Copper-Catalyzed Synthesis of Coumarins. A Mini-Review
Abstract
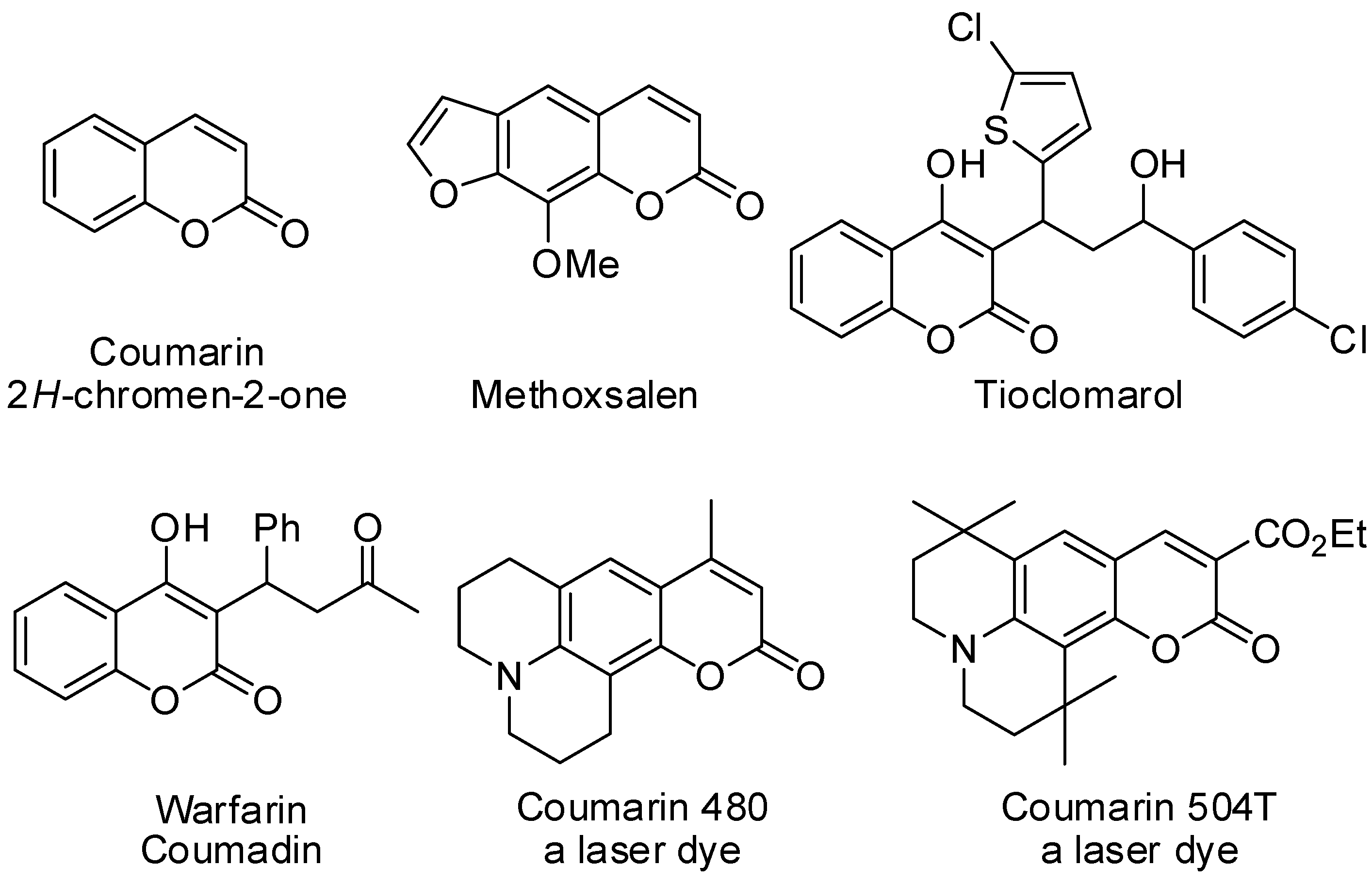
:1. Introduction
2. Synthesis of the Coumarin Core
3. Coumarin Functionalization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MOM | Methoxymethyl |
| MOF | Metal-organic framework |
| BHQ | Bull, Hutchings, Quayle |
| NHC | N-Heterocyclic carbene |
| DIPEA | Diisopropylethyl amine |
| DMF | Dimethylformamide |
| DMSO | Dimethylsulfoxide |
| TBPB | t-Butyl peroxybenzoate |
References
- Carneiro, A.; Matos, M.J.; Uriarte, E.; Santana, L. Trending Topics on Coumarin and Its Derivatives in 2020. Molecules 2021, 26, 501. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Liu, Y.; Zeng, Y.; Wu, G. A Review on Anti-Tumor Mechanisms of Coumarins. Front. Oncol. 2020, 10, 592853. [Google Scholar] [CrossRef]
- Qin, H.L.; Zhang, Z.W.; Ravindar, L.; Rakesh, K.P. Antibacterial activities with the structure-activity relationship of coumarin derivatives. Eur. J. Med. Chem. 2020, 207, 112832. [Google Scholar] [CrossRef] [PubMed]
- Prusty, J.S.; Kumar, A. Coumarins: Antifungal effectiveness and future therapeutic scope. Mol. Divers. 2020, 24, 1367–1383. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.A.; Spillere, A.R.; das Neves, G.M.; Kagami, L.P.; von Poser, G.L.; Canto, R.F.S.; Eifler-Lima, V. Natural and synthetic coumarins as antileishmanial agents: A review. Eur. J. Med. Chem. 2020, 203, 112514. [Google Scholar] [CrossRef] [PubMed]
- Pires, C.T.A.; Scodro, R.B.L.; Cortez, D.A.G.; Brenzan, M.A.; Siqueira, V.L.D.; Caleffi-Ferracioli, K.R.; Vieira, L.C.C.; Monteiro, J.L.; Corrêa, A.G.; Cardoso, R.F. Structure–activity relationship of natural and synthetic coumarin derivatives against Mycobacterium tuberculosis. Future Med. Chem. 2020, 12, 1533–1546. [Google Scholar] [CrossRef]
