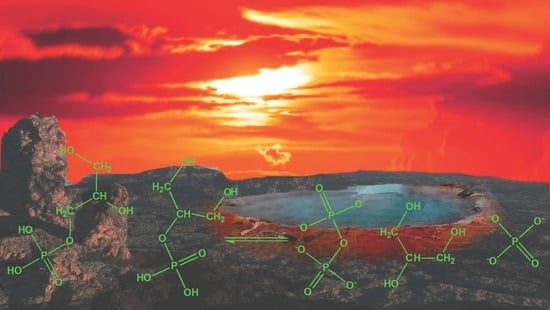
Catalytic Prebiotic Formation of Glycerol Phosphate Esters and an Estimation of Their Steady State Abundance under Plausible Early Earth Conditions
Abstract
:1. Introduction
2. Results and Discussions
3. Materials and Methods
3.1. Phosphorylation of Glycerol
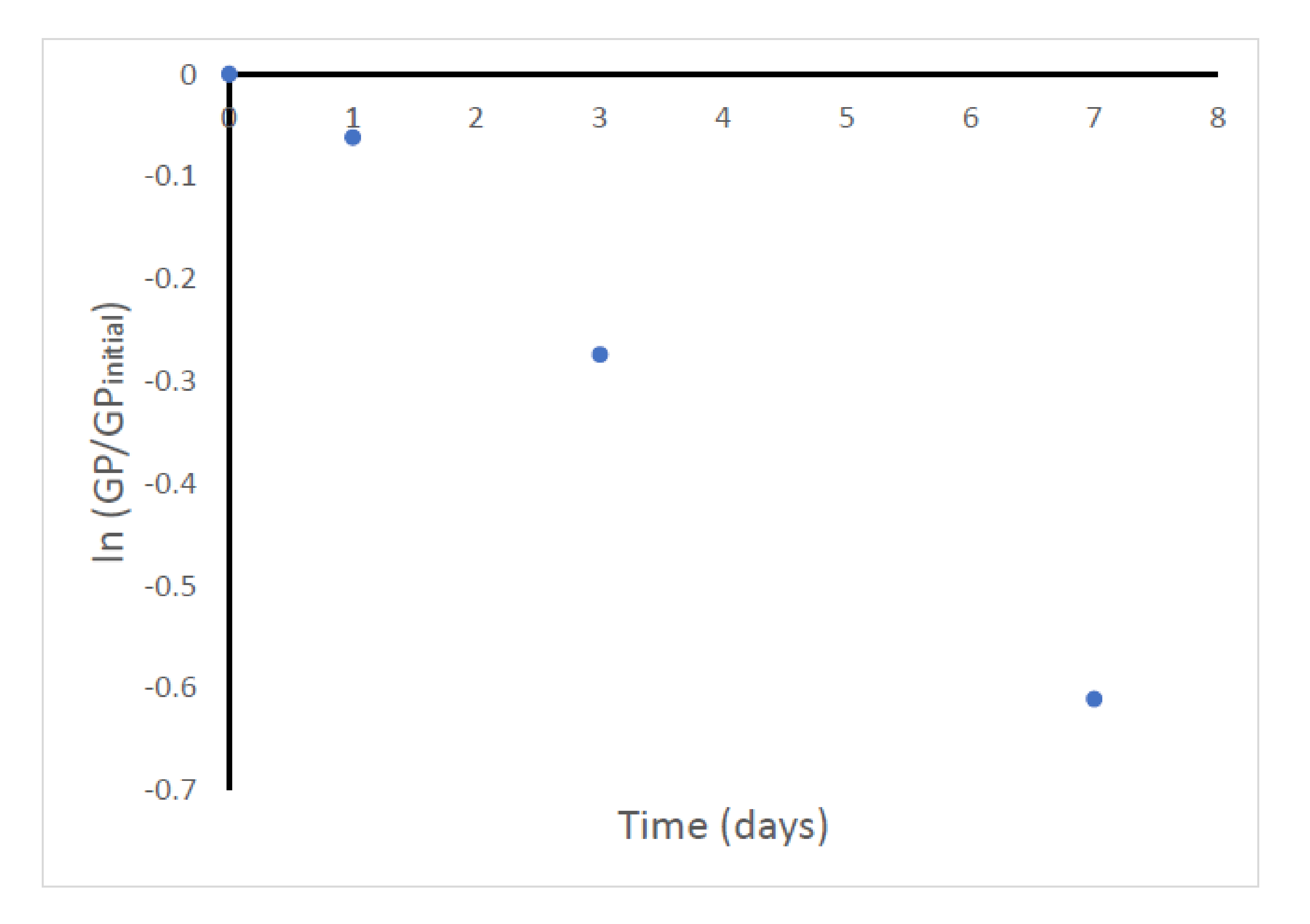
3.2. Decomposition Reaction Studies of the Glycerol Phosphates
3.3. 31P-NMR and Mass Spectrometry Analyses of GP Reactions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pasek, M.A.; Kee, T.P. On the origin of phosphorylated biomolecules. In Origins of Life: The Primal Self-Organization; Egel, R., Lankenau, D.-H., Mulkidjanian, A.Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 57–84. [Google Scholar]
- Lombard, J.; Lopez-Garcia, P.; Moreira, D. The early evolution of lipid membranes and the three domains of life. Nat. Rev. Genet. 2012, 10, 507–515. [Google Scholar] [CrossRef]
- Peretó, J.; Lopez-Garcia, P.; Moreira, D. Ancestral lipid biosynthesis and early membrane evolution. Trends Biochem. Sci. 2004, 29, 469–477. [Google Scholar] [CrossRef]
- Gull, M.; Pasek, M.A. The Role of Glycerol and Its Derivatives in the Biochemistry of Living Organisms, and Their Prebiotic Origin and Significance in the Evolution of Life. Catalysts 2021, 11, 86. [Google Scholar] [CrossRef]
- Epps, D.E.; Nooner, D.W.; Eichberg, J.; Sherwood, E.; Oró, J. Cyanamide mediated synthesis under plausible primitive earth conditions. VI. The synthesis of glycerol and glycerophos-phates. J. Mol. Evol. 1979, 14, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Gull, M.; Ge, T.; Yingwu, W.; Chao, H.; Zhan, S.; Hongming, Y.; Shouhua, F. Resolving the enigma of prebiotic C-O-P bond formation: Prebiotic hydrothermal synthesis of important biological phosphate esters. Heteroat. Chem. 2010, 21, 161–167. [Google Scholar]
