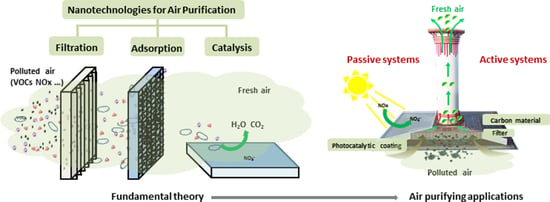
Ambient Air Purification by Nanotechnologies: From Theory to Application
Abstract
:1. Introduction
1.1. Major Sources of NOx and Common VOCs
1.2. Conventional Control Methods for Air Pollutants
| Type of Pollutant | Method | Mechanisms and Usage in China | Efficiency | Advantages and Drawbacks | Applications | Reference |
|---|---|---|---|---|---|---|
| Particulate matter/dust | Electrostatic precipitators | Particle collection by electrostatic forces, more than 90% coal-fired power plants installed | Poor performance for PM2.5 removal | Effective for large (>1 μm) and ultrafine (<0.1 μm) particle removal, low cost and energy consumption, affected by temperature and humidity | Coal-fired power plants, cement and metallurgy industries; household dust removal | [2,28] |
| Wet scrubbers | Particle adsorption by liquid sprays, dominant technology in China | Low efficiency for PM2.5 removal (~50%) | Simultaneous removal of some gaseous pollutants; high power consumption, metal corrosion, need for effluent treatment | Coal-fired power plants and cement industries | [2,19] | |
| Cyclones | Particle collection by centrifugal forces, typical pre-cleaners for large particles in most industries | Low efficiency for PM2.5 removal | Low cost and simple structure, not recommended for PM2.5 removal | Coal-fired power plants, cement and chemical industries | [2,19] | |
| Baghouse (or fabric filters) | Particle collection by filtration through porous textile fabrics, less than 10% thermal power plants installed | High efficiency for PM2.5 removal (>99%) | High efficiency for dust, simple structure, low investment; regeneration of filter media, temperature sensitivity | Coal-fired power plants, cement and iron–steel industries | [2,29] | |
| Nitrogen oxides | Selective catalytic reduction | Catalytic reduction by NH3 or hydrocarbon over metal oxides at high temperatures to form N2 and H2O nearly 90% thermal power plants installed | High deNOx efficiency (80–90%) | High conversion rate, adaptable for high dust conditions (particulate levels 1 to 30 g/m3), NH3 leaks and high cost of catalysts (i.e., Pt/Al2O3) | Coal-fired power plants, cement and iron–steel plants, industrial boilers, diesel engines | [26] |
| Selective non-catalytic reduction | Reduction by NH3 or aqueous urea at very high temperatures (850 to 1100 °C), nearly 90% thermal power plants installed | Low deNOx efficiency (30–75%) | Simplicity; catalyst free; low capital and operational costs; NH3 leaks, high temperature and narrow temperature window | All types of stationary fired equipment | [13] | |
| Three-way catalyst | Conversion of CO into CO2 and NOx into N2 and O2 over a catalyst surface | High deNOx efficiency (>95%) | High efficiency; high cost of catalysts (i.e., Pt/Rh/Pd); not suitable for diesel engines | Gasoline-powered motor vehicle engines | [30] | |
| Volatile organic compounds | Adsorption (combined with condensation or recovery) | Gas adsorption over zeolite and carbonaceous material (activated carbon, biochar, carbon nanotube and grapheme); widely used | High efficiency (>90%) | Economical, recover useful solvents, suitable for highly diluted exhaust gas stream with large volume; high regeneration cost and secondary pollution due to desorption | Petroleum-related industries, chemical industries, packing and printing industries, spraying and painting in car industries and indoor environments | [18,31] |
| Regenerative thermal oxidation | Incineration at extremely high temperatures (>1000 °C); widely used | High efficiency (>99%) | High efficiency in removing VOCs from flue gas streams with high flow rates and high VOC concentration; high energy consumption; undesirable by-products, such as dioxins and CO; possible explosion | Petroleum and coke industries, chemical industries, printing industries, spraying and painting industries, pharmaceutical plants, textile industries | [26] | |
| Regenerative catalytic oxidation | Catalytic oxidation over noble metals or non-noble metal oxides at moderate temperatures (250 to 500 °C), widely used | High efficiency (>99%) | Energy efficient, suitable for dilute effluent streams of VOCs (<1% VOCs) with moderate flow rates, lower formation of dioxins and by-products, high cost of catalysts and catalyst deactivation | Printing industries, spraying and painting industries, pharmaceutical plants, textile industries, petroleum and coke industries | [26] |
2. Ambient Air Purification by Nanotechnologies
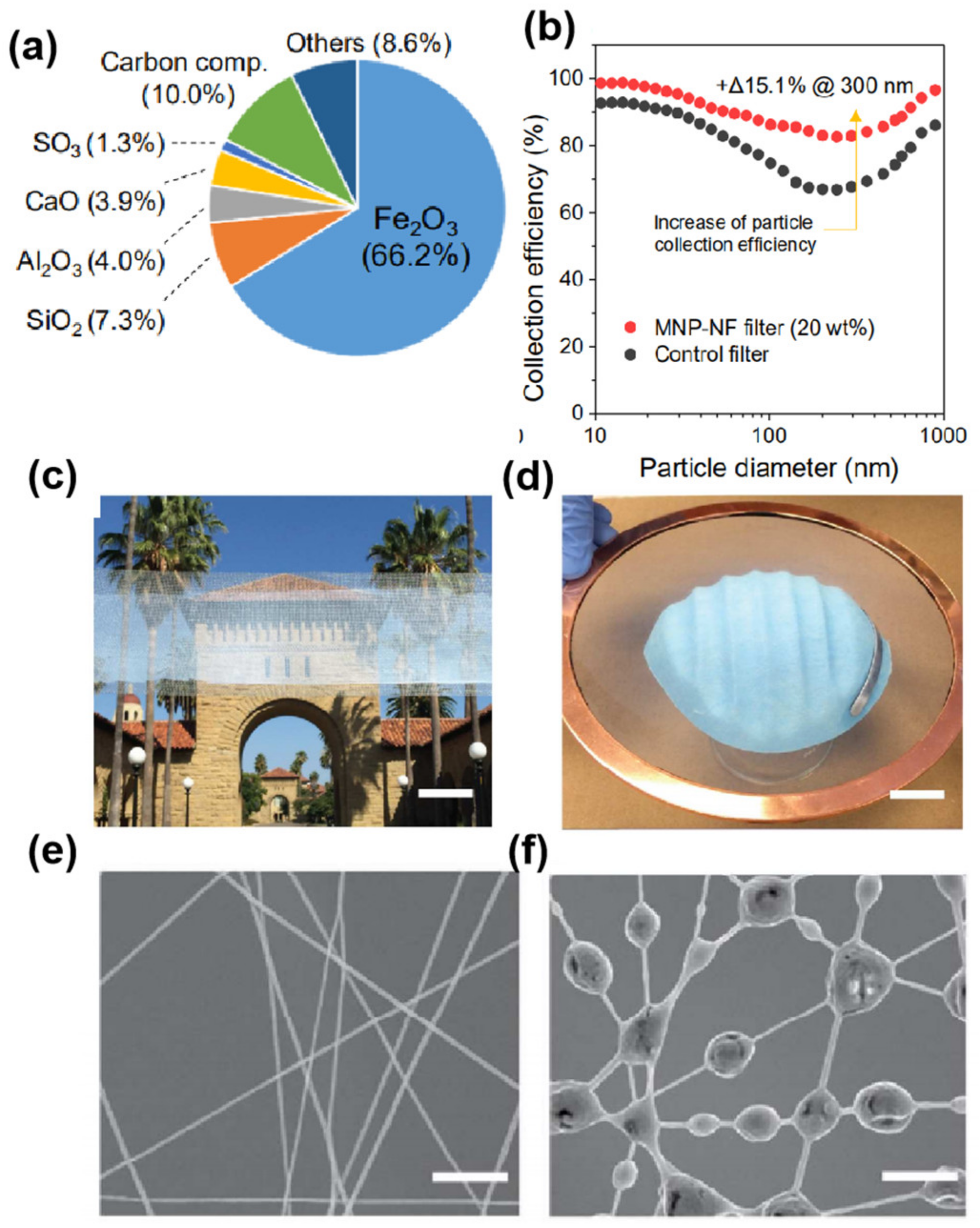
3. Filtration
4. Adsorption
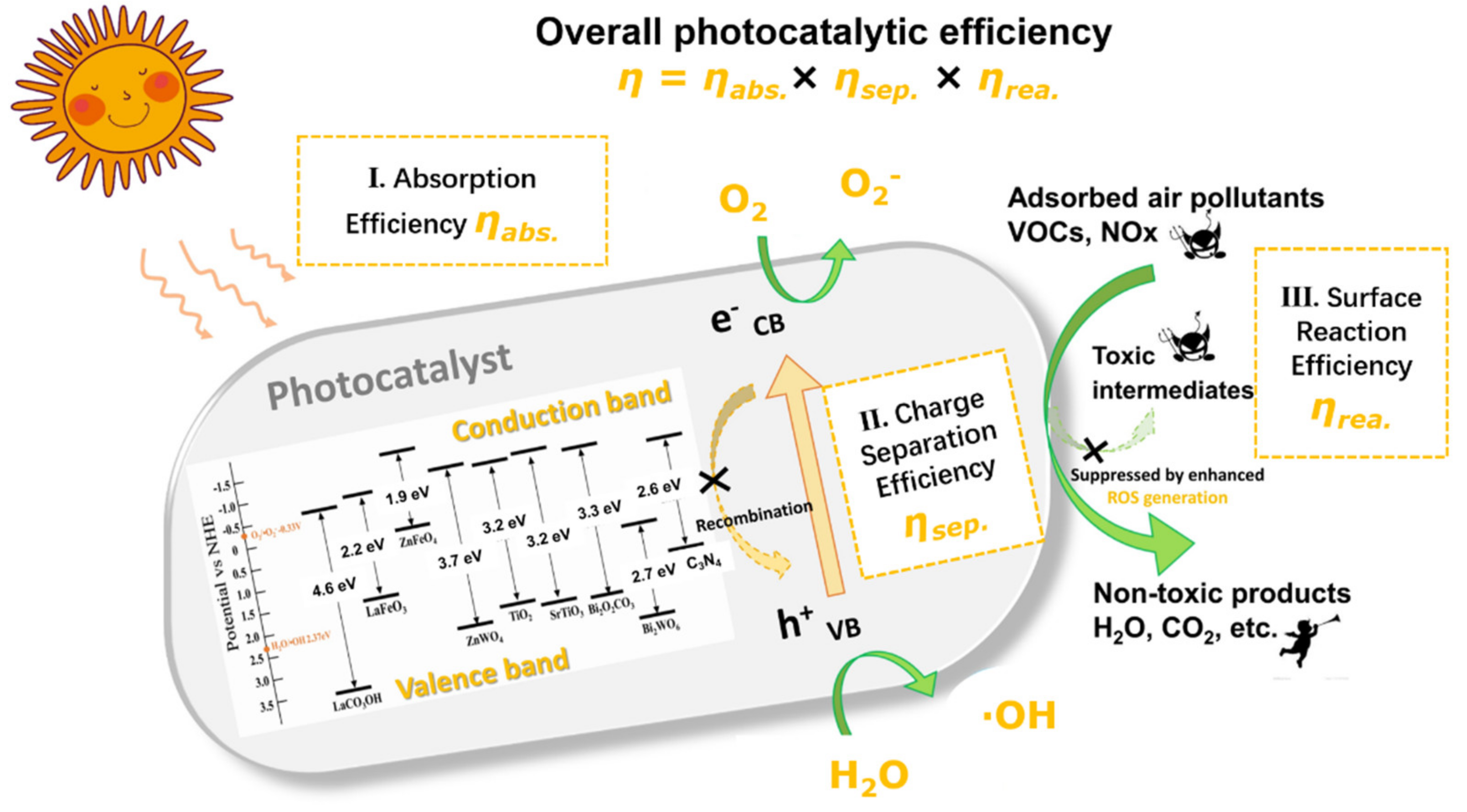
5. Photocatalysis
5.1. Principles of Photocatalysis and Major Nanomaterials
5.2. Key Aspects Underpinning Photocatalytic Efficiency
5.2.1. Light Absorption
5.2.2. Charge Separation
5.2.3. Surface Adsorption Properties
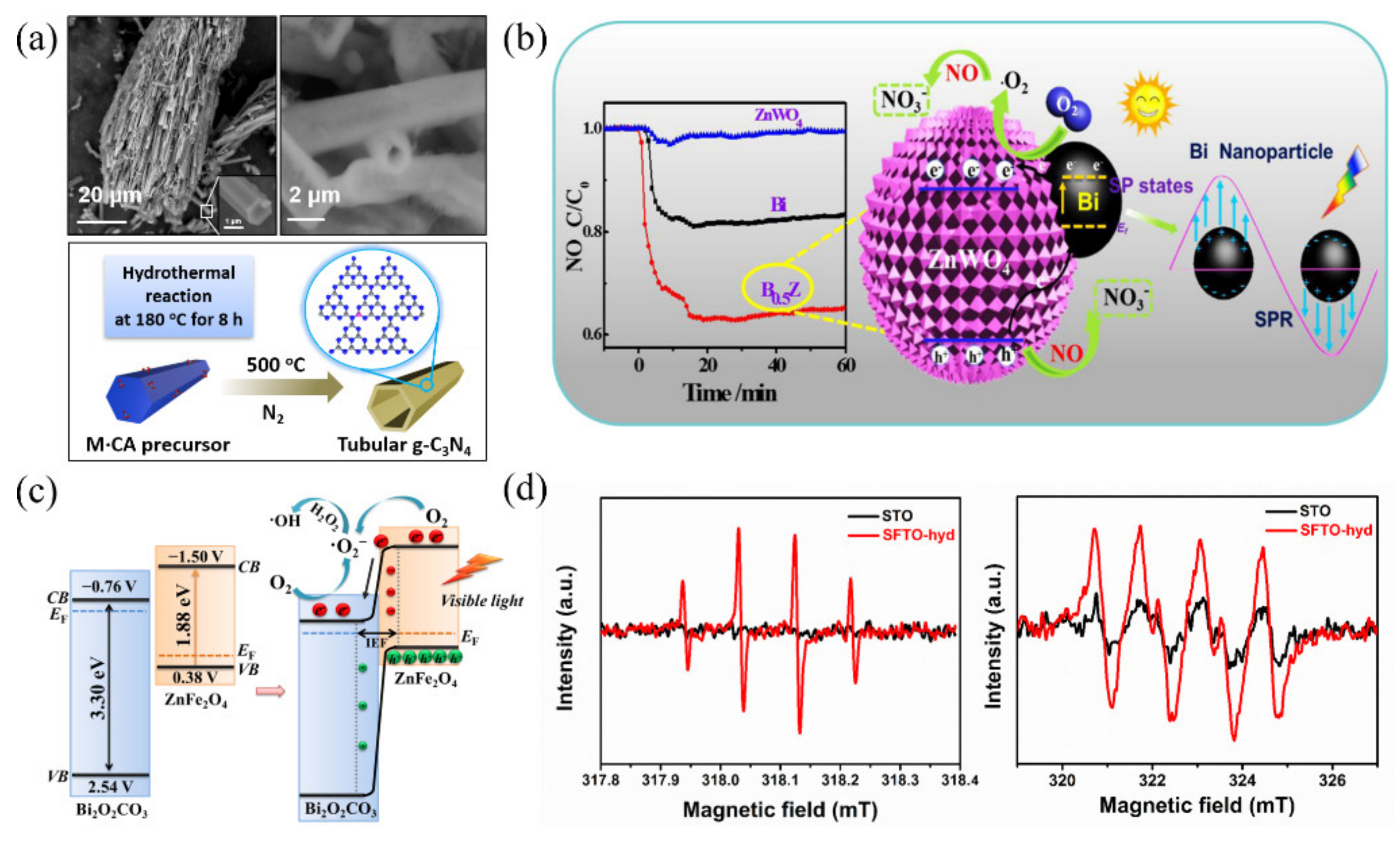
5.2.4. Effects of Environmental Conditions
- (1)
- Light intensity
- (2)
- Pollutant concentration
- (3)
- Relative humidity
- (4)
- Coexisting pollutants
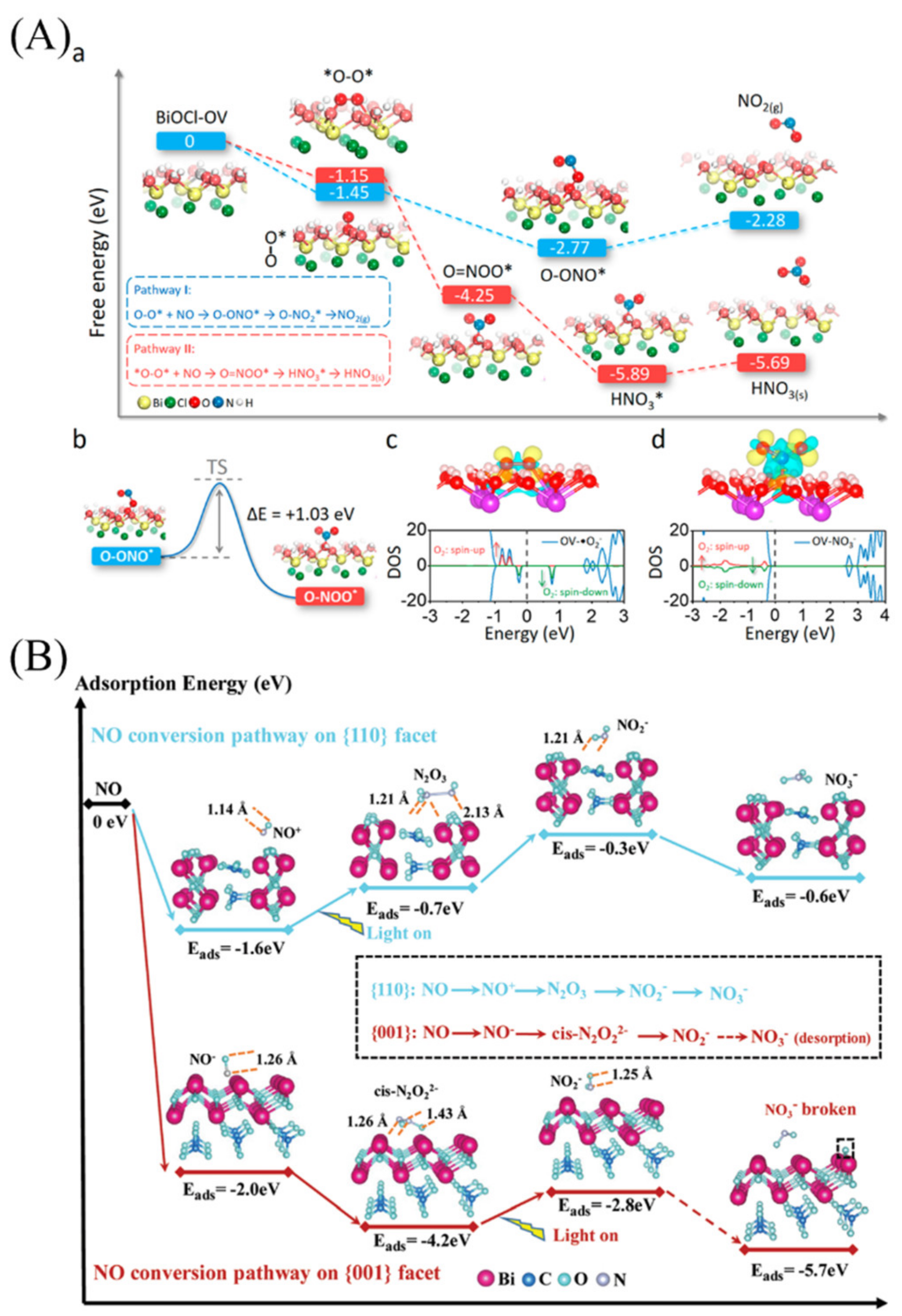
5.3. Generation of Intermediates, Catalyst Deactivation, and Regeneration
6. Room Temperature Catalysis
6.1. Key Variables Underpinning Catalytic Efficiency
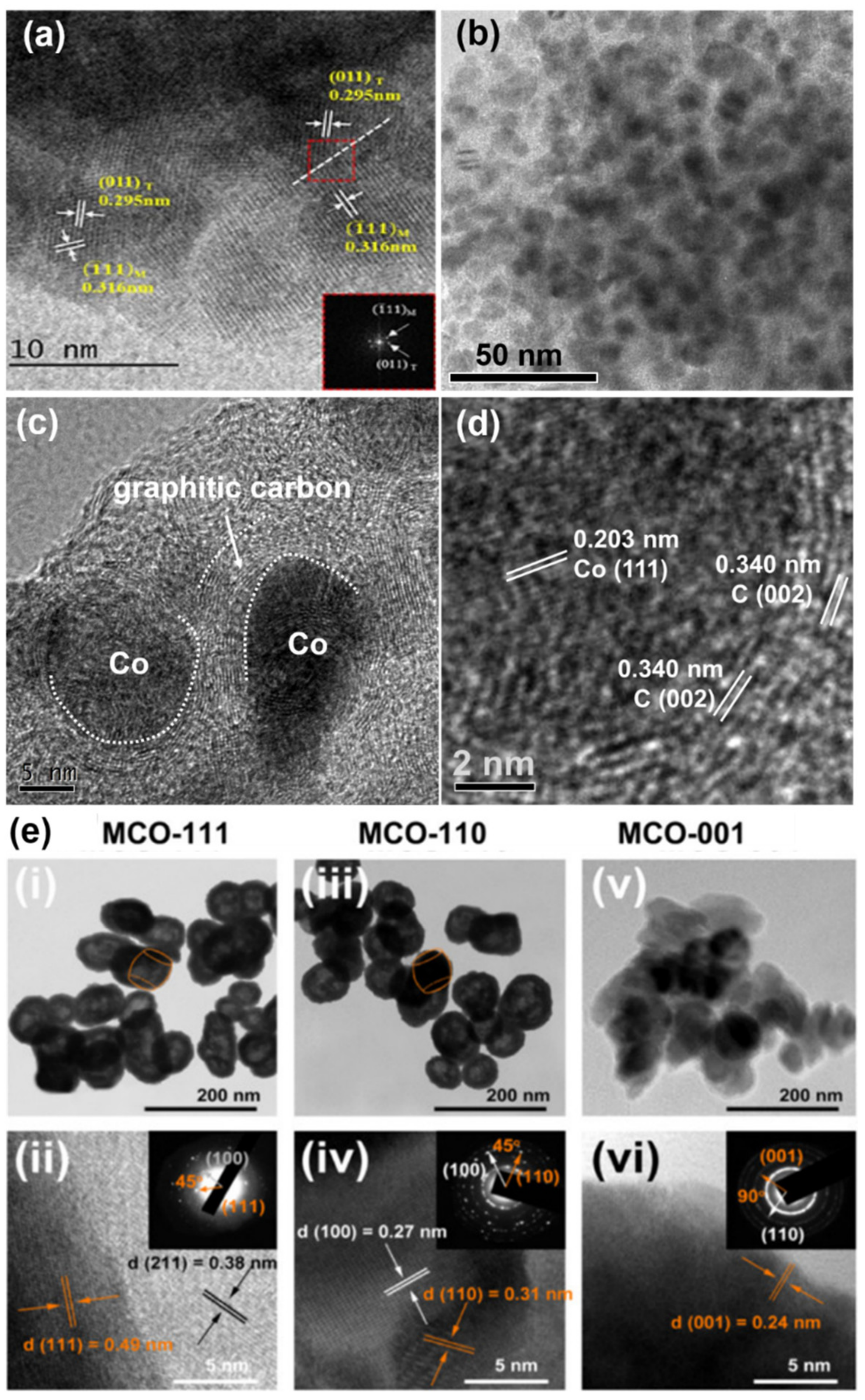
6.1.1. Structure, Crystallinity, and Surface/Interface State
6.1.2. Noble Metal Loading
6.1.3. Gas Hourly Space Velocity
6.1.4. Relative Humidity
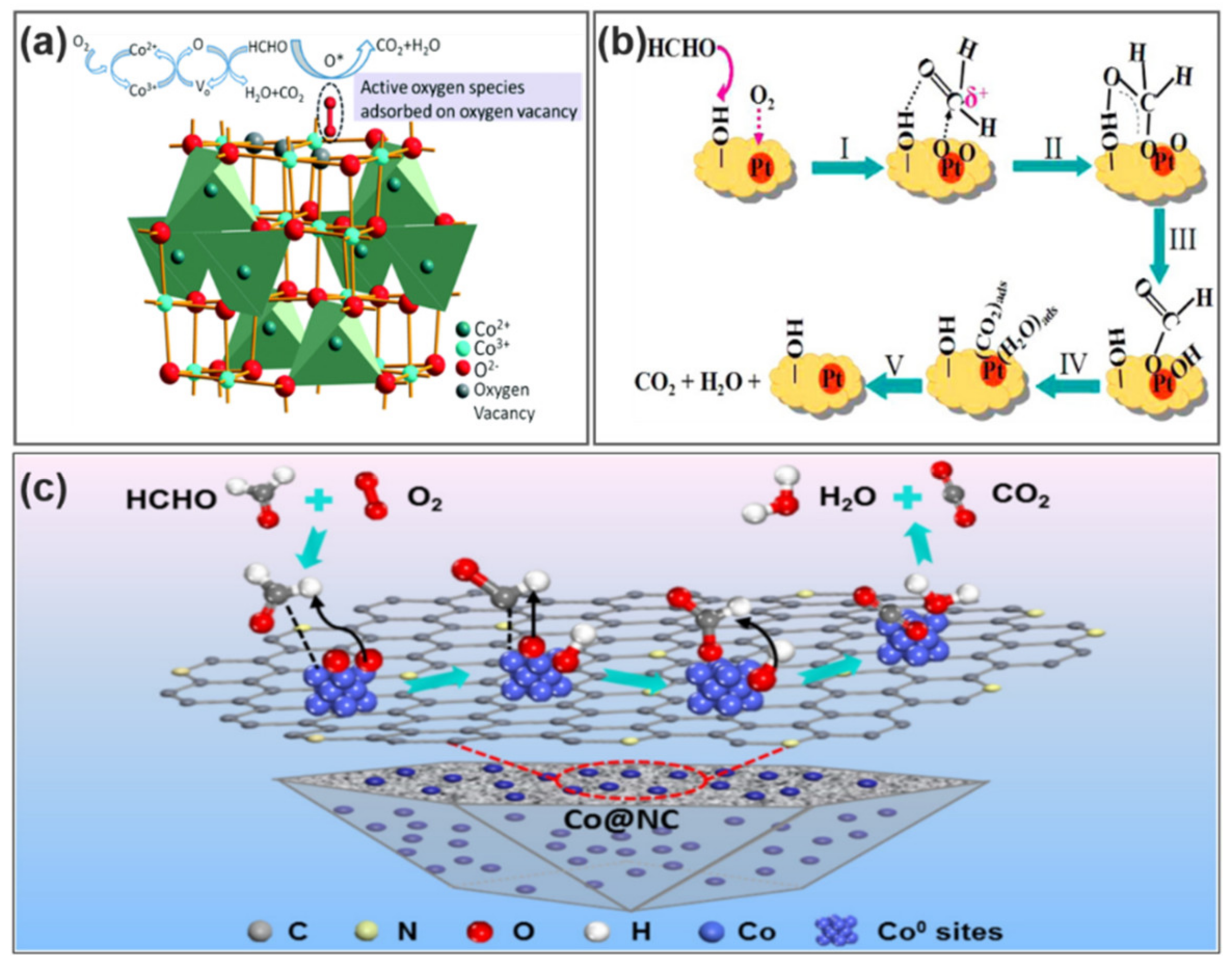
6.2. Catalytic Conversion Mechanisms
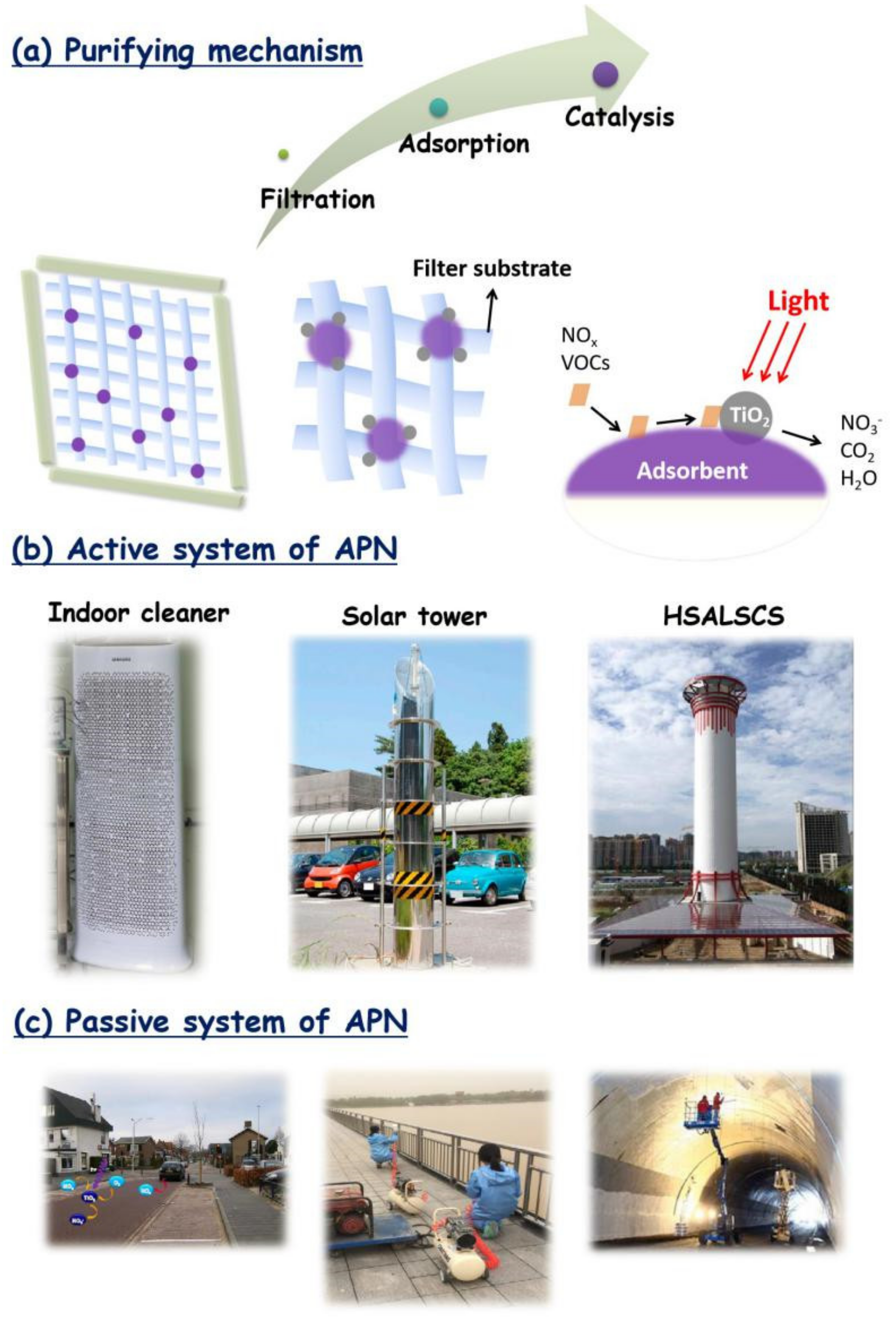
7. Applications of Ambient Air Purification by Nanotechnologies (APN)
7.1. Catalyst Immobilization
7.2. Passive Systems for APN
7.2.1. Roadside
7.2.2. Indoor Environment
7.2.3. Tunnels and Indoor Parking Lots
7.3. Active Systems for APN
7.3.1. Air Cleaners for Indoor Environment
7.3.2. Tunnel
7.3.3. Photocatalytic Solar Tower for an Industrial Zone
7.3.4. Catalytic Street Lamps
7.3.5. Hybrid Solar-Assisted Large-Scale Cleaning System (HSALSCS) for Large-Scale Air Cleaning
8. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations and Explanations
| PM2.5 | Particulate matter of less than 2.5 μm in size |
| VOCs | volatile organic compounds |
| SOA | secondary inorganic aerosol |
| LNBs | low NOx burners |
| MEIC | multi-resolution emission inventory for China |
| BTEX | benzene, toluene, ethylbenzene and xylene |
| SCR | Selective catalytic reduction |
| SNCR | selective non-catalytic reduction |
| TWC | three-way catalysis |
| APN | ambient air purification by nanotechnologies |
| TENG | triboelectric nanogenerator |
| AC | activated carbon |
| AOPs | advanced oxidation processes |
| ROS | reactive oxygen species |
| VB/CB | valence/conduction band |
| UV | ultraviolet |
| SPR | surface plasmon resonance |
| CDs | carbon nanodots |
| OVs | oxygen vacancies |
| RH | relative humidity |
| GC-MS | gas chromatography-mass spectrometry |
| in situ DRIFTS | In situ diffuse reflectance infrared fourier transform spectroscopy |
| SMSI | strong metal-support interaction |
| GHSV | gas hourly space velocity |
| L-H | Langmuir-Hinshelwood |
| E-R | Eley-Rideal |
| MvK | Mars-van-Krevelen |
| HSALSCS | hybrid solar-assisted large-scale cleaning system |
References
- Wang, L. The battle against PM2.5 is on. Natl. Sci. Rev. 2014, 1, 315–317. [Google Scholar] [CrossRef]
- Pui, D.Y.; Chen, S.-C.; Zuo, Z. PM2.5 in China: Measurements, sources, visibility and health effects, and mitigation. Particuology 2014, 13, 1–26. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef] [Green Version]
- An, Z.; Huang, R.-J.; Zhang, R.; Tie, X.; Li, G.; Cao, J.; Zhou, W.; Shi, Z.; Han, Y.; Gu, Z.; et al. Severe haze in northern China: A synergy of anthropogenic emissions and atmospheric processes. Proc. Natl. Acad. Sci. USA 2019, 116, 8657–8666. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.-J.; Zhang, Y.; Bozzetti, C.; Ho, K.F.; Cao, J.-J.; Han, Y.; Daellenbach, K.R.; Slowik, J.G.; Platt, S.; Canonaco, F.; et al. High secondary aerosol contribution to particulate pollution during haze events in China. Nature 2014, 514, 218–222. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Hu, M.; Zamora, M.L.; Peng, J.; Shang, D.; Zheng, J.; Du, Z.; Wu, Z.; Shao, M.; Zeng, L.; et al. Elucidat-ing severe urban haze formation in China. Proc. Natl. Acad. Sci. USA 2014, 111, 17373–17378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, E.G.; Wortham, H.; Strekowski, R.; Zetzsch, C.; Gligorovski, S. Atmospheric Photosensitized Heterogeneous and Multiphase Reactions: From Outdoors to Indoors. Environ. Sci. Technol. 2012, 46, 1955–1963. [Google Scholar] [CrossRef]
- WHO Guidelines for Indoor Air Quality: Selected Pollutants; World Health Organization: Geneva, Switzerland, 2010.
