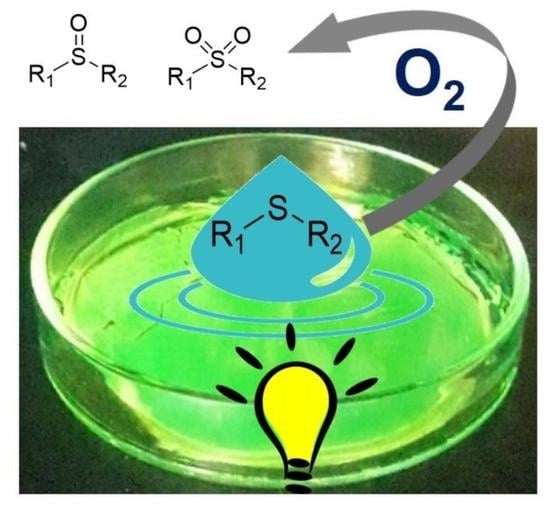
Solvent-Free Visible Light Photocatalytic Oxidation Processes Mediated by Transparent Films of an Imine-Based Organic Polymer
Abstract
:1. Introduction
2. Results and Discussion
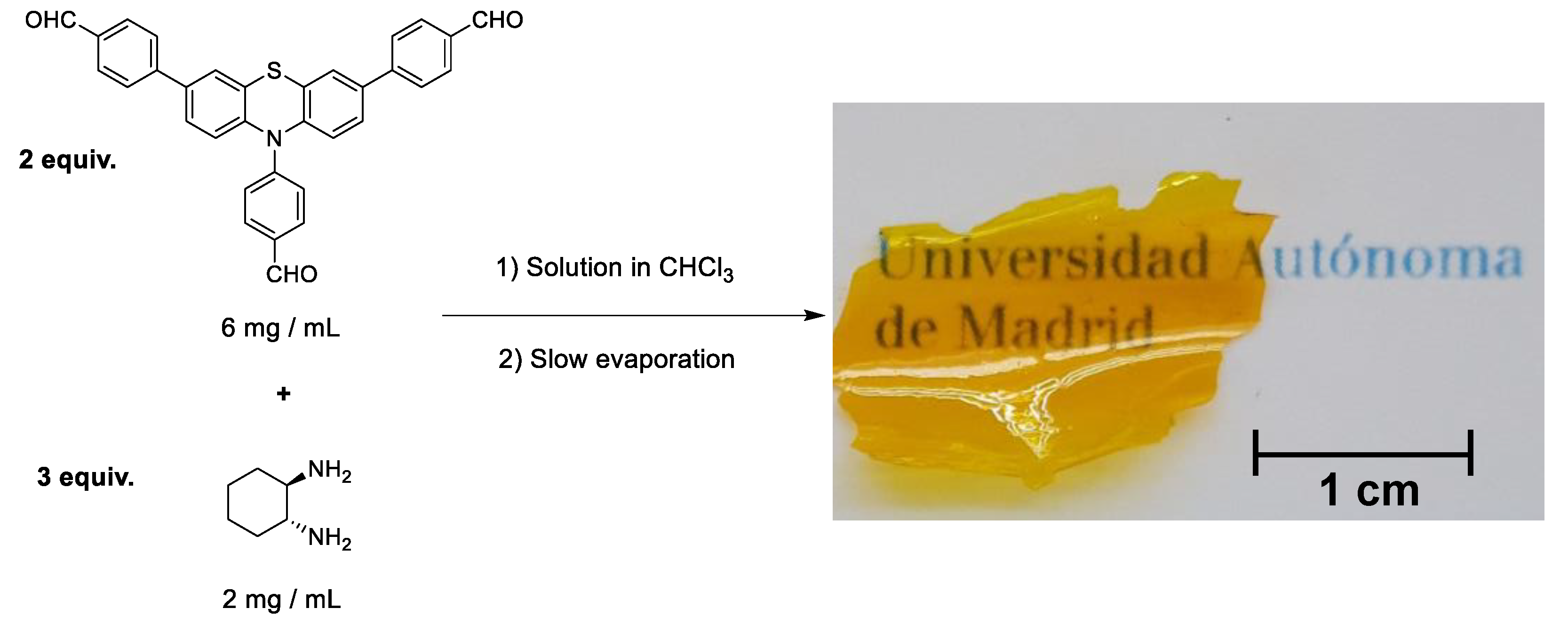
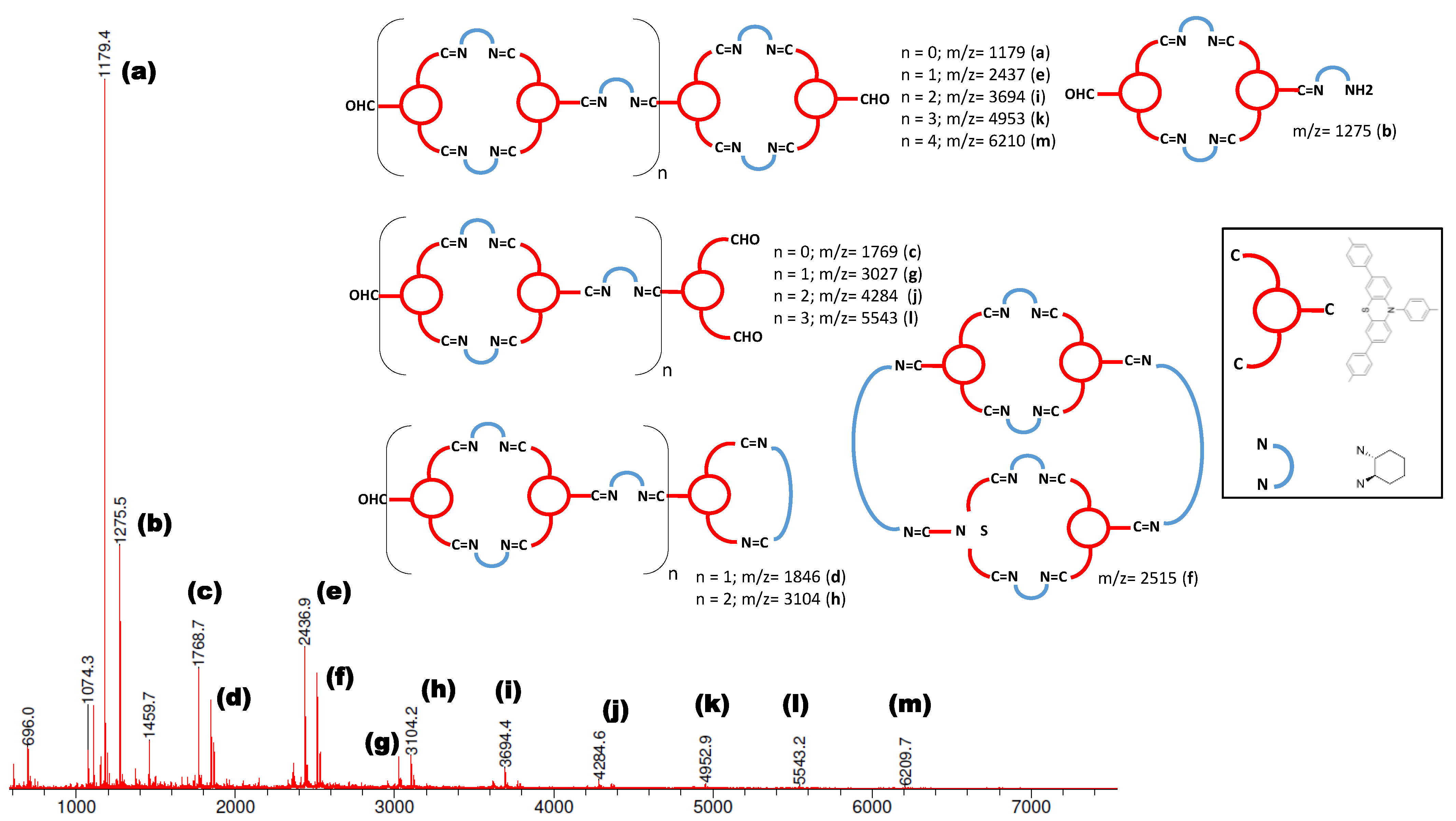
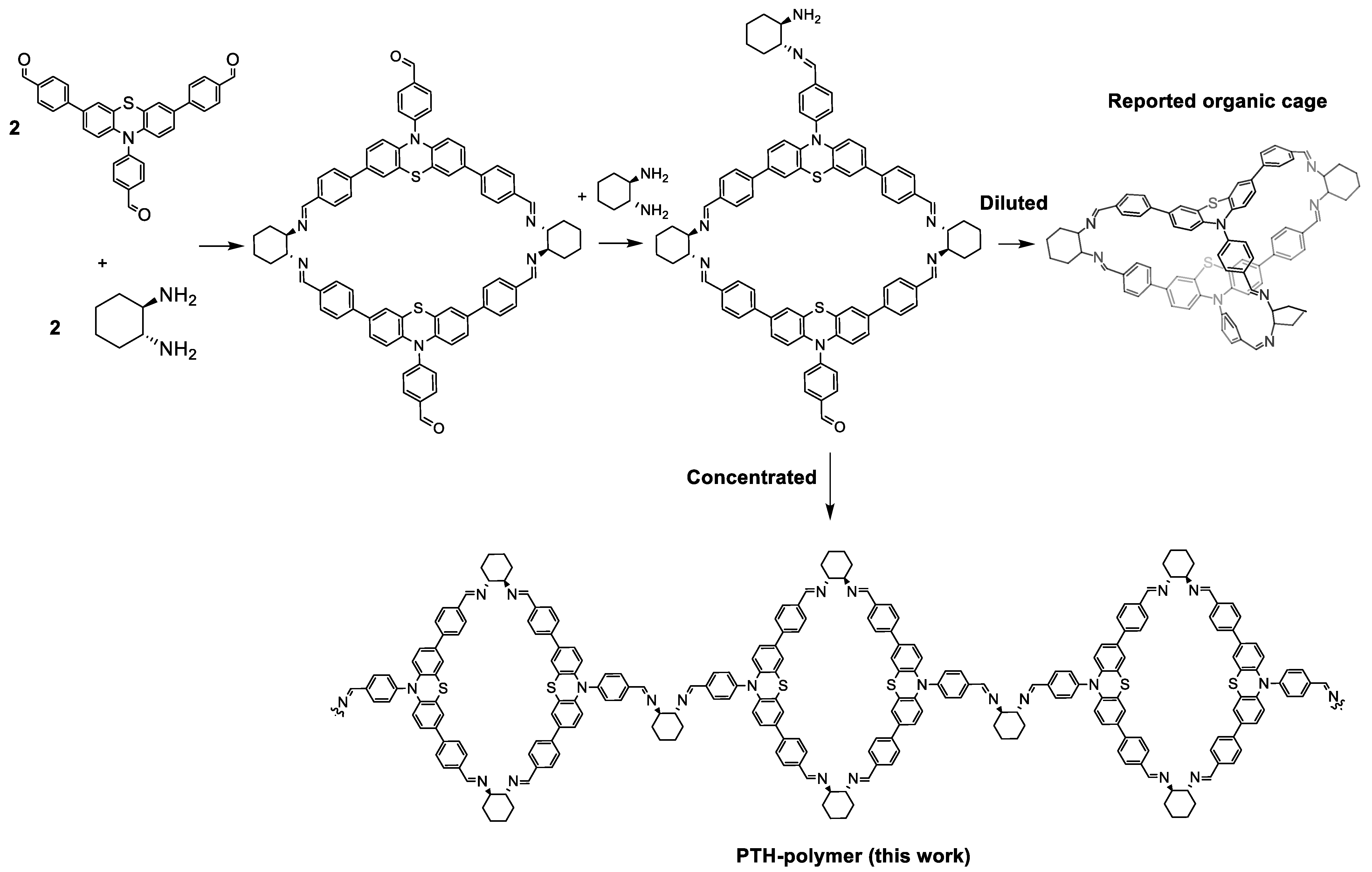
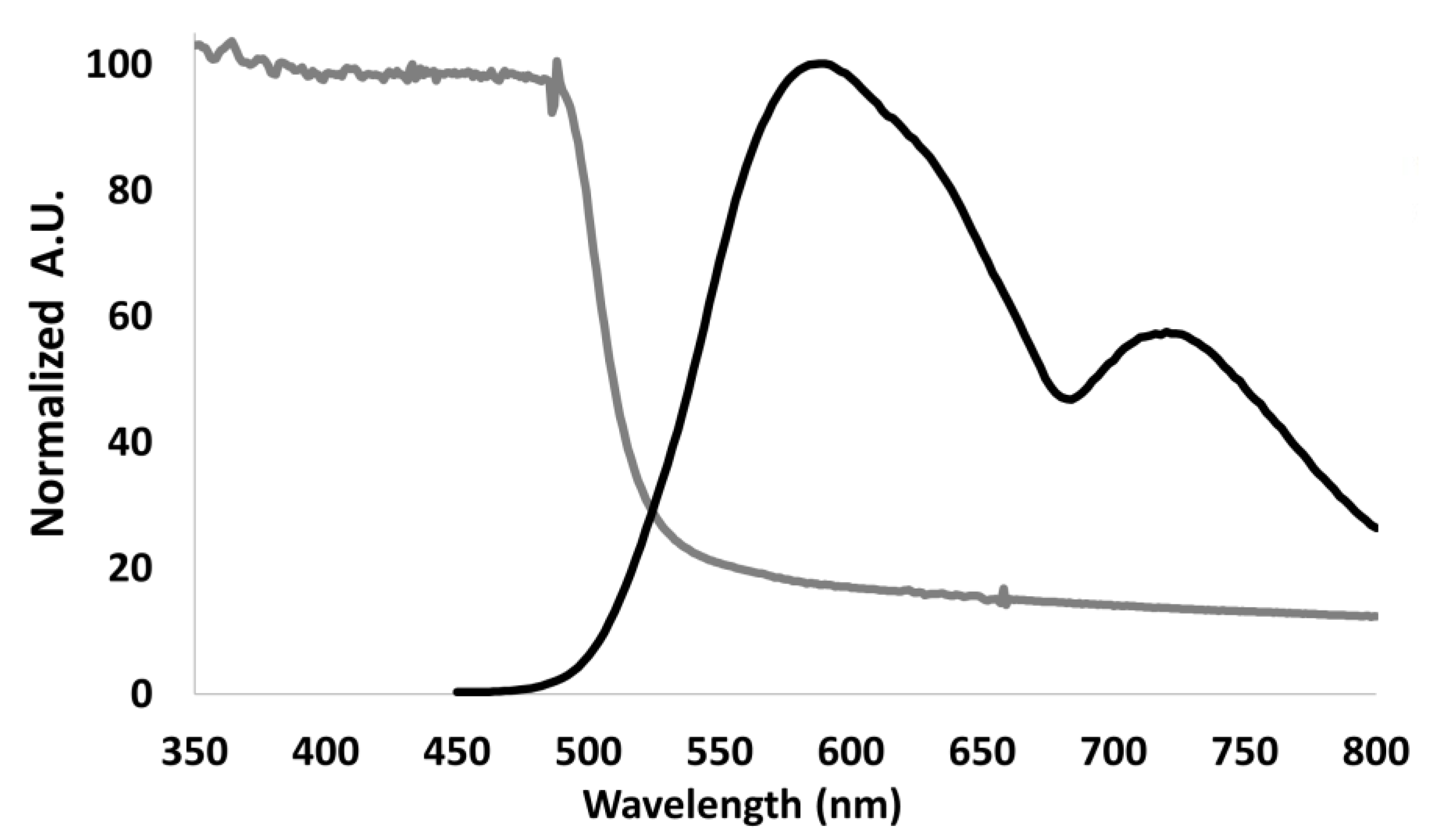
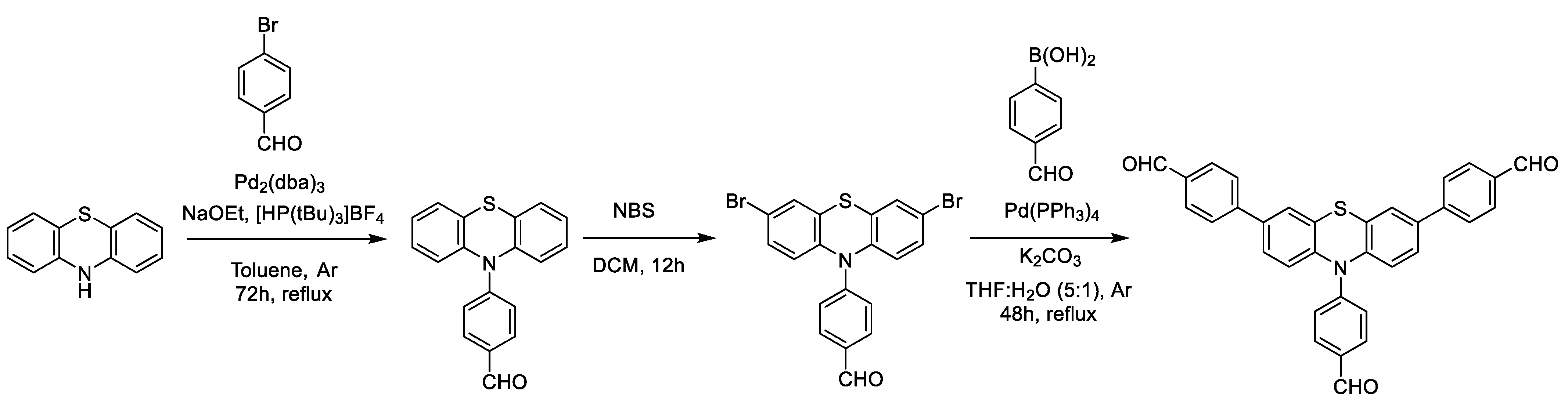
2.1. Synthesis and Characterization of Photoactive Polymer
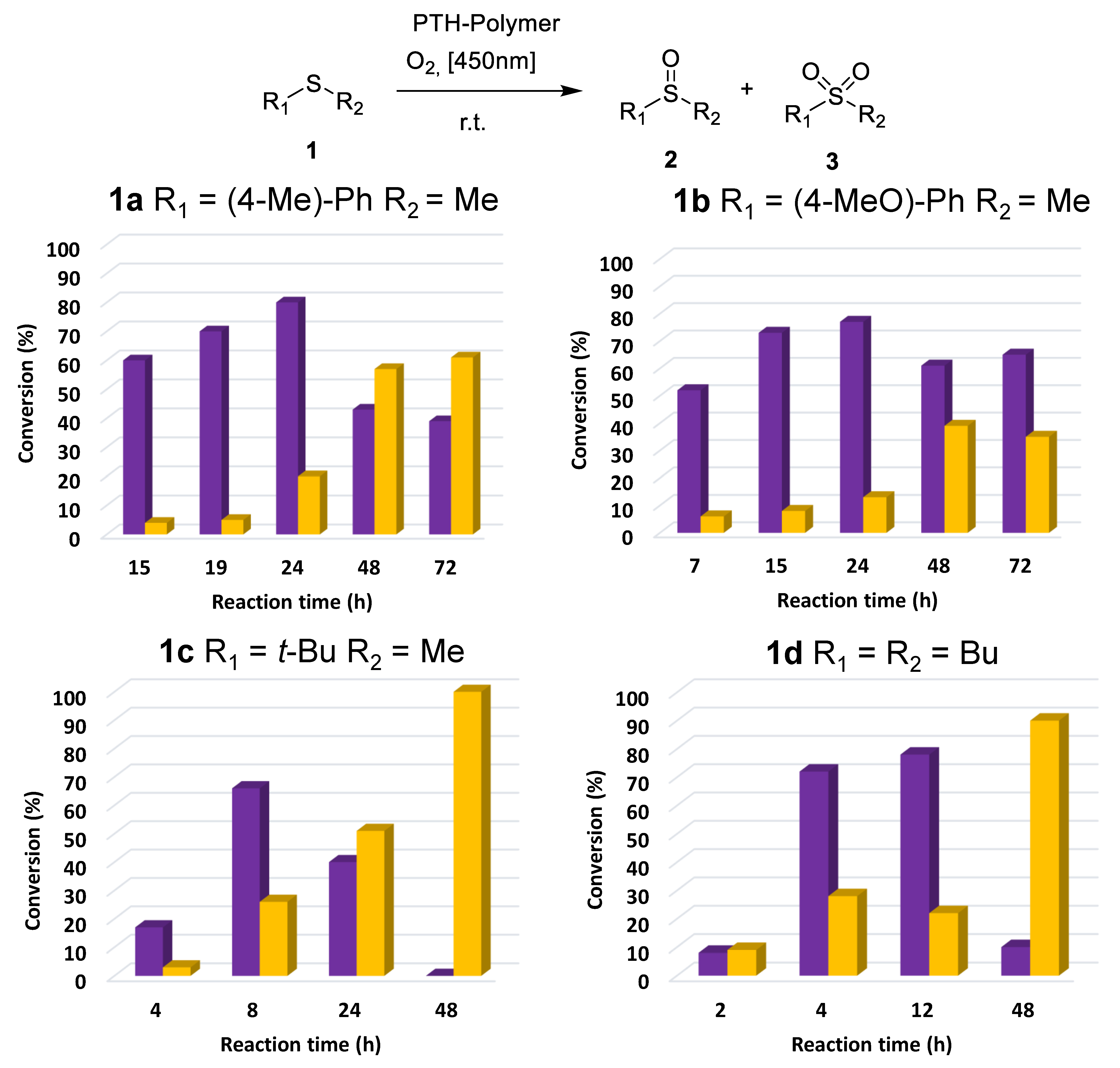
2.2. Photocatalytic Activity
3. Materials and Methods
3.1. Materials and General Methods
3.2. Synthesis of (4,4′,4″-(10-Phenothiazine-3,7,10-Triyl)Tribenzaldehyde) Building Block
3.3. Preparation of PTH-Polymer
3.4. General Procedure for the Photocatalytic Oxidations
3.5. Evaluation of the Recyclability of the Material
3.6. Mechanistic Experiments for the Sulfoxidation Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hoffmann, N. Photochemical Reactions as Key Steps in Organic Synthesis. Chem. Rev. 2008, 108, 1052–1103. [Google Scholar] [CrossRef]
- Garrido-Castro, A.F.; Maestro, C.M.; Aleman, J. α-Functionalization of Imines via Visible Light Photoredox Catalysis. Catalyst 2020, 10, 562. [Google Scholar] [CrossRef]
- Garrido-Castro, A.F.; Maestro, M.C.; Alemán, J. Asymmetric induction in photocatalysis—Discovering a new side to light-driven chemistry. Tetrahedron Lett. 2018, 59, 1286–1294. [Google Scholar] [CrossRef] [Green Version]
