Figures of Merit for Photocatalysis: Comparison of NiO/La-NaTaO3 and Synechocystis sp. PCC 6803 as a Semiconductor and a Bio-Photocatalyst for Water Splitting
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of Figures of Merit
2.2. Influence of Photocatalyst Concentration
2.3. Influence of Light Spectrum and Intensity
2.3.1. Sunlight Spectrum
2.3.2. Full Lamp Light Spectrum
2.4. Figures of Merit for the Comparison of NiO/La-NTO and Synechocystis
2.4.1. Maximum Product Evolution Rate
2.4.2. Similar Illumination Conditions
3. Materials and Methods
3.1. Synthesis of La-Doped NaTaO3 Loaded with NiO
3.2. Preparation of Synechocystis sp. PCC 6803
3.3. Photocatalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kan, S.; Chen, B.; Chen, G. Worldwide energy use across global supply chains: Decoupled from economic growth? Appl. Energy 2019, 250, 1235–1245. [Google Scholar] [CrossRef]
- Benveniste, H.; Boucher, O.; Guivarch, C.; Le Treut, H.; Criqui, P. Impacts of nationally determined contributions on 2030 global greenhouse gas emissions: Uncertainty analysis and distribution of emissions. Environ. Res. Lett. 2018, 13, 14022. [Google Scholar] [CrossRef]
- Malik, A.; Lan, J.; Lenzen, M. Trends in Global Greenhouse Gas Emissions from 1990 to 2010. Environ. Sci. Technol. 2016, 50, 4722–4730. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.; Kumar, P.; Kumar, S.; Adelodun, A.A.; Kim, K.-H. Solar energy: Potential and future prospects. Renew. Sustain. Energy Rev. 2018, 82, 894–900. [Google Scholar] [CrossRef]
- Elavarasan, R.M. The Motivation for Renewable Energy and its Comparison with Other Energy Sources: A Review. Eur. J. Sustain. Dev. Res. 2019, 3, em0076. [Google Scholar] [CrossRef]
- Shen, R.; Lu, X.; Zheng, Q.; Chen, Q.; Ng, Y.H.; Zhang, P.; Li, X. Tracking S-Scheme Charge Transfer Pathways in Mo2C/CdSH2-Evolution Photocatalysts. Sol. RRL 2021, 5, 2100177. [Google Scholar] [CrossRef]
- Ahmadi, M.H.; Ghazvini, M.; Sadeghzadeh, M.; Alhuyi Nazari, M.; Kumar, R.; Naeimi, A.; Ming, T. Solar power technology for electricity generation: A critical review. Energy. Sci. Eng. 2018, 6, 340–361. [Google Scholar] [CrossRef] [Green Version]
- Tuller, H.L. Solar to fuels conversion technologies: A perspective. Mater. Renew. Sustain. Energy 2017, 6, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafique, M.; Mubashar, R.; Irshad, M.; Gillani, S.S.A.; Tahir, M.B.; Khalid, N.R.; Yasmin, A.; Shehzad, M.A. A Comprehensive Study on Methods and Materials for Photocatalytic Water Splitting and Hydrogen Production as a Renewable Energy Resource. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3837–3861. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energ. 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- He, F.; Jeon, W.; Choi, W. Photocatalytic air purification mimicking the self-cleaning process of the atmosphere. Nat. Commun. 2021, 12, 2528. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Nguyen, T.N.; van Tran, V.; Hur, J.; Kim, I.T.; Park, D.; Lee, Y.-C. Photocatalytic materials for indoor air purification systems: An updated mini-review. Environ. Technol. Innov. 2021, 22, 101471. [Google Scholar] [CrossRef]
