An Alkalothermophilic Amylopullulanase from the Yeast Clavispora lusitaniae ABS7: Purification, Characterization and Potential Application in Laundry Detergent
Abstract
1. Introduction
2. Results and Discussion
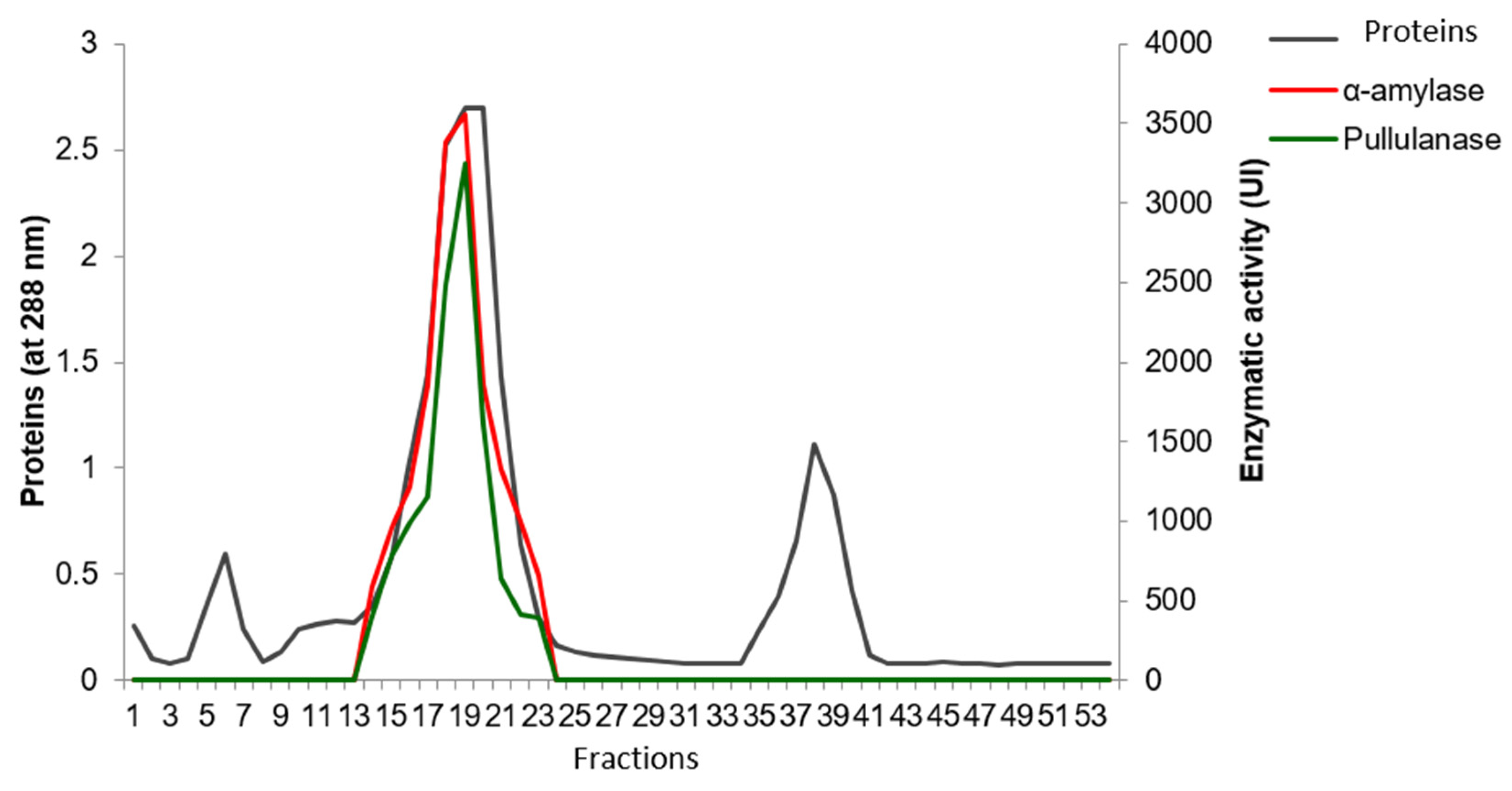
2.1. Purification of Enzymes
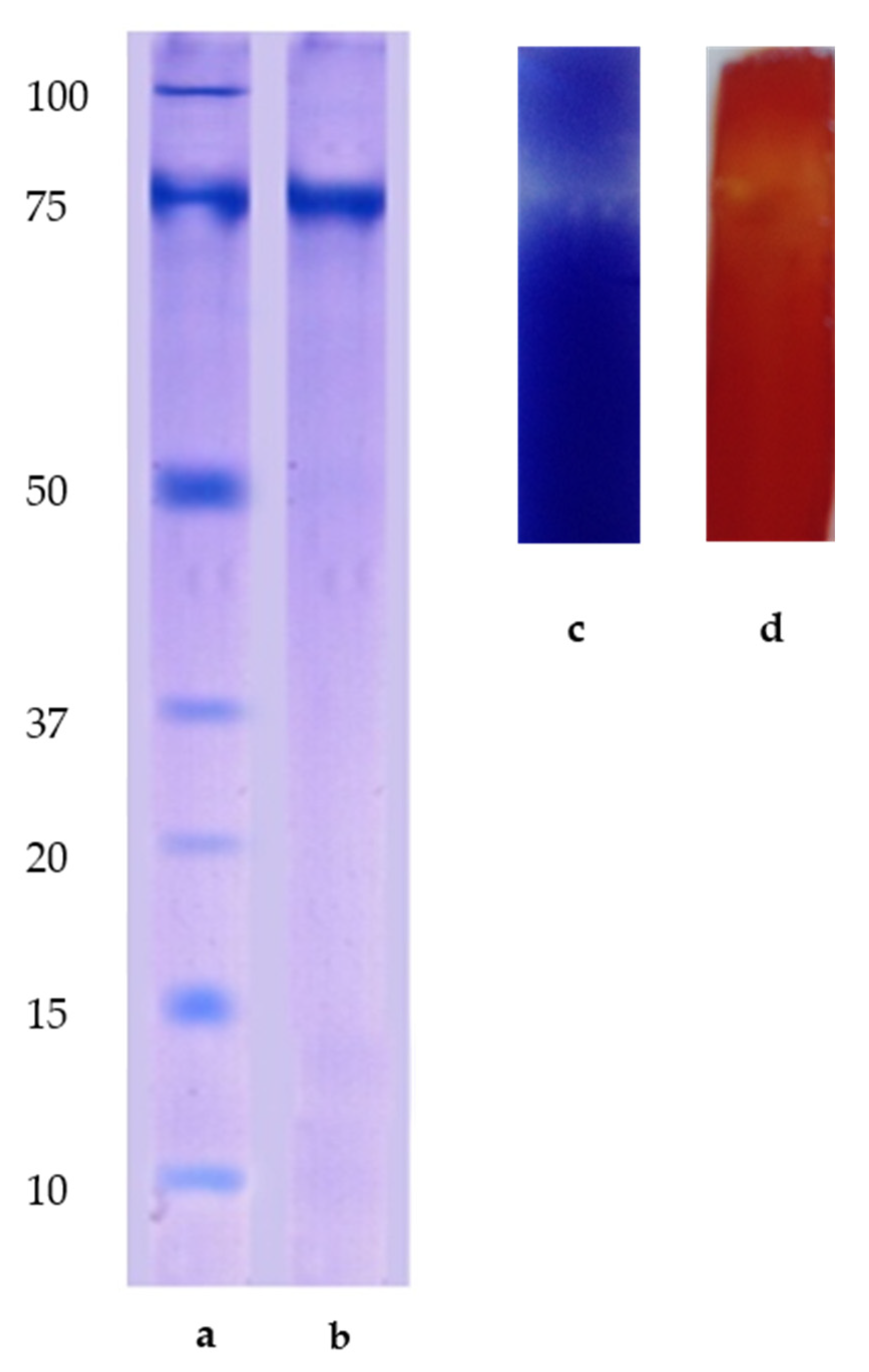
2.2. SDS-PAGE Analysis
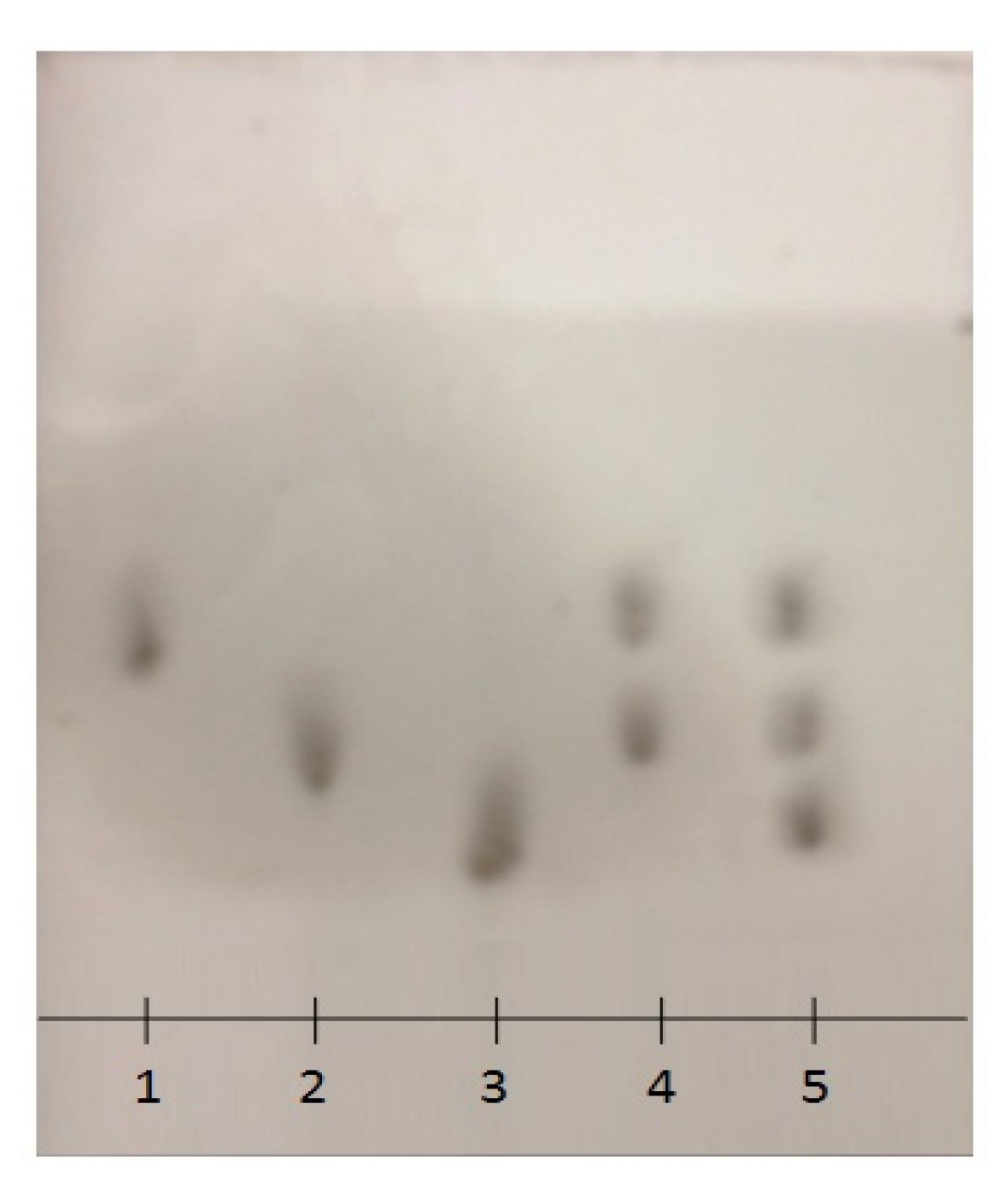
2.3. Thin Layer Chromatography (TLC)
2.4. Physicochemical Parameters of the Studied Enzymes
