The Study on the Active Site Regulated RuOx/Sn0.2Ti0.8O2 Catalysts with Different Ru Precursors for the Catalytic Oxidation of Dichloromethane
Abstract
:1. Introduction
2. Experimental Section
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Catalytic Oxidation Performance Evaluation
3. Results and Discussion
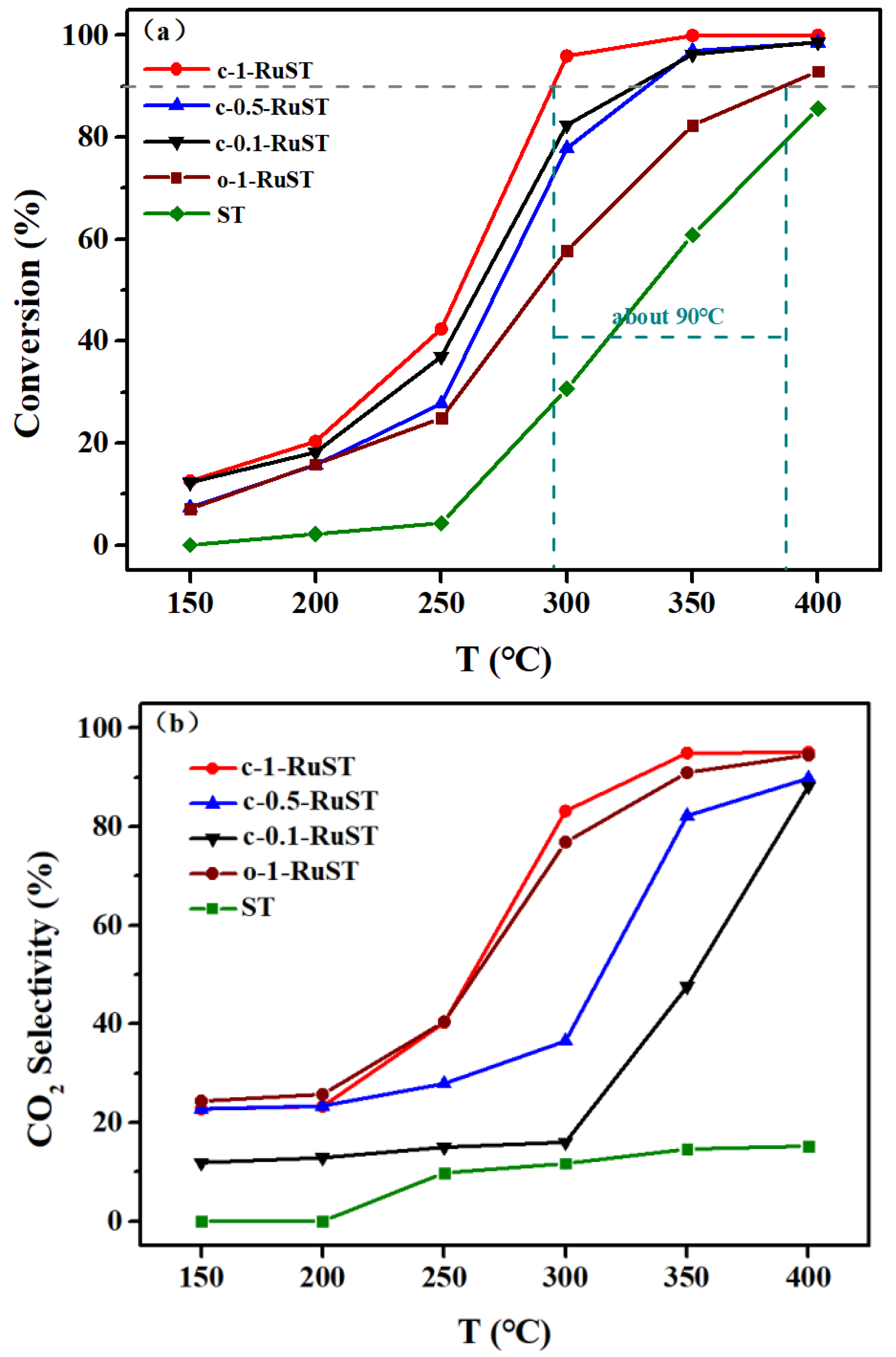
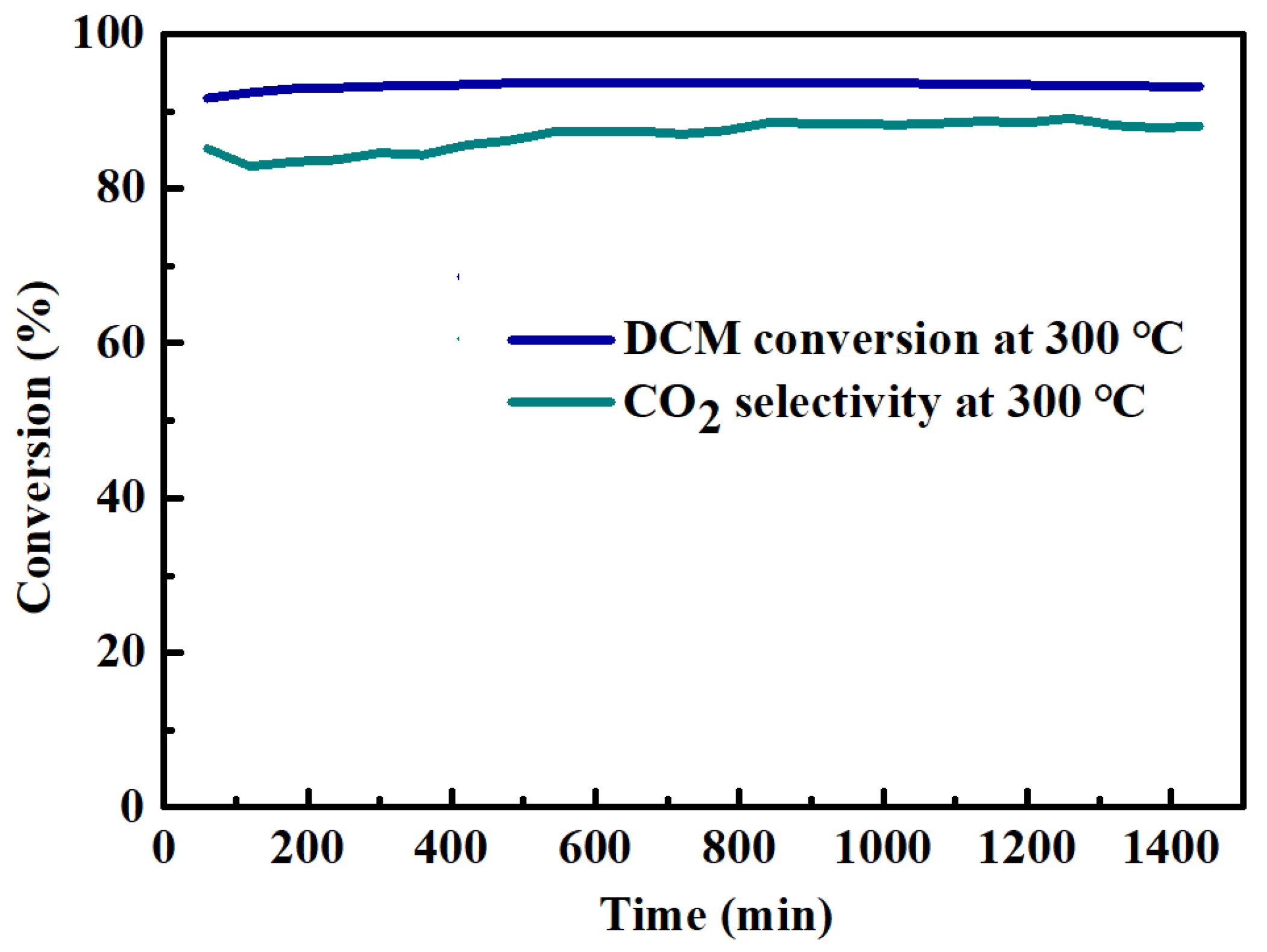
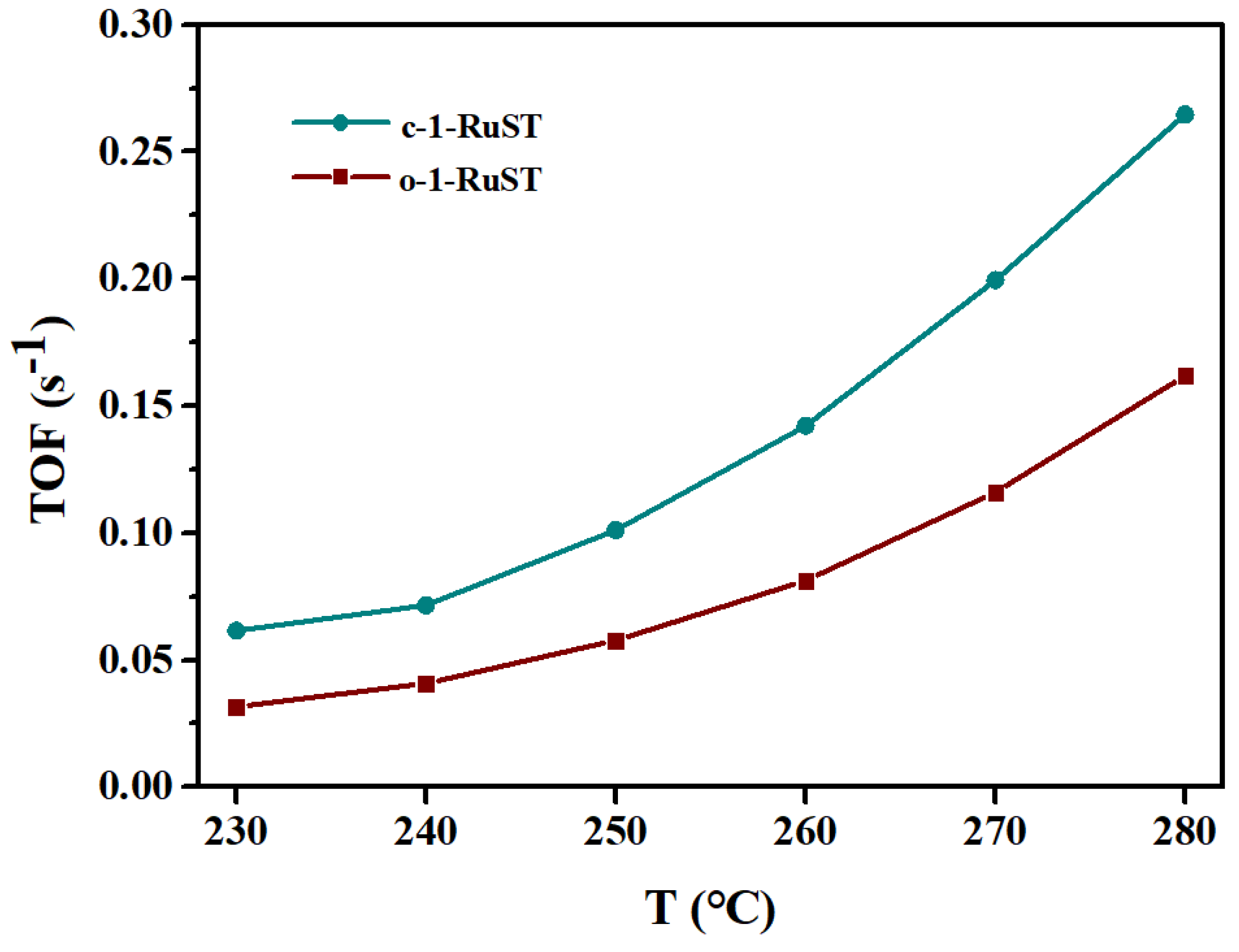
3.1. DCM Catalytic Oxidation Performance Test Results
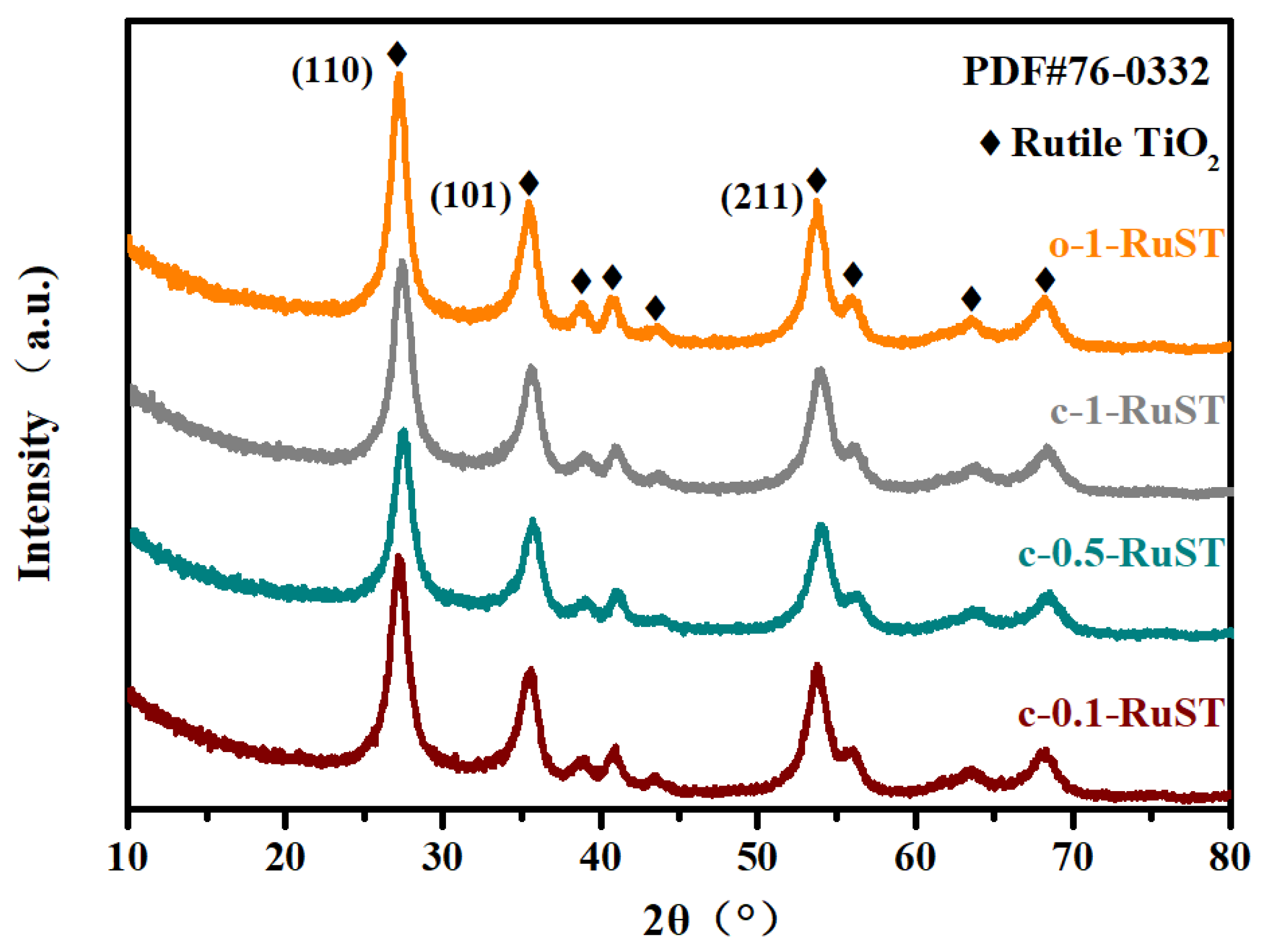
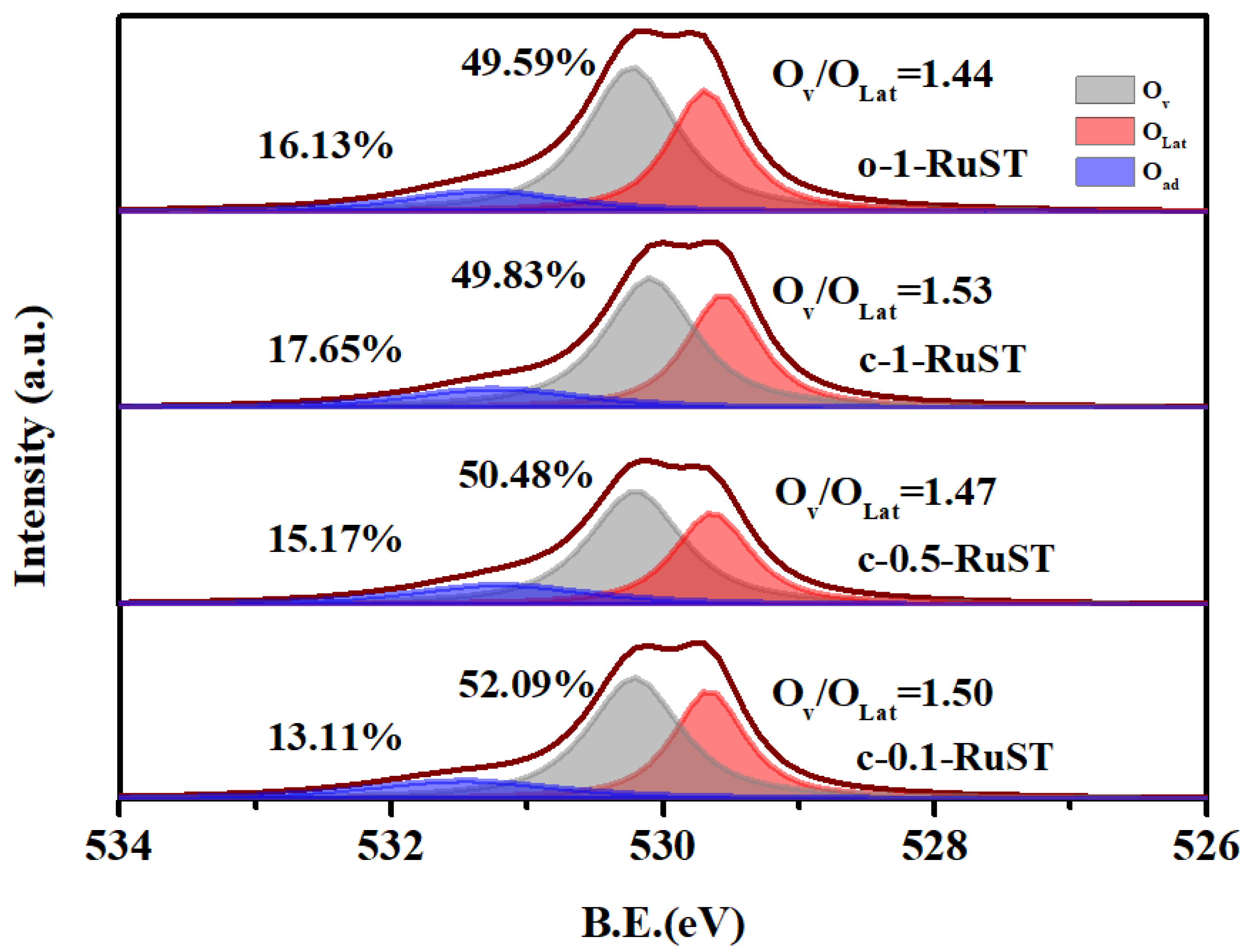
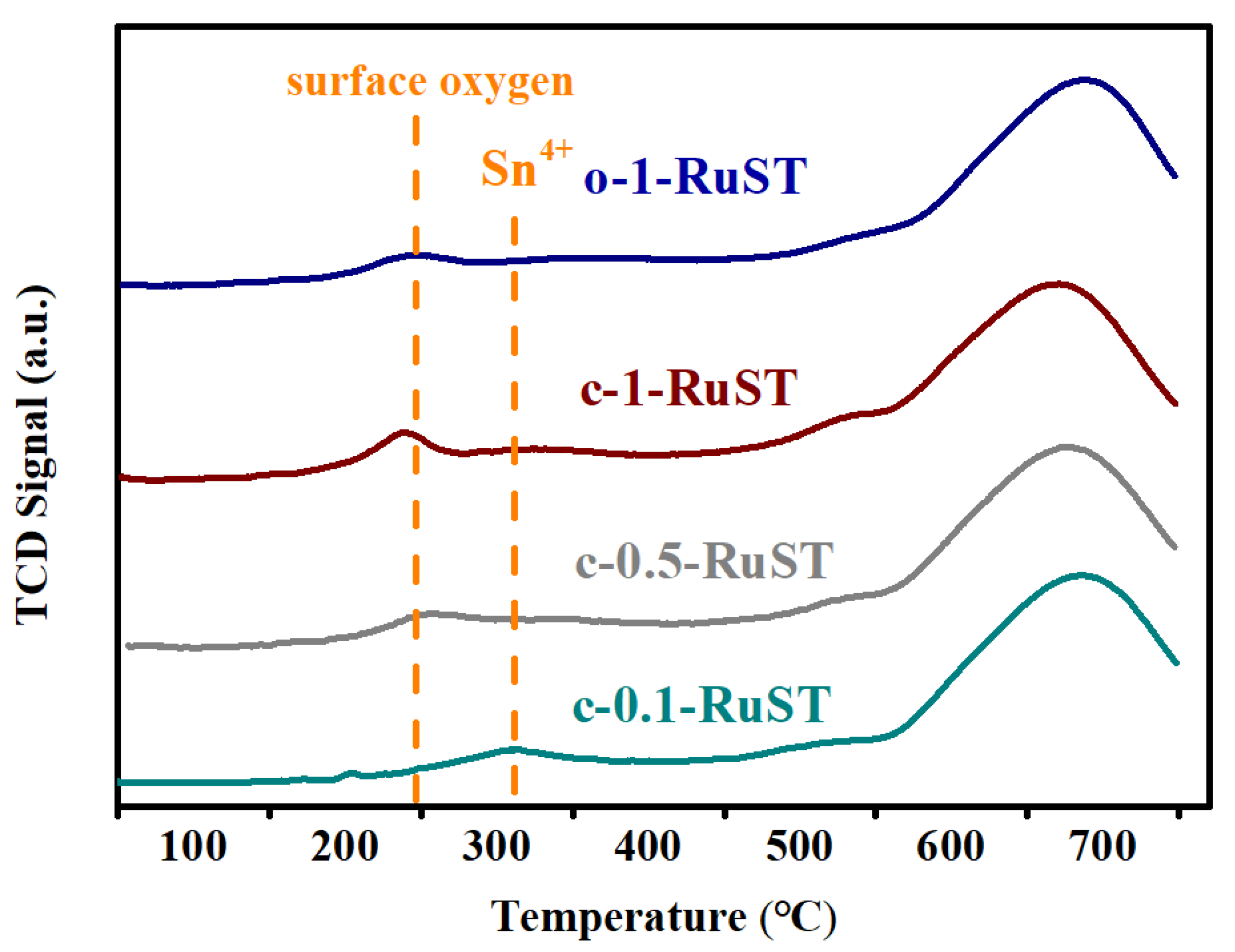
3.2. The Analysis of Physico-Chemical Properties of the Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Geng, C.; Yang, W.; Sun, X.; Wang, X.; Bai, Z.; Zhang, X. Emission factors, ozone and secondary organic aerosol formation potential of volatile organic compounds emitted from industrial biomass boilers. J. Environ. Sci. 2019, 83, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Amnuaylojaroen, T.; Macatangay, R.C.; Khodmanee, S. Modeling the effect of VOCs from biomass burning emissions on ozone pollution in upper Southeast Asia. Heliyon 2019, 5, e02661. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Song, M.; Liu, X.; Zhang, Y.; Hui, L.; Kong, L.; Zhang, Y.; Zhang, C.; Qu, Y.; An, J.; et al. Characterization and sources of volatile organic compounds (VOCs) and their related changes during ozone pollution days in 2016 in Beijing, China. Environ. Pollut. 2019, 257, 113599. [Google Scholar] [CrossRef] [PubMed]
- Doucette, W.J.; Wetzel, T.A.; Dettenmaier, E.; Gorder, K. Emission rates of chlorinated volatile organics from new and used consumer products found during vapor intrusion investigations: Impact on indoor air concentrations. Environ. Forensics 2018, 19, 185–190. [Google Scholar] [CrossRef]
- Hossaini, R.; Chipperfield, M.; Montzka, S.A.; Leeson, A.; Dhomse, S.S.; Pyle, J.A. The increasing threat to stratospheric ozone from dichloromethane. Nat. Commun. 2017, 8, 15962. [Google Scholar] [CrossRef]
- Blanch-Raga, N.; Palomares, A.E.; Triguero, J.M.; Puche, M.; Fetter, G.; Bosch, P. The oxidation of trichloroethylene over different mixed oxides derived from hydrotalcites. Appl. Catal. B Environ. 2014, 160–161, 129–134. [Google Scholar] [CrossRef]
- Dobrzyńska, E.; Pośniak, M.; Szewczynska, M.; Buszewski, B. Chlorinated Volatile Organic Compounds—Old, However, Actual Analytical and Toxicological Problem. Crit. Rev. Anal. Chem. 2010, 40, 41–57. [Google Scholar] [CrossRef]