- Wang, C.; Cai, M.; Liu, Y.; Yang, F.; Zhang, H.; Liu, J.; Li, S. Facile construction of novel organic-inorganic tetra (4-carboxyphenyl) porphyrin/Bi2MoO6 heterojunction for tetracycline degradation: Performance, degradation pathways, intermediate toxicity analysis and mechanism insight. J. Colloid Interface Sci. 2022, 605, 727–740. [Google Scholar] [CrossRef]
- Brisbin, R.; Zhou, J.; Bond, T.; Voss, L.; Simon, A.J.; Baxter, R.; Chang, A.S. Plasmonics-Enhanced UV Photocatalytic Water Purification. J. Phys. Chem. C 2021, 125, 9730–9735. [Google Scholar] [CrossRef]
- Berkani, M.; Smaali, A.; Kadmi, Y.; Almomani, F.; Vasseghian, Y.; Lakhdari, N.; Alyane, M. Photocatalytic degradation of Penicillin G in aqueous solutions: Kinetic, degradation pathway, and microbioassays assessment. J. Hazard. Mater. 2022, 421, 126719. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Liu, Y.; Xue, B.; Chen, J.; Wang, H.; Liu, Y. Facile Preparation of a Novel Bi2WO6/Calcined Mussel Shell Composite Photocatalyst with Enhanced Photocatalytic Performance. Catalysts 2020, 10, 1166. [Google Scholar] [CrossRef]
- He, D.; Zhang, Z.; Xing, Y.; Zhou, Y.; Yang, H.; Liu, H.; Qu, J.; Yuan, X.; Guan, J.; Zhang, Y. Black phosphorus/graphitic carbon nitride: A metal-free photocatalyst for “green” photocatalytic bacterial inactivation under visible light. Chem. Eng. J. 2020, 384, 123258. [Google Scholar] [CrossRef]
- Liu, N.; Ming, J.; Sharma, A.; Sun, X.; Kawazoe, N.; Chen, G.; Yang, Y. Sustainable photocatalytic disinfection of four representative pathogenic bacteria isolated from real water environment by immobilized TiO2-based composite and its mechanism. Chem. Eng. J. 2021, 426, 131217. [Google Scholar] [CrossRef]
- Ainas, M.; Hasnaoui, S.; Bouarab, R.; Abdi, N.; Drouiche, N.; Mameri, N. Hydrogen production with the cyanobacterium Spirulina platensis. Int. J. Hydrogen Energ. 2017, 42, 4902–4907. [Google Scholar] [CrossRef]
- Sadvakasova, A.K.; Kossalbayev, B.D.; Zayadan, B.K.; Bolatkhan, K.; Alwasel, S.; Najafpour, M.M.; Tomo, T.; Allakhverdiev, S.I. Bioprocesses of hydrogen production by cyanobacteria cells and possible ways to increase their productivity. Renew. Sustain. Energy Rev. 2020, 133, 110054. [Google Scholar] [CrossRef]