- Gull, M.; Cafferty, B.J.; Hud, N.V.; Pasek, M.A. Silicate-Promoted Phosphorylation of Glycerol in Non-Aqueous Solvents: A Prebiotically Plausible Route to Organophosphates. Life 2017, 7, 29. [Google Scholar] [CrossRef]
- Gull, M.; Zhou, M.; Fernández, F.M.; Pasek, M.A. Prebiotic Phosphate Ester Syntheses in a Deep Eutectic Solvent. J. Mol. Evol. 2013, 78, 109–117. [Google Scholar] [CrossRef]
- Burcar, B.; Pasek, M.; Gull, M.; Cafferty, B.J.; Velasco, F.; Hud, N.V.; Menor-Salván, C. Darwin’s Warm Little Pond: A One-Pot Reaction for Prebiotic Phosphorylation and the Mobilization of Phosphate from Minerals in a Urea-Based Solvent. Angew. Chem. Int. Ed. 2016, 55, 13249–13253. [Google Scholar] [CrossRef]
- Gibard, C.; Bhowmik, S.; Karki, M.; Kim, E.-K.; Krishnamurthy, R. Phosphorylation, oligomerization and self-assembly in water under potential prebiotic conditions. Nat. Chem. 2017, 10, 212–217. [Google Scholar] [CrossRef]
- Maguire, O.R.; Smokers, I.; Huck, W.T. A physicochemical orthophosphate cycle via a kinetically stable thermodynamically activated intermediate enables mild prebiotic phosphorylations. Nat. Commun. 2021, 12, 15517. [Google Scholar]
- Pasek, M.A.; Harnmeijer, J.P.; Buick, R.; Gull, M.; Atlas, Z. Evidence for reactive reduced phosphorus species in the early Archean ocean. Proc. Natl. Acad. Sci. USA 2013, 110, 10089–10094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Turner, A.M.; Abplanalp, M.J.; Kaiser, R.I.; Webb, B.; Siuzdak, G.; Fortenberry, R.C. An interstellar synthesis of glycerol phosphates. Astrophys. J. Lett. 2020, 899, L3. [Google Scholar] [CrossRef]
