The Study of C3H6 Impact on Selective Catalytic Reduction by Ammonia (NH3-SCR) Performance over Cu-SAPO-34 Catalysts
Abstract
:1. Introduction
2. Results
2.1. Structure and Physical Properties of Cu-SAPO-34 Catalysts
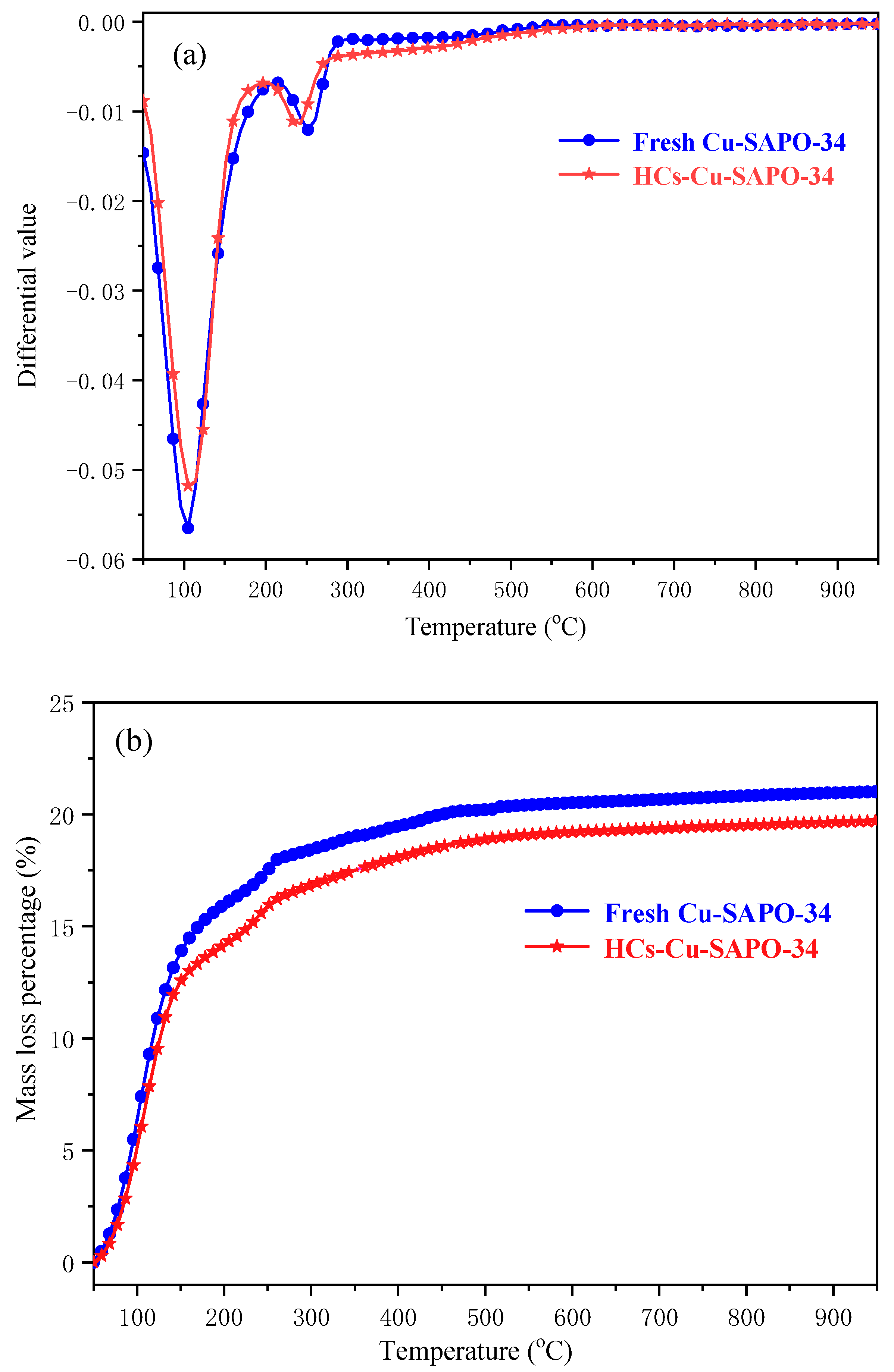
2.2. The TG Experiments
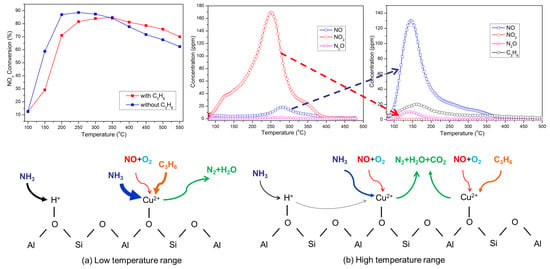
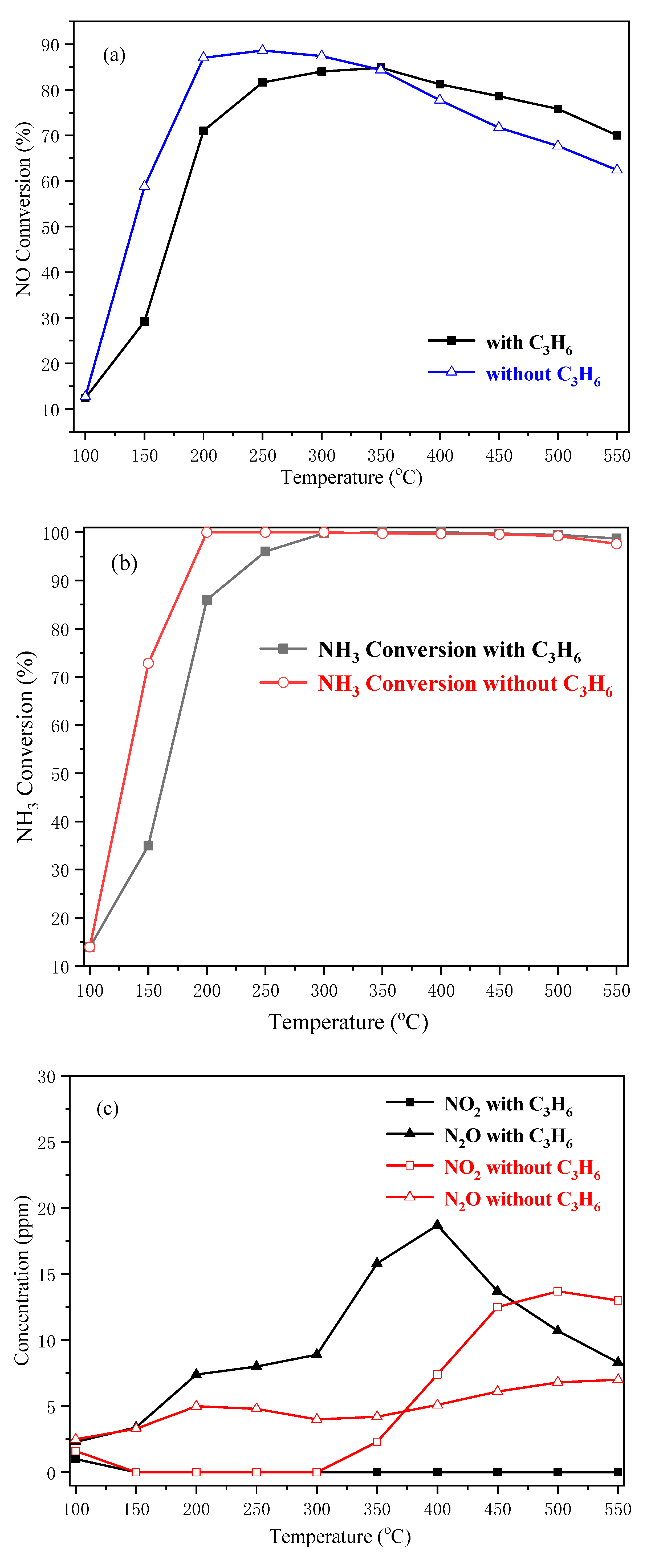
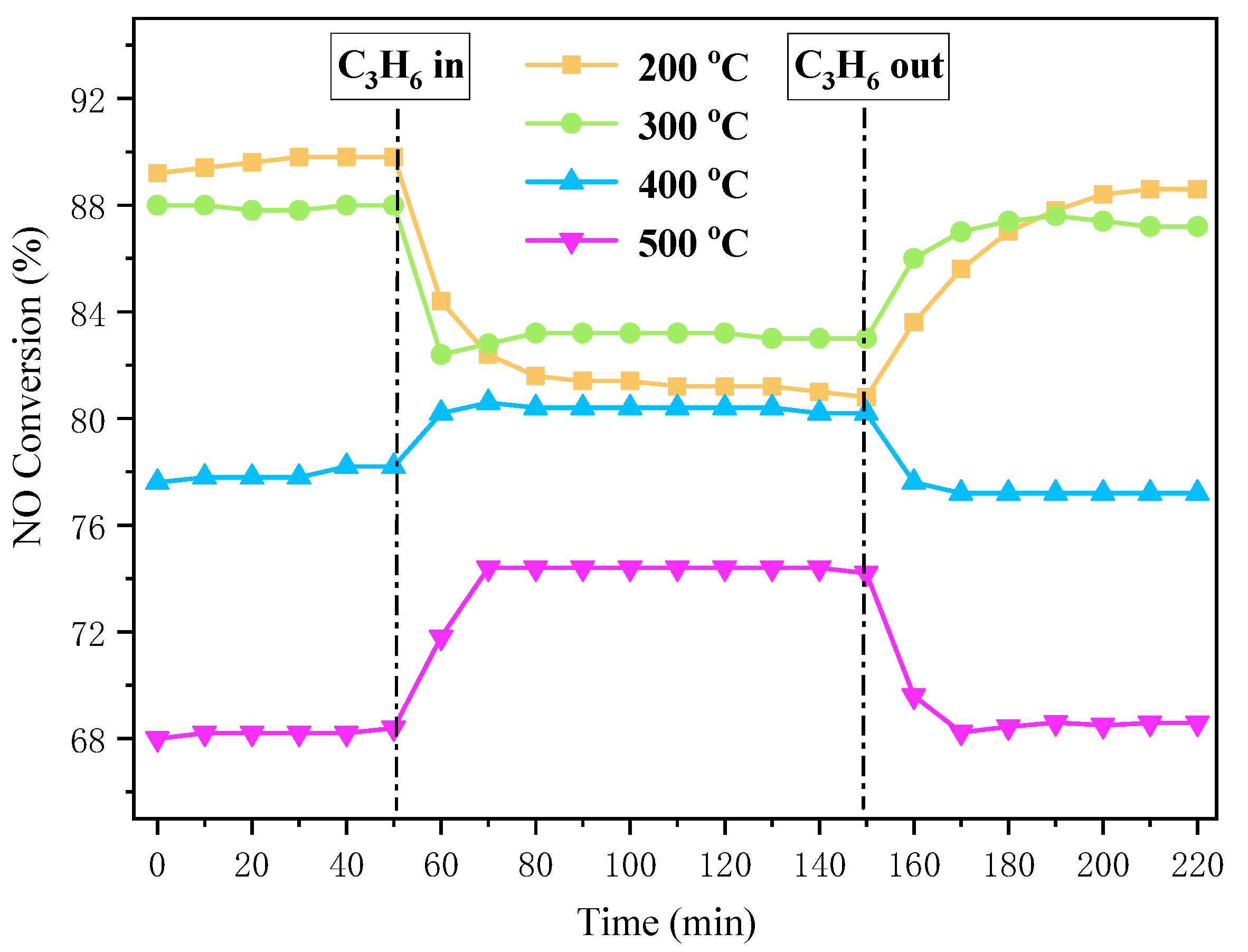
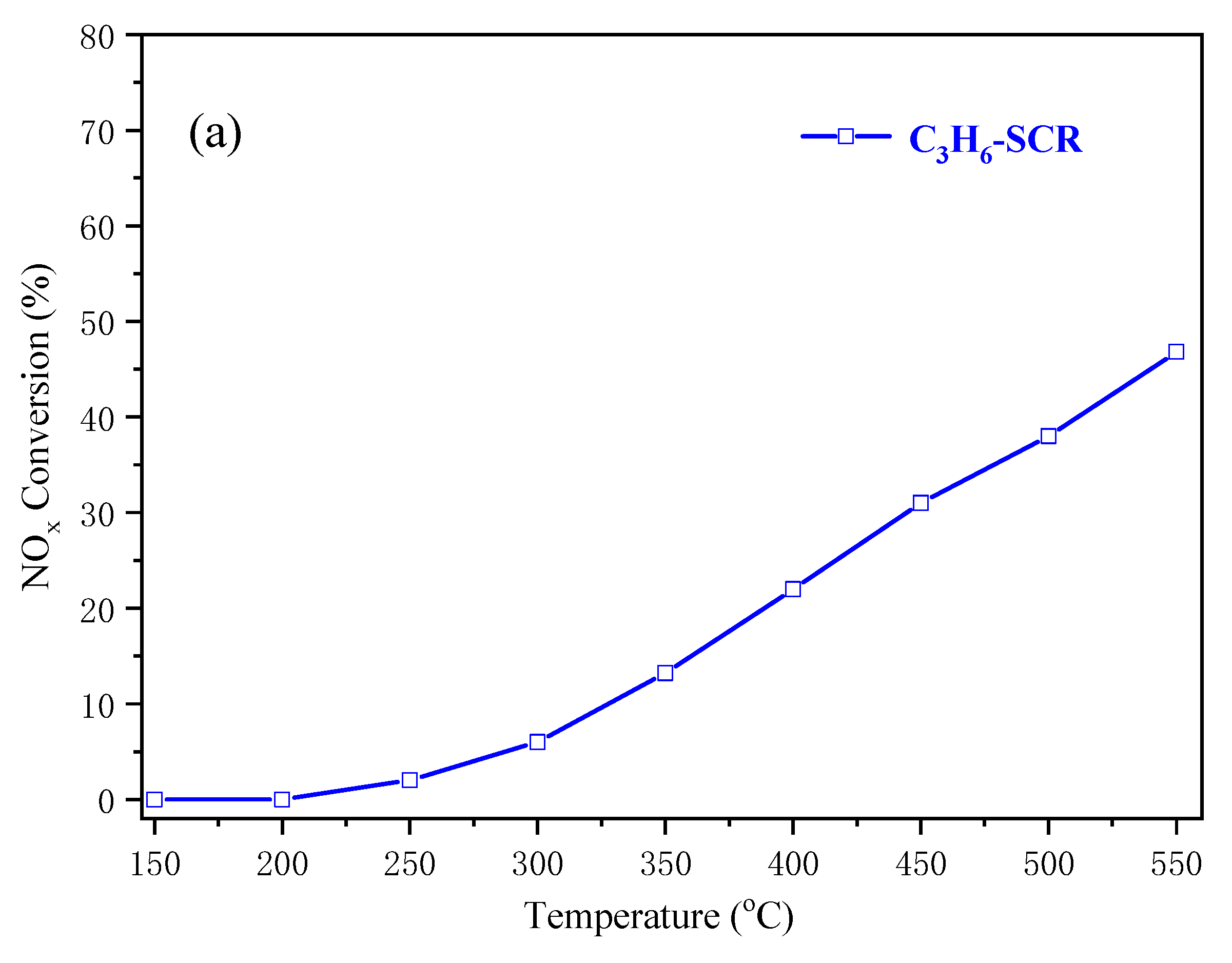
2.3. NH3-SCR Activity of Cu-SAPO-34 Catalysts
2.4. The Adsorption Performance of Reactants
2.4.1. NH3-TPD
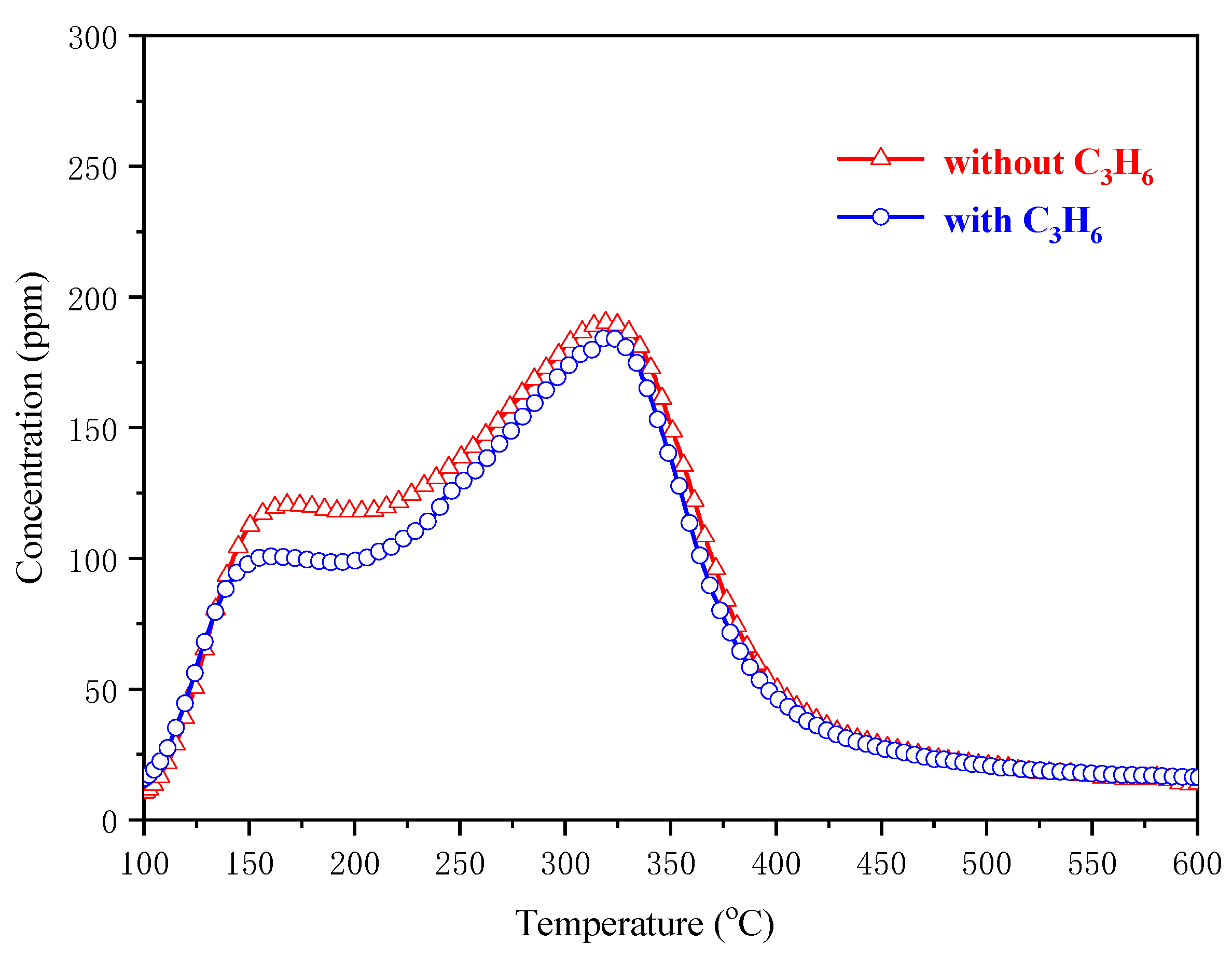
2.4.2. NOx-TPD Experiments
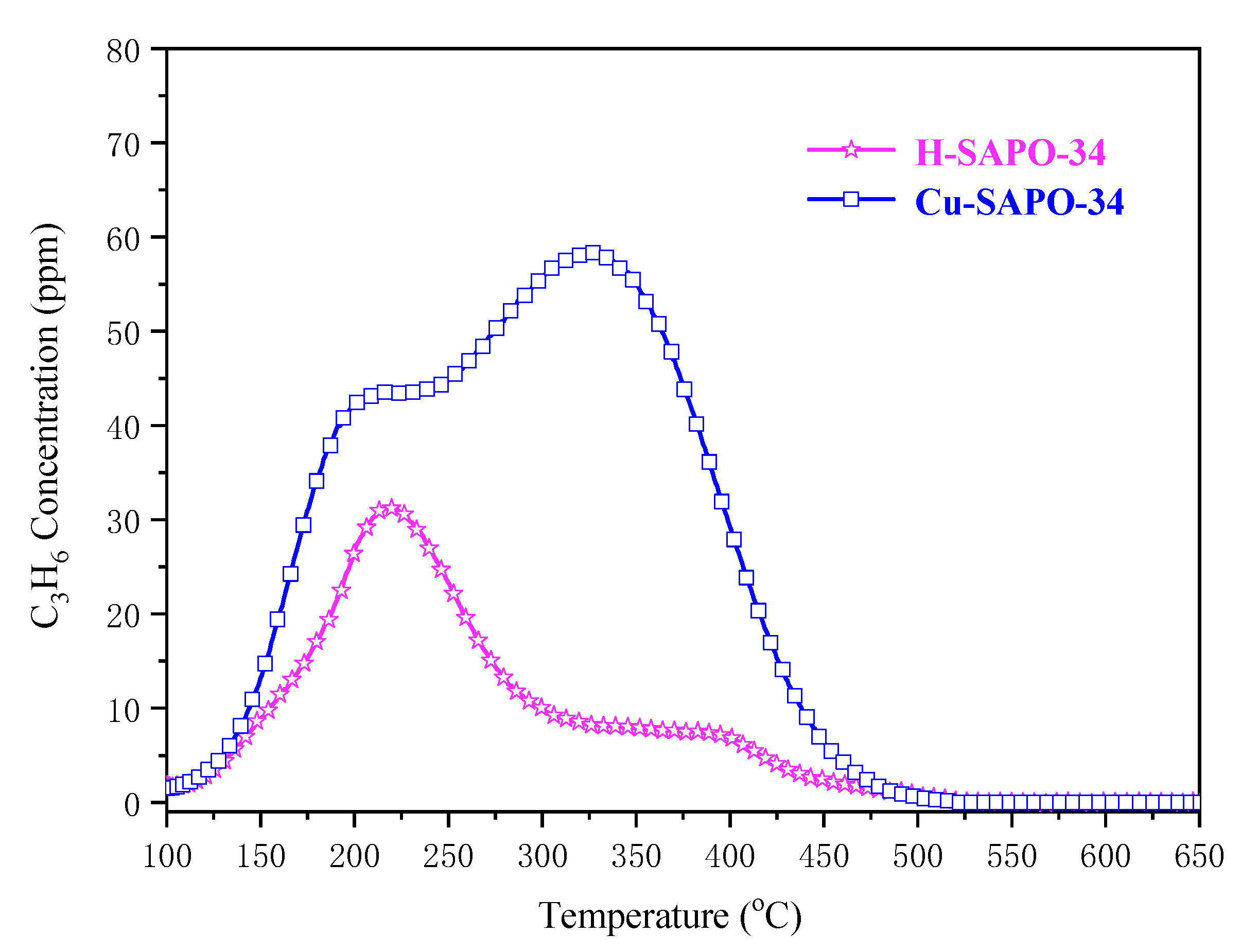
2.4.3. C3H6-TPD Experiments
2.5. The DRIFTS Experiments
2.5.1. Co-Adsorption of NH3 and C3H6 Species
2.5.2. Co-Adsorption of NO and C3H6 Species
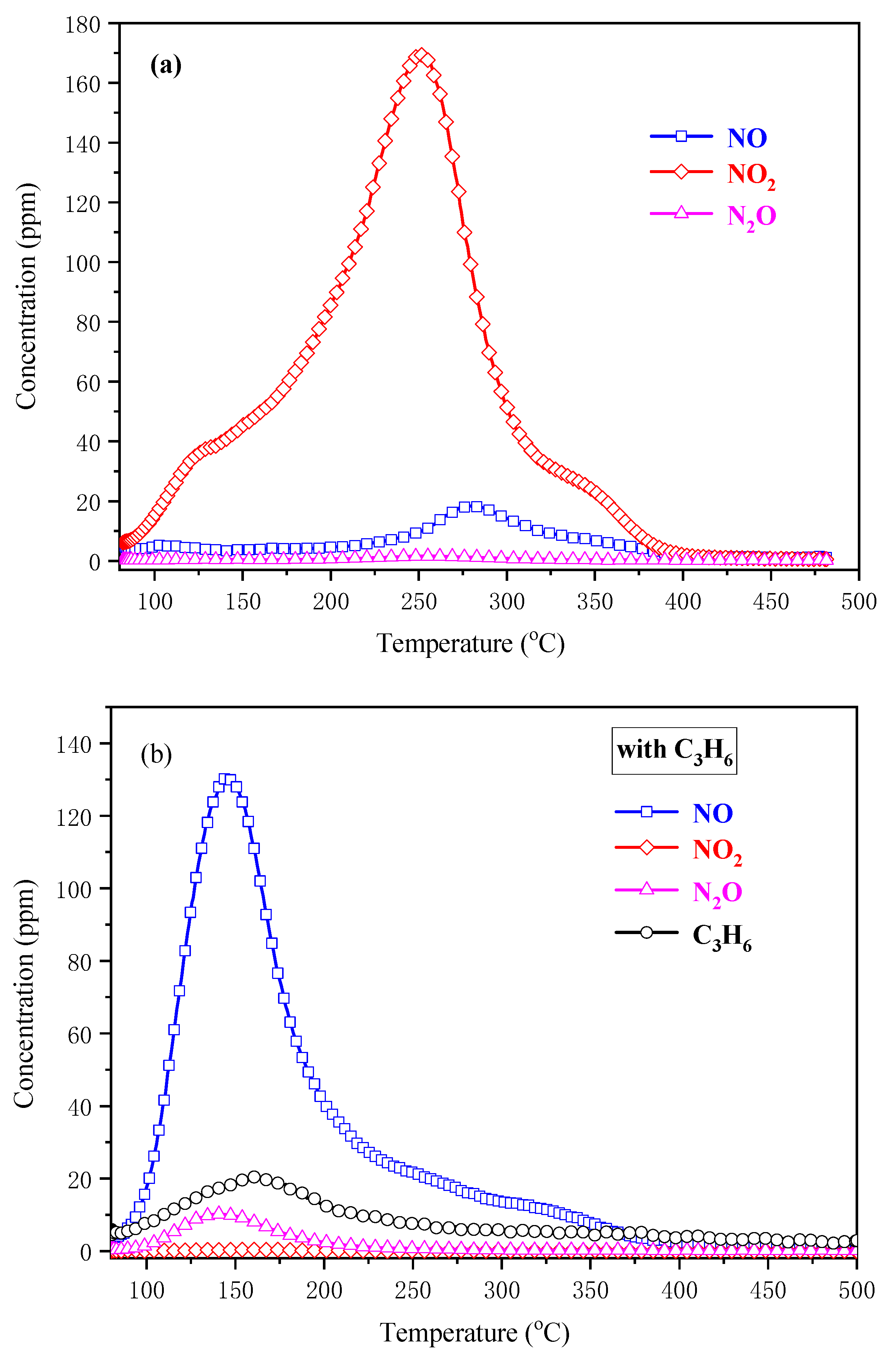
2.5.3. The In Situ DRIFTS Experiments
2.6. The Contribution of C3H6 to NH3-SCR Performance
3. Experiments
3.1. Catalyst Preparation
3.2. Catalytic Performance Measurement
3.3. Catalyst Characterization
3.4. In Situ DRIFTS Experiments
4. Discussion
4.1. The Effect of C3H6 Treatment on Catalyst Structure
4.2. The C3H6 Effect on the Adsorption Performance of NH3, NO Species
4.3. The C3H6 Effect on the SCR Performance over Cu-SAPO-34 Catalyst
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, B.; Zhao, S.; Wang, Y.; Wenren, Y.; Zhu, Z.; Harding, J.; Zhang, X.; Tu, X.; Zhang, X. Plasma-enhanced low temperature NH3-SCR of NOx over a Cu-Mn/SAPO-34 catalyst under oxygen-rich conditions. Appl. Catal. B 2021, 286, 119886. [Google Scholar] [CrossRef]