- Wang, S.; Ang, H.; Tade, M.O. Volatile organic compounds in indoor environment and photocatalytic oxidation: State of the art. Environ. Int. 2007, 33, 694–705. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Gómez-García, M.A.; Pitchon, V.; Kiennemann, A. Pollution by nitrogen oxides: An approach to NOx abatement by using sorbing catalytic materials. Environ. Int. 2005, 31, 445–467. [Google Scholar] [CrossRef] [PubMed]
- Skalska, K.; Miller, J.S.; Ledakowicz, S. Trends in NOx abatement: A review. Sci. Total Environ. 2010, 408, 3976–3989. [Google Scholar] [CrossRef] [PubMed]
- Perring, A.; Pusede, S.; Cohen, R.C. An Observational Perspective on the Atmospheric Impacts of Alkyl and Multifunctional Nitrates on Ozone and Secondary Organic Aerosol. Chem. Rev. 2013, 113, 5848–5870. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, H.; Geng, G.; Hong, C.; Liu, F.; Song, Y.; Tong, D.; Zheng, B.; Cui, H.; Man, H.; et al. Anthropogenic emission inventories in China: A review. Natl. Sci. Rev. 2018, 5, 603. [Google Scholar] [CrossRef]
- Kurtenbach, R.; Becker, K.; Gomes, J.; Kleffmann, J.; Lörzer, J.; Spittler, M.; Wiesen, P.; Ackermann, R.; Geyer, A.; Platt, U. Investigations of emissions and heterogeneous formation of HONO in a road traffic tunnel. Atmos. Environ. 2001, 35, 3385–3394. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Q.; Kurokawa, J.-I.; Woo, J.-H.; He, K.; Lu, Z.; Ohara, T.; Song, Y.; Streets, D.G.; Carmichael, G.R.; et al. MIX: A mosaic Asian anthropogenic emission inventory under the international collaboration framework of the MICS-Asia and HTAP. Atmos. Chem. Phys. Discuss. 2017, 17, 935–963. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, M.; Fu, L.; Lu, S.; Zeng, L.; Tang, D. Source profiles of volatile organic compounds (VOCs) measured in China: Part I. Atmos. Environ. 2008, 42, 6247–6260. [Google Scholar] [CrossRef]
- Darcovich, K.; Jonasson, K.; Capes, C. Developments in the control of fine particulate air emissions. Adv. Powder Technol. 1997, 8, 179–215. [Google Scholar] [CrossRef] [Green Version]
- Lasek, J.; Yu, Y.-H.; Wu, J.C.S. Removal of NOx by photocatalytic processes. J. Photochem. Photobiol. C Photochem. Rev. 2013, 14, 29–52. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, L.; Ji, J.; Wang, Q.; Fang, M. comprehensive CFD combustion model for supercritical CFB boilers. Particuology 2019, 43, 29–37. [Google Scholar] [CrossRef]
- Armesto, L.; Bahillo, A.; Cabanillas, A.; Veijonen, K.; Otero, J.; Plumed, A.; Salvador, L. Co-combustion of coal and olive oil in-dustry residues in fluidised bed. Fuel 2003, 82, 993–1000. [Google Scholar] [CrossRef]
- Krzywanski, J.; Czakiert, T.; Shimizu, T.; Majchrzak-Kuceba, I.; Shimazaki, Y.; Zylka, A.; Grabowska, K.; Sosnowski, M. NOx Emissions from Regenerator of Calcium Looping Process. Energy Fuels 2018, 32, 6355–6362. [Google Scholar] [CrossRef]
- Krzywanski, J.; Żyłka, A.; Czakiert, T.; Kulicki, K.; Jankowska, S.; Nowak, W. A 1.5D model of a complex geometry laboratory scale fuidized bed clc equipment. Powder Technol. 2017, 316, 592–598. [Google Scholar] [CrossRef]
- Heck, R.M. Catalytic abatement of nitrogen oxides–stationary applications. Catal. Today 1999, 53, 519–523. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Mudliar, S.; Giri, B.S.; Padoley, K.; Satpute, D.; Dixit, R.; Bhatt, P.; Pandey, R.; Juwarkar, A.; Vaidya, A. Bioreactors for treatment of VOCs and odours—A review. J. Environ. Manag. 2010, 91, 1039–1054. [Google Scholar] [CrossRef]
- Mizuno, A. Electrostatic precipitation. IEEE Trans. Dielectr. Electr. Insul. 2000, 7, 615–624. [Google Scholar] [CrossRef]
- Mukhopadhyay, A. Pulse-jet filtration: An effective way to control industrial pollution Part I: Theory, selection and design of pulse-jet filter. Text. Prog. 2009, 41, 195–315. [Google Scholar] [CrossRef]
- Granger, P.; Parvulescu, V.I. Catalytic NOx abatement systems for mobile sources: From three-way to lean burn after-treatment technologies. Chem. Rev. 2011, 111, 3155–3207. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.-Y.; Lin, X.-D.; Chen, S.-X.; Wei, X.-Q. Adsorption of VOC on modified activated carbon fiber. J. Porous Mater. 2008, 16, 521–526. [Google Scholar] [CrossRef]
- Liu, C.; Hsu, P.-C.; Lee, H.-W.; Ye, M.; Zheng, G.; Liu, N.; Li, W.; Cui, Y. Transparent air filter for high-efficiency PM2.5 capture. Nat. Commun. 2015, 6, 6205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Ling, L.; Nie, J.; Han, K.; Chen, X.; Bian, Z.; Li, H.; Wang, Z.L. Self-powered electrostatic filter with enhanced photo-catalytic degradation of formaldehyde based on built-in triboelectric nanogenerators. ACS Nano 2017, 11, 12411–12418. [Google Scholar] [CrossRef]
- Xu, J.; Liu, C.; Hsu, P.-C.; Liu, K.; Zhang, R.; Liu, Y.; Cui, Y. Roll-to-Roll Transfer of Electrospun Nanofiber Film for High-Efficiency Transparent Air Filter. Nano Lett. 2016, 16, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, X.; Yin, X.; Yu, J.; Ding, B. Electret Polyvinylidene Fluoride Nanofibers Hybridized by Polytetrafluoroethylene Nanoparticles for High-Efficiency Air Filtration. ACS Appl. Mater. Interfaces 2016, 8, 23985–23994. [Google Scholar] [CrossRef]
- Kim, J.; Hong, S.C.; Bae, G.N.; Jung, J.H. Electrospun Magnetic Nanoparticle-Decorated Nanofiber Filter and Its Applications to High-Efficiency Air Filtration. Environ. Sci. Technol. 2017, 51, 11967–11975. [Google Scholar] [CrossRef] [PubMed]
- Kadam, V.; Truong, Y.B.; Easton, C.; Mukherjee, S.; Wang, L.; Padhye, R.; Kyratzis, I.L. Electrospun polyacryloni-trile/β-cyclodextrin composite membranes for simultaneous air filtration and adsorption of volatile organic compounds. ACS Appl. Nano Mater. 2018, 1, 4268–4277. [Google Scholar] [CrossRef]
- Lv, D.; Wang, R.; Tang, G.; Mou, Z.; Lei, J.; Han, J.; de Smedt, S.; Xiong, R.; Huang, C. Ecofriendly electrospun membranes loaded with visible-light responding nanoparticles for multifunctional usages: Highly efficient air filtration, dye scavenging, and bactericidal activity. ACS Appl. Mater. Interfaces 2019, 11, 12880–12889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, W.; Gao, B.; Ok, Y.S.; Dong, L. Integrated adsorption and photocatalytic degradation of volatile organic compounds (VOCs) using carbon-based nanocomposites: A critical review. Chemosphere 2018, 218, 845–859. [Google Scholar] [CrossRef]
- Yao, H.C.; Shelef, M. The surface interaction of O2 and NO with manganous oxide. J. Catal. 1973, 31, 377–383. [Google Scholar] [CrossRef]
- Winter, E. The catalytic decomposition of nitric oxide by metallic oxides. J. Catal. 1971, 22, 158–170. [Google Scholar] [CrossRef]
- Otto, K.; Shelef, M. The adsorption of nitric oxide on iron oxides. J. Catal. 1970, 18, 184–192. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Chiang, P.-C.; Huang, C.-P. Effects of pore structure and temperature on VOC adsorption on activated carbon. Carbon 2001, 39, 523–534. [Google Scholar] [CrossRef]
- Lillo-Rodenas, M.A.; Cazorla-Amoros, D.; Linares-Solano, A. Behaviour of activated carbons with different pore size distribu-tions and surface oxygen groups for benzene and toluene adsorption at low concentrations. Carbon 2005, 43, 1758–1767. [Google Scholar] [CrossRef]
- Wang, H.; Lashaki, M.J.; Fayaz, M.; Hashisho, Z.; Philips, J.H.; Anderson, J.E.; Nichols, M. Adsorption and desorption of mix-tures of organic vapors on beaded activated carbon. Environ. Sci. Technol. 2012, 46, 8341–8350. [Google Scholar] [CrossRef]
- Lee, K.J.; Shiratori, N.; Lee, G.H.; Miyawaki, J.; Mochida, I.; Yoon, S.-H.; Jang, J. Activated carbon nanofiber produced from electrospun polyacrylonitrile nanofiber as a highly efficient formaldehyde adsorbent. Carbon 2010, 48, 4248–4255. [Google Scholar] [CrossRef]
- Baur, G.B.; Yuranov, I.; Kiwi-Minsker, L. Activated carbon fibers modified by metal oxide as effective structured adsorbents for acetaldehyde. Catal. Today 2015, 249, 252–258. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Shih, Y.-H.; Li, M.-S. Adsorption of selected volatile organic vapors on multiwall carbon nanotubes. J. Hazard. Mater. 2008, 154, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, N.; Lu, X.; Elimelech, M.; Tufenkji, N. Environmental performance of graphene-based 3D macrostructures. Nat. Nanotechnol. 2019, 14, 107–119. [Google Scholar] [CrossRef]
- Shen, Y.; Fang, Q.; Chen, B. Environmental Applications of Three-Dimensional Graphene-Based Macrostructures: Adsorption, Transformation, and Detection. Environ. Sci. Technol. 2014, 49, 67–84. [Google Scholar] [CrossRef]
- Chabot, V.; Higgins, D.; Yu, A.; Xiao, X.; Chen, Z.; Zhang, J. A review of graphene and graphene oxide sponge: Material synthesis and applications to energy and the environment. Energy Environ. Sci. 2014, 7, 1564–1596. [Google Scholar] [CrossRef]
- Hu, M.; Hui, K.S. Role of graphene in MnO2/graphene composite for catalytic ozonation of gaseous toluene. Chem. Eng. J. 2014, 254, 237–244. [Google Scholar] [CrossRef]