- Touloupakis, E.; Rontogiannis, G.; Silva Benavides, A.M.; Cicchi, B.; Ghanotakis, D.F.; Torzillo, G. Hydrogen production by immobilized Synechocystis sp. PCC 6803. Int. J. Hydrogen Energ. 2016, 41, 15181–15186. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic Water Splitting-The Untamed Dream: A Review of Recent Advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, J.; Gong, J. Tantalum-based semiconductors for solar water splitting. Chem. Soc. Rev. 2014, 43, 4395–4422. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K. Photocatalytic water splitting using semiconductor particles: History and recent developments. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 237–268. [Google Scholar] [CrossRef]
- Parmar, A.; Singh, N.K.; Pandey, A.; Gnansounou, E.; Madamwar, D. Cyanobacteria and microalgae: A positive prospect for biofuels. Bioresour. Technol. 2011, 102, 10163–10172. [Google Scholar] [CrossRef]
- Mangan, N.M.; Flamholz, A.; Hood, R.D.; Milo, R.; Savage, D.F. pH determines the energetic efficiency of the cyanobacterial CO2 concentrating mechanism. Proc. Natl. Acad. Sci. USA 2016, 113, E5354–E5362. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, T.; Sato, S.; Kotani, H.; Tanaka, A.; Asamizu, E.; Nakamura, Y.; Miyajima, N.; Hirosawa, M.; Sugiura, M.; Sasamoto, S.; et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996, 3, 109–136. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, T.; Tabata, S. Complete genome structure of the unicellular cyanobacterium Synechocystis sp. PCC6803. Plant. Cell Physiol. 1997, 38, 1171–1176. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, T.; Nakamura, Y.; Sasamoto, S.; Watanabe, A.; Kohara, M.; Matsumoto, M.; Shimpo, S.; Yamada, M.; Tabata, S. Structural analysis of four large plasmids harboring in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. DNA Res. 2003, 10, 221–228. [Google Scholar] [CrossRef]
- Kato, H.; Kudo, A. New tantalate photocatalysts for water decomposition into H2 and O2. Chem. Phys. Lett. 1998, 295, 487–492. [Google Scholar] [CrossRef]
- Kato, H.; Asakura, K.; Kudo, A. Highly Efficient Water Splitting into H2 and O2 over Lanthanum-Doped NaTaO3 Photocatalysts with High Crystallinity and Surface Nanostructure. J. Am. Chem. Soc. 2003, 125, 3082–3089. [Google Scholar] [CrossRef]
- Kudo, A.; Kato, H. Effect of lanthanide-doping into NaTaO3 photocatalysts for efficient water splitting. Chem. Phys. Lett. 2000, 331, 373–377. [Google Scholar] [CrossRef]
- Kato, H.; Kudo, A. Photocatalytic water splitting into H2 and O2 over various tantalate photocatalysts. Catal. Today 2003, 78, 561–569. [Google Scholar] [CrossRef]
- Kato, H.; Kudo, A. Highly efficient decomposition of pure water into H2 and O2 over NaTaO3 photocatalysts. Catal. Lett. 1999, 58, 153–155. [Google Scholar] [CrossRef]
- Kramm, U.I.; Marschall, R.; Rose, M. Pitfalls in Heterogeneous Thermal, Electro- and Photocatalysis. ChemCatChem 2019, 11, 2563–2574. [Google Scholar] [CrossRef]
