Bimetallic Cu/Fe Catalysts for Ibuprofen Mineralization
Abstract
:1. Introduction
2. Results
2.1. Catalyst Characterization
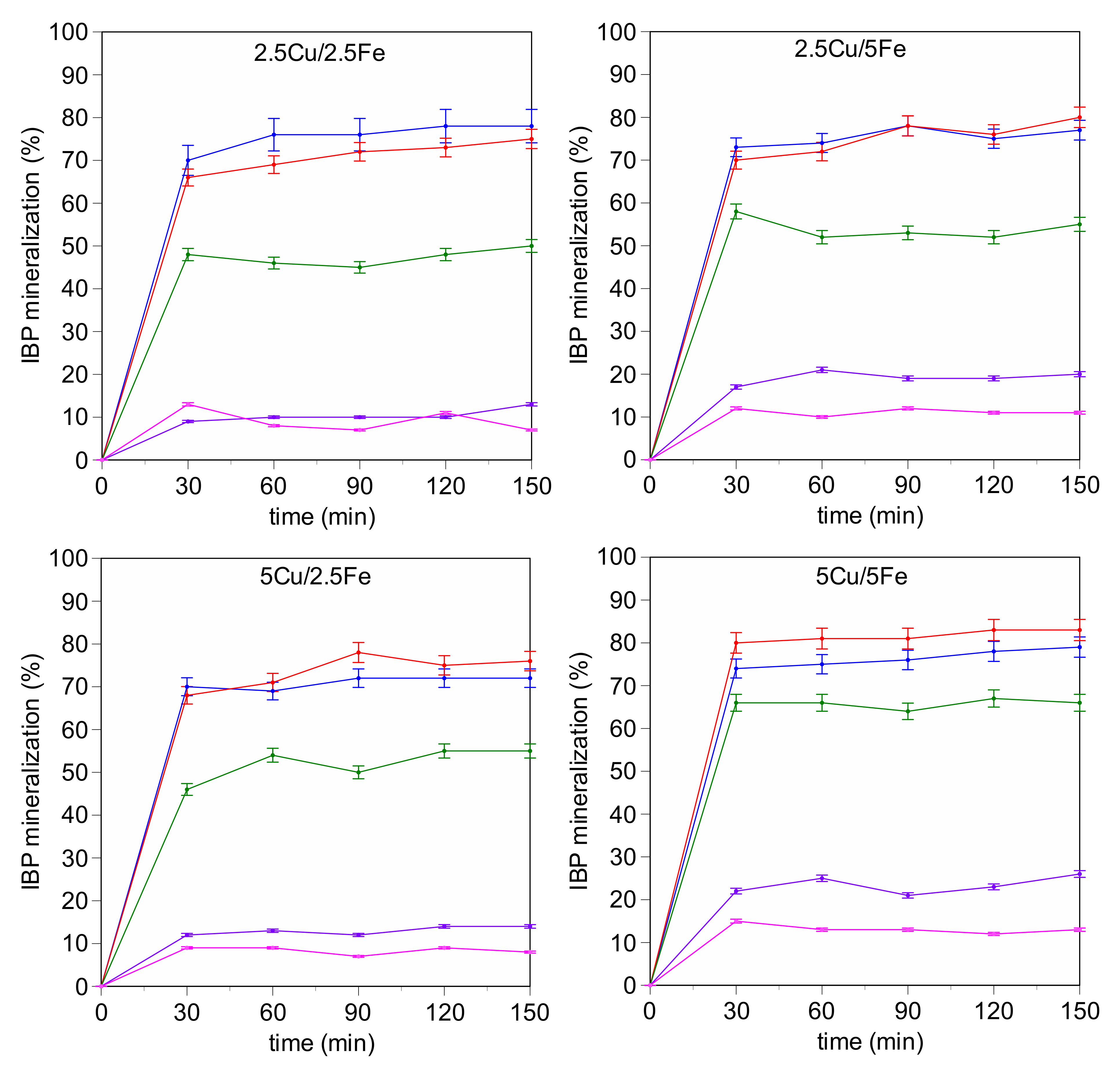
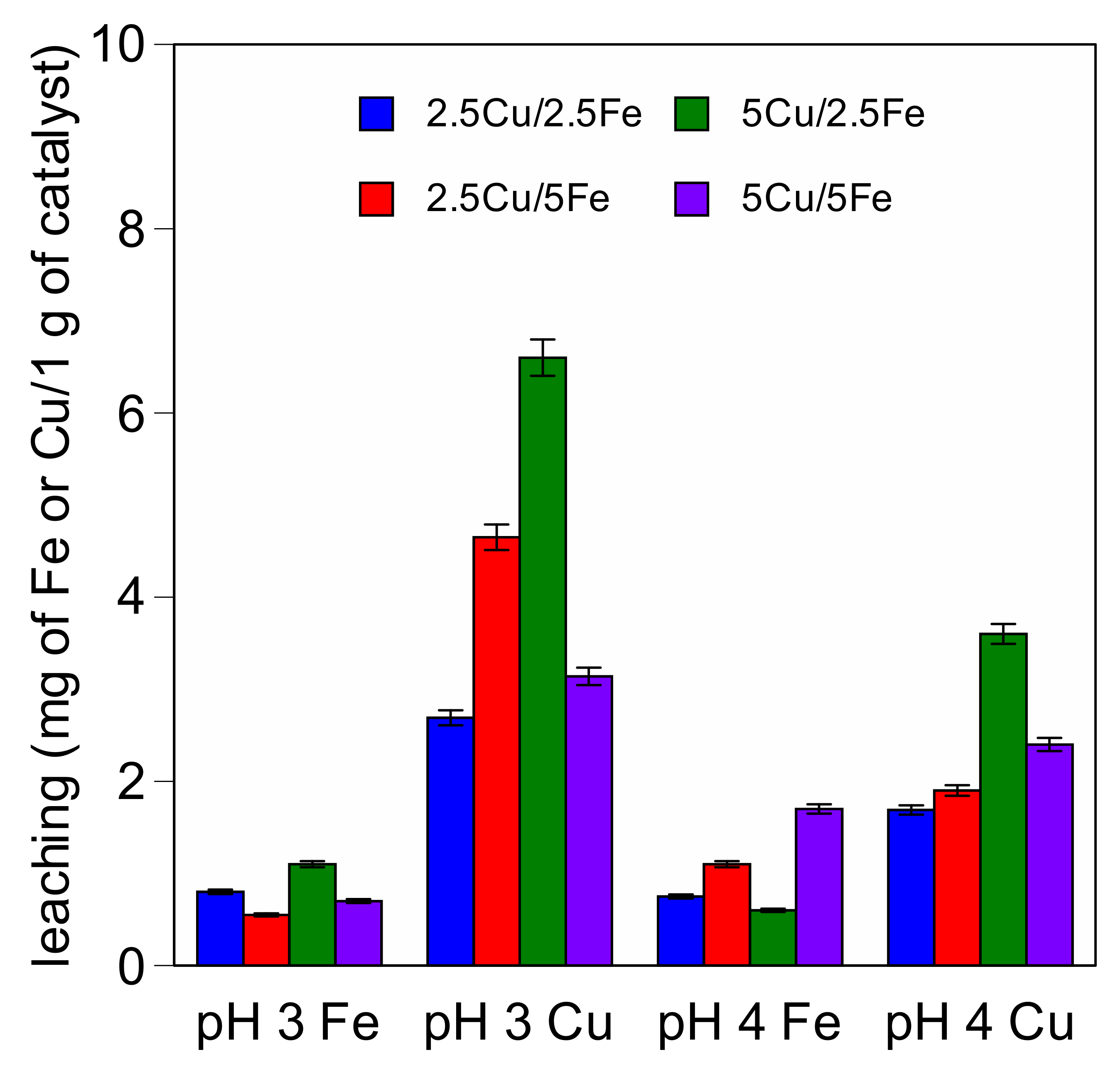
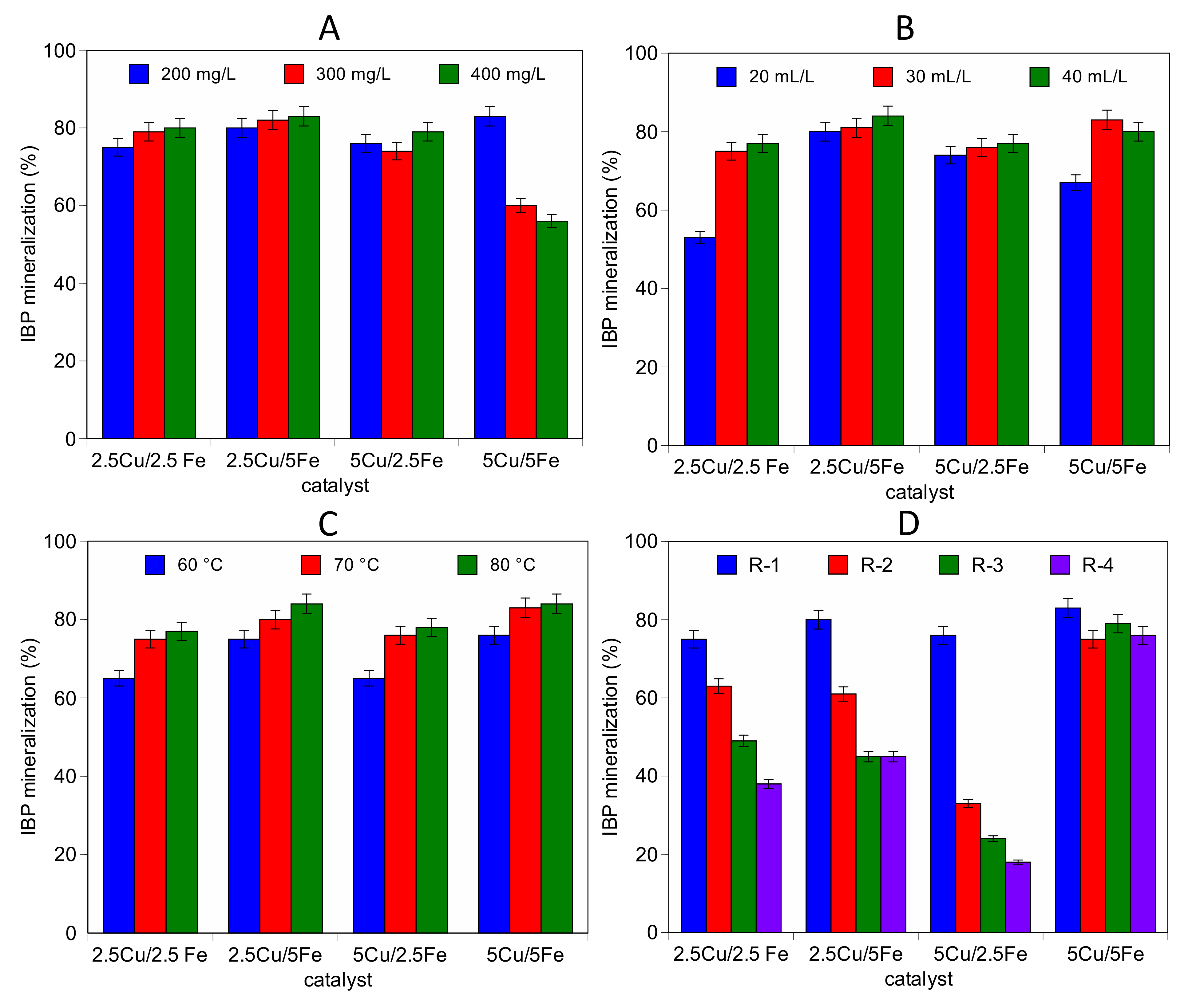
2.2. Catalytic Activity
3. Materials and Methods
3.1. Catalyst Preparation and Characterization
3.2. Catalytic Activity Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Shea, K.E.; Dionysiou, D.D. Advanced Oxidation Processes for Water Treatment. J. Phys. Chem. Lett. 2012, 3, 2112–2113. [Google Scholar] [CrossRef]
- Richardson, S.D.; Ternes, T.A. Water analysis: Emerging contaminants and current issues. Anal. Chem. 2014, 86, 2813–2848. [Google Scholar] [CrossRef] [PubMed]
- Jurado, A.; Vàzquez-Suné, E.; Carrera, J.; López de Alda, M.; Pujades, E.; Barceló, D. Emerging organic contaminants in groundwater in Spain: A review of sources, recent occurrence and fate in a European context. Sci. Total Environ. 2012, 440, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Song, H.; Li, Q.; Li, A. Fe/Cu bimetallic catalysis for reductive degradation of nitrobenzene under oxic conditions. Chem. Eng. J. 2016, 283, 366–374. [Google Scholar] [CrossRef]
- Lam, F.L.; Hu, X. pH-insensitive bimetallic catalyst for the abatement of dye pollutants by photo-fenton oxidation. Ind. Eng. Chem. Res. 2013, 52, 6639–6646. [Google Scholar] [CrossRef]