- Takeuchi, K.; Ibusuk, T. Heterogeneous photochemical reactions of a propylene-nitrogen dioxide-metal oxide-dry air system. Atmos. Environ. 1986, 20, 1155–1160. [Google Scholar] [CrossRef]
- Ibusuki, T.; Takeuchi, K. Removal of low concentration nitrogen oxides through photoassisted heterogeneous catalysis. J. Mol. Catal. 1994, 88, 93–102. [Google Scholar] [CrossRef]
- Paz, Y. Application of TiO2 photocatalysis for air treatment: Patents’ overview. Appl. Catal. B Environ. 2010, 99, 448–460. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Xu, C.; Anusuyadevi, P.R.; Aymonier, C.; Luque, R.; Marre, S. Nanostructured materials for photocatalysis. Chem. Soc. Rev. 2019, 48, 3868–3902. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Z.-R.; Fu, X.; Xu, Y.-J. Nanocomposite of Ag-AgBr-TiO2 as a photoactive and durable catalyst for degradation of volatile organic compounds in the gas phase. Appl. Catal. B Environ. 2011, 106, 445–452. [Google Scholar] [CrossRef]
- Zhu, X.; Jin, C.; Li, X.-S.; Liu, J.-L.; Sun, Z.; Shi, C.; Li, X.; Zhu, A.-M. Photocatalytic Formaldehyde Oxidation over Plasmonic Au/TiO2 under Visible Light: Moisture Indispensability and Light Enhancement. ACS Catal. 2017, 7, 6514–6524. [Google Scholar] [CrossRef]
- Li, J.; Dong, X.; Sun, Y.; Cen, W.; Dong, F. Facet-dependent interfacial charge separation and transfer in plasmonic photo-catalysts. Appl. Catal. B Environ. 2018, 226, 269–277. [Google Scholar] [CrossRef]
- Weon, S.; Choi, E.; Kim, H.; Kim, J.Y.; Park, H.-J.; Kim, S.-m.; Kim, W.; Choi, W. Active {001} facet exposed TiO2 nanotubes pho-tocatalyst filter for volatile organic compounds removal: From material development to commercial indoor air cleaner appli-cation. Environ. Sci. Technol. 2018, 52, 9330–9340. [Google Scholar] [CrossRef]
- Li, P.; Kim, S.; Jin, J.; Chun, D.H.; Park, J.H. Efficient photodegradation of volatile organic compounds by iron-based met-al-organic frameworks with high adsorption capacity. Appl. Catal. B Environ. 2020, 263, 118284–118293. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, M.; Huang, Y.; Shi, X.; Zhang, Y.; Huang, T.; Cao, J.; Ho, W.; Lee, S.-C. Self-assembly synthesis of boron-doped graphitic carbon nitride hollow tubes for enhanced photocatalytic NOx removal under visible light. Appl. Catal. B Environ. 2018, 239, 352–361. [Google Scholar] [CrossRef]
- Weon, S.; Kim, J.; Choi, W. Dual-components modified TiO2 with Pt and fluoride as deactivation-resistant photocatalyst for the degradation of volatile organic compound. Appl. Catal. B Environ. 2018, 220, 1–8. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, Y.; Rao, Y.; Zhu, D.; Cao, J.-J.; Shen, Z.; Ho, W.; Lee, S.-C. Environment-Friendly Carbon Quantum Dots/ZnFe2O4 Photocatalysts: Characterization, Biocompatibility, and Mechanisms for NO Removal. Environ. Sci. Technol. 2017, 51, 2924–2933. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Huang, Y.; Li, Y.; Zhang, Q.; Cao, J.; Ho, W.; Lee, S.C. Plasmonic Bi/ZnWO4 microspheres with improved photocata-lytic activity on NO removal under visible light. ACS Sustain. Chem. Eng. 2016, 4, 6912–6920. [Google Scholar] [CrossRef]
- Luo, S.; Ke, J.; Yuan, M.; Zhang, Q.; Xie, P.; Deng, L.; Wang, S. CuInS2 quantum dots embedded in Bi2WO6 nanoflowers for enhanced visible light photocatalytic removal of contaminants. Appl. Catal. B Environ. 2018, 221, 215–222. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, D.; Zhang, Q.; Zhang, Y.; Cao, J.-J.; Shen, Z.; Ho, W.; Lee, S.-C. Synthesis of a Bi2O2CO3/ZnFe2O4 heterojunction with enhanced photocatalytic activity for visible light irradiation-induced NO removal. Appl. Catal. B Environ. 2018, 234, 70–78. [Google Scholar] [CrossRef]
- Zhou, H.; Wen, Z.; Liu, J.; Ke, J.; Duan, X.; Wang, S. Z-scheme plasmonic Ag decorated WO3/Bi2WO6 hybrids for enhanced photocatalytic abatement of chlorinated-VOCs under solar light irradiation. Appl. Catal. B Environ. 2018, 242, 76–84. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Huang, Y.; Cao, J.; Huang, T.; Li, R.; Zhang, Q.; Lee, S.C.; Ho, W.K. Exploring the photocatalytic conversion mechanism of gaseous formaldehyde degradation on TiO2−x-OV surface. J. Hazard. Mater. 2021, 424, 127217–127226. [Google Scholar] [CrossRef]
- Rao, Z.; Xie, X.; Wang, X.; Mahmood, A.; Tong, S.; Ge, M.; Sun, J. Defect Chemistry of Er3+-Doped TiO2 and Its Photocata-lytic Activity for the Degradation of Flowing Gas-Phase VOCs. J. Phys. Chem. C 2019, 123, 12321–12334. [Google Scholar] [CrossRef]
- Roso, M.; Boaretti, C.; Pelizzo, M.G.; Lauria, A.; Modesti, M.; Lorenzetti, A. Nanostructured Photocatalysts Based on Different Oxidized Graphenes for VOCs Removal. Ind. Eng. Chem. Res. 2017, 56, 9980–9992. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, G.; Meng, Y.; Yang, C.; Ni, Z.; Hu, J. Kinetic and mechanistic analysis for the photodegradation of gaseous formaldehyde by core-shell CeO2@LDHs. Appl. Catal. B Environ. 2020, 278, 119266–119279. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, R.; Lin, J.; Zhu, Y. Enhancement of photocurrent and photocatalytic activity of ZnO hybridized with graphite-like C3N4. Energy Environ. Sci. 2011, 4, 2922–2929. [Google Scholar] [CrossRef]
- Chen, M.; Huang, Y.; Yao, J.; Cao, J.; Liu, Y. Visible-light-driven N-(BiO)2CO3/Graphene oxide composites with improved photocatalytic activity and selectivity for NOx removal. Appl. Surf. Sci. 2018, 430, 137–144. [Google Scholar] [CrossRef]
- Huang, Y.; Cao, J.-J.; Kang, F.; You, S.-J.; Chang, C.-W.; Wang, Y.-F. High selectivity of visible-light-driven La-doped TiO2 pho-tocatalysts for NO removal. Aerosol Air Qual. Res. 2017, 17, 2555–2565. [Google Scholar] [CrossRef]
- Liu, M.; Qiu, X.; Miyauchi, M.; Hashimoto, K. Energy-Level Matching of Fe(III) Ions Grafted at Surface and Doped in Bulk for Efficient Visible-Light Photocatalysts. J. Am. Chem. Soc. 2013, 135, 10064–10072. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.-M.; Disdier, J.; Pichat, P. Effect of chromium doping on the electrical and catalytic properties of powder titania under UV and visible illumination. Chem. Phys. Lett. 1984, 108, 618–622. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Y.; Xu, L.; Cao, J.; Ho, W.; Lee, S.C. Visible-light-active plasmonic Ag-SrTiO3 nanocomposites for the deg-radation of NO in air with high selectivity. ACS Appl. Mater. Interfaces 2016, 8, 4165–4174. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, T.; Zhang, H.; Liu, G.; Li, P.; Liu, L.; Hao, D.; Ren, J.; Chang, K.; Meng, X.; et al. Room-temperature driven and visible light enhanced dehydrogenation reactions catalyzed by basic Au/SrTiO3. J. Mater. Chem. A 2016, 4, 1941–1946. [Google Scholar] [CrossRef]
- Demchenko, A.P.; Dekaliuk, M.O. Novel fluorescent carbonic nanomaterials for sensing and imaging. Methods Appl. Fluoresc. 2013, 1, 042001. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Gao, Y.; Zhang, Q.; Zhang, Y.; Cao, J.-j.; Ho, W.; Lee, S.C. Biocompatible FeOOH-Carbon quantum dots nanocompo-sites for gaseous NOx removal under visible light: Improved charge separation and high selectivity. J. Hazard. Mater. 2018, 354, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liu, J.; Yu, Y.; Zuo, S.; Li, B. Preparation and visible light photocatalytic activity of carbon quantum dots/TiO2 nanosheet composites. Carbon 2014, 68, 718–724. [Google Scholar] [CrossRef]
- Hutton, G.A.M.; Martindale, B.C.M.; Reisner, E. Carbon dots as photosensitisers for solar-driven catalysis. Chem. Soc. Rev. 2017, 46, 6111–6123. [Google Scholar] [CrossRef] [Green Version]
- Dong, F.; Xiong, T.; Sun, Y.; Zhao, Z.; Zhou, Y.; Feng, X.; Wu, Z. A semimetal bismuth element as a direct plasmonic photocatlyst. Chem. Commun. 2014, 50, 10386–10389. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced nanoarchitectures for solar photocatalytic applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric Graphitic Carbon Nitride for Heterogeneous Photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Li, Y.; Yu, Y. Synthesis and internal electric field dependent photoreactivity of Bi3O4Cl single-crystalline nanosheets with high {001} facet exposure percentages. Nanoscale 2013, 6, 167–171. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, W.; Zhang, Q.; Cao, J.; Huang, R.; Ho, W.; Lee, S.C. In situ fabrication of α-Bi2O3/(BiO)2CO3 nanoplate heterojunctions with tunable optical property and photocatalytic activity. Sci. Rep. 2016, 6, 23435. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Huang, Y.; Chen, L.; Chen, M.; Cao, J.; Ho, W.; Lee, S.C. In situ g-C3N4 self-sacrificial synthesis of a g-C3N4/LaCO3OH heterostructure with strong interfacial charge transfer and separation for photocatalytic NO removal. J. Mater. Chem. A 2017, 6, 972–981. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Ho, W.; Cao, J.; Shen, Z.; Lee, S.C. Fabrication of Bi2O2CO3/g-C3N4 heterojunctions for efficiently photo-catalytic NO in air removal: In-situ self-sacrificial synthesis, characterizations and mechanistic study. Appl. Catal. B Environ. 2016, 199, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, Y.; Peng, S.; Zhang, Y.; Shen, Z.; Cao, J.-J.; Ho, W.; Lee, S.-C.; Pui, D.Y. Perovskite LaFeO3-SrTiO3 composite for synergistically enhanced NO removal under visible light excitation. Appl. Catal. B Environ. 2016, 204, 346–357. [Google Scholar] [CrossRef] [Green Version]
