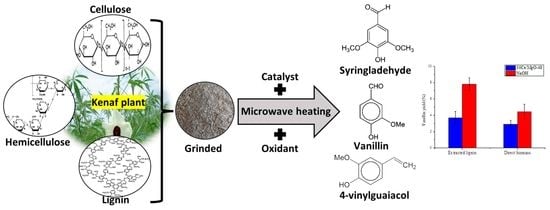
Synthesis of Ce/MgO Catalysts for Direct Oxidation of Hibiscus cannabinus Stalks to Vanillin
Abstract
1. Introduction
2. Result
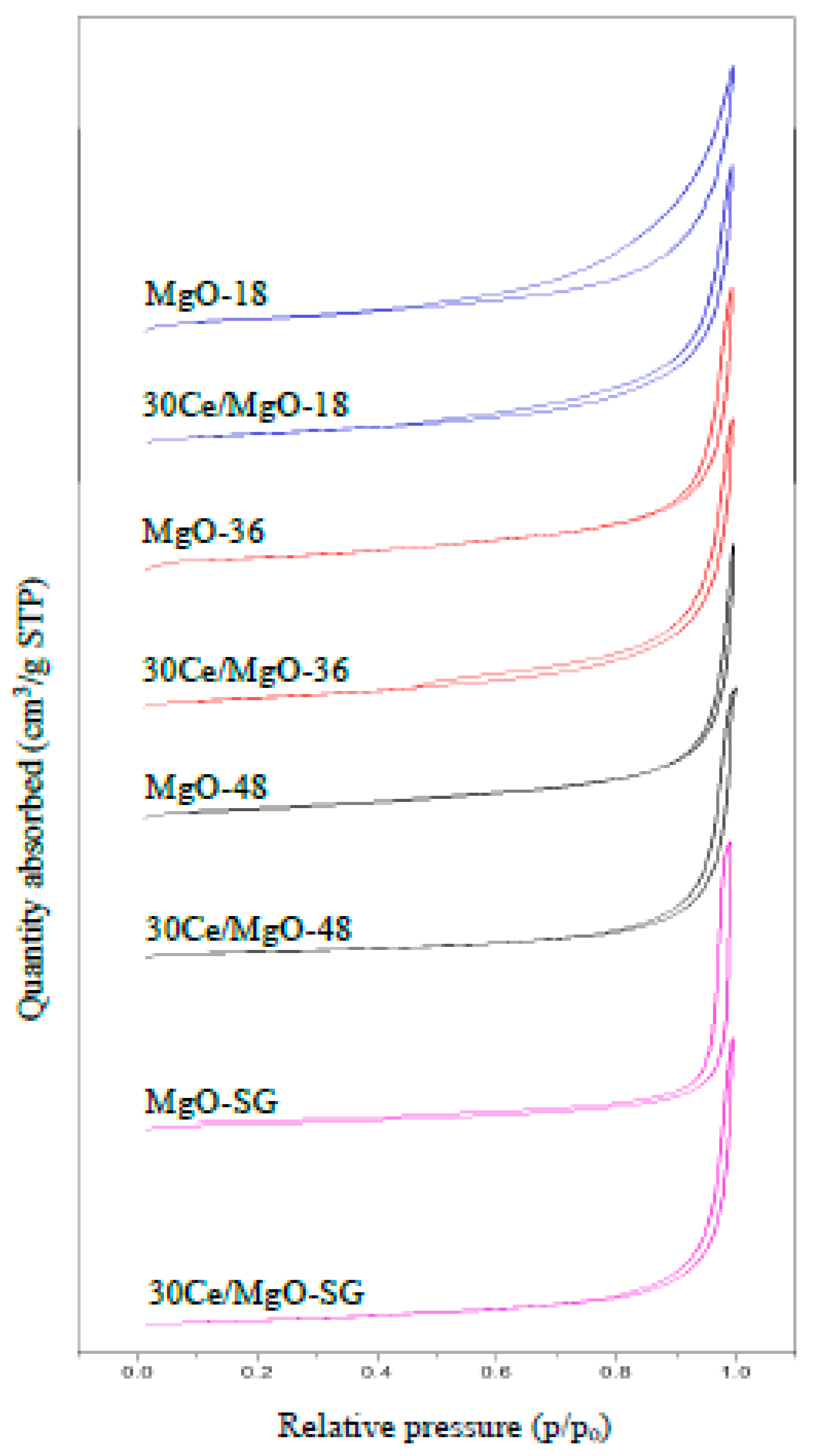
2.1. Catalyst Characterization
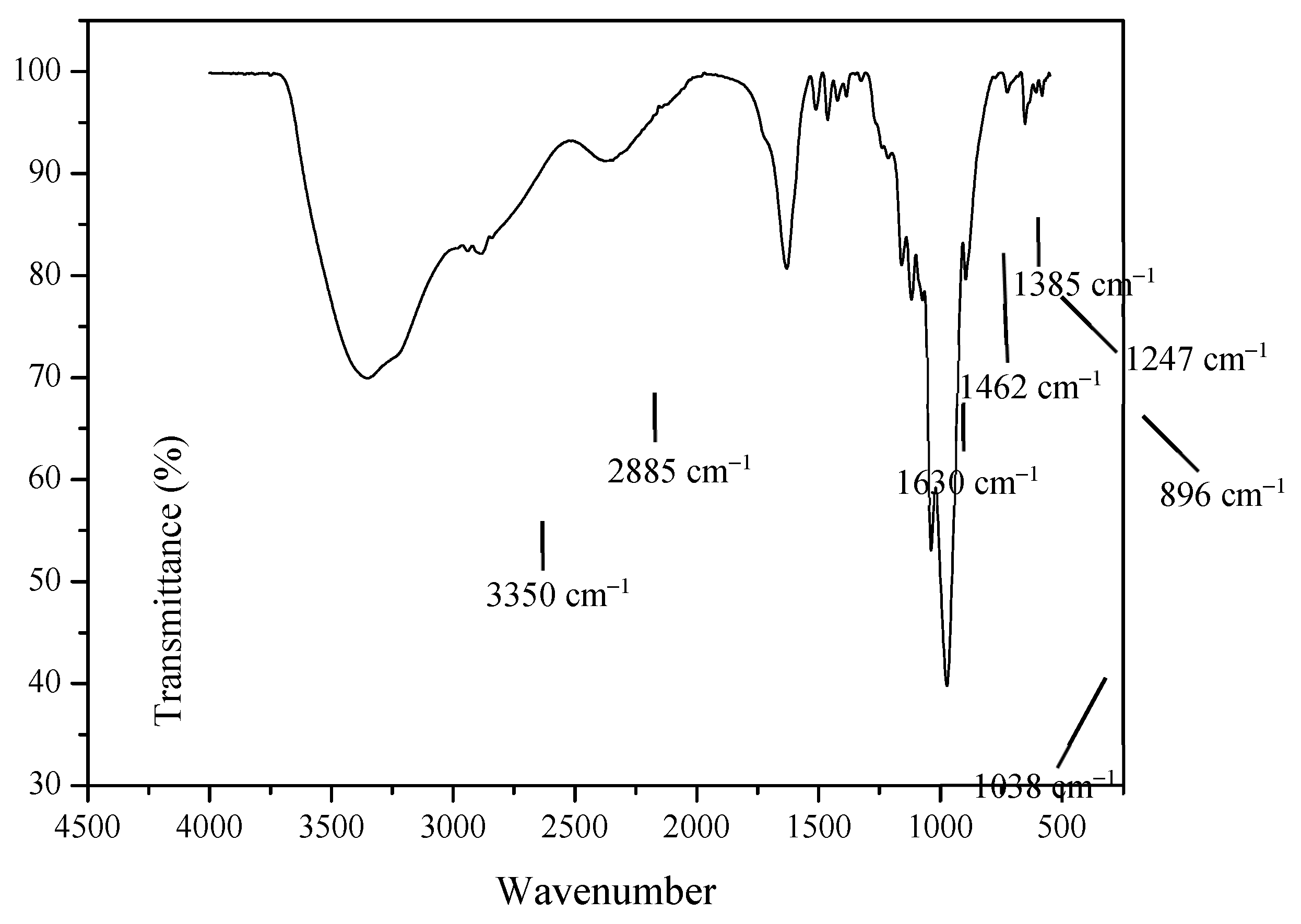
2.2. Lignin Extraction
| IR Band (cm−1) | Assignment | ||
|---|---|---|---|
| Literature Data | [ref] | Data Obtained | |
| 3500–3100 | [35] | 3350 | Stretching vibration of O–H bonds |
| 2900–2800 | [38] | 2886 | C–H stretching vibration in the methyl and methylene groups |
| 1650–1590 | [40] | 1630 | C=C aromatic vibrations |
| 1464–1424 | [39] | 1462 | C–H bending vibration in the methyl groups |
| 1272–1265 | [40] | 1247 | C=O stretching vibration in guaiacyl propane units (G) |
| 1340–1330 | [40] | 1385 | C=O stretching vibration in syringyl propane units (S) |
| 1130–1035 | [41] | 1038 | C=O deformation in secondary and primary alcohol or aliphaticesters |
| 897–889 | [42] | 896 | C–O–C stretching at the β-(1, 4)-glycosidic linkage in cellulose |
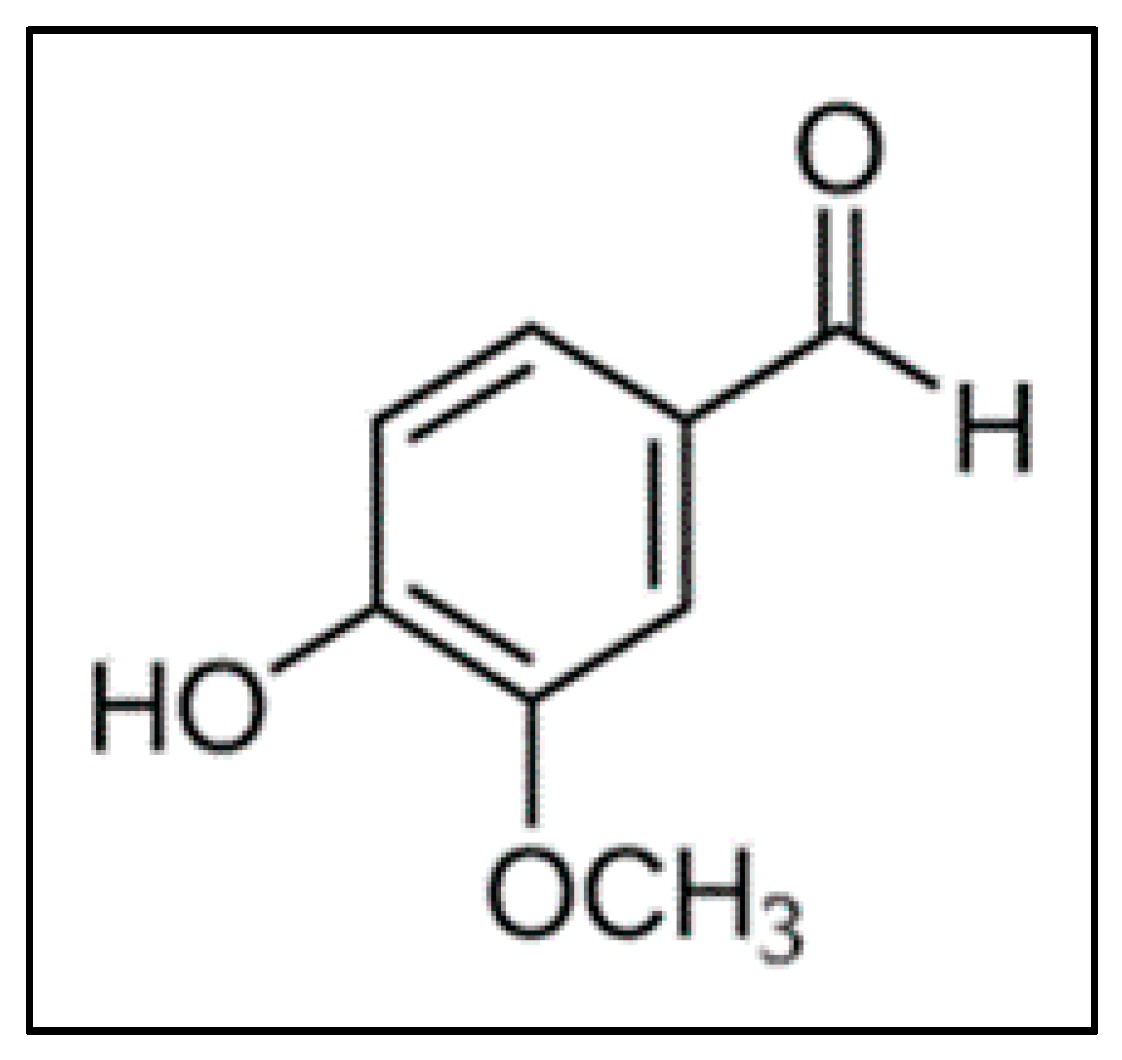
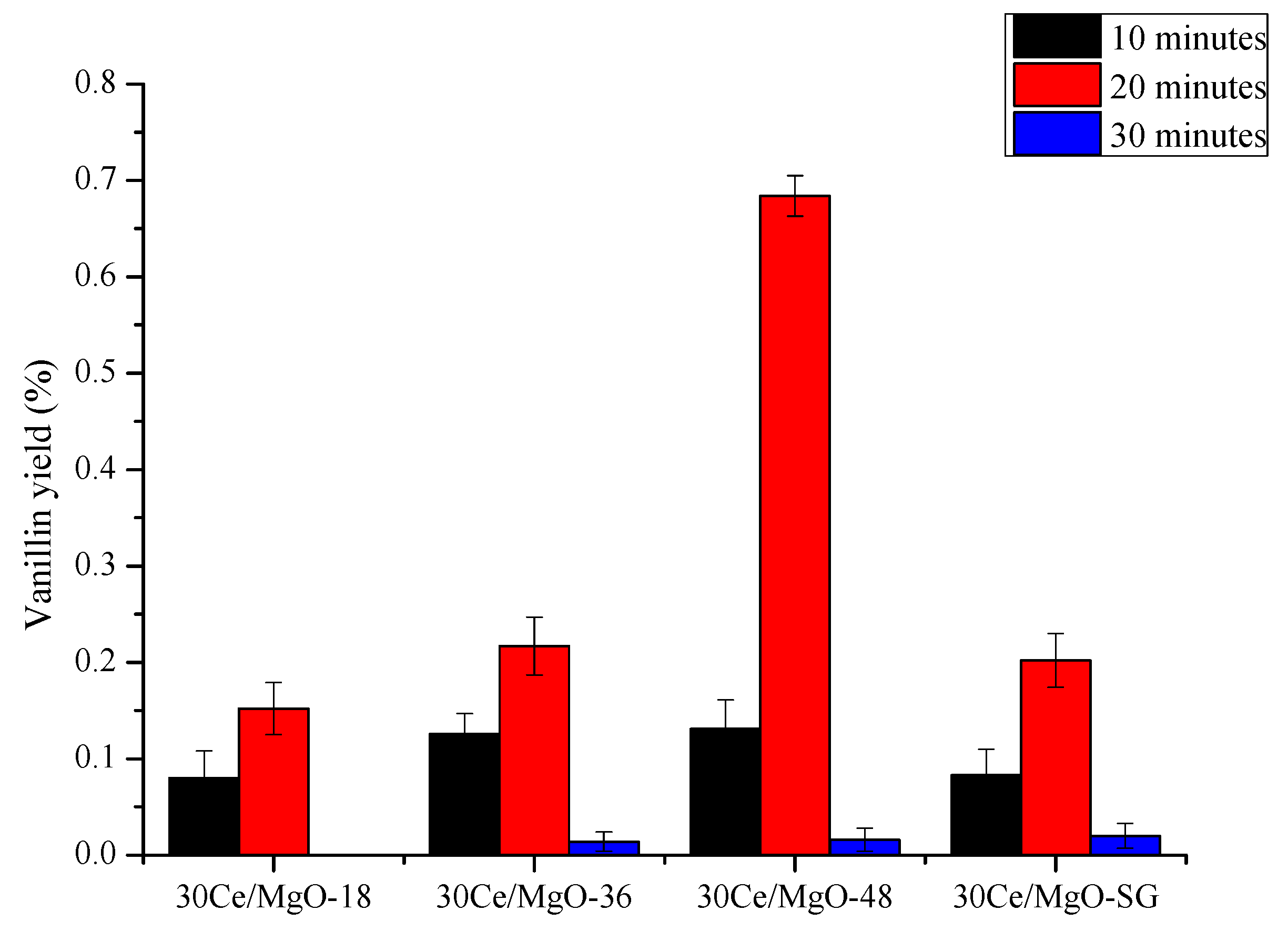
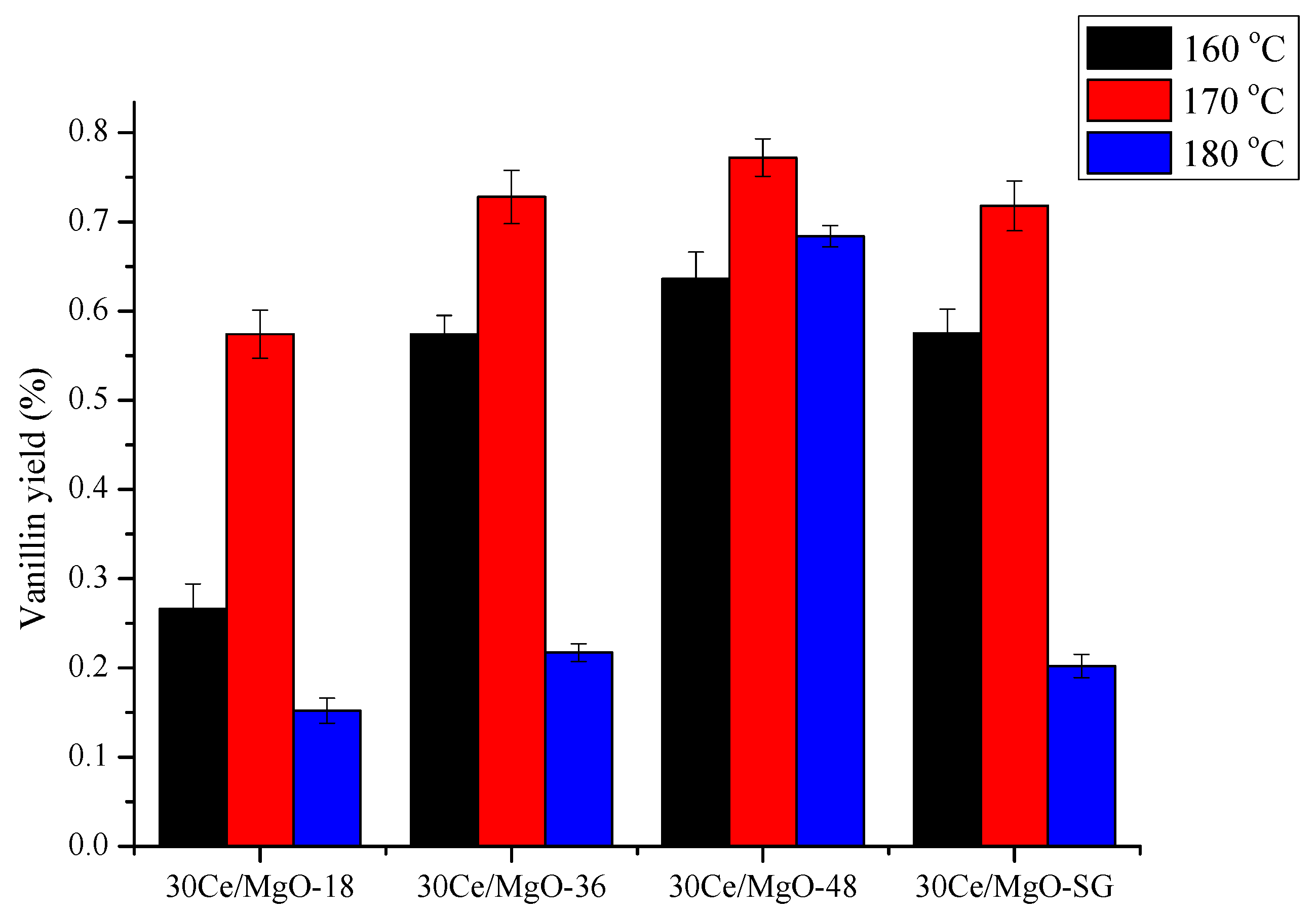
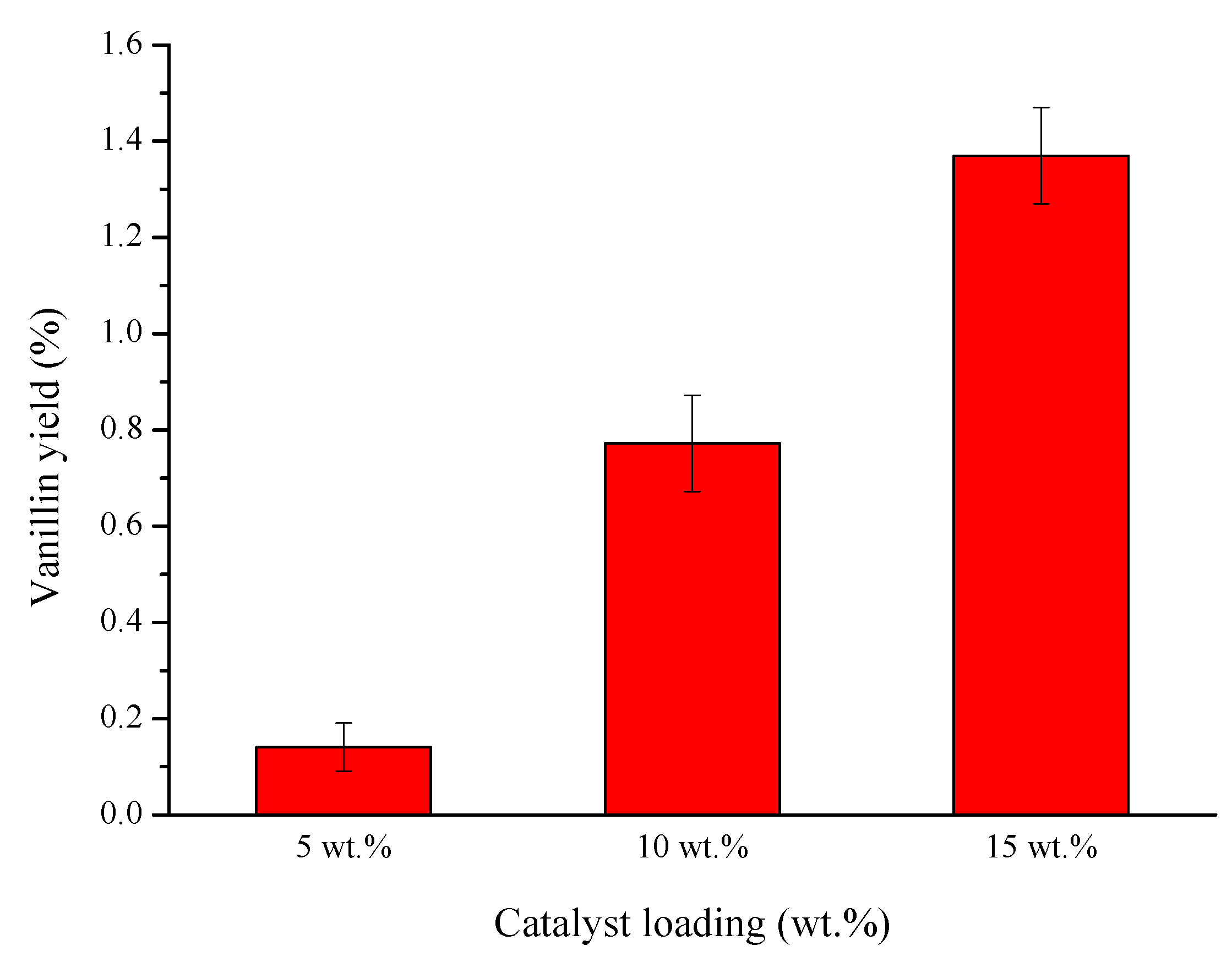
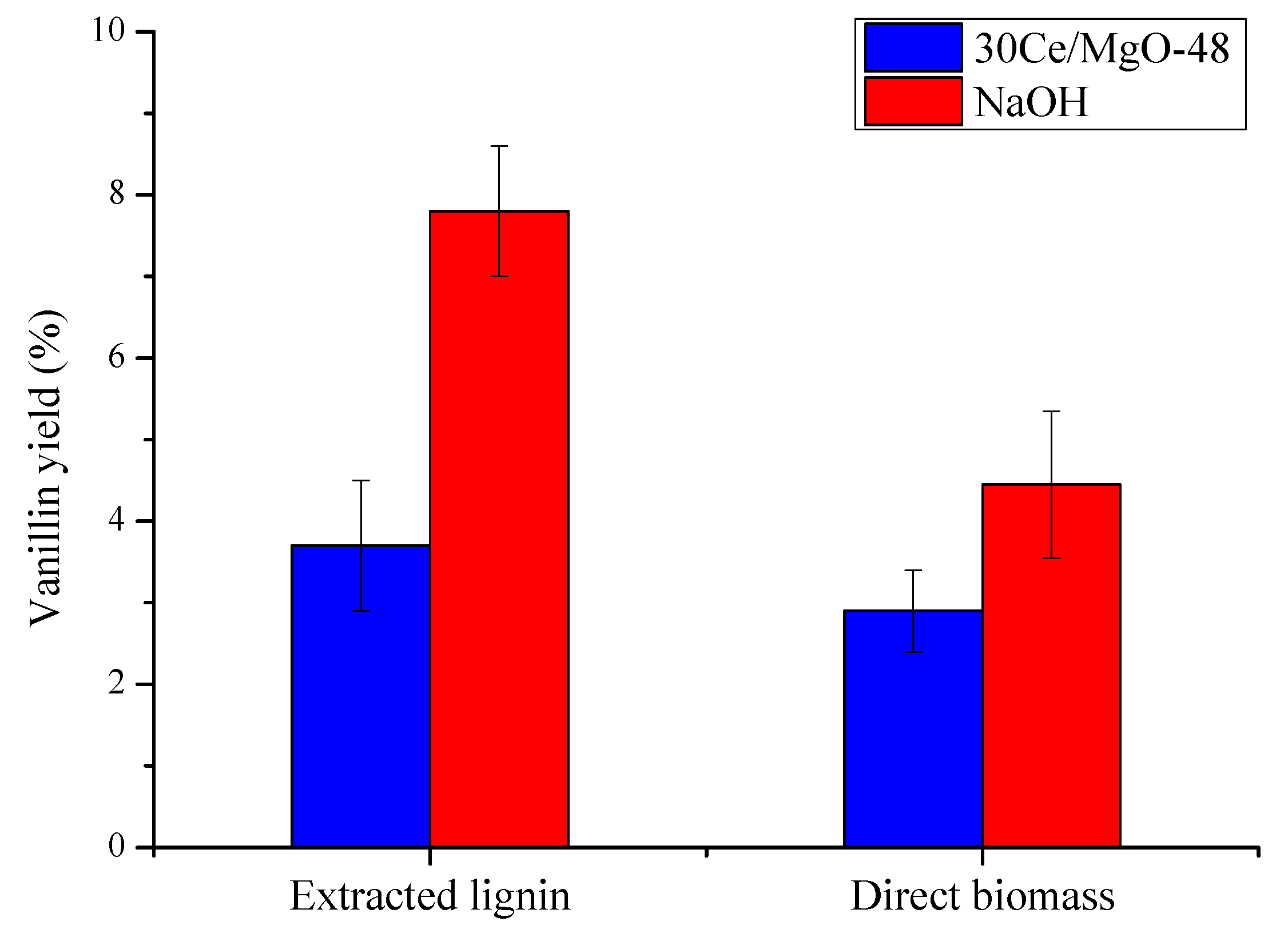
2.3. Catalyst Testing
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Lignin Extraction and Characterization
3.4. Catalytic Oxidation of Raw Kenaf
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maxence, F.; Bernard, B.; Sylvain, C. Vanillin production from lignin and its use as a renewable chemical. ACS Sustain. Chem. Eng. 2016, 4, 35–46. [Google Scholar]
- Akriti, A.; Nirmala, K.; Soumitra, B. Derivatives and applications of lignin—An insight. Scitech J. 2014, 1, 30–36. [Google Scholar]
- Nicholas, J.W.; Melinda, J.M.; Arjan, N. Vanillin. Phytochemistry 2003, 63, 505–515. [Google Scholar]
- Pesach, L.; Matthew, V.D.; Alex, V.D. Pollination of vanilla and evolution in the Orchidaceae. Lindleyana 2006, 75, 926–929. [Google Scholar]