- Moustakas, N.G.; Strunk, J. Photocatalytic CO2 Reduction on TiO2 -Based Materials under Controlled Reaction Conditions: Systematic Insights from a Literature Study. Chemistry 2018, 24, 12739–12746. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photokatalytische Reduktion von CO2 an TiO2 und anderen Halbleitern. Angew. Chem. 2013, 125, 7516–7557. [Google Scholar] [CrossRef]
- Loh, J.Y.Y.; Sharma, G.; Kherani, N.P.; Ozin, G.A. Post-Illumination Photoconductivity Enables Extension of Photo-Catalysis after Sunset. Adv. Energy Mater. 2021, 11, 2101566. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P. Updates on the Roadmap for Photocatalysis. ACS Catal. 2020, 10, 5493–5501. [Google Scholar] [CrossRef] [Green Version]
- Ohtani, B. Preparing Articles on Photocatalysis—Beyond the Illusions, Misconceptions, and Speculation. Chem. Lett. 2008, 37, 216–229. [Google Scholar] [CrossRef] [Green Version]
- Ohtani, B. Design and Development of Active Titania and Related Photocatalysts. In Photocatalysis and Water Purification: From Fundamentals to Recent Applications; Pichat, P., Ed.; Wiley-VCH: Weinheim, Bergstr, 2013; pp. 73–102. ISBN 9783527645404. [Google Scholar]
- Vignolo-González, H.A.; Laha, S.; Jiménez-Solano, A.; Oshima, T.; Duppel, V.; Schützendübe, P.; Lotsch, B.V. Toward Standardized Photocatalytic Oxygen Evolution Rates Using RuO2@TiO2 as a Benchmark. Matter 2020, 3, 464–486. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.; Takanabe, K. Insights on Measuring and Reporting Heterogeneous Photocatalysis: Efficiency Definitions and Setup Examples. Chem. Mater. 2017, 29, 158–167. [Google Scholar] [CrossRef]
- Sundar, K.P.; Kanmani, S. Progression of Photocatalytic reactors and it’s comparison: A Review. Chem. Eng. Res. Des. 2020, 154, 135–150. [Google Scholar] [CrossRef]
- McDaniel, N.D.; Bernhard, S. Solar fuels: Thermodynamics, candidates, tactics, and figures of merit. Dalton Trans. 2010, 39, 10021–10030. [Google Scholar] [CrossRef] [PubMed]
- Wachs, I.E.; Phivilay, S.P.; Roberts, C.A. Reporting of Reactivity for Heterogeneous Photocatalysis. ACS Catal. 2013, 3, 2606–2611. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Serpone, N.; Terzian, R.; Lawless, D.; Kennepohl, P.; Sauvé, G. On the usage of turnover numbers and quantum yields in heterogeneous photocatalysis. J. Photochem. Photobiol. A: Chem. 1993, 73, 11–16. [Google Scholar] [CrossRef]
- Gritzner, G.; Kreysa, G. Nomenclature, symbols and definitions in electrochemical engineering (IUPAC Recommendations 1993). Pure Appl. Chem. 1993, 65, 1009–1020. [Google Scholar] [CrossRef]
- Kisch, H.; Bahnemann, D. Best Practice in Photocatalysis: Comparing Rates or Apparent Quantum Yields? J. Phys. Chem. Lett. 2015, 6, 1907–1910. [Google Scholar] [CrossRef]
