A Review and Experimental Revisit of Alternative Catalysts for Selective Oxidation of Methanol to Formaldehyde
Abstract
:1. Introduction
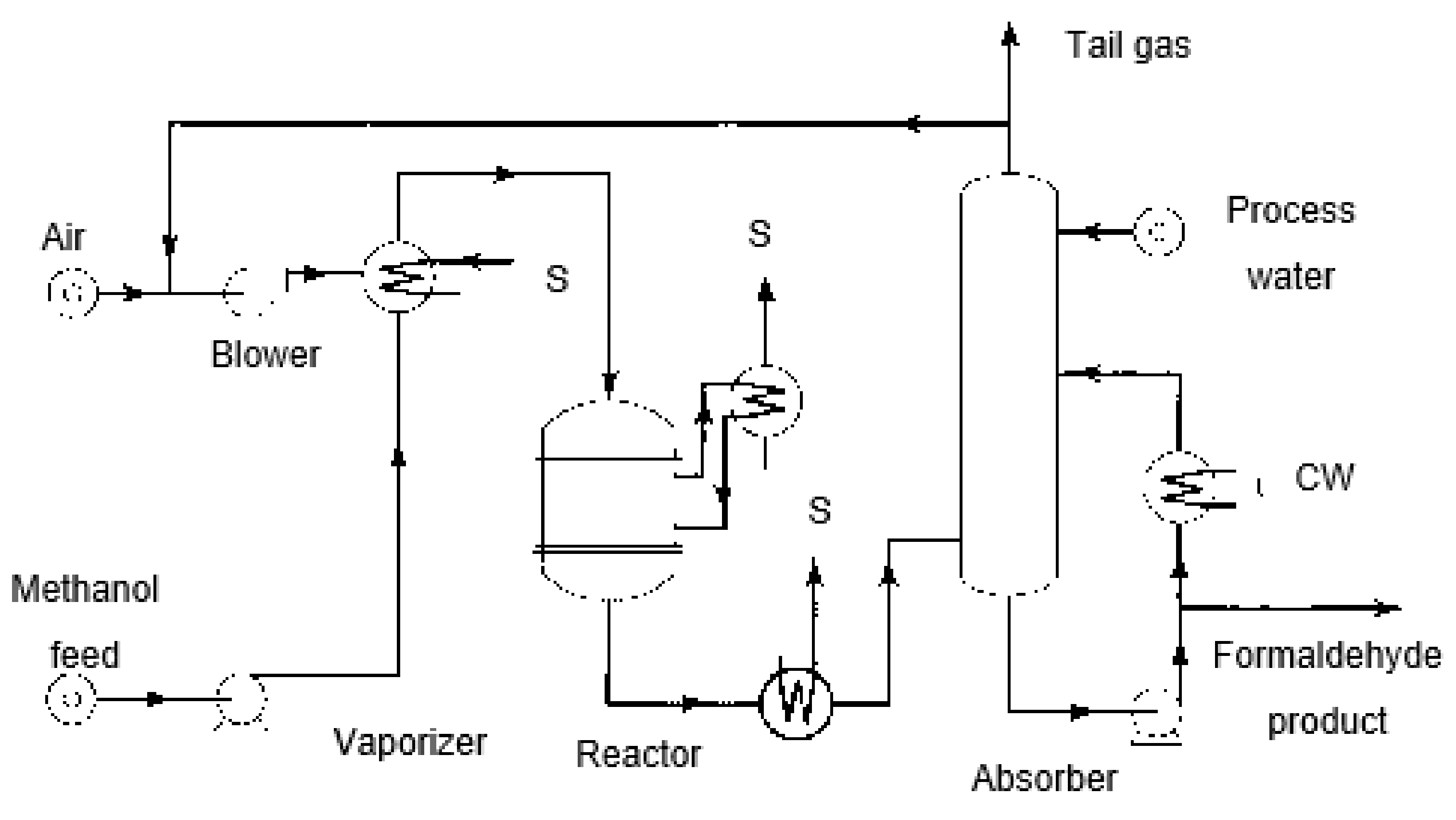
Processes
2. Literature Survey
2.1. Iron-Molybdenum Catalysts
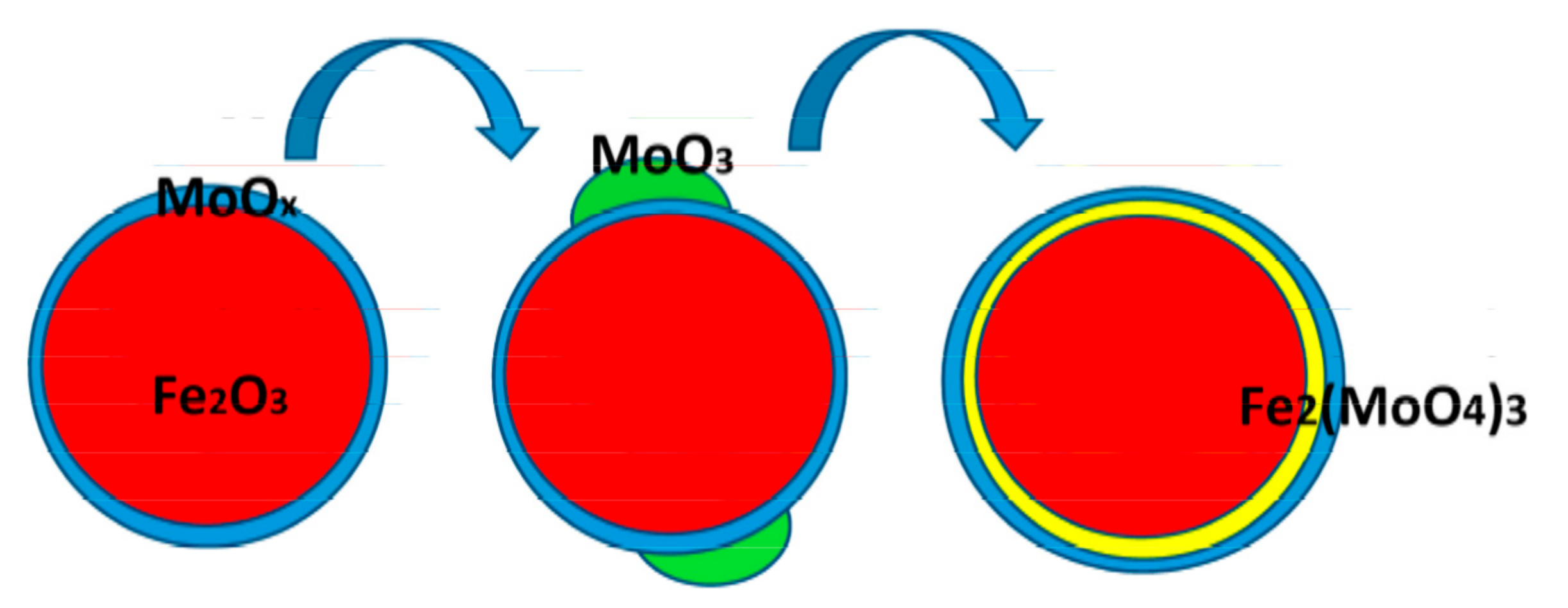
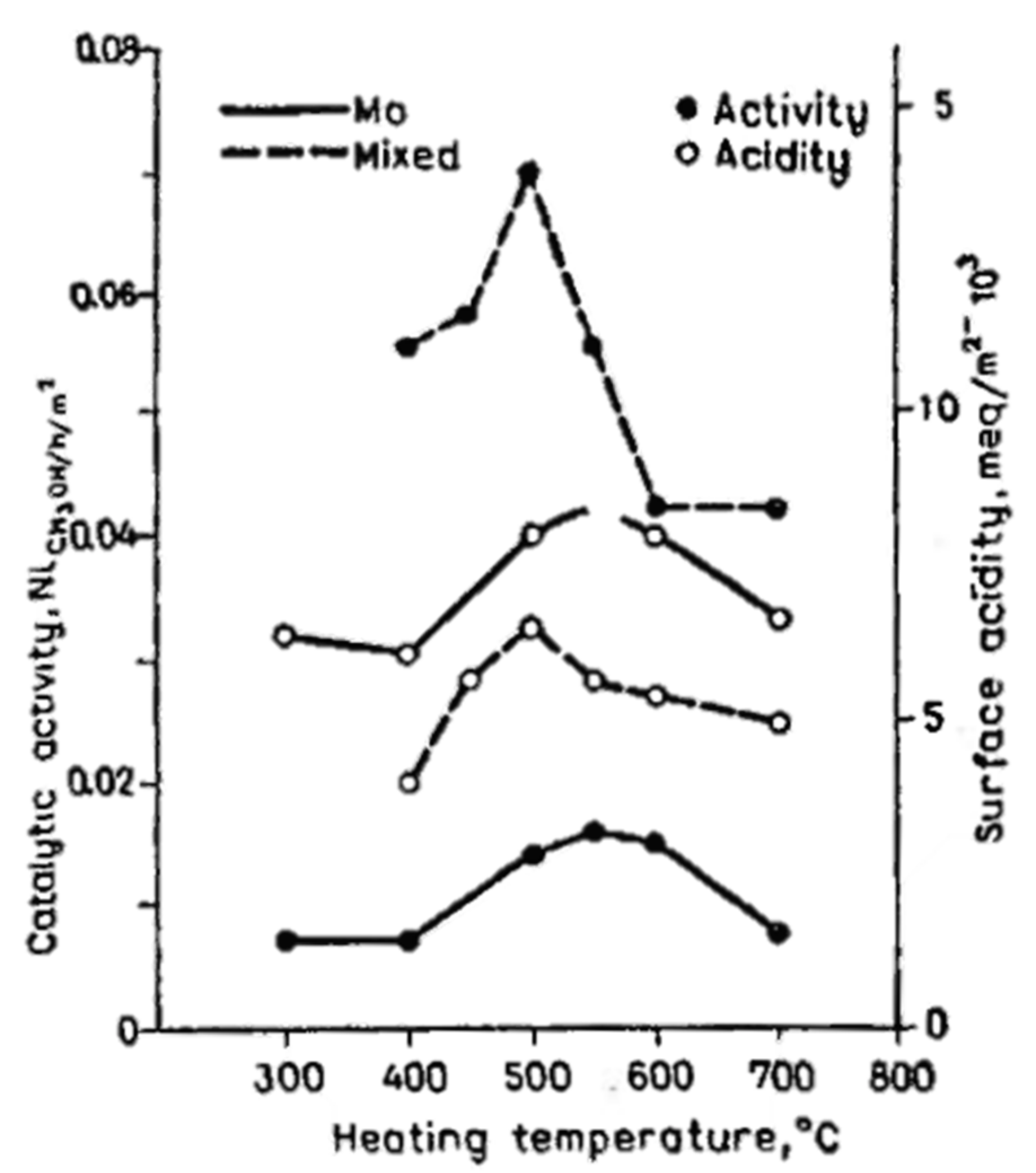
2.1.1. Structure
2.1.2. The Active Site
2.1.3. Influence of Catalyst Acidity/Basicity on Reaction Pathways
2.1.4. Reaction Mechanism and Kinetics
2.1.5. Reaction Engineering
2.1.6. Deactivation
2.2. Alternative Catalysts
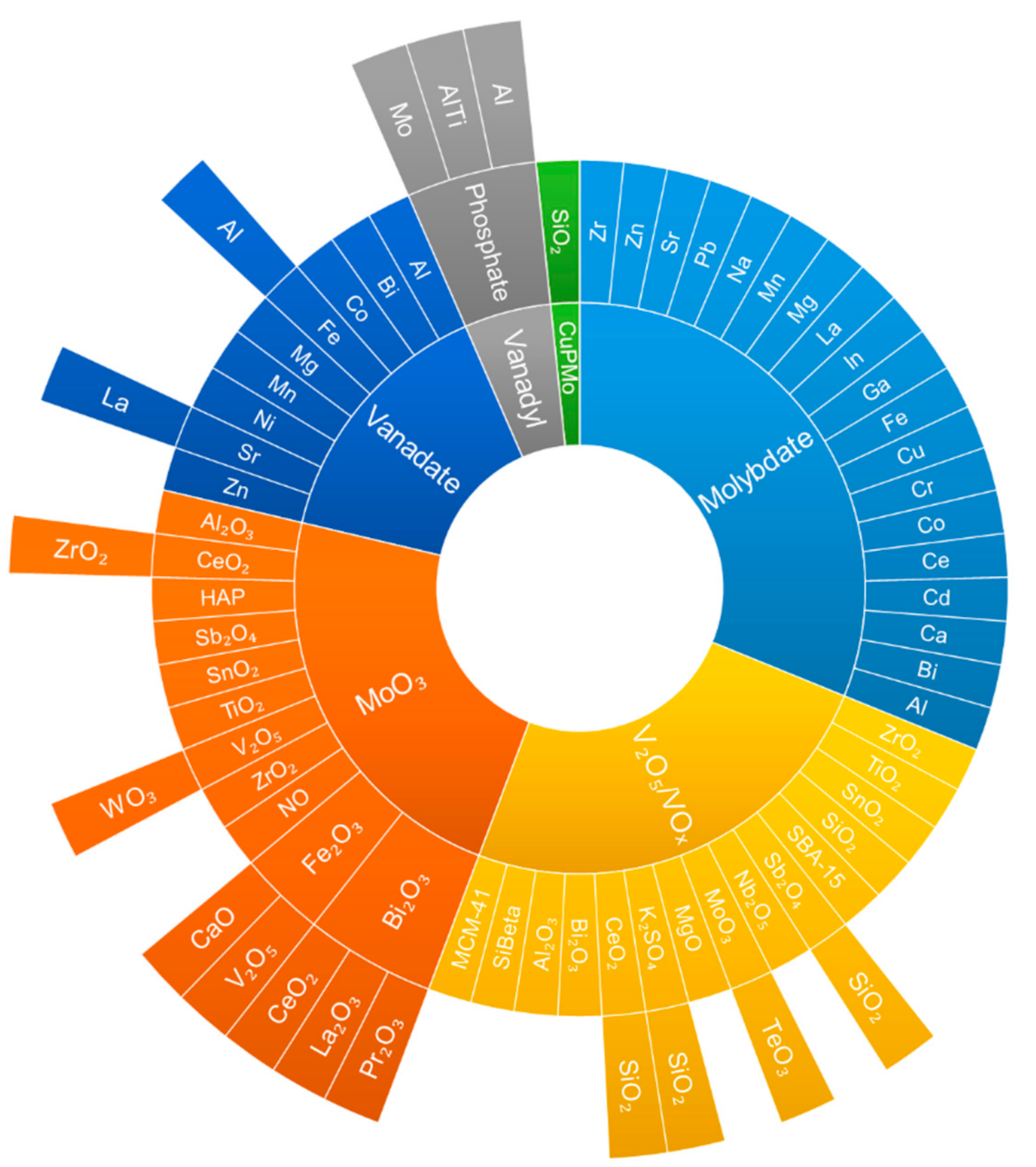
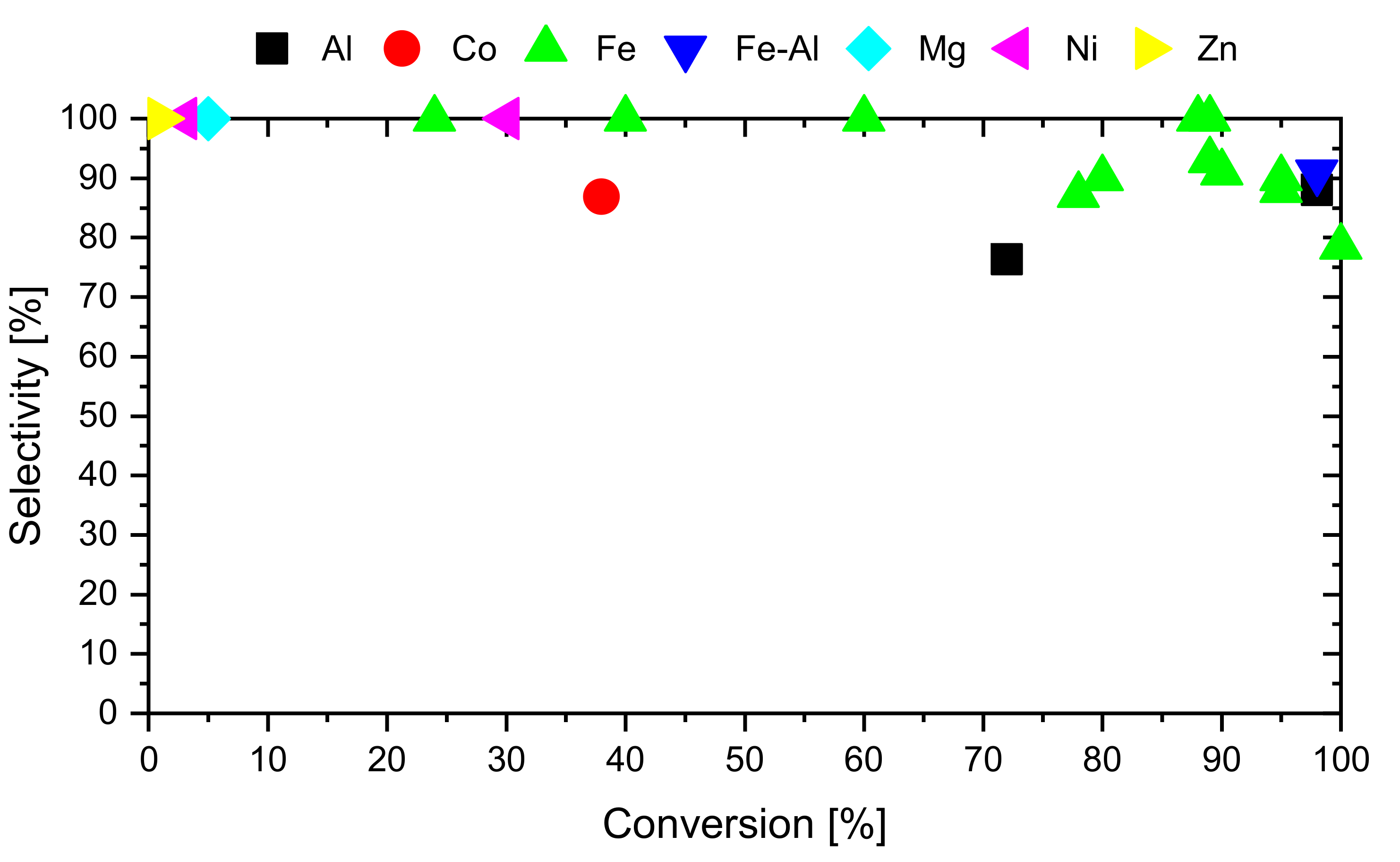
2.2.1. Overview of Catalysts Reported in the Literature
2.2.2. Mo Containing Catalysts
2.2.3. V Containing Catalysts
2.3. Other Types of Catalysts
Volatility of the Active Phase