- Rigotti, T.; Alemán, J. Visible light photocatalysis—from racemic to asymmetric activation strategies. Chem. Commun. 2020, 56, 11169. [Google Scholar] [CrossRef]
- Sicignano, M.; Rodríguez, R.I.; Alemán, J. Recent Visible Light and Metal Free Strategies in [2+2] and [4+2] Photocycloadditions. Eur. J. Org. Chem. 2021, 3303–3321. [Google Scholar] [CrossRef]
- Narayanam, J.M.R.; Stephenson, C.R.J. Visible light photoredox catalysis: Applications in organic synthesis. Chem. Soc. Rev. 2011, 40, 102–113. [Google Scholar] [CrossRef]
- Twilton, J.; Le, C.C.; Zhang, P.; Shaw, M.H.; Evans, R.W.; MacMillan, D.W.C. The Merger of Transition Metal and Photocatalysis. Nat. Rev. Chem. 2017, 1, 52. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Soc. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, A.S.; Bikram, C.; Subbaiyan, N.K.; Karr, P.A.; D’Souza, F. Phenothiazine-Sensitized Organic Solar Cells: Effect of Dye Anchor Group Positioning on the Cell Performance. App. Mater. Interfaces 2012, 4, 5813–5820. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Pastore, M.; Marotta, G.; Reddy, M.A.; Chandrasekharam, M.; De Angelis, F. Optical Properties and Aggregation of Phenothiazine-Based Dye-Sensitizers for Solar Cells Applications: A Combined Experimental and Computational Investigation. J. Phys. Chem. 2013, 117, 9613–9622. [Google Scholar] [CrossRef] [Green Version]
- DiMarco, B.N.; O’Donell, R.M.; Meyer, G.J. Cation-Dependent Charge Recombination to Organic Mediators in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2015, 119, 21599–21640. [Google Scholar] [CrossRef]