- Hisatomi, T.; Domen, K. Introductory lecture: Sunlight-driven water splitting and carbon dioxide reduction by heterogeneous semiconductor systems as key processes in artificial photosynthesis. Faraday Discuss. 2017, 198, 11–35. [Google Scholar] [CrossRef]
- Leblebici, M.E.; Stefanidis, G.D.; van Gerven, T. Comparison of photocatalytic space-time yields of 12 reactor designs for wastewater treatment. Chem. Eng. Process. 2015, 97, 106–111. [Google Scholar] [CrossRef]
- Buriak, J.M.; Kamat, P.V.; Schanze, K.S. Best practices for reporting on heterogeneous photocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 11815–11816. [Google Scholar] [CrossRef]
- Maschmeyer, T.; Che, M. Catalytic aspects of light-induced hydrogen generation in water with TiO2 and other photocatalysts: A simple and practical way towards a normalization? Angew. Chem. Int. Ed. 2010, 49, 1536–1539. [Google Scholar] [CrossRef]
- Maschmeyer, T.; Che, M. Intrinsic Catalytic Activity versus Effective Light Usage-A Reply to Professor Kisch’s Comments. Angew. Chem. Int. Ed. 2010, 49, 9590–9591. [Google Scholar] [CrossRef]
- Kisch, H. On the problem of comparing rates or apparent quantum yields in heterogeneous photocatalysis. Angew. Chem. Int. Ed. 2010, 49, 9588–9589. [Google Scholar] [CrossRef]
- Kunz, L.Y.; Diroll, B.T.; Wrasman, C.J.; Riscoe, A.R.; Majumdar, A.; Cargnello, M. Artificial inflation of apparent photocatalytic activity induced by catalyst-mass-normalization and a method to fairly compare heterojunction systems. Energy Environ. Sci. 2019, 12, 1657–1667. [Google Scholar] [CrossRef]
- Takanabe, K. Photocatalytic Water Splitting: Quantitative Approaches toward Photocatalyst by Design. ACS Catal. 2017, 7, 8006–8022. [Google Scholar] [CrossRef]
- Nadeem, M.A.; Khan, M.A.; Ziani, A.A.; Idriss, H. An Overview of the Photocatalytic Water Splitting over Suspended Particles. Catalysts 2021, 11, 60. [Google Scholar] [CrossRef]
- Ballari, M.d.l.M.; Alfano, O.M.; Cassano, A.E. Mass transfer limitations in slurry photocatalytic reactors: Experimental validation. Chem. Eng. Sci. 2010, 65, 4931–4942. [Google Scholar] [CrossRef]
- Amaechi, I.C.; Katoch, R.; Kolhatkar, G.; Sun, S.; Ruediger, A. Particle size effect on the photocatalytic kinetics of barium titanate powders. Catal. Sci. Technol. 2020, 10, 6274–6284. [Google Scholar] [CrossRef]
- Li, D.; Song, H.; Meng, X.; Shen, T.; Sun, J.; Han, W.; Wang, X. Effects of Particle Size on the Structure and Photocatalytic Performance by Alkali-Treated TiO2. Nanomaterials 2020, 10, 546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kröger, J.; Jiménez-Solano, A.; Savasci, G.; Lau, V.W.h.; Duppel, V.; Moudrakovski, I.; Küster, K.; Scholz, T.; Gouder, A.; Schreiber, M.-L.; et al. Morphology Control in 2D Carbon Nitrides: Impact of Particle Size on Optoelectronic Properties and Photocatalysis. Adv. Funct. Mater. 2021, 31, 2102468. [Google Scholar] [CrossRef]