- Lago, J.L.; Burcar, B.T.; Hud, N.V.; Febrian, R.; Mehta, C.; Bracher, P.J.; Atlas, Z.D.; Pasek, M.A. The Prebiotic Provenance of Semi-Aqueous Solvents. Orig. Life Evol. Biosph. 2020, 50, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.W. Phosphorus in prebiotic chemistry-an update and a note on plausibility. In Handbook of Astrobiology; Kolb, V., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 355–359. [Google Scholar]
- Österberg, R.; Orgel, L.E.; Lohrmann, R. Further studies of urea-catalyzed phosphorylation reactions. J. Mol. Evol. 1973, 2, 231–234. [Google Scholar] [CrossRef]
- Fiore, M.; Strazewski, P. Prebiotic Lipidic Amphiphiles and Condensing Agents on the Early Earth. Life 2016, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Gull, M.; Omran, A.; Feng, T.; Pasek, M.A. Silicate-, magnesium ion-, and urea-induced prebiotic phosphorylation of uridine via pyrophosphate; revisiting the hot drying water pool scenario. Life 2020, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Gull, M. Prebiotic Phosphorylation Reactions on the Early Earth. Challenges 2014, 5, 193–212. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, Y.; Horiuchi, M.; Kakegawa, T. Selective Stabilization of Ribose by Borate. Orig. Life Evol. Biosph. 2013, 43, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Silva, J.A.L. Boron in Prebiological Evolution. Angew. Chem. Int. Ed. 2020, 60, 10458–10468. [Google Scholar] [CrossRef]
- Scorei, R. Is Boron a Prebiotic Element? A Mini-review of the Essentiality of Boron for the Appearance of Life on Earth. Orig. Life Evol. Biosph. 2012, 42, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Grew, E.S.; Bada, J.L.; Hazen, R.M. Borate Minerals and Origin of the RNA World. Orig. Life Evol. Biosph. 2011, 41, 307–316. [Google Scholar] [CrossRef]
