On the Performance of a Sustainable Rice Husk Biochar for the Activation of Persulfate and the Degradation of Antibiotics
Abstract
:1. Introduction
2. Results and Discussion
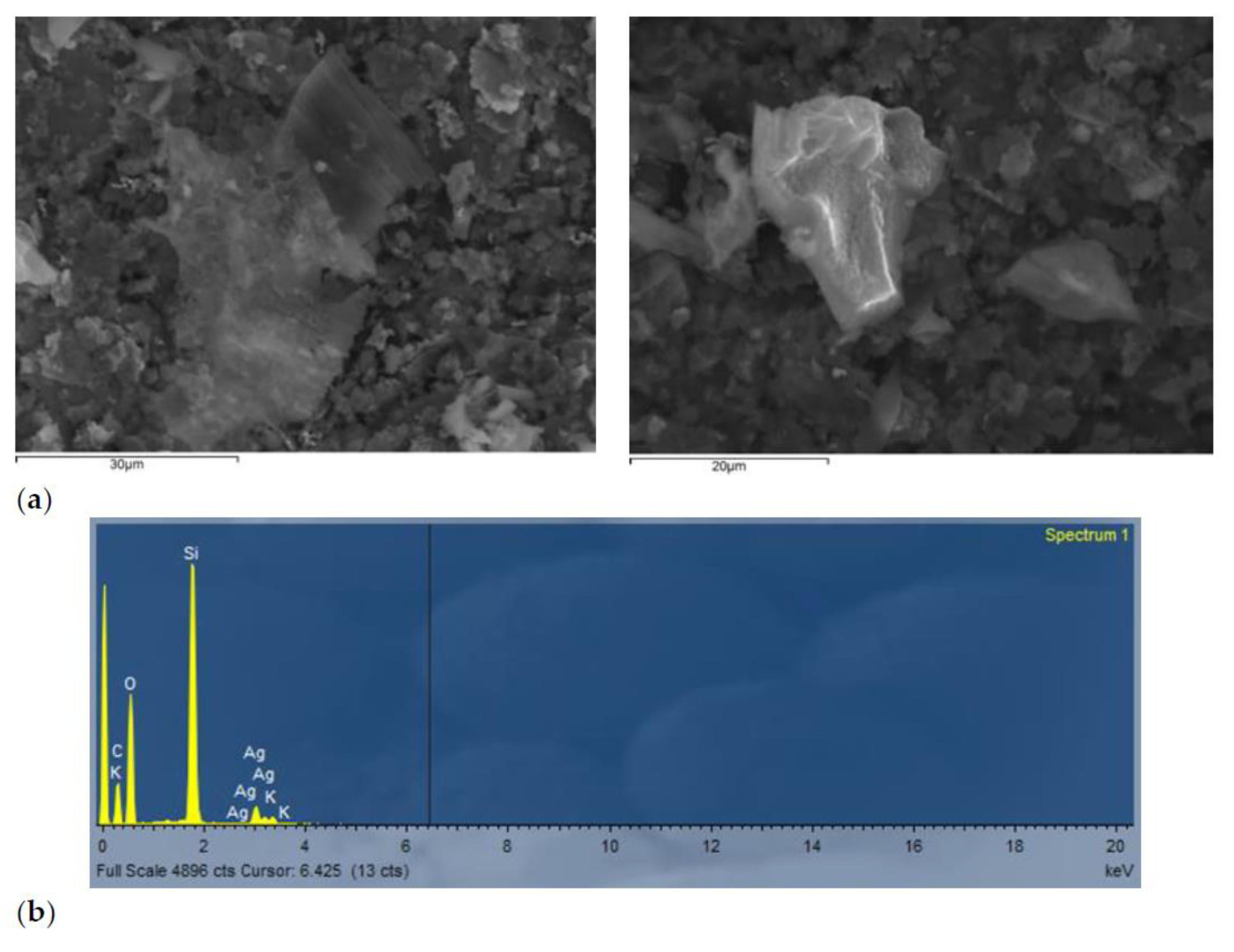
2.1. Physicochemical Characterization
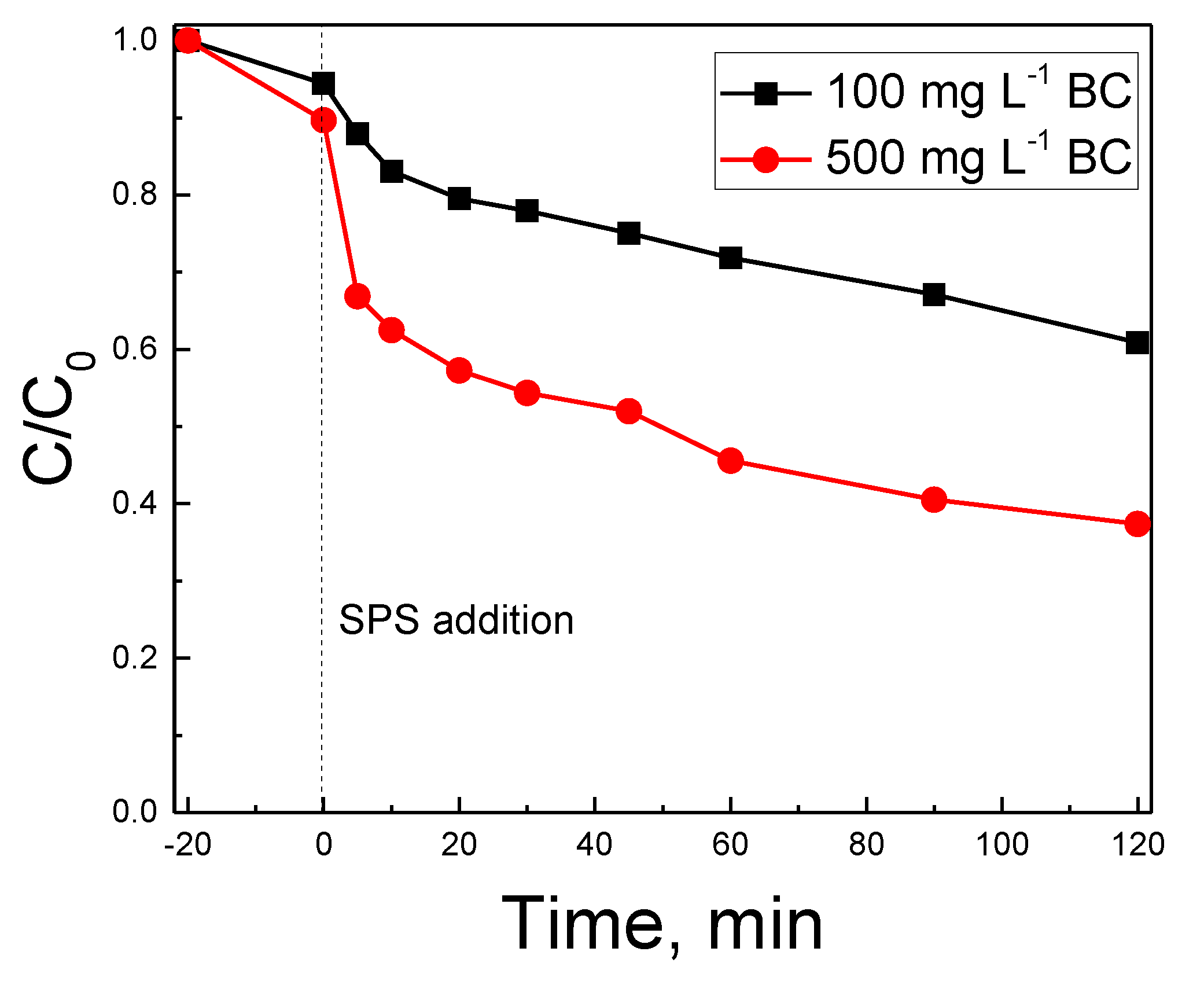
2.2. Oxidative Degradation of Antibiotics
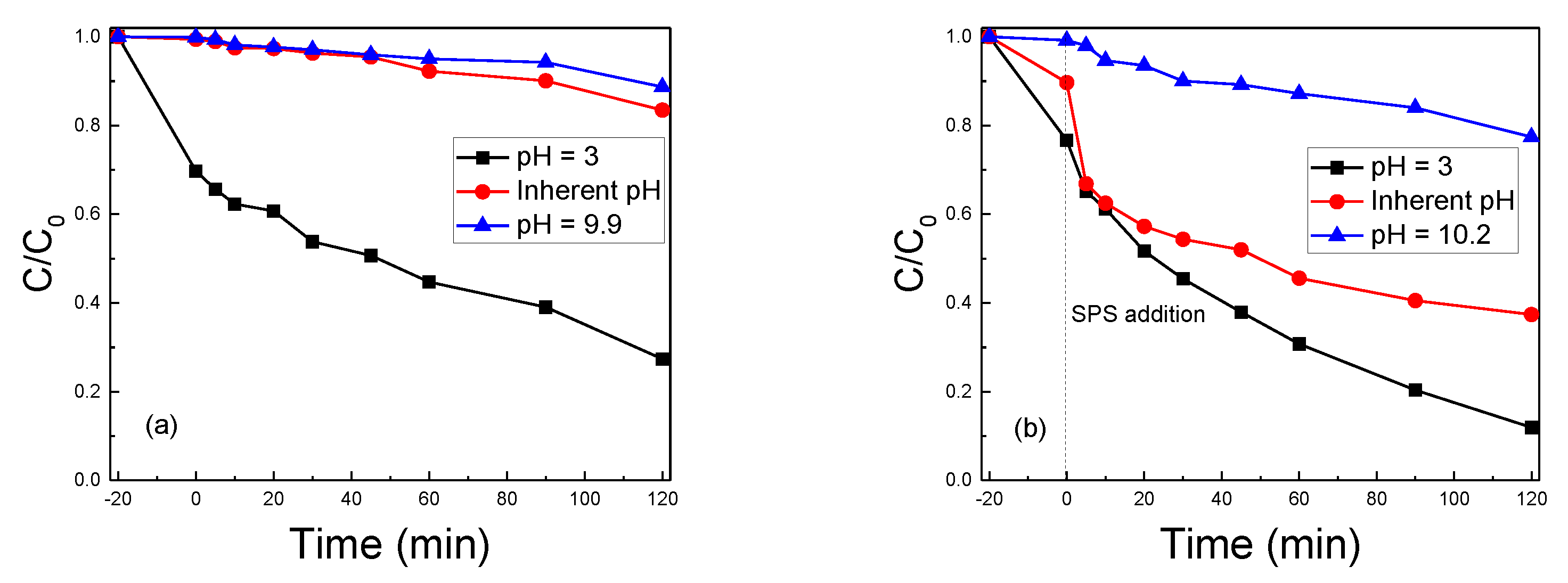
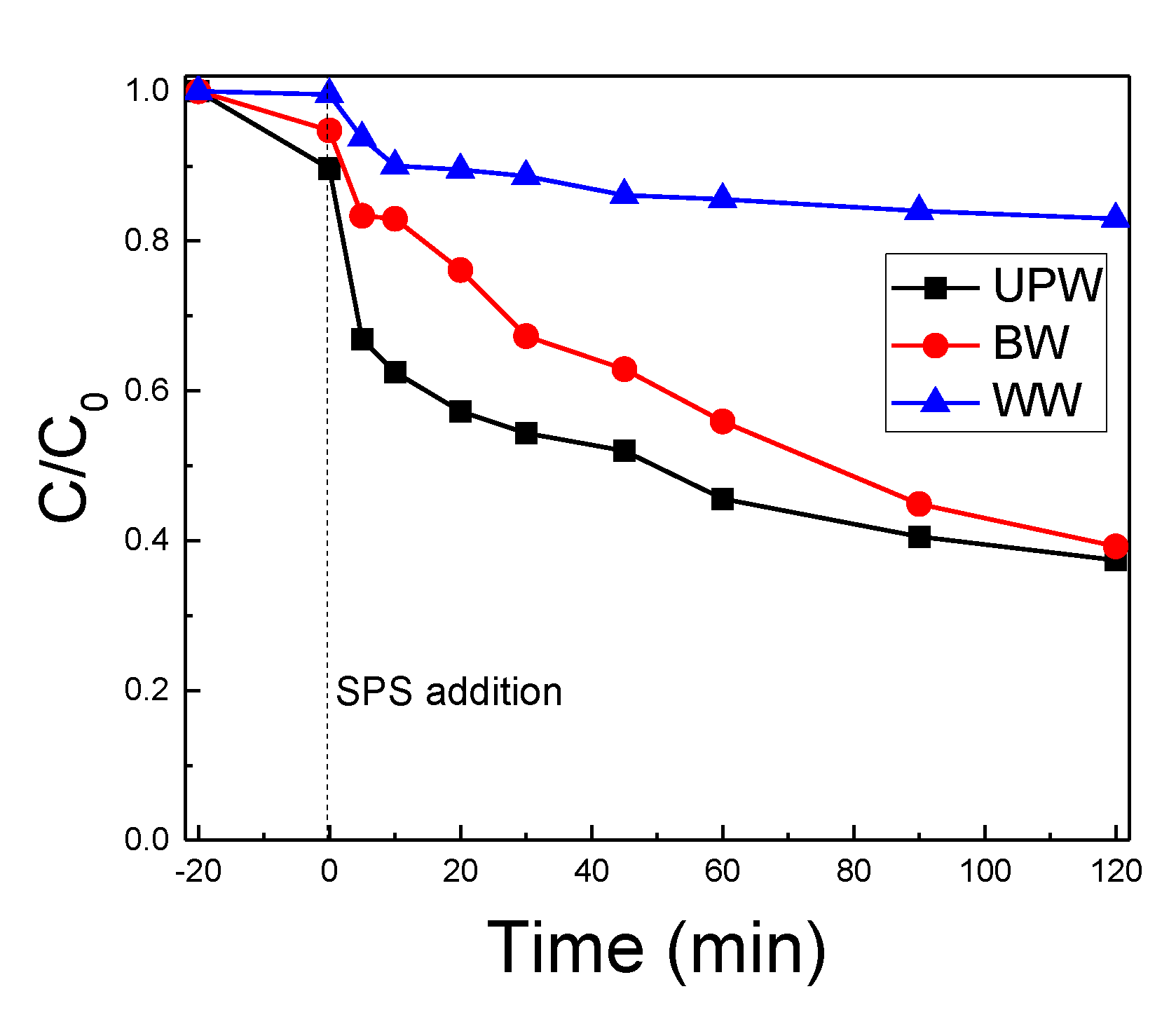
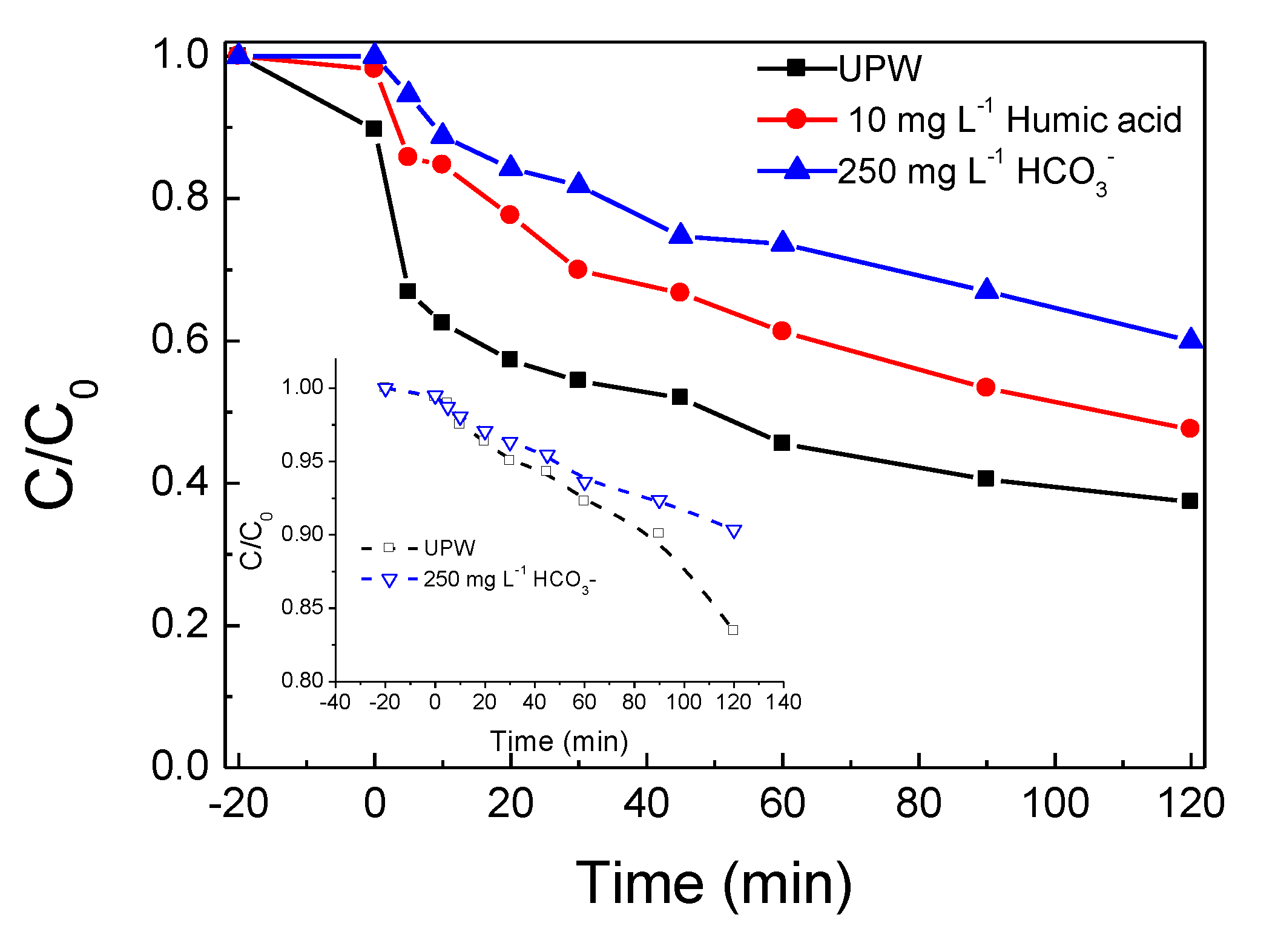
2.3. The Water Matrix Effect
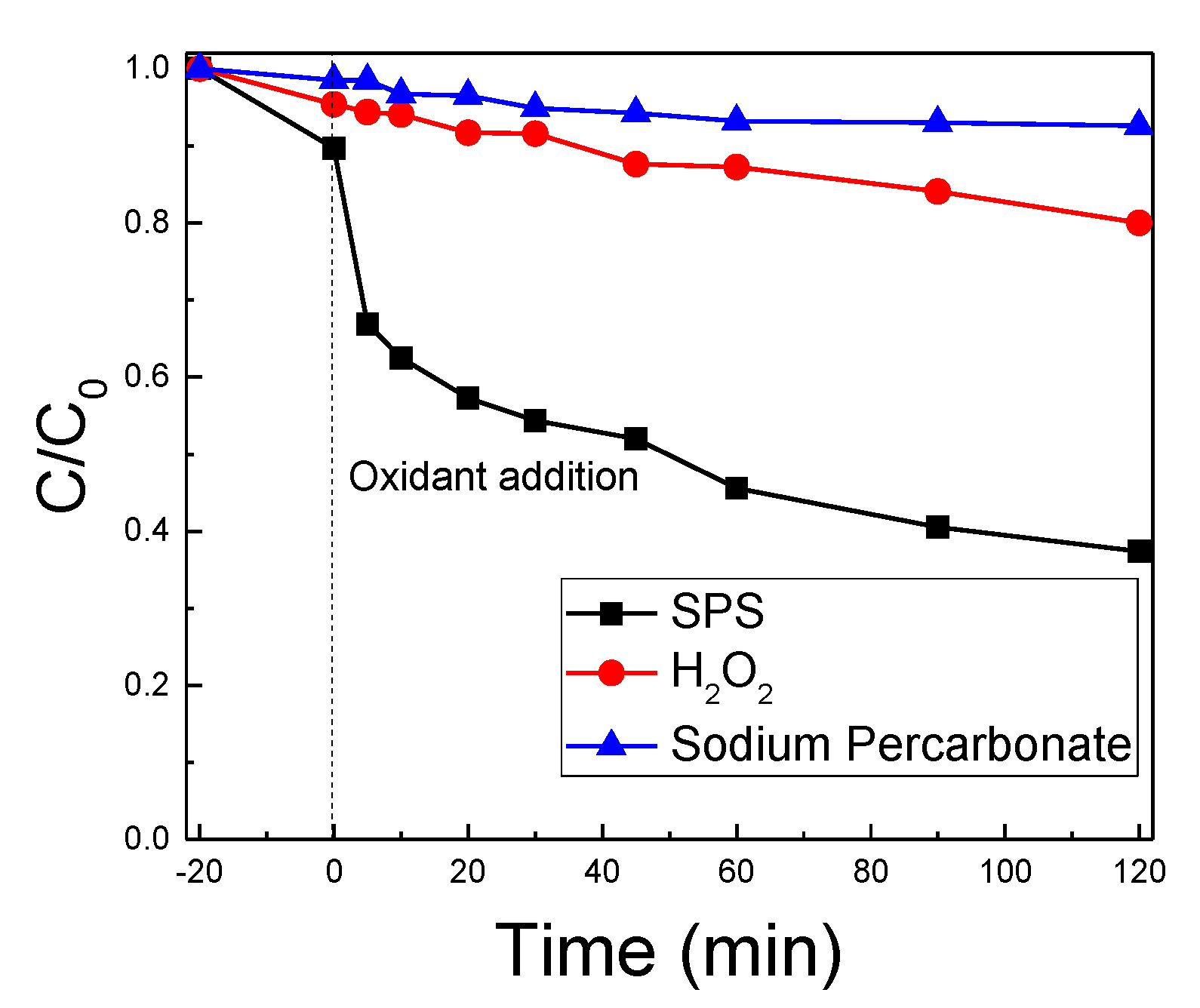
2.4. The Role of Radical Scavengers and Oxidant
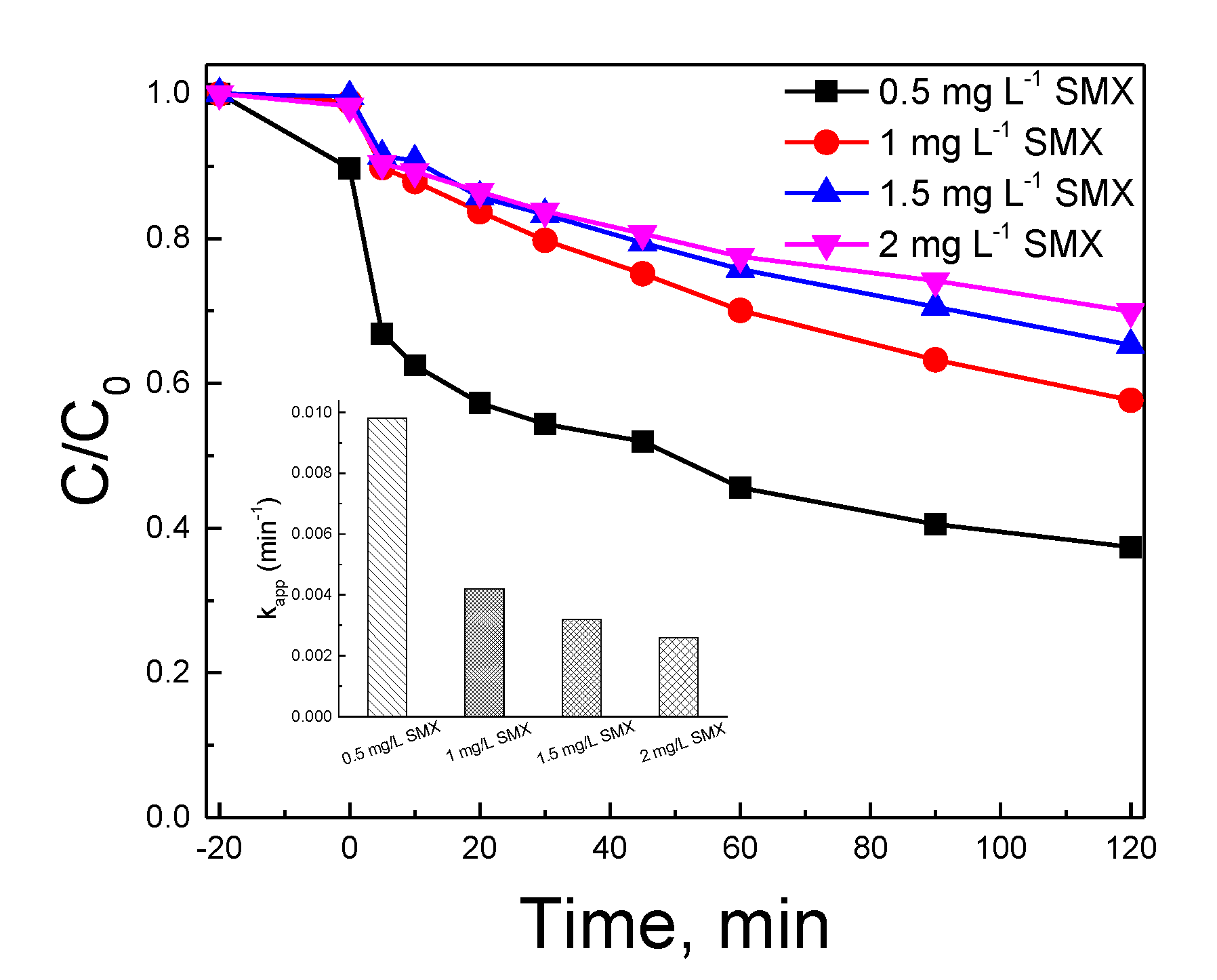
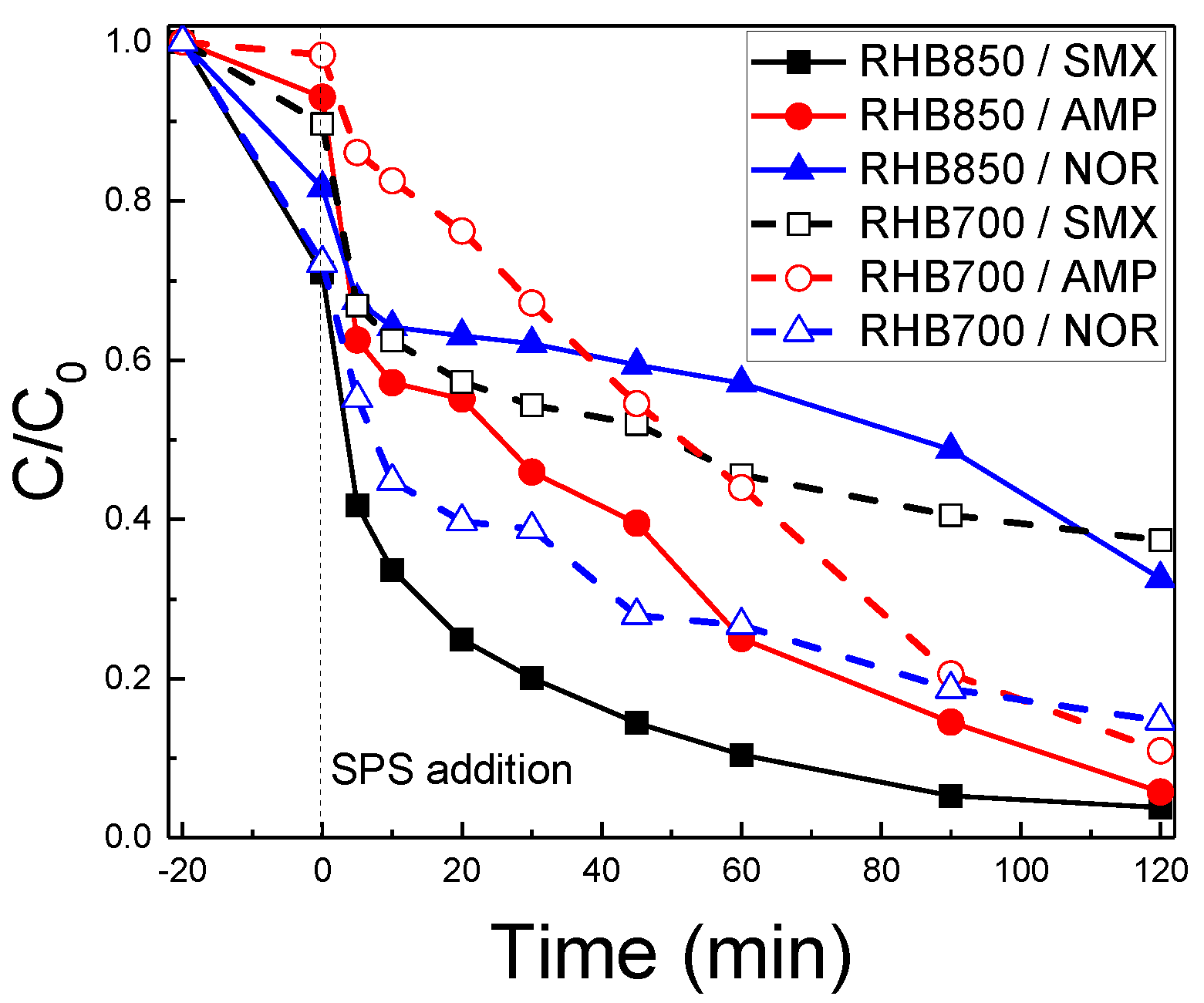
2.5. The Effect of the Antibiotic Type
3. Materials and Methods
3.1. Chemicals and Water Matrices
3.2. Preparation and Characterization of Biochar
3.3. Experimental Procedures
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tongcumpou, C.; Usapein, P.; Tuntiwiwattanapun, N. Complete Utilization of Wet Spent Coffee Grounds Waste as a Novel Feedstock for Antioxidant, Biodiesel, and Bio-Char Production. Ind. Crop. Prod. 2019, 138, 111484. [Google Scholar] [CrossRef]
- Goodman, B.A. Utilization of Waste Straw and Husks from Rice Production: A Review. J. Bioresour. Bioprod. 2020, 5, 143–162. [Google Scholar] [CrossRef]
- Manariotis, I.D.; Fotopoulou, K.N.; Karapanagioti, H.K. Preparation and Characterization of Biochar Sorbents Produced from Malt Spent Rootlets. Ind. Eng. Chem. Res. 2015, 54, 9577–9584. [Google Scholar] [CrossRef]
- Zeng, H.; Qi, W.; Zhai, L.; Wang, F.; Zhang, J.; Li, D. Preparation and Characterization of Sludge-Based Magnetic Biochar by Pyrolysis for Methylene Blue Removal. Nanomaterials 2021, 11, 2473. [Google Scholar] [CrossRef] [PubMed]
- El-Azazy, M.; Nabil, I.; Hassan, S.S.; El-Shafie, A.S. Adsorption Characteristics of Pristine and Magnetic Olive Stones Biochar with Respect to Clofazimine. Nanomaterials 2021, 11, 963. [Google Scholar] [CrossRef] [PubMed]
