Effect of the Type of Heterostructures on Photostimulated Alteration of the Surface Hydrophilicity: TiO2/BiVO4 vs. ZnO/BiVO4 Planar Heterostructured Coatings
Abstract
:1. Introduction
2. Results
2.1. Sample Characterization
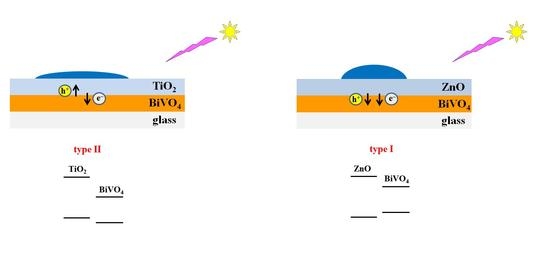
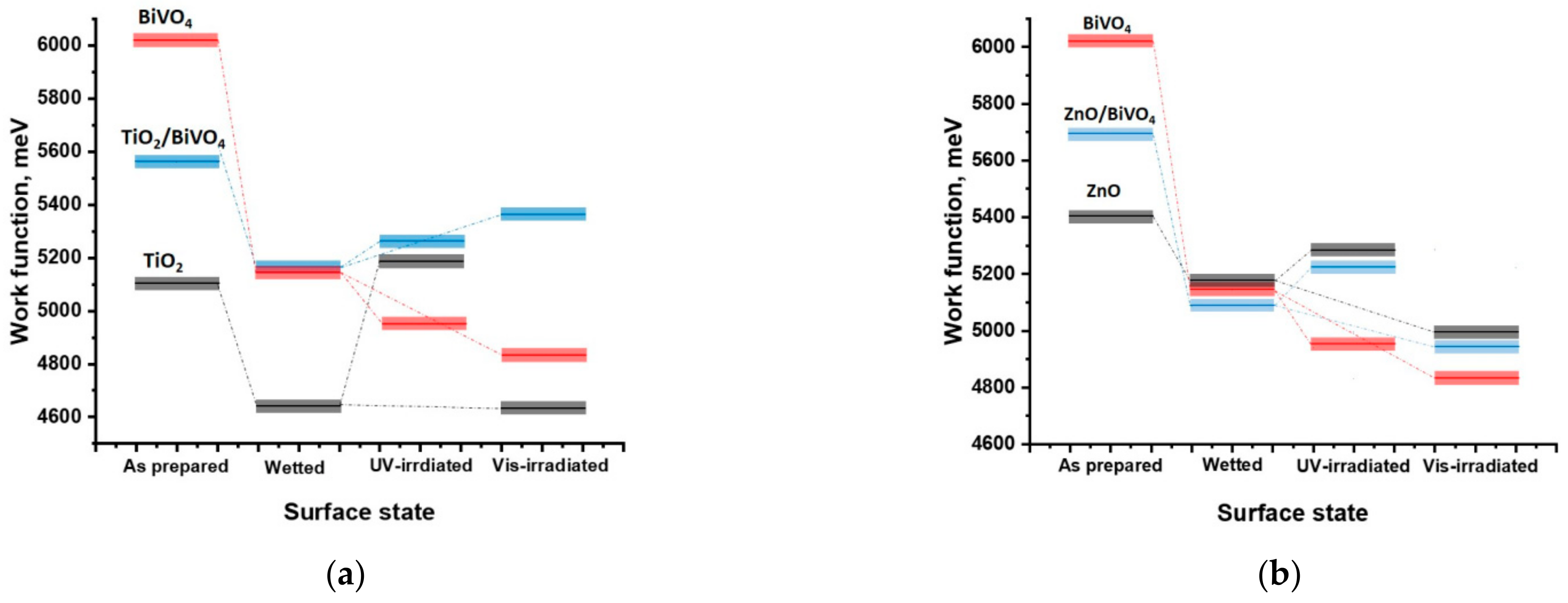
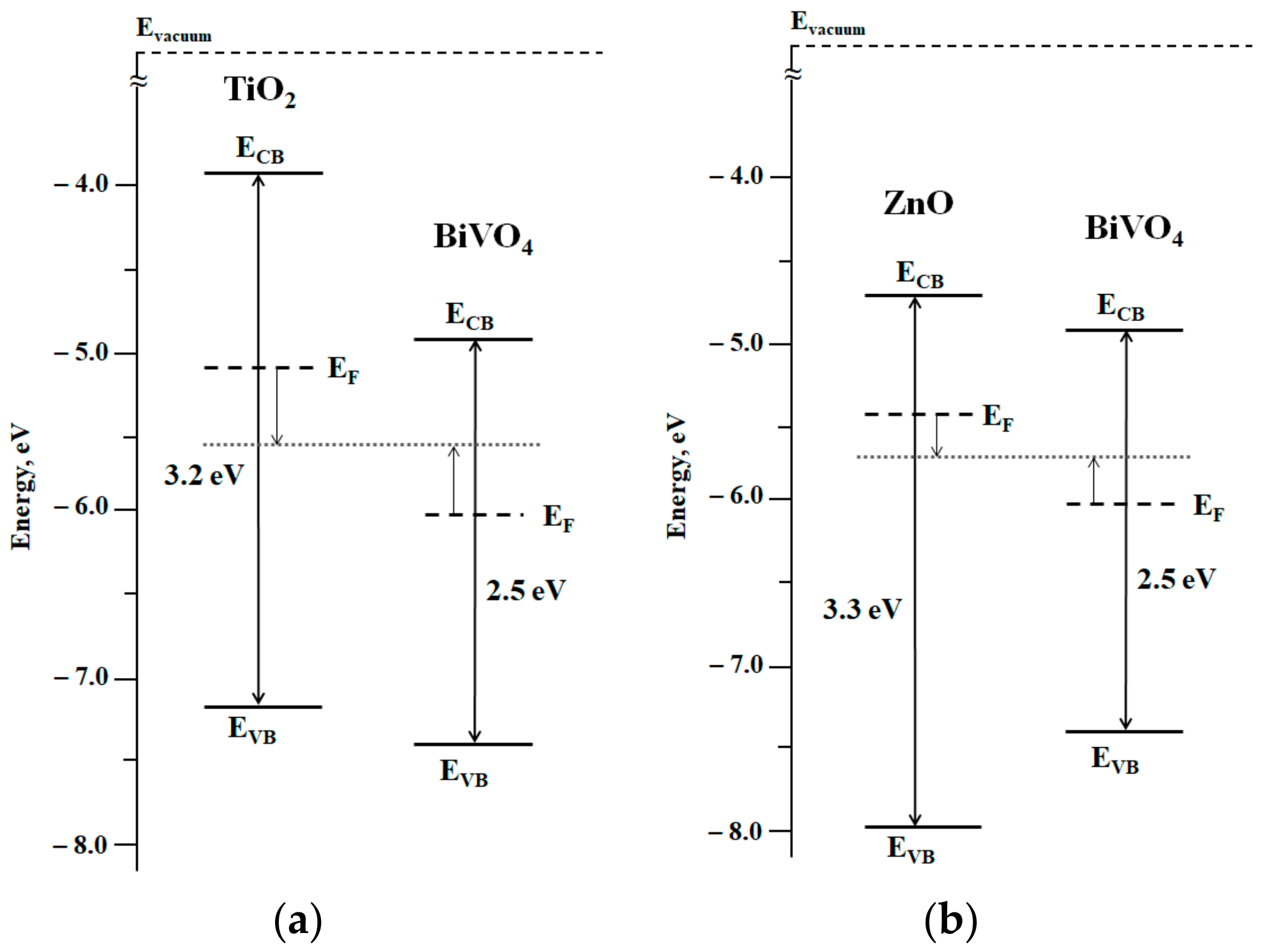
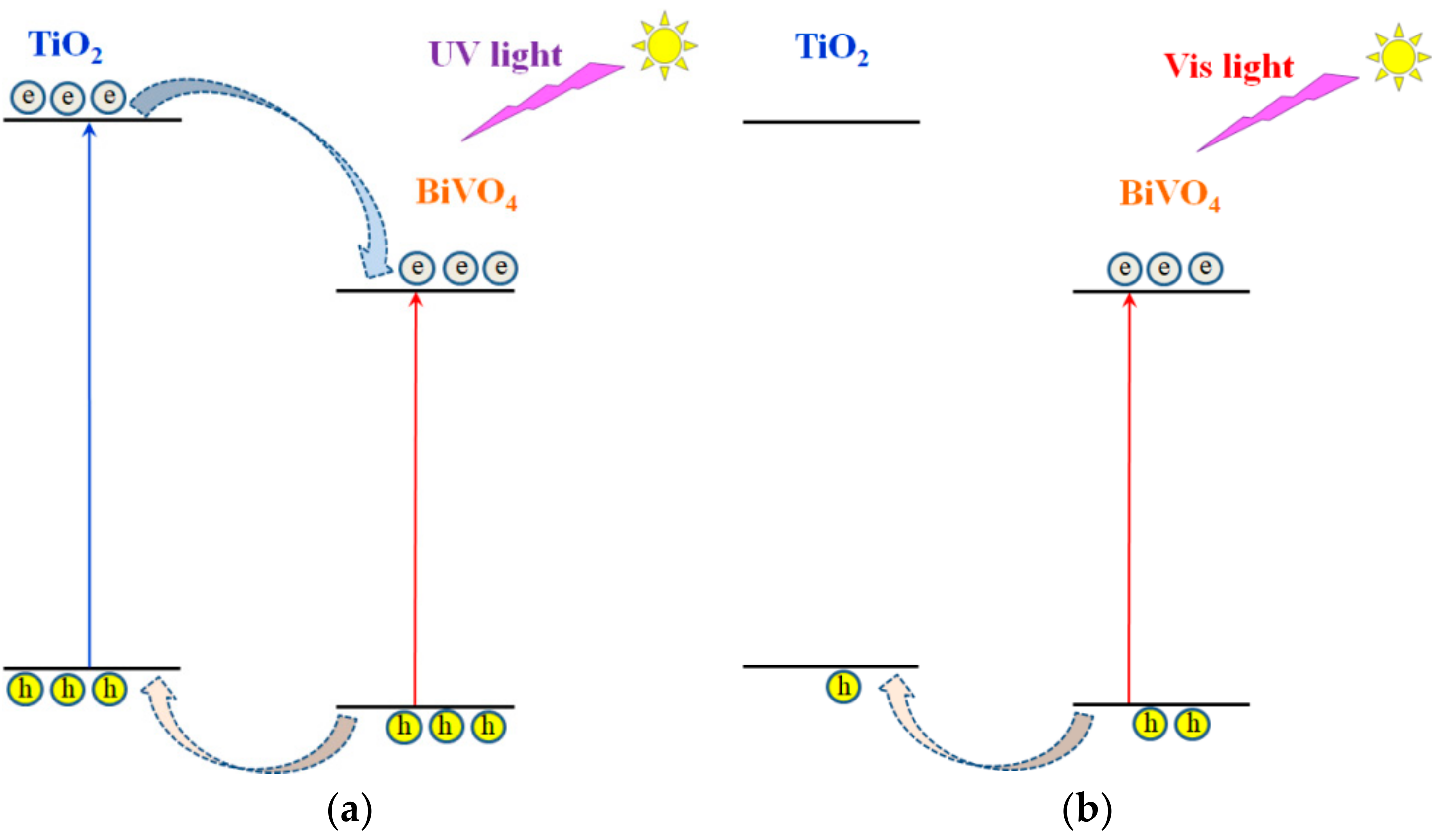
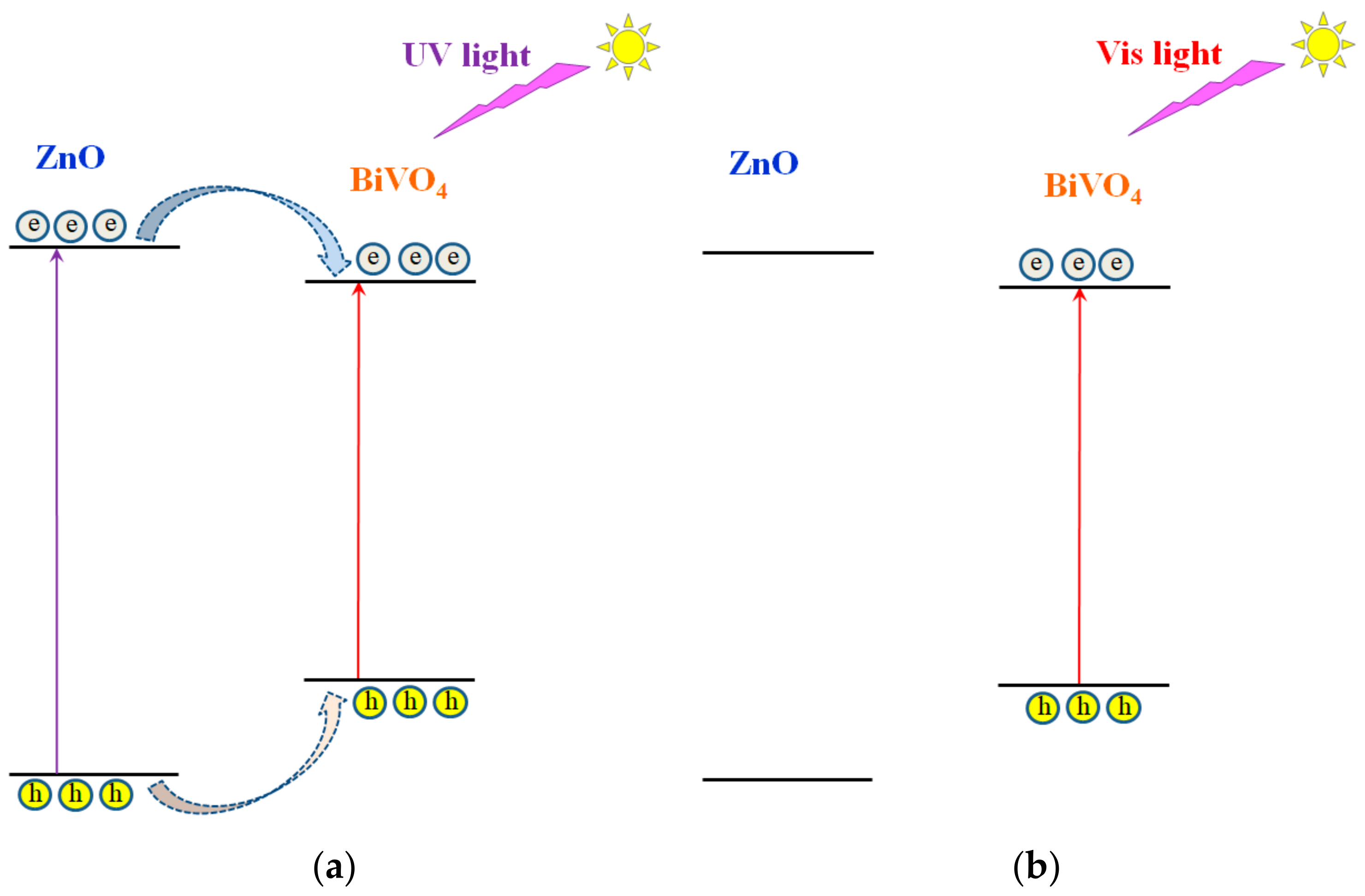
2.2. Electronic Properties of the Heterostructure Components
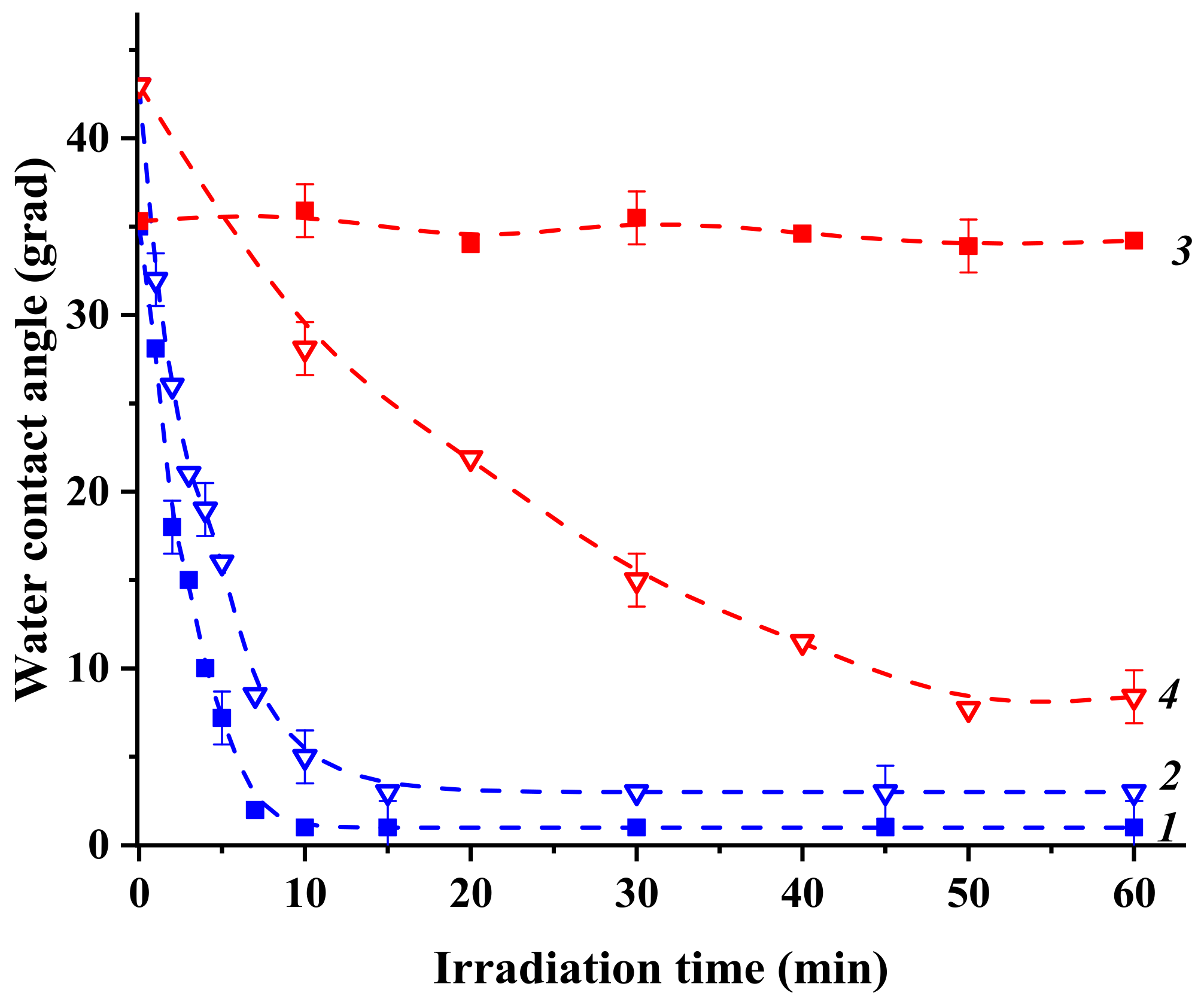
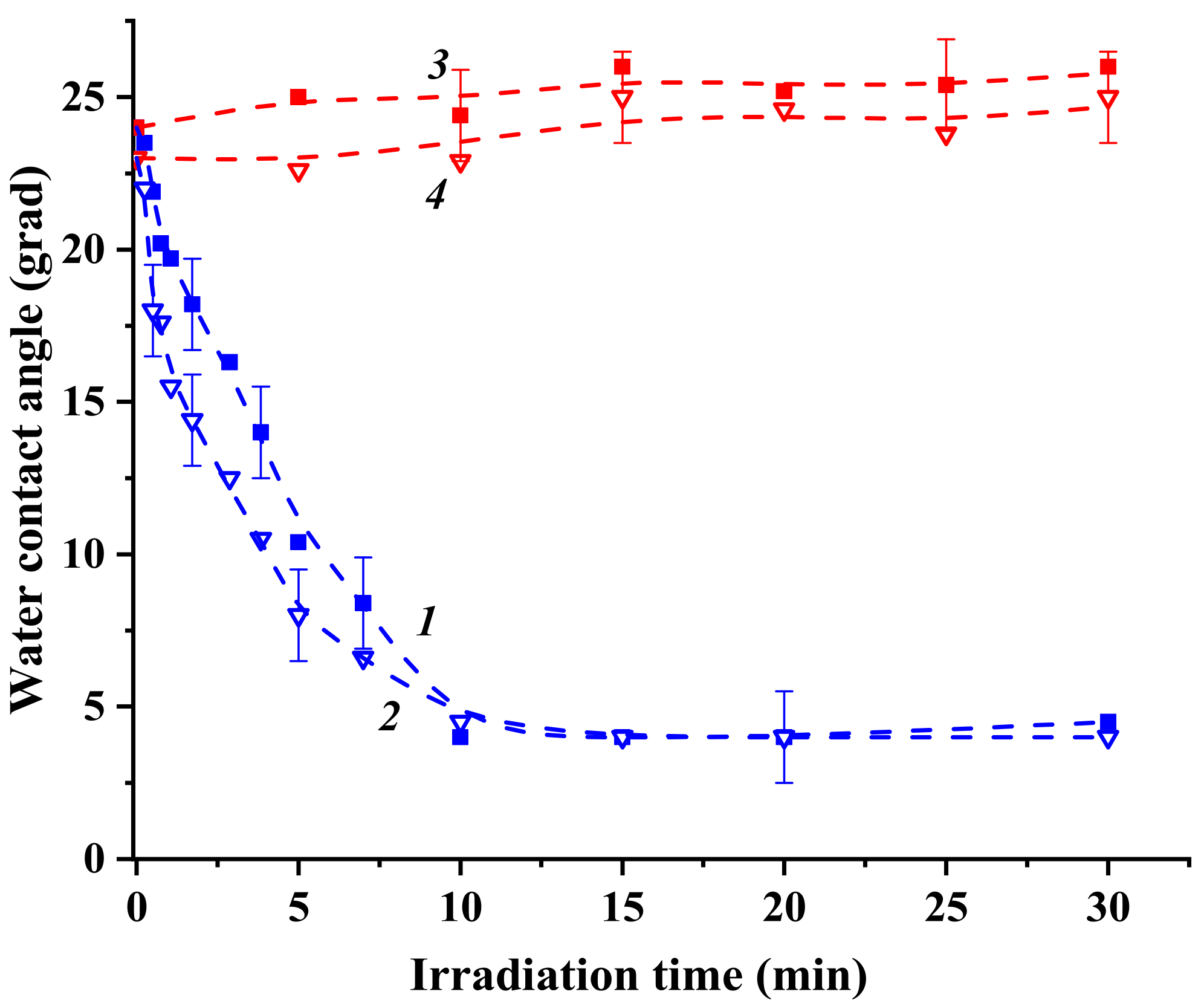
2.3. Photoinduced Alterations in Surface Hydrophilicity of Heterostructured Coatings
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Emeline, A.V.; Rudakova, A.V.; Mikhaylov, R.V.; Ryabchuk, V.K.; Serpone, N. Electron transfer processes in heterostructured photocatalysts. In Springer Handbook of Inorganic Photochemistry, 1st ed.; Bahnemann, D., Patrocinio, A.O.T., Eds.; Springer: Basel, Switzerland, 2022; in press. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor composites: Strategies for enhancing charge carrier separation to improve photocatalytic activity. Adv. Funct. Mater. 2013, 24, 2421–2440. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdara, M.; He, J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-H.; Chang, T.