- Mao, C.; Cheng, H.; Tian, H.; Li, H.; Xiao, W.-J.; Xu, H.; Zhao, J.; Zhang, L. Visible light driven selective oxidation of amines to imines with BiOCl: Does oxygen vacancy concentration matter? Appl. Catal. B Environ. 2018, 228, 87–96. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Y.; Peng, S.; Huang, T.; Cao, J.-J.; Ho, W.; Lee, S.-C. Synthesis of SrFexTi1-xO3-δ nanocubes with tunable oxygen vacancies for selective and efficient photocatalytic NO oxidation. Appl. Catal. B Environ. 2018, 239, 1–9. [Google Scholar] [CrossRef]
- Tasbihi, M.; Štangar, U.L.; Černigoj, U.; Jirkovsky, J.; Bakardjieva, S.; Tušar, N.N. Photocatalytic oxidation of gaseous toluene on titania/mesoporous silica powders in a fluidized-bed reactor. Catal. Today 2011, 161, 181–188. [Google Scholar] [CrossRef]
- Ahmad, R.; Kim, J.K.; Kim, J.H.; Kim, J. Well-organized, mesoporous nanocrystalline TiO2 on alumina membranes with hierarchical architecture: Antifouling and photocatalytic activities. Catal. Today 2017, 282, 2–12. [Google Scholar] [CrossRef]
- Gomez, S.; Marchena, C.L.; Renzini, M.S.; Pizzio, L.; Pierella, L. In situ generated TiO2 over zeolitic supports as reusable photo-catalysts for the degradation of dichlorvos. Appl. Catal. B Environ. 2015, 162, 167–173. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Z.-R.; Fu, X.; Xu, Y.-J. TiO2-graphene nanocomposites for gas-phase photocatalytic degradation of volatile aromatic pollutant: Is TiO2-graphene truly different from other TiO2-carbon composite materials? ACS Nano 2010, 4, 7303–7314. [Google Scholar] [CrossRef]
- Li, M.; Lu, B.; Ke, Q.-F.; Guo, Y.-J. Synergetic effect between adsorption and photodegradation on nanostructured TiO2/activated carbon fiber felt porous composites for toluene removal. J. Hazard. Mater. 2017, 333, 88–98. [Google Scholar] [CrossRef]
- Martins, A.C.; Cazetta, A.L.; Pezoti, O.; de Souza, J.R.B.; Zhang, T.; Pilau, E.; Asefa, T.; Almeida, V.C. Sol-gel synthesis of new TiO2/activated carbon photocatalyst and its application for degradation of tetracycline. Ceram. Int. 2017, 43, 4411–4418. [Google Scholar] [CrossRef]
- Shan, A.Y.; Ghazi, T.I.M.; Rashid, S.A. Immobilization of titanium dioxide onto supporting materials in heterogeneous photo-catalysis: A review. Appl. Catal. A Gen. 2010, 389, 1–8. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Hamzavi, R. Kinetic study on photocatalytic degradation of C.I. Acid Yellow 23 by ZnO photocatalyst. J. Hazard. Mater. 2006, 133, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Stafford, U.; Gray, K.A.; Kamat, P. Photocatalytic Degradation of 4-Chlorophenol: The Effects of Varying TiO2 Concentration and Light Wavelength. J. Catal. 1997, 167, 25–32. [Google Scholar] [CrossRef]
- Ohko, Y.; Hashimoto, K.; Fujishima, A. Kinetics of Photocatalytic Reactions under Extremely Low-Intensity UV Illumination on Titanium Dioxide Thin Films. J. Phys. Chem. A 1997, 101, 8057–8062. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Emeline, A.V.; Ryabchuk, V.K.; Serpone, N. Dogmas and Misconceptions in Heterogeneous Photocatalysis. Some Enlightened Reflections. J. Phys. Chem. B 2005, 109, 18515–18521. [Google Scholar] [CrossRef] [PubMed]
- Moulis, F.; Krýsa, J. Photocatalytic degradation of several VOCs (n-hexane, n-butyl acetate and toluene) on TiO2 layer in a closed-loop reactor. Catal. Today 2012, 209, 153–158. [Google Scholar] [CrossRef]
- Zhong, L.; Haghighat, F.; Lee, C.-S.; Lakdawala, N. Performance of ultraviolet photocatalytic oxidation for indoor air applications: Systematic experimental evaluation. J. Hazard. Mater. 2013, 261, 130–138. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, Y.; Xu, Q.; Yang, R. Effect of TiO2/adsorbent hybrid photocatalysts for toluene decomposition in gas phase. J. Hazard. Mater. 2009, 168, 276–281. [Google Scholar] [CrossRef]
- Debono, O.; Thevenet, F.; Gravejat, P.; Hequet, V.; Raillard, C.; LE Coq, L.; Locoge, N. Toluene photocatalytic oxidation at ppbv levels: Kinetic investigation and carbon balance determination. Appl. Catal. B Environ. 2011, 106, 600–608. [Google Scholar] [CrossRef]
- Zhang, L.; Anderson, W.A.; Sawell, S.; Moralejo, C. Mechanistic analysis on the influence of humidity on photocatalytic decomposition of gas-phase chlorobenzene. Chemosphere 2007, 68, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Peral, J.; Ollis, D.F. Heterogeneous photocatalytic oxidation of gas-phase organics for air purification: Acetone, 1-butanol, butyraldehyde, formaldehyde, and m-xylene oxidation. J. Catal. 1992, 136, 554–565. [Google Scholar] [CrossRef]
- Cao, L.; Spiess, F.-J.; Huang, A.; Suib, S.L.; Obee, T.N.; Hay, S.O.; Freihaut, J.D. Heterogeneous Photocatalytic Oxidation of 1-Butene on SnO2 and TiO2 Films. J. Phys. Chem. B 1999, 103, 2912–2917. [Google Scholar] [CrossRef]
- Sleiman, M.; Conchon, P.; Ferronato, C.; Chovelon, J.-M. Photocatalytic oxidation of toluene at indoor air levels (ppbv): To-wards a better assessment of conversion, reaction intermediates and mineralization. Appl. Catal. B Environ. 2009, 86, 159–165. [Google Scholar] [CrossRef]
- Lichtin, N.N.; Avudaithai, M.; Berman, E.; Grayfer, A. TiO2-photocatalyzed oxidative degradation of binary mixtures of va-porized organic compounds. Sol. Energy 1996, 56, 377–385. [Google Scholar] [CrossRef]
- Ao, C.; Lee, S.; Yu, J.Z.; Xu, J. Photodegradation of formaldehyde by photocatalyst TiO2: Effects on the presences of NO, SO2 and VOCs. Appl. Catal. B Environ. 2004, 54, 41–50. [Google Scholar] [CrossRef]
- Stucchi, M.; Galli, F.; Bianchi, C.; Pirola, C.; Boffito, D.C.; Biasioli, F.; Capucci, V. Simultaneous photodegradation of VOC mixture by TiO2 powders. Chemosphere 2018, 193, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Debono, O.; Hequet, V.; Le Coq, L.; Locoge, N.; Thevenet, F. VOC ternary mixture effect on ppb level photocatalytic oxidation: Removal kinetic, reaction intermediates and mineralization. Appl. Catal. B Environ. 2017, 218, 359–369. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Gao, Z.; Suib, S.L.; Obee, T.N.; Hay, S.O.; Freihaut, J.D. Photocatalytic Oxidation of Toluene on Nanoscale TiO2 Catalysts: Studies of Deactivation and Regeneration. J. Catal. 2000, 196, 253–261. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Yang, L.; Ren, L.; Wang, D.; Ye, J. Metal nanoparticles induced photocatalysis. Natl. Sci. Rev. 2017, 4, 761–780. [Google Scholar] [CrossRef] [Green Version]
- Cui, W.; Li, J.; Dong, F.; Sun, Y.; Jiang, G.; Cen, W.; Lee, S.C.; Wu, Z. Highly efficient performance and conversion pathway of photocatalytic NO oxidation on SrO-clusters@amorphous carbon nitride. Environ. Sci. Technol. 2017, 51, 10682–10690. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Li, J.; Jiang, G.; Zhou, Y.; Lee, S.; Dong, F. Transformation pathway and toxic intermediates inhibition of pho-tocatalytic NO removal on designed Bi metal@defective Bi2O2SiO3. Appl. Catal. B Environ. 2019, 241, 187–195. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Zhang, C.; Wang, H.; Song, Y.; Zhang, W.; Li, C. H2O2-assisted hydrothermal synthesis of TiO2-SiO2 and its en-hanced photocatalytic-adsorptive desulfurization performance for model fuel. Fuel 2018, 226, 527–535. [Google Scholar] [CrossRef]
- Li, H.; Shang, H.; Cao, X.; Yang, Z.; Ai, Z.; Zhang, L. Oxygen Vacancies Mediated Complete Visible Light NO Oxidation via Side-On Bridging Superoxide Radicals. Environ. Sci. Technol. 2018, 52, 8659–8665. [Google Scholar] [CrossRef]
- Chen, P.; Sun, Y.; Liu, H.; Zhou, Y.; Jiang, G.; Lee, S.C.; Zhang, Y.; Dong, F. Facet-dependent photocatalytic NO conversion pathways predetermined by adsorption activation patterns. Nanoscale 2019, 11, 2366–2373. [Google Scholar] [CrossRef]
- Dong, X.; Li, J.; Xing, Q.; Zhou, Y.; Huang, H.; Dong, F. The activation of reactants and intermediates promotes the selective photocatalytic NO conversion on electron-localized Sr-intercalated g-C3N4. Appl. Catal. B Environ. 2018, 232, 69–76. [Google Scholar] [CrossRef]
- Weon, S.; Choi, W. TiO2 Nanotubes with Open Channels as Deactivation-Resistant Photocatalyst for the Degradation of Volatile Organic Compounds. Environ. Sci. Technol. 2016, 50, 2556–2563. [Google Scholar] [CrossRef]