- Goud, N.S.; Kumar, P.; Bharath, R.D. Recent Developments of Target Based Coumarin Derivatives as Potential Anticancer Agents. Mini Rev. Med. Chem. 2020, 20, 1754–1766. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Udayabhanu; Alghamdi, A.A.; Mahadevan, K.M.; Nagaraju, G. Solvent free and green synthesis of efficient solvochromism based coumarin moieties for quick visualization of LFPs and OLEDs applications. J. Mol. Struct. 2021, 1223, 129208. [Google Scholar] [CrossRef]
- Sun, X.Y.; Liu, T.; Sun, J.; Wang, X.J. Synthesis and application of coumarin fluorescence probes. RSC Adv. 2020, 10, 10826–10847. [Google Scholar] [CrossRef]
- Jung, J.W.; Kim, N.J.; Yun, H.; Han, Y.T. Recent Advances in Synthesis of 4-Arylcoumarins. Molecules 2018, 23, 2417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, M.A.; Helal, M.H.; Gouda, M.A.; Ammar, Y.A.; El-Gaby, M.S.A.; Abbas, S.Y. An overview on synthetic strategies to coumarins. Synth. Commun. 2018, 48, 1534–1550. [Google Scholar] [CrossRef]
- Lončarić, M.; Gašo-Sokač, D.; Jokić, S.; Molnar, M. Recent Advances in the Synthesis of Coumarin Derivatives from Different Starting Materials. Biomolecules 2020, 10, 151. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, Y.F.; Bashir, M.K.; Oglah, M.K. Original and Innovative Advances in the Synthetic Schemes of Coumarin-Based Derivatives: A Review. Syst. Rev. Pharm. 2020, 11, 598–612. [Google Scholar]
- Gouda, M.A.; Salem, M.A.; Helal, M.H. A Review on Synthesis and Pharmacological Activity of Coumarins and Their Analogs. Curr. Bioact. Compd. 2020, 16, 818–836. [Google Scholar] [CrossRef]
- Zeydi, M.M.; Kalantarian, S.J.; Kazeminejad, Z. Overview on developed synthesis procedures of coumarin heterocycles. J. Iran. Chem. Soc. 2020, 17, 3031–3094. [Google Scholar] [CrossRef]