- Lee, J.; Kwak, J.; Ko, K.C.; Park, J.H.; Ko, J.H.; Park, N.; Kim, E.; Ryu, D.H.; Ahn, T.K.; Lee, J.Y.; et al. Phenothiazine-based organic dyes with two anchoring groups on TiO2 for highly efficient visible light-induced water splitting. Chem. Commun. 2012, 48, 11431–11433. [Google Scholar] [CrossRef]
- Watanabe, M.; Hagiwara, H.; Iribe, A.; Ogata, Y.; Shiomi, K.; Staykov, A.; Ida, S.; Tanaka, S.; Ishihara, T. Spacer effects in metal-free organic dyes for visible-light-driven dye-sensitized photocatalytic hydrogen production. J. Mater. Chem. A 2014, 2, 12952–12961. [Google Scholar] [CrossRef]
- Cecconi, B.; Manfredi, N.; Ruffo, R.; Montini, T.; Romero-Ocaña, I.; Fornasiero, P.; Abbotto, A. Tuning Thiophene-Based Phenothiazines for Stable Photocatalytic Hydrogen Production. ChemSusChem 2015, 8, 4216–4228. [Google Scholar] [CrossRef]
- Manfredi, N.; Cecconi, B.; Calabrese, V.; Minotti, A.; Peri, F.; Ruffo, R.; Monai, M.; Romero-Ocaña, I.; Montini, T.; Fornasiero, P.; et al. Dye-sensitized photocatalytic hydrogen production: Distinct activity in a glucose derivative of a phenothiazine dye. Chem. Commun. 2016, 52, 6977–6980. [Google Scholar] [CrossRef] [Green Version]
- Mandredi, N.; Monai, M.; Montini, T.; Salamone, M.; Ruffo, R.; Fornasiero, P.; Abbotto, A. Enhanced photocatalytic hydrogen generation using carbazole-based sensitizers. Sustain. Energy Fuels 2017, 1, 694–698. [Google Scholar] [CrossRef]