- Krol, M.C.; Lelieveld, J.; Oram, D.E.; Sturrock, G.A.; Penkett, S.A.; Brenninkmeijer, C.A.M.; Gros, V.; Williams, J.; Scheeren, H.A. Continuing emissions of methyl chloroform from Europe. Nature 2003, 421, 131–135. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Y.; Song, H.; Li, H. A review on recent advances in catalytic combustion of chlorinated volatile organic compounds. J. Chem. Technol. Biotechnol. 2019, 95, 2069–2082. [Google Scholar] [CrossRef]
- Zheng, Z.; Yang, Y.; Li, H.; Xin, Q.; Zhang, S.; Liu, Y.; Liu, S.; Zheng, C.; Song, H.; Gao, X. Effect of multi-pollutant on the catalytic oxidation of dichloromethane over RuO2-WO3/Sn0.2Ti0.8O2 catalyst. Fuel 2020, 278, 118207. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S.; Zhao, H.; Li, H.; Qu, R.; Zhang, S.; Zhu, X.; Zheng, C.; Gao, X. Promotional effect of doping Cu into cerium-titanium binary oxides catalyst for deep oxidation of gaseous dichloromethane. Chemosphere 2018, 214, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Guo, X.; Dong, R.; Guo, Z.; Shi, J.; Yu, Y.; Cheng, M.; Albilali, R.; He, C. Insight into the boosted catalytic performance and chlorine resistance of nanosphere-like meso-macroporous CrOx/MnCo3Ox for 1,2-dichloroethane destruction. Appl. Catal. B Environ. 2019, 259, 118018. [Google Scholar] [CrossRef]
