueous solutions: A review. Chemosphere 2021, 274, 129957. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Paumo, H.K.; Dalhatou, S.; Katata-Seru, L.M.; Kamdem, B.P.; Tijani, J.O.; Vishwanathan, V.; Kane, A.; Bahadur, I. TiO2 assisted photocatalysts for degradation of emerging organic pollutants in water and wastewater. J. Mol. Liq. 2021, 331, 115458. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Letifi, H.; Dridi, D.; Litaiem, Y.; Ammar, S.; Dimassi, W.; Chtourou, R. High efficient and cost effective titanium doped tin dioxide based photocatalysts synthesized via co-precipitation approach. Catalysts 2021, 11, 803. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, L.; Mou, X.; Xu, H.; Liu, J.; Miao, Y.; Wang, X.C.; Li, X. Characterization and evolution of antibiotic resistance of Salmonella in municipal wastewater treatment plants. J. Environ. Manag. 2019, 251, 109547. [Google Scholar] [CrossRef]
- Lhoutellier, C.; Ducray, F.; Delgene, J. Behaviour of pathogenic and indicator bacteria during urban wastewater treatment and sludge composting, as revealed by quantitative PCR. Water Res. 2008, 42, 53–62. [Google Scholar]
- Lin, Y.; Li, D.; Gu, A.Z.; Zeng, S.; He, M. Bacterial regrowth in water reclamation and distribution systems revealed by viable bacterial detection assays. Chemosphere 2016, 144, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Duan, X.; Zhou, X.; Wang, R.; Wang, S.; Ren, N.; Ho, S.-H. Advanced oxidation processes for water disinfection: Features, mechanisms and prospects. Chem. Eng. J. 2021, 409, 128207. [Google Scholar] [CrossRef]
- Srikanth, B.; Goutham, R.; Badri Narayan, R.; Ramprasath, A.; Gopinath, K.P.; Sankaranarayanan, A.R. Recent advancements in supporting materials for immobilised photocatalytic applications in waste water treatment. J. Environ. Manag. 2017, 200, 60–78. [Google Scholar] [CrossRef]
- Lee, J.; Gunten, U.V.; Kim, J. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Lu, J.; Zhuo, Q.; Ren, X.; Qiu, Y.; Li, Y.; Chen, Z.; Huang, K. Treatment of wastewater from adhesive-producing industries by electrocoagulation and electrochemical oxidation. Process Saf. Environ. Prot. 2021. [Google Scholar] [CrossRef]
- Mergenbayeva, S.; Atabaev, T.; Poulopoulos, S.G. Ti2O3/TiO2-Assisted Solar Photocatalytic Degradation of 4-tert-Butylphenol in Water. Catalysts 2021, 11, 1379. [Google Scholar] [CrossRef]
- Ivanov, M.; Vukušić Pavičić, T.; Kraljić, K.; Grgas, D.; Landeka Dragičević, T.; Herceg, Z. Effects of High Voltage Electrical Discharge Plasma on Olive Mill Wastewater Treatment. Sustainability 2021, 13, 1552. [Google Scholar] [CrossRef]
- Yu, G.; Wang, Y.; Cao, H.; Zhao, H.; Xie, Y. Reactive Oxygen Species and Catalytic Active Sites in Heterogeneous Catalytic Ozonation for Water Purification. Environ. Sci. Technol. 2020, 54, 5931–5946. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wang, L.; Yang, T.; Yang, G.; Wang, D.; Ni, H.; Wu, M. Tuning Lewis acidity of iron-based metal-organic frameworks for enhanced catalytic ozonation. Chem. Eng. J. 2021, 404, 127075. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, D.; Hong, S.; Khan, S.; Darya, B.; Lee, J.-Y.; Chung, J.; Cho, S.-H. Investigation of the Synergistic Effect of Sonolysis and Photocatalysis of Titanium Dioxide for Organic Dye Degradation. Catalysts 2020, 10, 500. [Google Scholar] [CrossRef]