- Jumal, J.; Norhanis, S. Synthesis, Characterization, and Applications of Coumarin Derivatives: A Short Review. Malays. J. Sci. Health Technol. 2021, 7, 62–68. [Google Scholar] [CrossRef]
- Alwan, E.S.; Mohareb, R.M. Synthesis of biologically active chromene, coumarin, azole, azine and thiophene derivatives from 1,3-diketone. Org. Commun. 2021, 14, 163–227. [Google Scholar] [CrossRef]
- Bouhaoui, A.; Eddahmi, M.; Dib, M.; Khouili, M.; Aires, A.; Catto, M.; Bouissane, L. Synthesis and Biological Properties of Coumarin Derivatives. A Review. ChemistrySelect 2021, 6, 5848–5870. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, A. Visible Light-Induced Synthesis of Functionalized Coumarins. Adv. Synth. Catal. 2021, 363, 3411–3438. [Google Scholar] [CrossRef]
- Molnar, M.; Lončarić, M.; Kovač, M. Green Chemistry Approaches to the Synthesis of Coumarin Derivatives. Curr. Org. Chem. 2020, 24, 4–43. [Google Scholar] [CrossRef]
- Gulati, S.; Singh, R.; Sangwan, S. A review on convenient synthesis of substituted coumarins using reusable solid acid catalysts. RSC Adv. 2021, 11, 29130–29155. [Google Scholar] [CrossRef]
- Rullo, M.; Pisani, L. 4-Hydroxycoumarins as Michael donors in asymmetric routes to polycyclic coumarins (microreview). Chem. Heterocycl. Compd. 2018, 54, 394–396. [Google Scholar] [CrossRef]
- Moreira, M.; Martelli, L.S.R.; Corrêa, A.G. Asymmetric organocatalyzed synthesis of coumarin derivatives. Beilstein J. Org. Chem. 2021, 17, 1952–1980. [Google Scholar] [CrossRef] [PubMed]
- Dalpozzo, R.; Mancuso, R.; Liu, Y.K. Recent Advances in the Asymmetric Synthesis of Chromane Derivatives. Targets Heterocycl. Syst. 2021, 24, 226–273. [Google Scholar]
- Priyanka; Sharma, R.K.; Katiyar, D. Recent Advances in Transition-Metal-Catalyzed Synthesis of Coumarins. Synthesis 2016, 48, 2303–2322. [Google Scholar] [CrossRef]