2.4.1. Effect of Temperature on the Amylopullulanase Activity
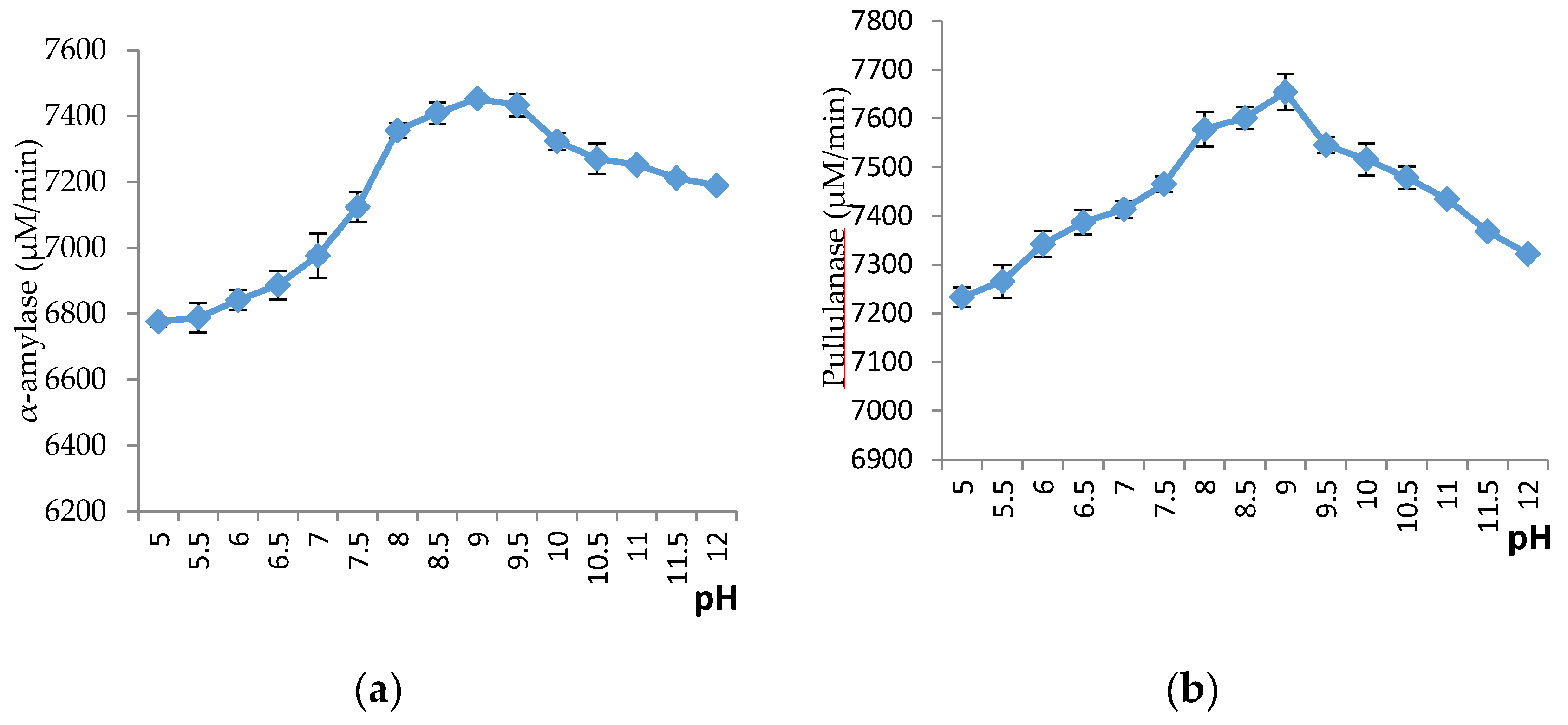
2.4.2. Effect of pH on α-Amylase and Pullulanase Activities
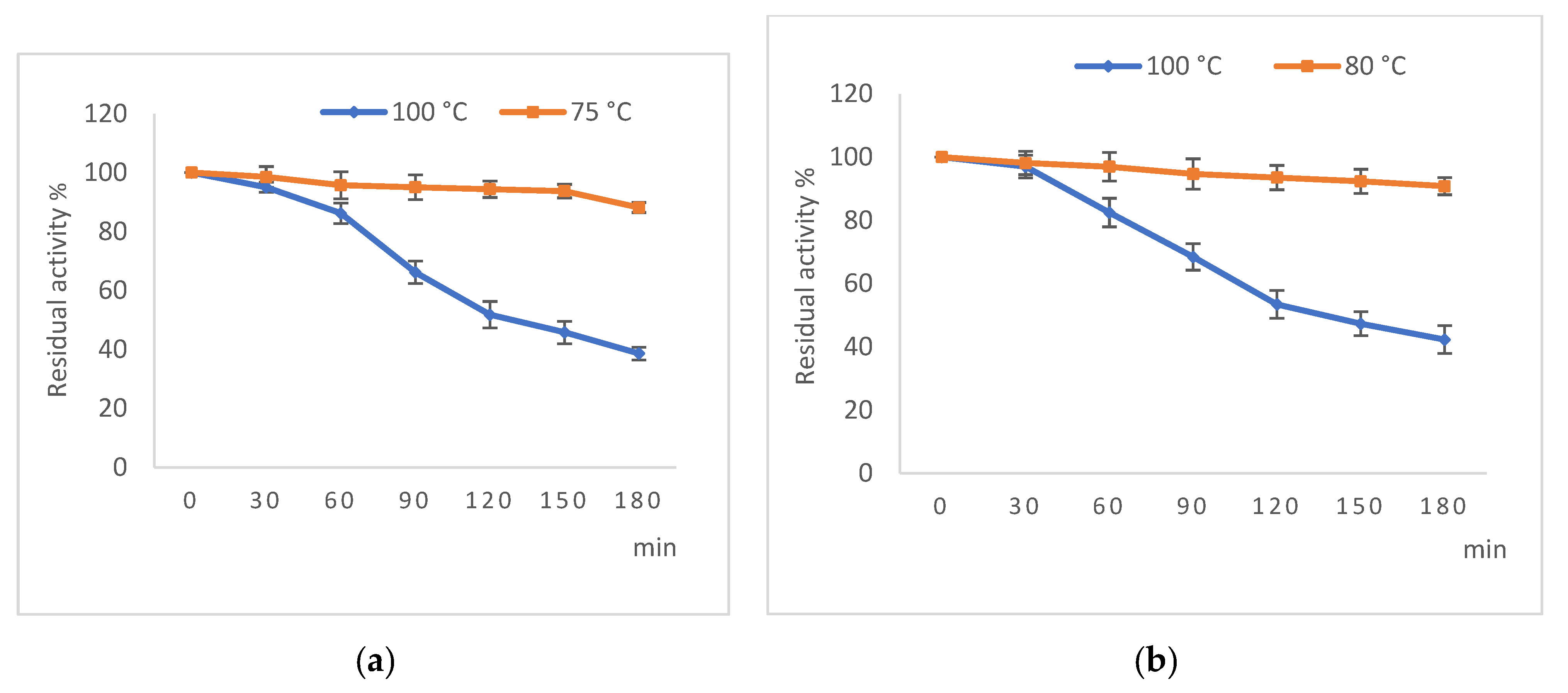
2.4.3. Study of Thermal Stability of α-Amylase and Pullulanase Activities
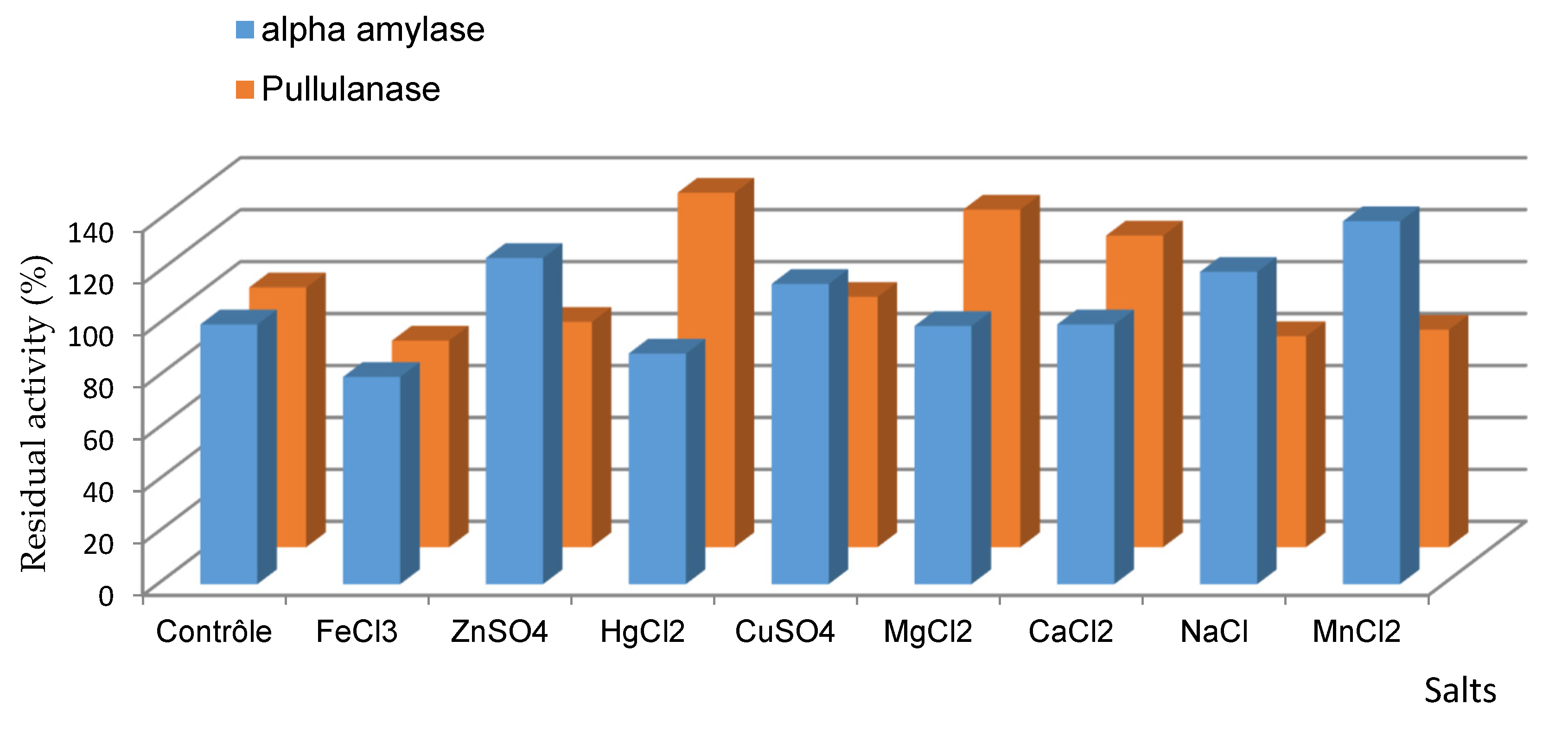
2.5. Effect of Different Salts and Chemical Reagents on the α-Amylase and Pullulanase Activities of C. lusitaniae ABS7
2.5.1. Effect of Salts
2.5.2. Influence of Different Chemical Reagents