- Vakros, J.; Manariotis, I.D.; Dracopoulos, V.; Mantzavinos, D.; Lianos, P. Biochar from Spent Malt Rootlets and Its Application to an Energy Conversion and Storage Device. Chemosensors 2021, 9, 57. [Google Scholar] [CrossRef]
- Deng, J.; Li, J.; Song, S.; Zhou, Y.; Li, L. Electrolyte-Dependent Supercapacitor Performance on Nitrogen-Doped Porous Bio-Carbon from Gelatin. Nanomaterials 2020, 10, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsavatopoulou, V.D.; Vakros, J.; Manariotis, I.D. Lipid Conversion of Scenedesmus Rubescens Biomass into Biodiesel Using Biochar Catalysts from Malt Spent Rootlets. J. Chem. Technol. Biotechnol. 2020, 95, 2421–2429. [Google Scholar] [CrossRef]
- Shi, C.; Li, Y.; Feng, H.; Jia, S.; Xue, R.; Li, G.; Wang, G. Removal of P-Nitrophenol Using Persulfate Activated by Biochars Prepared from Different Biomass Materials. Chem. Res. Chin. Univ. 2018, 34, 39–43. [Google Scholar] [CrossRef]
- Varela Milla, O.; Rivera, E.B.; Huang, W.-J.; Chien, C.-C.; Wang, Y.-M. Agronomic Properties and Characterization of Rice Husk and Wood Biochars and Their Effect on the Growth of Water Spinach in a Field Test. J. Soil Sci. Plant Nutr. 2013, 13, 251–266. [Google Scholar] [CrossRef] [Green Version]
- Grilla, E.; Vakros, J.; Konstantinou, I.; Manariotis, I.D.; Mantzavinos, D. Activation of Persulfate by Biochar from Spent Malt Rootlets for the Degradation of Trimethoprim in the Presence of Inorganic Ions. J. Chem. Technol. Biotechnol. 2020, 95, 2348–2358. [Google Scholar] [CrossRef]
- Han, R.; Fang, Y.; Sun, P.; Xie, K.; Zhai, Z.; Liu, H.; Liu, H. N-Doped Biochar as a New Metal-Free Activator of Peroxymonosulfate for Singlet Oxygen-Dominated Catalytic Degradation of Acid Orange 7. Nanomaterials 2021, 11, 2288. [Google Scholar] [CrossRef]
- Wang, C.; Huang, R.; Ruirui Sun, R.; Yang, J.; Sillanpää, M. A review on persulfates activation by functional biochar for organic contaminants removal: Synthesis, characterizations, radical determination, and mechanism. J. Environ. Chem. Eng. 2021, 9, 106267. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, F.; Wu, Y.; Hu, H.; Dai, X. Progress of Potato Staple Food Research and Industry Development in China. J. Integr. Agric. 2017, 16, 2924–2932. [Google Scholar] [CrossRef]