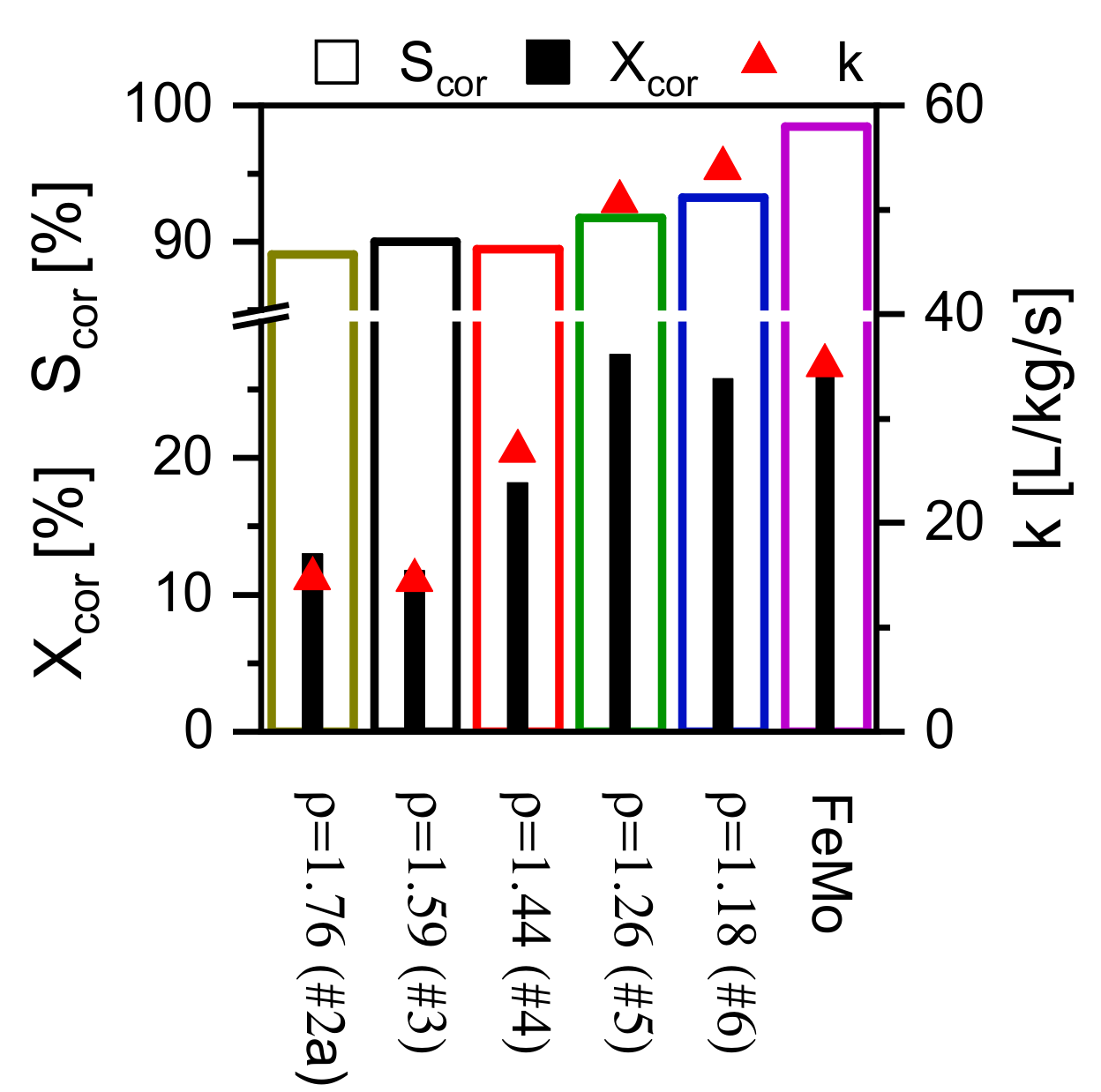
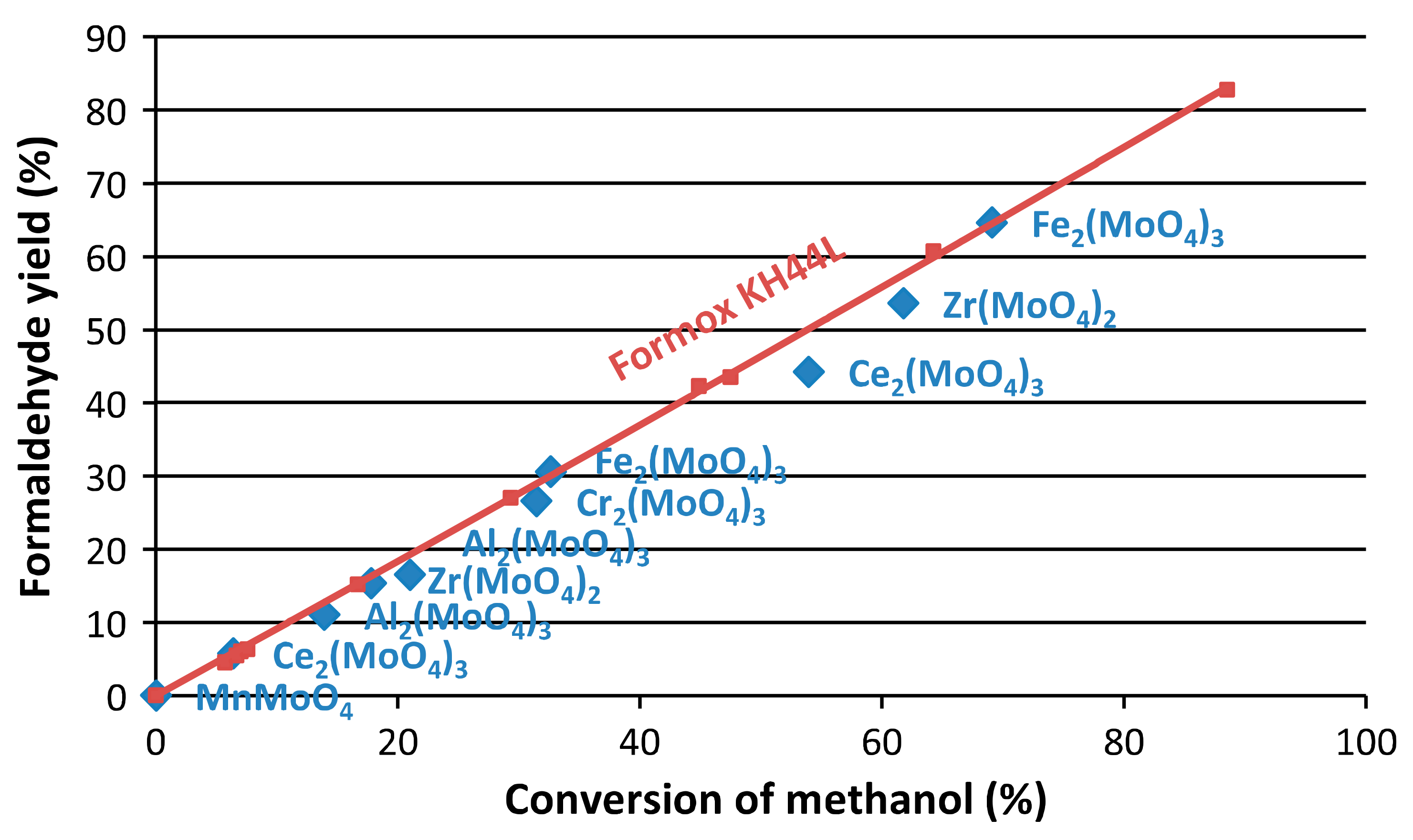
3. Summary of the Experimental Reproductions of the Most Interesting Alternative Catalysts
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Franz, A.W.; Kronemayer, H.; Pfeiffer, D.; Pilz, R.D.; Reuss, G.; Disteldorf, W.; Gamer, A.O.; Hilt, A. Formaldehyde. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2016; pp. 1–34. [Google Scholar]
- Soares, A.P.V.; Portela, M.F.; Kiennemann, A. Methanol Selective Oxidation to Formaldehyde over Iron-Molybdate Catalysts. Catal. Rev. Sci. Eng. 2005, 47, 125–174. [Google Scholar] [CrossRef]
- IHS Markit Formaldehyde. Available online: https://www.ihs.com/products/formaldehyde-chemical-economics-handbook.html (accessed on 28 September 2017).