- Manfredi, N.; Monai, M.; Montini, T.; Peri, F.; De Angelis, F.; Fornasiero, P.; Abbotto, A. Dye-Sensitized Photocatalytic Hydrogen Generation: Efficiency Enhancement by Organic Photosensitizer–Coadsorbent Intermolecular Interaction. ACS Energy Lett. 2018, 3, 85–91. [Google Scholar] [CrossRef]
- González-Muñoz, D.; Gómez-Avilés, A.; Molina, C.B.; Bedia, J.; Belver, C.; Alemán, J.; Cabrera, S. Anchoring of 10-phenylphenothiazine to mesoporous silica materials: A water compatible organic photocatalyst for the degradation of pollutants. J. Mater. Sci. Technol. 2022, 102, 134. [Google Scholar] [CrossRef]
- Martínez-Gualda, A.M.; Cano, R.; Marzo, L.; Pérez-Ruiz, R.; Luis-Barrera, J.; Mas-Ballesté, R.; Fraile, A.; de la Peña O’Shea, V.A.; Alemán, J. Chromoselective access to Z- or E- allylated amines and heterocycles by a photocatalytic allylation reaction. Nat. Commun. 2019, 10, 2634. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Ko, K.C.; Kim, E.; Park, N.; Ko, J.H.; Ryu, D.H.; Ahn, T.K.; Lee, J.Y.; Son, S.U. Photocatalysis by Phenothiazine Dyes: Visible-Light-Driven Oxidative Coupling of Primary Amines at Ambient Temperature. Org. Lett. 2012, 14, 5502–5505. [Google Scholar] [CrossRef]
- Boyington, A.J.; Seath, C.P.; Zearfoss, A.M.; Xu, Z.; Jiu, N.T. Catalytic Strategy for Regioselective Arylethylamine Synthesis. J. Am. Chem. Soc. 2019, 141, 4147–4153. [Google Scholar] [CrossRef]