- Beale, A.M.; Gao, F.; Lezcano-Gonzalez, I.; Peden, C.H.; Szanyi, J. Recent advances in automotive catalysis for NOx emission control by small-pore microporous. Chem. Soc. Rev. 2015, 44, 7371–7405. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Jiang, H.; Peng, X.; Wei, Y.; Liu, Z.; Chen, T.; Lin, H.; Huang, Z. Promotional effect and mechanism of the modification of Ce on the enhanced NH3-SCR efficiency and the low temperature hydrothermal stability over Cu/SAPO-34 catalysts. Appl. Catal. A 2021, 617, 118110. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Haller, G.; Li, Y. Recent advances in the selective catalytic reduction of NOx with NH3 on Cu-Chabazite catalysts. Appl. Catal. B 2017, 202, 346–354. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Zhao, Z.; Liao, J.; Chen, C.; Li, Q. Recent progress of metal-exchanged zeolites for selective catalytic reduction of NOx with NH3 in diesel exhaust. Fuel 2021, 305, 121482. [Google Scholar] [CrossRef]
- Mi, Y.; Li, G.; Zheng, Y.; Luo, Y.; Liu, W.; Li, Z.; Wu, D.; Peng, H. Insights into novel mesoporous Cu-SAPO-34 with enhanced de-NOx performance for diesel emission control. Microporous Mesoporous Mater. 2021, 323, 111245. [Google Scholar] [CrossRef]
- Chapman, D.M. Behavior of titania-supported vanadia and tungsta SCR catalysts at high temperatures in reactant streams: Tungsten and vanadium oxide and hydroxide vapor pressure reduction by surficial stabilization. Appl. Catal. A 2011, 392, 143–150. [Google Scholar] [CrossRef]
- Casanova, M.; Schermanz, K.; Llorca, J.; Trovarelli, A. Improved high temperature stability of NH3-SCR catalysts based on rare earth vanadates supported on TiO2WO3SiO2. Catal. Today 2012, 184, 227–236. [Google Scholar] [CrossRef]
- Shi, A.; Wang, X.; Yu, T.; Shen, M. The effect of zirconia additive on the activity and structure stability of V2O5/WO3-TiO2 ammonia SCR catalysts. Appl. Catal. B 2011, 106, 359–369. [Google Scholar] [CrossRef]
- Kim, J.; Jentys, A.; Maier, S.M.; Lercher, J.A. Characterization of Fe-Exchanged BEA Zeolite Under NH3 Selective Catalytic Reduction Conditions. J. Phys. Chem. C 2013, 117, 986–993. [Google Scholar] [CrossRef]
