Highly Sensitive and Selective Copper (II)-Catalyzed Dual-DNAzyme Colorimetric Biosensor Based on Exonuclease III-Mediated Cyclical Assembly
Abstract
:1. Introduction
2. Results
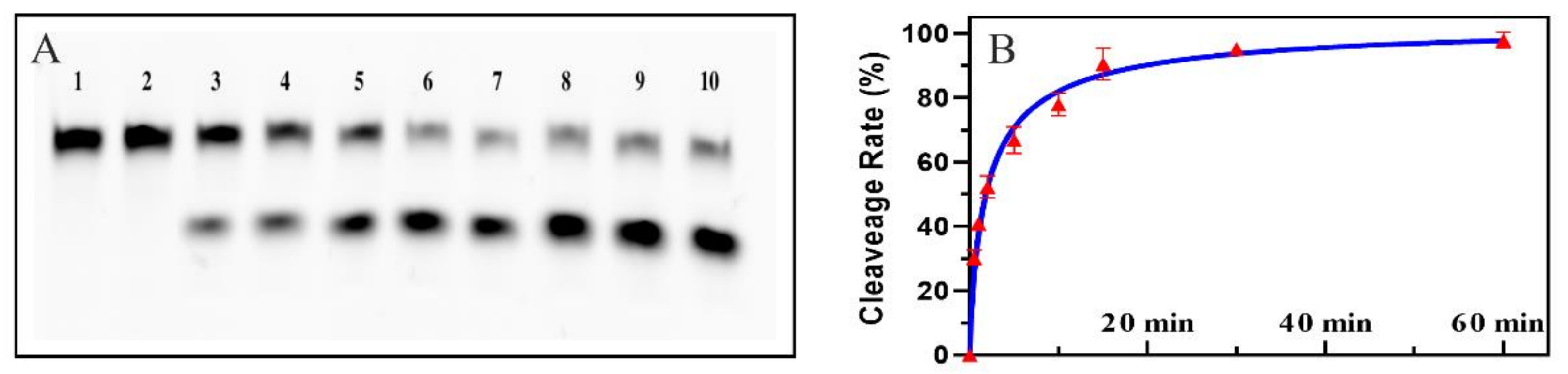
2.1. Feasibility of the Colorimetric Biosensor Based EMCA
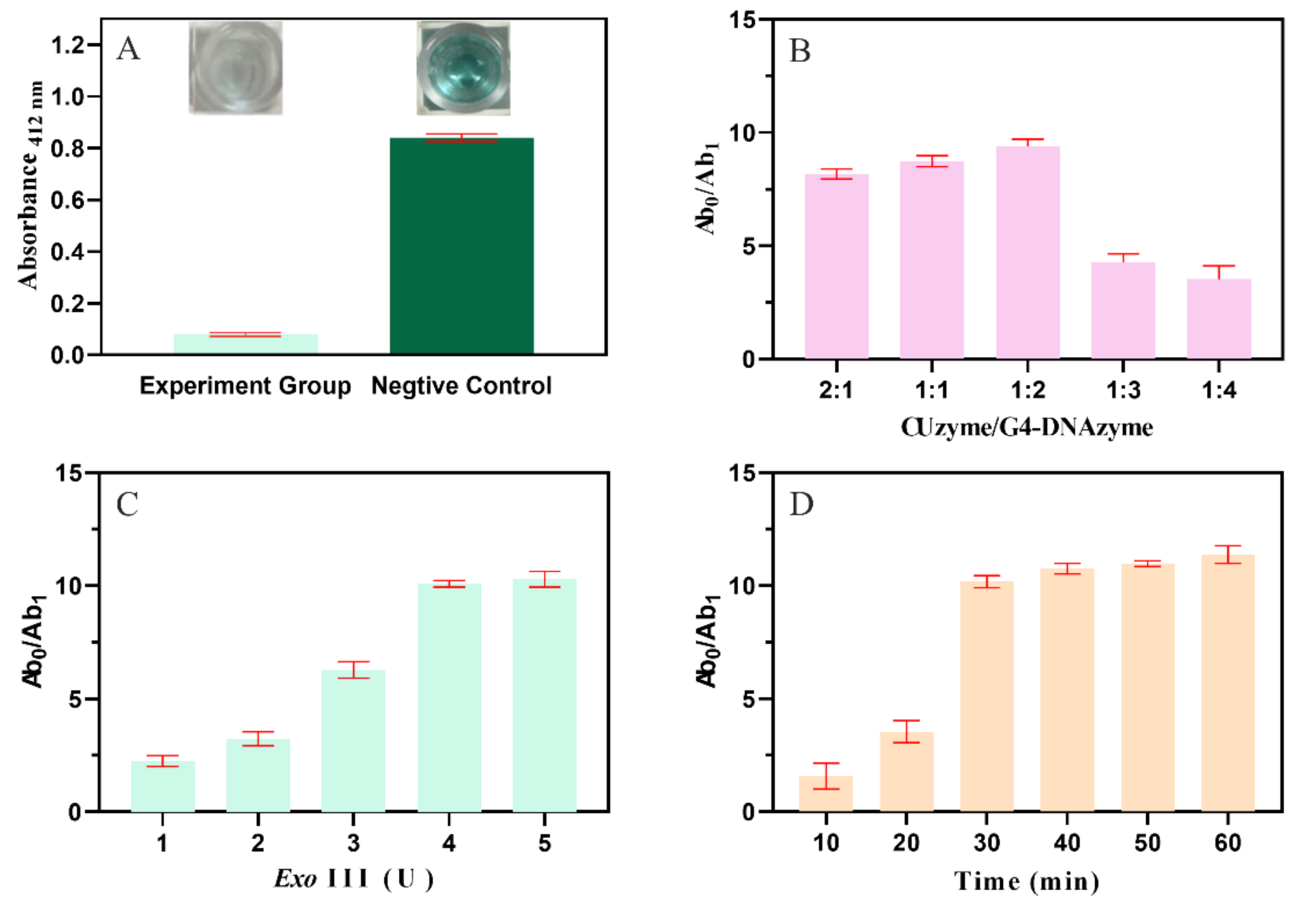
2.2. Develop and Optimize the Condition of the Biosensor
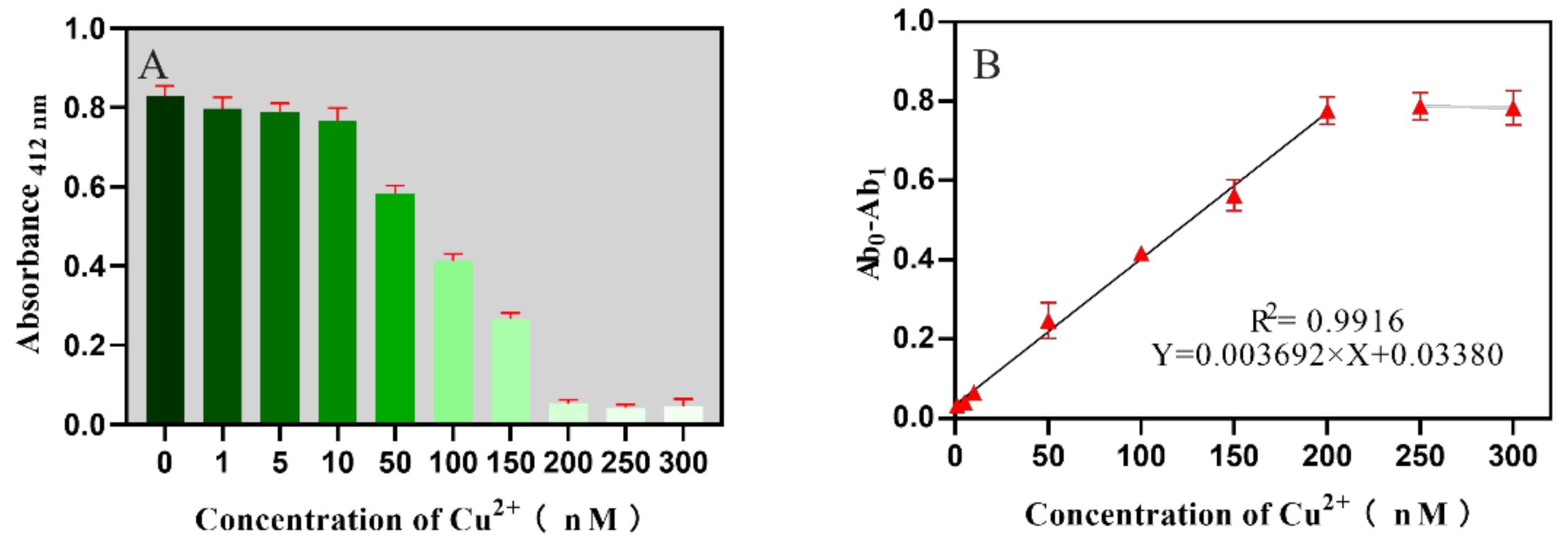
2.3. Sensitivity for the Biosensor
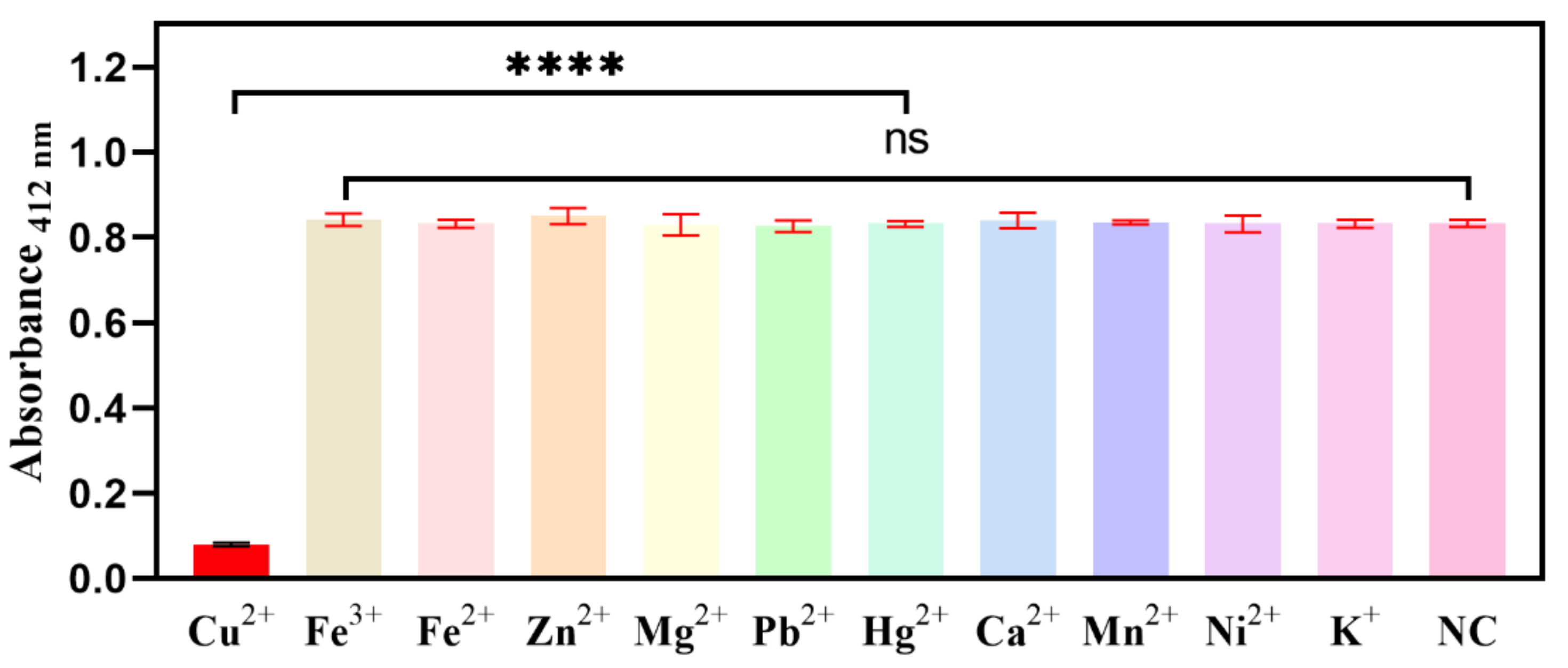
2.4. Selectivity for the Biosensor
2.5. Application of the Biosensor in Tap Water Samples
3. Materials and Methods
3.1. Materials and Reagents
3.2. Cu-DNAzyme Activity Assays
3.3. The Procedure of EMCA and Dual-DNAzyme Colorimetric Biosensor
3.4. Application of the Biosensor in a Real Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Festa, R.A.; Thiele, D.J. Copper: An essential metal in biology. Curr. Biol. 2011, 21, R877–R883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotruvo, J.J.A.; Aron, A.T.; Ramos-Torres, K.M.; Chang, C.J. Synthetic fluorescent probes for studying copper in biological systems. Chem. Soc. Rev. 2015, 44, 4400–4414. [Google Scholar] [CrossRef] [Green Version]
- Säbel, C.E.; Neureuther, J.M.; Siemann, S. A spectrophotometric method for the determination of zinc, copper, and cobalt ions in metalloproteins using Zincon. Anal. Biochem. 2010, 397, 218–226. [Google Scholar] [CrossRef]