- Xu, S.; Zhu, H.; Cao, W.; Wen, Z.; Wang, J.; Francois-Xavier, C.P.; Wintgens, T. Cu-Al2O3-g-C3N4 and Cu-Al2O3-C-dots with dual-reaction centres for simultaneous enhancement of Fenton-like catalytic activity and selective H2O2 conversion to hydroxyl radicals. Appl. Catal. B Environ. 2018, 234, 223–233. [Google Scholar] [CrossRef]
- Duan, H.; Liu, Y.; Yin, X.; Bai, J.; Qi, J. Degradation of nitrobenzene by Fenton-like reaction in a H2O2/schwertmannite system. Chem. Eng. J. 2016, 283, 873–879. [Google Scholar] [CrossRef]
- Dükkancı, M.; Gündüz, G.; Yılmaz, S.; Prihod’Ko, R. Heterogeneous Fenton-like degradation of Rhodamine 6G in water using CuFeZSM-5 zeolite catalyst prepared by hydrothermal synthesis. J. Hazard. Mater. 2010, 181, 343–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crane, R.A.; Scott, T.B. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211, 112–125. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248. [Google Scholar] [CrossRef]
- Sashkina, K.; Labko, V.; Rudina, N.; Parmon, V.; Parkhomchuk, E. Hierarchical zeolite FeZSM-5 as a heterogeneous Fenton-type catalyst. J. Catal. 2013, 299, 44–52. [Google Scholar] [CrossRef]
- Cruz, A.; Couto, L.; Esplugas, S.; Sans, C. Study of the contribution of homogeneous catalysis on heterogeneous Fe (III)/alginate mediated photo-Fenton process. Chem. Eng. J. 2017, 318, 272–280. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Kurosu, S.; Suzuki, M.; Kawase, Y. Hydroxyl radical generation by zero-valent iron/Cu (ZVI/Cu) bimetallic catalyst in wastewater treatment: Heterogeneous Fenton/Fenton-like reactions by Fenton reagents formed in-situ under oxic conditions. Chem. Eng. J. 2018, 334, 1537–1549. [Google Scholar] [CrossRef]