- Wang, P.; Yu, D.; Zhang, L.; Ren, Y.; Jin, M.; Lei, L. Evolution mechanism of NOx in NH3-SCR reaction over Fe-ZSM-5 catalyst: Species-performance relationships. Appl. Catal. A 2020, 607, 117806. [Google Scholar] [CrossRef]
- Wang, X.T.; Hu, H.P.; Zhang, X.Y.; Su, X.X.; Yang, X.D. Effect of iron loading on the performance and structure of Fe/ZSM-5 catalyst for the selective catalytic reduction of NO with NH3. Environ. Sci. Pollut. Res. Int. 2019, 26, 1706–1715. [Google Scholar] [CrossRef]
- Brandenberger, S.; Kröcher, O.; Casapu, M.; Tissler, A.; Althoff, R. Hydrothermal deactivation of Fe-ZSM-5 catalysts for the selective catalytic reduction of NO with NH3. Appl. Catal. B 2011, 101, 649–659. [Google Scholar] [CrossRef]
- Shwan, S.; Nedyalkova, R.; Jansson, J.; Korsgren, J.; Olsson, L.; Skoglundh, M. Hydrothermal Stability of Fe-BEA as an NH3-SCR Catalyst. Ind. Eng. Chem. Res. 2012, 51, 12762–12772. [Google Scholar] [CrossRef]
- Shwan, S.; Nedyalkova, R.; Jansson, J.; Korsgren, J.; Olsson, L.; Skoglundh, M. Influence of Hydrothermal Ageing on NH3-SCR Over Fe-BEA-Inhibition of NH3-SCR by Ammonia. Top. Catal. 2013, 56, 80–88. [Google Scholar] [CrossRef]
- Zhang, S.; Pang, L.; Chen, Z.; Ming, S.; Dong, Y.; Liu, Q.; Liu, P.; Cai, W.; Li, T. Cu/SSZ-13 and Cu/SAPO-34 catalysts for deNOx in diesel exhaust: Current status, challenges, and future perspectives. Appl. Catal. A 2020, 607, 117855. [Google Scholar] [CrossRef]
- Mohan, S.; Dinesha, P.; Kumar, S. NOx reduction behaviour in copper zeolite catalysts for ammonia SCR systems: A review. Chem. Eng. J. 2020, 384, 123253. [Google Scholar] [CrossRef]
- Schmieg, S.J.; Oh, S.H.; Kim, C.H.; Brown, D.B.; Lee, J.H.; Peden, C.H.F.; Kim, D.H. Thermal durability of Cu-CHA NH3-SCR catalysts for diesel NOx reduction. Catal. Today 2012, 184, 252–261. [Google Scholar] [CrossRef]