- Schwartz, A.W. Phosphorus in prebiotic chemistry. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1743–1749. [Google Scholar] [CrossRef] [Green Version]
- Tollari, N.; Toplis, M.; Barnes, S.-J. Predicting phosphate saturation in silicate magmas: An experimental study of the effects of melt composition and temperature. Geochim. Cosmochim. Acta 2006, 70, 1518–1536. [Google Scholar] [CrossRef] [Green Version]
- Walton, C.R.; Shorttle, O.; Jenner, F.E.; Williams, H.M.; Golden, J.; Morrison, S.M.; Downs, R.T.; Zerkle, A.; Hazen, R.M.; Pasek, M. Phosphorus mineral evolution and prebiotic chemistry: From minerals to microbes. Earth-Sci. Rev. 2021, 221, 103806. [Google Scholar] [CrossRef]
- Guo, F.-F.; Svetov, S.; Maier, W.D.; Hanski, E.; Yang, S.-H.; Rybnikova, Z. Geochemistry of komatiites and basalts in Archean greenstone belts of Russian Karelia with emphasis on platinum-group elements. Miner. Deposita 2019, 55, 971–990. [Google Scholar] [CrossRef] [Green Version]
- Schulte, M.; Blake, D.; Hoehler, T.; McCollom, T. Serpentinization and Its Implications for Life on the Early Earth and Mars. Astrobiology 2006, 6, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Pasek, M.; Omran, A.; Lang, C.; Gull, M.; Abbatiello, J.; Feng, T.; Garong, L.; Abbott-Lyon, H. Serpentinization as a Route to Liberating Phosphorus on Habitable Worlds. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Barnes, S.; Arndt, N.T. Distribution and Geochemistry of Komatiites and Basalts Through the Archean. In Earth’s Oldest Rocks; Elsevier: Amsterdam, The Netherlands, 2018; pp. 103–132. [Google Scholar] [CrossRef]