- González-Muñoz, D.; Nova-Fernández, J.L.; Martinelli, A.; Pascual-Coca, G.; Cabrera, S.; Alemán, J. Visible Light Photocatalytic Synthesis of Tetrahydroquinolines under Batch and Flow Conditions. Eur. J. Org. Chem. 2020, 2020, 5995–5999. [Google Scholar] [CrossRef]
- Gisbertz, S.; Pieber, B. Heterogeneous Photocatalysis in Organic Synthesis. ChemPhotoChem 2020, 4, 456–475. [Google Scholar] [CrossRef] [Green Version]
- López-Magano, A.; Jiménez-Almarza, A.; Alemán, J.; Mas-Ballesté, R. Metal–Organic Frameworks (MOFs) and Covalent Organic Frameworks (COFs) Applied to Photocatalytic Organic Transformations. Catalysts 2020, 10, 720. [Google Scholar] [CrossRef]
- Sharma, R.K.; Yadav, P.; Yadav, M.; Gupta, R.; Rana, P.; Srivastava, A.; Zbořil, R.; Varma, R.S.; Antonietti, M.; Gawande, M.B. Recent development of covalent organic frameworks (COFs): Synthesis and catalytic (organic-electro-photo) applications. Mater. Horiz. 2020, 7, 411–454. [Google Scholar] [CrossRef]
- Romero, N.; Bofill, R.; Francàs, L.; García-Antón, J.; Sala, X. Light-Driven Hydrogen Evolution Assisted by Covalent Organic Frameworks. Catalysts 2021, 11, 754. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, X.; Gardner, A.M.; Wang, X.; Chong, S.Y.; Neri, G.; Cowan, A.J.; Liu, L.; Li, X.; Vogel, A.; et al. A stable covalent organic framework for photocatalytic carbon dioxide reduction. Chem. Sci. 2019, 11, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Obst, M.; König, B. Organic Synthesis without Conventional Solvents. Organic Synthesis without Conventional Solvents. Eur. J. Org. Chem. 2018, 2018, 4213–4232. [Google Scholar] [CrossRef]