- Bathia, R.; Pathania, S.; Singh, V.; Rawal, R.K. Metal-catalyzed synthetic strategies toward coumarin derivatives. Chem. Heterocycl. Comp. 2018, 54, 280–291. [Google Scholar]
- Kanchana, U.S.; Diana, E.J.; Mathew, T.V.; Anilkumar, G. Palladium-catalyzed cross-coupling reactions of coumarin derivatives: An overview. Appl. Organomet. Chem. 2020, 34, e5983. [Google Scholar] [CrossRef]
- Seoane, A.; Casanova, N.; Quiñones, N.; Mascareñas, J.L.; Gulías, M. Straightforward Assembly of Benzoxepines by Means of a Rhodium(III)-Catalyzed C−H Functionalization of o-Vinylphenols. J. Am. Chem. Soc. 2014, 136, 834–837. [Google Scholar] [CrossRef]
- Tan, H.; Li, H.; Wang, J.; Wang, L. Ru-Catalyzed Decarboxylative Annulations of a-Keto Acids with Internal Alkynes: Dual Roles of COOH as Directing Group and Leaving Group. Chem. Eur. J. 2015, 21, 1904–1907. [Google Scholar] [CrossRef]
- Li-Jie Cheng, L.J.; Mankad, N.P. C–C and C–X coupling reactions of unactivated alkyl electrophiles using copper catalysis. Chem. Soc. Rev. 2020, 49, 8036–8064. [Google Scholar] [CrossRef]
- Das, A.; Ren, Y.; Hessin, C.; Desage-El Murr, M. Copper catalysis with redox-active ligands. Beilstein J. Org. Chem. 2020, 16, 858–870. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, S.; Xiao, H.; Zhang, G. Recent Advances in Visible-Light-Promoted Copper Catalysis in Organic Reactions. Synthesis 2021, 53. in press. [Google Scholar]
- Souza de Araújo, F.H.; Rojas de Figueiredo, D.; Auharek, S.A.; Pesarini, J.R.; Meza, A.; da Silva Gomes, R.; Monreal, A.C.D.; Antoniolli-Silva, A.C.M.D.; Pires de Lima, D.; Kassuya, C.A.L.; et al. In vivo chemotherapeutic insight of a novel isocoumarin (3-hexyl-5,7-dimethoxy-isochromen-1-one): Genotoxicity, cell death induction, leukometry and phagocytic evaluation. Genet. Mol. Biol. 2017, 40, 665–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