- Pillai, U.R.; Sahle-Demessie, E. Selective Oxidation of Alcohols in Gas Phase Using Light-Activated Titanium Dioxide. J. Catal. 2002, 211, 434–444. [Google Scholar] [CrossRef]
- Piera, E.; Ayllon, J.A.; Doménech, X.; Peral, J. TiO2 deactivation during gas-phase photocatalytic oxidation of ethanol. Catal. Today 2002, 76, 259–270. [Google Scholar] [CrossRef]
- Ameen, M.; Raupp, G.B. Reversible Catalyst Deactivation in the Photocatalytic Oxidation of Diluteo-Xylene in Air. J. Catal. 1999, 184, 112–122. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, G. Sepiolite nanofiber-supported platinum nanoparticle catalysts toward the catalytic oxidation of formalde-hyde at ambient temperature: Efficient and stable performance and mechanism. Chem. Eng. J. 2016, 288, 70–78. [Google Scholar] [CrossRef]
- Jiaguo, Y.; Xinyang, L.; Zhihua, X.; Wei, X. NaOH-modified ceramic honeycomb with enhanced formaldehyde adsorption and removal performance. Environ. Sci. Technol. 2013, 47, 9928–9933. [Google Scholar]
- Pei, J.; Zhang, J.S. On the performance and mechanisms of formaldehyde removal by chemi-sorbents. Chem. Eng. J. 2011, 167, 59–66. [Google Scholar] [CrossRef]
- Nakayama, H.; Hayashi, A.; Eguchi, T.; Nakamura, N.; Tsuhako, M. Adsorption of formaldehyde by polyamine-intercalated α-zirconium phosphate. Solid State Sci. 2002, 4, 1067–1070. [Google Scholar] [CrossRef]
- Bai, B.; Arandiyan, H.; Li, J. Comparison of the performance for oxidation of formaldehyde on nano-Co3O4, 2D-Co3O4, and 3D-Co3O4 catalysts. Appl. Catal. B Environ. 2013, 142–143, 677–683. [Google Scholar] [CrossRef]
- Chen, H.; He, J.; Zhang, A.C.; He, H. Self-Assembly of Novel Mesoporous Manganese Oxide Nanostructures and Their Application in Oxidative Decomposition of Formaldehyde. J. Phys. Chem. C 2007, 111, 18033–18038. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Li, J.; Jiang, C.; Yunus, R.; Kim, J. Room-temperature oxidation of formaldehyde by layered manganese ox-ide: Effect of water. Environ. Sci. Technol. 2015, 49, 12372. [Google Scholar] [CrossRef]
- Rong, S.; Zhang, P.; Yang, Y.; Lin, Z.; Wang, J.; Fang, L. MnO2 framework for instantaneous mineralization of carcinogenic air-borne formaldehyde at room temperature. ACS Catal. 2017, 7, 1057–1067. [Google Scholar] [CrossRef]
- Sidheswaran, M.A.; Destaillats, H.; Sullivan, D.P.; Larsen, J.; Fisk, W.J. Quantitative room-temperature mineralization of air-borne formaldehyde using manganese oxide catalysts. Appl. Catal. B Environ. 2011, 107, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Foster, J.J.; Masel, R.I. Formaldehyde oxidation on nickel oxide. Ind. Eng. Chem. Prod. Res. Dev. 1986, 25, 563–568. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, Z.; Yang, C.; Bebensee, F.; Heissler, S.; Nefedov, A.; Tang, M.; Ge, Q.; Long, C.; Kay, B.D. Interaction of formalde-hyde with the rutile TiO2(110) surface: A combined experimental and theoretical study. J. Phys. Chem. C 2016, 120, 12626–12636. [Google Scholar] [CrossRef]
- Yu, X.; He, J.; Wang, D.; Hu, Y.; Hua, T.; He, Z. Facile controlled synthesis of Pt/MnO2 nanostructured catalysts and their cata-lytic performance for oxidative decomposition of formaldehyde. J. Phys. Chem. C 2011, 116, 851–860. [Google Scholar] [CrossRef]
- Zhang, C.; He, H. A comparative study of TiO2 supported noble metal catalysts for the oxidation of formaldehyde at room temperature. Catal. Today 2007, 126, 345–350. [Google Scholar] [CrossRef]
- Changbin, Z.; Fudong, L.; Yanping, Z.; Hiroko, A.; Nan, Y.; Yongchun, L.; Kiyotaka, A.; Maria, F.S.; Hong, H. Alka-li-metal-promoted Pt/TiO2 opens a more efficient pathway to formaldehyde oxidation at ambient temperatures. Angew. Chem. Int. 2012, 51, 9628–9632. [Google Scholar]
- Bai, B.; Li, J. Positive Effects of K+ Ions on Three-Dimensional Mesoporous Ag/Co3O4 Catalyst for HCHO Oxidation. ACS Catal. 2014, 4, 2753–2762. [Google Scholar] [CrossRef]
- Chen, B.B.; Zhu, X.B.; Crocker, M.; Yu, W.; Shi, C. FeOx-supported gold catalysts for catalytic removal of formaldehyde at room temperature. Appl. Catal. B Environ. 2014, 154-155, 73–81. [Google Scholar] [CrossRef]
- Huang, H.Y.; Yang, R.T. Removal of NO by Reversible Adsorption on Fe−Mn Based Transition Metal Oxides. Langmuir 2001, 17, 4997–5003. [Google Scholar] [CrossRef]
- Sekine, Y. Oxidative decomposition of formaldehyde by metal oxides at room temperature. Atmos. Environ. 2002, 36, 5543–5547. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Wang, L.; Zhang, C.; He, H. Catalytic oxidation of formaldehyde over manganese oxides with different crystal structures. Catal. Sci. Technol. 2015, 5, 2305–2313. [Google Scholar] [CrossRef]
- Bai, B.; Qiao, Q.; Arandiyan, H.; Li, J.; Hao, J. Three-Dimensional Ordered Mesoporous MnO2-Supported Ag Nanoparticles for Catalytic Removal of Formaldehyde. Environ. Sci. Technol. 2016, 50, 2635–2640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, H.; Tanaka, K.-I. Catalytic performance and mechanism of a Pt/TiO2 catalyst for the oxidation of formaldehyde at room temperature. Appl. Catal. B Environ. 2006, 65, 37–43. [Google Scholar] [CrossRef]
- Li, G.; Li, L. Highly efficient formaldehyde elimination over meso-structured M/CeO2 (M = Pd, Pt, Au and Ag) catalyst under ambient conditions. RSC Adv. 2015, 5, 36428–36433. [Google Scholar] [CrossRef]
- An, N.; Wu, P.; Li, S.; Jia, M.; Zhang, W. Catalytic oxidation of formaldehyde over Pt/Fe2O3 catalysts prepared by different method. Appl. Surf. Sci. 2013, 285, 805–809. [Google Scholar] [CrossRef]
- Ikegami, M.; Matsumoto, T.; Kobayashi, Y.; Jikihara, Y.; Nakayama, T.; Ohashi, H.; Honma, T.; Takei, T.; Haruta, M. Air purifica-tion by gold catalysts supported on PET nonwoven fabric. Appl. Catal. B Environ. 2013, 134–135, 130–135. [Google Scholar] [CrossRef]
- Shu, Z.; Huang, W.; Hua, Z.; Zhang, L.; Cui, X.; Chen, Y.; Chen, H.; Wei, C.; Wang, Y.; Fan, X.; et al. Template-free synthesis of mesoporous X–Mn (X = Co, Ni, Zn) bimetal oxides and catalytic application in the room temperature removal of low-concentration NO. J. Mater. Chem. A 2013, 1, 10218–10227. [Google Scholar] [CrossRef]
- Shu, Z.; Chen, Y.; Huang, W.; Cui, X.; Zhang, L.; Chen, H.; Zhang, G.; Fan, X.; Wang, Y.; Tao, G.; et al. Room-temperature catalytic removal of low-concentration NO over mesoporous Fe–Mn binary oxide synthesized using a template-free approach. Appl. Catal. B Environ. 2013, 140–141, 42–50. [Google Scholar] [CrossRef]
- Huang, W.; Shi, J. Water-promoted low-concentration NO removal at room temperature by Mg-doped manganese oxides OMS-2. Appl. Catal. A Gen. 2015, 507, 65–74. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, J.; Zhou, X.; Du, Y.; Huang, W.; Liu, J.; Zhang, W.; Shi, J.; Chen, H. Nanoflower-like weak crystallization manga-nese oxide for efficient removal of low-concentration NO at room temperature. J. Mater. Chem. A 2015, 3, 7631–7638. [Google Scholar] [CrossRef]
- Du, Y.; Hua, Z.; Huang, W.; Wu, M.; Wang, M.; Wang, J.; Cui, X.; Zhang, L.; Chen, H.; Shi, J. Mesostructured amorphous man-ganese oxides: Facile synthesis and highly durable elimination of low-concentration NO at room temperature in air. Chem. Commun. 2015, 51, 5887–5889. [Google Scholar] [CrossRef] [PubMed]
- Mochida, I.; Kawabuchi, Y.; Kawano, S.; Matsumura, Y.; Yoshikawa, M. High catalytic activity of pitch-based activated carbon fibres of moderate surface area for oxidation of NO to NO2 at room temperature. Fuel 1997, 76, 543–548. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Xu, Y.; Chen, Y.; Li, X. Adsorption-Oxidation Reaction Mechanism of NO on Na-ZSM-5 Molecular Sieves with a High Si/Al Ratio at Ambient Temperature. Chin. J. Catal. 2010, 31, 1233–1241. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, M.; Huang, Y.; Zhao, K.; Gao, Z.; Wu, M.; Dong, Y.; Wang, T.; Shi, J.; He, D. A novel chromic oxide catalyst for NO oxidation at ambient temperature. RSC Adv. 2014, 4, 29180–29186. [Google Scholar] [CrossRef]
- Wang, A.; Guo, Y.; Gao, F.; Peden, C.H.F. Ambient-temperature NO oxidation over amorphous CrOx-ZrO2 mixed oxide cata-lysts: Significant promoting effect of ZrO2. Appl. Catal. B Environ. 2017, 202, 706–714. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.; Lin, B.; Zhang, H.; Engelhard, M.H.; Guo, Y.; Lu, G.; Peden, C.H.F.; Gao, F. Ambient temperature NO oxidation over Cr-based amorphous mixed oxide catalysts: Effects from the second oxide components. Catal. Sci. Technol. 2017, 7, 2362–2370. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Jiang, C.; Zhou, P.; Zhang, P.; Yu, J. The effect of manganese vacancy in birnessite-type MnO2 on room-temperature oxidation of formaldehyde in air. Appl. Catal. B Environ. 2017, 204, 147–155. [Google Scholar] [CrossRef]