2.6. Compatibility Test with Various Commercial Laundry Detergents
2.7. Wash Performance Analysis
3. Materials and Methods
3.1. Yeast
3.1.1. Ability of Yeasts to Produce Amylolytic Enzymes
3.1.2. Study of the Inoculum
3.2. Culture Media
3.2.1. Base Medium
3.2.2. Whey Processing
3.3. Production of α-Amylase and Pullulanase in Fermenter
3.4. Enzyme Activity and Protein Concentration Assays
3.5. Purification of the Enzyme
3.5.1. Protein Precipitation with Acetone
3.5.2. Sephacryl S200 Chromatography
3.5.3. Ion Exchange Chromatography
3.6. Electrophoresis SDS PAGE
3.7. Thin Layer Chromatography (TLC)
3.8. Enzyme Characterization
3.8.1. Effect of Temperature on Amylase Activity
3.8.2. Effects of pH on Amylase Activity
3.8.3. Thermostability
3.9. Effect of Metals Ions and Chelating Agent
3.10. Compatibility Test with Various Commercial Laundry Detergents
3.11. Analysis of the Wash Performance
- (A)
- 25 mL of tap water (control).
- (B)
- 20 mL of tap water and 5.0 mL of the purified alkaline amylopullulanase (500 U/mL).