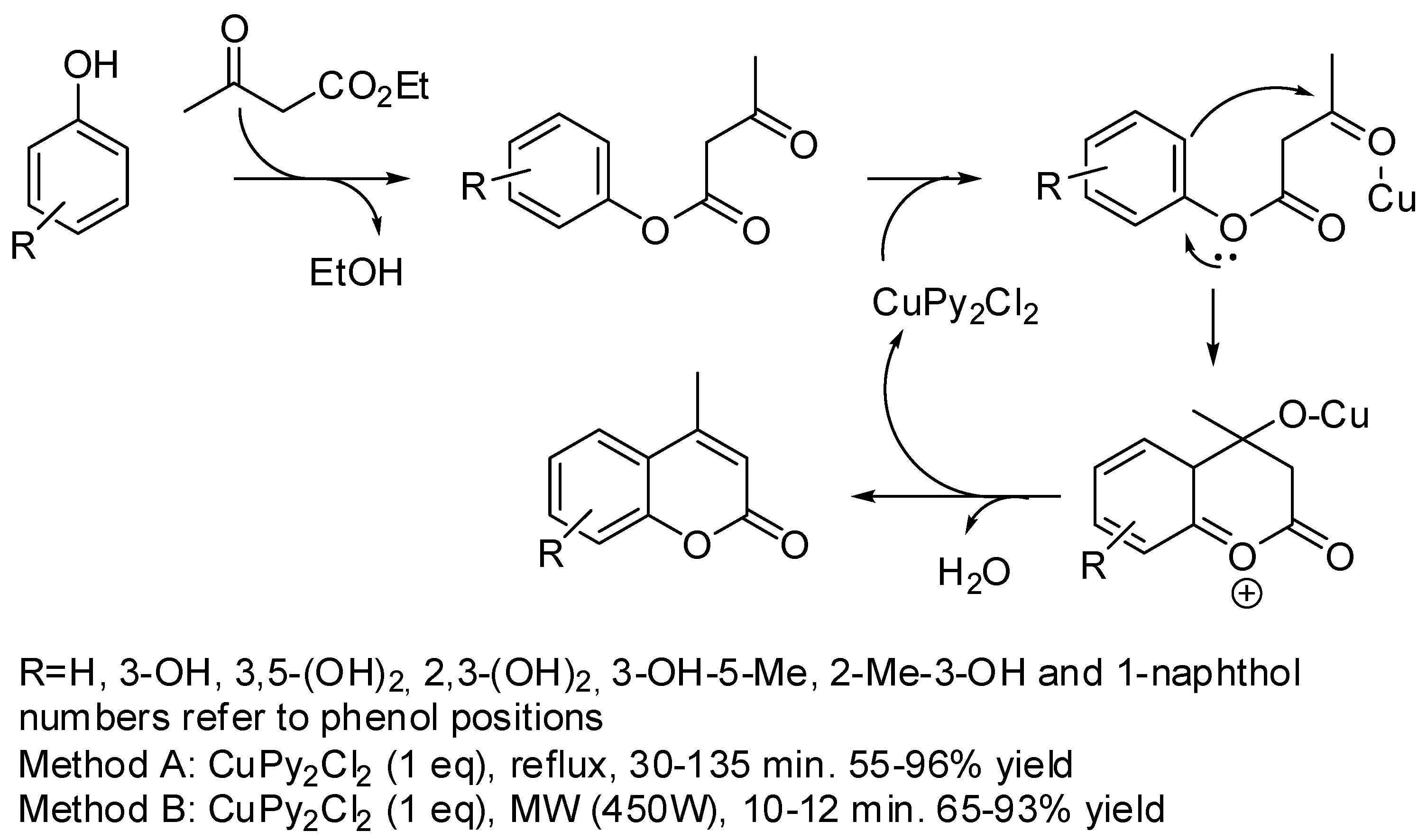
- Rajitha, B.; Naveen Kumara, V.; Someshwara, P.; Venu Madhava, J.; Narsimha Reddy, P.; Thirupathi Reddy, Y. Dipyridine copper chloride catalyzed coumarin synthesis via Pechmann condensation under conventional heating and microwave irradiation. ARKIVOC 2006, 2006, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Opanasenko, M.; Shamzhy, M.; Čejka, J. Solid Acid Catalysts for Coumarin Synthesis by the Pechmann Reaction: MOFs versus Zeolites. ChemCatChem 2013, 5, 1024–1031. [Google Scholar] [CrossRef]
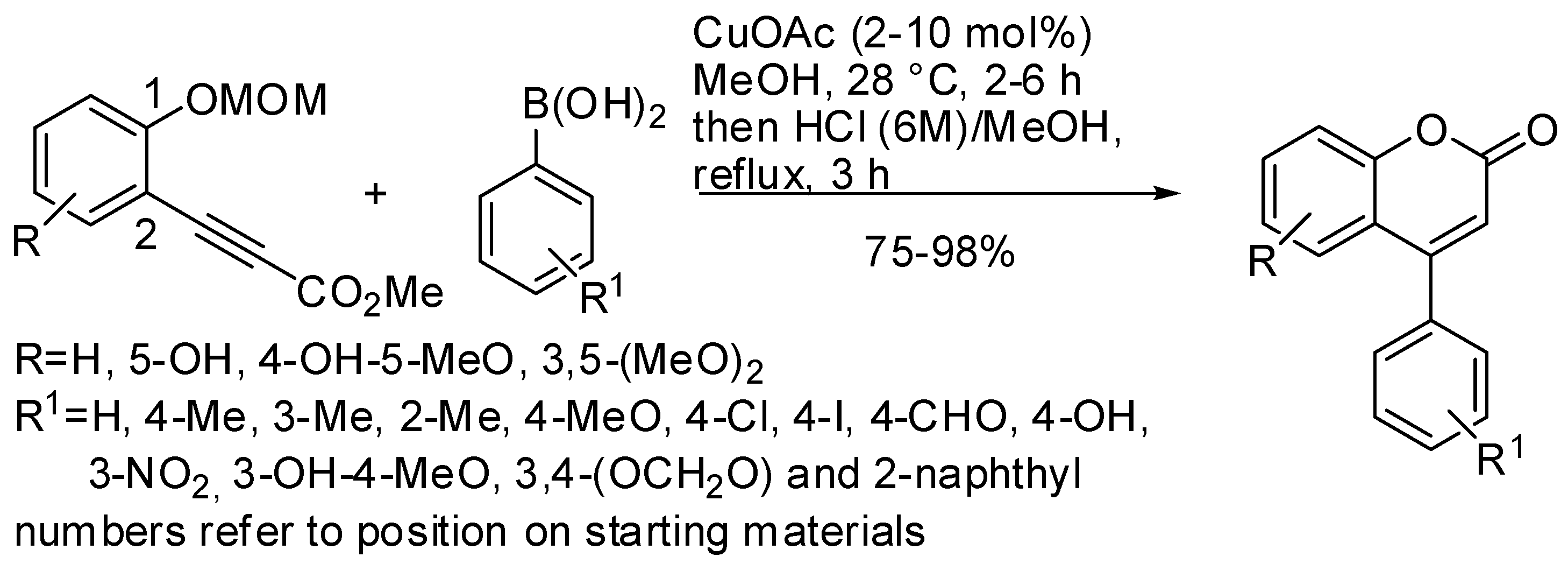
- Yamamoto, Y.; Kirai, N. Synthesis of 4-Arylcoumarins via Cu-Catalyzed Hydroarylation with Arylboronic Acids. Org. Lett. 2008, 10, 5513–5516. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kirai, N.; Harada, Y. Cu-catalyzed stereoselective conjugate addition of arylboronic acids to alkynoates. Chem. Commun. 2008, 2010–2012. [Google Scholar] [CrossRef]
- Reddy, M.S.; Thirupathi, N.; Haribabu, M. Tandem aldehyde–alkyne–amine coupling/cycloisomerization: A new synthesis of coumarins. Beilstein J. Org. Chem. 2013, 9, 180–184. [Google Scholar] [CrossRef] [PubMed]
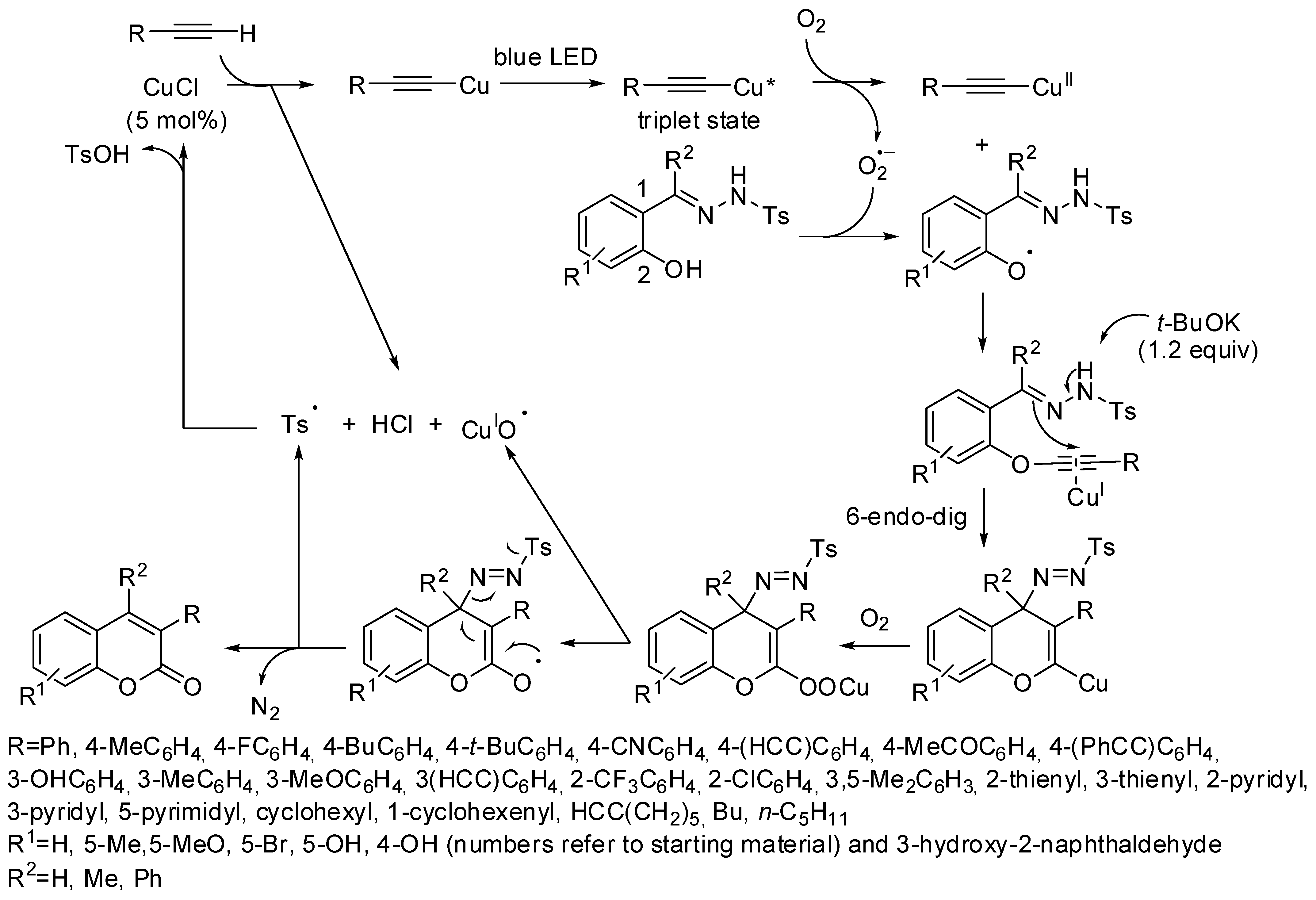
- Ragupathi, A.; Sagadevan, A.; Charpe, V.P.; Lin, C.C.; Hwu, J.R.; Hwang, K.C. Visible-light-driven copper-catalyzed aerobic oxidative cascade cyclization of N-tosylhydrazones and terminal alkynes: Regioselective synthesis of 3-arylcoumarins. Chem. Commun. 2019, 55, 5151–5154. [Google Scholar] [CrossRef]