- Barber, D.J.; Scott, E. Origin of supposedly biogenic magnetite in the Martian meteorite Allan Hills 84001. Proc. Natl. Acad. Sci. USA 2002, 99, 6556–6561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasek, M.A. Phosphorus NMR of Natural Samples; Amazon: Seattle, WA, USA, 2018. [Google Scholar]
- Cleaves, H.J., II; Scott, A.M.; Hill, F.C.; Leszczynski, J.; Sahai, N.; Hazen, R. Mineral-organic interfacial processes: Potential roles in the origins of life. Chem. Soc. Rev. 2012, 41, 5502–5525. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L. Production of Some Organic Compounds under Possible Primitive Earth Conditions1. J. Am. Chem. Soc. 1955, 77, 2351–2361. [Google Scholar] [CrossRef]
- Pasek, M.; Dworkin, J.; Lauretta, D. A radical pathway for phosphorylation during schreibersite corrosion with implications for the origin of life. Geochim. Cosmochim. Acta 2007, 71, 1721–1736. [Google Scholar] [CrossRef]
- Pasek, M.A.; Kee, T.P.; Bryant, D.E.; Pavlov, A.A.; Lunine, J.I. Production of potentially prebiotic condensed phosphates by phosphorus redox chemistry. Angew. Chem. Int. Ed. Engl. 2008, 47, 7918–7920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamagata, Y.; Watanabe, H.; Saitoh, M.; Namba, T. Volcanic production of polyphosphate under primitive Earth conditions. Nature 1991, 204, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Hazen, R.M.; Sverjensky, D.A. Mineral Surfaces, Geochemical Complexities, and the Origins of Life. Cold Spring Harb. Perspect. Biol. 2010, 2, a002162. [Google Scholar] [CrossRef] [Green Version]
- Lambert, J.B.; Gurusamy-Thangavelu, S.A.; Ma, K. The silicate-mediated formose reaction: Bottom-up synthesis of sugar silicates. Science 2010, 327, 984–986. [Google Scholar] [CrossRef] [Green Version]
- Allen, C.C.; Westall, F.; Schelble, R.T. Importance of a Martian Hematite Site for Astrobiology. Astrobiology 2001, 1, 111–123. [Google Scholar] [CrossRef]