- Hussain, S.; Aneggi, E.; Trovarelli, A.; Goi, D. Heterogeneous Fenton-like oxidation of ibuprofen over zirconia-supported iron and copper catalysts: Effect of process variables. J. Water Process Eng. 2021, 44, 102343. [Google Scholar] [CrossRef]
- Vindedahl, A.M.; Strehlau, J.H.; Arnold, W.A.; Penn, R.L. Organic matter and iron oxide nanoparticles: Aggregation, interactions, and reactivity. Environ. Sci. Nano 2016, 3, 494–505. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]
- Ziylan, A.; Ince, N.H. Catalytic ozonation of ibuprofen with ultrasound and Fe-based catalysts. Catal. Today 2015, 240, 2–8. [Google Scholar] [CrossRef]
- Madhavan, J.; Grieser, F.; Ashokkumar, M. Combined advanced oxidation processes for the synergistic degradation of ibuprofen in aqueous environments. J. Hazard. Mater. 2010, 178, 202–208. [Google Scholar] [CrossRef]
- Adityosulindro, S.; Barthe, L.; González-Labrada, K.; Jáuregui Haza, U.J.; Delmas, H.; Julcour, C. Sonolysis and sono-Fenton oxidation for removal of ibuprofen in (waste)water. Ultrason. Sonochem. 2017, 39, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gągol, M.; Przyjazny, A.; Boczkaj, G. Wastewater treatment by means of advanced oxidation processes based on cavitation—A review. Chem. Eng. J. 2018, 338, 599–627. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Zhang, Y.; Wang, Y.; Sun, S.