Upcycling Waste Plastics into Multi-Walled Carbon Nanotube Composites via NiCo2O4 Catalytic Pyrolysis
Abstract
:1. Introduction
2. Results and Discussion
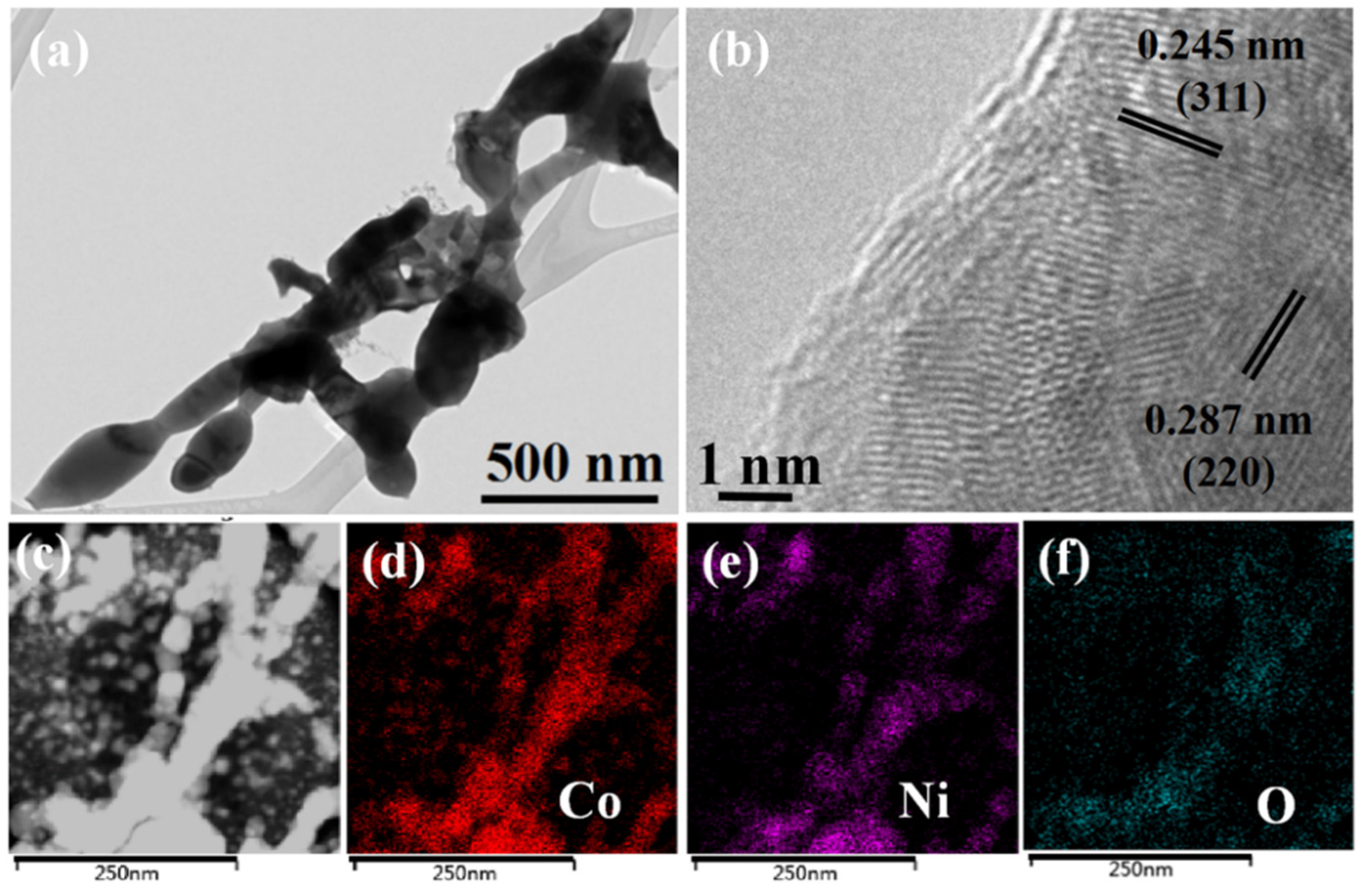
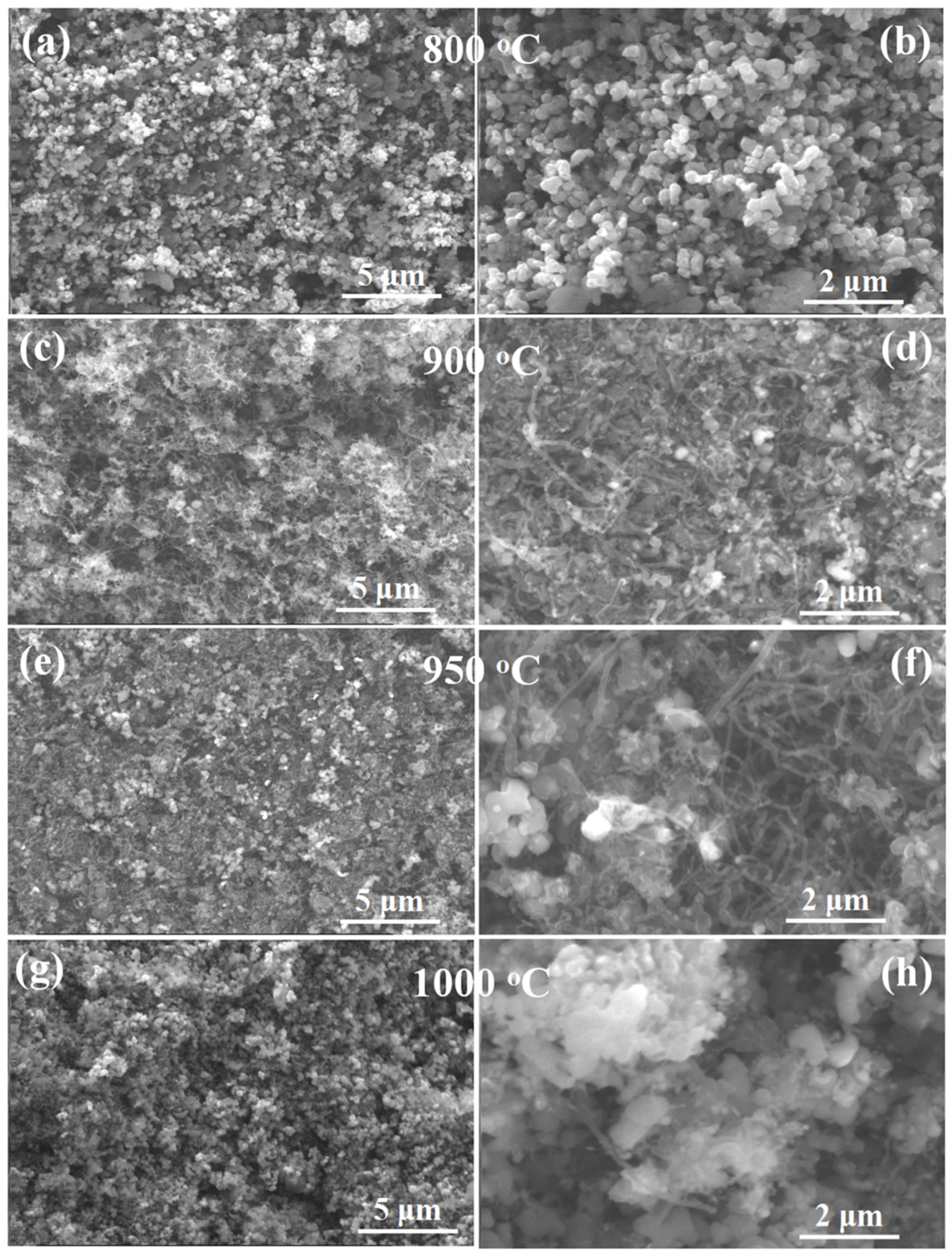
2.1. Microstructure of Catalysts
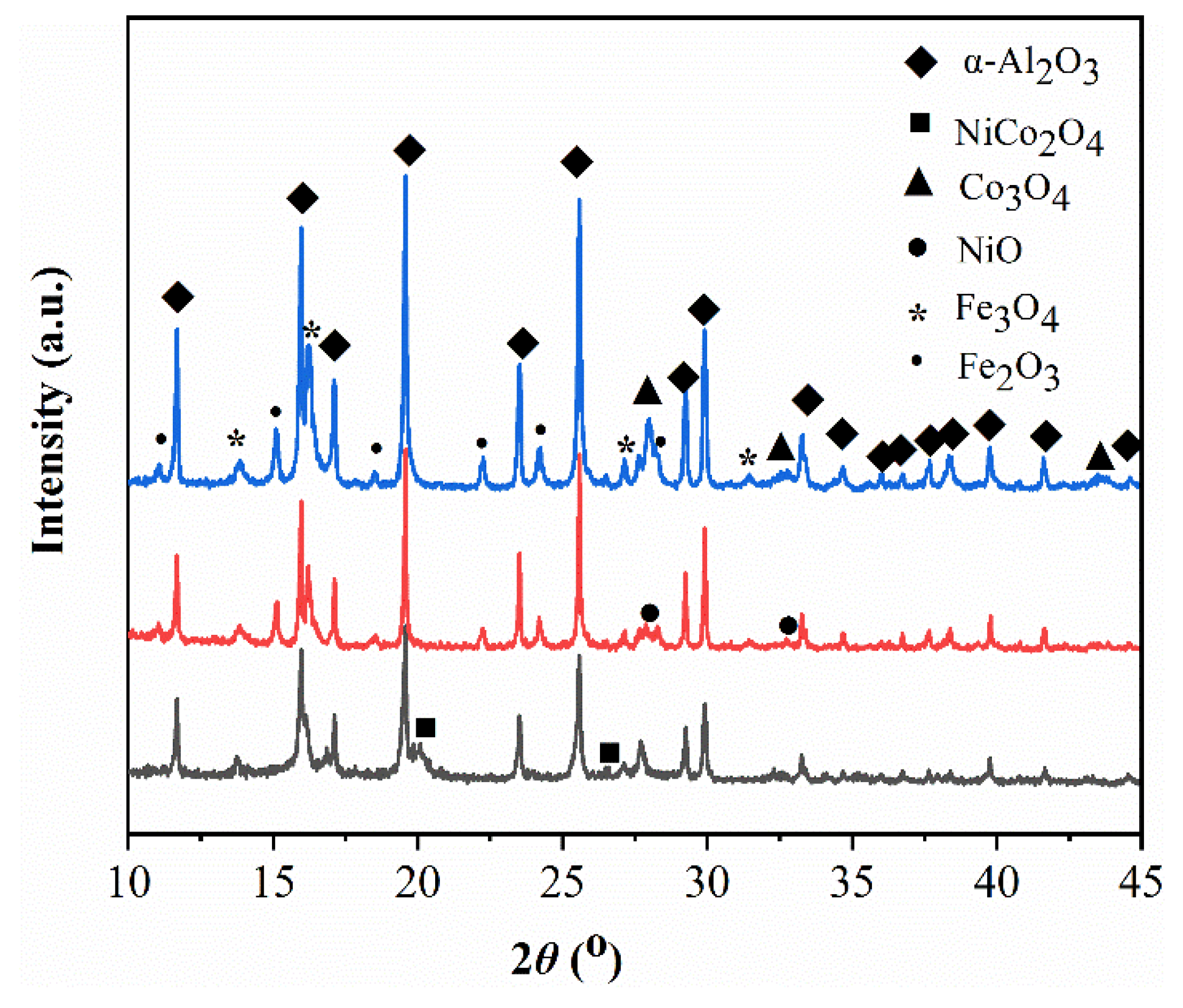
2.2. Crystal Structure of Catalysts
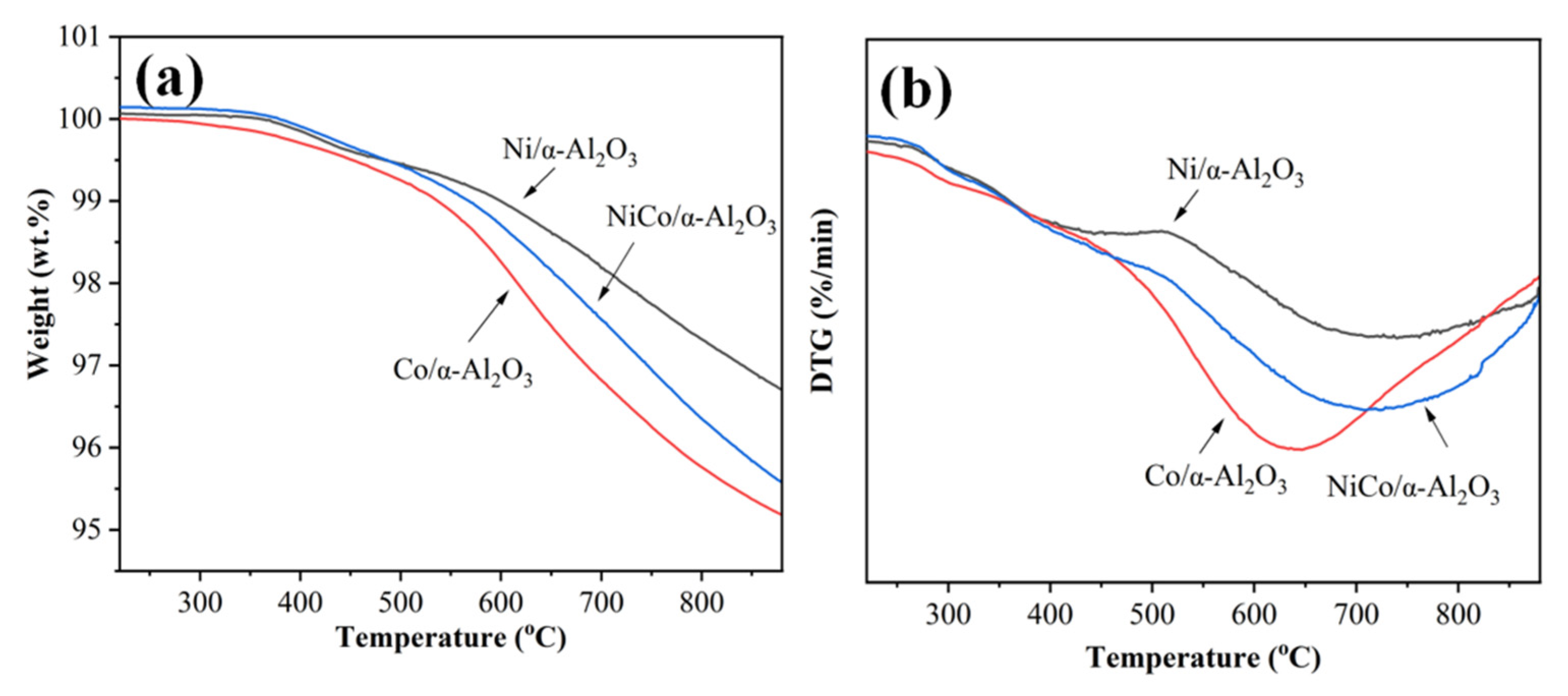
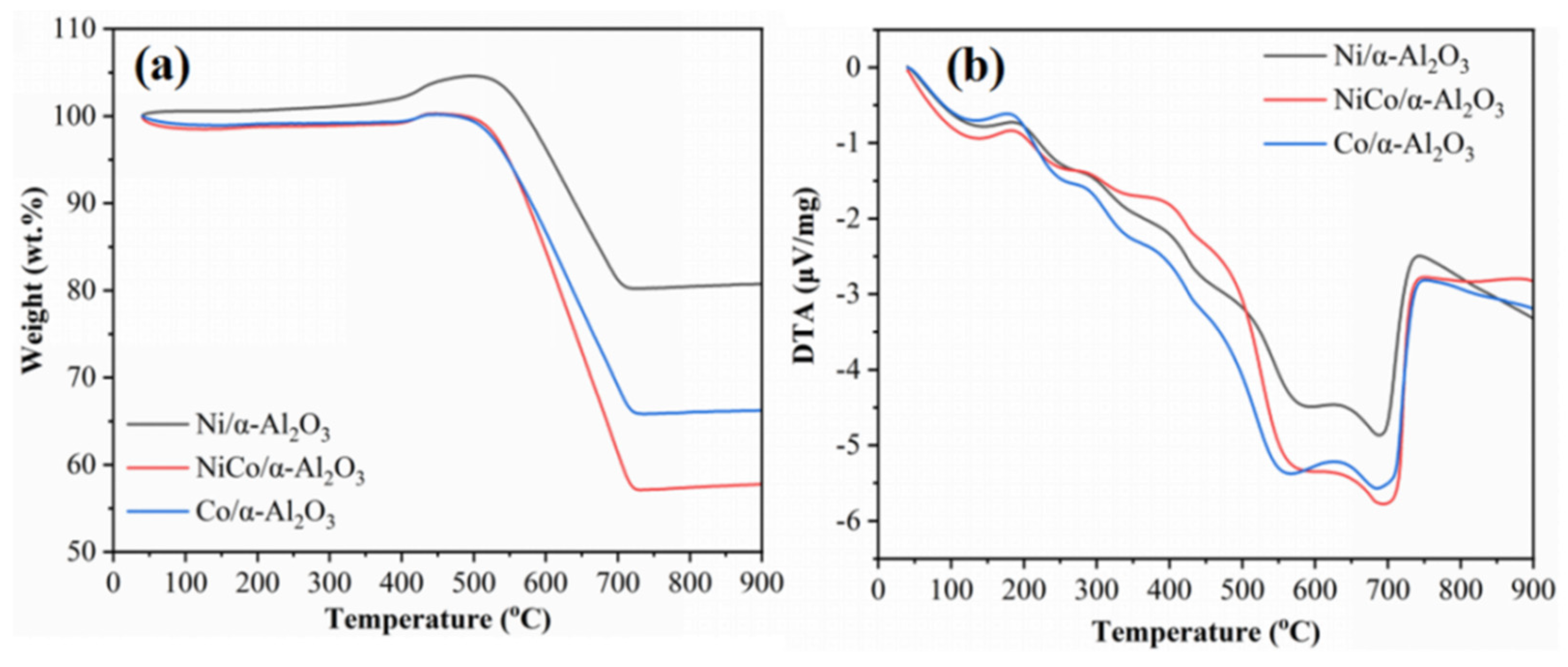
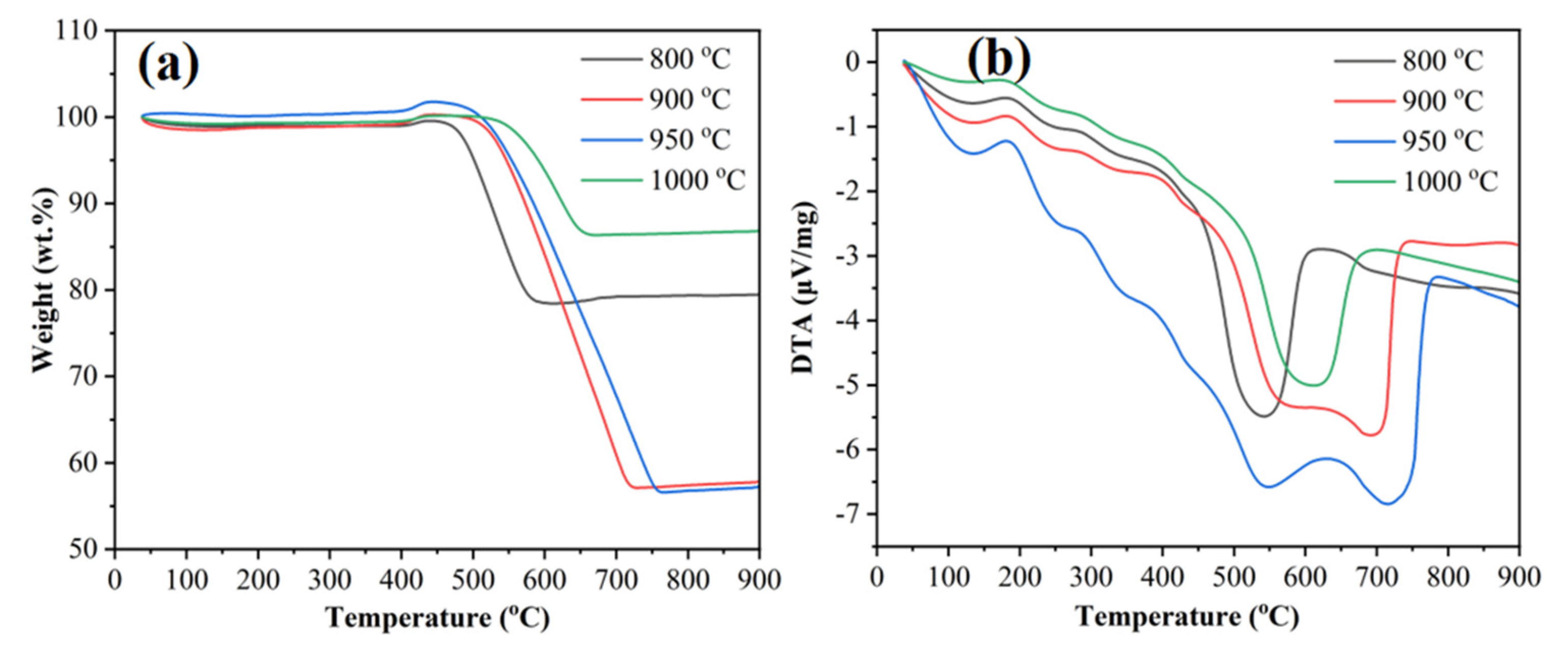
2.3. Thermochemistry of Catalysts
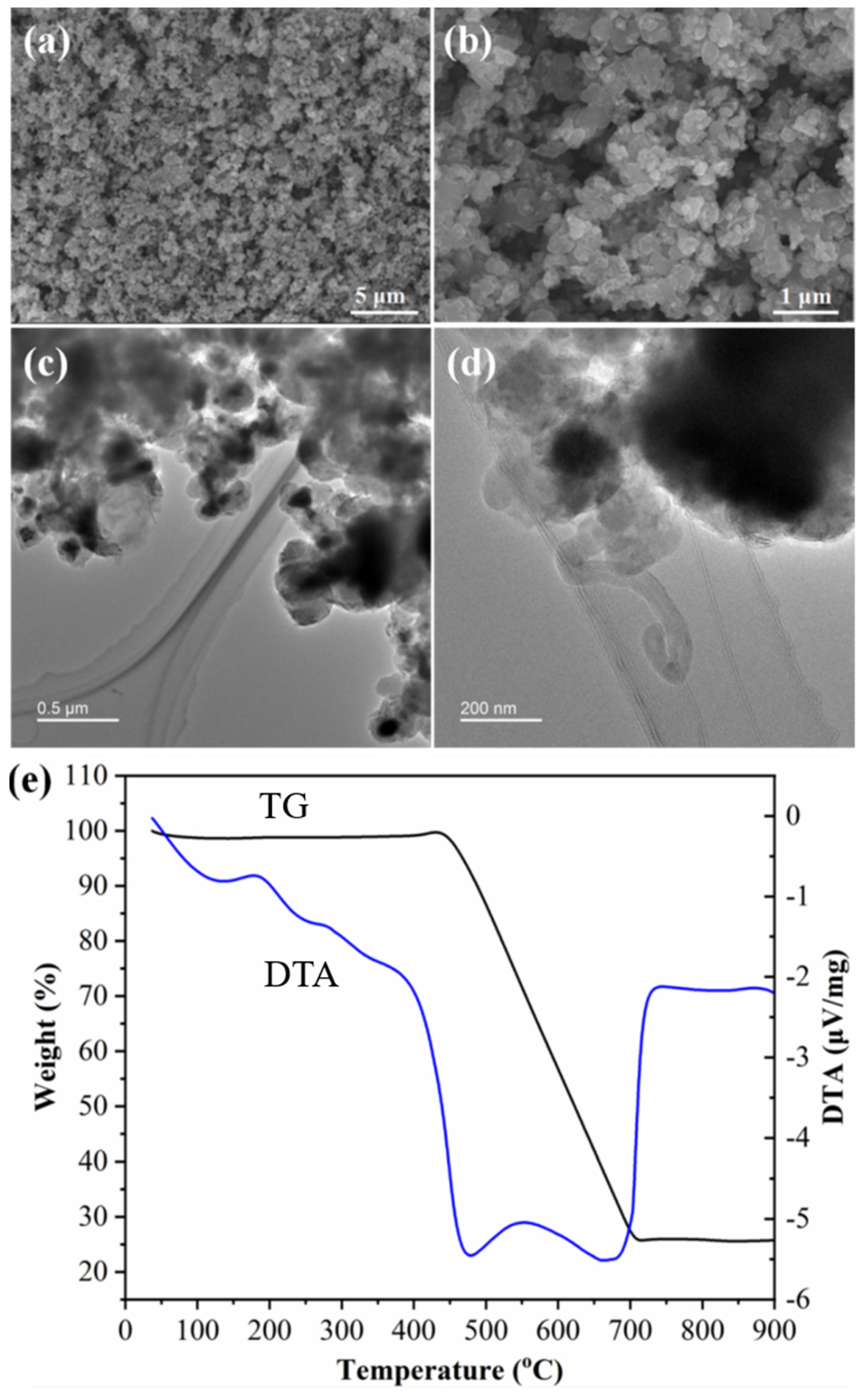
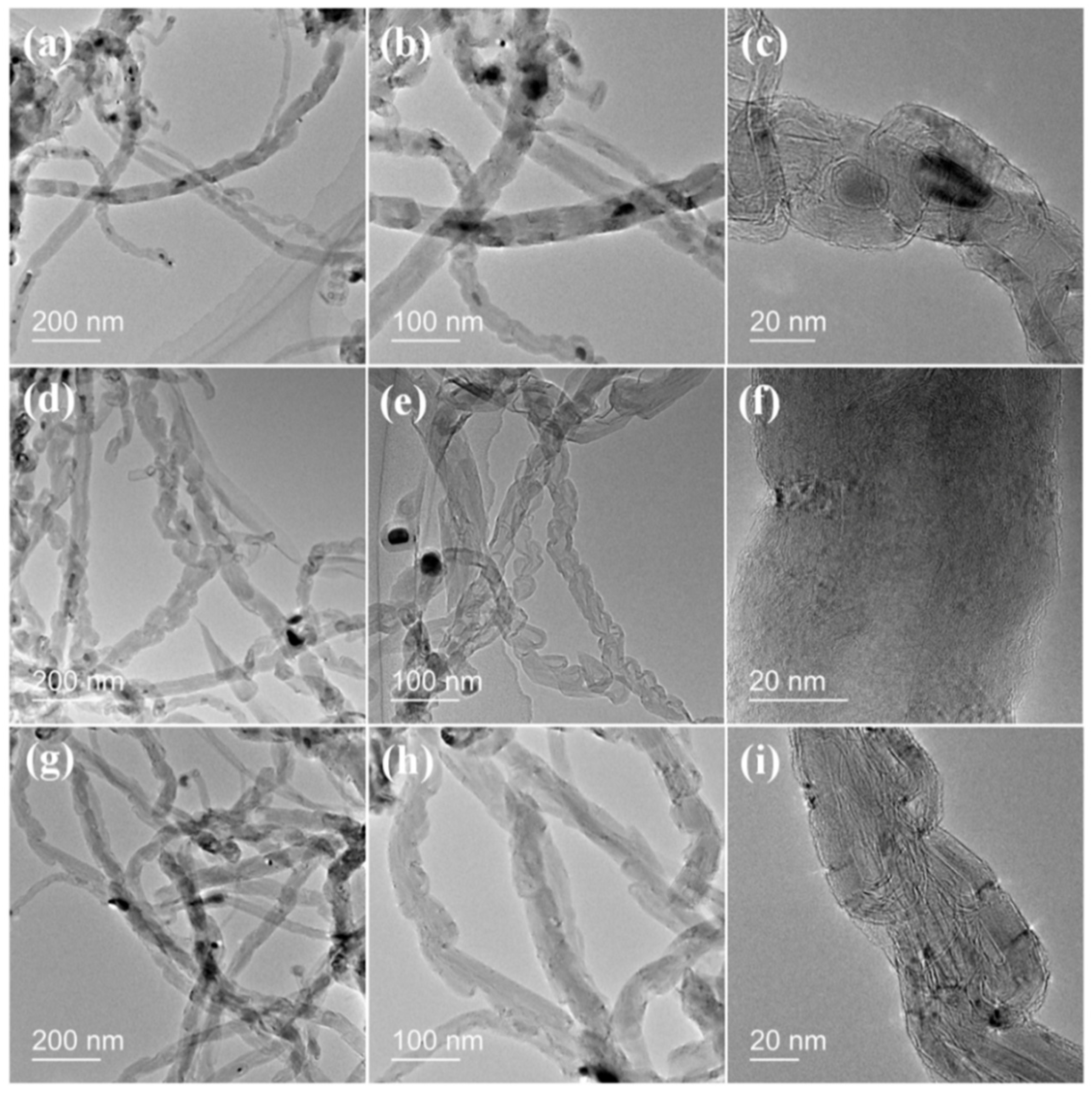
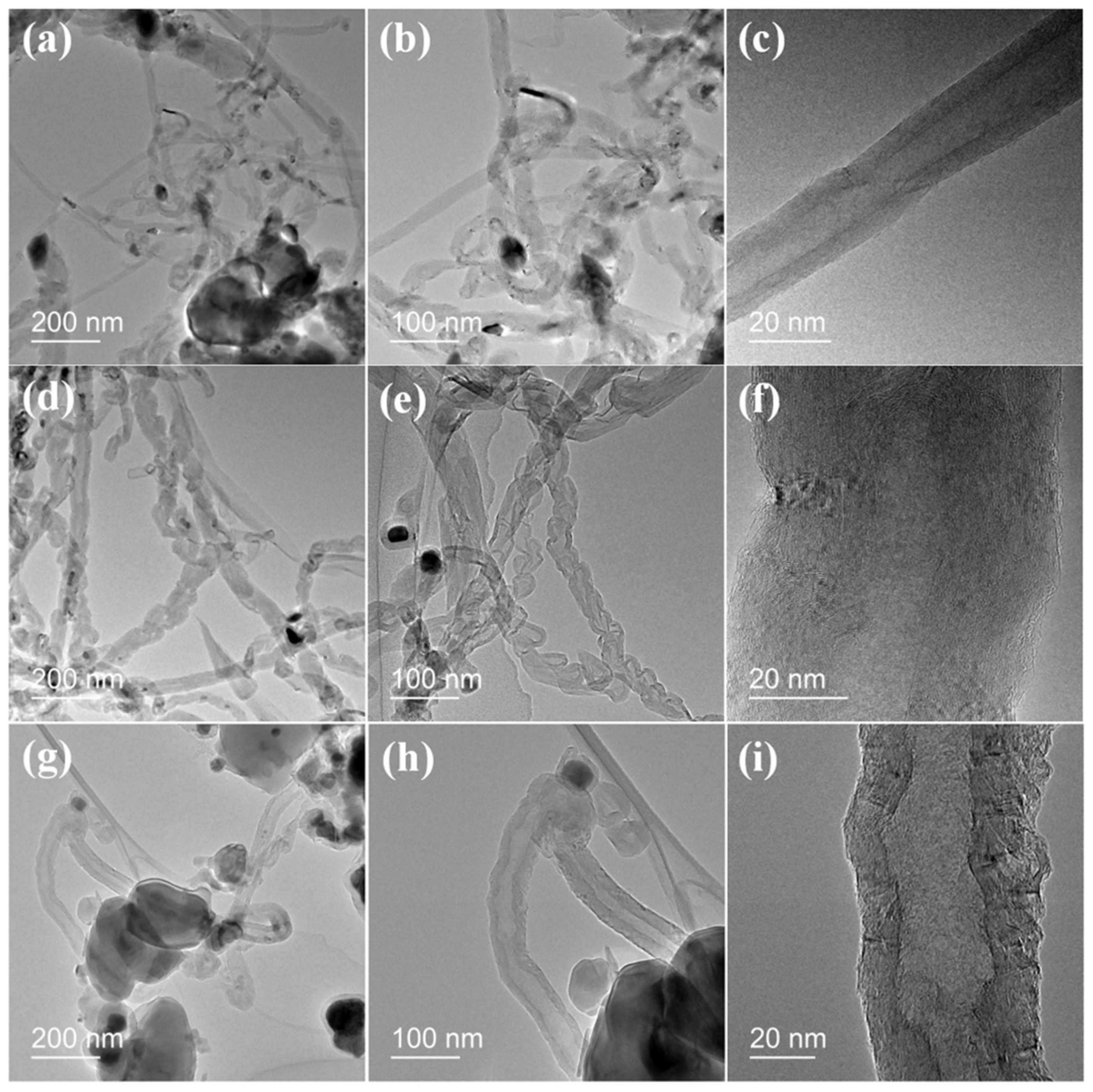
2.4. Growth of Carbon Nanotubes Composites
3. Experimental Part
3.1. Catalyst Preparation
3.2. Experimental Setup and Procedure
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Zhu, H.; Yao, D.; Williams, P.T.; Wu, C.; Xu, D.; Qiang, H.; George, M.; Lu, Y.; Ming, Z.; et al. Thermo-chemical conversion of carbonaceous waste for CNT and hydrogen productions: A review. Sustain. Energy Fuels 2021, 5, 4173–4208. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Ahmed, J.; Ubaidullah, M.; Shaikh, S.F.; Ahamad, T.; Naushad, M.; Zheng, G. Utilization of waste polyethylene terephthalate bottles to develop metal-organic frameworks for energy applications: A clean and feasible approach. J. Clean. Prod. 2020, 248, 119251. [Google Scholar] [CrossRef]
- Vanapalli, K.R.; Sharma, H.B.; Ranjan, V.P.; Samal, B.; Bhattacharya, J.; Dubey, B.K.; Goel, S. Challenges and strategies for effective plastic waste management during and post COVID-19 pandemic. Sci. Total Environ. 2021, 750, 141514. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Lehmann, A. Microplastic in terrestrial ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Ubaidullah, M.; Ahmed, J.; Ahamad, T.; Ahmad, T.; Shaikh, S.F.; Naushad, M. Synthesis of NiOx@ NPC composite for high-performance supercapacitor via waste PET plastic-derived Ni-MOF. Compos. Part B. Eng. 2020, 183, 107655. [Google Scholar] [CrossRef]