- Williams, A.J.; Sumner, D.Y.; Alpers, C.N.; Karunatillake, S.; Hofmann, B.A. Preserved Filamentous Microbial Biosig-natures in the Brick Flat Gossan, Iron Mountain, California. Astrobiology 2015, 15, 637–668. [Google Scholar] [CrossRef] [Green Version]
- Russell, M.J.; Ponce, A. Six ‘Must-Have’ Minerals for Life’s Emergence: Olivine, Pyrrhotite, Bridgmanite, Serpentine, Fougerite and Mackinawite. Life 2020, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Santosh, M.; Arai, T.; Maruyama, S. Hadean Earth and primordial continents: The cradle of prebiotic life. Geosci. Front. 2016, 8, 309–327. [Google Scholar] [CrossRef] [Green Version]
- Sleep, N.H.; Bird, D.K.; Pope, E.C. Serpentinite and the dawn of life. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2857–2869. [Google Scholar] [CrossRef] [Green Version]
- Larralde, R.; Robertson, M.P.; Miller, S.L. Rates of decomposition of ribose and other sugars: Implications for chemical evolution. Proc. Natl. Acad. Sci. USA 1995, 92, 8158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, B.C.; Kolb, V.M. Macrobiont: Cradle for the Origin of Life and Creation of a Biosphere. Life 2020, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Damer, B.; Deamer, D. The Hot Spring Hypothesis for an Origin of Life. Astrobiology 2020, 20, 429–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deamer, D.W. Assembling Life: How Can Life Begin on Earth and Other Habitable Planets? Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Sutherland, J.D. The Origin of Life-Out of the Blue. Angew. Chem. Int. Ed. 2015, 55, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Ritson, D.J.; Battilocchio, C.; Ley, S.V.; Sutherland, J.D. Mimicking the surface and prebiotic chemistry of early Earth using flow chemistry. Nat. Commun. 2018, 9, 1821. [Google Scholar] [CrossRef] [Green Version]