- American Chemistry Council Formaldehyde. Available online: https://formaldehyde.americanchemistry.com/Applications/ (accessed on 28 September 2017).
- ICIS Formaldehyde Production and Manufacturing Process. Available online: https://www.icis.com/resources/news/2007/11/05/9076014/formaldehyde-production-and-manufacturing-process/ (accessed on 28 September 2017).
- Andersson, A.; Holmberg, J.; Häggblad, R. Process Improvements in Methanol Oxidation to Formaldehyde: Application and Catalyst Development. Top. Catal. 2016, 59, 1589–1599. [Google Scholar] [CrossRef]
- Cision Global Formaldehyde Market 2018–2022. Available online: https://www.prnewswire.com/news-releases/global-formaldehyde-market-2018-2022-300633054.html (accessed on 25 April 2019).
- Kortewille, B.; Wachs, I.E.; Cibura, N.; Pfingsten, O.; Bacher, G.; Muhler, M.; Strunk, J. Proof of Equivalent Catalytic Functionality upon Photon-Induced and Thermal Activation of Supported Isolated Vanadia Species in Methanol Oxidation. ChemCatChem 2018, 10, 2360–2364. [Google Scholar] [CrossRef]
- Wang, C.T.; Willey, R.J. Mechanistic aspects of methanol partial oxidation over supported iron oxide aerogels. J. Catal. 2001, 202, 211–219. [Google Scholar] [CrossRef]
- Sukumar, M.; Kennedy, L.J. Catalytic Conversion of Methanol to Formaldehyde Over La2CuO4 Nanoparticles. J. Nanosci. Nanotechnol. 2019, 19, 826–832. [Google Scholar] [CrossRef]
- Espinosa, M.R.; Charboneau, D.J.; Garcia de Oliveira, A.; Hazari, N. Controlling Selectivity in the Hydroboration of Carbon Dioxide to the Formic Acid, Formaldehyde, and Methanol Oxidation Levels. ACS Catal. 2019, 9, 301–314. [Google Scholar] [CrossRef]
- Siebert, M.; Seibicke, M.; Siegle, A.; Kräh, S.; Trapp, O. Selective Ruthenium-Catalyzed Transformation of Carbon Dioxide: An Alternative Approach towards Formaldehyde. J. Am. Chem. Soc. 2019, 141, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.S.; Pan, W.X.; Pan, W.X.; Li, J.L.; Zhu, Q.M.; Tin, K.C.; Wong, N.B. Preparation and effect of Mo-V-Cr-Bi-Si oxide catalysts on controlled oxidation of methane to methanol and formaldehyde. Korean J. Chem. Eng. 1998, 15, 496–499. [Google Scholar] [CrossRef]
- Santos, O.S.; Mascarenhas, A.J.S.; Andrade, H.M.C. N2O-assisted methanol selective oxidation to formaldehyde on cobalt oxide catalysts derived from layered double hydroxides. Catal. Commun. 2018, 113, 32–35. [Google Scholar] [CrossRef]
- Koivikko, N.; Laitinen, T.; Mouammine, A.; Ojala, S.; Keiski, R.L. Catalytic activity studies of Vanadia/Silica-Titania catalysts in SVOC partial oxidation to formaldehyde: Focus on the catalyst composition. Catalysts 2018, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Gerberich, H.R.; Seaman, G.C. Hoechst-Celanese Corporation Formaldehyde. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 24–26. ISBN 9780471238966. [Google Scholar]
- Söderhjelm, E.; House, M.P.; Cruise, N.; Holmberg, J.; Bowker, M.; Bovin, J.-O.; Andersson, A. On the Synergy Effect in MoO3–Fe2(MoO4)3 Catalysts for Methanol Oxidation to Formaldehyde. Top. Catal. 2008, 50, 145–155. [Google Scholar] [CrossRef]
- Methanex Methanex Monthly Average Regional Posted Contract Price History. Available online: https://www.methanex.com/sites/default/files/methanol-price/MxAvgPrice_Sept29%2C2017.pdf (accessed on 29 September 2017).
- Andersson, L.-O. New Green Policies in China. In Informally Speaking A Formaldehyde Magazine from Johnson Matthey; Johnson Matthey: London, UK, 2019; pp. 12–13. [Google Scholar]
- Andersson, A.; Hernelind, M.; Augustsson, O. A study of the ageing and deactivation phenomena occurring during operation of an iron molybdate catalyst in formaldehyde production. Catal. Today 2006, 112, 40–44. [Google Scholar] [CrossRef]
- Abaulina, L.I.; Kustova, G.N.; Klevtsova, R.F.; Popov, B.I.; Bibin, V.N.; Melekhina, V.A.; Kolomiichuk, V.N.; Boreskov, G.K. Stydy of an iron-molybdenum oxide catalyst for oxidation of methanol to formaldehyde V. Formation of a solid-solution of molybdenum trioxide in iron molybdate and nature of catalytically active component. Kinet. Catal. 1976, 17, 1126–1132. [Google Scholar]
- Chapman, S.; Brookes, C.; Bowker, M.; Gibson, E.K.; Wells, P.P. Design and stabilisation of a high area iron molybdate surface for the selective oxidation of methanol to formaldehyde. Faraday Discuss 2016, 188, 115–129. [Google Scholar] [CrossRef] [Green Version]
- Brookes, C.; Wells, P.P.; Dimitratos, N.; Jones, W.; Gibson, E.K.; Morgan, D.J.; Cibin, G.; Nicklin, C.; Mora-Fonz, D.; Scanlon, D.O.; et al. The Nature of the Molybdenum Surface in Iron Molybdate. The Active Phase in Selective Methanol Oxidation. J. Phys. Chem. C 2014, 118, 26155–26161. [Google Scholar] [CrossRef]
- Brookes, C.; Bowker, M.; Gibson, E.K.; Gianolio, D.; Mohammed, K.M.H.; Parry, S.; Rogers, S.M.; Silverwood, I.P.; Wells, P.P. In situ spectroscopic investigations of MoOx/Fe2O3 catalysts for the selective oxidation of methanol. Catal. Sci. Technol. 2016, 6, 722–730. [Google Scholar] [CrossRef] [Green Version]
- Brookes, C.; Wells, P.P.; Cibin, G.; Dimitratos, N.; Jones, W.; Morgan, D.J.; Bowker, M. Molybdenum Oxide on Fe2O3 Core–Shell Catalysts: Probing the Nature of the Structural Motifs Responsible for Methanol Oxidation Catalysis. ACS Catal. 2014, 4, 243–250. [Google Scholar] [CrossRef]
- Uhlrich, J.J.; Sainio, J.; Lei, Y.; Edwards, D.; Davies, R.; Bowker, M.; Shaikhutdinov, S.; Freund, H.J. Preparation and characterization of iron–molybdate thin films. Surf. Sci. 2011, 605, 1550–1555. [Google Scholar] [CrossRef]
- House, M.P.; Shannon, M.D.; Bowker, M. Surface segregation in iron molybdate catalysts. Catal. Lett. 2008, 122, 210–213. [Google Scholar] [CrossRef]
- Brookes, C.; Bowker, M.; Wells, P. Catalysts for the Selective Oxidation of Methanol. Catalysts 2016, 6, 92. [Google Scholar] [CrossRef] [Green Version]
- Routray, K.; Zhou, W.; Kiely, C.J.; Grünert, W.; Wachs, I.E. Origin of the synergistic interaction between MoO3 and iron molybdate for the selective oxidation of methanol to formaldehyde. J. Catal. 2010, 275, 84–98. [Google Scholar] [CrossRef]
- Bowker, M.; Brookes, C.; Carley, A.F.; House, M.P.; Kosif, M.; Sankar, G.; Wawata, I.; Wells, P.P.; Yaseneva, P. Evolution of active catalysts for the selective oxidative dehydrogenation of methanol on Fe2O3 surface doped with Mo oxide. Phys. Chem. Chem. Phys. 2013, 15, 12056–12067. [Google Scholar] [CrossRef]
- Bowker, M.; House, M.; Alshehri, A.; Brookes, C.; Gibson, E.K.; Wells, P.P. Selectivity determinants for dual function catalysts: Applied to methanol selective oxidation on iron molybdate. Catal. Struct. React. 2015, 1, 95–100. [Google Scholar] [CrossRef]
- Bowker, M.; Gibson, E.K.; Silverwood, I.P.; Brookes, C. Methanol oxidation on Fe2O3 catalysts and the effects of surface Mo. Faraday Discuss. 2016, 188, 387–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowker, M.; Holroyd, R.; Elliott, A.; Morrall, P.; Alouche, A.; Entwistle, C.; Toerncrona, A. The selective oxidation of methanol to formaldehyde on iron molybdate catalysts and on component oxides. Catal. Lett. 2002, 83, 165–176. [Google Scholar] [CrossRef]
- Yamada, H.; Niwa, M.; Murakami, Y. Methanol oxidation on a molybdena monolayer supported on iron oxide. Appl. Catal. A Gen. 1993, 96, 113–123. [Google Scholar] [CrossRef]
- Busca, G. On the mechanism of methanol oxidation over vanadia-based catalysts: A FT-IR study of the adsorption of methanol, formaldehyde and formic acid on vanadia-silica. J. Mol. Catal. 1989, 50, 241–249. [Google Scholar] [CrossRef]
- Chung, J.S.; Miranda, R.; Bennett, C.O. Mechanism of Partial Oxidation of Methanol over MoO3. J. Catal. 1988, 114, 398–410. [Google Scholar] [CrossRef]
- Louis, C.; Tatibouët, J.M.; Che, M. Catalytic properties of silica-supported molybdenum catalysts in methanol oxidation: The influence of molybdenum dispersion. J. Catal. 1988, 109, 354–366. [Google Scholar] [CrossRef]
- Bowker, M.; Carley, A.F.; House, M. Contrasting the behaviour of MoO3 and MoO2 for the oxidation of methanol. Catal. Lett. 2008, 120, 34–39. [Google Scholar] [CrossRef]
- Bowker, M.; Holroyd, R.; House, M.; Bracey, R.; Bamroongwongdee, C.; Shannon, M.; Carley, A. The selective oxidation of methanol on iron molybdate catalysts. Top. Catal. 2008, 48, 158–165. [Google Scholar] [CrossRef]
- Trifirò, F.; Notarbartolo, S.; Pasquon, I. The nature of the active component in a Fe2O3-MoO3 catalyst. II. Study of the variations occurring during high temperature treatment. J. Catal. 1971, 22, 324–332. [Google Scholar] [CrossRef]
- Dias, A.P.S.; Montemor, F.; Portela, M.F.; Kiennemann, A. The role of the suprastoichiometric molybdenum during methanol to formaldehyde oxidation over Mo-Fe mixed oxides. J. Mol. Catal. A Chem. 2015, 397, 93–98. [Google Scholar] [CrossRef]
- Hummadi, K.K.; Hassan, K.H.; Mitchell, P.C.H. Selectivity and activity of iron molybdate catalysts in oxidation of methanol. J. Eng. Res. 2009, 6, 1–7. [Google Scholar]
- Raun, K.V.; Lundegaard, L.F.; Chevallier, J.; Beato, P.; Appel, C.C.; Nielsen, K.; Thorhauge, M.; Jensen, A.D.; Høj, M. Deactivation behavior of an iron-molybdate catalyst during selective oxidation of methanol to formaldehyde. Catal. Sci. Technol. 2018, 8, 4626–4637. [Google Scholar] [CrossRef] [Green Version]
- Pernicone, N.; Liberti, G.; Ersini, L. Catalytic Activity of Pure MoO3 and of Mixtures of MoO3 with Trivalent Metal Molybdates in the Oxidation of CH3OH to CH2O. In Proceedings of the Fourth International Congress on Catalysis, Moscow, Russia, 23–29 June 1968; pp. 287–296. [Google Scholar]