- Aouad, S.; Abi-Aad, E.; Aboukaïs, A. Simultaneous oxidation of carbon black and volatile organic compounds over Ru/CeO2 catalysts. Appl. Catal. B Environ. 2009, 88, 249–256. [Google Scholar] [CrossRef]
- Okal, J.; Zawadzki, M. Influence of Catalyst Pretreatments on Propane Oxidation Over Ru/gamma-Al2O3. Catal. Lett. 2009, 132, 225–234. [Google Scholar] [CrossRef]
- Joo, S.H.; Park, J.Y.; Renzas, J.; Butcher, D.R.; Huang, W.; Somorjai, G.A. Size Effect of Ruthenium Nanoparticles in Catalytic Carbon Monoxide Oxidation. Nano Lett. 2010, 10, 2709–2713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.-T. First-Principles Study of CO Adsorption and Oxidation on Ru-Doped CeO2(111) Surface. J. Phys. Chem. C 2012, 116, 6239–6246. [Google Scholar] [CrossRef]
- Hevia, M.A.; Amrute, A.P.; Schmidt, T.; Pérez-Ramírez, J. Transient mechanistic study of the gas-phase HCl oxidation to Cl2 on bulk and supported RuO2 catalysts. J. Catal. 2010, 276, 141–151. [Google Scholar] [CrossRef]
- Sun, W.; Gong, B.; Pan, J.; Wang, Y.; Xia, H.; Zhang, H.; Dai, Q.; Wang, L.; Wang, X. Catalyticcombustion of CVOCs over CrxTi1−x oxide catalysts. J. Catal. 2020, 391, 132–144. [Google Scholar] [CrossRef]