- Weckhuysen, B.M. Creating value from plastic waste. Science 2020, 370, 400–401. [Google Scholar] [CrossRef]
- Yao, D.; Zhang, Y.; Williams, P.T.; Yang, H.; Chen, H. Co-production of hydrogen and carbon nanotubes from real-world waste plastics: Influence of catalyst composition and operational parameters. Appl. Catal. B Environ. 2018, 221, 584–597. [Google Scholar] [CrossRef]
- Yao, D.; Yang, H.; Hu, Q.; Chen, Y.; Chen, H.; Williams, P.T. Carbon nanotubes from post-consumer waste plastics: Investigations into catalyst metal and support material characteristics. Appl. Catal. B Environ. 2021, 280, 119413. [Google Scholar] [CrossRef]
- Yao, D.; Wu, C.; Yang, H.; Zhang, Y.; Nahil, M.A.; Chen, Y.; Chen, H. Co-production of hydrogen and carbon nanotubes from catalytic pyrolysis of waste plastics on Ni-Fe bimetallic catalyst. Energy Convers. Manag. 2017, 148, 692–700. [Google Scholar] [CrossRef]
- De Volder, M.F.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Herrera, A.V.; González-Curbelo, M.Á.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á. Carbon nanotubes applications in separation science: A review. Anal. Chim. Acta 2012, 734, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Thostenson, E.T.; Ren, Z.; Chou, T.W. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Ando, Y. Chemical vapor deposition of carbon nanotubes: A review on growth mechanism and mass production. J. Nanosci. Nanotechnol. 2010, 10, 3739–3758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteves, L.M.; Oliveira, H.A.; Passos, F.B. Carbon nanotubes as catalyst support in chemical vapor deposition reaction: A review. J. Ind. Eng. Chem. 2018, 65, 1–12. [Google Scholar] [CrossRef]
- Weidenkaff, A.; Ebbinghaus, S.G.; Lippert, T. Ln1−xAxCoO3 (Ln = Er, La; A = Ca, Sr)/Carbon Nanotube Composite Materials Applied for Rechargeable Zn/Air Batteries. Chem. Mater. 2002, 14, 1797–1805. [Google Scholar] [CrossRef]