- Ferris, J.P.; Hill, A.R.; Liu, R.; Orgel, L.E. Synthesis of long prebiotic oligomers on mineral surfaces. Nature 1996, 381, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ferris, J.P. One-step, regioselective synthesis of up to 50-mers of RNA oligomers by montmorillonite catalysis. J. Am. Chem. Soc. 2006, 128, 8914–8919. [Google Scholar] [CrossRef]
- Pedreira-Segade, U.; Hao, J.; Razafitianamaharavo, A.; Pelletier, M.; Marry, V.; Le Crom, S.; Michot, L.J.; Daniel, I. How do Nucleotides Adsorb Onto Clays? Life 2018, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Hashizume, H. Adsorption of Nucleic Acid Bases, Ribose, and Phosphate by Some Clay Minerals. Life 2015, 5, 637–650. [Google Scholar] [CrossRef] [Green Version]
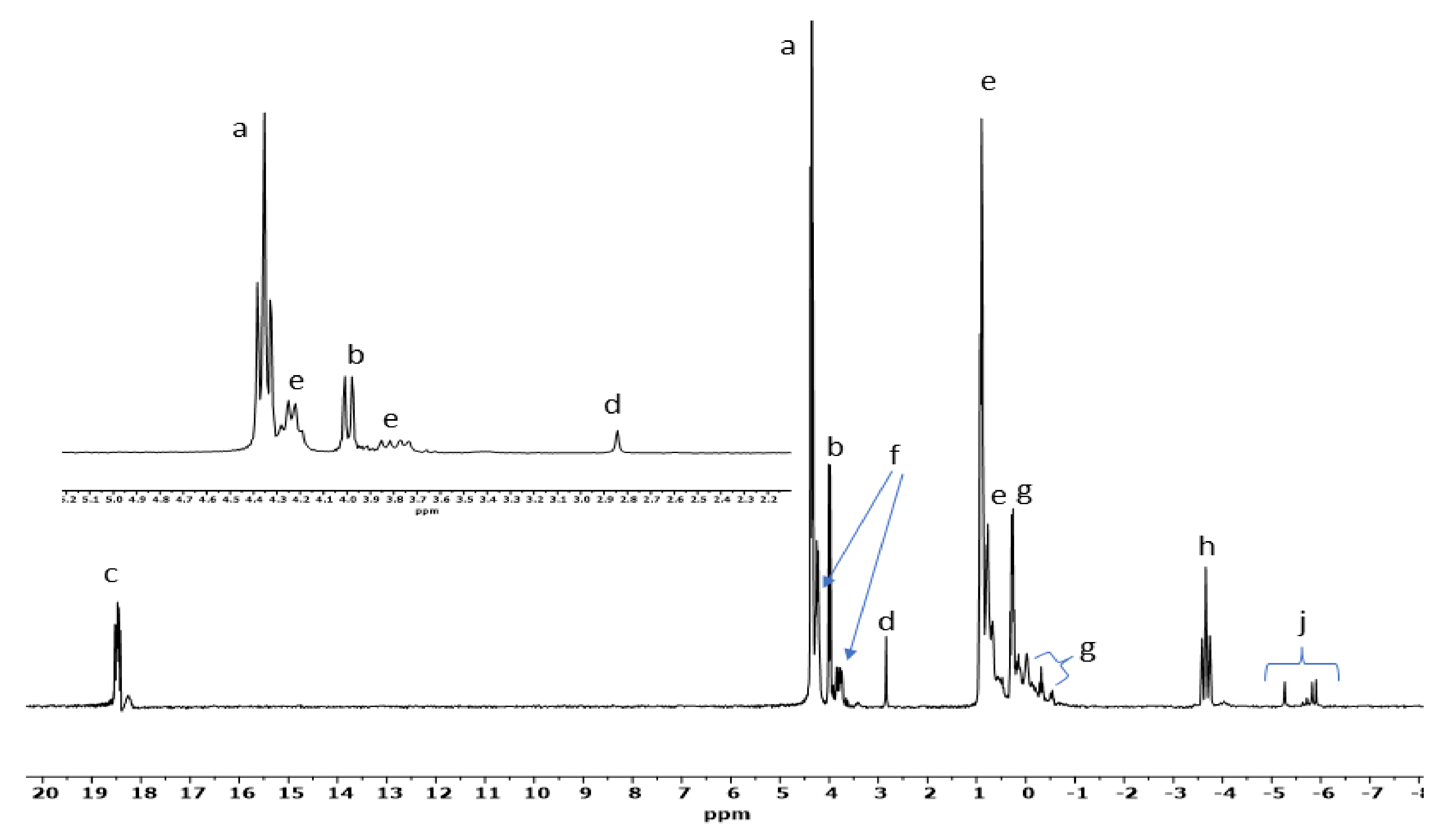
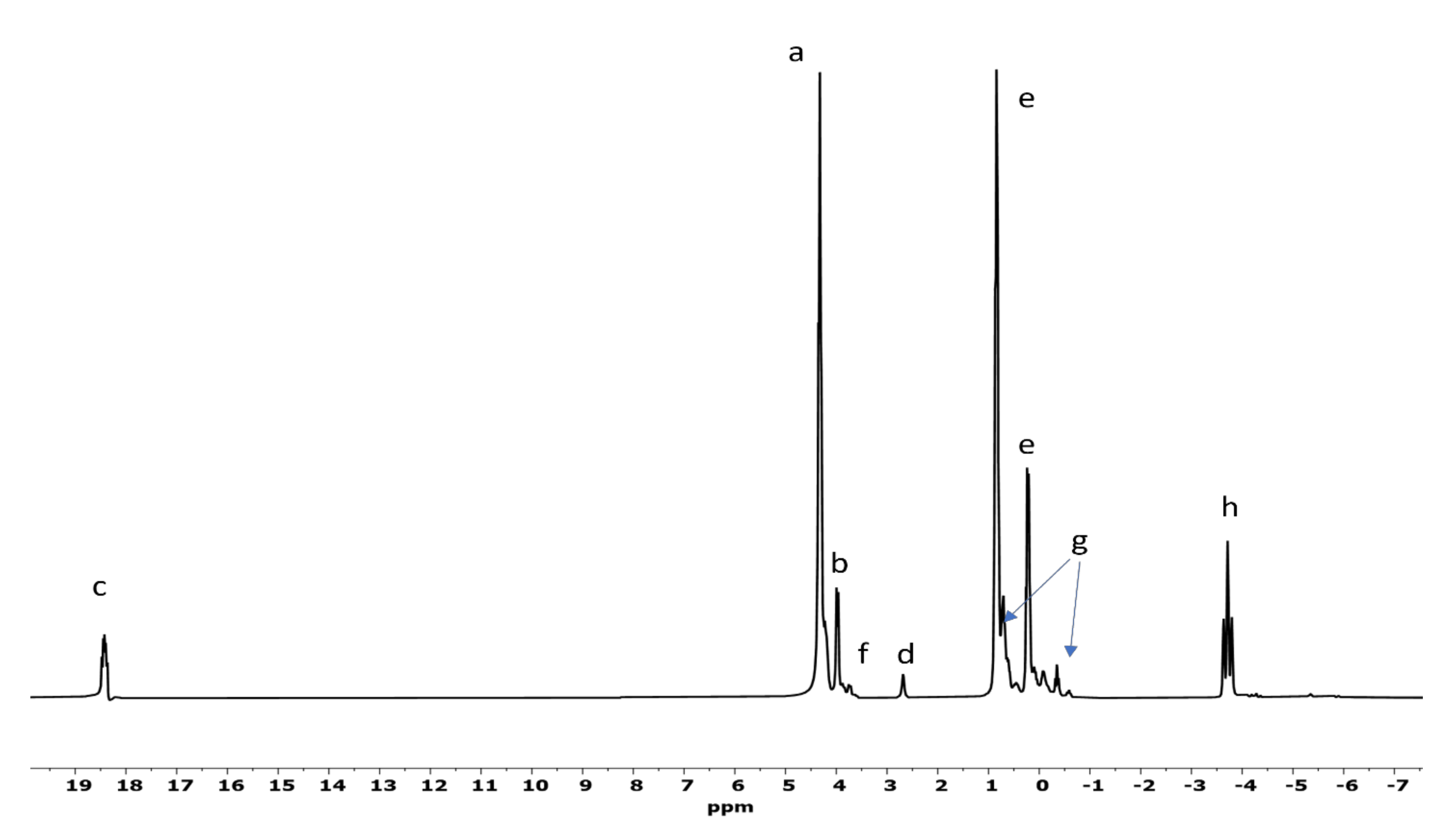

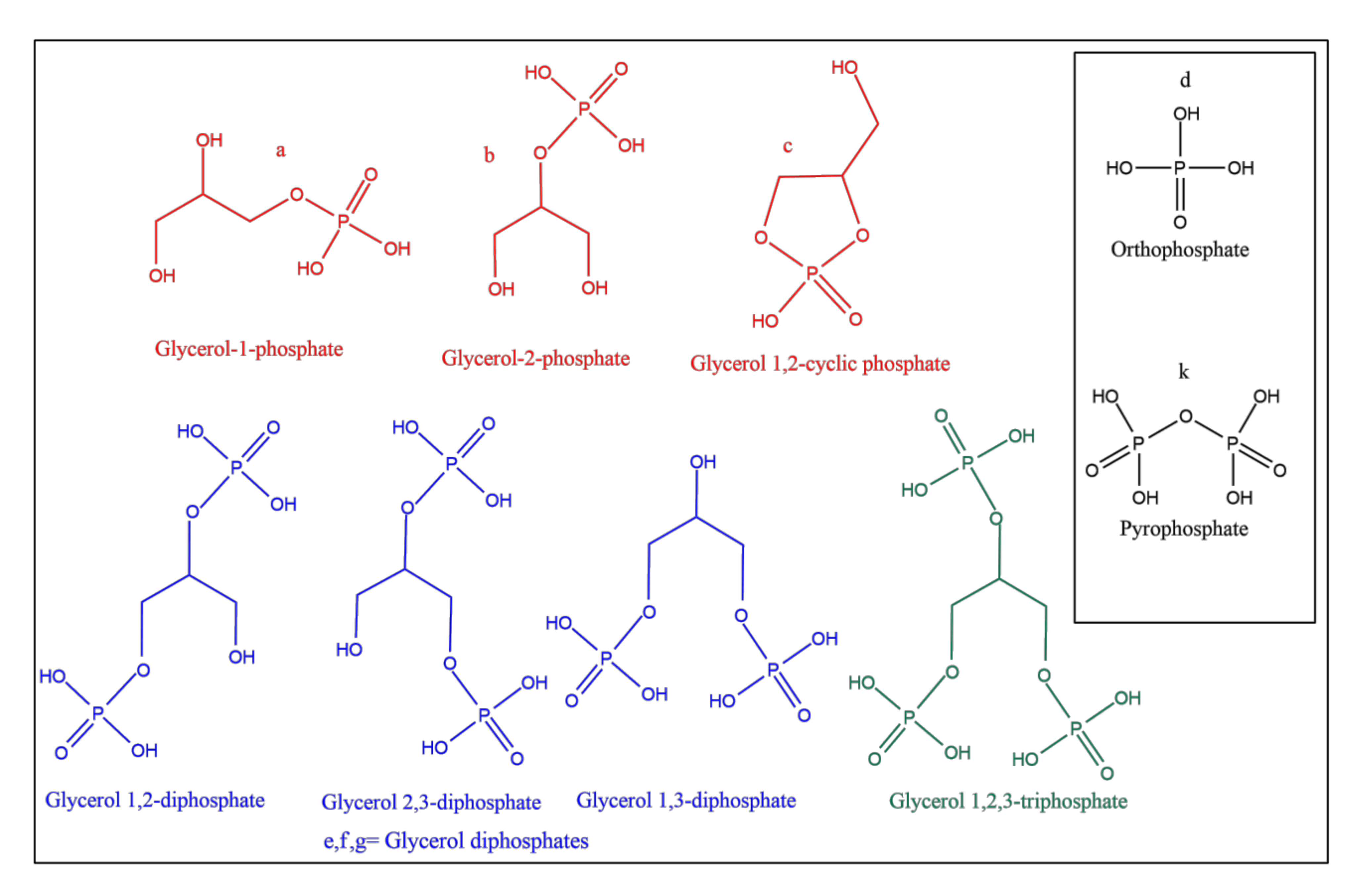
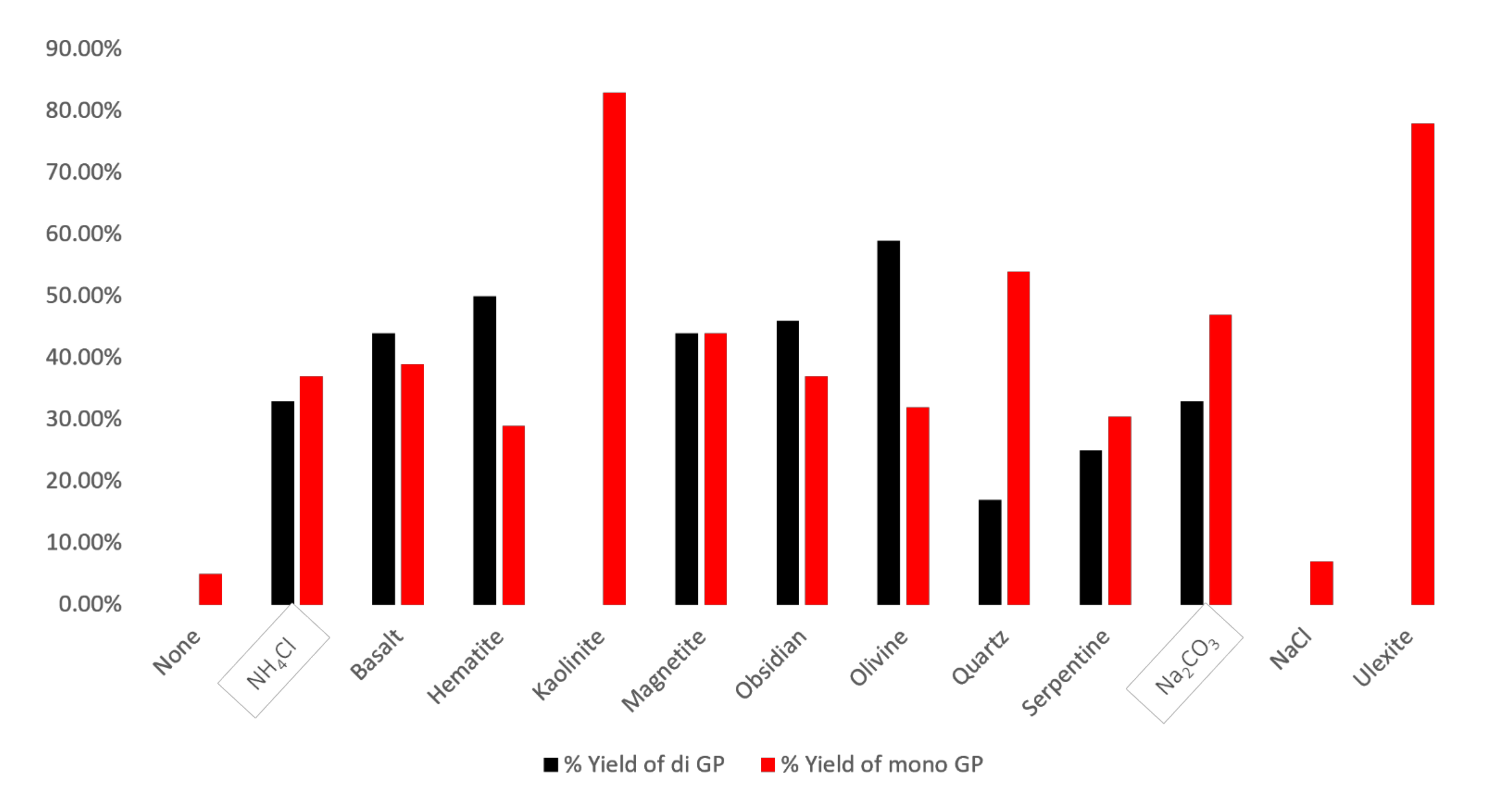
| Sample | Catalyst | a | b | c | d | e | f | g | h | Net Org. PO4 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | None | 3 | 2 | ND | 95 | ND | ND | ND | ND | 5 |
| 2 | Na2CO3 | 26 | 21 | ND | 13 | 18 | 8 | 7 | 7 | 80 |
| 3 | Serpentine | 21 | 8.5 | 1 | 33 | 25 | ND | ND | 11.5 | 55.5 |
| 4 | Hematite | 18 | 10 | 1 | 8 | 50 | ND | ND | 13 | 79 |
| 5 | Obsidian | 22 | 14 | 1 | 9 | 42 | 2 | 2 | 8 | 83 |
| 6 | NaCl | 4 | 3 | ND | 93 | ND | ND | ND | ND | 7 |
| 7 | Basalt | 30 | 5 | 4 | 8 | 40 | 2 | 2 | 9 | 83 |
| 8 | Magnetite | 31 | 7 | 6 | 5 | 42 | 2 | ND | 7 | 88 |
| 9 | NH4Cl | 22 | 15 | ND | 20 | 20 | 13 | ND | 10 | 70 |
| 10 | Ulexite | 57 | 17 | 4 | 20 | ND | ND | ND | 2 | 78 |