-P.; Wu, W.D.; Wu, Z. Nanostructured semiconductor supported iron catalysts for heterogeneous photo-Fenton oxidation: A review. J. Mater. Chem. A 2020, 8, 15513–15546. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; Di Somma, I.; Marotta, R.; Andreozzi, R. Homogeneous photo-Fenton processes at near neutral pH: A review. Appl. Catal. B Environ. 2017, 209, 358–371. [Google Scholar] [CrossRef]
- Adityosulindro, S.; Julcour, C.; Riboul, D.; Barthe, L. Degradation of ibuprofen by photo-based advanced oxidation processes: Exploring methods of activation and related reaction routes. Int. J. Environ. Sci. Technol. 2021, 1–14. [Google Scholar] [CrossRef]
- Tetorou, A.; Makhatova, A.; Poulopoulos, S.G. Photochemical mineralization of Ibuprofen medicinal product by means of UV, hydrogen peroxide, titanium dioxide and iron. Water Sci. Technol. 2020, 80, 2200–2205. [Google Scholar] [CrossRef]
- Singh, L.; Rekha, P.; Chand, S. Cu-impregnated zeolite Y as highly active and stable heterogeneous Fenton-like catalyst for degradation of Congo red dye. Sep. Purif. Technol. 2016, 170, 321–336. [Google Scholar] [CrossRef]
- Hussain, S.; Aneggi, E.; Briguglio, S.; Mattiussi, M.; Gelao, V.; Cabras, I.; Zorzenon, L.; Trovarelli, A.; Goi, D. Enhanced ibuprofen removal by heterogeneous-Fenton process over Cu/ZrO2 and Fe/ZrO2 catalysts. J. Environ. Chem. Eng. 2020, 8, 103586. [Google Scholar] [CrossRef]