- Züttel, A.; Nützenadel, C.; Sudan, P.; Mauron, P.; Emmenegger, C.; Rentsch, S.; Schlapbach, L.; Weidenkaff, A.; Kiyobayashi, T. Hydrogen sorption by carbon nanotubes and other carbon nanostructures. J. Alloys Compd. 2002, 330, 676–682. [Google Scholar] [CrossRef]
- Manawi, Y.M.; Samara, A.; Al-Ansari, T.; Atieh, M.A. A review of carbon nanomaterials’ synthesis via the chemical vapor deposition (CVD) method. Materials 2018, 11, 822. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Nahil, M.A.; Miskolczi, N.; Huang, J.; Williams, P.T. Production and application of carbon nanotubes, as a co-product of hydrogen from the pyrolysis-catalytic reforming of waste plastic. Process Saf. Environ. Prot. 2016, 103, 107–114. [Google Scholar] [CrossRef]
- Borsodi, N.; Szentes, A.; Miskolczi, N.; Wu, C.; Liu, X. Carbon nanotubes synthetized from gaseous products of waste polymer pyrolysis and their application. J. Anal. Appl. Pyrol. 2016, 120, 304–313. [Google Scholar] [CrossRef]
- Wen, X.; Chen, X.; Tian, N.; Gong, J.; Liu, J.; Rümmeli, M.H.; Tang, T. Nanosized carbon black combined with Ni2O3 as “universal” catalysts for synergistically catalyzing carbonization of polyolefin wastes to synthesize carbon nanotubes and application for supercapacitors. Environ. Sci. Technol. 2014, 48, 4048–4055. [Google Scholar] [CrossRef]
- Acomb, J.C.; Wu, C.; Williams, P.T. The use of different metal catalysts for the simultaneous production of carbon nanotubes and hydrogen from pyrolysis of plastic feedstocks. Appl. Catal. B Environ. 2016, 180, 497–510. [Google Scholar] [CrossRef] [Green Version]
- Lua, A.C.; Wang, H.Y. Hydrogen production by catalytic decomposition of methane over Ni-Cu-Co alloy particles. Appl. Catal. B Environ. 2014, 156, 84–93. [Google Scholar] [CrossRef]