- Bates, S.A.; Verma, A.A.; Paolucci, C.; Parekh, A.A.; Anggara, T.; Yezerets, A.; Schneider, W.F.; Miller, J.T.; Delgass, W.N.; Ribeiro, F.H. Identification of the active Cu site in standard selective catalytic reduction with ammonia on Cu-SSZ-13. J. Catal. 2014, 312, 87–97. [Google Scholar] [CrossRef]
- Deka, U.; Juhin, A.; Eilertsen, E.A.; Emerich, H.; Green, M.A.; Korhonen, S.T.; Weckhuysen, B.M.; Beale, A.M. Confirmation of Isolated Cu2+Ions in SSZ-13 Zeolite as Active Sites in NH3-Selective Catalytic Reduction. J. Phys. Chem. C 2012, 116, 4809–4818. [Google Scholar] [CrossRef]
- Wang, J.; Yu, T.; Wang, X.; Qi, G.; Xue, J.; Shen, M.; Li, W. The influence of silicon on the catalytic properties of Cu/SAPO-34 for NOx reduction by ammonia-SCR. Appl. Catal. B 2012, 127, 137–147. [Google Scholar] [CrossRef]
- Paolucci, C.; Verma, A.A.; Bates, S.A.; Kispersky, V.F.; Miller, J.T.; Gounder, R.; Delgass, W.N.; Ribeiro, F.H.; Schneider, W.F. Isolation of the Copper Redox Steps in the Standard Selective Catalytic Reduction on Cu-SSZ-13. Angew. Chem. Int. Ed. 2014, 53, 11828–11833. [Google Scholar] [CrossRef]
- Zhu, H.; Kwak, J.H.; Peden, C.H.F.; Szanyi, J. In situ DRIFTS-MS studies on the oxidation of adsorbed NH3 by NOx over a Cu-SSZ-13 zeolite. Catal. Today 2013, 205, 16–23. [Google Scholar] [CrossRef]
- Su, W.; Chang, H.; Peng, Y.; Zhang, C.; Li, J. Reaction Pathway Investigation on the Selective Catalytic Reduction of NO with NH3 over Cu/SSZ-13 at Low Temperatures. Environ. Sci. Technol. 2015, 49, 467–473. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, J.; Yu, T.; Shen, M.; Wang, J. The role and activity of various adsorbed ammonia species on Cu/SAPO-34 catalyst during passive-SCR process. RSC Adv. 2015, 5, 14103–14113. [Google Scholar] [CrossRef]