- Hisaindee, S.; Meetani, M.A.; Rauf, M.A. Application of LC-MS to the analysis of advanced oxidation process (AOP) degradation of dye products and reaction mechanisms. TrAC Trends Anal. Chem. 2013, 49, 31–44. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, G.; Yi, J.; Cheng, M.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y.; Zhou, C.; Xue, W.; et al. Progress and challenges of metal-organic frameworks-based materials for SR-AOPs applications in water treatment. Chemosphere 2021, 263, 127672. [Google Scholar] [CrossRef]
- Xing, Z.; Zhang, J.; Cui, J.; Yin, J.; Zhao, T.; Kuang, J.; Xiu, Z.; Wan, N.; Zhou, W. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B Environ. 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, F.; Xu, W.; Cao, S.; Zhu, H. Recent progress in enhancing photocatalytic efficiency of TiO2-based materials. Appl. Catal. A Gen. 2015, 495, 131–140. [Google Scholar] [CrossRef]
- Wang, W.; Huang, G.; Yu, J.C.; Wong, P.K. Advances in photocatalytic disinfection of bacteria: Development of photocatalysts and mechanisms. J. Environ. Sci. 2015, 34, 232–247. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; binti Mohd Noor, N.H.; Umar, K.; Adnan, R.; Ibrahim, M.N.M.; Rashid, M. Graphene oxide–ZnO nanocomposite: An efficient visible light photocatalyst for degradation of rhodamine B. Appl. Nanosci. 2021, 11, 1291–1302. [Google Scholar] [CrossRef]
- Khasawneh, O.F.S.; Palaniandy, P.; Palaniandy, P.; Ahmadipour, M.; Mohammadi, H.; Bin Hamdan, M.R. Removal of acetaminophen using Fe2O3-TiO2 nanocomposites by photocatalysis under simulated solar irradiation: Optimization study. J. Environ. Chem. Eng. 2021, 9, 104921. [Google Scholar] [CrossRef]
- Akel, S.; Dillert, R.; Balayeva, N.O.; Boughaled, R.; Koch, J.; El Azzouzi, M.; Bahnemann, D.W. Ag/Ag2O as a Co-Catalyst in TiO2 Photocatalysis: Effect of the Co-Catalyst/Photocatalyst Mass Ratio. Catalysts 2018, 8, 647. [Google Scholar] [CrossRef] [Green Version]
- Umar, K.; Dar, A.A.; Haque, M.M.; Mir, N.A.; Muneer, M. Photocatalysed decolourization of two textile dye derivatives, Martius Yellow and Acid Blue 129, in UV-irradiated aqueous suspensions of Titania. Desalin. Water Treat. 2012, 46, 205–214. [Google Scholar] [CrossRef]
- Mir, N.; Khan, A.; Umar, K.; Muneer, M. Photocatalytic Study of a Xanthene Dye Derivative, Phloxine B in Aqueous Suspension of TiO 2: Adsorption Isotherm and Decolourization Kinetics. Energy Environ. Focus 2013, 2, 208–216. [Google Scholar] [CrossRef]
- Shao, J.; Sheng, W.; Wang, M.; Li, S.; Chen, J.; Zhang, Y.; Cao, S. In situ synthesis of carbon-doped TiO2 single-crystal nanorods with a remarkably photocatalytic efficiency. Appl. Catal. B Environ. 2017, 209, 311–319. [Google Scholar] [CrossRef]
- Saravanan, R.; Sacari, E.; Gracia, F.; Khan, M.M.; Mosquera, E.; Gupta, V.K. Conducting PANI stimulated ZnO system for visible light photocatalytic degradation of coloured dyes. J. Mol. Liq. 2016, 221, 1029–1033. [Google Scholar] [CrossRef]
- Choi, S.W.; Shahbaz, H.M.; Kim, J.U.; Kim, D.-H.; Yoon, S.; Jeong, S.H.; Park, J.; Lee, D.-U. Photolysis and TiO2 Photocatalytic Treatment under UVC/VUV Irradiation for Simultaneous Degradation of Pesticides and Microorganisms. Appl. Sci. 2020, 10, 4493. [Google Scholar] [CrossRef]
- Sujatha, G.; Shanthakumar, S.; Chiampo, F. UV light-irradiated photocatalytic degradation of coffee processing wastewater using tio2 as a catalyst. Environments 2020, 7, 47. [Google Scholar] [CrossRef]