-F.M.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Mechanistic insights into photodegradation of organic dyes using heterostructure photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Zhang, C. Recent advances in structure design for enhancing photocatalysis. J. Mater. Sci. 2019, 54, 8831–8851. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.-X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting—A critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Emeline, A.V.; Rudakova, A.V.; Mikhaylov, R.V.; Bulanin, K.M.; Bahnemann, D.W. Photoactive heterostructures: How they are made and explored. Catalysts 2021, 11, 294. [Google Scholar] [CrossRef]
- Rudakova, A.V.; Emeline, A.V. Photoinduced hydrophilicity of surfaces of thin film. Colloid J. 2021, 83, 20–48. [Google Scholar] [CrossRef]
- Rudakova, A.V.; Emeline, A.V.; Bahnemann, D.W. Effect of the TiO2–ZnO heterostructure on the photoinduced hydrophilic conversion of TiO2 and ZnO Surfaces. J. Phys. Chem. C 2019, 123, 8884–8891. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.N. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Henderson, M.A. A surface science perspective on TiO2 photocatalysis. Surf. Sci. Rep. 2011, 66, 185–297. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Bahnemann, D.W. Photoelectrocatalytic materials for environmental applications. J. Mater. Chem. 2009, 19, 5089–5121. [Google Scholar] [CrossRef]