- Cartwright, R.; Esconjauregui, S.; Hardeman, D.; Bhardwaj, S.; Weatherup, R.; Guo, Y.; Robertson, J. Low temperature growth of carbon nanotubes on tetrahedral amorphous carbon using Fe–Cu catalyst. Carbon 2015, 81, 639–649. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. Investigation of coke formation on Ni-Mg-Al catalyst for hydrogen production from the catalytic steam pyrolysis-gasification of polypropylene. Appl. Catal. B Environ. 2010, 96, 198–207. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. Hydrogen production by steam gasification of polypropylene with various nickel catalysts. Appl. Catal. B Environ. 2009, 87, 152–161. [Google Scholar] [CrossRef]
- Joshi, R.; Engstler, J.; Houben, L.; Bar Sadan, M.; Weidenkaff, A.; Mandaliev, P.; Issanin, A.; Schneider, J.J. Catalyst Composition, Morphology and Reaction Pathway in the Growth of “Super-Long” Carbon Nanotubes. ChemCatChem 2010, 2, 1069–1073. [Google Scholar] [CrossRef]
- Weidenkaff, A.; Ebbinghaus, S.G.; Mauron, P.; Reller, A.; Zhang, Y.; Züttel, A. Metal nanoparticles for the production of carbon nanotube composite materials by decomposition of different carbon sources. Mater. Sci. Eng. C 2002, 19, 119–123. [Google Scholar] [CrossRef]
- Nahil, M.; Wu, C.; Williams, P.T. Influence of metal addition to Ni-based catalysts for the co-production of carbon nanotubes and hydrogen from the thermal processing of waste polypropylene. Fuel Process. Technol. 2015, 130, 46–53. [Google Scholar] [CrossRef]
- Yao, D.; Wang, C. Pyrolysis and in-line catalytic decomposition of polypropylene to carbon nanomaterials and hydrogen over Fe-and Ni-based catalysts. Appl. Energy 2020, 265, 114819. [Google Scholar] [CrossRef]
- Yao, D.; Li, H.; Dai, Y.; Wang, C.H. Impact of temperature on the activity of Fe-Ni catalysts for pyrolysis and decomposition processing of plastic waste. Chem. Eng. J. 2021, 408, 127268. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Mamba, B.B.; Msagati, T.A. Application of spinel ferrite nanoparticles in water and wastewater treatment: A review. Sep. Purif. Technol. 2017, 188, 399–422. [Google Scholar] [CrossRef]