| 11 | Quartz | 31 | 23 | ND | 12 | 12 | 5 | ND | 17 | 71 |
| 12 | Olivine | 21 | 6 | 5 | 1 | 32 | 14 | 13 | 8 | 91 |
| 13 | Kaolinite | 65 | 15 | 3 | 11 | ND | ND | ND | 6 | 83 |
| Sample | a | b | c | d | e | f | k | Net | Org. PO4 |
|---|---|---|---|---|---|---|---|---|---|
| Set | 1 | 46 | 35 | ND | ND | ND | 19 | ND | 100 |
| Set | 2 | 49.50 | 30 | ND | 3.5 | 0.5 | 14 | 2.5 | 94 |
| Set | 3 | 39 | 19 | ND | 5 | 1 | 17 | 19 | 76 |
| Set | 4 | 28.50 | 12 | ND | 4.7 | 0.8 | 13 | 41 | 54.3 |
| Set | 5 | 46 | 35 | ND | ND | 1 | 18 | ND | 100 |
| Set | 6 | 46 | 32 | ND | 0.5 | 0.5 | 21 | ND | 99.5 |
| Set | 7 | 36 | 20 | ND | 4 | 0.5 | 15.5 | 24 | 72 |
| Set | 8 | 45 | 14 | ND | 7 | 1 | 5 | 28 | 65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gull, M.; Pasek, M.A. Catalytic Prebiotic Formation of Glycerol Phosphate Esters and an Estimation of Their Steady State Abundance under Plausible Early Earth Conditions. Catalysts 2021, 11, 1384. https://doi.org/10.3390/catal11111384
Gull M, Pasek MA. Catalytic Prebiotic Formation of Glycerol Phosphate Esters and an Estimation of Their Steady State Abundance under Plausible Early Earth Conditions. Catalysts. 2021; 11(11):1384. https://doi.org/10.3390/catal11111384
Chicago/Turabian StyleGull, Maheen, and Matthew A. Pasek. 2021. "Catalytic Prebiotic Formation of Glycerol Phosphate Esters and an Estimation of Their Steady State Abundance under Plausible Early Earth Conditions" Catalysts 11, no. 11: 1384. https://doi.org/10.3390/catal11111384
APA StyleGull, M., & Pasek, M. A. (2021). Catalytic Prebiotic Formation of Glycerol Phosphate Esters and an Estimation of Their Steady State Abundance under Plausible Early Earth Conditions. Catalysts, 11(11), 1384. https://doi.org/10.3390/catal11111384