- Yu, T.; Hao, T.; Fan, D.; Wang, J.; Shen, M.; Li, W. Recent NH3-SCR Mechanism Research over Cu/SAPO-34 Catalyst. J. Phys. Chem. C 2014, 118, 6565–6575. [Google Scholar] [CrossRef]
- Yao, D.; Liu, B.; Wu, F.; Li, Y.; Hu, X.; Jin, W.; Wang, X. N2O Formation Mechanism During Low-Temperature NH3-SCR over Cu-SSZ-13 Catalysts with Different Cu Loadings. Ind. Eng. Chem. Res. 2021, 60, 10083–10093. [Google Scholar] [CrossRef]
- Xi, Y.; Ottinger, N.A.; Keturakis, C.J.; Liu, Z.G. Dynamics of low temperature N2O formation under SCR reaction conditions over a Cu-SSZ-13 catalyst. Appl. Catal. B 2021, 294, 120245. [Google Scholar] [CrossRef]
- Feng, Y.; Janssens, T.V.W.; Vennestrøm, P.N.R.; Jansson, J.; Skoglundh, M.; Grönbeck, H. The Role of H+- and Cu+-Sites for N2O Formation during NH3-SCR over Cu-CHA. J. Phys. Chem. C 2021, 125, 4595–4601. [Google Scholar] [CrossRef]
- Zhang, T.; Qin, X.; Peng, Y.; Wang, C.; Chang, H.; Chen, J.; Li, J. Effect of Fe precursors on the catalytic activity of Fe/SAPO-34 catalysts for N2O decomposition. Catal. Commun. 2019, 128, 105706. [Google Scholar] [CrossRef]
- Meng, T.; Ren, N.; Ma, Z. Effect of copper precursors on the catalytic performance of Cu-ZSM-5 catalysts in N2O decomposition. Chin. J. Chem. Eng. 2018, 26, 1051–1058. [Google Scholar] [CrossRef]
- Wang, L.; Gaudet, J.R.; Li, W.; Weng, D. Migration of Cu species in Cu/SAPO-34 during hydrothermal aging. J. Catal. 2013, 306, 68–77. [Google Scholar] [CrossRef]
- Ma, L.; Cheng, Y.; Cavataio, G.; McCabe, R.W.; Fu, L.; Li, J. Characterization of commercial Cu-SSZ-13 and Cu-SAPO-34 catalysts with hydrothermal treatment for NH3-SCR of NOx in diesel exhaust. Chem. Eng. J. 2013, 225, 323–330. [Google Scholar] [CrossRef]