- Ohkubo, K.; Suga, K.; Fukuzumi, S. Solvent-free selective photocatalytic oxidation of benzyl alcohol to benzaldehyde by molecular oxygen using 9-phenyl-10-methylacridinium. Chem. Commun. 2006, 2018–2020. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, K.; Hirose, K.; Fukuzumi, S. Solvent-free one-step photochemical hydroxylation of benzene derivatives by the singlet excited state of 2,3-dichloro-5,6-dicyano-p-benzoquinone acting as a super oxidant. Chem. Eur. J. 2014, 21, 2855–2861. [Google Scholar] [CrossRef]
- Bayer, P.; Wangelin, A.J. An entirely solvent-free photooxygenation of olefins under continuous flow conditions. Green Chem. 2020, 22, 2359–2364. [Google Scholar] [CrossRef]
- Feng, W.; Wu, G.; Li, L.; Guan, N. Solvent-free selective photocatalytic oxidation of benzyl alcohol over modified TiO2. Green Chem. 2011, 13, 3265–3272. [Google Scholar] [CrossRef]
- Yuan, R.; Fan, S.; Zhou, H.; Ding, Z.; Lin, S.; Li, Z.; Zhang, Z.; Xu, C.; Wu, L.; Wang, X.; et al. Chlorine-Radical-Mediated Photocatalytic Activation of C-H Bonds with Visible Light. Angew. Chem. Int. Ed. 2013, 52, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Štrukil, V.; Sajkoa, I. Mechanochemically-assisted solid-state photocatalysis. Chem. Commun. 2017, 53, 9101–9104. [Google Scholar] [CrossRef]