- Aulakh, D.S.; Singh, J.; Kumar, S. The Effect of Utilizing Rice Husk Ash on Some Properties of Concrete—A Review. Curr. World Environ. 2018, 13, 224–231. [Google Scholar] [CrossRef]
- Kraehmer, H.; Thomas, C.; Vidotto, F. Rice Production in Europe. In Rice Production Worldwide; Chauhan, B.S., Jabran, K., Mahajan, G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 93–116. ISBN 978-3-319-47514-1. [Google Scholar]
- Mirmohamadsadeghi, S.; Karimi, K. Recovery of silica from rice straw and husk. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 411–433. ISBN 978-0-444-64321-6. [Google Scholar]
- Meng, H.; Nie, C.; Li, W.; Duan, X.; Lai, B.; Ao, Z.; Wang, S.; An, T. Insight into the Effect of Lignocellulosic Biomass Source on the Performance of Biochar as Persulfate Activator for Aqueous Organic Pollutants Remediation: Epicarp and Mesocarp of Citrus Peels as Examples. J. Hazard. Mater. 2020, 399, 123043. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Liang, S.; Feng, Z.; Zhao, J. Mechanism of Persulfate Activation by Biochar for the Catalytic Degradation of Antibiotics: Synergistic Effects of Environmentally Persistent Free Radicals and the Defective Structure of Biochar. Sci. Total. Environ. 2021, 794, 148707. [Google Scholar] [CrossRef]
- Moles, S.; Mosteo, R.; Gómez, J.; Szpunar, J.; Gozzo, S.; Castillo, J.R.; Ormad, M.P. Towards the Removal of Antibiotics Detected in Wastewaters in the POCTEFA Territory: Occurrence and TiO2 Photocatalytic Pilot-Scale Plant Performance. Water 2020, 12, 1453. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Santos, L. Antibiotics in the Aquatic Environments: A Review of the European Scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- Danner, M.-C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic Pollution in Surface Fresh Waters: Occurrence and Effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhuan, R.; Chu, L. The Occurrence, Distribution and Degradation of Antibiotics by Ionizing Radiation: An Overview. Sci. Total Environ. 