- Chen, J.; Teo, K.C. Determination of cadmium, copper, lead and zinc in water samples by flame atomic absorption spectrometry after cloud point extraction. Anal. Chim. Acta 2001, 450, 215–222. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, N.; Zhou, X.; Han, J.; Bu, H. Cu(I)-Catalyzed Click Reaction-Triggered 3D DNA Walker for Constructing an “OFF–ON” Fluorescent Biosensor for Cu2+ Detection. ACS Appl. Bio Mater. 2021, 4, 3571–3578. [Google Scholar] [CrossRef]
- Tian, J.; Du, Z.; Zhu, L.; Shao, X.; Li, X.; Xu, W. Fluorescent detection of Cu (II) ions based on DNAzymatic cascaded cyclic amplification. Microchim. Acta 2020, 187, 443. [Google Scholar] [CrossRef]
- Stebunov, Y.V.; Yakubovsky, D.I.; Fedyanin, D.Y.; Arsenin, A.V.; Volkov, V.S. Superior Sensitivity of Copper-Based Plasmonic Biosensors. Langmuir 2018, 34, 4681–4687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safran, V.; Göktürk, I.; Derazshamshir, A.; Yılmaz, F.; Sağlam, N.; Denizli, A. Rapid sensing of Cu+2 in water and biological samples by sensitive molecularly imprinted based plasmonic biosensor. Microchem. J. 2019, 148, 141–150. [Google Scholar] [CrossRef]
- Korkmaz, N.; Hwang, C.; Kessler, K.K.; Silina, Y.E.; Müller, L.; Park, J. A novel copper (II) binding peptide for a colorimetric biosensor system design. Talanta 2021, 232, 122439. [Google Scholar] [CrossRef]
- Aktara, M.N.; Das, S.; Nayim, S.; Sahoo, N.K.; Beg, M.; Jana, G.C.; Maji, A.; Jha, P.K.; Hossain, M. A sensorial colorimetric detection method for Hg2+ and Cu2+ ions using single probe sensor based on 5-methyl-1,3,4-thiadiazole-2-thiol stabilized gold nanoparticles and its application in real water sample analysis. Microchem. J. 2019, 147, 1163–1172. [Google Scholar] [CrossRef]