- Lv, X.; Cai, S.; Chen, J.; Yan, D.; Jiang, M.; Jia, H. Tuning the degradation activity and pathways of chlorinated organic pollutants over CeO2 catalyst with acid sites: Synergistic effect of Lewis and Brønsted acid sites. Catal. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Yang, Y.; Li, H.; Zhao, H.; Qu, R.; Zhang, S.; Hu, W.; Yu, X.; Zhu, X.; Liu, S.; Zheng, C.; et al. Structure and crystal phase transition effect of Sn doping on anatase TiO2 for dichloromethane decomposition. J. Hazard. Mater. 2019, 371, 156–164. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Z.; Zhou, Y.; Zhu, Q.; Pan, Z.; Lu, H. A facile route for spraying preparation of Pt/TiO2 monolithic catalysts toward VOCs combustion. Appl. Catal. A Gen. 2018, 566, 190–199. [Google Scholar] [CrossRef]
- Todorova, S.; Blin, J.; Naydenov, A.; Lebeau, B.; Kolev, H.; Gaudin, P.; Dotzeva, A.; Velinova, R.; Filkova, D.; Ivanova, I.; et al. Co3O4-MnOx oxides supported on SBA-15 for CO and VOCs oxidation. Catal. Today 2019, 357, 602–612. [Google Scholar] [CrossRef]
- Topka, P.; Dvořáková, M.; Kšírová, P.; Perekrestov, R.; Čada, M.; Balabánová, J.; Koštejn, M.; Jirátová, K.; Kovanda, F. Structured cobalt oxide catalysts for VOC abatement: The effect of preparation method. Environ. Sci. Pollut. Res. 2019, 27, 7608–7617. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.; Wang, H.; Chen, M.; Zhang, C.; Ning, P.; He, H. Nano-sized Ag rather than single-atom Ag determines CO oxidation activity and stability. Nano Res. 2021, 1–5. [Google Scholar] [CrossRef]
- Veisi, H.; Azizi, S.; Mohammadi, P. Green synthesis of the silver nanoparticles mediated by Thymbra spicata extract and its application as a heterogeneous and recyclable nanocatalyst for catalytic reduction of a variety of dyes in water. J. Clean. Prod. 2018, 170, 1536–1543. [Google Scholar] [CrossRef]
- Xu, L.; Chen, D.; Qu, J.; Wang, L.; Tang, J.; Liu, H.; Yang, J. Replacement reaction-based synthesis of supported palladium catalysts with atomic dispersion for catalytic removal of benzene. J. Mater. Chem. A 2018, 6, 17032–17039. [Google Scholar] [CrossRef]
- Agarwal, N.; Freakley, S.J.; McVicker, R.U.; Althahban, S.M.; Dimitratos, N.; He, Q.; Morgan, D.J.; Jenkins, R.L.; Willock, D.J.; Taylor, S.H.; et al. Aqueous Au-Pd colloids catalyze selective CH4 oxidation to CH3OH with O2 under mild conditions. Science 2017, 358, 223–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Jia, L.; Medrano, J.A.; Ross, J.R.H.; Lefferts, L. Supported Pd Catalysts Prepared via Colloidal Method: The Effect of Acids. ACS Catal. 2013, 3, 2341–2352. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, D.; Liu, J.; He, D.; Cao, X.; Han, C.; Lu, J.; Luo, Y. Tuning the metal-support interaction on chromium-based catalysts for catalytically eliminate methyl mercaptan: Anchored active chromium species through surface hydroxyl groups. Chem. Eng. J. 2020, 389, 124384. [Google Scholar] [CrossRef]
- Li, H.; Wang, G.; Zhang, F.; Zou, L.; Zou, Z.; Yang, H. An Atomically Dispersed Pt Catalyst Anchored on an Fe/N/C Support for Enhanced Hydrogen Evolution Reaction. J. Phys. Chem. C 2020, 124, 11760–11766. [Google Scholar] [CrossRef]
- Seitsonen, A.P.; Over, H. Oxidation of HCl over TiO2-Supported RuO2: A Density Functional Theory Study. J. Phys. Chem. C 2010, 114, 22624–22629. [Google Scholar] [CrossRef]
- Nong, S.; Dong, W.; Yin, J.; Dong, B.; Lu, Y.; Yuan, X.; Wang, X.; Bu, K.; Chen, M.; Jiang, S.; et al. Well-Dispersed Ruthenium in Mesoporous Crystal TiO2 as an Advanced Electrocatalyst for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2018, 140, 5719–5727. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Standards and Technology. NIST X-ray Photoelectron Spectroscopy Database, Version 4.1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012. Available online: http://srdata.nist.gov/xps/ (accessed on 20 April 2020).