- Hernández, J.G. Mechanochemical borylation of aryldiazonium salts; merging light and ball milling. Beilstein J. Org. Chem. 2017, 13, 1463–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obst, M.; Shaikh, R.S.; König, B. Solvent-free coupling of aryl halides with pyrroles applying visible-light photocatalysis. React. Chem. Eng. 2017, 2, 472–478. [Google Scholar] [CrossRef]
- Jiménez-Almarza, A.; López-Magano, A.; Marzo, L.; Cabrera, S.; Mas-Ballesté, R.; Alemán, J. Imine-Based Covalent Organic Frameworks as Photocatalysts for Metal Free Oxidation Processes under Visible Light Conditions. ChemCatChem 2019, 11, 4916. [Google Scholar] [CrossRef]
- López-Magano, A.; Platero-Prats, A.E.; Cabrera, S.; Mas-Ballesté, R.; Alemán, J. Incorporation of Photocatalytic Pt(II) Complexes into Imine-Based Layered Covalent Organic Frameworks (COFs) through Monomer Truncation Strategy. Appl. Catal. B Environ. 2020, 272, 119027. [Google Scholar] [CrossRef]
- López-Magano, A.; Ortín-Rubio, B.; Imaz, I.; Maspoch, D.; Alemán, J.; Mas-Ballesté, R. Photoredox Heterobimetallic Dual Catalysis Using Engineered Covalent Organic Frameworks. ACS Catal. 2021, 11, 12344. [Google Scholar] [CrossRef]
- González-Muñoz, D.; Casado-Sánchez, A.; del Hierro, I.; Gómez-Ruiz, S.; Cabrera, S.; Alemán, J. Size-selective mesoporous silica-based Pt(II) complex as efficient and reusable photocatalytic material. J. Catal. 2019, 373, 374–383. [Google Scholar] [CrossRef]