- Pinto, P.; Da Silva, E.A.B.; Rodrigues, A.E. Lignin as source of fine chemicals: Vanillin and syringaldehyde. Methods Mol. Biol. 2012, 381–420. [Google Scholar]
- Alireza, S.; Sepideh, M.R.; Ali, G. Oxidative production of vanillin from industrial lignin using oxygen and nitrobenzene: A comparative study. Int. J. Farming Appl. Sci. 2013, 2, 1165–1171. [Google Scholar]
- Donostia, S.S. Lignin extraction, purification & depolymerization study. Scitech J. 2012, 1, 30–36. [Google Scholar]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Sales, F.G.; Maranhão, L.C.; Filho, N.M.L.; Abreu, C.A. Experimental evaluation and continuous catalytic process for fine aldehyde production from lignin. Chem. Eng. Sci. 2007, 62, 5386–5391. [Google Scholar] [CrossRef]
- Araújo, J.; Grande, C.; Rodrigues, A.E. Vanillin production from lignin oxidation in a batch reactor. Chem. Eng. Res. Des. 2010, 88, 1024–1032. [Google Scholar] [CrossRef]
- Valery, E.T.; Konstantin, L.K.; Ekaterina, A.S.; Nikolay, T.; Yulia, V.C.; Olga, V.B.; Boris, N.K.; Laurent, D. Processing pine wood into vanillin and glucose by sequential catalytic oxidation and enzymatic hydrolysis. J. Wood Chem. Technol. 2016, 37, 43–51. [Google Scholar]
- Chen, Q.; Masakazu, K.; Keiichiro, K.; Kanade, T.; Suguru, O.; Takashi, W. direct production of vanillin from wood particles by copper oxide–peroxide reaction production of vanillin from wood particles by copper oxide–peroxide reaction. ACS Sustain. Chem. Eng. 2017, 5, 11551–11557. [Google Scholar]
- Chaudhary, R.; Dhepe, P.L. Solid base catalyzed depolymerization of lignin into low molecular weight products. Green Chem. 2017, 19, 778–788. [Google Scholar] [CrossRef]
- Long, J.; Zhang, Q.; Wang, T.; Zhang, X.; Xu, Y.; Ma, L. An Efficient and Economical Process for Lignin Depolymerization in Biomass-derived Solvent Tetrahydrofuran. Bioresour. Technol. 2014, 154, 10–17. [Google Scholar] [CrossRef]
- Biswas, B.; Kumar, A.; Krishna, B.B.; Bhaskar, T. Effects of solid base catalysts on depolymerization of alkali lignin for the production of phenolic monomer compounds. Renew. Energy 2021, 175, 270–280. [Google Scholar] [CrossRef]