- Liu, K.; Chen, K.S.; Sen, D.; Yu, H.-Z. Ultrasensitive detection of total copper with an electrochemical biosensor built on the in cis coupling of hexynyl CLICK-17 DNAzyme with azido self-assembled monolayers. Electrochim. Acta 2021, 379, 138125. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, L.; Shao, X.; Huang, K.; Luo, Y. An electrochemical biosensor based on nucleic acids enzyme and nanochannels for detecting copper (II) ion. Biosens. Bioelectron. 2018, 120, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Atapour, M.; Amoabediny, G.; Ahmadzadeh-Raji, M. Integrated optical and electrochemical detection of Cu2+ ions in water using a sandwich amino acid–gold nanoparticle-based nano-biosensor consisting of a transparent-conductive platform. RSC Adv. 2019, 9, 8882–8893. [Google Scholar] [CrossRef] [Green Version]
- Cuenoud, B.; Szostak, J.W. A DNA metalloenzyme with DNA ligase activity. Nature 1995, 375, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Zhang, W.; Du, Q.; Liu, G. An in-situ plasmonic spectroscopy based biosensor for detection of copper (II) ions highlighting analytical specifications. Sensor. Actuat. B Chem. 2021, 329, 129103. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Liu, S.; Zhang, X.; Wang, J.; Wang, H.; Zhang, F.; Yu, J.; Huang, J. A triply amplified electrochemical lead(II) sensor by using a DNAzyme and via formation of a DNA-gold nanoparticle network induced by a catalytic hairpin assembly. Microchim. Acta 2019, 186, 559. [Google Scholar] [CrossRef]
- Fu, L.; Lu, Q.; Liu, X.; Chen, X.; Wu, X.; Xie, S. Combining whispering gallery mode optofluidic microbubble resonator sensor with GR-5 DNAzyme for ultra-sensitive lead ion detection. Talanta 2020, 213, 120815. [Google Scholar] [CrossRef]