- Narang, S.B.; Pubby, K. Nickel spinel ferrites: A review. J. Magn. Magn. Mater. 2021, 519, 167163. [Google Scholar] [CrossRef]
- Ganesh, I. A review on magnesium aluminate (MgAl2O4) spinel: Synthesis, processing and applications. Int. Mater. Rev. 2013, 58, 63–112. [Google Scholar] [CrossRef]
- Zhao, X.; Mao, L.; Cheng, Q.; Li, J.; Liao, F.; Yang, G.; Chen, L. Two-dimensional spinel structured Co-based materials for high performance supercapacitors: A critical review. Chem. Eng. J. 2020, 387, 124081. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Z.; Meng, F. Spinel-Type Materials Used for Gas Sensing: A Review. Sensors 2020, 20, 5413. [Google Scholar] [CrossRef]
- Mameli, V.; Angotzi, M.S.; Cara, C.; Cannas, C. Liquid phase synthesis of nanostructured spinel ferrites—A review. J. Nanosci. Nanotechnol. 2019, 19, 4857–4887. [Google Scholar] [CrossRef]
- Dou, S. Review and prospects of Mn-based spinel compounds as cathode materials for lithium-ion batteries. Ionics 2015, 21, 3001–3030. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Xu, Q.Z.; Wang, J.Y.; Li, N.; Guo, S.H.; Su, Y.Z.; Chen, S. Facile hydrothermal synthesis of urchin-like NiCo2O4 spheres as efficient electrocatalysts for oxygen reduction reaction. Int. J. Hydrog. Energy 2013, 38, 6657–6662. [Google Scholar] [CrossRef]
- Gu, Y.J.; Chen, Y.B.; Liu, H.Q.; Wang, Y.M.; Wang, C.L.; Wu, H.K. Structural characterization of layered LiNi0.85−xMnxCo0.15O2 with x = 0, 0.1, 0.2 and 0.4 oxide electrodes for Li batteries. J. Alloys Compd. 2011, 509, 7915–7921. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Cherepanova, S.V.; Tsybulya, S.V. In situ XRD investigation of Co3O4 reduction. In Eleventh European Powder Diffraction Conference; Oldenbourg Wissenschaftsverlag: München, Germany, 2015; pp. 329–334. [Google Scholar]