- Uma, H.B.; Ananda, S.; Nandaprakash, M.B. High efficient photocatalytic treatment of textile dye and antibacterial activity via electrochemically synthesized Ni-doped ZnO nano photocatalysts. Chem. Data Collect. 2019, 24, 100301. [Google Scholar] [CrossRef]
- Dhandole, L.K.; Seo, Y.S.; Kim, S.G.; Kim, A.; Cho, M.; Jang, J.S. A mechanism study on the photocatalytic inactivation of: Salmonella typhimurium bacteria by CuxO loaded rhodium-antimony co-doped TiO2 nanorods. Photochem. Photobiol. Sci. 2019, 18, 1092–1100. [Google Scholar] [CrossRef]
- Fiorentino, A.; Rizzo, L.; Guilloteau, H.; Bellanger, X.; Merlin, C. Comparing TiO2 photocatalysis and UV-C radiation for inactivation and mutant formation of Salmonella typhimurium TA102. Environ. Sci. Pollut. Res. 2017, 24, 1871–1879. [Google Scholar] [CrossRef]
- Garvey, M.; Panaitescu, E.; Menon, L.; Byrne, C.; Dervin, S.; Hinder, S.J.; Pillai, S.C. Titania nanotube photocatalysts for effectively treating waterborne microbial pathogens. J. Catal. 2016, 344, 631–639. [Google Scholar] [CrossRef]
- Pino-Sandoval, D.; Villanueva-Rodríguez, M.; Cantú-Cárdenas, M.E.; Hernández-Ramírez, A. Performance of Ag-Cu/TiO2 photocatalyst prepared by sol-gel method on the inactivation of Escherichia coli and Salmonella typhimurium. J. Environ. Chem. Eng. 2020, 8, 104539. [Google Scholar] [CrossRef]
- Gayán, E.; Serrano, M.J.; Raso, J.; Álvarez, I.; Condón, S. Inactivation of Salmonella enterica by UV-C light Alone and in combination with mild temperatures. Appl. Environ. Microbiol. 2012, 78, 8353–8361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismael, M. Latest progress on the key operating parameters affecting the photocatalytic activity of TiO2-based photocatalysts for hydrogen fuel production: A comprehensive review. Fuel 2021, 303, 121207. [Google Scholar] [CrossRef]
- Galedari, M.; Mehdipour Ghazi, M.; Mirmasoomi, S.R. Novel visible-driven Ag2O/Fe2O3/TiO2 nano sized hetero-structured photocatalyst: Synthesis, characterization and photo-degradation of tetracycline. Chem. Eng. Res. Des. 2021, 170, 248–255. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, C. Mesoporous Cu-Cu2O@TiO2 heterojunction photocatalysts derived from metal-organic frameworks. RSC Adv. 2020, 10, 14550–14555. [Google Scholar] [CrossRef] [Green Version]
- Gogoi, D.; Namdeo, A.; Golder, A.K.; Peela, N.R. Ag-doped TiO2 photocatalysts with effective charge transfer for highly efficient hydrogen production through water splitting. Int. J. Hydrogen Energy 2020, 45, 2729–2744. [Google Scholar] [CrossRef]
- Mollavali, M.; Rohani, S.; Elahifard, M.; Behjatmanesh-Ardakani, R.; Nourany, M. Band gap reduction of (Mo+N) co-doped TiO2 nanotube arrays with a significant enhancement in visible light photo-conversion: A combination of experimental and theoretical study. Int. J. Hydrogen Energy 2021, 46, 21475–21498. [Google Scholar] [CrossRef]
- Bakar, S.A.; Ribeiro, C. Nitrogen-doped titanium dioxide: An overview of material design and dimensionality effect over modern applications. J. Photochem. Photobiol. C Photochem. Rev. 2016, 27, 1–29. [Google Scholar] [CrossRef]
- Changanaqui, K.; Brillas, E.; Alarcón, H.; Sirés, I. ZnO/TiO2/Ag2Se nanostructures as photoelectrocatalysts for the degradation of oxytetracycline in water. Electrochim. Acta 2020, 331, 135194. [Google Scholar] [CrossRef]
- Hu, X.; Hu, X.; Tang, C.; Wen, S.; Wu, X.; Long, J.; Yang, X.; Wang, H.; Zhou, L. Mechanisms underlying degradation pathways of microcystin-LR with doped TiO2 photocatalysis. Chem. Eng. J. 2017, 330, 355–371. [Google Scholar] [CrossRef]