- Hussain, S.; Aneggi, E.; Goi, D. Catalytic activity of metals in heterogeneous Fenton-like oxidation of wastewater contaminants: A review. Environ. Chem. Lett. 2021, 19, 2405–2424. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Nidheesh, P.V. Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: A review. RSC Adv. 2015, 5, 40552–40577. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef] [Green Version]
- Aneggi, E.; Trovarelli, A.; Goi, D. Degradation of phenol in wastewaters via heterogeneous Fenton-like Ag/CeO2 catalyst. J. Environ. Chem. Eng. 2017, 5, 1159–1165. [Google Scholar] [CrossRef]
- Adityosulindro, S.; Julcour, C.; Barthe, L. Heterogeneous Fenton oxidation using Fe-ZSM5 catalyst for removal of ibuprofen in wastewater. J. Environ. Chem. Eng. 2018, 6, 5920–5928. [Google Scholar] [CrossRef] [Green Version]
- Xia, M.; Long, M.; Yang, Y.; Chen, C.; Cai, W.; Zhou, B. A highly active bimetallic oxides catalyst supported on Al-containing MCM-41 for Fenton oxidation of phenol solution. Appl. Catal. B Environ. 2011, 110, 118–125. [Google Scholar] [CrossRef]
- Zhang, H.; Choi, H.J.; Huang, C.P. Optimization of Fenton process for the treatment of landfill leachate. J. Hazard. Mater. 2005, 125, 166–174. [Google Scholar] [CrossRef]
- Zhou, X.; Su, T.; Jiang, Y.; Qin, Z.; Ji, H.; Guo, Z. CuO-Fe2O3-CeO2/HZSM-5 bifunctional catalyst hydrogenated CO2 for enhanced dimethyl ether synthesis. Chem. Eng. Sci. 2016, 153, 10–20. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, K.; Chen, T.; Su, H. Influence of crystallite size and interface on the catalytic performance over the CeO2/CuO catalysts. Int. J. Hydrogen Energy 2013, 38, 14542–14549. [Google Scholar] [CrossRef]