- (C)
- 20 mL of tap water and 5.0 mL of heated detergent (7 mg/mL).
- (D)
- 20 mL of tap water and 5.0 mL of heated detergent (7 mg/mL), containing 500 U/mL of the purified alkaline enzyme.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tao, Z.; Dong, B.; Teng, Z.; Zhao, Y. The Classification of Enzymes by Deep Learning. IEEE Access 2020, 8, 89802–89811. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Jeevarathinam, G.; Kumar, S.K.S.; Muniraj, I.; Uthandi, S. Optimization and scale-up of α-amylase production by Aspergillus oryzae using solid-state fermentation of edible oil cakes. BMC Biotechnol. 2021, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Munguambe, D.M.; Tharmavaram, M.; Rawtani, D.; Agrawal, Y.K. Halloysite nanotubes—An efficient ‘nano-support’ for the immobilization of α-amylase. Appl. Clay Sci. 2017, 136, 184–191. [Google Scholar] [CrossRef]
- De Souza, P.M.; Magalhães, P.O. Application of microbial α-amylase in industry—A review. Braz. J. Microbiol. 2010, 41, 850–861. [Google Scholar] [CrossRef] [PubMed]
- BBC Research, Global Markets for Enzymes in Industrial Applications. Available online: https://www.bccresearch.com/market-research/biotechnology/global-markets-for-enzymes-in-industrial-applications-bio030k.html (accessed on 1 October 2018).
- Kim, T.U.; Gu, B.G.; Jeong, J.Y.; Byun, S.M.; Shin, Y.C. Purification and characterization of maltotetraose forming alkaline Bacillus strain GM 8901. Appl. Environ. Microbiol. 1995, 61, 3105–3112. [Google Scholar] [CrossRef]
- Far, B.E.; Ahmadi, Y.; Khosroushahi, A.Y.; Dilmaghani, A. Microbial Alpha-Amylase Production: Progress, Challenges and Perspectives. Adv. Pharm. Bull. 2020, 10, 350–358. [Google Scholar] [CrossRef]
- Hmidet, N.; Jemil, N.; Nasri, M. Simultaneous production of alkaline amylase and biosurfactant by Bacillus methylotrophicus DCS1: Application as detergent additive. Biodegradation 2018, 30, 247–258. [Google Scholar] [CrossRef]
- Ito, S.; Kobayashi, T.; Ozaki, K. Development of Detergent Enzymes. J. Appl. Glycosci. 2000, 47, 243–251. [Google Scholar] [CrossRef][Green Version]
- Mitidieri, S.; Souza Martinelli, A.H.; Schrank, A.; Vainstein, M.H. Enzymatic detergent formulation containing amylase from Aspergillus niger: A comparative study with commercial detergent formulations. Bioresour. Technol. 2006, 97, 1217–1224. [Google Scholar] [CrossRef]
- Raul, D.; Biswas, T.; Mukhopadhyay, S.; Das, S.K.; Gupta, S. Production and partial purification of alpha Amylase from Bacillus subtilis (MTCC 121) using solid state fermentation. Biochem. Res. Int. 2014, 2014, 568141. [Google Scholar] [CrossRef] [PubMed]
- Djekrif, D.S.; Gheribi, A.Z.; Meraihi, Z.; Bennamoun, L. Application of a statistical design to the optimization of culture medium for α-amylase production by Aspergillus niger ATCC 16404 grown on orange waste powder. J. Food Eng. 2006, 73, 190–197. [Google Scholar] [CrossRef]
- Siroosi, M.; Borjian, B.F.; Amoozegar, M.A.; Babavalian, H.; Hassanshahian, M. Halophilic Amylase Production and Purification from Haloarcula sp. Strain D61. Biointerface Res. Appl. Chem. 2021, 11, 7382–7392. [Google Scholar]
- Naidu, K.; Maseko, S.; Kruger, G.; Lin, J. Purification and characterization of α-amylase from Paenibacillus sp. D9 and Escherichia coli recombinants. Biocatal. Biotransf. 2020, 38, 124–134. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Esmail, G.A.; Ghilan, A.K.M.; Arasu, M.V.; Duraipandiyan, V.; Ponmurugan, K. Isolation and purification of starch hydrolysing amylase from Streptomyces sp. Al-Dhabi-46 obtained from the Jazan region of Saudi Arabia with industrial applications. J. King Saud Univ. Sci. 2020, 32, 1226–1232. [Google Scholar] [CrossRef]
- Simair, A.A.; Qureshi, A.S.; Khushk, I.; Ali, C.H.; Lashari, S.; Bhutto, M.A.; Mangrio, G.S.; Lu, C. Production and Partial Characterization of α-Amylase Enzyme from Bacillus sp. BCC 01-50 and Potential Applications. BioMed Res. Int. 2017, 2017, 9173040. [Google Scholar] [CrossRef]
- Lin, L.L.; Chyau, C.C.; Hsu, W.H. Production and properties of a raw starch degrading amylase from the thermophilic and alkaliphilic Bacillus sp. TS23. Biotechnol. Appl. Biochem. 1998, 28, 61–68. [Google Scholar]
- Moubasher, H.; Wahsh, S.S.; El-Kassem, N.A. Purification of pullulanase from Aureobasidium pullulans. Microbiology 2010, 79, 759–766. [Google Scholar] [CrossRef]
- Ramachandran, N. Amylolytic enzymes from the yeast Lipomyces kononenkoae. Biologia 2005, 60, 103–110. [Google Scholar]
- Iefuji, H.; Chino, M.; Kato, M.; Iimura, Y. Raw-starch-digesting and thermostable α-amylase from the yeast Cryptococcus sp. S-2: Purification, characterization, cloning and sequencing. Biochem. J. 1996, 318, 989–996. [Google Scholar] [CrossRef]
- Vishnu, C.; Naveena, B.J.; Altaf, M.D.; Venkateshwar, M.; Reddy, G. Amylopullulanase—A novel enzyme of L. amylophilus GV6 in direct fermentation of starch to L(+) lactic acid. Enzyme Microb. Technol. 2006, 38, 545–550. [Google Scholar] [CrossRef]