- Meng, Y.; Song, W.; Huang, H.; Ren, Z.; Chen, S.-Y.; Suib, S.L. Structure-property relationship of bifunctional MnO2 nanostruc-tures: Highly efficient, ultra-stable electrochemical water oxidation and oxygen reduction reaction catalysts identified in alka-line media. J. Am. Chem. Soc. 2014, 136, 11452–11464. [Google Scholar] [CrossRef]
- Chen, T.; Dou, H.; Li, X.; Tang, X.; Li, J.; Hao, J. Tunnel structure effect of manganese oxides in complete oxidation of formalde-hyde. Microporous Mesoporous Mater. 2009, 122, 270–274. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, P.; Chen, L. The effect of morphology of α-MnO2 on catalytic decomposition of gaseous ozone. Catal. Sci. Technol. 2016, 6, 5841–5847. [Google Scholar] [CrossRef]
- Wang, F.; Dai, H.; Deng, J.; Bai, G.; Ji, K.; Liu, Y. Manganese Oxides with Rod-, Wire-, Tube-, and Flower-Like Morphologies: Highly Effective Catalysts for the Removal of Toluene. Environ. Sci. Technol. 2012, 46, 4034–4041. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Lee, S.C.; Cao, J.-J. The mechanism of room temperature catalytic C–H dissociation and oxygenation of formaldehyde over nano-zirconia phase-junction. Chem. Eng. J. 2019, 380, 122498. [Google Scholar] [CrossRef]
- Zhu, D.; Huang, Y.; Cao, J.-J.; Lee, S.C.; Chen, M.; Shen, Z. Cobalt nanoparticles encapsulated in porous nitrogen-doped carbon: Oxygen activation and efficient catalytic removal of formaldehyde at room temperature. Appl. Catal. B Environ. 2019, 258. [Google Scholar] [CrossRef]
- Li, H.; Ho, W.; Cao, J.; Park, D.; Lee, S.-c.; Huang, Y. Active complexes on engineered crystal facets of MnOx-CeO2 and scale-up demonstration on an air cleaner for indoor formaldehyde removal. Environ. Sci. Technol. 2019, 53, 10906–10916. [Google Scholar] [CrossRef]
- Du, Y.; Huang, W.; Hua, Z.; Wang, Y.; Cui, X.; Wu, M.; Shu, Z.; Zhang, L.; Wang, J.; Chen, H.; et al. A facile ultrasonic process for the preparation of Co3O4 nanoflowers for room-temperature removal of low-concentration NOx. Catal. Commun. 2014, 57, 73–77. [Google Scholar] [CrossRef]
- Wang, M.-X.; Huang, Z.-H.; Shimohara, T.; Kang, F.; Liang, K. NO removal by electrospun porous carbon nanofibers at room temperature. Chem. Eng. J. 2011, 170, 505–511. [Google Scholar] [CrossRef]
- Fujiwara, K.; Okuyama, K.; Pratsinis, S.E. Metal–support interactions in catalysts for environmental remediation. Environ. Sci. Nano 2017, 4, 2076–2092. [Google Scholar] [CrossRef]
- Rui, Z.; Chen, L.; Chen, H.; Ji, H. Strong Metal-Support Interaction in Pt/TiO2 Induced by Mild HCHO and NaBH4 Solution Reduction and Its Effect on Catalytic Toluene Combustion. Ind. Eng. Chem. Res. 2014, 53, 15879–15888. [Google Scholar] [CrossRef]
- Xu, Q.; Lei, W.; Li, X.; Qi, X.; Yu, J.; Liu, G.; Wang, J.; Zhang, P. Efficient Removal of Formaldehyde by Nanosized Gold on Well-Defined CeO2 Nanorods at Room Temperature. Environ. Sci. Technol. 2014, 48, 9702–9708. [Google Scholar] [CrossRef] [PubMed]
- Vayssilov, G.N.; Lykhach, Y.; Migani, A.; Staudt, T.; Petrova, G.P.; Tsud, N.; Skála, T.; Bruix, A.; Illas, F.; Prince, K.C.; et al. Support nanostructure boosts oxygen transfer to catalytically active platinum nanoparticles. Nat. Mater. 2011, 10, 310–315. [Google Scholar] [CrossRef]
- Li, H.; Shang, J.; Yang, Z.; Shen, W.; Ai, Z.; Zhang, L. Oxygen Vacancy Associated Surface Fenton Chemistry: Surface Structure Dependent Hydroxyl Radicals Generation and Substrate Dependent Reactivity. Environ. Sci. Technol. 2017, 51, 5685–5694. [Google Scholar] [CrossRef]
- Huang, H.; Leung, D.Y. Complete elimination of indoor formaldehyde over supported Pt catalysts with extremely low Pt content at ambient temperature. J. Catal. 2011, 280, 60–67. [Google Scholar] [CrossRef]
- Liu, B.-T.; Hsieh, C.-H.; Wang, W.-H.; Huang, C.-C.; Huang, C.-J. Enhanced catalytic oxidation of formaldehyde over dual-site supported catalysts at ambient temperature. Chem. Eng. J. 2013, 232, 434–441. [Google Scholar] [CrossRef]
- Park, S.J.; Bae, I.; Nam, I.-S.; Cho, B.K.; Jung, S.M.; Lee, J.-H. Oxidation of formaldehyde over Pd/Beta catalyst. Chem. Eng. J. 2012, 195–196, 392–402. [Google Scholar] [CrossRef]
- Chen, B.-B.; Shi, C.; Crocker, M.; Wang, Y.; Zhu, A.-M. Catalytic removal of formaldehyde at room temperature over supported gold catalysts. Appl. Catal. B Environ. 2013, 132–133, 245–255. [Google Scholar] [CrossRef]
- Chen, B.-B.; Zhu, X.; Crocker, M.; Wang, Y.; Shi, C. Complete oxidation of formaldehyde at ambient temperature over γ-Al2O3 supported Au catalyst. Catal. Commun. 2013, 42, 93–97. [Google Scholar] [CrossRef]
- Grbic, B.; Radic, N.; Terlecki-Baricevic, A. Kinetics of deep oxidation of n-hexane and toluene over Pt/Al2O3 catalysts: Oxidation of mixture. Appl. Catal. B Environ. 2004, 50, 161–166. [Google Scholar] [CrossRef]
- Mars, P.; van Krevelen, D. Oxidations carried out by means of vanadium oxide catalysts. Chem. Eng. Sci. 1954, 3, 41–59. [Google Scholar] [CrossRef]
- Garetto, T.; Apesteguıa, C. Oxidative catalytic removal of hydrocarbons over Pt/Al2O3 catalysts. Catal. Today 2000, 62, 189–199. [Google Scholar] [CrossRef]
- Doornkamp, C.; Ponec, V. The universal character of the Mars and Van Krevelen mechanism. J. Mol. Catal. A Chem. 2000, 162, 19–32. [Google Scholar] [CrossRef]
- Ma, C.; Wang, D.; Xue, W.; Dou, B.; Wang, H.; Hao, Z. Investigation of Formaldehyde Oxidation over Co3O4−CeO2 and Au/Co3O4−CeO2 Catalysts at Room Temperature: Effective Removal and Determination of Reaction Mechanism. Environ. Sci. Technol. 2011, 45, 3628–3634. [Google Scholar] [CrossRef]
- Wu, H.; Ma, S.; Song, W.; Hensen, E.J.M. Density Functional Theory Study of the Mechanism of Formaldehyde Oxidation on Mn-Doped Ceria. J. Phys. Chem. C 2016, 120, 13071–13077. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Le, Y.; Jiang, C.; Cheng, B. Ultrathin Bi2WO6 nanosheet decorated with Pt nanoparticles for efficient formaldehyde removal at room temperature. Appl. Surf. Sci. 2018, 441, 429–437. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Zhang, L.; Jiang, D. Surface oxygen vacancies on Co3O4 mediated catalytic formaldehyde oxidation at room temperature. Catal. Sci. Technol. 2015, 6, 3845–3853. [Google Scholar] [CrossRef]
- Yan, Z.; Xu, Z.; Yu, J.; Jaroniec, M. Highly Active Mesoporous Ferrihydrite Supported Pt Catalyst for Formaldehyde Removal at Room Temperature. Environ. Sci. Technol. 2015, 49, 6637–6644. [Google Scholar] [CrossRef]
- Jin, J.; Sun, N.; Hu, W.; Yuan, H.; Wang, H.; Hu, P. Insight into Room-Temperature Catalytic Oxidation of Nitric oxide by Cr2O3: A DFT Study. ACS Catal. 2018, 8, 5415–5424. [Google Scholar] [CrossRef]
- Zhu, X.; Bei, C.; Yu, J.; Ho, W. Halogen poisoning effect of Pt-TiO2 for formaldehyde catalytic oxidation performance at room temperature. Appl. Surf. Sci. 2016, 364, 808–814. [Google Scholar] [CrossRef]
- Chen, B.B.; Zhu, X.B.; Wang, Y.D.; Yu, L.M.; Lu, J.Q.; Shi, C. Nano-sized gold particles dispersed on HZSM-5 and SiO2 sub-strates for catalytic oxidation of HCHO. Catal. Today 2017, 281, 512–519. [Google Scholar] [CrossRef]
- Hauff, K.; Dubbe, H.; Tuttlies, U.; Eigenberger, G.; Nieken, U. Platinum oxide formation and reduction during NO oxidation on a diesel oxidation catalyst—Macrokinetic simulation. Appl. Catal. B Environ. 2013, 129, 273–281. [Google Scholar] [CrossRef]
- Wang, H.-F.; Guo, Y.-L.; Lu, G.; Hu, P. NO Oxidation on Platinum Group Metals Oxides: First Principles Calculations Combined with Microkinetic Analysis. J. Phys. Chem. C 2009, 113, 18746–18752. [Google Scholar] [CrossRef]
- Dong, F.; Wang, Z.; Li, Y.; Ho, W.-K.; Lee, S.C. Immobilization of Polymeric g-C3N4 on Structured Ceramic Foam for Efficient Visible Light Photocatalytic Air Purification with Real Indoor Illumination. Environ. Sci. Technol. 2014, 48, 10345–10353. [Google Scholar] [CrossRef]
- Maynard, A.D.; Aitken, R.J.; Butz, T.; Colvin, V.; Donaldson, K.; Oberdörster, G.; Philbert, M.A.; Ryan, J.; Seaton, A.; Stone, V.; et al. Safe handling of nanotechnology. Nature 2006, 444, 267–269. [Google Scholar] [CrossRef]
- Peill, N.J.; Hoffmann, M.R. Development and optimization of a TiO2-coated fiber-optic cable reactor: Photocatalytic degrada-tion of 4-chlorophenol. Environ. Sci. Technol. 1995, 29, 2974–2981. [Google Scholar] [CrossRef]
- Rachel, A.; Lavedrine, B.; Subrahmanyam, M.; Boule, P. Use of porous lavas as supports of photocatalysts. Catal. Commun. 2002, 3, 165–171. [Google Scholar] [CrossRef]
- Liu, Z.; Fang, P.; Wang, S.; Gao, Y.; Chen, F.; Zheng, F.; Liu, Y.; Dai, Y. Photocatalytic degradation of gaseous benzene with CdS-sensitized TiO2 film coated on fiberglass cloth. J. Mol. Catal. A Chem. 2012, 363, 159–165. [Google Scholar] [CrossRef]
- Habibi, M.H.; Mikhak, M. Titania/zinc oxide nanocomposite coatings on glass or quartz substrate for photocatalytic degrada-tion of direct blue 71. Appl. Surf. Sci. 2012, 258, 6745–6752. [Google Scholar] [CrossRef]