- Qu, Z.; Miao, L.; Wang, H.; Fu, Q. Highly dispersed Fe2O3 on carbon nanotubes for low-temperature selective catalytic reduction of NO with NH3. Chem. Commun. 2015, 51, 956–958. [Google Scholar] [CrossRef]
- Zhang, L.; Nie, Y.; Hu, C.; Qu, J. Enhanced Fenton degradation of Rhodamine B over nanoscaled Cu-doped LaTiO3 perovskite. Appl. Catal. B Environ. 2012, 125, 418–424. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Zhao, G. Iron-copper bimetallic nanoparticles embedded within ordered mesoporous carbon as effective and stable heterogeneous Fenton catalyst for the degradation of organic contaminants. Appl. Catal. B Environ. 2015, 164, 396–406. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, K.; Dai, C.; Zhou, X.; Si, H. An enhanced Fenton reaction catalyzed by natural heterogeneous pyrite for nitrobenzene degradation in an aqueous solution. Chem. Eng. J. 2014, 244, 438–445. [Google Scholar] [CrossRef]
- Sabhi, S.; Kiwi, J. Degradation of 2, 4-dichlorophenol by immobilized iron catalysts. Water Res. 2001, 35, 1994–2002. [Google Scholar] [CrossRef]
- Lam, F.L.; Yip, A.C.; Hu, X. Copper/MCM-41 as a highly stable and pH-insensitive heterogeneous photo-Fenton-like catalytic material for the abatement of organic wastewater. Ind. Eng. Chem. Res. 2007, 46, 3328–3333. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Z.; Tian, P.; Sheng, Y.; Xu, J.; Han, Y.-F. Oxidative degradation of nitrobenzene by a Fenton-like reaction with Fe-Cu bimetallic catalysts. Appl. Catal. B Environ. 2019, 244, 1–10. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Li, J.; Luo, R.; Hu, X.; Sun, X.; Shen, J.; Han, W.; Wang, L. In-situ incorporation of iron-copper bimetallic particles in electrospun carbon nanofibers as an efficient Fenton catalyst. Appl. Catal. B Environ. 2017, 207, 316–325. [Google Scholar] [CrossRef]
- Cihanoğlu, A.; Gündüz, G.; Dükkancı, M. Degradation of acetic acid by heterogeneous Fenton-like oxidation over iron-containing ZSM-5 zeolites. Appl. Catal. B Environ. 2015, 165, 687–699. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, J.; Crittenden, J.C.; Shen, C. Novel RGO/α-FeOOH supported catalyst for Fenton oxidation of phenol at a wide pH range using solar-light-driven irradiation. J. Hazard. Mater. 2017, 329, 321–329. [Google Scholar] [CrossRef]
- Yang, X.; Tian, P.-F.; Zhang, C.; Deng, Y.-Q.; Xu, J.; Gong, J.; Han, Y.-F. Au/carbon as Fenton-like catalysts for the oxidative degradation of bisphenol A. Appl. Catal. B Environ. 2013, 134, 145–152. [Google Scholar] [CrossRef]
- Ramirez, J.H.; Costa, C.A.; Madeira, L.M. Experimental design to optimize the degradation of the synthetic dye Orange II using Fenton’s reagent. Catal. Today 2005, 107, 68–76. [Google Scholar] [CrossRef]