- Li, W.; Yang, Y.; Chen, J.; Zhang, Q.; Wang, Y.; Wang, F.; Yu, C. Detection of lead(II) ions with a DNAzyme and isothermal strand displacement signal amplification. Biosens. Bioelectron. 2014, 53, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, L.; Li, G.; Du, Z.; Tian, J.; Luo, Y.; Huang, K.; Xu, W. A “turn-off” ultra-sensitive fluorescent quantitative biosensor driven by zinc ion DNAzyme. Sensor. Actuat. B Chem. 2019, 285, 173–178. [Google Scholar] [CrossRef]
- Li, S.; Li, G.; Du, Z.; Zhu, L.; Tian, J.; Luo, Y.; Huang, K.; Xu, W. The ultra-sensitive visual biosensor based on thermostatic triple step functional nucleic acid cascade amplification for detecting Zn2+. Food Chem. 2019, 290, 95–100. [Google Scholar] [CrossRef]
- Zhao, X.-H.; Zhang, L.-Z.; Zhao, S.-Y.; Cui, X.-H.; Gong, L.; Zhao, R.; Yu, B.-F.; Xie, J. Silver-ion-mediated Mg2+-dependent DNAzyme activity for amplified fluorescence detection of cysteine. Analyst 2019, 144, 1982–1987. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, R.; Chu, S.; Tang, W.; Lin, N.; Fu, J.; Yang, J.; Gao, B. A quencher-free DNAzyme beacon for fluorescently sensing uranyl ions via embedding 2-aminopurine. Biosens. Bioelectron. 2019, 135, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Miao, M.; Shao, X.; Du, Z.; Huang, K.; Luo, Y.; Xu, W. A Universal Electrochemical Biosensor Using Nick-HCR Nanostructure as Molecular Gate of Nanochannel for Detecting Chromium(III) Ions and MicroRNA. Anal. Chem. 2019, 91, 14992–14999. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, X.; Lu, Z.; Luo, H.; Dong, L.; Ji, Z.; Xu, F.; Huo, D.; Hou, C. Ultra-sensitive detection of Pb2+ based on DNAzymes coupling with multi-cycle strand displacement amplification (M-SDA) and nano-graphene oxide. Sens. Actuat. B Chem. 2020, 311, 127898. [Google Scholar] [CrossRef]
- Tang, D.; Xia, B.; Tang, Y.; Zhang, J.; Zhou, Q. Metal-ion-induced DNAzyme on magnetic beads for detection of lead(II) by using rolling circle amplification, glucose oxidase, and readout of pH changes. Microchim. Acta 2019, 186, 318. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, Q.; Qi, L.; Lin, J.-M.; Yu, L. Liquid crystal-based sensing platform for detection of Pb2+ assisted by DNAzyme and rolling circle amplification. J. Hazard. Mater. 2020, 400, 123218. [Google Scholar] [CrossRef]
- Zhou, F.; Li, B. Exonuclease III-Assisted Target Recycling Amplification Coupled with Liposome-Assisted Amplification: One-Step and Dual-Amplification Strategy for Highly Sensitive Fluorescence Detection of DNA. Anal. Chem. 2015, 87, 7156–7162. [Google Scholar] [CrossRef]
- Bayindir, S.; Toprak, M. A novel pyrene-based selective colorimetric and ratiometric turn-on sensing for copper. Spectrochim. Acta. A 2019, 213, 6–11. [Google Scholar] [CrossRef]
- Zhu, X.; Duan, Y.; Li, P.; Fan, H.; Han, T.; Huang, X. A highly selective and instantaneously responsive Schiff base fluorescent sensor for the “turn-off” detection of iron(iii), iron(ii), and copper(ii) ions. Anal. Methods 2019, 11, 642–647. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Y.; Liu, J. G-quadruplex DNA for construction of biosensors. TrAC Trends Anal. Chem. 2020, 132, 116060. [Google Scholar] [CrossRef]