- Ivanova, I.; Schneider, J.; Gutzmann, H.; Kliemann, J.O.; Gärtner, F.; Klassen, T.; Bahnemann, D.; Mendive, C.B. Photocatalytic degradation of oxalic and dichloroacetic acid on TiO2 coated metal substrates. Catal. Today 2013, 209, 84–90. [Google Scholar] [CrossRef]
- Wenhua, L.; Hong, L.; Sao’an, C.; Jianqing, Z.; Chunan, C. Kinetics of photocatalytic degradation of aniline in water over TiO2 supported on porous nickel. J. Photochem. Photobiol. A Chem. 2000, 131, 125–132. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, X.; Yuan, D.; Wang, W.; Gao, L. Preparation and characterization of TiO2 based on wood templates. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Yadav, H.M.; Otari, S.V.; Bohara, R.A.; Mali, S.S.; Pawar, S.H.; Delekar, S.D. Synthesis and visible light photocatalytic antibacterial activity of nickel-doped TiO2 nanoparticles against Gram-positive and Gram-negative bacteria. J. Photochem. Photobiol. A Chem. 2014, 294, 130–136. [Google Scholar] [CrossRef]
- Liu, B.; Mu, L.; Han, B.; Zhang, J.; Shi, H. Fabrication of TiO2/Ag2O heterostructure with enhanced photocatalytic and antibacterial activities under visible light irradiation. Appl. Surf. Sci. 2017, 396, 1596–1603. [Google Scholar] [CrossRef]
- Mangayayam, M.; Kiwi, J.; Giannakis, S.; Pulgarin, C.; Zivkovic, I.; Magrez, A.; Rtimi, S. FeOx magnetization enhancing E. coli inactivation by orders of magnitude on Ag-TiO2 nanotubes under sunlight. Appl. Catal. B Environ. 2017, 202, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Sethi, D.; Sakthivel, R. ZnO/TiO2 composites for photocatalytic inactivation of Escherichia coli. J. Photochem. Photobiol. B Biol. 2017, 168, 117–123. [Google Scholar] [CrossRef]
- Nararom, M.; Thepa, S.; Kongkiattikajorn, J.; Songprakorp, R. Disinfection of water containing Escherichia coli by use of a compound parabolic concentrator: Effect of global solar radiation and reactor surface treatment. Res. Chem. Intermed. 2015, 41, 6543–6558. [Google Scholar] [CrossRef]
- Varnagiris, S.; Urbonavicius, M.; Sakalauskaite, S.; Daugelavicius, R.; Pranevicius, L.; Lelis, M.; Milcius, D. Floating TiO2 photocatalyst for efficient inactivation of E. coli and decomposition of methylene blue solution. Sci. Total Environ. 2020, 720, 137600. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhou, R.; Zhang, X.; Li, J.; Wang, X.; Chen, Q.; Yang, S.; Chen, Z.; Bazaka, K.; Ostrikov, K. Synergistic Effect of Atmospheric-pressure Plasma and TiO2 Photocatalysis on Inactivation of Escherichia coli Cells in Aqueous Media. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bui, V.K.H.; Van Tran, V.; Moon, J.Y.; Park, D.; Lee, Y.C. Titanium dioxide microscale and macroscale structures: A mini-review. Nanomaterials 2020, 10, 1190. [Google Scholar] [CrossRef]
- Venieri, D.; Fraggedaki, A.; Kostadima, M.; Chatzisymeon, E.; Binas, V.; Zachopoulos, A.; Kiriakidis, G.; Mantzavinos, D. Solar light and metal-doped TiO2 to eliminate water-transmitted bacterial pathogens: Photocatalyst characterization and disinfection performance. Appl. Catal. B Environ. 2014, 154–155, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Fu, P.; Zhang, P.; Li, J. Photocatalytic degradation of low concentration formaldehyde and simultaneous elimination of ozone by-product using palladium modified TiO2 films under UV254+185nm irradiation. Appl. Catal. B Environ. 2011, 105, 220–228. [Google Scholar] [CrossRef]