- Kayal, U.; Karmakar, R.; Banerjee, D.; Maiti, G. Copper oxide catalyzed domino process for the synthesis of substituted 2H-pyran-2-ones and polyhydroxy coumarin derivatives. Tetrahedron 2014, 70, 7016–7021. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Qiu, G.; Ding, Q. Copper-Catalyzed Direct Trifluoromethylation of Propiolates: Construction of Trifluoromethylated Coumarins. Org. Lett. 2014, 16, 4240–4243. [Google Scholar] [CrossRef]
- Bull, J.A.; Luján, C.; Hutchings, M.G.; Quayle, P. Application of the BHQ benzannulation reaction to the synthesis of benzo-fused coumarins. Tetrahedron Lett. 2009, 50, 3617–3620. [Google Scholar] [CrossRef]
- Cheng, G.; Hu, Y. One-pot synthesis of furocoumarins through cascade addition–cyclization–oxidation. Chem. Commun. 2007, 38, 3285–3287. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Hu, Y. Two Efficient Cascade Reactions to Synthesize Substituted Furocoumarins. J. Org. Chem. 2008, 73, 4732–4735. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Xu, M.H. One-pot synthesis of furocoumarins via sequential Pd/Cu-catalyzed alkynylation and intramolecular hydroalkoxylation. Org. Biomol. Chem. 2010, 8, 3073–3077. [Google Scholar] [CrossRef]
- Zhu, R.; Wei, J.; Shi, Z. Benzofuran synthesis via copper-mediated oxidative annulation of phenols and unactivated internal alkynes. Chem. Sci. 2013, 4, 3706–3711. [Google Scholar] [CrossRef]
- Zhang, W.L.; Yue, S.N.; Shen, Y.M.; Hu, H.Y.; Meng, Q.H.; Wu, H.; Liu, Y. Copper(II) bromide-catalyzed intramolecular decarboxylative functionalization to form a C(sp3)–O bond for the synthesis of furo[3,2-c]-coumarins. Org. Biomol. Chem. 2015, 13, 3602–3609. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Hu, L.L.; Shen, Z.; Chen, Z.Z.; Xu, Z.G.; Li, S.Q.; Xie, J.W.; Cui, H.L. Copper-Catalyzed Synthesis of Furo[3,2-c]coumarins and Dihydrofuro[3,2-c]coumarins through a Propargylation/Alkyne Oxacyclization/Isomerization Cascade under Microwave Irradiation. Synlett 2015, 26, 2821–2825. [Google Scholar] [CrossRef]