- Bartůněk, V.; Huber, Š.; Sedmidubský, D.; Sofer, Z.; Šimek, P.; Jankovský, O. CoO and Co3O4 nanoparticles with a tunable particle size. Ceram. Int. 2014, 40, 12591–12595. [Google Scholar] [CrossRef]
- Xu, J.; Wu, L.; Liu, Y.; Zhang, J.; Liu, J.; Shu, S.; Hu, Y. NiO-rGO composite for supercapacitor electrode. Surf. Interfaces 2020, 18, 100420. [Google Scholar] [CrossRef]
- Rahdar, A.; Aliahmad, M.; Azizi, Y. NiO nanoparticles: Synthesis and characterization. J. Nanostruct. 2015, 5, 145–151. [Google Scholar]
- Kong, L.; Yin, X.; Yuan, X.; Zhang, Y.; Liu, X.; Cheng, L.; Zhang, L. Electromagnetic wave absorption properties of graphene modified with carbon nanotube/poly (dimethyl siloxane) composites. Carbon 2014, 73, 185–193. [Google Scholar] [CrossRef]
- Liu, X.; Yin, X.; Kong, L.; Li, Q.; Liu, Y.; Duan, W.; Cheng, L. Fabrication and electromagnetic interference shielding effectiveness of carbon nanotube reinforced carbon fiber/pyrolytic carbon composites. Carbon 2014, 68, 501–510. [Google Scholar] [CrossRef]
- Du, G.; Feng, S.; Zhao, J.; Song, C.; Bai, S.; Zhu, Z. Particle–wire–tube mechanism for carbon nanotube evolution. J. Am. Chem. Soc. 2006, 128, 15405–15414. [Google Scholar] [CrossRef]
- Deck, C.P.; Vecchio, K. Growth mechanism of vapor phase CVD-grown multi-walled carbon nanotubes. Carbon 2005, 43, 2608–2617. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Xie, F.; Fasel, C.; Yin, X.; Riedel, R. Highly flexible and ultrathin Mo2C film via in-situ growth on graphene oxide for electromagnetic shielding application. Carbon 2020, 163, 254–264. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Xie, F.; Yin, X.; Riedel, R. Light-weight and highly flexible TaC modified PyC fiber fabrics derived from cotton fiber textile with excellent electromagnetic shielding effectiveness. Chem. Eng. J. 2020, 387, 124085. [Google Scholar] [CrossRef]