2019, 646, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Straub, J.O. Aquatic Environmental Risk Assessment for Human Use of the Old Antibiotic Sulfamethoxazole in Europe: Aquatic Environmental Risk Assessment for Human Sulfamethoxazole in Europe. Environ. Toxicol. Chem. 2016, 35, 767–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lykoudi, A.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Degradation of Sulfamethoxazole with Persulfate Using Spent Coffee Grounds Biochar as Activator. J. Environ. Manag. 2020, 271, 111022. [Google Scholar] [CrossRef]
- Schott, H.; Astigarrabia, E. Isoelectric Points of Some Sulfonamides: Determination by Microelectrophoresis and by Calculations Involving Acid-Base Strength. J. Pharm. Sci. 1988, 77, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Avisar, D.; Primor, O.; Gozlan, I.; Mamane, H. Sorption of Sulfonamides and Tetracyclines to Montmorillonite Clay. Water Air Soil Pollut. 2010, 209, 439–450. [Google Scholar] [CrossRef]
- Xie, B.; Tang, X.; Ng, H.Y.; Deng, S.; Shi, X.; Song, W.; Huang, S.; Li, G.; Liang, H. Biological Sulfamethoxazole Degradation along with Anaerobically Digested Centrate Treatment by Immobilized Microalgal-Bacterial Consortium: Performance, Mechanism and Shifts in Bacterial and Microalgal Communities. Chem. Eng. J. 2020, 388, 124217. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of Antibiotics by Advanced Oxidation Processes: An Overview. Sci. Total. Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Liu, S.; Peng, W.; Sun, H.; Wang, S. Physical and Chemical Activation of Reduced Graphene Oxide for Enhanced Adsorption and Catalytic Oxidation. Nanoscale 2014, 6, 766–771. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Tade, M.; Wang, S. Metal-Free Activation of Persulfate by Cubic Mesoporous Carbons for Catalytic Oxidation via Radical and Nonradical Processes. Catal. Today 2018, 307, 140–146. [Google Scholar] [CrossRef]