- He, M.; Yan, Z.; Wang, W.; Zhu, F.; Lin, S. Copper-catalyzed radical/radical cross-coupling of ketoxime carboxylates with 4-hydroxycoumarins: A novel synthesis of furo[3,2-c]-coumarins. Tetrahedron Lett. 2018, 59, 3706–3712. [Google Scholar] [CrossRef]
- To, T.A.; Vo, Y.H.; Nguyen, A.T.; Phan, A.N.Q.; Truong, T.; Phan, N.T.S. A new route to substituted furocoumarins via copper-catalyzed cyclization between 4-hydroxycoumarins and ketoximes. Org. Biomol. Chem. 2018, 16, 5086–5089. [Google Scholar] [CrossRef]
- Naik, M.M.; Kamat, V.P.; Tilve, S.G. Copper-mediated synthesis of coumestans via C(sp2)-H functionalization: Protective group free route to coumestrol and 4’-O-methylcoumestrol. Tetrahedron 2017, 73, 5528–5536. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Wang, E. A highly selective “turn-on” fluorescent probe for detecting Cu2+ in two different sensing mechanisms. Dyes Pigments 2019, 163, 533–537. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Mondal, S. A new strategy for the synthesis of coumarin- and quinolone-annulated pyrroles via Pd(0) mediated cross-coupling followed by Cu(I) catalyzed heteroannulation. Tetrahedron Lett. 2008, 49, 2418–2420. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Taher, A.; Nandi, R.K. Copper(II) Acetate Promoted Intramolecular Carboamination of Alkenes: An Efficient Synthesis of Pentacyclic Sultams. Synlett 2010, 21, 1389–1393. [Google Scholar] [CrossRef]
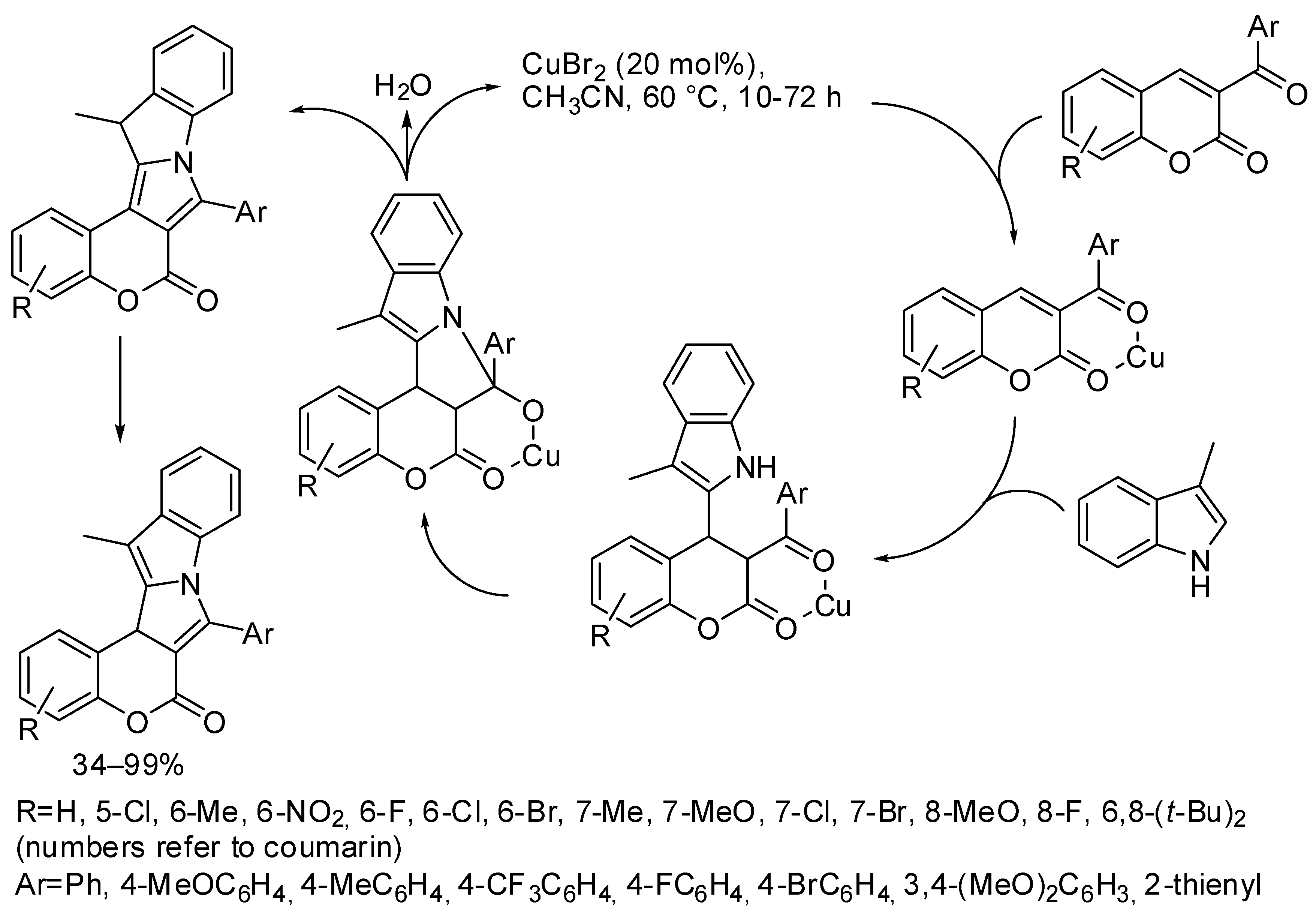
- Jin, S.J.; Guo, J.M.; Zhu, Y.S.; Wang, Q.L.; Bu, Z.W. A copper-catalyzed tandem reaction for the construction of coumarin fused 9H-pyrrolo[1,2-a]indoles. Org. Biomol. Chem. 2017, 15, 8729–8737. [Google Scholar] [CrossRef] [PubMed]