- Zareian, S.; Khajeh, K.; Ranjbar, B.; Dabirmanesh, B.; Ghollasi, M.; Mollania, N. Purification and characterization of a novel amylopullulanase that converts pullulan to glucose, maltose, and maltotriose and starch to glucose and maltose. Enzyme Microb. Technol. 2010, 46, 57–63. [Google Scholar] [CrossRef]
- Wasko, A.; Polak-Berecka, M.; Targonski, Z. Purification and characterization of Pullulanase from lactococcus lactis. Prep. Biochem. Biotechnol. 2011, 41, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Ray, R.C.; Mohapatra, U.B. Purification, characterization and application of thermostable amylopullulanase from Streptomyces erumpens MTCC 7317 under submerged fermentation. Ann. Microbiol. 2012, 62, 931–937. [Google Scholar] [CrossRef]
- Ara, K.; Saeki, K.; Igarashi, K.; Takaiwa, M.; Uemura, T.; Hagihara, H.; Kawai, S.; Ito, S. Purification and characterization of an alkaline amylopullulanase with both α-1,4 and α-1,6 hydrolytic activiy from alkalophilic Bacillus sp. KSM-1378. Biochim. Biophys. Acta (BBA) Gen. Subj. 1995, 1243, 315–324. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, Y.S. Substrate specificity and detailed characterization of a bifunctional amylase-pullulanase enzyme from Bacillus circulans F2 having two different active sites on one polypeptide. Eur. J. Biochem. 1995, 227, 687–693. [Google Scholar] [CrossRef]
- Melasniemi, H. Characterization of a-amylase and pullulanase activities of Clostridium thermohydrosulfuricum. Biochem. J. 1987, 246, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Kim, D.-S.; Taniguchi, H.; Maruyama, Y. Purification of a amylase-pullulanase bifunctional enzyme by high-performance size-exclusion and hydrophobic-interaction chromatography. J. Chromatogr. A 1990, 512, 131–137. [Google Scholar] [CrossRef]
- Mathupala, S.P.; Lowe, S.E.; Podkovyrov, S.M.; Zeikus, J.G. Sequencing of the amylopullulanase (apu) gene of Thermoanaerobacter ethanolicus 39E, and identification of the active site by site-directed mutagenesis. J. Biol. Chem. 1993, 268, 16332–16344. [Google Scholar] [CrossRef]
- Ara, K.; Igarashi, K.; Saeki, K.; Ito, S. An Alkaline Amylopullulanase from Alkalophilic Bacillus sp. KSM-1378; Kinetic Evidence for Two Independent Active Sites for the α-1,4 and α-1,6 Hydrolytic Reactions. Biosci. Biotechnol. Biochem. 1995, 59, 662–666. [Google Scholar] [CrossRef]
- Hii, L.S.; Ling, T.C.; Mohamad, R.; Ariff, A.B. Characterization of Pullulanase Type II from Bacillus cereus H1.5. Am. J. Biochem. Biotechnol. 2009, 5, 170–179. [Google Scholar] [CrossRef]
- Mrudula, S.; Gopal, R.; Seenayya, G. Purification and characterization of highly thermostable amylopullulanase from a thermophilic, anaerobic bacterium Clostridium thermosulfurogenes SVM17. Malays. J. Microbiol. 2011, 7, 97–106. [Google Scholar] [CrossRef]
- Lévêque, E.; Janeček, Š.; Haye, B.; Belarbi, A. Thermophilic archaeal amylolytic enzymes. Enzyme Microb. Technol. 2000, 26, 3–14. [Google Scholar] [CrossRef]
- Nakamura, Y.; Fukuhara, H.; Sano, K. Secreted Phytase Activities of Yeasts. Biosci. Biotechnol. Biochem. 2000, 64, 841–844. [Google Scholar] [CrossRef]
- Prieto, J.A.; Bort, B.R.; Martínez, J.; Randez-Gil, F.; Buesa, C.; Sanz, P. Purification and characterization of a new alpha-amylase of intermediate thermal stability from the yeast Lipomyces kononenkoae. Biochem. Cell Biol. 1995, 73, 41–49. [Google Scholar] [CrossRef]
- Simões-Mendes, B. Purification and characterization of the extracellular amylases of the yeast Schwanniomyces alluvius. Can. J. Microbiol. 1984, 30, 1163–1170. [Google Scholar] [CrossRef]
- Wanderley, K.J.; Torres, F.A.G.; Moraes, L.M.P.; Ulhoa, C.J. Biochemical characterization of α-amylase from the yeast Cryptococcus flavus. FEMS Microbiol. Lett. 2004, 231, 165–169. [Google Scholar] [CrossRef]
- Kwon, Y.M.; Choi, H.S.; Lim, J.Y.; Jang, H.S.; Chung, D. Characterization of Amylolytic Activity by a Marine-Derived Yeast Sporidiobolus pararoseus PH-Gra1. Mycobiology 2020, 48, 195–203. [Google Scholar] [CrossRef]
- Demirkan, E. Production, purification, and characterization of α-amylase by Bacillus subtilis and its mutant derivates. Turk. J. Biol. 2011, 35, 705–712. [Google Scholar]
- Abdel-Fattah, Y.R.; Soliman, N.A.; El-Toukhy, N.M.; El-Gendi, H.; Ahmed, R.S. Production, Purification, and Characterization of Thermostable α-Amylase Produced by Bacillus licheniformis Isolate AI20. J. Chem. 2013, 2013, 673173. [Google Scholar] [CrossRef]
- Rodríguez-Saavedra, C.; Rodríguez-Sanoja, R.; Guillén, D.; Wacher, C.; Díaz-Ruiz, G. Streptococcus infantarius 25124 isolated from pozol produces a high molecular weight amylopullulanase, a key enzyme for niche colonization. Amylase 2021, 5, 1–12. [Google Scholar] [CrossRef]
- Saha, B.C.; Lamed, R.; Lee, C.Y.; Mathupala, S.P.; Zeikus, J.G. Characterization of an endo-Acting Amylopullulanase from Thermoanaerobacter Strain B6A. Appl. Environ. Microbiol. 1990, 56, 881–886. [Google Scholar] [CrossRef]