- Ververis, C.; Georghiou, K.; Christodoulakis, N.; Santas, P.; Santas, R. Fiber Dimensions, Lignin and Cellulose Content of Various Plant Materials and Their Suitability for Paper Production. Ind. Crops Prod. 2004, 19, 245–254. [Google Scholar] [CrossRef]
- Badr, M.M.; Mobasherpoura, I.; Marzban, E.R.; Mortazavic, G. Synthesis of cubic MgO nanostructure by an easy hydrothermal-calcinations method. J. Ceram. Process. Res. 2014, 15, 88–92. [Google Scholar]
- Wahab, R.; Ansari, S.; Dar, M.; Kim, Y.S.; Shin, H.S. Synthesis of magnesium oxide nanoparticles by sol-gel process. Mater. Sci. Forum 2007, 558–559, 983–986. [Google Scholar] [CrossRef]
- Bing, W.; Xingaoyuan, X.; Ren, H.; Zhi, Y.H. Preparation of MgO nanocrystals and catalytic mechanism on phenol ozonation. RSC Adv. 2017, 7, 43464–43473. [Google Scholar]
- Li, X.; Xiao, W.; He, G.; Zheng, W.; Yu, N.; Tan, M. Pore size and surface area control of MgO nanostructures using a surfac-tant-templated hydrothermal process: High adsorption capability to azo dyes. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 408, 79–86. [Google Scholar] [CrossRef]
- Widiyandari, H.; Sukmawati, A.N.; Sutanto, S.; Yudha, C.; Purwanto, A. Synthesis of LiNi0.8Mn0.1Co0.1O2 cathode material by Hydrothermal Method for High Energy Density Lithium Ion Batery. In Proceedings of the 9th International Conference on Physics and Its Applications: Journal of Physics, Surakarta, Indonesia, 14 August 2019. [Google Scholar]
- Wang, J.A.; Novaro, O.; Bokhimi, X.; López, T.; Gómez, R.; Navarrete, J.; Llanos, M.E.; López-Salinas, E. Structural defects and acidic and basic sites in Sol−Gel MgO. J. Phys. Chem. B 1997, 101, 7448–7451. [Google Scholar] [CrossRef]
- Ahmed, S. Development of Modified Si-MCM-41 and Studies on Its Behaviour for CO2 Adsorption. Ph.D. Thesis, Universiti Teknologi PETRONAS, Seri Iskandar, Malaysia, 2014. [Google Scholar]