- Guo, Y.; Mei, S.; Yuan, K.; Wang, D.J.; Liu, H.C.; Yan, C.H.; Zhang, Y.W. Low-Temperature CO2 Methanation over CeO2-Supported Ru Single Atoms, Nanoclusters, and Nanoparticles Competitively Tuned by Strong Metal–Support Interactions and H-Spillover Effect. ACS Catal. 2018, 8, 6203–6215. [Google Scholar] [CrossRef]
- Yang, Y.; Li, H.; Zhang, S.; Yu, X.; Liu, S.; Qu, R.; Zheng, C.; Gao, X. Different reactive behaviours of dichloromethane over anatase TiO2 supported RuO2 and V2O5. Catal. Today 2019, 355, 349–357. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Cullen, D.A.; Hu, W.; Huang, J.; Yao, L.; Peng, Z.; Liao, P.; Wang, R. Distribution and Valence State of Ru Species on CeO2 Supports: Support Shape Effect and Its Influence on CO Oxidation. ACS Catal. 2019, 9, 11088–11103. [Google Scholar] [CrossRef]
- Liotta, L.; Ousmane, M.; Di Carlo, G.; Pantaleo, G.; Deganello, G.; Marcì, G.; Retailleau, L.; Giroir-Fendler, A. Total oxidation of propene at low temperature over Co3O4–CeO2 mixed oxides: Role of surface oxygen vacancies and bulk oxygen mobility in the catalytic activity. Appl. Catal. A Gen. 2008, 347, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Pu, Z.-Y.; Liu, X.-S.; Jia, A.-P.; Xie, Y.-L.; Lu, J.-Q.; Luo, M.-F. Enhanced Activity for CO Oxidation over Pr- and Cu-Doped CeO2 Catalysts: Effect of Oxygen Vacancies. J. Phys. Chem. C 2008, 112, 15045–15051. [Google Scholar] [CrossRef]
- Dai, Q.; Wu, J.; Deng, W.; Hu, J.; Wu, Q.; Guo, L.; Sun, W.; Zhan, W.; Wang, X. Comparative studies of P/CeO2 and Ru/CeO2 catalysts for catalytic combustion of dichloromethane: From effects of H2O to distribution of chlorinated by-products. Appl. Catal. B Environ. 2019, 249, 9–18. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Wang, R. Support structure and reduction treatment effects on CO oxidation of SiO2 nanospheres and CeO2 nanorods supported ruthenium catalysts. J. Colloid Interface Sci. 2018, 531, 204–215. [Google Scholar] [CrossRef]


| Samples | Metal Dispersion (%) | Metallic Surface Area (m2/g) |
|---|---|---|
| c-0.1-RuST | 15.86 | 75.59 |
| c-0.5-RuST | 7.18 | 34.20 |
| c-1-RuST | 2.35 | 11.19 |
| Samples | Snmol:Timol a | Ru (wt%) a | BET (m2/g) | Pore Size (nm) |
|---|---|---|---|---|
| o-1-RuST | 0.250 | 1.13 | 64.87 | 13.44 |
| c-1-RuST | 0.248 | 0.857 | 80.61 | 11.88 |
| c-0.5-RuST | 0.246 | 0.415 | 83.51 | 10.66 |
| c-0.1-RuST | 0.250 | 0.141 | 85.31 | 10.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zheng, Z.; Kong, M.; Hua, Z.; Yang, Z.; Jiang, Y.; Liu, S.; Yan, X.; Gao, X. The Study on the Active Site Regulated RuOx/Sn0.2Ti0.8O2 Catalysts with Different Ru Precursors for the Catalytic Oxidation of Dichloromethane. Catalysts 2021, 11, 1306. https://doi.org/10.3390/catal11111306
Yang Y, Zheng Z, Kong M, Hua Z, Yang Z, Jiang Y, Liu S, Yan X, Gao X. The Study on the Active Site Regulated RuOx/Sn0.2Ti0.8O2 Catalysts with Different Ru Precursors for the Catalytic Oxidation of Dichloromethane. Catalysts. 2021; 11(11):1306. https://doi.org/10.3390/catal11111306
Chicago/Turabian StyleYang, Yang, Zhong Zheng, Mengyue Kong, Zhesheng Hua, Zhengda Yang, Ye Jiang, Shaojun Liu, Xinhuan Yan, and Xiang Gao. 2021. "The Study on the Active Site Regulated RuOx/Sn0.2Ti0.8O2 Catalysts with Different Ru Precursors for the Catalytic Oxidation of Dichloromethane" Catalysts 11, no. 11: 1306. https://doi.org/10.3390/catal11111306
APA StyleYang, Y., Zheng, Z., Kong, M., Hua, Z., Yang, Z., Jiang, Y., Liu, S., Yan, X., & Gao, X. (2021). The Study on the Active Site Regulated RuOx/Sn0.2Ti0.8O2 Catalysts with Different Ru Precursors for the Catalytic Oxidation of Dichloromethane. Catalysts, 11(11), 1306. https://doi.org/10.3390/catal11111306