- Sun, R.-D.; Nakajima, A.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Photoinduced surface wettability conversion of ZnO and TiO2 thin films. J. Phys. Chem. B 2001, 105, 1984–1990. [Google Scholar] [CrossRef]
- Miyauchi, M.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Photocatalysis and photoinduced hydrophilicity of various metal oxide thin films. Chem. Mater. 2002, 14, 2812–2816. [Google Scholar] [CrossRef]
- Miyauchi, M.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Photoinduced hydrophilic conversion of TiO2/WO3 layered thin films. Chem. Mater. 2002, 14, 4714–4720. [Google Scholar] [CrossRef]
- Kim, H.-M.; Kim, D.; Kim, B. Photoinduced hydrophilicity of TiO2/WO3 double layer films. Surf. Coat. Technol. 2015, 271, 18–21. [Google Scholar] [CrossRef]
- Maevskaya, M.V.; Sivokhina, M.M.; Rudakova, A.V.; Emeline, A.V. Photocatalytic properties of layered TiO2/CdS, TiO2/ZnO, ZnO/TiO2 heterostructures. In Proceedings of the 6th International Conference on Semiconductor and Photochemistry, Oldenburg, Germany, 11–14 September 2017. [Google Scholar]
- Grishina, A.E.; Maevskaya, M.V.; Rudakova, A.V.; Emeline, A.V. Photoinduced hydrophilic behavior of hydrated surfaces of TiO2/ZnO, TiO2/CdS, TiO2/WO3 composite films. In Proceedings of the 9th European Meeting on Solar Chemistry and Photocatalysis, Strasburg, France, 13–17 June 2016. [Google Scholar]
- Maevskaya, M.V.; Rudakova, A.V.; Emeline, A.V.; Bahnemann, D.W. Effect of Cu2O substrate on photoinduced hydrophilicity of TiO2 and ZnO nanocoatings. Nanomaterials 2021, 11, 1526. [Google Scholar] [CrossRef] [PubMed]
- Rudakova, A.V.; Emeline, A.V.; Romanychev, A.I.; Bahnemann, D.W. Photoinduced hydrophilic behavior of TiO2 thin film on Si substrate. J. Alloy. Compd. 2021, 872, 159746. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X. Titanium dioxide photocatalysis: Present situation and future approaches. Comptes Rendus Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Sufian, S.; Hameed, B.H. Epigrammatic progress and perspective on the photocatalytic properties of BiVO4-based photocatalyst in photocatalytic water treatment technology: A review. J. Mol. Liq. 2018, 268, 438–459. [Google Scholar] [CrossRef]
- Wetchakun, N.; Chainet, S.; Phanichphant, S.; Wetchakun, K. Efficient photocatalytic degradation of methylene blue over BiVO4/TiO2 nanocomposites. Ceram. Int. 2015, 41, 5999–6004. [Google Scholar] [CrossRef]