- Li, Z.P.; Wan, Y.Y.; Ji, F.W.; Jun, C.; Chuan, G.F.; Qian, F.Z. Low temperature synthesis of magnesium oxide and spinel powders by a Sol-gel Process. Mater. Res. 2010, 13, 339–343. [Google Scholar]
- Krishnakanth, R.; Jayakumar, G.; Irudayaraj, A.A.; Raj, A.D. Structural and magnetic properties of NiO and Fe-doped NiO nanoparticles synthesized by chemical co-precipitation method. Mater. Today Proc. 2016, 3, 1370–1377. [Google Scholar] [CrossRef]
- Setoudeh, N.; Zamani, C.; Sajjadnejad, M. Mechanochemical synthesis of nanostructured MgXNi1−XO compound by Mg-NiO mixture. Mater. Sci. 2017, 50, 51–59. [Google Scholar]
- Bindu, P.; Thomas, S. Estimation of lattice strain in ZnO nanoparticles: X-Ray peak profile analysis. J. Theory Appl. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef]
- Soo-Min, P.; Yoon-Chae, N.; Chunghee, N. Effects of hydrothermal treatment duration on morphology of WO3 nanostructures. J. Nanosci. Nanotechnol. 2017, 17, 7719–7722. [Google Scholar]
- Chary, K.V.; Naresh, D.; Vishwanathan, V.; Sadakane, M.; Ueda, W. Vapour phase hydrogenation of phenol over Pd/C catalysts: A relationship between dispersion, metal area and hydrogenation activity. Catal. Commun. 2007, 8, 471–477. [Google Scholar] [CrossRef]
- Roy, M.; Ghosh, S.; Naskar, M.K. Synthesis of morphology controllable porous Co3O4 nanostructures with tunable textural properties and their catalytic application. Dalton Trans. 2014, 43, 10248–10257. [Google Scholar] [CrossRef]
- Matthias, T.; Katsumi, K.; Alexander, V.N.; James, P.O.; Francisco Rodriguez, E.R.; Jean, R.; Kenneth, S.W.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar]
- Zhang, H.; Hu, J.; Xie, J.; Wang, S.; Cao, Y. A solid-state chemical method for synthesizing MgO nanoparticles with superior adsorption properties. RSC Adv. 2019, 9, 2011–2017. [Google Scholar] [CrossRef]
- Chowdhury, I.H.; Bose, P.; Mandal, S.; Naskar, M.K. Effect of anion type on the synthesis of mesoporous nanostructured MgO, and its excellent adsorption capacity for the removal of toxic heavy metal ions from water. RSC Adv. 2016, 6, 6038–6047. [Google Scholar] [CrossRef]