- Kumar, A.; Smith, M.A.; Kamasamudram, K.; Currier, N.W.; An, H.; Yezerets, A. Impact of different forms of feed sulfur on small-pore Cu-zeolite SCR catalyst. Catal. Today 2014, 231, 75–82. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Liu, Y.; Kamasamudram, K.; Li, J.; Epling, W. SO2 poisoning impact on the NH3-SCR reaction over a commercial Cu-SAPO-34 SCR catalyst. Appl. Catal. B 2014, 156–157, 371–377. [Google Scholar] [CrossRef]
- Ma, L.; Su, W.; Li, Z.; Li, J.; Fu, L.; Hao, J. Mechanism of propene poisoning on Cu-SSZ-13 catalysts for SCR of NOx with NH3. Catal. Today 2014, 245, 16–21. [Google Scholar] [CrossRef]
- Malpartida, I.; Marie, O.; Bazin, P.; Daturi, M.; Jeandel, X. An operando IR study of the unburnt HC effect on the activity of a commercial automotive catalyst for NH3-SCR. Appl. Catal. B 2011, 102, 190–200. [Google Scholar] [CrossRef]
- Sultana, A.; Nanba, T.; Sasaki, M.; Haneda, M.; Suzuki, K.; Hamada, H. Selective catalytic reduction of NOx with NH3 over different copper exchanged zeolites in the presence of decane. Catal. Today 2011, 164, 495–499. [Google Scholar] [CrossRef]
- Nanba, T.; Sultana, A.; Masukawa, S.; Haneda, M.; Uchisawa, J.; Obuchi, A.; Hamada, H. High Resistance of Cu–Ferrierite to Coke Formation During NH3-SCR in the Presence of n-Decane. Top. Catal. 2009, 52, 1766–1770. [Google Scholar] [CrossRef]
- Ma, L.; Li, J.; Cheng, Y.; Lambert, C.K.; Fu, L. Propene poisoning on three typical Fe-zeolites for SCR of NOx with NH3: From mechanism study to coating modified architecture. Environ. Sci. Technol. 2012, 46, 1747–1754. [Google Scholar] [CrossRef]
- Li, J.; Zhu, R.; Cheng, Y.; Lambert, C.K.; Yang, R.T. Mechanism of Propene Poisoning on Fe-ZSM-5 for Selective Catalytic Reduction of NOx with Ammonia. Environ. Sci. Technol. 2010, 44, 1799–1805. [Google Scholar] [CrossRef]