- Thomas, J.; Yoon, M. Facile synthesis of pure TiO2(B) nanofibers doped with gold nanoparticles and solar photocatalytic activities. Appl. Catal. B Environ. 2012, 111–112, 502–508. [Google Scholar] [CrossRef]
- Wattanawikkam, C.; Pecharapa, W. Structural studies and photocatalytic properties of Mn and Zn co-doping on TiO2 prepared by single step sonochemical method. Radiat. Phys. Chem. 2020, 171, 108714. [Google Scholar] [CrossRef]
- Niishiro, R.; Kato, H.; Kudo, A. Nickel and either tantalum or niobium-codoped TiO2 and SrTiO3 photocatalysts with visible-light response for H2 or O2 evolution from aqueous solutions. Phys. Chem. Chem. Phys. 2005, 7, 2241–2245. [Google Scholar] [CrossRef]
- Hu, H.; Xiao, W.J.; Yuan, J.; Shi, J.W.; Chen, M.X.; Guan, S.; Feng, W. Preparations of TiO2 film coated on foam nickel substrate by sol-gel processes and its photocatalytic activity for degradation of acetaldehyde. J. Environ. Sci. 2007, 19, 80–85. [Google Scholar] [CrossRef]
- Davidsdóttir, S.; Canulescu, S.; Dirscherl, K.; Schou, J.; Ambat, R. Investigation of photocatalytic activity of titanium dioxide deposited on metallic substrates by DC magnetron sputtering. Surf. Coat. Technol. 2013, 216, 35–45. [Google Scholar] [CrossRef]
- Urbonavicius, M.; Varnagiris, S.; Sakalauskaite, S.; Demikyte, E.; Tuckute, S.; Lelis, M. Application of Floating TiO2 Photocatalyst for Methylene Blue Decomposition and Salmonella typhimurium Inactivation. Catalysts 2021, 11, 794. [Google Scholar] [CrossRef]
- Varnagiris, S.; Urbonavicius, M.; Sakalauskaite, S.; Demikyte, E.; Tuckute, S.; Lelis, M. Photocatalytic Inactivation of Salmonella typhimurium by Floating Carbon-Doped TiO2 Photocatalyst. Materials 2021, 14, 5681. [Google Scholar] [CrossRef]
- Ohno, T.; Tokieda, K.; Higashida, S.; Matsumura, M. Synergism between rutile and anatase TiO2 particles in photocatalytic oxidation of naphthalene. Appl. Catal. A Gen. 2003, 244, 383–391. [Google Scholar] [CrossRef]
- Kleiman, A.; Meichtry, J.M.; Vega, D.; Litter, M.I.; Márquez, A. Photocatalytic activity of TiO2 films prepared by cathodic arc deposition: Dependence on thickness and reuse of the photocatalysts. Surf. Coat. Technol. 2020, 382, 125154. [Google Scholar] [CrossRef]
- Kamiko, M.; Aotani, K.; Suenaga, R.; Koo, J.-W.; Nose, K.; Kyuno, K.; Ha, J.-G. The influence of Ta underlayers on the structure of TiO2 thin films deposited on an unheated glass substrate. Appl. Surf. Sci. 2012, 258, 8764–8768. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.X.; Bu, Y.; Zhang, J.; Wang, X.X.; Huang, J.; Chen, J.; Zhao, J. Preparation, characterization, and photocatalytic activity evaluation of Fe–N-codoped TiO2/fly ash cenospheres floating photocatalyst. Environ. Sci. Pollut. Res. 2016, 23, 22793–22802. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Wang, X.; Song, J.; Huang, J.; Louangsouphom, B.; Zhao, J. Floating photocatalysts based on loading Bi/N-doped TiO2 on expanded graphite C/C (EGC) composites for the visible light degradation of diesel. RSC Adv. 2015, 5, 71922–71931. [Google Scholar] [CrossRef]
- Valdez-Castillo, M.; Saucedo-Lucero, J.O.; Arriaga, S. Photocatalytic inactivation of airborne microorganisms in continuous flow using perlite-supported ZnO and TiO2. Chem. Eng. J. 2019, 374, 914–923. [Google Scholar] [CrossRef]