- Erra-Pujada, M.; Chang-Pi-Hin, F.; Debeire, P.; Duchiron, F.; O’Donohue, J. Purification and properties of the catalytic domain of the thermostable pullulanase Type II from Thermococcus hydrothermalis. Biotechnol. Lett. 2001, 23, 1273–1277. [Google Scholar] [CrossRef]
- Ueda, S.; Ohba, R. Purification, Crystallization and Some Properties o-Extracellular Pullulanase from Aerobacter aerogenes. Agric. Biol. Chem. 1972, 36, 2381–2391. [Google Scholar] [CrossRef][Green Version]
- Kim, C.H.; Choi, H.I.; Lee, D.S. Purification and Biochemical Properties of an Alkaline Pullulanase from Alkalophilic Bacillus sp. S-l. Biosci. Biotechnol. Biochem. 1993, 57, 1632–1637. [Google Scholar] [CrossRef]
- Qiao, Y.; Peng, Q.; Yan, J.; Wang, H.; Ding, H.; Shi, B. Gene cloning and enzymatic characterization of alkali-tolerant Type I pullulanase from Exiguobacterium acetylicum. Lett. Appl. Microbiol. 2015, 60, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Hii, S.L.; Tan, J.S.; Ling, T.C.; Ariff, A.B. Pullulanase: Role in Starch Hydrolysis and Potential Industrial Applications. Enzyme Res. 2012, 2012, 921362. [Google Scholar] [CrossRef]
- Asha, R.; Niyonzima, F.N.; Sunil, S.M. Purification and properties of pullulanase from Bacillus halodurans. Int. Res. J. Biol. Sci. 2013, 2, 35–43. [Google Scholar]
- Lim, S.J.; Hazwani-Oslan, S.N.; Oslan, S.N. Purification and characterisation of thermostable α-amylases from microbial sources. BioResources 2019, 15, 2005–2029. [Google Scholar] [CrossRef]
- Xian, L.; Wang, F.; Luo, X.; Feng, Y.L.; Feng, J.X. Purification and Characterization of a Highly Efficient Calcium-Independent α-Amylase from Talaromyces pinophilus 1-95. PLoS ONE 2015, 10, e0121531. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Azhar, E.I.; Ba-Akdah, M.M.; Tashk, N.R.; Kumosani, T.A. Production, purification and characterization of α-amylase from Trichoderma harzianum grown on mandarin peel. Afr. J. Microbiol. Res. 2011, 5, 930–940. [Google Scholar] [CrossRef][Green Version]
- Saini, R.; Saini, H.S.; Dahiya, A. Amylases: Characteristics and industrial applications. J. Pharmacogn. Phytochem. 2017, 6, 1865–1871. [Google Scholar]
- Larpent, J.P.; Larpent-Gourgaud, M. Mémento Technique de Microbiologie, 3rd ed.; Lavoisier-Tec & Doc: Paris, France, 1997; Volume 8, pp. 217–240. [Google Scholar]
- Declerck, N.; Machius, M.; Joyet, P.; Wiegand, G.; Huber, R.; Gaillardin, C. Hyperthermostabilization of Bacillus licheniformis α-amylase and modulation of its stability over a 50°C temperature range. Protein Eng. Des. Sel. 2003, 16, 287–293. [Google Scholar] [CrossRef]
- Lonhienne, T.; Zoidakis, J.; Vorgias, C.E.; Feller, G.; Gerday, C.; Bouriotis, V. Modular structure, local flexibility and cold-activity of a novel chitobiase from a psychrophilic antarctic bacterium. J. Mol. Biol. 2001, 310, 291–297. [Google Scholar] [CrossRef]
- Unsworth, L.D.; van der Oost, J.; Koutsopoulos, S. Hyperthermophilic enzymes—Stability, activity and implementation strategies for high temperature applications: Properties and applications of hyperthermozymes. FEBS J. 2007, 274, 4044–4056. [Google Scholar] [CrossRef]
- Koutsopoulos, S.; van der Oost, J.; Norde, W. Temperature-dependent structural and functional features of a hyperthermostable enzyme using elastic neutron scattering: Dynamics of a Hyperthermostable Enzyme. Proteins 2005, 61, 377–384. [Google Scholar] [CrossRef]
- Al-Quadan, F.; Akel, H.; Natshi, R. Characteristics of a novel, highly acid- and thermo-stable amylase from thermophilic Bacillus strain HUTBS62 under different environmental conditions. Ann. Microbiol. 2011, 61, 887–892. [Google Scholar] [CrossRef]
- Galdino, A.S.; Silva, R.N.; Lottermann, M.T.; Alvares, A.C.M.; Moraes, L.M.P.D.; Torres, F.A.G.; Freitas, S.M.D.; Ulhoa, C.J. Biochemical and Structural Characterization of Amy1: An Alpha-Amylase from Cryptococcus flavus Expressed in Saccharomyces cerevisiae. Enzyme Res. 2011, 2011, 157294. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Nakashima, K.; Okai, N.; Okazaki, F.; Miyake, M.; Harazono, K.; Ogino, C.; Kondo, A. Thermal Stability and Starch Degradation Profile of α-Amylase from Streptomyces avermitilis. Biosci. Biotechnol. Biochem. 2013, 77, 2449–2453. [Google Scholar] [CrossRef] [PubMed]
- Bernhardsdotter, E.C.M.J.; Ng, J.D.; Garriott, O.K.; Pusey, M.L. Enzymic properties of an alkaline chelator-resistant α-amylase from an alkaliphilic Bacillus sp. isolate L1711. Process Biochem. 2005, 40, 2401–2408. [Google Scholar] [CrossRef]
- Suzuki, Y.; Imai, T. Bacillus stearothermophilus KP 1064 pullulan hydrolase. Appl. Microbiol. Biotechnol. 1985, 21, 20–26. [Google Scholar] [CrossRef]
- Rüdiger, A.; Jorgensen, P.L.; Antranikian, G. Isolation and characterization of a heat-stable pullulanase from the hyperthermophilic archaeon Pyrococcus woesei after cloning and expression of its gene in Escherichia coli. Appl. Environ. Microbiol. 1995, 61, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Zohra, R.R.; Qader, S.A.; Pervez, S.; Aman, A. Influence of different metals on the activation and inhibition of α-amylase from thermophilic Bacillus firmus KIBGE-IB28. Pak. J. Pharm. Sci. 2016, 29, 1275–1278. [Google Scholar] [PubMed]