- Mu, Q.; Zhang, Q.; Wang, H.; Li, Y. Facile growth of vertically aligned BiOCl nanosheet arrays on conductive glass substrate with high photocatalytic properties. J. Mater. Chem. 2012, 22, 16851–16857. [Google Scholar] [CrossRef]
- Yu, J.C.; Ho, W.; Lin, J.; Yip, H.; Wong, P.K. Photocatalytic Activity, Antibacterial Effect, and Photoinduced Hydrophilicity of TiO2 Films Coated on a Stainless Steel Substrate. Environ. Sci. Technol. 2003, 37, 2296–2301. [Google Scholar] [CrossRef]
- Ohko, Y.; Nakamura, Y.; Fukuda, A.; Matsuzawa, S.; Takeuchi, K. Photocatalytic Oxidation of Nitrogen Dioxide with TiO2 Thin Films under Continuous UV-Light Illumination. J. Phys. Chem. C 2008, 112, 10502–10508. [Google Scholar] [CrossRef]
- Anderson, C.; Bard, A.J. An Improved Photocatalyst of TiO2/SiO2 Prepared by a Sol-Gel Synthesis. J. Phys. Chem. 1995, 99, 9882–9885. [Google Scholar] [CrossRef]
- Kato, K.; Tsuzuki, A.; Taoda, H.; Torii, Y.; Kato, T.; Butsugan, Y. Crystal structures of TiO2 thin coatings prepared from the alkoxide solution via the dip-coating technique affecting the photocatalytic decomposition of aqueous acetic acid. J. Mater. Sci. 1994, 29, 5911–5915. [Google Scholar] [CrossRef]
- Puma, G.L.; Bono, A.; Krishnaiah, D.; Collin, J.G. Preparation of titanium dioxide photocatalyst loaded onto activated carbon support using chemical vapor deposition: A review paper. J. Hazard. Mater. 2008, 157, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q. Synthesis and characterization of Fe-doped TiO2 films by electrophoretic method and its photocatalytic ac-tivity toward methyl orange. Solid State Sci. 2013, 16, 16–20. [Google Scholar] [CrossRef]
- Damodar, R.A.; Swaminathan, T. Performance evaluation of a continuous flow immobilized rotating tube photocatalytic re-actor (IRTPR) immobilized with TiO2 catalyst for azo dye degradation. Chem. Eng. J. 2008, 144, 59–66. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, P.; Wang, Z.; Rao, Y.; Cao, J.; Pu, S.; Ho, W.; Lee, S.C. Protonated g-C3N4/Ti3+ self-doped TiO2 nanocompo-site films: Room-temperature preparation, hydrophilicity, and application for photocatalytic NOx removal. Appl. Catal. B Environ. 2019, 240, 122–131. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Huang, W.; Xiu, T.; Zhuang, C.; Shi, J. The catalytic oxidation removal of low-concentration HCHO at high space velocity by partially crystallized mesoporous MnOx. Chem. Eng. J. 2017, 320, 667–676. [Google Scholar] [CrossRef]
- Zhou, L.; He, J.; Zhang, J.; He, Z.; Hu, Y.; Zhang, C.; He, H. Facile In-Situ Synthesis of Manganese Dioxide Nanosheets on Cellulose Fibers and their Application in Oxidative Decomposition of Formaldehyde. J. Phys. Chem. C 2011, 115, 16873–16878. [Google Scholar] [CrossRef]
- Miyawaki, J.; Lee, G.-H.; Yeh, J.; Shiratori, N.; Shimohara, T.; Mochida, I.; Yoon, S.-H. Development of carbon-supported hybrid catalyst for clean removal of formaldehyde indoors. Catal. Today 2012, 185, 278–283. [Google Scholar] [CrossRef]
- Li, J.; Zhang, P.; Wang, J.; Wang, M. Birnessite-Type Manganese Oxide on Granular Activated Carbon for Formaldehyde Removal at Room Temperature. J. Phys. Chem. C 2016, 120, 24121–24129. [Google Scholar] [CrossRef]
- Liu, Q.; Ke, M.; Liu, F.; Yu, P.; Hu, H.; Li, C. High-performance removal of methyl mercaptan by nitrogen-rich coconut shell activated carbon. RSC Adv. 2017, 7, 22892–22899. [Google Scholar] [CrossRef] [Green Version]
- Sikkema, J.K.; Ong, S.K.; Alleman, J.E. Photocatalytic concrete pavements: Laboratory investigation of NO oxidation rate un-der varied environmental conditions. Constr. Build. Mater. 2015, 100, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Todorova, N.; Giannakopoulou, T.; Karapati, S.; Petridis, D.; Vaimakis, T.; Trapalis, C. Composite TiO2/clays materials for pho-tocatalytic NOx oxidation. Appl. Surf. Sci. 2014, 319, 113–120. [Google Scholar] [CrossRef]
- Folli, A.; Pade, C.; Hansen, T.B.; De Marco, T.; Macphee, D.E. TiO2 photocatalysis in cementitious systems: Insights into self-cleaning and depollution chemistry. Cem. Concr. Res. 2012, 42, 539–548. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Pirola, C.; Galli, F.; Vitali, S.; Minguzzi, A.; Stucchi, M.; Manenti, F.; Capucci, V. NOx degradation in a continuous large-scale reactor using full-size industrial photocatalytic tiles. Catal. Sci. Technol. 2016, 6, 2261–2267. [Google Scholar] [CrossRef] [Green Version]
- Maggos, T.; Bartzis, J.; Liakou, M.; Gobin, C. Photocatalytic degradation of NOx gases using TiO2-containing paint: A real scale study. J. Hazard. Mater. 2007, 146, 668–673. [Google Scholar] [CrossRef]
- Gallus, M.; Ciuraru, R.; Mothes, F.; Akylas, V.; Barmpas, F.; Beeldens, A.; Bernard, F.; Boonen, E.; Boréave, A.; Cazaunau, M.; et al. Photocatalytic abatement results from a model street canyon. Environ. Sci. Pollut. Res. 2015, 22, 18185–18196. [Google Scholar] [CrossRef]
- Gallus, M.; Akylas, V.; Barmpas, F.; Beeldens, A.; Boonen, E.; Boreave, A.; Cazaunau, M.; Chen, H.; Daele, V.; Doussin, J.F.; et al. Photocatalytic de-pollution in the Leopold II tunnel in Brus-sels: NOx abatement results. Build. Environ. 2015, 84, 125–133. [Google Scholar] [CrossRef]
- Ballari, M.; Brouwers, H. Full scale demonstration of air-purifying pavement. J. Hazard. Mater. 2013, 254–255, 406–414. [Google Scholar] [CrossRef] [Green Version]
- Folli, A.; Strøm, M.; Madsen, T.P.; Henriksen, T.; Lang, J.; Emenius, J.; Klevebrant, T.; Nilsson, A. Field study of air purifying paving elements containing TiO2. Atmos. Environ. 2015, 107, 44–51. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, J.; Wang, Z.; Liu, Y.; Wang, P.; Cao, J.J.; Ho, W.K. g-C3N4/TiO2 composite film in the fabrication of a photo-catalytic air-purifying pavements. Sol. RRL 2020, 4, 2000170. [Google Scholar] [CrossRef]
- Lorencik, S.; Yu, Q.L.; Brouwers, H.J.H. Design and performance evaluation of the functional coating for air purification under indoor conditions. Appl. Catal. B Environ. 2015, 168, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Gandolfo, A.; Bartolomei, V.; Alvarez, E.G.; Tlili, S.; Gligorovski, S.; Kleffmann, J.; Wortham, H. The effectiveness of indoor photocatalytic paints on NOx and HONO levels. Appl. Catal. B Environ. 2015, 166-167, 84–90. [Google Scholar] [CrossRef]
- Boonen, E.; Akylas, V.; Barmpas, F.; Bottalico, L.; Boreave, A.; Cazaunau, M.; Chen, H.; Daele, V.; de Marco, T.; Doussin, J.F.; et al. Construction of a photocatalytic de-polluting field site in the Leopold II tunnel in Brussels. J. Environ. Manag. 2015, 155, 136–144. [Google Scholar] [CrossRef]
- Guerrini, G.L. Photocatalytic performances in a city tunnel in Rome: NOx monitoring results. Constr. Build. Mater. 2012, 27, 165–175. [Google Scholar] [CrossRef]
- Maggos, T.; Plassais, A.; Bartzis, J.G.; Vasilakos, C.; Moussiopoulos, N.; Bonafous, L. Photocatalytic degradation of NOx in a pilot street canyon configuration using TiO2-mortar panels. Environ. Monit. Assess. 2007, 136, 35–44. [Google Scholar] [CrossRef]
- Ao, C.; Lee, S.-C. Indoor air purification by photocatalyst TiO2 immobilized on an activated carbon filter installed in an air cleaner. Chem. Eng. Sci. 2005, 60, 103–109. [Google Scholar] [CrossRef]
- Denny, F.; Permana, E.; Scott, J.; Wang, J.; Pui, D.Y.H.; Amal, R. Integrated Photocatalytic Filtration Array for Indoor Air Quality Control. Environ. Sci. Technol. 2010, 44, 5558–5563. [Google Scholar] [CrossRef]
- Jo, W.-K.; Tayade, R.J. New Generation Energy-Efficient Light Source for Photocatalysis: LEDs for Environmental Applications. Ind. Eng. Chem. Res. 2014, 53, 2073–2084. [Google Scholar] [CrossRef]
- Slimen, H.; Ochiai, T.; Nakata, K.; Murakami, T.; Houas, A.; Morito, Y.; Fujishima, A. Photocatalytic Decomposition of Cigarette Smoke Using a TiO2-Impregnated Titanium Mesh Filter. Ind. Eng. Chem. Res. 2011, 51, 587–590. [Google Scholar] [CrossRef]
- Negishi, N.; Sano, T. Photocatalytic Solar Tower Reactor for the Elimination of a Low Concentration of VOCs. Molecules 2014, 19, 16624–16639. [Google Scholar] [CrossRef] [Green Version]
- Cyranoski, D. China tests giant air cleaner to combat urban smog. Nature 2018, 555, 152–153. [Google Scholar] [CrossRef] [PubMed]
- Cao, J. The Importance of Aerosols in the Earth System: Science and Engineering Perspectives. Aerosol Sci. Eng. 2017, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Kuehn, T.H.; Shen, L.; Chen, S.-C.; Zhang, N.; Huang, Y.; Cao, J.; Pui, D.Y. Urban-scale SALSCS, Part I: Experimental Evaluation and Numerical Modeling of a Demonstration Unit. Aerosol Air Qual. Res. 2018, 18, 2865–2878. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Huang, M.; Kuehn, T.H.; Shen, L.; Tao, W.-Q.; Cao, J.; Pui, D.Y. Urban-scale SALSCS, Part II: A Parametric Study of System Performance. Aerosol Air Qual. Res. 2018, 18, 2879–2894. [Google Scholar] [CrossRef]