- Qian, B.; Xu, Q.; Wu, Y.; Zhang, Y.; Li, H.; Wang, Y.; Wang, B.; Li, S.; Song, X.-M. A highly efficient photocatalytic methanol fuel cell based on non-noble metal photoelectrodes: Study on its energy band engineering via experimental and density functional theory method. J. Power Sources 2020, 78, 228756. [Google Scholar] [CrossRef]
- Hu, K.; Lei, E.; Li, Y.; Zhao, X.; Zhao, D.; Zhao, W.; Rong, H. Photocatalytic degradation mechanism of the visible-light responsive BiVO4/TiO2 core–shell heterojunction photocatalyst. J. Inorg. Organomet. Polym. 2020, 30, 775–788. [Google Scholar] [CrossRef]
- Balachandran, S.; Prakash, N.; Thirumalai, K.; Muruganandham, M.; Sillanpaa, M.; Swaminathan, M. Facile construction of heterostructured BiVO4−ZnO and its dual application of greater solar photocatalytic activity and self-cleaning property. Ind. Eng. Chem. Res. 2014, 53, 8346–8356. [Google Scholar] [CrossRef]
- Rudakova, A.V.; Oparicheva, U.G.; Grishina, A.E.; Murashkina, A.A.; Emeline, A.V.; Bahnemann, D.W. Photoinduced hydrophilic conversion of hydrated ZnO surfaces. J. Colloid Interface Sci. 2016, 466, 452–460. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Safaei, J.; Ismail, A.F.; Khalid, M.N.; Jailani, M.F.A.M.; Noh, M.F.M.; Arzaee, N.A.; Zhou, D.; Sagu, J.S.; Teridi, M.A.M. Boosting photocatalytic activities of BiVO4 by creation of g-C3N4/ZnO@ BiVO4 heterojunction. Mater. Res. Bull. 2020, 125, 110779. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, L. Synthesis and sonophotocatalytic activities of ZnO\BiVO4\Co3O4 composites. Chem. Phys. Lett. 2021, 775, 138660. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Liang, Y. Lotus Leaf-inspired hydrothermal synthesis of composite nanoparticles and application for photocatalytic oil denitrification. Catal. Lett. 2020, 150, 2474–2486. [Google Scholar] [CrossRef]
- Watanabe, T.; Fukayama, S.; Miyauchi, M.; Fujishima, A.; Hashimoto, K. Photocatalytic activity and photo-induced wettability conversion of TiO2. Thin film prepared by sol-gel process on a soda-lime glass. J. Sol-Gel Sci. Technol. 2000, 19, 71–76. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, X. Effect of substrates on the photocatalytic activity of nanometer TiO2 thin films. Mater. Res. Bull. 2000, 35, 1293–1301. [Google Scholar] [CrossRef]
- Emeline, A.V.; Rudakova, A.V.; Sakai, M.; Murakami, T.; Fujishima, A. Factors affecting UV-induced superhydrophilic conversion of a TiO2 surface. J. Phys. Chem. C 2013, 117, 12086–12092. [Google Scholar] [CrossRef]
| Coating | Crystalline Phase | Thickness, nm | Average Particle Diameter, nm | Ra, nm |