- Sui, C.; Xing, L.; Cai, X.; Wang, Y.; Zhou, Q.; Li, M. Co-supported CeO2Nanoparticles for CO catalytic oxidation: Effects of different synthesis methods on catalytic performance. Catalysts 2020, 10, 243. [Google Scholar] [CrossRef]
- Mohamad Aini, N.A.; Othman, N.; Hussin, M.H.; Sahakaro, K.; Hayeemasae, N. Effect of extraction methods on the mo-lecular structure and thermal stability of kenaf (Hibiscus cannabinus core) biomass as an alternative bio-filler for rubber composites. Int. J. Biol. Macromol. 2020, 154, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wei, J.; Yuanming, Z.; He, W.; Fangwei, Z.; Kai, Y.; Guangting, H. A novel process of nanocellulose extraction from Kenaf Bast. Mater. Res. Express 2018, 5. [Google Scholar]
- Yuting, Z.; Yuhe, L.; Wei, L.; Jing, L.; Xiangbo, S.; Lungang, C.; Chenguang, W.; Bert, F.S.; Longlong, M. complementing vanillin and cellulose production by oxidation of lignocellulose with stirring control. ACS Sustain. Chem. Eng. 2020, 8, 2361–2374. [Google Scholar]
- Khalil, H.P.A.; Ismail, H.; Rozman, H.D.; Ahmad, M.N. The Effect of acetylation on interfacial shear strength between plant fiber and various matrices. Eur. Polym. J. 2001, 37, 1037–1045. [Google Scholar] [CrossRef]
- Rodrigues, J.; Faix, O.; Pereira, H. Determination of lignin content of Eucalyptus globulus wood using FTIR spectroscopy. Holzforsch 1998, 52, 46–50. [Google Scholar] [CrossRef]
- Hassan, N.S.; Badri, K.H. Lignin recovery from alkaline hydrolysis and glycerolysis of oil palm fiber. In Proceedings of the 2014 Universiti Kebangsaan Malaysia, Faculty of Science and Technology 2014 Postgraduate Colloquium, Selangor, Malaysia, 9–11 April 2014. [Google Scholar]
- Zhao, J.; Xiuwen, W.; Hu, J.; Liu, Q.; Shen, D.; Xiao, R. Thermal degradation of softwood lignin and hardwood lignin by TG-FTIR and Py-GC/MS. Polym. Degrad. Stab. 2014, 108, 133–138. [Google Scholar] [CrossRef]