- Guedes, A.M.; Madeira, L.M.; Boaventura, R.A.; Costa, C.A. Fenton oxidation of cork cooking wastewater—Overall kinetic analysis. Water Res. 2003, 37, 3061–3069. [Google Scholar] [CrossRef]
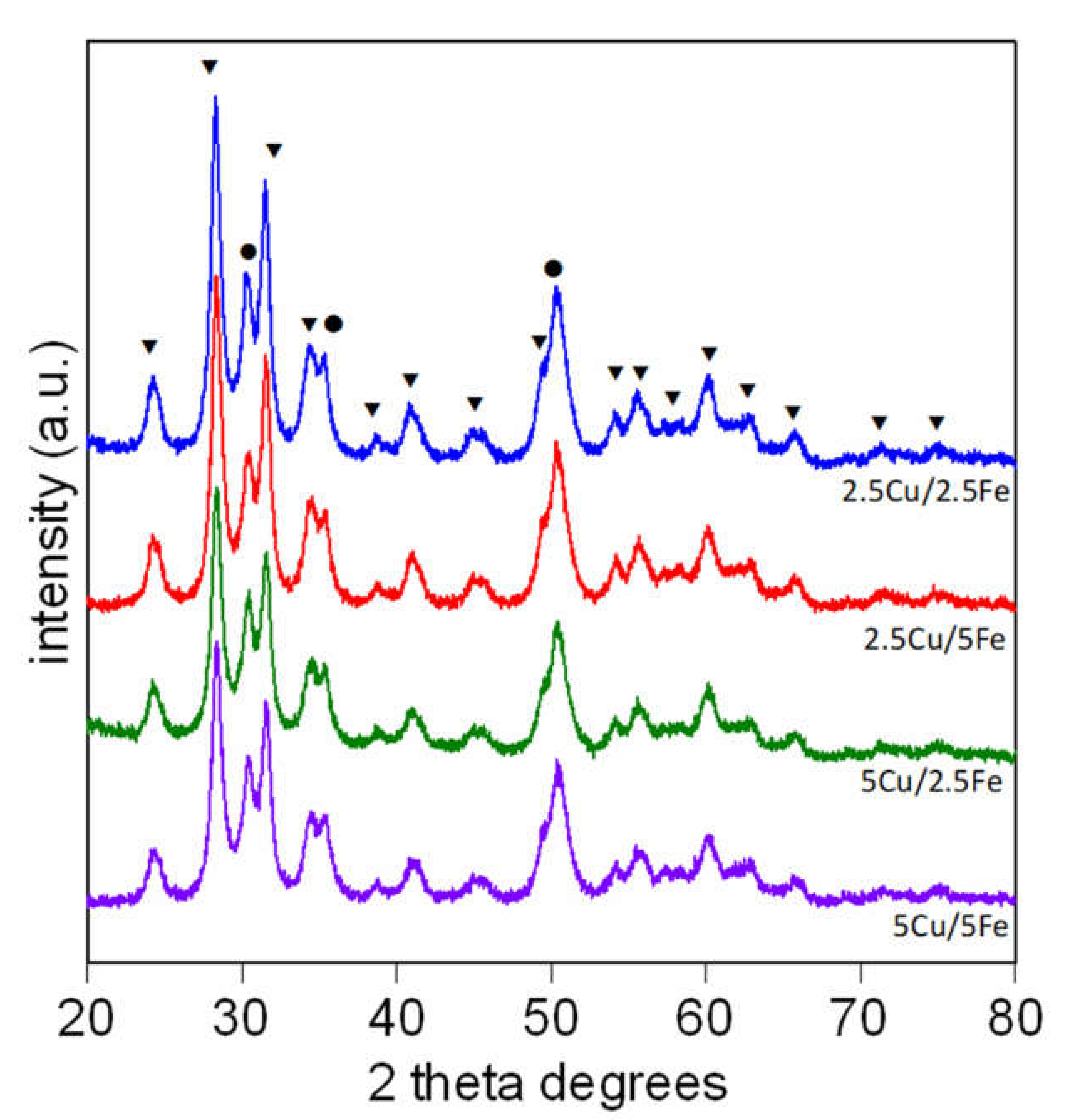

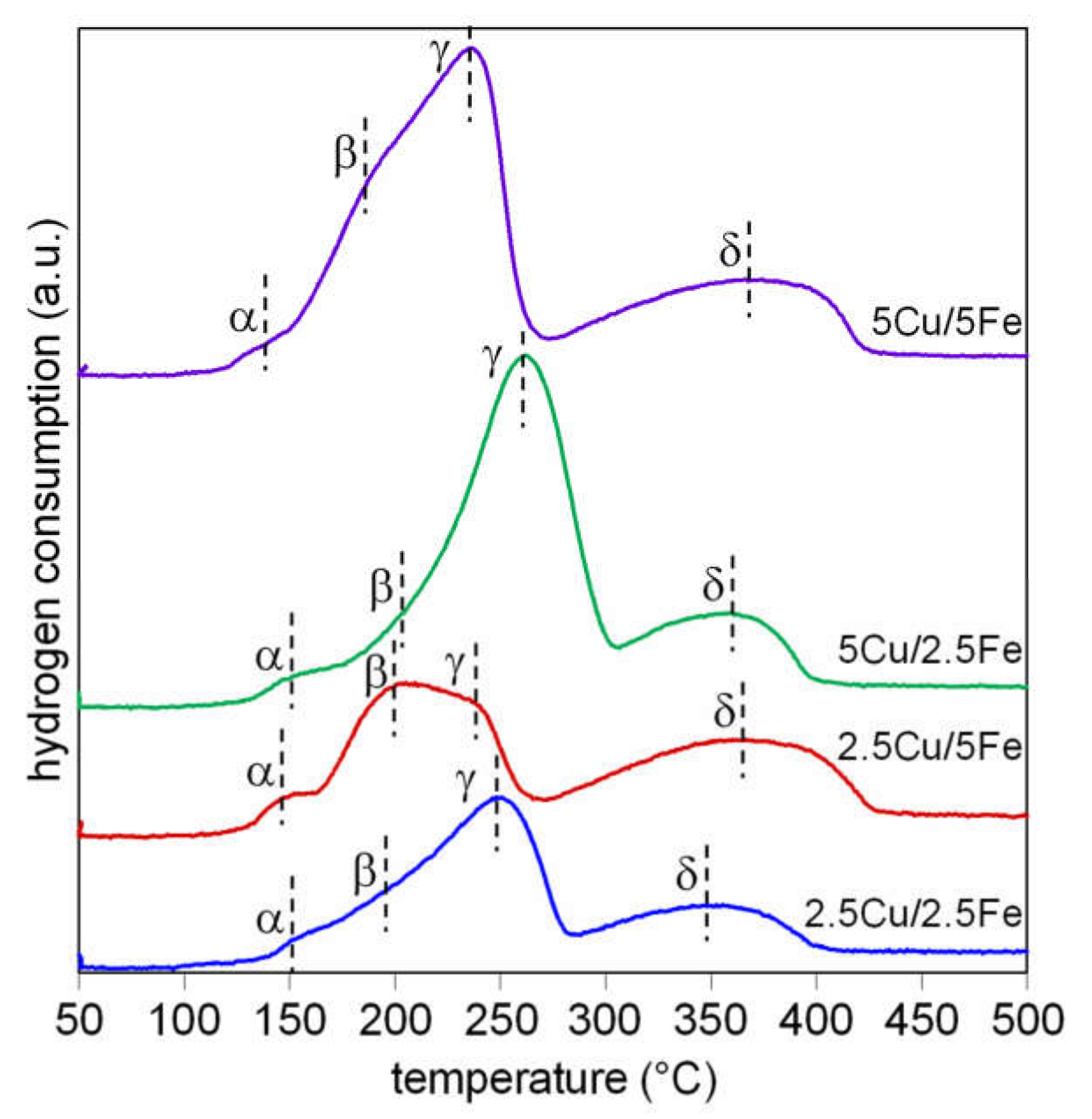
| Sample | Composition | Crystallyte Size a (nm) | Surface Area (m2/g) |
|---|---|---|---|
| 2.5Cu/2.5Fe | Cu(2.5 wt.%)-Fe(2.5 wt.%)/ZrO2 | 13 | 59 |
| 2.5Cu/5Fe | Cu(2.5 wt.%)-Fe(5 wt.%)//ZrO2 | 12 | 55 |
| 5Cu/2.5Fe | Cu(5 wt.%)-Fe(2.5 wt.%)/ZrO2 | 13 | 59 |
| 5Cu/5Fe | Cu(5 wt.%)-Fe(5 wt.%)/ZrO2 | 12 | 55 |
| 2θ (°) | Phase |
|---|---|
| 24.3 | ZrO2; Fe2O3 |
| 28.2 | ZrO2 |
| 34.2 | ZrO2; |
| 35.6 | ZrO2; CuO; Fe2O3 |
| 54.1 | ZrO2; Fe2O3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Aneggi, E.; Goi, D.; Trovarelli, A. Bimetallic Cu/Fe Catalysts for Ibuprofen Mineralization. Catalysts 2021, 11, 1383. https://doi.org/10.3390/catal11111383
Hussain S, Aneggi E, Goi D, Trovarelli A. Bimetallic Cu/Fe Catalysts for Ibuprofen Mineralization. Catalysts. 2021; 11(11):1383. https://doi.org/10.3390/catal11111383
Chicago/Turabian StyleHussain, Sajid, Eleonora Aneggi, Daniele Goi, and Alessandro Trovarelli. 2021. "Bimetallic Cu/Fe Catalysts for Ibuprofen Mineralization" Catalysts 11, no. 11: 1383. https://doi.org/10.3390/catal11111383
APA StyleHussain, S., Aneggi, E., Goi, D., & Trovarelli, A. (2021). Bimetallic Cu/Fe Catalysts for Ibuprofen Mineralization. Catalysts, 11(11), 1383. https://doi.org/10.3390/catal11111383