- Tatibouët, J.M. Methanol oxidation as a catalytic surface probe. Appl. Catal. A Gen. 1997, 148, 213–252. [Google Scholar] [CrossRef]
- Ivanov, K.; Dimitrov, D. On the mechanism of the selective oxidation of methanol over iron-molybdate catalysts. Oxid. Commun. 2008, 31, 444–455. [Google Scholar]
- AI, M. Catalytic Activity for the Oxidation of Methanol and the Acid-Base Properties of Metal Oxides. J. Catal. 1978, 54, 426–435. [Google Scholar] [CrossRef]
- Nikolenko, N.V.; Kalashnikov, Y.V.; Kostyniuk, A.O.; Poloz, A.Y.; Aksenenko, E.V. Difference in adsorption properties of Fe(III), Mo(VI) oxides and Fe(III) molybdate as a cause of high selectivity of methanol oxidation on iron molybdate catalyst. Vopr. Khimii Khimicheskoi Tekhnologii 2019, 3, 35–45. [Google Scholar]
- Wachs, I.E. Raman and IR studies of surface metal oxide species on oxide supports: Supported metal oxide catalysts. Catal. Today 1996, 27, 437–455. [Google Scholar] [CrossRef]
- Pernicone, N.; Lazzerin, F.; Lanzavecchia, G. The effect of water on the catalytic oxidation of methanol to formaldehyde. J. Catal. 1968, 10, 83–84. [Google Scholar] [CrossRef]
- Pernicone, N.; Lazzerin, F.; Liberti, G.; Lanzavecchia, G. On the mechanism of CH3OH oxidation to CH2O over MoO3-Fe2(MoO4)3 catalyst. J. Catal. 1969, 14, 293–302. [Google Scholar] [CrossRef]
- Rellán-Piñeiro, M.; López, N. The active molybdenum oxide phase in the methanol oxidation to formaldehyde (Formox process): A DFT study. ChemSusChem 2015, 8, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Liberti, G.; Pernicone, N.; Soattini, S. Pulse microreactor study of methanol oxidation over MoO3-Fe2(MoO4)3 catalyst. J. Catal. 1972, 27, 52–55. [Google Scholar] [CrossRef]
- Holstein, W.L.; Machiels, C.J. Inhibition of Methanol Oxidation by Water Vapor—Effect on Measured Kinetics and Relevance to the Mechanism. J. Catal. 1996, 162, 118–124. [Google Scholar] [CrossRef]
- Drăgan, S.; Kulic, I. A macrokinetic study of the oxidation of methanol to formaldehyde on Fe2O3—MoO3 oxide catalyst. Stud. Univ. Babes-Bolyai Chem. 2016, LXI, 155–166. [Google Scholar]
- Bhattacharyya, S.K.; Janakiram, K.; Ganguly, N.D. Kinetics of the Vapor-Phase Oxidation of Methyl Alcohol on Vanadium Pentoxide Catalyst. J. Catal. 1967, 18, 128–136. [Google Scholar] [CrossRef]
- Bibin, V.N.; Popov, B.I. Kinetics of methanol oxidation by air on iron-molybdenum oxide catalysts. Kinet. Catal. 1969, 10, 1091–1098. [Google Scholar]
- Evmenenko, N.P.; Gorokhovatskii, Y.B. Kinetics of methanol oxidation at a ferromolybdenum catalyst. Kinet. Katal. 1968, 10, 1299–1304. [Google Scholar]
- Santacesaria, E.; Morbidelli, M.; Carrà, S. Kinetics of the catalytic oxidation of methanol to formaldehyde. Chem. Eng. Sci. 1981, 36, 909–918. [Google Scholar] [CrossRef]
- Deshmukh, S.A.R.K.; van Sint Annaland, M.; Kuipers, J.A.M. Kinetics of the partial oxidation of methanol over a Fe-Mo catalyst. Appl. Catal. A Gen. 2005, 289, 240–255. [Google Scholar] [CrossRef]
- Ulukardesler, A.H.; Atalay, S.; Atalay, F.S. Determination of Optimum Conditions and the Kinetics of Methanol Oxidation. Chem. Eng. Technol. 2010, 33, 167–176. [Google Scholar] [CrossRef]
- Tesser, R.; Di Serio, M.; Santacesaria, E. Catalytic oxidation of methanol to formaldehyde: An example of kinetics with transport phenomena in a packed-bed reactor. Catal. Today 2003, 77, 325–333. [Google Scholar] [CrossRef]
- Machiels, C.; Sleight, A.W. Kinetic isotope effect in the selective oxidation of methanol to formaldehyde over some molybdate catalysts. J. Catal. 1982, 76, 238–239. [Google Scholar] [CrossRef]
- Machiels, C. Development of a pulse reactor with online MS analysis to study the oxidation of methanol. Acs Symp. Ser. 1982, 178, 240–251. [Google Scholar]
- Yang, T.-J.; Lunsford, J.H. Partial oxidation of Methanol to Formaldehyde over Molybdenum Oxide on Silica. Catal. Lett. 1987, 103, 55–64. [Google Scholar] [CrossRef]
- Routray, K.; Briand, L.E.; Wachs, I.E. Is there a relationship between the M=O bond length (strength) of bulk mixed metal oxides and their catalytic activity? J. Catal. 2008, 256, 145–153. [Google Scholar] [CrossRef]
- Lafyatis, D.S.; Creten, G.; Froment, G.F. TAP reactor study of the partial oxidation of methanol to formaldehyde using an industrial Fe-Cr-Mo oxide catalyst. Appl. Catal. A Gen. 1994, 120, 85–103. [Google Scholar] [CrossRef]
- Szabo, A.; Urda, A.; Alifanti, M. Aspects concerning the mechanism of the partial oxidation reactions. Analele Univ. Bucureşti Chim. Anul. 2006, II, 85–91. [Google Scholar]
- O’Brien, M.G.; Beale, A.M.; Jacques, S.D.M.; Buslaps, T.; Honkimaki, V.; Weckhuysen, B.M. On the Active Oxygen in Bulk MoO3 during the Anaerobic Dehydrogenation of Methanol. J. Phys. Chem. C 2009, 113, 4890–4897. [Google Scholar] [CrossRef]
- House, M.P.; Carley, A.F.; Bowker, M. Selective oxidation of methanol on iron molybdate catalysts and the effects of surface reduction. J. Catal. 2007, 252, 88–96. [Google Scholar] [CrossRef]
- Choksi, T.; Greeley, J. Partial Oxidation of Methanol on MoO3(010): A DFT and Microkinetic Study. ACS Catal. 2016, 6, 7260–7277. [Google Scholar] [CrossRef]
- Peyrovi, M.H.; Parsafard, N.; Hasanpour, H. Catalytic Study of the Partial Oxidation Reaction of Methanol to Formaldehyde in the Vapor Phase. Bull. Chem. React. Eng. Catal. 2018, 13, 520. [Google Scholar] [CrossRef]
- Ivanov, K.; Dimitrov, D.; Boyanov, B. Optimization of the methanol oxidation over iron-molybdate catalysts. Chem. Eng. J. 2009, 154, 189–195. [Google Scholar] [CrossRef]
- Partopour, B.; Dixon, A.G. Effect of particle shape on methanol partial oxidation in a fixed bed using CFD reactor modeling. AIChE J. 2019, 1–13. [Google Scholar] [CrossRef]
- Popov, B.I.; Bibin, V.N.; Boreskov, G.K. Study of an Iron-Molybdenum oxide catalyst for the oxidation of methanol to formaldehyde. IV. Entrainment of Molybdenum from the catalyst, the main reason for the decrease in its activity during use. Kinet. Katal. 1976, 17, 371–377. [Google Scholar]
- Carbucicchio, M.; Forzatti, P.; Trifiro’, F.; Tronconi, E.; Villa, P.L.L. Deactivation of silica supported Fe2O3-MoO3 catalyst for the oxidation of methanol. Stud. Surf. Sci. Catal. 1980, 6, 103–113. [Google Scholar]
- Popov, B.I.; Skomorokhova, N.G. Changes in activity, selectivity and surface area along an iron-molybdenum catalyst bed after its industrial application. React. Kinet. Catal. Lett. 1982, 18, 101–105. [Google Scholar] [CrossRef]
- Burriesci, N.; Garbassi, F.; Petrera, M.; Petrini, G.; Pernicone, N. Solid state reactions in Fe-Mo oxide catalysts for methanol oxidation during aging in industrial plants. Stud. Surf. Sci. Catal. 1980, 6, 115–126. [Google Scholar]
- Ma, Y.H.; Kmiotek, S.J. Deactivation kinetics of ferric molybdate catalysts. J. Catal. 1988, 109, 132–142. [Google Scholar] [CrossRef]
- Smith, R.L.; Rohrer, G.S. The morphological evolution of the MoO3(010) surface during reactions in methanol-air mixtures. J. Catal. 1998, 180, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Mccarron, E.M.; Staley, R.H.; Sleight, A.W. Oxy-Methoxy Compounds of Hexavalent Molybdenum. Inorg. Chem. 1984, 23, 1043–1045. [Google Scholar] [CrossRef]
- Ivanov, K.I.; Dimitrov, D.Y. Deactivation of an industrial iron-molybdate catalyst for methanol oxidation. Catal. Today 2010, 154, 250–255. [Google Scholar] [CrossRef]
- O’Brien, M.G.; Beale, A.M.; Jacques, S.D.M.; Di Michiel, M.; Weckhuysen, B.M. Spatiotemporal multitechnique imaging of a catalytic solid in action: Phase variation and volatilization during molybdenum oxide reduction. ChemCatChem 2009, 1, 99–102. [Google Scholar] [CrossRef]
- Soares, A.P.V.; Portela, M.F.; Kiennemann, A.; Millet, J.M.M. Iron-molybdate deactivation during methanol to formaldehyde oxidation: Effect of water. React. Kinet. Catal. Lett. 2002, 75, 13–20. [Google Scholar] [CrossRef]
- Soares, A.P.V.; Portela, M.F.; Kiennemann, A.; Hilaire, L. Mechanism of deactivation of iron-molybdate catalysts prepared by coprecipitation and sol-gel techniques in methanol to formaldehyde oxidation. Chem. Eng. Sci. 2003, 58, 1315–1322. [Google Scholar] [CrossRef]
- Soares, A.P.V.; Farinha Portela, M.; Kiennemann, A.; Hilaire, L.; Millet, J.M.M. Iron molybdate catalysts for methanol to formaldehyde oxidation: Effects of Mo excess on catalytic behaviour. Appl. Catal. A Gen. 2001, 206, 221–229. [Google Scholar] [CrossRef]
- Raun, K.V.; Schumann, M.; Høj, M.; Thorhauge, M.; Beato, P.; Damsgaard, C.D.; Chevallier, J.; Nielsen, K.; Grundwaldt, J.-D.; Jensen, A.D. Studies of Deactivation of Methanol to Formaldehyde Selective Oxidation Catalyst. In Proceedings of the 13th European Congress on Catalysis, Florence, Italy, 27–31 August 2017. [Google Scholar]
- Raun, K.V.; Lundegaard, L.F.; Beato, P.; Appel, C.C.; Nielsen, K.; Thorhauge, M.; Schumann, M.; Jensen, A.D.; Grunwaldt, J.D.; Høj, M. Stability of Iron-Molybdate Catalysts for Selective Oxidation of Methanol to Formaldehyde: Influence of Preparation Method. Catal. Lett. 2020, 150, 1434–1444. [Google Scholar] [CrossRef]
- Gaur, A.; Schumann, M.; Raun, K.V.; Stehle, M.; Beato, P.; Jensen, A.D.; Grunwaldt, J.-D.; Høj, M. Operando XAS/XRD and Raman spectroscopy study of structural changes of the iron molybdate catalyst during selective oxidation of methanol. ChemCatChem 2019, 11, 4871–4883. [Google Scholar] [CrossRef]