- Amano, F.; Ishinaga, E.; Yamakata, A. Effect of Particle Size on the Photocatalytic Activity of WO3 Particles for Water Oxidation. J. Phys. Chem. C 2013, 117, 22584–22590. [Google Scholar] [CrossRef]
- Carbajo, J.; Tolosana-Moranchel, A.; Casas, J.A.; Faraldos, M.; Bahamonde, A. Analysis of photoefficiency in TiO2 aqueous suspensions: Effect of titania hydrodynamic particle size and catalyst loading on their optical properties. Appl. Catal. B 2018, 221, 1–8. [Google Scholar] [CrossRef]
- Gerischer, H. Photocatalysis in aqueous solution with small TiO2 particles and the dependence of the quantum yield on particle size and light intensity. Electrochim. Acta 1995, 40, 1277–1281. [Google Scholar] [CrossRef]
- Broddrick, J.T.; Rubin, B.E.; Welkie, D.G.; Du, N.; Mih, N.; Diamond, S.; Lee, J.J.; Golden, S.S.; Palsson, B.O. Unique attributes of cyanobacterial metabolism revealed by improved genome-scale metabolic modeling and essential gene analysis. Proc. Natl. Acad. Sci. USA 2016, 113, E8344–E8353. [Google Scholar] [CrossRef] [Green Version]
- Hoque, M.A.; Guzman, M.I. Photocatalytic Activity: Experimental Features to Report in Heterogeneous Photocatalysis. Materials 2018, 11, 1990. [Google Scholar] [CrossRef] [Green Version]
- Long, S.P.; Humphries, S.; Falkowski, P.G. Photoinhibition of Photosynthesis in Nature. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1994, 45, 633–662. [Google Scholar] [CrossRef]
- Liang, F.; Lindblad, P. Synechocystis PCC 6803 overexpressing RuBisCO grow faster with increased photosynthesis. Metab. Eng. Commun. 2017, 4, 29–36. [Google Scholar] [CrossRef]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Tabata, S.; Ohnishi, H.; Yagasaki, E.; Ippommatsu, M.; Domen, K. Light-intensity dependence in photocatalytic decomposition of water over K4Nb6O17 catalyst. Catal. Lett. 1994, 28, 417–422. [Google Scholar] [CrossRef]
- Bloh, J.Z. A Holistic Approach to Model the Kinetics of Photocatalytic Reactions. Front. Chem. 2019, 7, 128. [Google Scholar] [CrossRef]
- Touloupakis, E.; Cicchi, B.; Torzillo, G. A bioenergetic assessment of photosynthetic growth of Synechocystis sp. PCC 6803 in continuous cultures. Biotechnol Biofuels 2015, 8, 133. [Google Scholar] [CrossRef] [Green Version]
- Cui, M.; Liu, Y.; Zhang, J. The Variation of Growth Rate, Photosynthetic Activity, and Biodiesel Productivity in Synechocystis sp. PCC 6803 under Antibiotic Exposure. Bioenerg. Res. 2020, 13, 955–962. [Google Scholar] [CrossRef]
- Lai, B.; Schneider, H.; Tschörtner, J.; Schmid, A.; Krömer, J.O. Technical-scale biophotovoltaics for long-term photo-current generation from Synechocystis sp. PCC6803. Biotechnol. Bioeng. 2021, 118, 2637–2648. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, F.; Wang, L.; Yang, C. Design of Photobioreactors for Mass Cultivation of Photosynthetic Organisms. Engineering 2017, 3, 318–329. [Google Scholar] [CrossRef]