- Das, R.; Vecitis, C.D.; Schulze, A.; Cao, B.; Ismail, A.F.; Lu, X.; Chen, J.; Ramakrishna, S. Recent advances in nanomaterials for water protection and monitoring. Chem. Soc. Rev. 2017, 46, 6946–7020. [Google Scholar] [CrossRef] [PubMed]
- Abidi, M.; Assadi, A.; Bouzaza, A.; Hajjaji, A.; Bessais, B.; Rtimi, S. Photocatalytic indoor/outdoor air treatment and bacterial inactivation on CuxO/TiO2 prepared by HiPIMS on polyester cloth under low intensity visible light. Appl. Catal. B Environ. 2019, 259, 118074. [Google Scholar] [CrossRef]
- Castillo, M.V.; Lucerob, J.O.S.; Arriagaa, S. Photocatalytic inactivation of airborne microorganisms in continuous flow using perlite-supported ZnO and TiO2. Chem. Eng. J. 2019, 374, 914–923. [Google Scholar] [CrossRef]
- Chen, Y.; Liao, C.; Shen, W.; Su, C.; Wu, Y.; Tsai, M.; Hsiao, S.; Yu, K.; Tseng, C. Effective disinfection of airborne microbial contamination in hospital wards using a zero-valent nano-silver/TiO2-chitosan composite. Indoor Air 2019, 29, 439–449. [Google Scholar] [CrossRef]
- Mamba, G.; Gangashe, G.; Moss, L.; Hariganesh, S.; Thakur, S.; Vadivel, S.; Mishra, A.K.; Vilakati, G.D.; Muthuraj, V.; Nkambule, T.T.I. State of the art on the photocatalytic applications of graphene based nanostructures: From elimination of hazardous pollutants to disinfection and fuel generation. J. Environ. Chem. Eng. 2020, 8, 103505. [Google Scholar] [CrossRef]

| Photocatalyst | Synthesis | Reaction Conditions | Performances | Reference | ||
|---|---|---|---|---|---|---|
| Target Pollutant | Initial Concentration | Light Source | ||||
| B-Doped g-C3N4 | Hydrothermal reaction and calcination in N2 | NO | 400 ppb (continuous flow mode) | 300 W Xenon lamp (λ > 420 nm) | NO removal efficiency (30.4%) | [64] |
| Ag-AgBr-TiO2 | Stirring under room temperature | Benzene and acetone | 250 ppm (fixed-bed mode) | Visible light (λ > 400 nm) | Removal efficiency: benzene (47.2%) acetone (70.4%) | [59] |
| Au-TiO2 | Calcination in air | HCHO | 50 ± 2 ppmv (continuous flow mode) | Visible light | Conversion rate: 83.3% (RH = 44.0%) | [60] |
| F-TiO2/Pt | Photodeposition for Pt and NaF soaking (pH = 3.5) for surface fluorination | Toluene | 50 ppmv (closed reactor) | 370 nm UV light | Removal efficiency: 53.0% (after the fifth cycle) | [65] |
| Carbon nanodots/ZnFe2O4 | Hydrothermal reaction | NO | 400 ppb (continuous flow mode) | Visible light (λ > 420 nm) | Removal efficiency: 38.0% | [66] |
| Bi/ZnWO4 | Hydrothermal reaction | NO | 400 ppb (continuous flow mode) | Visible light (λ > 420 nm) | Removal efficiency: 63.0% | [67] |
| CuInS2/Bi2WO6 | Solvothermal and hydrothermal synthesis | Toluene | 1 μL (fixed-bed mode) | Visible light (λ > 420 nm) | Removal efficiency: 63.0% | [68] |
| Bi2O2CO3/ZnFe2O4 | Hydrothermal reaction | NO | 400 ppb (continuous flow mode) | Visible light (λ > 420 nm) | Removal efficiency: 32.0% | [69] |
| Ag-decorated WO3/Bi2WO6 | Hydrothermal reaction | Chlorobenzene | 2 μL in a closed 120 mL reactor | Xe lamp | Removal efficiency: 80.0% | [70] |
| BiOCl (001) and (010) | Hydrothermal reaction | NO | 600 ppb (continuous flow mode) | Visible light (λ > 420 nm) | Removal efficiency: BiOCl (001): 50.1% BiOCl (010): 60.4% | [61] |
| (001) TiO2 nanotubes | Electrochemical anodization and NaF treatment | Toluene; HCHO; Aldehyde | 10 ppmv (closed reactor) | 370 nm UV light | Removal efficiency: toluene (32.7%) HCHO (97.2%) aldehyde (58.7%) | [62] |
| Sr-doped defective TiO2 | Hydrothermal reaction | HCHO | 1 ppm (continuous flow mode) | UV light (λ = 365 nm, 90 mW cm−1) | Removal efficiency: 50.85%, QE: 5.53% | [71] |
| Er3+-Doped TiO2 | Sol-gel method | Acetaldehyde; o-xylene; ethylene | o-xylene and ethylene (25 ppm flow mode); acetaldehyde (25 and 500 ppm flow mode) | Visible light (λ > 420 nm) | Removal efficiency: Acetaldehyde (99.2%) o-xylene (84.6%) ethylene (22.4%) | [72] |
| Graphene-TiO2 composite mats | Hydrothermal reaction and Electrospinning method | Gas-phase methanol | 4000 ± 200 ppm (static mode) | UV light | Removal efficiency: 100% | [73] |
| Fe-based metal-organic-frameworks | Solvothermal method | Toluene | 460 ppm (closed-circulation reactor) | Simulated sunlight (100 mW cm−2) | 100% adsorption and photodegradation removal efficiency | [63] |
| Core-shell CeO2@LDHs | Reverse precipitation and hydrothermal method | Formaldehyde | 8.0, 16.0, 24.0, 32.0 and 40.0 mg m−3 (continuous flow mode) | Visible light | Removal efficiency: 86.9% | [74] |
| Catalyst | Pollutant | Temperature | Experimental Conditions | Catalytic Activity | Reference |
|---|---|---|---|---|---|
| MnOx | HCHO | Room temperature | Fixed-bed reactor; (HCHO) = 30 ppb to 200 ppb; Catalyst: 0.11 g | 80% conversion efficiency, 100% mineralization | [143] |
| MnO2 | HCHO | 21 to 25 °C | Glass vessel (1.16 L); (HCHO) = 520 ppm; Catalyst: 0.5 g | 94% of HCHO conversion into CO2 at 21 °C | [152] |
| MnO2 (α, β, γ and δ phase) | HCHO | 50 to 200 °C | Fixed-bed reactor; (HCHO) = 170 ppm; 25% RH; Space velocity = 100,000 mL/g/h | 100% HCHO conversion: δ-MnO2 > α-MnO2 > γ-MnO2 > β-MnO2 (80 °C, 125 °C, 150 °C and 200 °C) | [153] |
| Birnessite | HCHO | Room temperature | Glass bottle (3.5 L); (HCHO) = 200 ppm; | 84.7% HCHO conversion over S-30 sample (dried at the temperature of 30 °C) | [141] |
| Ag/MnO2 | HCHO | 20 to 120 °C | Fixed-bed reactor; (HCHO) = 500 ppm, 1300 ppm; GHSV a = 60,000 h−1, 150,000 h−1 | 8.9% Ag/MnO2 shows a high normalised rate (10.1 nmol·s−1·m−2) and TOF = 0.007 s−1 at 110 °C under 1300 ppm of HCHO and 150,000 h−1 of GHSV | [154] |
| 1 wt % Pt (Rh, Pd and Au)/TiO2 | HCHO | 20 to 120 °C | Fixed-bed flow reactor; (HCHO) = 100 ppm; GHSV = 50,000 h−1, 100,000 h−1, 200,000 h−1 | Pt/TiO2 (100% HCHO conversion) > Rh/TiO2 > Pd/TiO2 = Au/TiO2 | [155] |
| 0.78–0.98 wt % M/CeO2 (M = Pt, Pd, Au and Ag) | HCHO | 20 to 120 °C | Fixed-bed flow reactor; (HCHO) = 600 ppm; GHSV = 120,000 h−1, 240,000 h−1, 360,000 h−1 | ~100% over Pd/CeO2 at 20 °C | [156] |
| 1 wt % Pt/Fe2O3 | HCHO | 25 to 100 °C | Fixed-bed reactor; (HCHO) = 100 ppm to 500 ppm; Catalyst: 0.2 g | 100% HCHO conversion overPt/Fe2O3-CD | [157] |
| Au/ZrO2/PET | HCHO | Room temperature | Fixed-bed reactor; (HCHO) = 0.5 ppm, GHSV = 8000 h−1 | Close to 100% HCHO conversion | [158] |
| X-Mn bimetal oxides (X = Co, Ni, Zn) | NO | 25 °C | Fixed-bed flow reactor; (NO) = 10 ppm; space velocity = 120,000 mL/g/h | 100% NO removal in time duration up to 1.8 h over Co1Mn6-300 catalyst | [159] |
| Fe–Mn binary oxide | NO | 25 °C | Fixed-bed flow reactor; (NO) = 10 ppm; GHSV = 40,000 h−1 | 4 h for 100% NO removal over Fe1Mn4-300 sample | [160] |
| Mg-SC-OMS-2 | NO | Room temperature | Fixed-bed flow reactor; (NO) = 10 ppm; space velocity = 120,000 mL/g/h | 10% Mg-SC-OMS-2; 99% NO removal for 8 h in dry atmosphere; 96% NO removal for approximately 25 h (70% relative humidity) | [161] |
| Weak crystallization manganese oxide (WMO) | NO | 25 °C | Fixed-bed flow reactor; (NO) = 10 ppm; GHSV = 40,000 h−1 | 100% NO removal capability for approximately 20 h; >70 h for >80% NO removal (60% relative humidity) | [162] |
| Amorphous manganese oxides | NO | 25 °C | Fixed-bed flow reactor; (NO) = 10 ppm; space velocity = 120,000 mL/g/h | 10 and 21 h for 100% and 60% NO removal, respectively; 237 h for 90% NO removal (50–90% RH) | [163] |
| Active carbon | NO | Room temperature | (NO) = 380 ppm, space velocity = 6000 mL/g/h | ~50% removal for 20 h | [164] |
| Zeolite | NO | 30 °C | (NO) = 500 ppm, space velocity = 32,432 mL/g/h | ~30% removal for 20 min | [165] |
| Chromic oxide | NO | 30 °C | Fixed-bed flow reactor; (NO) = 15 ppm, space velocity y = 86,400 mL/g/h | >65% NO conversion for 500 min | [166] |
| CrOx-ZrO2 | NO | 25 °C | Fixed-bed flow reactor; (NO) = 12 ppm; space velocity = 45,000 mL/g/h | 100% NO conversion for nearly 26 h over Cr8Zr1-300 catalyst | [167] |
| Cr-M mixed oxides (M=Co, Fe, Ni) | NO | 25 °C | Fixed-bed flow reactor; (NO) = 12 ppm; space velocity = 45,000 mL/g/h | 100% NO conversion for over 30 h on Cr8Co1-300 sample | [168] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.-j.; Huang, Y.; Zhang, Q. Ambient Air Purification by Nanotechnologies: From Theory to Application. Catalysts 2021, 11, 1276. https://doi.org/10.3390/catal11111276
Cao J-j, Huang Y, Zhang Q. Ambient Air Purification by Nanotechnologies: From Theory to Application. Catalysts. 2021; 11(11):1276. https://doi.org/10.3390/catal11111276
Chicago/Turabian StyleCao, Jun-ji, Yu Huang, and Qian Zhang. 2021. "Ambient Air Purification by Nanotechnologies: From Theory to Application" Catalysts 11, no. 11: 1276. https://doi.org/10.3390/catal11111276
APA StyleCao, J.-j., Huang, Y., & Zhang, Q. (2021). Ambient Air Purification by Nanotechnologies: From Theory to Application. Catalysts, 11(11), 1276. https://doi.org/10.3390/catal11111276