- Li, M.; Chai, N.; Liu, X.; Xie, W.; Wang, G.; Qu, F.; Riedel, R. Sustainable Paper Templated Ultrathin, Light-Weight and Flexible Niobium Carbide Based Films against Electromagnetic Interference. Carbon 2021, 183, 929–939. [Google Scholar] [CrossRef]
- Vaitkus, A.; Merkys, A.; Gražulis, S. Validation of the Crystallography Open Database using the Crystallographic Information Framework. J. App. Crystallogr. 2021, 54, 661–672. [Google Scholar] [CrossRef]
- Quirós, M.; Gražulis, S.; Girdzijauskaitė, S.; Merkys, A.; Vaitkus, A. Using SMILES strings for the description of chemical connectivity in the Crystallography Open Database. J. Cheminform. 2018, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Merkys, A.; Vaitkus, A.; Butkus, J.; Okulič-Kazarinas, M.; Kairys, V.; Gražulis, S. COD::CIF::Parser: An error-correcting CIF parser for the Perl language. J. App. Crystallogr. 2016, 249, 92–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gražulis, S.; Merkys, A.; Vaitkus, A.; Okulič-Kazarinas, M. Computing stoichiometric molecular composition from crystal structures. J. App. Crystallogr. 2015, 48, 85–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quirós, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; LeBail, A. Crystallography Open Database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2012, 40, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Grazulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.T.; Quiros, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database—An open-access collection of crystal structures. J. App. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef]
- Downs, R.T.; Hall-Wallace, M. The American Mineralogist Crystal Structure Database. Am. Mineral. 2003, 88, 247–250. [Google Scholar]




| Catalysts | Active Content | Synthesis Method | Feedstock | Reaction Temperature | Catalysts to Feedstock Weight Ratio | Ref. |
|---|---|---|---|---|---|---|
| Ni-Al | Molar ratio 1:2 | Precipitation | PP | 800 °C | 1:2 | [25] |