- Mohd Warid, M.N.; Ariffin, H.; Hassan, M.A.; Shirai, Y. Optimization of Superheated Steam Treatment to Improve Surface Modification of Oil Palm Biomass Fiber. BioResources 2016, 11, 5780–5796. [Google Scholar] [CrossRef]
- Haridass, K. Synthesis of Renewable Vanillin from Pineapple Leaves Lignin. In Final Year Project Dissertation, Degree; Universiti Teknologi PETRONAS: Seri Iskandar, Malaysia, 2018. [Google Scholar]
- Das, L.; Xu, S.; Shi, J. Catalytic oxidation and depolymerization of lignin in aqueous ionic liquid. Front. Energy Res. 2017, 5, 5. [Google Scholar] [CrossRef]
- Nolan, M.; Fearon, J.E.; Watson, G.W. Oxygen vacancy formation and migration in ceria. Solid State Ion. 2006, 177, 3069–3074. [Google Scholar] [CrossRef]
- Keating, P.R.; Scanlon, D.O.; Watson, G.W. The nature of oxygen states on the surfaces of CeO2 and La-doped CeO2. Chem. Phys. Lett. 2014, 608, 239–243. [Google Scholar] [CrossRef]
- Gu, T.; Liu, Y.; Weng, X.; Wang, H.; Wu, Z. The enhanced performance of ceria with surface sulfation for selective catalytic reduction of NO by NH3. Catal. Commun. 2010, 12, 310–313. [Google Scholar] [CrossRef]
- Tarabanko, V.E.; Tarabanko, N. Catalytic oxidation of lignins into the aromatic aldehydes: General process trends and development prospects. Int. J. Mol. Sci. 2017, 18, 2421. [Google Scholar] [CrossRef] [PubMed]
- Tarabanko, V.E.; Gulbis, G.R.; Ivanchenko, N.M.; Kuznetsov, B.N.; Kudryashev, A.V. Study of wood and lignosulfonates processing into fine products. Chem. Sustain. Dev. 1996, 4, 405–417. [Google Scholar]
- Amarasekara, A. Acidic ionic liquids. Chem. Rev. 2016, 116, 6133–6183. [Google Scholar] [CrossRef]
- Prado, R.; Erdocia, X.; de Gregorio, G.F.; Labidi, J.; Welton, T. Willow lignin oxidation and depolymerization under low cost ionic liquid. ACS Sustain. Chem. Eng. 2016, 4, 5277–5288. [Google Scholar] [CrossRef]
- Lu, J.; Wang, M.; Zhang, X.; Heyden, A.; Wang, F. β-O-4 bond cleavage mechanism for lignin model compounds over Pd catalysts identified by combination of first-principles calculations and experiments. ACS Catal. 2016, 6, 5589–5598. [Google Scholar] [CrossRef]