- Heo, I.; Lee, Y.; Nam, I.-S.; Choung, J.W.; Lee, J.-H.; Kim, H.-J. Effect of hydrocarbon slip on NO removal activity of Cu-ZSM-5, Fe-ZSM-5 and V2O5/TiO2 catalysts by NH3. Microporous Mesoporous Mater. 2011, 141, 8–15. [Google Scholar] [CrossRef]
- Wang, J.; Ji, Y.; He, Z.; Crocker, M.; Dearth, M.; McCabe, R.W. A non-NH3 pathway for NOx conversion in coupled LNT-SCR systems. Appl. Catal. B 2012, 111–112, 562–570. [Google Scholar] [CrossRef]
- Kim, D.J.; Wang, J.; Crocker, M. Adsorption and desorption of propene on a commercial Cu-SSZ-13 SCR catalyst. Catal. Today 2014, 231, 83–89. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, D.; Yang, Y.; Jiang, X.; Tian, Y.; Ding, T.; Li, X. Influence of Copper Locations on Catalytic Properties and Activities of Cu/SAPO-34 in C3H6-SCR. Ind. Eng. Chem. Res. 2021, 60, 6940–6949. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, H.; Wang, C.; Zhao, H.; Wen, N.; Huang, S.; Zhou, X.; Zhang, H.; Deng, W.; Su, Y. NO selective catalytic reduction with propylene over one-pot synthesized Fe-SAPO-34 catalyst under diesel exhaust conditions. Fuel 2021, 290, 119822. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Matyshak, V.A. Selective Catalytic Reduction of Nitrogen Oxides by Hydrocarbons in an Excess of Oxygen. Russ. J. Phys. Chem. A 2021, 95, 475–491. [Google Scholar] [CrossRef]
- Luo, J.Y.; Oh, H.; Henry, C.; Epling, W. Effect of C3H6 on selective catalytic reduction of NOx by NH3 over a Cu/zeolite catalyst. A mechanistic study. Appl. Catal. B 2012, 123–124, 296–305. [Google Scholar] [CrossRef]
- Lin, B.; Zhang, J.; Shi, H.; Chen, Z.; Jiang, B. Mechanism of the hydrocarbon resistance of selective catalytic reduction catalysts supported on different zeolites. Catal. Sci. Technol. 2021, 11, 1758–1765. [Google Scholar] [CrossRef]
- Kumar, A.; Kamasamudram, K.; Yezerets, A. Hydrocarbon Storage on Small-Pore Cu-Zeolite SCR Catalyst. SAE Int. 2013, 6, 680–687. [Google Scholar] [CrossRef]