- Liu, X.; He, S.; Yang, Y.; Yao, B.; Tang, Y.; Luo, L.; Zhi, D.; Wan, Z.; Wang, L.; Zhou, Y. A Review on Percarbonate-Based Advanced Oxidation Processes for Remediation of Organic Compounds in Water. Environ. Res. 2021, 200, 111371. [Google Scholar] [CrossRef]
- Fang, G.; Gao, J.; Liu, C.; Dionysiou, D.D.; Wang, Y.; Zhou, D. Key Role of Persistent Free Radicals in Hydrogen Peroxide Activation by Biochar: Implications to Organic Contaminant Degradation. Environ. Sci. Technol. 2014, 48, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-M.; Huang, C.-P.; Chen, C.-W.; Wu, C.-H.; Lin, Y.-L.; Dong, C.-D. Activation of Percarbonate by Water Treatment Sludge–Derived Biochar for the Remediation of PAH-Contaminated Sediments. Environ. Pollut. 2020, 265, 114914. [Google Scholar] [CrossRef]
- Ntzoufra, P.; Vakros, J.; Frontistis, Z.; Tsatsos, S.; Kyriakou, G.; Kennou, S.; Manariotis, I.D.; Mantzavinos, D. Effect of Sodium Persulfate Treatment on the Physicochemical Properties and Catalytic Activity of Biochar Prepared from Spent Malt Rootlets. J. Environ. Chem. Eng. 2021, 9, 105071. [Google Scholar] [CrossRef]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Wu, Q.; Chang, J.-S.; Ren, N. Singlet Oxygen-Dominated Peroxydisulfate Activation by Sludge-Derived Biochar for Sulfamethoxazole Degradation through a Nonradical Oxidation Pathway: Performance and Mechanism. Chem. Eng. J. 2019, 357, 589–599. [Google Scholar] [CrossRef]
- Li, F.; Duan, F.; Ji, W.; Gui, X. Biochar-Activated Persulfate for Organic Contaminants Removal: Efficiency, Mechanisms and Influencing Factors. Ecotoxicol. Environ. Saf. 2020, 198, 110653. [Google Scholar] [CrossRef] [PubMed]
- Huong, P.T.; Jitae, K.; Al Tahtamouni, T.M.; Le Minh Tri, N.; Kim, H.-H.; Cho, K.H.; Lee, C. Novel Activation of Peroxymonosulfate by Biochar Derived from Rice Husk toward Oxidation of Organic Contaminants in Wastewater. J. Water Process. Eng. 2020, 33, 101037. [Google Scholar] [CrossRef]
- Avramiotis, E.; Frontistis, Z.; Manariotis, I.D.; Vakros, J.; Mantzavinos, D. Oxidation of Sulfamethoxazole by Rice Husk Biochar-Activated Persulfate. Catalysts 2021, 11, 850. [Google Scholar] [CrossRef]