|---|---|---|---|---|
| TiO2 | anatase | 50 | 15 | ±1 |
| TiO2/BiVO4 | anatase/monoclinic | 50/50 | 10 | ±5 |
| ZnO | zincite | 80 | 15 | ±3 |
| ZnO/BiVO4 | zincite/monoclinic | 70/50 | 20 | ±6 |
| Coating Component | Ebg, eV | EF, eV | EVB, eV | ECB, eV |
|---|---|---|---|---|
| TiO2 | 3.2 | −5.1 | −7.2 | −3.9 |
| ZnO | 3.3 | −5.4 | −8.0 | −4.7 |
| BiVO4 | 2.5 | −6.0 | −7.4 | −4.9 |
| Coating | WF, eV (±0.02 eV) | |||
|---|---|---|---|---|
| As Prepared | Wetted | UV-Irradiated | Vis-Irradiated | |
| BiVO4 | 6.02 | 5.14 | 4.95 | 4.83 |
| TiO2 | 5.10 | 4.65 | 5.19 | 4.63 |
| TiO2/BiVO4 | 5.56 | 5.17 | 5.26 | 5.36 |
| ZnO | 5.40 | 5.18 | 5.29 | 5.00 |
| ZnO/BiVO4 | 5.69 | 5.09 | 5.22 | 4.94 |
| Coating | SFE, mN/m (±0.02 eV) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Wetted | UV-Irradiated | Vis-Irradiated | |||||||
| t | d | p | t | d | p | t | d | p | |
| TiO2 | 65.7 | 39.6 | 26.1 | 79.8 | 47.6 | 32.2 | 67.0 | 40.1 | 26.8 |
| TiO2/BiVO4 | 62.0 | 40.1 | 21.9 | 80.1 | 48.1 | 32.0 | 77.8 | 44.2 | 33.6 |
| ZnO | 77.8 | 44.2 | 33.6 | 80.8 | 50.0 | 30.8 | 74.1 | 47.2 | 26.9 |