- Gramigni, F.; Iacobone, U.; Nasello, N.D.; Selleri, T.; Usberti, N.; Nova, I. Review of Hydrocarbon Poisoning and Deactivation Effects on Cu-Zeolite, Fe-Zeolite, and Vanadium-Based Selective Catalytic Reduction Catalysts for NOx Removal from Lean Exhausts. Ind. Eng. Chem. Res. 2021, 60, 6403–6420. [Google Scholar] [CrossRef]
- Xue, J.; Wang, X.; Qi, G.; Wang, J.; Shen, M.; Li, W. Characterization of copper species over Cu/SAPO-34 in selective catalytic reduction of NOx with ammonia: Relationships between active Cu sites and de-NOx performance at low temperature. J. Catal. 2013, 297, 56–64. [Google Scholar] [CrossRef]
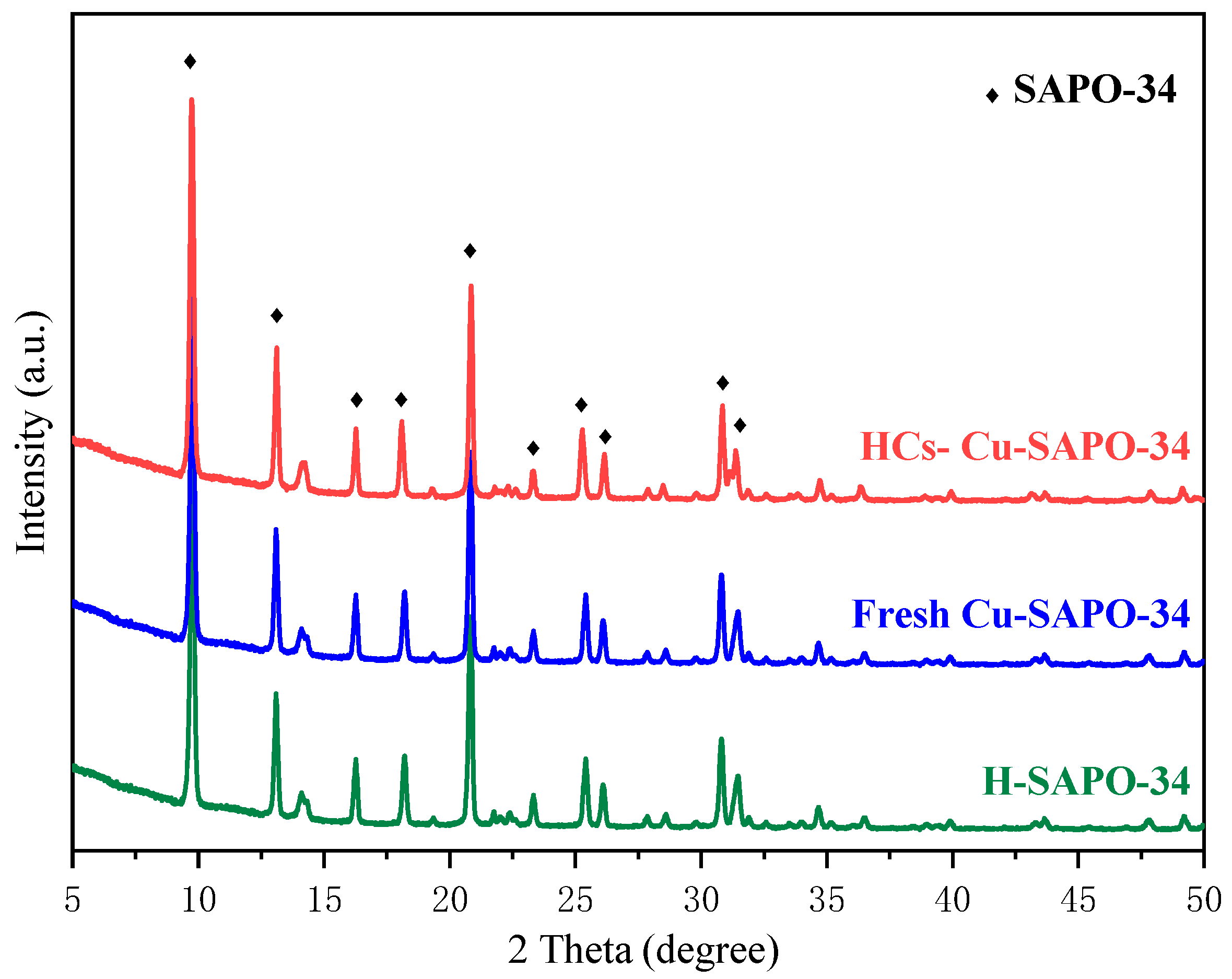






| Catalysts | Fresh Cu-SAPO-34 | HCs-Cu-SAPO-34 |
|---|---|---|
| BET specific surface area (m2/g) | 417 | 415 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Y.; Wang, L.; Zhang, Y.; Du, W.; Zhang, Y. The Study of C3H6 Impact on Selective Catalytic Reduction by Ammonia (NH3-SCR) Performance over Cu-SAPO-34 Catalysts. Catalysts 2021, 11, 1327. https://doi.org/10.3390/catal11111327
Duan Y, Wang L, Zhang Y, Du W, Zhang Y. The Study of C3H6 Impact on Selective Catalytic Reduction by Ammonia (NH3-SCR) Performance over Cu-SAPO-34 Catalysts. Catalysts. 2021; 11(11):1327. https://doi.org/10.3390/catal11111327
Chicago/Turabian StyleDuan, Yingfeng, Lina Wang, Yagang Zhang, Wei Du, and Yating Zhang. 2021. "The Study of C3H6 Impact on Selective Catalytic Reduction by Ammonia (NH3-SCR) Performance over Cu-SAPO-34 Catalysts" Catalysts 11, no. 11: 1327. https://doi.org/10.3390/catal11111327
APA StyleDuan, Y., Wang, L., Zhang, Y., Du, W., & Zhang, Y. (2021). The Study of C3H6 Impact on Selective Catalytic Reduction by Ammonia (NH3-SCR) Performance over Cu-SAPO-34 Catalysts. Catalysts, 11(11), 1327. https://doi.org/10.3390/catal11111327