- Biswas, R.K.; Khan, P.; Mukherjee, S.; Mukhopadhyay, A.K.; Ghosh, J.; Muraleedharan, K. Study of Short Range Structure of Amorphous Silica from PDF Using Ag Radiation in Laboratory XRD System, RAMAN and NEXAFS. J. Non-Cryst. Solids 2018, 488, 1–9. [Google Scholar] [CrossRef]
- Qian, L.; Guo, F.; Jia, X.; Zhan, Y.; Zhou, H.; Jiang, X.; Tao, C. Recent Development in the Synthesis of Agricultural and Forestry Biomass-Derived Porous Carbons for Supercapacitor Applications: A Review. Ionics 2020, 26, 3705–3723. [Google Scholar] [CrossRef]
- Andrade, T.S.; Vakros, J.; Mantzavinos, D.; Lianos, P. Biochar Obtained by Carbonization of Spent Coffee Grounds and Its Application in the Construction of an Energy Storage Device. Chem. Eng. J. Adv. 2020, 4, 100061. [Google Scholar] [CrossRef]
- Cho, D.-W.; Yoon, K.; Kwon, E.E.; Biswas, J.K.; Song, H. Fabrication of Magnetic Biochar as a Treatment Medium for As(V) via Pyrolysis of FeCl3 -Pretreated Spent Coffee Ground. Environ. Pollut. 2017, 229, 942–949. [Google Scholar] [CrossRef]
- Niu, H.; Liu, S.; Cai, Y.; Wu, F.; Zhao, X. MOF Derived Porous Carbon Supported Cu/Cu2O Composite as High Performance Non-Noble Catalyst. Microporous Mesoporous Mater. 2016, 219, 48–53. [Google Scholar] [CrossRef]
- Tran, T.N.; Pham, T.V.A.; Le, M.L.P.; Nguyen, T.P.T.; Tran, V.M. Synthesis of Amorphous Silica and Sulfonic Acid Functionalized Silica Used as Reinforced Phase for Polymer Electrolyte Membrane. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 045007. [Google Scholar] [CrossRef] [Green Version]
- Magioglou, E.; Frontistis, Z.; Vakros, J.; Manariotis, I.; Mantzavinos, D. Activation of Persulfate by Biochars from Valorized Olive Stones for the Degradation of Sulfamethoxazole. Catalysts 2019, 9, 419. [Google Scholar] [CrossRef] [Green Version]
- Ntaflou, M.; Vakros, J. Transesterification activity of modified biochars from spent malt rootlets using triacetin. J. Clean. Prod. 2020, 259, 120931. [Google Scholar] [CrossRef]