- Gaur, A.; Stehle, M.; Raun, K.V.; Thrane, J.; Jensen, A.D.; Grunwaldt, J.-D.; Høj, M. Structural dynamics of an iron molybdate catalyst under redox cycling conditions studied with in situ multi edge XAS and XRD. Phys. Chem. Chem. Phys. 2020, 22, 11713–11723. [Google Scholar] [CrossRef] [PubMed]
- Carbucicchio, M.; Trifirò, F. Redox processes at the surfaces of Fe2O3-MoO3/SiO2 catalysts. J. Catal. 1980, 62, 13–18. [Google Scholar] [CrossRef]
- Carbucicchio, M.; Trifirò, F. Surface and bulk redox processes in iron-molybdate-based catalysts. J. Catal. 1976, 45, 77–85. [Google Scholar] [CrossRef]
- Pernicone, N. Deactivation of Fe-Mo oxide catalyst in industrial plant and simulation tests on laboratory scale. Catal. Today 1991, 11, 85–91. [Google Scholar] [CrossRef]
- Mitov, I.; Asenov, S.; Tomov, T.; Klissurski, D. In situ mössbauer study of the interaction of methanol with an iron-molybdenum oxide catalyst. J. Phys. Chem. C 2007, 111, 5389–5393. [Google Scholar] [CrossRef]
- O’Brien, M.G.; Beale, A.M.; Jacques, S.D.M.; Di Michiel, M.; Weckhuysen, B.M. Closing the operando gap: The application of high energy photons for studying catalytic solids at work. Appl. Catal. A Gen. 2011, 391, 468–476. [Google Scholar] [CrossRef]
- Raun, K.V.; Johannessen, J.; McCormack, K.; Appel, C.C.; Baier, S.; Thorhauge, M.; Høj, M.; Jensen, A.D. Modeling of the molybdenum loss in iron molybdate catalyst pellets for selective oxidation of methanol to formaldehyde. Chem. Eng. J. 2019, 361, 1285–1295. [Google Scholar] [CrossRef]
- Raun, K.V.; Thorhauge, M.; Høj, M.; Jensen, A.D. Modeling of molybdenum transport and pressure drop increase in fixed bed reactors used for selective oxidation of methanol to formaldehyde using iron molybdate catalysts. Chem. Eng. Sci. 2019, 202, 347–356. [Google Scholar] [CrossRef]
- Wachs, I.E.; Briand, L.E. In Situ Regeneration of Metal-Molybdate Catalysts for Methanol Oxidation to Formaldehyde. U.S. Patent No. 6,037,290, 14 March 2000. [Google Scholar]
- Burcham, L.J.; Briand, L.E.; Wachs, I.E. Quantification of Active Sites for the Determination of Methanol Oxidation Turn-over Frequencies Using Methanol Chemisorption and in Situ Infrared Techniques. 2. Bulk Metal Oxide Catalysts. Langmuir 2001, 17, 6175–6184. [Google Scholar] [CrossRef]
- Burcham, L.J.; Briand, L.E.; Wachs, I.E. Quantification of Active Sites for the Determination of Methanol Oxidation Turn-over Frequencies Using Methanol Chemisorption and in Situ Infrared Techniques. 1. Supported Metal Oxide Catalysts. Langmuir 2001, 17, 6164–6174. [Google Scholar] [CrossRef]
- Popov, B.I.; Shkuratova, L.N.; Orlova, L.B. Effect of excess molybdenum trioxide on the activity and selectivity of some molybdates in methanol oxidation. React. Kinet. Catal. Lett. 1976, 4, 323–328. [Google Scholar] [CrossRef]
- Shkuratova, L.N.; Pankratiev, Y.D.; Popov, B.I.; Turkov, V.M. Steady state catalytic properties and oxygen biding energy of cadmium-molybdenum catalysts in methanol oxidation. React. Kinet. Catal. Lett. 1977, 7, 229–233. [Google Scholar] [CrossRef]
- Sutula, V.D.; Zeif, A.P.; Popov, B.I.; Vadash, P.I. Indo study of the interaction of methanol with molybdates. React. Kinet. Catal. Lett. 1978, 9, 79–83. [Google Scholar] [CrossRef]
- Sutula, V.D.; Zeif, A.P.; Popov, B.I.; Chernyavskii, L.I. Interaction of methanol and propene with iron and gallium molybdates by the extended Hüchel method. J. Struct. Chem. 1979, 20, 204–212. [Google Scholar] [CrossRef]
- Popov, B.I.; Shkuratova, L.N.; Maksimov, Y.V.; Gustov, V.V. Catalytic properties and radiothermoluminescence of calcium molybdate with MoO3 additives. React. Kinet. Catal. Lett. 1982, 20, 293–297. [Google Scholar] [CrossRef]
- Malka, K.; Tatibouet, J. A Two-Step Preparation of Silica-Supported Calcium-Molybdenum Catalysts. J. Catal. 1998, 175, 204–212. [Google Scholar] [CrossRef]
- Thrane, J.; Lundegaard, L.F.; Beato, P.; Mentzel, U.V.; Thorhauge, M.; Jensen, A.D.; Høj, M. Alkali Earth Metal Molybdates as Catalysts for the Selective Oxidation of Methanol to Formaldehyde—Selectivity, Activity, and Stability. Catalysts 2020, 10, 82. [Google Scholar] [CrossRef] [Green Version]
- Said, A.E.A.A.; El-Wahab, M.M.M.A.; Alian, A.M. Selective Oxidation of Methanol to Formaldehyde over Active Molybdenum Oxide Supported on Hydroxyapatite Catalysts. Catal. Lett. 2016, 146, 82–90. [Google Scholar] [CrossRef]
- Said, A.A.; El-Wahab, M.M.A.; Alian, A.M. New approach on the catalytic oxidation of methanol to formaldehyde over MoO3 supported on nano hydroxyapatite catalysts. IOP Conf. Ser. Mater. Sci. Eng. 2014, 64, 012058. [Google Scholar] [CrossRef] [Green Version]
- Thrane, J.; Elvebakken, C.F.; Juelsholt, M.; Christiansen, T.L.; Jensen, K.M.Ø.; Hansen, L.P.; Lundegaard, L.F.; Mentzel, U.V.; Thorhauge, M.; Jensen, A.D.; et al. Highly Stable Apatite Supported Molybdenum Oxide Catalysts for Selective Oxidation of Methanol to Formaldehyde: Structure, Activity and Stability. ChemCatChem Accepted Author Manuscript. 2021. [Google Scholar] [CrossRef]
- Thrane, J. Investigation of Copper Based Catalysts by Chemisorption Methods; Technical University of Denmark: Lyngby, Denmark, 2015. [Google Scholar]
- Thrane, J.; Mentzel, U.V.; Thorhauge, M.; Høj, M.; Jensen, A.D. Hydroxyapatite Supported Molybdenum Oxide Catalyst for Selective Oxidation of Methanol to Formaldehyde: Studies of Industrial Sized Catalyst Pellets. Catal. Sci. Technol. 2021, 11, 970–983. [Google Scholar] [CrossRef]
- Kostynyuk, A.; Nikolenko, M. Iron molybdate catalyst stabilized by calcium oxide for methanol to formaldehyde conversion. Chem. Chem. Technol. 2011, 5, 89–93. [Google Scholar] [CrossRef]
- Popov, B.I.; Shkuratova, L.N.; Skorokhova, N.G. Influence of sodium salts on the catalytic properties of iron-molybdenum oxide catalysts in the oxidation of methanol to formaldehyde. React. Kinet. Catal. Lett. 1975, 3, 463–469. [Google Scholar] [CrossRef]
- Popov, T.S.; Popov, B.I.; Bibin, V.N.; Bliznakov, G.M.; Boreskov, G.K. Catalytic properties of chromium-molybdenum oxide catalysts in methanol oxidation. React. Kinet. Catal. Lett. 1975, 3, 169–175. [Google Scholar] [CrossRef]
- Popov, T.S.; Klissurski, D.G.; Ivanov, K.I.; Pesheva, J. Effect of ultrasonic treatment on the physicochemical properties of Cr-Mo-O catalysts for methanol oxidation. Stud. Surf. Sci. Catal. 1987, 31, 191–197. [Google Scholar]
- Cheshkova, K.T.; Bibin, V.N.; Popov, B.I. Kinetics of oxidation of methanol with air on a chromium-molybdenum oxide catalyst supported on porous a-Al2O3. React. Kinet. Catal. Lett. 1976, 4, 307–313. [Google Scholar] [CrossRef]
- Ivanov, K.; Krustev, S.; Litcheva, P. Oxidation of methanol on sodium modified chromium-molybdenum catalysts. J. Alloys Compd. 1998, 279, 132–135. [Google Scholar] [CrossRef]
- Ivanov, K.I.; Mitov, I.G.; Krustev, S.V.; Boyanov, B.S. Mössbauer study of modified iron-molybdenum catalysts for methanol oxidation. J. Phys. Conf. Ser. 2010, 217, 012046. [Google Scholar] [CrossRef] [Green Version]
- Meyer, A.; Renken, A. Sodium Compounds as Catalysts for Methanol Dehydrogenation to Water-Free Formaldehyde. Chem. Eng. Technol. 1990, 13, 145–149. [Google Scholar] [CrossRef]
- Del Arco, M.; Martin, C.; Rives, V.; Estevez, A.M.; Marquez, M.C.; Tena, A.F. Effect of doping with chromium on the physicochemical properties of iron-molybdenum oxide systems. J. Mater. Sci. 1989, 24, 3750–3755. [Google Scholar] [CrossRef]
- Klissurski, D.; Rives, V.; Pesheva, Y.; Mitov, I.; Abadzhjieva, N. Iron-chromium-molybdenum oxide catalysts for methanol oxidation. Catal. Lett. 1993, 18, 265–271. [Google Scholar] [CrossRef]
- Pesheva, Y.; Nemska, S.; Stefanov, P.; Klissurski, D.; Rives, V. Surface reduction of iron-chromium-molybdenum oxide catalysts for methanol oxidation. J. Mater. Sci. Lett. 1994, 13, 1567–1569. [Google Scholar] [CrossRef]
- Popov, B.I.; Bibin, V.N. Catalytic properties of bismuth molybdate and its constituent oxides in methanol oxidation. React. Kinet. Catal. Lett. 1975, 3, 337–341. [Google Scholar] [CrossRef]
- Arora, N.; Deo, G.; Wachs, I.E.; Hirt, A.M. Surface aspects of bismuth-metal oxide catalysts. J. Catal. 1996, 159, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Mann, R.S.; Hahn, K.W. Kinetics of vapor-phase oxidation of methyl alcohol on Manganese Dioxide-Molybdenum Trioxide Catalyst. J. Catal. 1969, 15, 329–341. [Google Scholar] [CrossRef]
- Mann, R.S.; Hahn, K.W. Oxidation of Methanol Over Manganese Dioxide-Molybdenum Trioxide Catalyst. Ind. Eng. Chem. Process Des. Dev. 1970, 9, 43–46. [Google Scholar] [CrossRef]
- Ivanov, K.; Litcheva, P.; Popov, T. Thermal Stability of MnMoO4-MoO3 Catalysts for Methanol Oxidation. J. Therm. Anal. 1990, 36, 1361–1368. [Google Scholar] [CrossRef]
- Ivanov, K.; Litcheva, P.; Klissurski, D. Mn-Mo-O Catalysts for Methanol Oxidation. II. Oxidation of Methanol. Collect. Czechoslov. Chem. Commun. 1992, 57, 2539–2547. [Google Scholar] [CrossRef]
- Ivanov, K.; Litcheva, P. Mn-Mo-O Catalysts for methanol oxidation. I. Preparation and Characterization of catalysts. Collect. Czechoslov. Chem. Commun. 1992, 57, 2529–2538. [Google Scholar] [CrossRef]
- Thorhauge, M. Internal Reports—Haldor Topsoe A/S 2017; Haldor Topsøe: Lyngby, Denmark, 2017. [Google Scholar]
- Mann, R.S.; Jain, S.K.; Dosi, M.K.; Mann, S.; Jain, S.K.; Dosi, M.K. Catalytic Oxidation of Methanol over Molybdenum Oxide-Tungsten Oxide. J. Appl. Chem. Biotechnol. 1977, 27, 198–204. [Google Scholar] [CrossRef]
- Machiels, C.J.; Cheng, W.H.; Chowdhry, U.; Farneth, W.E.; Hong, F.; Mc Carron, E.M.; Sleight, A.W. The effect of the structure of molybdenum oxides on the selective oxidation of methanol. Appl. Catal. 1986, 25, 249–256. [Google Scholar] [CrossRef]