- Iwase, A.; Kato, H.; Okutomi, H.; Kudo, A. Formation of Surface Nano-step Structures and Improvement of Photocatalytic Activities of NaTaO3 by Doping of Alkaline Earth Metal Ions. Chem. Lett. 2004, 33, 1260–1261. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Deruelles, J.; Rippka, R.; Herdman, M.; Waterbury, J.B. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]

| Figure of Merit | Equation | Advantages/Disadvantages | Transferable | Citation |
|---|---|---|---|---|
| material-based | ||||
| Reaction Rate | + Widely used + Can include information about catalyst + Easily accessible (Reaction Rate) + Suitable to evaluate, if the reaction is a catalytic phenomenon (TON) − Completely neglects influence of light − Can depend on the measurement time − Can be limited by other factors (Co-catalyst concentration, catalyst concentration) − Requires knowledge about the active sites (TOF/TON) | Yes | [37,45] | |
| Reaction Rate Related to Catalyst Mass | Yes (based on cell dry weight) | |||
| Turnover Frequency (TOF) | Yes | [37,45,46] | ||
| Turnover Number (TON) | Yes | [37,46,47,48] | ||
| Space-Time-Yield (STY) | Yes | [49] | ||
| light-based | ||||
| Quantum Yield (QY) | + Takes the light source into account + Takes information about reactor geometry into account (STC/STH) +/− Does not take losses of light due to catalytic setup into account and allows comparison of catalytic setups (AQY, AQE, , STC/STH, PSTY) +/− Takes losses of light due to catalytic setup into account and gives information about the intrinsic catalytic activity (QY, QE, ) − Completely neglects the catalyst (concentration/amount, active sites) − Requires knowledge about the absorbed photons (QY, QE) − Requires monochromatic light (QY, PY, AQY) − Only applicable to AM 1.5G light (STC/STH) − Only applicable to H2 (STH) | Yes | [37,39,43,50] | |
| Apparent Quantum Yield (AQY) | Yes | [43,47] | ||
| Quantum Efficiency (QE)/Internal Quantum Efficiency (IQE) | Yes (based on cell number) | [43] | ||
| Photonic Efficiency (PE)/External Quantum Efficiency (EQE) | Yes | [37,43,50] | ||
| Photonic Yield (PY) | Yes | [43] | ||
| Power Conversion Efficiency | Yes | [37] | ||
| Solar to Chemical Conversion Effiency (STC)/Solar to Hydrogen Efficiency (STH) | Yes | [43,51] | ||
| Photocatalytic Space-Time-Yield (PTSY) | Yes | [52] | ||
| Figure of Merit | Synechocystis | NiO/La-NTO | |
|---|---|---|---|
| material-based | |||
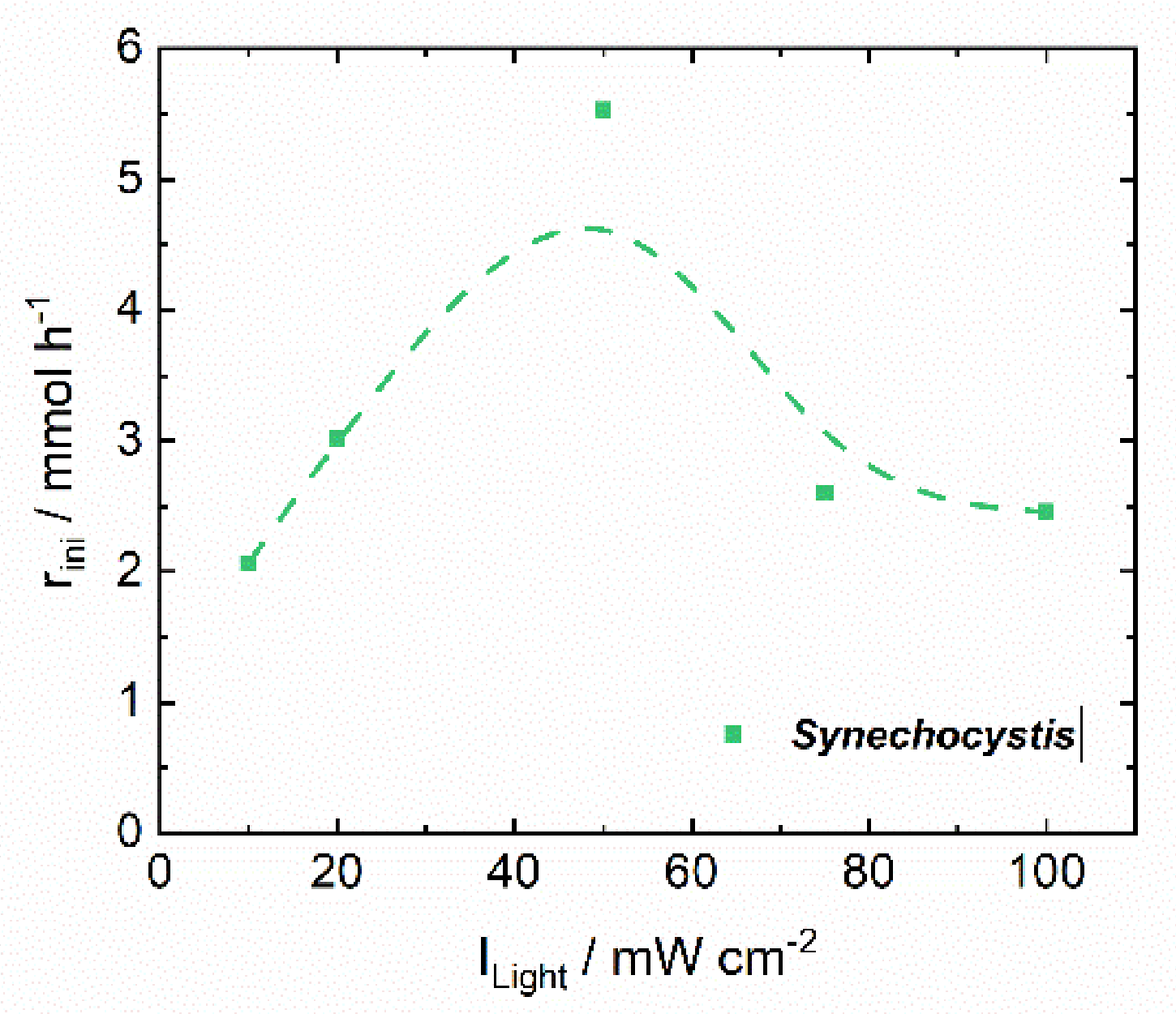
| Initial reaction rate | rini/mmol h−1 | 5.529 | 0.226 |
| Mass-related initial reaction rate | rini,m/mmol h−1 g−1 | 339.202 | 2.194 |
| Average reaction rate | rave/mmol h−1 | 4.815 | 0.215 |
| Mass related average reaction rate | rave,m/mmol h−1 g−1 | 295.395 | 2.092 |