- Jiang, L.; Guo, H.; Li, C.; Zhou, P.; Zhang, Z. Selective cleavage of lignin and lignin model compounds without external hydrogen, catalyzed by heterogeneous nickel catalysts. Chem. Sci. 2019, 10, 4458–4468. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Lopes, A.M.; Gomes, J.R.B.; Coutinho, J.A.P.; Silvestre, A.J.D. Novel insights into biomass delignification with acidic deep eutectic solvents: A mechanistic study of β-O-4 ether bond cleavage and the role of the halide counterion in the catalytic performance. Green Chem. 2020, 22, 2474–2487. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, H.; Wu, X.; Li, R.; Zhang, Q.; Wang, Y. Oxidative conversion of lignin and lignin model compounds catalyzed by CeO2-supported Pd nanoparticles. Green Chem. 2015, 17, 5009–5018. [Google Scholar] [CrossRef]
- Ma, Y.; Du, Z.; Liu, J.; Xia, F.; Xu, J. Selective oxidative C–C bond cleavage of a lignin model compound in the presence of acetic acid with a vanadium catalyst. Green Chem. 2015, 17, 4968–4973. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Lu, J.; Zeng, S.; Wang, M.; Luo, N.; Xu, S.; Wang, F. Photocatalytic cleavage of C–C Bond in lignin models under visible light on mesoporous graphitic carbon nitride through π–π stacking interaction. ACS Catal. 2018, 8, 4761–4771. [Google Scholar] [CrossRef]
- Fan, H.; Yang, Y.; Song, J.; Ding, G.; Wu, C.; Yang, G.; Han, B. One-pot sequential oxidation and aldol-condensation reactions of veratryl alcohol catalyzed by the Ru@ZIF-8 + CuO/basic ionic liquid system. Green Chem. 2014, 16, 600–604. [Google Scholar] [CrossRef]
- Xu, C.; Arancon, R.A.D.; Labidi, J.; Luque, R. Lignin depolymerisation strategies: Towards valuable chemicals and fuels. Chem. Soc. Rev. 2014, 43, 7485–7500. [Google Scholar] [CrossRef] [PubMed]
- Shilpy, M.; Ehsan, M.A.; Ali, T.H.; Abd Hamid, S.B.; Ali, M.E. Performance of Cobalt Titanate Towards H2O2 Based Catalytic Oxidation of Lignin Model Compound. RSC Adv. 2015, 5, 9644–79653. [Google Scholar] [CrossRef]