- Ivanov, K.; Mitov, I.; Krustev, S. Selective oxidation of methanol on Fe-Mo-W catalysts. J. Alloys Compd. 2000, 309, 57–60. [Google Scholar] [CrossRef]
- Ramachandra, B.; Choi, J.S.; Choo, K.Y.; Sung, J.S.; Song, S.D.; Kim, T.H. Partial oxidation of methanol to formaldehyde on molybdenum based mixed oxide catalyst. Catal. Lett. 2005, 105, 23–27. [Google Scholar] [CrossRef]
- Klissurski, D.; Pesheva, Y.; Abadjieva, N.; Mitov, I.; Filkova, D.; Petrov, L. Multicomponent oxide catalysts for the oxidation of methanol to formaldehyde. Appl. Catal. 1991, 77, 55–66. [Google Scholar] [CrossRef]
- Klissurski, D.; Petridis, D.; Abadzhieva, N.; Hadjiivanov, K. MoO3 supported on montmorillonite type pillared clays: Characterization, surface acidity and catalytic properties towards the oxidation of methanol. Appl. Clay Sci. 1996, 10, 451–459. [Google Scholar] [CrossRef]
- Mann, R.S.; Dosi, M.K. Kinetics of the Vapor-Phase Oxidation of Methyl Alcohol on Vanadium Pentoxide-Molybdenum Trioxide Catalyst. J. Catal. 1973, 28, 282–288. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Niwa, M.; Murakami, Y. Morphology of molybdena supported on various oxides and its activity for methanol oxidation. J. Phys. Chem. 1990, 94, 1477–1482. [Google Scholar] [CrossRef]
- Burcham, L.J.; Badlani, M.; Wachs, I.E. The Origin of the Ligand Effect in Metal Oxide Catalysts: Novel Fixed-Bed in Situ Infrared and Kinetic Studies during Methanol Oxidation. J. Catal. 2001, 203, 104–121. [Google Scholar] [CrossRef] [Green Version]
- Banares, M.A.; Hu, H.; Wachs, I.E. Molybdena on Silica Catalysts: Role of Preparation Methos on the Structure-Selectivity Properties for the Oxidation of Methanol. J. Catal. 1994, 150, 407–420. [Google Scholar] [CrossRef]
- Inokawa, H.; Zaman, S.; Driss, H.; Daous, M.; Al-zahrani, A. Partial Oxidation of Methanol over Oxides of Cr, Mo and W Supported on Mixed CeO2-ZrO2 Carrier. In Proceedings of the EUROPA CAT, Basel, Switzerland, 7–11 August 2017. [Google Scholar]
- Niwa, M.; Mizutani, M.; Murakami, Y. Measurement of M+5 spectra during methanol oxidation. Chem. Lett. 1975, 4, 1295–1298. [Google Scholar] [CrossRef] [Green Version]
- Niwa, M.; Mizutani, M.; Murakami, Y. Oxidation of Methanol over SnO2-MoO3-Catalysts. Nippun Kagaku Kaishi 1977, 6, 757–760. [Google Scholar] [CrossRef] [Green Version]
- Niwa, M.; Mizutani, M.; Takahashi, M.; Murakami, Y. Mechanism of methanol oxidation over oxide catalysts containing MoO3. J. Catal. 1981, 70, 14–23. [Google Scholar] [CrossRef]
- Niwa, M.; Igarashi, J.Y. Role of the solid acidity on the MoO3 loaded on SnO2 in the methanol oxidation into formaldehyde. Catal. Today 1999, 52, 71–81. [Google Scholar] [CrossRef]
- Niwa, M.; Habuta, Y.; Okumura, K.; Katada, N. Solid acidity of metal oxide monolayer and its role in catalytic reactions. Catal. Today 2003, 87, 213–218. [Google Scholar] [CrossRef]
- Niwa, M.; Yamada, H.; Murakami, Y. Activity for the oxidation of methanol of a molybdena monolayer supported on tin oxide. J. Catal. 1992, 134, 331–339. [Google Scholar] [CrossRef]
- Narishige, N.; Niwa, M. Adsorbed species of methanol on zirconia support and molybdenum oxide monolayer. Its role in the methanol oxidation. Catal. Lett. 2001, 71, 63–67. [Google Scholar] [CrossRef]
- Valente, N.G.; Arrúa, L.A.; Cadús, L.E. Structure and activity of Sn-Mo-O catalysts: Partial oxidation of methanol. Appl. Catal. A Gen. 2001, 205, 201–214. [Google Scholar] [CrossRef]
- Mann, R.S.; Diaz-Real, R.A. Oxidation of Methanol to Formaldehyde over Antimony-Molybdenum Oxide. In Proceedings of the 10th International Congress on Catalysis, Budapest, Hungary, 19–24 July 1993; pp. 1991–1994. [Google Scholar]
- Castillo, R.; Dewaele, K.; Ruiz, P.; Delmon, B. Mechanical mixtures of alfa-Sb2O4 and MoO3 as highly selective catalysts for the oxidation of methanol to formaldehyde. Appl. Catal. A Gen. 1997, 153, L1–L8. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Cadus, L.E.; Daza, L.; Bertrand, P.; Ladri, J. Solid-state reactivity of iron molybdate artificially contaminated by antimony ions and its relation with catalytic activity in the selective oxidation of isobutene to methacrolein. Top. Catal. 2000, 12, 167–180. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Weng, L.T.; Bertrand, P.; Ladrière, J.; Daza, L.; Ruiz, P.; Delmon, B. Synergy between α-SB2O4 and Fe2(MoO4)3 during the first hours of the catalytic oxidation of isobutene to methacrolein. J. Mol. Catal. A Chem. 2000, 155, 59–71. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Castillo, R.; Papadopoulou, C.; Daza, L.; Ladrière, J.; Ruiz, P.; Delmon, B. The Protecting Role of Antimony Oxide Against Deactivation of Iron Molybdate in Oxidation Catalysts. Stud. Surf. Sci. Catal. 1991, 68, 425–432. [Google Scholar]
- Chernyshkova, F.A. Heteropolyacids and their salts as new promising catalysts for petrochemical and organic synthesis. Pet. Chem. 1991, 31, 571–584. [Google Scholar]
- Whiting, G.T.; Bartley, J.K.; Dummer, N.F.; Hutchings, G.J.; Taylor, S.H. Vanadium promoted molybdenum phosphate catalysts for the vapour phase partial oxidation of methanol to formaldehyde. Appl. Catal. A Gen. 2014, 485, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Harrison, W.T.A.; Cheetham, A.K.; Faber, J. The Crystal Determined Structure of Aluminum by Time-of-Flight Powder Neutron. J. Solid State Chem. 1988, 76, 328–333. [Google Scholar] [CrossRef] [Green Version]
- Bowker, M.; Hellier, P.; Decarolis, D.; Gianolio, D.; Mohammed, K.M.H.; Stenner, A.; Hulthwelker, T.; Wells, P.P. Al-doped Fe2O3 as a support for molybdenum oxide methanol oxidation catalysts. Phys. Chem. Chem. Phys. 2020, 22, 18911–18918. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghanem, H. Evaluation of Nanoparticles Iron-Molybdate Catalyst Using Physical Properties Measurements; Wichita State University: Wichita, KS, USA, 2006. [Google Scholar]
- Pham, T.T.P.; Nguyen, P.H.D.; Vo, T.T.; Luu, C.L.; Nguyen, H.H.P. Preparation of NO-doped β-MoO3 and its methanol oxidation property. Mater. Chem. Phys. 2016, 184, 5–11. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Keller, D.E. Chemistry, spectroscopy and the role of supported vanadium oxides in heterogeneous catalysis. Catal. Today 2003, 78, 25–46. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, M.P.; Raj, A. Novel synthesis of a vanadium—Titanium aluminophosphate molecular sieve of MFI structure (VTAPO-5) and catalytic activity for the partial oxidation of methanol. Chem. Commun. 1999, 15, 1409–1410. [Google Scholar] [CrossRef]
- Behera, G.C.; Parida, K. Selective gas phase oxidation of methanol to formaldehyde over aluminum promoted vanadium phosphate. Chem. Eng. J. 2012, 180, 270–276. [Google Scholar] [CrossRef]
- Behera, G.C.; Parida, K.; Dummer, N.F.; Whiting, G.; Sahu, N.; Carley, A.F.; Conte, M.; Hutchings, G.J.; Bartley, J.K. Tungstate promoted vanadium phosphate catalysts for the gas phase oxidation of methanol to formaldehyde. Catal. Sci. Technol. 2013, 3, 1558–1564. [Google Scholar] [CrossRef]
- Klissurski, D.; Pesheva, Y. Comparative Study of the Catalytic Properties of V2O5, Nb2O5 and Ta2O5 in the Oxidation of Methanol to Formaldehyde. React. Kinet. Catal. Lett. 1986, 32, 77–82. [Google Scholar] [CrossRef]
- Smith, M.A.; Zoelle, A.; Yang, Y.; Rioux, R.M.; Hamilton, N.G.; Amakawa, K.; Nielsen, P.K.; Trunschke, A. Surface roughness effects in the catalytic behavior of vanadia supported on SBA-15. J. Catal. 2014, 312, 170–178. [Google Scholar] [CrossRef]
- Smith, M.A.; Lobo, R.F. A fractal description of pore structure in block-copolymer templated mesoporous silicates. Microporous Mesoporous Mater. 2010, 131, 204–209. [Google Scholar] [CrossRef]
- Hess, C. Characterization of the synthesis and reactivity behavior of nanostructured vanadia model catalysts using XPS and vibrational spectroscopy. Surf. Sci. 2006, 600, 3695–3701. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Du, G.; Lim, S.; Haller, G.L. Radius of curvature effect of V-MCM-41 probed by methanol oxidation. J. Catal. 2005, 234, 318–327. [Google Scholar] [CrossRef]
- Yang, Y.; Lim, S.; Wang, C.; Harding, D.; Haller, G. Multivariate correlation and prediction of the synthesis of vanadium substituted mesoporous molecular sieves. Microporous Mesoporous Mater. 2004, 67, 245–257. [Google Scholar] [CrossRef]
- Bronkema, J.L.; Bell, A.T. Mechanistic Studies of Methanol Oxidation to Formaldehyde on Isolated Vanadate Sites Supported on MCM-48. J. Phys. Chem. C 2007, 111, 420–430. [Google Scholar] [CrossRef]
- Bronkema, J.L.; Bell, A.T.; Leo, D.C.; Bell, A.T. Mechanistic Studies of Methanol Oxidation to Formaldehyde on Isolated Vanadate Sites Supported on High Surface Area Anatase. J. Phys. Chem. C 2007, 111, 14530–14540. [Google Scholar] [CrossRef]
- Döbler, J.; Pritzsche, M.; Sauer, J. Oxidation of methanol to formaldehyde on supported vanadium oxide catalysts compared to gas phase molecules. J. Am. Chem. Soc. 2005, 127, 10861–10868. [Google Scholar] [CrossRef]
- Trejda, M.; Millot, Y.; Chalupka, K.; Dzwigaj, S. Preparation of two series of VxSiBeta zeolite catalysts with V centres in framework and extra-framework positions and their application in selective oxidation of methanol. Appl. Catal. A Gen. 2019, 579, 1–8. [Google Scholar] [CrossRef]