- Freer, S.N. Purification and characterization of the extracellular alpha-amylase from Streptococcus bovis JB1. Appl. Environ. Microbiol. 1993, 59, 1398–1402. [Google Scholar] [CrossRef]
- Divakaran, D.; Chandran, A.; Pratap Chandran, R. Comparative study on production of a-Amylase from Bacillus licheniformis strains. Braz. J. Microbiol. 2011, 42, 1397–1404. [Google Scholar] [CrossRef]
- Pompeyo, C.; Gomez, M.; Gasparian, S.; Morlon-Guyot, J. Comparison of amylolytic properties of Lactobacillus amylovorus and of Lactobacillus amylophilus. Appl. Microbiol. Biotechnol. 1993, 40, 266–269. [Google Scholar] [CrossRef]
- Odibo, F.J.C.; Ulbrich-Hofmann, R. Thermostable α-Amylase and Glucoamylase from Thermomyces lanuginosus F1. Acta Biotechnol. 2001, 21, 141–153. [Google Scholar] [CrossRef]
- Arabacı, N.; Arıkan, B. Isolation and characterization of a cold-active, alkaline, detergent stable α-amylase from a novel bacterium Bacillus subtilis N8. Prep. Biochem. Biotechnol. 2018, 48, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.S.; Chang, C.S. Production of an oxidant and SDS-stable alkaline protease from an alkaophilic Bacillus clausii I-52 by submerged fermentation: Feasibility as a laundry detergent additive. Enzyme Microb. Technol. 2006, 38, 176–183. [Google Scholar] [CrossRef]
- Lorient, D.; Closs, B.; Courthaudon, J.L. Connaissances nouvelles sur les propriétés fonctionnelles des protéines du lait et des dérivés. Lait 1991, 71, 141–171. [Google Scholar] [CrossRef]
- DJekrif, D.S.; Gillmann, L.; Cochet, N.; Bennamoun, L.; Ait-Kaki, A.; Labbani, K.; Nouadri, T.; Meraihi, Z. Optimization of thermophilic pullulanase and α-amylase production by amylolytic yeast. Int. J. Micro. Biol. Res. 2014, 6, 559–569. [Google Scholar]
- Bernfeld, P. Amylase α and β. In Methods in Enzymology; Colowick, S.P., Kaplan, O.N., Eds.; Academic Press: New York, NY, USA, 1955; pp. 140–146. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.; Farr, A.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Arikan, B. Highly thermostable, thermophilic, alkaline, SDS and chelator resistant amylase from a thermophilic Bacillus sp. isolate A3-15. Bioresour. Technol. 2008, 99, 3071–3076. [Google Scholar] [CrossRef]
- Hmidet, N.; Ali, N.E.-H.; Haddar, A.; Kanoun, S.; Alya, S.K.; Nasri, M. Alkaline proteases and thermostable α-amylase co-produced by Bacillus licheniformis NH1: Characterization and potential application as detergent additive. Biochem. Eng. J. 2009, 47, 71–79. [Google Scholar] [CrossRef]
- Rameshkumar, A.; Sivasudha, T. Optimization of Nutritional Constitute for Enhanced Alpha amylase Production Using by Solid State Fermentation Technology. Int. J. Microbiol. Res. 2011, 2, 143–148. [Google Scholar]






| Purification Step | Total Protein (mg/mL) | Total Activity (IU) | Specific Activity IU/mg | Purification (Fold) | Yield (%) | |
|---|---|---|---|---|---|---|
| Lyophilized Extract | 4330.6 | α-amylase | 346,340 | 79,975 | 1 | 100 |
| Pullulanase | 325,900 | 75.25 | 1 | 100 | ||
| Acetone precipitation | 450 | α-amylase | 158,587 | 352.41 | 4.40 | 45.79 |
| Pullulanase | 131,102.5 | 291.33 | 3.87 | 40.23 | ||
| Sephacryl S 200 | 75.22 | α-amylase | 106,122 | 1410.82 | 17.64 | 30.64 |
| Pullulanase | 85,386.1 | 1135.15 | 15.08 | 26.2 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dakhmouche Djekrif, S.; Bennamoun, L.; Labbani, F.Z.K.; Ait Kaki, A.; Nouadri, T.; Pauss, A.; Meraihi, Z.; Gillmann, L. An Alkalothermophilic Amylopullulanase from the Yeast Clavispora lusitaniae ABS7: Purification, Characterization and Potential Application in Laundry Detergent. Catalysts 2021, 11, 1438. https://doi.org/10.3390/catal11121438
Dakhmouche Djekrif S, Bennamoun L, Labbani FZK, Ait Kaki A, Nouadri T, Pauss A, Meraihi Z, Gillmann L. An Alkalothermophilic Amylopullulanase from the Yeast Clavispora lusitaniae ABS7: Purification, Characterization and Potential Application in Laundry Detergent. Catalysts. 2021; 11(12):1438. https://doi.org/10.3390/catal11121438
Chicago/Turabian StyleDakhmouche Djekrif, Scheherazed, Leila Bennamoun, Fatima Zohra Kenza Labbani, Amel Ait Kaki, Tahar Nouadri, André Pauss, Zahia Meraihi, and Louisa Gillmann. 2021. "An Alkalothermophilic Amylopullulanase from the Yeast Clavispora lusitaniae ABS7: Purification, Characterization and Potential Application in Laundry Detergent" Catalysts 11, no. 12: 1438. https://doi.org/10.3390/catal11121438
APA StyleDakhmouche Djekrif, S., Bennamoun, L., Labbani, F. Z. K., Ait Kaki, A., Nouadri, T., Pauss, A., Meraihi, Z., & Gillmann, L. (2021). An Alkalothermophilic Amylopullulanase from the Yeast Clavispora lusitaniae ABS7: Purification, Characterization and Potential Application in Laundry Detergent. Catalysts, 11(12), 1438. https://doi.org/10.3390/catal11121438