- Saberi, F.; Rodriguez-Padrón, D.; Garcia, A.; Shaterian, H.; Luque, R. Unprecedented Proline-Based Heterogeneous Organocatalyst for Selective Production of Vanillin. Catalysts 2018, 8, 167. [Google Scholar] [CrossRef]
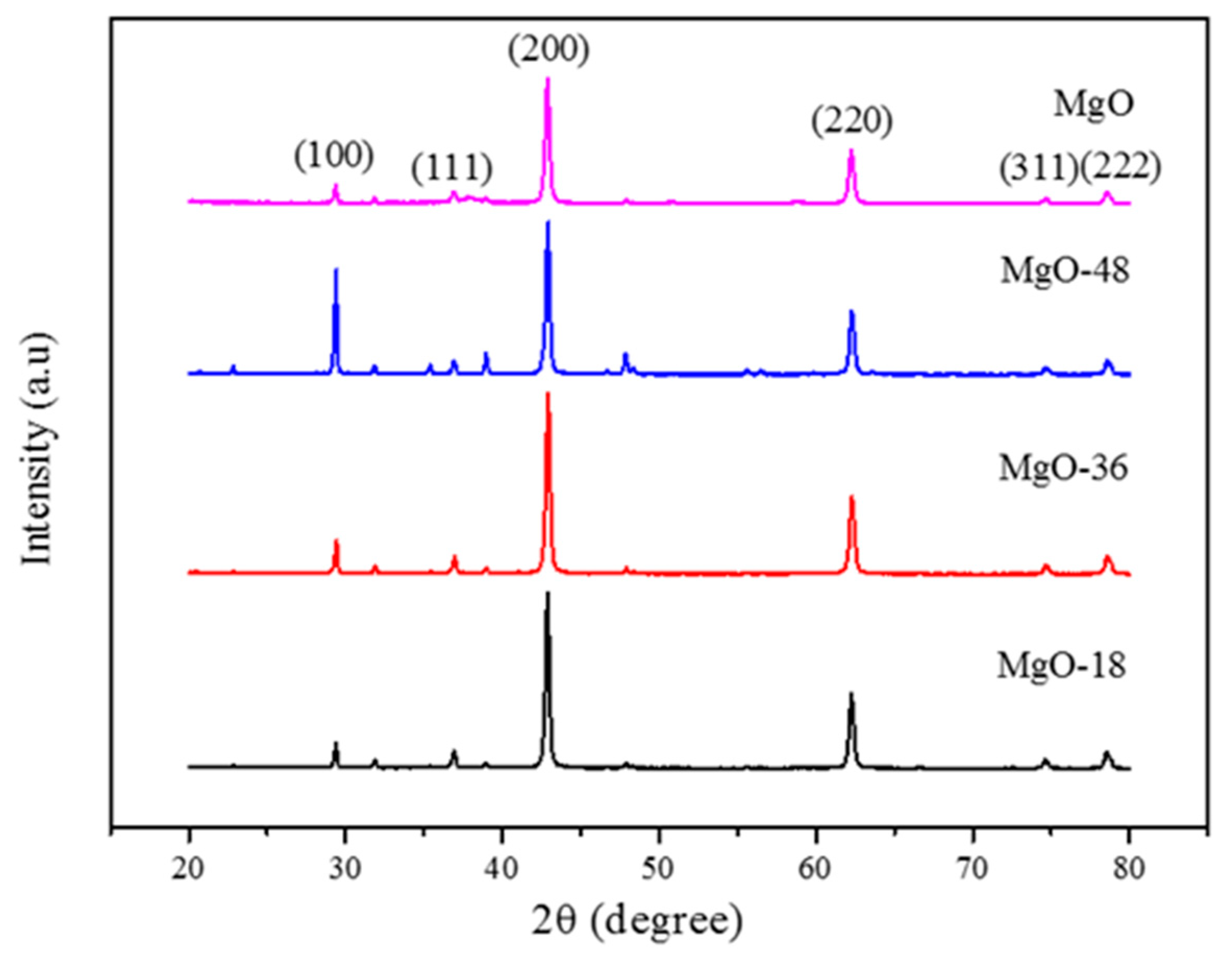
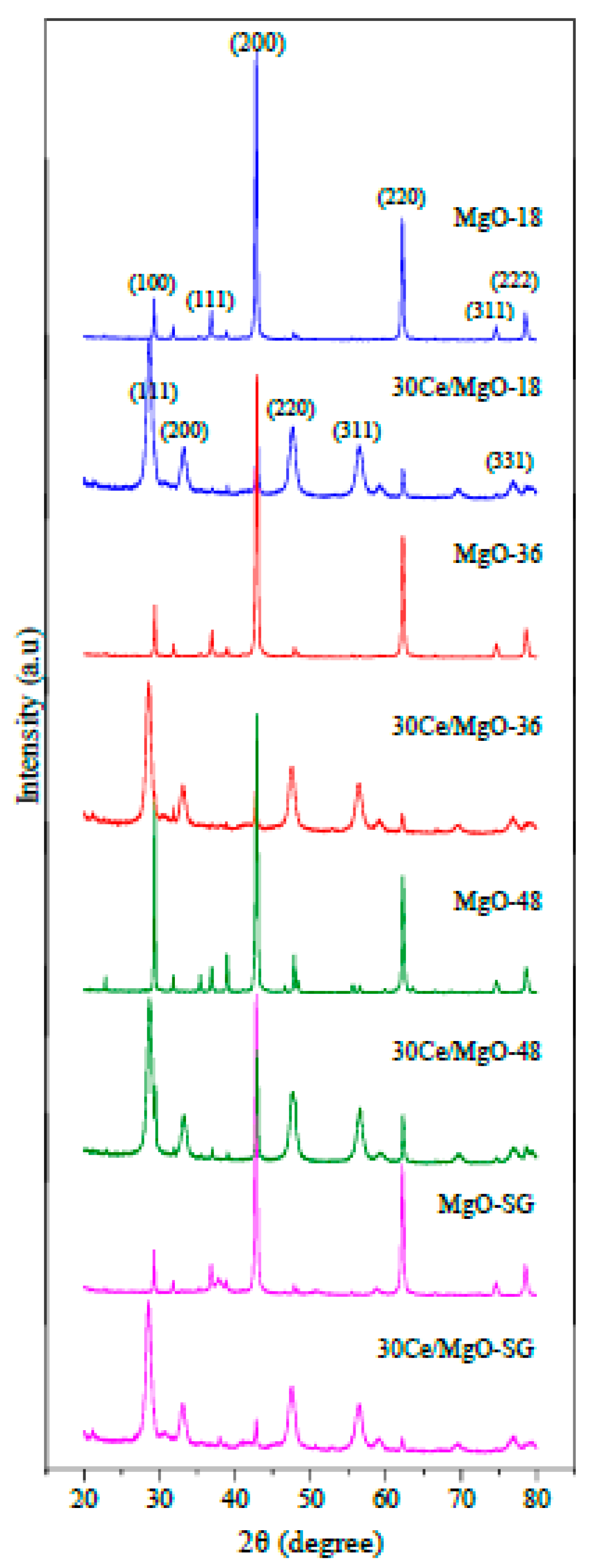


| Catalyst | Crystallite Size (nm) | |
|---|---|---|
| Mg (200) | Ce (111) | |
| MgO-18 | 36.5 | - |
| MgO-36 | 29.9 | - |
| MgO-48 | 48.4 | - |
| MgO-SG | 32.8 | - |
| 30Ce/MgO-18 | 43.9 | 3.9 |
| 30Ce/MgO-36 | 50.7 | 4 |
| 30Ce/MgO-48 | 40.0 | 14.4 |
| 30-Ce/MgO-SG | 45.3 | 7.1 |
| Catalyst | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) | Crystallite Size (nm) |
|---|---|---|---|---|
| MgO-18 | 28.4 | 0.193 | 21.7 | 36.5 |
| MgO-36 | 34.6 | 0.285 | 36.4 | 29.9 |
| MgO-48 | 13.4 | 0.076 | 14.4 | 48.4 |
| MgO-SG | 27.3 | 0.146 | 20.4 | 32.8 |
| 30Ce/MgO-18 | 27.5 | 0.165 | 18.8 | 43.9 |
| 30Ce/MgO-36 | 25.9 | 0.158 | 18.2 | 50.7 |
| 30Ce/MgO-48 | 29.3 | 0.293 | 32.3 | 40.0 |
| 30Ce/MgO-SG | 28.4 | 0.247 | 30.2 | 45.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khairul Anuar, N.A.S.I.; Ramli, A.; Jun Wei, L. Synthesis of Ce/MgO Catalysts for Direct Oxidation of Hibiscus cannabinus Stalks to Vanillin. Catalysts 2021, 11, 1449. https://doi.org/10.3390/catal11121449
Khairul Anuar NASI, Ramli A, Jun Wei L. Synthesis of Ce/MgO Catalysts for Direct Oxidation of Hibiscus cannabinus Stalks to Vanillin. Catalysts. 2021; 11(12):1449. https://doi.org/10.3390/catal11121449
Chicago/Turabian StyleKhairul Anuar, Nur Akila Syakida Idayu, Anita Ramli, and Lim Jun Wei. 2021. "Synthesis of Ce/MgO Catalysts for Direct Oxidation of Hibiscus cannabinus Stalks to Vanillin" Catalysts 11, no. 12: 1449. https://doi.org/10.3390/catal11121449
APA StyleKhairul Anuar, N. A. S. I., Ramli, A., & Jun Wei, L. (2021). Synthesis of Ce/MgO Catalysts for Direct Oxidation of Hibiscus cannabinus Stalks to Vanillin. Catalysts, 11(12), 1449. https://doi.org/10.3390/catal11121449