- Vieira, L.; Possato, L.; Chaves, T.; Lee, J.; Sulmonetti, T.; Jones, C.; Martins, L. This Insights into redox dynamics of vanadium species impregnated in layered siliceous zeolitic structures during methanol oxidation reactions. ChemCatChem Catal. 2020, 12, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wu, P.; Liu, G.; Zhu, Z.; Zeng, G. Co-Electrospun VTiOx Hollow Nanofibers for Selective Oxidation of Methanol to High Value Chemicals. ACS Appl. Nano Mater. 2019, 2, 5224–5232. [Google Scholar] [CrossRef]
- Agarwal, D.C.; Nigam, P.C.; Srivastava, R.D. Kinetics of Vapor Phase Oxidation of Methyl Alcohol over Supported V2O5-K2SO4 Catalyst. J. Catal. 1978, 55, 1–9. [Google Scholar] [CrossRef]
- Roozeboom, F.; Cordingley, P.D.; Gellings, P.J. Vanadium Oxide Monolayer Catalysts The Vapor-Phase Oxidation of Methanol. J. Catal. 1981, 68, 464–472. [Google Scholar] [CrossRef]
- Deo, G.; Wachs, I.E.; Goutam, D. Reactivity of Supported Vanadium Oxide Catalysts: The Partial Oxidation of Methanol. J. Catal. 1994, 146, 323–334. [Google Scholar] [CrossRef]
- Fievez, T.; Weckhuysen, B.M. Chemical reactivity indices as a tool for understanding the support-effect in supported metal oxide catalysts. J. Phys. Chem. C 2009, 11, 19905–19912. [Google Scholar] [CrossRef]
- Kaichev, V.V.; Popova, G.Y.; Chesalov, Y.A.; Saraev, A.A.; Andrushkevich, T.V.; Bukhtiyarov, V.I. Active component of supported vanadium catalysts in the selective oxidation of methanol. Kinet. Catal. 2016, 57, 82–94. [Google Scholar] [CrossRef]
- Andrushkevich, T.V.; Kaichev, V.V.; Chesalov, Y.A.; Saraev, A.A.; Buktiyarov, V.I. Selective oxidation of ethanol over vanadia-based catalysts: The influence of support material and reaction mechanism. Catal. Today 2017, 279, 95–106. [Google Scholar] [CrossRef]
- Burcham, L.J.; Wachs, I.E. The origin of the support effect in supported metal oxide catalysts: In situ infrared and kinetic studies during methanol oxidation. Catal. Today 1999, 49, 467–484. [Google Scholar] [CrossRef]
- Kim, T.; Wachs, I.E. CH3OH oxidation over well-defined supported V2O5/Al2O3 catalysts: Influence of vanadium oxide loading and surface vanadium-oxygen functionalities. J. Catal. 2008, 255, 197–205. [Google Scholar] [CrossRef]
- Khaliullin, R.Z.; Bell, A.T. A density functional theory study of the oxidation of methanol to formaldehyde over vanadia supported on silica, titania, and zirconia. J. Phys. Chem. B 2002, 106, 7832–7838. [Google Scholar] [CrossRef] [Green Version]
- Zhanpeisov, N.U. A density functional theory study of the oxidation of methanol to formaldehyde over vanadia supported on silica, titania and zirconia. Res. Chem. Intermed. 2004, 30, 133–141. [Google Scholar] [CrossRef]
- Makedonski, L.; Nikolov, V.; Anastasov, A.; Stancheva, M. Effect of calcination temperature on the properties of industrial V2O5-TiO2 (anatase) catalysts in methanol oxidation. React. Kinet. Catal. Lett. 2004, 81, 21–25. [Google Scholar] [CrossRef]
- Bronkema, J.L.; Bell, A.T. Mechanistic studies of methanol oxidation to formaldehyde on isolated vanadate sites supported on high surface area zirconia. J. Phys. Chem. C 2008, 112, 6404–6412. [Google Scholar] [CrossRef]
- Goodrow, A.; Bell, A.T. A Theoretical Investigation of the Selective Oxidation of Methanol to Formaldehyde on Isolated Vanadate Species Supported on Titania. J. Phys. Chem. C 2008, 112, 13204–13214. [Google Scholar] [CrossRef]
- González-Navarrete, P.; Gracia, L.; Calatayud, M.; Andrés, J. Unraveling the mechanisms of the selective oxidation of methanol to formaldehyde in vanadia supported on titania catalyst. J. Phys. Chem. C 2010, 114, 6039–6046. [Google Scholar] [CrossRef]
- Busca, G.; Elmi, A.S.; Forzatti, P. Mechanism of selective methanol oxidation over vanadium oxide-titanium oxide catalysts: A FT-IR and flow reactor study. J. Phys. Chem. 1987, 91, 5263–5269. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, H.M.; Pala, R.G.S.; Metiu, H. Oxidative dehydrogenation of methanol to formaldehyde by isolated vanadium, molybdenum, and chromium oxide clusters supported on rutile TiO2(110). J. Phys. Chem. C 2009, 113, 16083–16093. [Google Scholar] [CrossRef]
- Ganduglia-Pirovano, M.V.; Popa, C.; Sauer, J.; Abbott, H.; Uhl, A.; Baron, M.; Stacchiola, D.; Bondarchuk, O.; Shaikhutdinov, S.; Freund, H.J. Role of ceria in oxidative dehydrogenation on supported vanadia catalysts. J. Am. Chem. Soc. 2010, 132, 2345–2349. [Google Scholar] [CrossRef]
- Kropp, T.; Paier, J.; Sauer, J. Oxidative dehydrogenation of methanol at ceria-supported vanadia oligomers. J. Catal. 2017, 352, 382–387. [Google Scholar] [CrossRef]
- Kropp, T.; Paier, J.; Sauer, J. Support effect in oxide catalysis: Methanol oxidation on vanadia/ceria. J. Am. Chem. Soc. 2014, 136, 14616–14625. [Google Scholar] [CrossRef]
- Feng, T.; Vohs, J.M. A TPD study of the partial oxidation of methanol to formaldehyde on CeO2-supported vanadium oxide. J. Catal. 2004, 221, 619–629. [Google Scholar] [CrossRef]
- Vining, W.C.; Strunk, J.; Bell, A.T. Investigation of the structure and activity of VOx/CeO2/SiO2 catalysts for methanol oxidation to formaldehyde. J. Catal. 2012, 285, 160–167. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Feng, Z.; Li, C. Effective silica supported Sb-V mixed oxide catalyst for selective oxidation of methanol to formaldehyde. J. Catal. 2008, 260, 295–304. [Google Scholar] [CrossRef]
- Guerrero-Pérez, M.O.; Kim, T.; Ban, M.A.; Wachs, I.E. Nature of Catalytic Active Sites for Sb-V-O Mixed Metal Oxides. J. Phys. Chem. C 2008, 112, 16858–16863. [Google Scholar] [CrossRef]
- Isaguliants, G.V.; Belomestnykh, I.P. Selective oxidation of methanol to formaldehyde over V-Mg-O catalysts. Catal. Today 2005, 100, 441–445. [Google Scholar] [CrossRef]
- Nielsen, N.D.; Thrane, J.; Jensen, A.D.; Christensen, J.M. Bifunctional Synergy in CO Hydrogenation to Methanol with Supported Cu. Catal. Lett. 2020, 150, 1427–1433. [Google Scholar] [CrossRef]
- Lakshmi, L.J.; Narsimha, K.; Rao, P.K. Chemisorptive and Catalytic Properties of V2O5 Supported on Phosphate Modified Gamma-alumina. Appl. Catal. A Gen. 1993, 94, 61–70. [Google Scholar] [CrossRef]
- Bliznakov, G.; Pesheva, Y.; Klissurski, D.; Marinov, M.; Kozhukharov, V. Methanol Oxidation on V2O5-MoO3-TeO3. Appl. Catal. 1987, 29, 211–218. [Google Scholar] [CrossRef]
- Pesheva, Y.; Abadzhjieva, N.; Vrachnou, E.; Kovanis, Y.; Rives, V.; del Hoyo, C.; Klissurski, D. Selective oxidation of methanol on V2O5 and V2O5 MoO 3 supported on montrmorillonite. React. Kinet. Catal. Lett. 1994, 53, 283–288. [Google Scholar] [CrossRef]
- Sturm, J.M.; Göbke, D.; Kuhlenbeck, H.; Döbler, J.; Reinhardt, U.; Ganduglia-Pirovano, M.V.; Sauer, J.; Freund, H.-J. Partial oxidation of methanol on well-ordered V2O5(001)/Au(111) thin films. Phys. Chem. Chem. Phys. 2009, 11, 3010. [Google Scholar] [CrossRef]
- Reddy, B.M. Structure and Reactivity of Tin Oxide Supported Vanadium Oxide Catalysts. ACS Symp. Ser. 1993, 523, 204–216. [Google Scholar]
- Wang, C.-T.; Chen, M.-T.; Lai, D.-L. Vanadium-tin oxide nanoparticles with Gas-sensing and Catalytic Activity. J. Am. Ceram. Soc. 2011, 94, 4471–4477. [Google Scholar] [CrossRef]
- Malinski, R.; Akimoto, M.; Echigoya, E. Catalytic activity of Vanadates in oxidation of Methanol. J. Catal. 1976, 44, 101–106. [Google Scholar] [CrossRef]
- Maliński, R. The catalytic activity of Ni-V oxide catalysts. React. Kinet. Catal. Lett. 1976, 5, 265–271. [Google Scholar] [CrossRef]
- Salagre, P.; Sueiras, J.E. Hexagonal Orthovanadates as Catalysts in the Oxidation of Methanol to Formaldehyde. J. Chem. Soc. Chem. Commun. 1988, 16, 1084–1085. [Google Scholar] [CrossRef]
- Häggblad, R.; Wagner, J.B.; Hansen, S.; Andersson, A. Oxidation of methanol to formaldehyde over a series of Fe1-xAlx-V-oxide catalysts. J. Catal. 2008, 258, 345–355. [Google Scholar] [CrossRef]
- Häggblad, R.; Hansen, S.; Wallenberg, L.R.; Andersson, A. Stability and performance of vacant Fe3-x-yVx□yO4 spinel phase catalysts in methanol oxidation. J. Catal. 2010, 276, 24–37. [Google Scholar] [CrossRef]
- Massa, M.; Häggblad, R.; Andersson, A. Cation Vacant Fe3−x−yVx□yO4 Spinel-Type Catalysts for the Oxidation of Methanol to Formaldehyde. Top. Catal. 2011, 54, 685–697. [Google Scholar] [CrossRef]
- Massa, M.; Häggblad, R.; Hansen, S.; Andersson, A. Oxidation of methanol to formaldehyde on cation vacant Fe-V-Mo-oxide. Appl. Catal. A Gen. 2011, 408, 63–72. [Google Scholar] [CrossRef]
- Klissurski, D.; Abadzhieva, N.; Kassabov, S.; Stefanov, P.; Kovacheva, D.; Uzunov, I. Selective oxidation of methanol to formaldehyde on iron vanadate catalyst. Comptes Rendus L’Academie Bulg. Sci. 2009, 62, 1073–1078. [Google Scholar]
- Routray, K.; Zhou, W.; Kiely, C.J.; Wachs, I.E. Catalysis science of methanol oxidation over iron vanadate catalysts: Nature of the Catalytic Active sites. ACS Catal. 2011, 1, 54–66. [Google Scholar] [CrossRef]
- Wachs, I.E.; Routray, K. Catalysis science of bulk mixed oxides. ACS Catal. 2012, 2, 1235–1246. [Google Scholar] [CrossRef]
- Hellier, P.; Wells, P.P.; Gianolio, D.; Bowker, M. VOx/Fe2O3 Shell-Core Catalysts for the Selective Oxidation of Methanol to Formaldehyde. Top. Catal. 2018, 61, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Hellier, P.; Wells, P.P.; Bowker, M. Methanol oxidation over shell-core MOx/Fe2O3 (M=Mo, V, Nb) catalysts. Chin. J. Catal. 2019, 40, 1686–1692. [Google Scholar] [CrossRef]
- Malmusi, A.; Velasquez Ochoa, J.; Tabanelli, T.; Basile, F.; Lucarelli, C.; Agnoli, S.; Carraro, F.; Granozzi, G.; Cavani, F. Ethanol aerobic and anaerobic oxidation with FeVO4 and V2O5 catalysts. Appl. Catal. A Gen. 2018, 570, 139–147. [Google Scholar] [CrossRef]