- Mrozik, W.; Minofar, B.; Thongsamer, T.; Wiriyaphong, N.; Khawkomol, S.; Plaimart, J.; Vakros, J.; Karapanagioti, H.; Vinitnantharat, S.; Werner, D. Valorisation of Agricultural Waste Derived Biochars in Aquaculture to Remove Organic Micropollutants from Water—Experimental Study and Molecular Dynamics Simulations. J. Environ. Manag. 2021, 300, 113717. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.T.; Minh, T.D.; Nguyet, H.M.; Sharma, A.K. Ampicillin Adsorption onto Amine-Functionalized Magnetic Graphene Oxide: Synthesis, Characterization and Removal Mechanism. Korean J. Chem. Eng. 2021, 38, 22–31. [Google Scholar] [CrossRef]
- Yu, J.; Tang, L.; Pang, Y.; Zeng, G.; Wang, J.; Deng, Y.; Liu, Y.; Feng, H.; Chen, S.; Ren, X. Magnetic Nitrogen-Doped Sludge-Derived Biochar Catalysts for Persulfate Activation: Internal Electron Transfer Mechanism. Chem. Eng. J. 2019, 364, 146–159. [Google Scholar] [CrossRef]
- Guo, Y.; Zeng, Z.; Liu, Y.; Huang, Z.; Cui, Y.; Yang, J. One-Pot Synthesis of Sulfur Doped Activated Carbon as a Superior Metal-Free Catalyst for the Adsorption and Catalytic Oxidation of Aqueous Organics. J. Mater. Chem. A 2018, 6, 4055–4067. [Google Scholar] [CrossRef]
- Martínez, L.; Bilski, P.; Chignell, C.F. Effect of Magnesium and Calcium Complexation on the Photochemical Properties of Norfloxacin. Photochem. Photobiol. 1996, 64, 911–917. [Google Scholar] [CrossRef]
- Kłosińska-Szmurło, E.; Pluciński, F.A.; Grudzień, M.; Betlejewska-Kielak, K.; Biernacka, J.; Mazurek, A.P. Experimental and Theoretical Studies on the Molecular Properties of Ciprofloxacin, Norfloxacin, Pefloxacin, Sparfloxacin, and Gatifloxacin in Determining Bioavailability. J. Biol. Phys. 2014, 40, 335–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Vecchio, P.; Haro, N.K.; Souza, F.S.; Marcílio, N.R.; Féris, L.A. Ampicillin Removal by Adsorption onto Activated Carbon: Kinetics, Equilibrium and Thermodynamics. Water Sci. Technol. 2019, 79, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Bourikas, K.; Vakros, J.; Kordulis, C.; Lycourghiotis, A. Potentiometric Mass Titrations: Experimental and Theoretical Establishment of a New Technique for Determining the Point of Zero Charge (PZC) of Metal (Hydr)Oxides. J. Phys. Chem. B 2003, 107, 9441–9451. [Google Scholar] [CrossRef]