| Space time yield | STY/mmol h−1 dm−3 | 19.431 | 0.870 |
| light-based | |||
| External quantum efficiency | EQE | 3.5 × 10−6 (λ250–800) | 3.2 × 10−3 (λ250–800) |
| External quantum efficiency | EQE | 3.5 × 10−6 (λ305–780) | 6.6 × 10−3 (λ250–305) |
| Apparent quantum yield | AQY/% | 1.4 × 10−3 (λ250–800) | 1.3 × (λ250–800) |
| Apparent quantum yield | AQY/% | 1.4 × 10−4 (λ305–780) | 2.7 × (λ250–305) |
| Photocatalytic space time yield | PSTY/mmol h−1 dm−3 kW−1 | 1.1 × 10−5 | 4.8 × 10−7 |
| Figure of Merit | Synechocystis | NiO/La-NTO | |
|---|---|---|---|
| material-based | |||
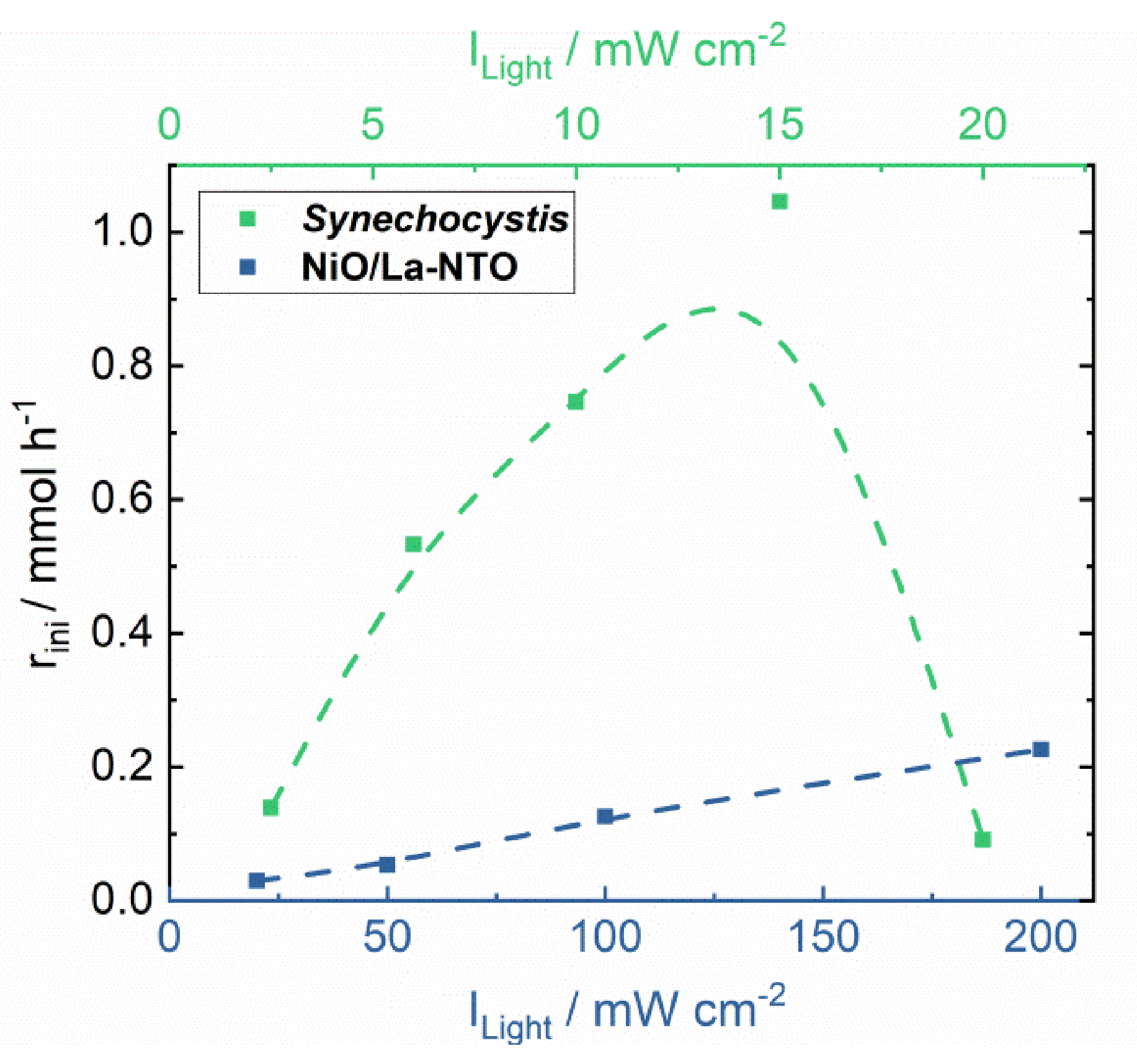
| Initial reaction rate | rini/mmol h−1 | 0.091 | 0.030 |
| Mass related initial reaction rate | rini,m/mmol h−1 g−1 | 5.558 | 0.300 |
| Average reaction rate | rave/mmol h−1 | 0.206 | 0.004 |
| Mass related average reaction rate | rave,m/mmol h−1 g−1 | 12.623 | 0.045 |
| Space time yield | STY/mmol h−1 dm−3 | 0.830 | 0.018 |
| Light-based Figures of Meritlight-based | |||
| External quantum efficiency | EQE | 1.3 × 10−2 (λ250–800) | 8.7 × 10−3 (λ250–800) |
| External quantum efficiency | EQE | 1.3 × 10−2 (λ305–780) | 8.8 × 10−3 (λ250–305) |
| Apparent quantum yield | AQY/% | 5.2 × (λ250–800) | 3.5 × (λ250–800) |
| Apparent quantum yield | AQY/% | 5.3 × (λ305–780) | 3.5 × (λ250–305) |
| Photocatalytic space time yield | PSTY/mmol h−1 dm−3 kW−1 | 4.6 × 10−7 | 9.9 × 10−9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welter, E.S.; Kött, S.; Brandenburg, F.; Krömer, J.; Goepel, M.; Schmid, A.; Gläser, R. Figures of Merit for Photocatalysis: Comparison of NiO/La-NaTaO3 and Synechocystis sp. PCC 6803 as a Semiconductor and a Bio-Photocatalyst for Water Splitting. Catalysts 2021, 11, 1415. https://doi.org/10.3390/catal11111415
Welter ES, Kött S, Brandenburg F, Krömer J, Goepel M, Schmid A, Gläser R. Figures of Merit for Photocatalysis: Comparison of NiO/La-NaTaO3 and Synechocystis sp. PCC 6803 as a Semiconductor and a Bio-Photocatalyst for Water Splitting. Catalysts. 2021; 11(11):1415. https://doi.org/10.3390/catal11111415
Chicago/Turabian StyleWelter, Eike S., Sebastian Kött, Fabian Brandenburg, Jens Krömer, Michael Goepel, Andreas Schmid, and Roger Gläser. 2021. "Figures of Merit for Photocatalysis: Comparison of NiO/La-NaTaO3 and Synechocystis sp. PCC 6803 as a Semiconductor and a Bio-Photocatalyst for Water Splitting" Catalysts 11, no. 11: 1415. https://doi.org/10.3390/catal11111415
APA StyleWelter, E. S., Kött, S., Brandenburg, F., Krömer, J., Goepel, M., Schmid, A., & Gläser, R. (2021). Figures of Merit for Photocatalysis: Comparison of NiO/La-NaTaO3 and Synechocystis sp. PCC 6803 as a Semiconductor and a Bio-Photocatalyst for Water Splitting. Catalysts, 11(11), 1415. https://doi.org/10.3390/catal11111415