- Kaminski, P.; Ziolek, M. Surface and catalytic properties of Ce-, Zr-, Au-, Cu-modified SBA-15. J. Catal. 2014, 312, 249–262. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Lunin, V.V.; Bogdanchikova, N.; Temkin, O.N.; Smolentseva, E. Active states of gold in small and big metal particles in CO and methanol selective oxidation. Fuel 2013, 110, 48–53. [Google Scholar] [CrossRef]
- Kaskow, I.; Wojtaszek-Gurdak, A.; Sobczak, I. Methanol oxidation on AuAg-Zn/MCM-36—The effect of catalyst components and pretreatment. Catal. Today 2019, 354, 1–10. [Google Scholar] [CrossRef]
- Deng, X.; Sorescu, D.C.; Lee, J. Methanol Oxidation to Formaldehyde Promoted at the Step Sites of Ultrathin ZnO. Top. Catal. 2018, 61, 499–508. [Google Scholar] [CrossRef]
- El-Molla, S.A.; Mahmoud, H.R. Synthesis, textural and catalytic properties of nanosized Fe2O3/MgO system. Mater. Res. Bull. 2013, 48, 4105–4111. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, H.; Imoto, H.; Shido, T.; Iwasawa, Y. Performance and Characterization of a New Crystalline SbRe2O6 Catalyst for Selective Oxidation of Methanol to Methylal. J. Catal. 2000, 195, 51–61. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Taylor, S.H. Designing oxidation catalysts. Catal. Today 1999, 49, 105–113. [Google Scholar] [CrossRef]
- Harrison, W.T.A.; Chowdhry, U.; Machiels, C.J.; Sleight, A.W.; Cheetham, A.K. Preparation of ferric tungstate and its catalytic behavior toward methanol. J. Solid State Chem. 1985, 60, 101–106. [Google Scholar] [CrossRef]
- Wachs, I.E.; Jehng, J.-M.; Ueda, W. Determination of the Chemical Nature of Active Surface Sites Present on Bulk Mixed Metal Oxide Catalysts. J. Phys. Chem. B 2005, 109, 2275–2284. [Google Scholar] [CrossRef]
- Badlani, M.; Wachs, I.E. Methanol: A “smart” chemical probe molecule. Catal. Lett. 2001, 75, 137–149. [Google Scholar] [CrossRef]
- Delgado, D.; Soriano, M.D.; Solsona, B.; Zamora, S.; Agouram, S.; Concepción, P.; López Nieto, J.M. Tungsten-titanium mixed oxide bronzes: Synthesis, characterization and catalytic behavior in methanol transformation. Appl. Catal. A Gen. 2019, 582, 117092. [Google Scholar] [CrossRef]
- Abadzhieva, N.; Klisurski, D.G. Oxidation of Methanol to Formaldehyde on Sb2O4. Kinet. Katal. 1987, 28, 735–736. [Google Scholar]
- Chen, Y.; Fierro, J.L.G.; Tanaka, T.; Wachs, I.E. Supported Tantalum Oxide Catalysts: Synthesis, Physical Characterization, and Methanol Oxidation Chemical Probe Reaction. J. Phys. Chem. B 2003, 107, 5243–5250. [Google Scholar] [CrossRef]
- Chen, Y.; Wachs, I.E. Tantalum oxide-supported metal oxide (Re2O7, CrO3, MoO3, WO3, V2O5, and Nb2O5) catalysts: Synthesis, Raman characterization and chemically probed by methanol oxidation. J. Catal. 2003, 217, 468–477. [Google Scholar] [CrossRef]
- Gao, X.; Wachs, I.E.; Wong, M.S.; Ying, J.Y. Structural and Reactivity Properties of Nb-MCM-41: Comparison with That of Highly Dispersed Nb2O5/SiO2 Catalysts. J. Catal. 2001, 203, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Davies, A.M.; Taylor, S.H. CATL 15 Nb-Phosphates as Catalysts for the Partial Oxidation og Methanol to Formaldehyde. In Proceedings of the 236th National Meeting and Exposition, Philadelphia, PA, USA, 17–21 August 2008; American Chemical Society: Philadelphia, PA, USA, 2008. [Google Scholar]
- Davies, A.M. Selective Oxidation and Oxidative Dehydrogenation Reactions Using Niobium Based Catalysts; Cardiff University: Cardiff, UK, 2009. [Google Scholar]
- Hashimoto, K.; Hanada, Y.; Minami, Y.; Kera, Y. Conversion of methanol to dimethyl ether and formaldehyde over alumina intercalated in a montmorillonite. Appl. Catal. A Gen. 1996, 141, 57–69. [Google Scholar] [CrossRef]
- Cairati, L.; Trifirò, F. SiO2 and Al2O3 as oxidation catalysts of methanol. J. Catal. 1983, 80, 25–30. [Google Scholar] [CrossRef]
- Gryaznova, Z.V.; Ponomareva, N.N.; Nefedova, A.R.; Yeschenko, L.S.; Dvoskina, R.N.; Yakovenko, Z.I. Methanol transformations on zirconium phosphate, CuX zeolite and their mixtures. React. Kinet. Catal. Lett. 1982, 19, 393–396. [Google Scholar] [CrossRef]
- Klissurski, D.; Rives, V.; Abadzhjieva, N.; Pesheva, Y.; Pomonis, P.; Sdoukos, T.; Petrakis, D. High performance of iron(III) phosphate for selective oxidation of methanol. J. Chem. Soc. Chem. Commun. 1993, 21, 1606–1607. [Google Scholar] [CrossRef]
- Abadzhjieva, N.; Tzokov, P.; Uzunov, I.; Minkov, V.; Klissurski, D.I.; Rives, V. Methanol Oxidation to Formaldehyde on Bismuth Phosphate-Based Catalysts. React. Kinet. Catal. Lett. 1994, 53, 413–418. [Google Scholar] [CrossRef]
- Nikolenko, N.V.; Kozhevnikov, I.V.; Kostyniuk, A.O.; Bayahia, H.; Kalashnykov, Y.V. Preparation of iron molybdate catalysts for methanol to formaldehyde oxidation based on ammonium molybdoferrate(II) precursor. J. Saudi Chem. Soc. 2018, 22, 372–379. [Google Scholar] [CrossRef]
- Zhao, H.; Bennici, S.; Shen, J.; Auroux, A. Influence of the host oxide of sulfated-titania catalysts on partial oxidation methanol reaction. Appl. Catal. A Gen. 2010, 385, 224–231. [Google Scholar] [CrossRef]
- Kropp, T.; Paier, J. Activity versus Selectivity of the Methanol Oxidation at Ceria Surfaces: A Comparative First-Principles Study. J. Phys. Chem. C 2015, 119, 23021–23031. [Google Scholar] [CrossRef]
- Sutton, J.E.; Danielson, T.; Beste, A.; Savara, A. Below-Room-Temperature C-H Bond Breaking on an Inexpensive Metal Oxide: Methanol to Formaldehyde on CeO2(111). J. Phys. Chem. Lett. 2017, 8, 5810–5814. [Google Scholar] [CrossRef] [PubMed]
- Capdevila-Cortada, M.; López, N. Descriptor Analysis in Methanol Conversion on Doped CeO2(111): Guidelines for Selectivity Tuning. ACS Catal. 2015, 5, 6473–6480. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; An, K.; Sapi, A.; Liu, F.; Somorjai, G.A. Effects of nanoparticle size and metal/support interactions in pt-catalyzed methanol oxidation reactions in gas and liquid phases. Catal. Lett. 2014, 144, 1930–1938. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Varma, A. Low-temperature selective oxidation over Pt-Bi bimetallic catalysts. J. Catal. 2018, 363, 144–153. [Google Scholar] [CrossRef]
- Sun, Y.K.; Lee, W.Y. Catalytic behavior of YBa2Cu3O7-x in the partial oxidation of methanol to formaldehyde. Korean J. Chem. Eng. 1995, 12, 36–38. [Google Scholar] [CrossRef]
- Velu, S.; Wang, L.; Okazaki, M.; Suzuki, K.; Tomura, S. Characterization of MCM-41 mesoporous molecular sieves containing copper and zinc and their catalytic performance in the selective oxidation of alcohols to aldehydes. Microporous Mesoporous Mater. 2002, 54, 113–126. [Google Scholar] [CrossRef]
- Li, C.-L.; Wang, C.-L.; Lin, Y.-C. Pd-integrated lanthanum-transition metal perovskites for methanol partial oxidation. Catal. Today 2011, 174, 135–140. [Google Scholar] [CrossRef]
- Li, C.L.; Jiang, B.S.; Fanchiang, W.L.; Lin, Y.C. The effect of Pd content in LaMnO3 for methanol partial oxidation. Catal. Commun. 2011, 16, 165–169. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, C.-B.; Wachs, I.E. Reaction induced spreading of metal oxides: In situ Raman spectroscopic studies during oxidation reactions. Stud. Surf. Sci. Catal. 1997, 110, 255–264. [Google Scholar]
- Gao, X.; Bare, S.R.; Weckhuysen, B.M.; Wachs, I.E. In Situ Spectroscopic Investigation of Molecular Structures of Highly Dispersed Vanadium Oxide on Silica under Various Conditions. J. Phys. Chem. B 1998, 102, 10842–10852. [Google Scholar] [CrossRef] [Green Version]
- Jehng, J.-M.; Hu, H.; Gao, X.; Wachs, I.E. The dynamic states of silica-supported metal oxide catalysts during methanol oxidation. Catal. Today 1996, 28, 335–350. [Google Scholar] [CrossRef]
- Bradley, D.C. Metal Alkoxides. In Progress in Inorganic Chemistry; Cotton, F.A., Ed.; John Wiley & Sons Inc.: New York, NY, USA; London, UK, 1960; Volume II, pp. 303–362. [Google Scholar]













































| Authors | Year | Expression | Comments |
|---|---|---|---|
| Pernicone et al. [51] (Bhattacharyya et al. [56]) a | 1969 (1967) | m = n = 0.5 (m = n = 1) | |
| Bibin and Popov [57] | 1969 | ||
| Evemenenko and Gorokhovatskii [58] | 1968 | ||
| Santacesaria et al. [59] | 1981 | ||
| Holstein and Machiels [54] | 1996 | x = 0.94 ± 0.06, y = 0.10 ± 0.05, z = −0.45 ± 0.07 | |
| Deshmukh et al. [60] | 2005 | ||
| Ulukardeslar et al. [61] | 2010 |
| Catalyst | Mo- and V-loss (%/m2 Surface Area) |
|---|---|
| MoO3/Fe2(MoO4)3 | 9.3 |
| Fe2(MoO4)3 | 2.3 |
| Cr2(MoO4)3 | 6.2 |
| Zr(MoO4)3 | 9.7 |
| FeVO4 | 1.9 |
| AlVO4 | 4.8 |
| Mn3(VO4)2 | 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thrane, J.; Mentzel, U.V.; Thorhauge, M.; Høj, M.; Jensen, A.D. A Review and Experimental Revisit of Alternative Catalysts for Selective Oxidation of Methanol to Formaldehyde. Catalysts 2021, 11, 1329. https://doi.org/10.3390/catal11111329
Thrane J, Mentzel UV, Thorhauge M, Høj M, Jensen AD. A Review and Experimental Revisit of Alternative Catalysts for Selective Oxidation of Methanol to Formaldehyde. Catalysts. 2021; 11(11):1329. https://doi.org/10.3390/catal11111329
Chicago/Turabian StyleThrane, Joachim, Uffe V. Mentzel, Max Thorhauge, Martin Høj, and Anker D. Jensen. 2021. "A Review and Experimental Revisit of Alternative Catalysts for Selective Oxidation of Methanol to Formaldehyde" Catalysts 11, no. 11: 1329. https://doi.org/10.3390/catal11111329
APA StyleThrane, J., Mentzel, U. V., Thorhauge, M., Høj, M., & Jensen, A. D. (2021). A Review and Experimental Revisit of Alternative Catalysts for Selective Oxidation of Methanol to Formaldehyde. Catalysts, 11(11), 1329. https://doi.org/10.3390/catal11111329