- Xue, H.; Jiang, Y.; Yuan, K.; Yang, T.; Hou, J.; Cao, C.; Feng, K.; Wang, X. Floating photocatalyst of B-N-TiO2/expanded perlite: A sol-gel synthesis with optimized mesoporous and high photocatalytic activity. Sci. Rep. 2016, 6, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, P.K.; Mahalingam, H. Novel Floating Ag+ -Doped TiO2/Polystyrene Photocatalysts for the Treatment of Dye Wastewater. Ind. Eng. Chem. Res. 2014, 53, 16332–16340. [Google Scholar] [CrossRef]
- Gualdrón-Reyes, A.F.; Meléndez, A.M.; González, I.; Lartundo-Rojas, L.; Niño-Gómez, M.E. Effect of Metal Substrate on Photo(electro)catalytic Activity of B-Doped Graphene Modified TiO2 Thin Films: Role of Iron Oxide Nanoparticles at Grain Boundaries of TiO2. J. Phys. Chem. C 2018, 122, 297–306. [Google Scholar] [CrossRef]
- Yazid, S.A.; Rosli, Z.M.; Juoi, J.M. Effect of titanium (IV) isopropoxide molarity on the crystallinity and photocatalytic activity of titanium dioxide thin film deposited via green sol-gel route. J. Mater. Res. Technol. 2019, 8, 1434–1439. [Google Scholar] [CrossRef]
- Wattanawikkam, C.; Pecharapa, W. Synthesis and Characterization of Zn-Doped TiO2 Nanoparticles via Sonochemical Method. Integr. Ferroelectr. 2015, 165, 167–175. [Google Scholar] [CrossRef]
- Abdulraheem, Y.M.; Ghoraishi, S.; Arockia-Thai, L.; Zachariah, S.K.; Ghannam, M. The effect of annealing on the structural and optical properties of titanium dioxide films deposited by electron beam assisted PVD. Adv. Mater. Sci. Eng. 2013, 2013, 574738. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.R.; Ting, C.H.; Wang, J.J.; Liu, W.C.; Lin, C.H. Characteristics of graded TiO2 and TiO2/ITO films prepared by twin DC magnetron sputtering technique. Surf. Coatings Technol. 2006, 200, 6030–6036. [Google Scholar] [CrossRef]
- Singh, P.; Kaur, D. Room temperature growth of nanocrystalline anatase TiO2 thin films by dc magnetron sputtering. Phys. B Condens. Matter 2010, 405, 1258–1266. [Google Scholar] [CrossRef]
- Hui, W.; Guodong, S.; Xiaoshu, Z.; Wei, Z.; Lin, H.; Ying, Y. In-situ synthesis of TiO2 rutile/anatase heterostructure by DC magnetron sputtering at room temperature and thickness effect of outermost rutile layer on photocatalysis. J. Environ. Sci. 2017, 60, 33–42. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Short Notes Rev. Pap. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Enache-Pommer, E.; Liu, B.; Aydil, E.S. Electron transport and recombination in dye-sensitized solar cells made from single-crystal rutile TiO2nanowires. Phys. Chem. Chem. Phys. 2009, 11, 9648–9652. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, I.; Gupta, A.K.; Kumar, P.P.; Sekhar, P.S.C.; Radha, K.; Padmanabham, G.; Sundararajan, G. Preparation and characterization of Ni-doped TiO2 materials for photocurrent and photocatalytic applications. Sci. World J. 2012, 2012, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Yin, S.; Dong, Q.; Guo, C.; Li, H.; Kimura, T.; Sato, T. Synthesis of high visible light active carbon doped TiO2 photocatalyst by a facile calcination assisted solvothermal method. Appl. Catal. B Environ. 2013, 142–143, 450–457. [Google Scholar] [CrossRef]
- Beranek, R.; Kisch, H. Tuning the optical and photoelectrochemical properties of surface-modified TiO2. Photochem. Photobiol. Sci. 2007, 7, 40–48. [Google Scholar] [CrossRef]
- NIST. NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database 20. Available online: https://srdata.nist.gov/xps/ (accessed on 25 November 2021).