- Özkal, C.B.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Mantzavinos, D.; Meriç, S. Removal of Antibiotics in a Parallel- Plate Thin-Film-Photocatalytic Reactor: Process Modeling and Evolution of Transformation by-Products and Toxicity. J. Environ. Sci. 2017, 60, 114–122. [Google Scholar] [CrossRef] [PubMed]



| RHB700 | RHB850 | |||
|---|---|---|---|---|
| Scavenger | kapp (min−1) | μg SMX/mg Biochar | kapp (min−1) | μg SMX/mg Biochar |
| UPW | 1.0 × 10−2 | 0.103 | 2.4 × 10−2 | 1.45 |
| MeOH | 7.1 × 10−3 | 0.022 | 1.6 × 10−2 | 1.14 |
| t-BuOH | 3.5 × 10−3 | 0.0099 | 1.5 × 10−2 | 0.56 |
| NaN3 | 5.2 × 10−3 | 0.036 | 8.6 × 10−3 | 1.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avramiotis, E.; Frontistis, Z.; Manariotis, I.D.; Vakros, J.; Mantzavinos, D. On the Performance of a Sustainable Rice Husk Biochar for the Activation of Persulfate and the Degradation of Antibiotics. Catalysts 2021, 11, 1303. https://doi.org/10.3390/catal11111303
Avramiotis E, Frontistis Z, Manariotis ID, Vakros J, Mantzavinos D. On the Performance of a Sustainable Rice Husk Biochar for the Activation of Persulfate and the Degradation of Antibiotics. Catalysts. 2021; 11(11):1303. https://doi.org/10.3390/catal11111303
Chicago/Turabian StyleAvramiotis, Efstathios, Zacharias Frontistis, Ioannis D. Manariotis, John Vakros, and Dionissios Mantzavinos. 2021. "On the Performance of a Sustainable Rice Husk Biochar for the Activation of Persulfate and the Degradation of Antibiotics" Catalysts 11, no. 11: 1303. https://doi.org/10.3390/catal11111303
APA StyleAvramiotis, E., Frontistis, Z., Manariotis, I. D., Vakros, J., & Mantzavinos, D. (2021). On the Performance of a Sustainable Rice Husk Biochar for the Activation of Persulfate and the Degradation of Antibiotics. Catalysts, 11(11), 1303. https://doi.org/10.3390/catal11111303