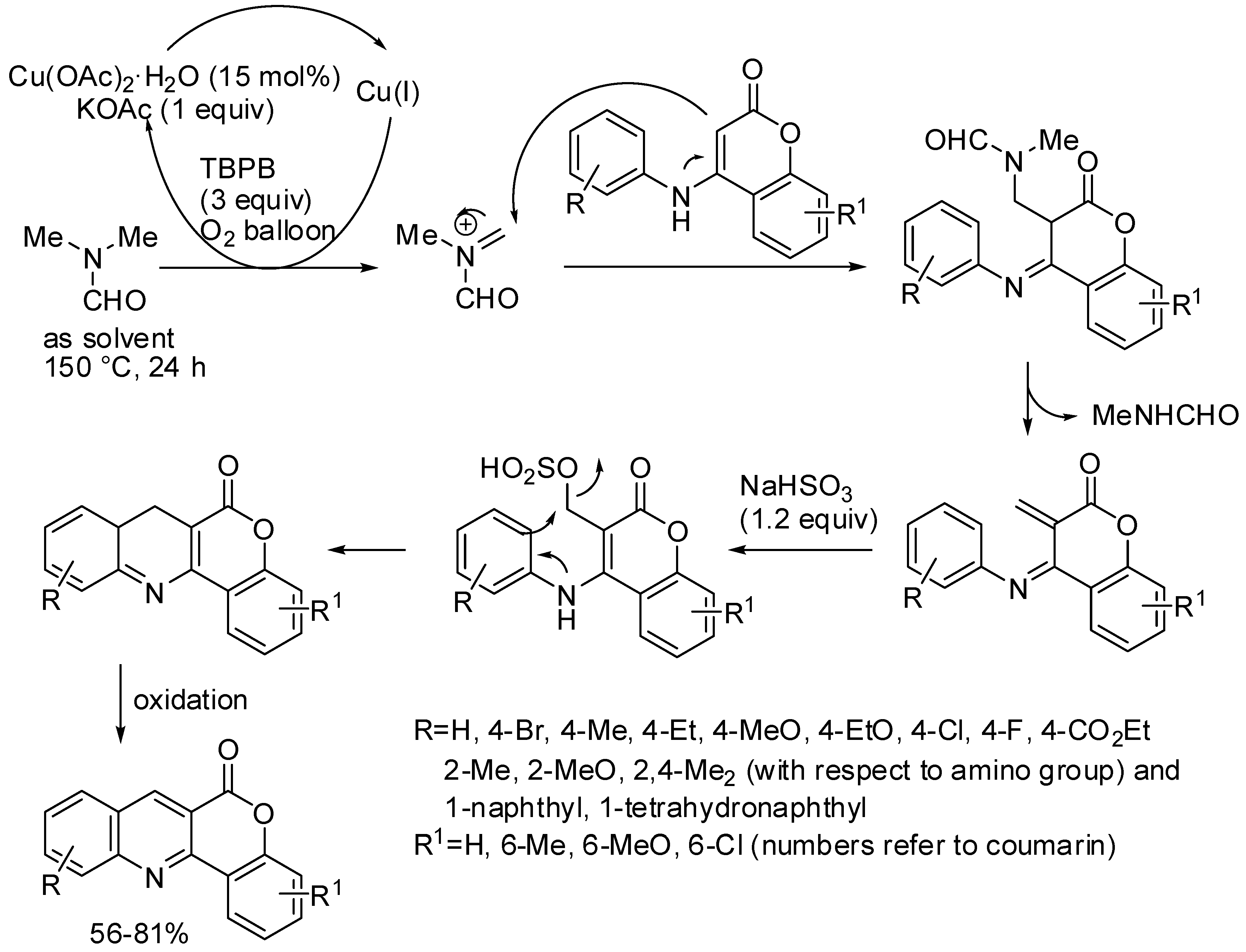
- Weng, Y.; Zhou, H.; Sun, C.; Xie, Y.; Su, W. Copper-Catalyzed Cyclization for Access to 6H-Chromeno[4,3-b]quinolin-6-ones Employing DMF as the Carbon Source. J. Org. Chem. 2017, 82, 9047–9053. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Shakoor, S.M.A.; Khullar, S.; Mandal, S.K.; Sakhuja, R. An unprecedented tandem synthesis of fluorescent coumarin-fused pyrimidines via copper-catalyzed cross-dehydrogenative C(sp3)–N bond coupling. Org. Biomol. Chem. 2018, 16, 3220–3228. [Google Scholar] [CrossRef]
- Seela, F.; Sirivolu, V.R. Pyrrolo-dC oligonucleotides bearing alkynyl side chains with terminal triple bonds: Synthesis, base pairing and fluorescent dye conjugates prepared by the azide–alkyne “click” reaction. Org. Biomol. Chem. 2008, 6, 1674–1687. [Google Scholar] [CrossRef] [PubMed]
- Olomola, T.O.; Klein, R.; Lobb, K.A.; Sayed, Y.; Kaye, P.T. Towards the synthesis of coumarin derivatives as potential dual-action HIV-1 protease and reverse transcriptase inhibitors. Tetrahedron Lett. 2010, 51, 6325–6328. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalpozzo, R.; Mancuso, R. Copper-Catalyzed Synthesis of Coumarins. A Mini-Review. Catalysts 2021, 11, 1382. https://doi.org/10.3390/catal11111382
Dalpozzo R, Mancuso R. Copper-Catalyzed Synthesis of Coumarins. A Mini-Review. Catalysts. 2021; 11(11):1382. https://doi.org/10.3390/catal11111382
Chicago/Turabian StyleDalpozzo, Renato, and Raffaella Mancuso. 2021. "Copper-Catalyzed Synthesis of Coumarins. A Mini-Review" Catalysts 11, no. 11: 1382. https://doi.org/10.3390/catal11111382
APA StyleDalpozzo, R., & Mancuso, R. (2021). Copper-Catalyzed Synthesis of Coumarins. A Mini-Review. Catalysts, 11(11), 1382. https://doi.org/10.3390/catal11111382