| Ni-Fe-Si/Al | 10 wt.% | Impregnation | HDPE (40 wt.%) LDPE (35 wt.%) PP (20 wt.%) PS (5 wt.%) | 800 °C | 1:2 | [8] |
| Ni-Metal-Al | Molar ratio 1:1:1 | Co-precipitation | PP | 800 °C | 1:2 | [28] |
| Ni-Fe-Al | 10 wt.% | Impregnation | HDPE (40 wt.%) LDPE (35 wt.%) PP (20 wt.%) PS (5 wt.%) | 700–900 °C | 1:2 | [8] |
| Ni-Fe-Al | 10 wt.% | Impregnation | HDPE (40 wt.%) LDPE (35 wt.%) PP (20 wt.%) PS (5 wt.%) | 800 °C | 1:2 | [9] |
| Ni-Fe-Al | 10 wt.% | Impregnation | Polypropylene | 800 °C | 2:5 | [29] |
| Ni-Fe-Al | 10 wt.% | Impregnation | Polypropylene | 600–800 °C | 2:5 | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Xie, W.; Widenmeyer, M.; Ding, H.; Chen, G.; De Carolis, D.M.; Lakus-Wollny, K.; Molina-Luna, L.; Riedel, R.; Weidenkaff, A. Upcycling Waste Plastics into Multi-Walled Carbon Nanotube Composites via NiCo2O4 Catalytic Pyrolysis. Catalysts 2021, 11, 1353. https://doi.org/10.3390/catal11111353
Liu X, Xie W, Widenmeyer M, Ding H, Chen G, De Carolis DM, Lakus-Wollny K, Molina-Luna L, Riedel R, Weidenkaff A. Upcycling Waste Plastics into Multi-Walled Carbon Nanotube Composites via NiCo2O4 Catalytic Pyrolysis. Catalysts. 2021; 11(11):1353. https://doi.org/10.3390/catal11111353
Chicago/Turabian StyleLiu, Xingmin, Wenjie Xie, Marc Widenmeyer, Hui Ding, Guoxing Chen, Dario M. De Carolis, Kerstin Lakus-Wollny, Leopoldo Molina-Luna, Ralf Riedel, and Anke Weidenkaff. 2021. "Upcycling Waste Plastics into Multi-Walled Carbon Nanotube Composites via NiCo2O4 Catalytic Pyrolysis" Catalysts 11, no. 11: 1353. https://doi.org/10.3390/catal11111353
APA StyleLiu, X., Xie, W., Widenmeyer, M., Ding, H., Chen, G., De Carolis, D. M., Lakus-Wollny, K., Molina-Luna, L., Riedel, R., & Weidenkaff, A. (2021). Upcycling Waste Plastics into Multi-Walled Carbon Nanotube Composites via NiCo2O4 Catalytic Pyrolysis. Catalysts, 11(11), 1353. https://doi.org/10.3390/catal11111353