| Sample | Addition (nM) | Biosensor (nM) | Recovery Rate (%) | ICP-MS (nM) | Recovery Rate (%) |
|---|---|---|---|---|---|
| 1 | 10 | 10.3 ± 0.3 | 103 ± 3 | 9.9 ± 0.5 | 99 ± 5 |
| 2 | 20 | 20.2 ± 0.5 | 101 ± 2.5 | 19.4 ± 0.7 | 97 ± 3.5 |
| 3 | 40 | 39.2 ± 0.7 | 98 ± 1.8 | 38.8 ± 0.8 | 97 ± 2 |
| 4 | 60 | 58.4 ± 1.6 | 97.3 ± 2.7 | 60.4 ± 1.9 | 100.7 ± 3.2 |
| 5 | 80 | 78.9 ± 1.4 | 98.6 ± 1.8 | 80.7 ± 1.6 | 100.9 ± 2 |
| 6 | 100 | 99.0 ± 1.3 | 99 ± 1.3 | 101.2 ± 2.8 | 101.2 ± 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, B.; Huang, K.; Wang, H.; Su, D.; Xu, Y. Highly Sensitive and Selective Copper (II)-Catalyzed Dual-DNAzyme Colorimetric Biosensor Based on Exonuclease III-Mediated Cyclical Assembly. Catalysts 2021, 11, 1352. https://doi.org/10.3390/catal11111352
Zhai B, Huang K, Wang H, Su D, Xu Y. Highly Sensitive and Selective Copper (II)-Catalyzed Dual-DNAzyme Colorimetric Biosensor Based on Exonuclease III-Mediated Cyclical Assembly. Catalysts. 2021; 11(11):1352. https://doi.org/10.3390/catal11111352
Chicago/Turabian StyleZhai, Baiqiang, Kunlun Huang, Hongtao Wang, Dongmin Su, and Yuancong Xu. 2021. "Highly Sensitive and Selective Copper (II)-Catalyzed Dual-DNAzyme Colorimetric Biosensor Based on Exonuclease III-Mediated Cyclical Assembly" Catalysts 11, no. 11: 1352. https://doi.org/10.3390/catal11111352
APA StyleZhai, B., Huang, K., Wang, H., Su, D., & Xu, Y. (2021). Highly Sensitive and Selective Copper (II)-Catalyzed Dual-DNAzyme Colorimetric Biosensor Based on Exonuclease III-Mediated Cyclical Assembly. Catalysts, 11(11), 1352. https://doi.org/10.3390/catal11111352