- Han, J.; Soloshonok, V.A.; Klika, K.D.; Drabowicz, J.; Wzorek, A. Chiral sulfoxides: Advances in asymmetric synthesis and problems with the accurate determination of the stereochemical outcome. Chem. Soc. Rev. 2018, 47, 1307–1350. [Google Scholar] [CrossRef]
- Mondal, B.; Mukherjee, P.S. Cage Encapsulated Gold Nanoparticles as Heterogeneous Photocatalyst for Facile and Selective Reduction of Nitroarenes to Azo Compounds. J. Am. Chem. Soc. 2018, 140, 12592–12601. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Fuente, M.; Jimenez-Almarza, A.; Alemán, J.; Mas-Ballesté, R. Solvent-Free Visible Light Photocatalytic Oxidation Processes Mediated by Transparent Films of an Imine-Based Organic Polymer. Catalysts 2021, 11, 1426. https://doi.org/10.3390/catal11121426
Sánchez-Fuente M, Jimenez-Almarza A, Alemán J, Mas-Ballesté R. Solvent-Free Visible Light Photocatalytic Oxidation Processes Mediated by Transparent Films of an Imine-Based Organic Polymer. Catalysts. 2021; 11(12):1426. https://doi.org/10.3390/catal11121426
Chicago/Turabian StyleSánchez-Fuente, Miguel, Alicia Jimenez-Almarza, José Alemán, and Rubén Mas-Ballesté. 2021. "Solvent-Free Visible Light Photocatalytic Oxidation Processes Mediated by Transparent Films of an Imine-Based Organic Polymer" Catalysts 11, no. 12: 1426. https://doi.org/10.3390/catal11121426
APA StyleSánchez-Fuente, M., Jimenez-Almarza, A., Alemán, J., & Mas-Ballesté, R. (2021). Solvent-Free Visible Light Photocatalytic Oxidation Processes Mediated by Transparent Films of an Imine-Based Organic Polymer. Catalysts, 11(12), 1426. https://doi.org/10.3390/catal11121426