| ZnO/BiVO4 | 76.1 | 46.8 | 29.3 | 80.8 | 49.9 | 30.9 | 75.0 | 48.3 | 26.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maevskaya, M.V.; Rudakova, A.V.; Koroleva, A.V.; Sakhatskii, A.S.; Emeline, A.V.; Bahnemann, D.W. Effect of the Type of Heterostructures on Photostimulated Alteration of the Surface Hydrophilicity: TiO2/BiVO4 vs. ZnO/BiVO4 Planar Heterostructured Coatings. Catalysts 2021, 11, 1424. https://doi.org/10.3390/catal11121424
Maevskaya MV, Rudakova AV, Koroleva AV, Sakhatskii AS, Emeline AV, Bahnemann DW. Effect of the Type of Heterostructures on Photostimulated Alteration of the Surface Hydrophilicity: TiO2/BiVO4 vs. ZnO/BiVO4 Planar Heterostructured Coatings. Catalysts. 2021; 11(12):1424. https://doi.org/10.3390/catal11121424
Chicago/Turabian StyleMaevskaya, Maria V., Aida V. Rudakova, Alexandra V. Koroleva, Aleksandr S. Sakhatskii, Alexei V. Emeline, and Detlef W. Bahnemann. 2021. "Effect of the Type of Heterostructures on Photostimulated Alteration of the Surface Hydrophilicity: TiO2/BiVO4 vs. ZnO/BiVO4 Planar Heterostructured Coatings" Catalysts 11, no. 12: 1424. https://doi.org/10.3390/catal11121424
APA StyleMaevskaya, M. V., Rudakova, A. V., Koroleva, A. V., Sakhatskii, A. S., Emeline, A. V., & Bahnemann, D. W. (2021). Effect of the Type of Heterostructures on Photostimulated Alteration of the Surface Hydrophilicity: TiO2/BiVO4 vs. ZnO/BiVO4 Planar Heterostructured Coatings. Catalysts, 11(12), 1424. https://doi.org/10.3390/catal11121424