- Cheng, M.; Fan, H.; Song, Y.; Cui, Y.; Wang, R. Interconnected hierarchical NiCo2O4 microspheres as high-performance electrode materials for supercapacitors. Dalt. Trans. 2017, 46, 9201–9209. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Zhao, Y.; Bai, Y.; Li, F.; Zhang, Y.; Chen, Y. Conductive Metal–Organic Frameworks with Extra Metallic Sites as an Efficient Electrocatalyst for the Hydrogen Evolution Reaction. Adv. Sci. 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Park, Y.; Kim, W.; Park, H.; Tachikawa, T.; Majima, T.; Choi, W. Carbon-doped TiO2 photocatalyst synthesized without using an external carbon precursor and the visible light activity. Appl. Catal. B Environ. 2009, 91, 355–361. [Google Scholar] [CrossRef]
- Moncayo-Lasso, A.; Mora-Arismendi, L.E.; Rengifo-Herrera, J.A.; Sanabria, J.; Benítez, N.; Pulgarin, C. The detrimental influence of bacteria (E. coli, Shigella and Salmonella) on the degradation of organic compounds (and vice versa) in TiO2 photocatalysis and near-neutral photo-Fenton processes under simulated solar light. Photochem. Photobiol. Sci. 2012, 11, 821–827. [Google Scholar] [CrossRef]
- Liu, N.; Ming, J.; Sharma, A.; Sun, X.; Kawazoe, N.; Chen, G.; Yang, Y. Sustainable photocatalytic disinfection of four representative pathogenic bacteria isolated from real water environment by immobilized TiO2-based composite and its mechanism. Chem. Eng. J. 2021, 426, 131217. [Google Scholar] [CrossRef]
- Li, G.; Nie, X.; Chen, J.; Jiang, Q.; An, T.; Wong, P.K.; Zhang, H.; Zhao, H.; Yamashita, H. Enhanced visible-light-driven photocatalytic inactivation of Escherichia coli using g-C3N4/TiO2 hybrid photocatalyst synthesized using a hydrothermal-calcination approach. Water Res. 2015, 86, 17–24. [Google Scholar] [CrossRef]
- Hajjaji, A.; Elabidi, M.; Trabelsi, K.; Assadi, A.A.; Bessais, B.; Rtimi, S. Bacterial adhesion and inactivation on Ag decorated TiO2-nanotubes under visible light: Effect of the nanotubes geometry on the photocatalytic activity. Colloids Surf. B Biointerfaces 2018, 170, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Wei, F.; Zhang, T.; Luo, L.; Pan, Y.; Yang, X.; Yu, H.; Zhou, S. Different antibacterial effect of Ag3PO4/TiO2 heterojunctions and the TiO2 polymorphs. J. Alloys Compd. 2021, 876, 160016. [Google Scholar] [CrossRef]
- Macomber, L.; Hausinger, R.P. Mechanisms of nickel toxicity in microorganisms. Metallomics 2011, 3, 1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, D.; Singh, A.; Khan, A.U. Nanoparticles as Efflux Pump and Biofilm Inhibitor to Rejuvenate Bactericidal Effect of Conventional Antibiotics. Nanoscale Res. Lett. 2017, 12, 1–6. [Google Scholar] [CrossRef]
- Rabiei, M.; Palevicius, A.; Monshi, A.; Nasiri, S.; Vilkauskas, A.; Janusas, G. Comparing methods for calculating nano crystal size of natural hydroxyapatite using X-ray diffraction. Nanomaterials 2020, 10, 1627. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, D.B.; Zhao, Z.; Pei, J.; Zhang, B.P. Enhanced thermoelectric properties of ZnO: C doping and band gap tuning. J. Eur. Ceram. Soc. 2020, 41, 1324–1331. [Google Scholar] [CrossRef]
- Kuliesiene, N.; Sakalauskaite, S.; Tuckute, S.; Urbonavicius, M.; Varnagiris, S.; Daugelavicius, R.; Lelis, M. TiO2 application for the photocatalytical inactivation of S. enterica, E. coli and M. luteus bacteria mixtures. Environ. Clim. Technol. 2020, 24, 418–429. [Google Scholar] [CrossRef]
- Sanders, E.R. Aseptic Laboratory Techniques: Plating Methods. J. Vis. Exp. 2012, 1–18. [Google Scholar] [CrossRef]
- Pal, S.; Misra, A.; Banerjee, S.; Dam, B. Adaptation of ethidium bromide fluorescence assay to monitor activity of efflux pumps in bacterial pure cultures or mixed population from environmental samples. J. King Saud Univ.-Sci. 2020, 32, 939–945. [Google Scholar] [CrossRef]
- Murakami, S. Structures and Transport Mechanisms of RND Efflux Pumps. In Efflux-Mediated Antimicrobial Resistance in Bacteria: Mechanisms, Regulation and Clinical Implications; Li, X.-Z., Elkins, C.A., Zgurskaya, H.I., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–28. ISBN 978-3-319-39658-3. [Google Scholar]
- Venter, H.; Mowla, R.; Ohene-Agyei, T.; Ma, S. RND-type Drug Efflux Pumps from Gram-negative bacteria: Molecular Mechanism and Inhibition. Front. Microbiol. 2015, 6, 377. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).