Characterization of a Novel Lutein Cleavage Dioxygenase, EhLCD, from Enterobacter hormaechei YT-3 for the Enzymatic Synthesis of 3-Hydroxy-β-ionone from Lutein
Abstract
:1. Introduction
2. Results and Discussion
2.1. Purification of EhLCD
2.2. Substrate Specificity and Kinetic Parameters of EhLCD
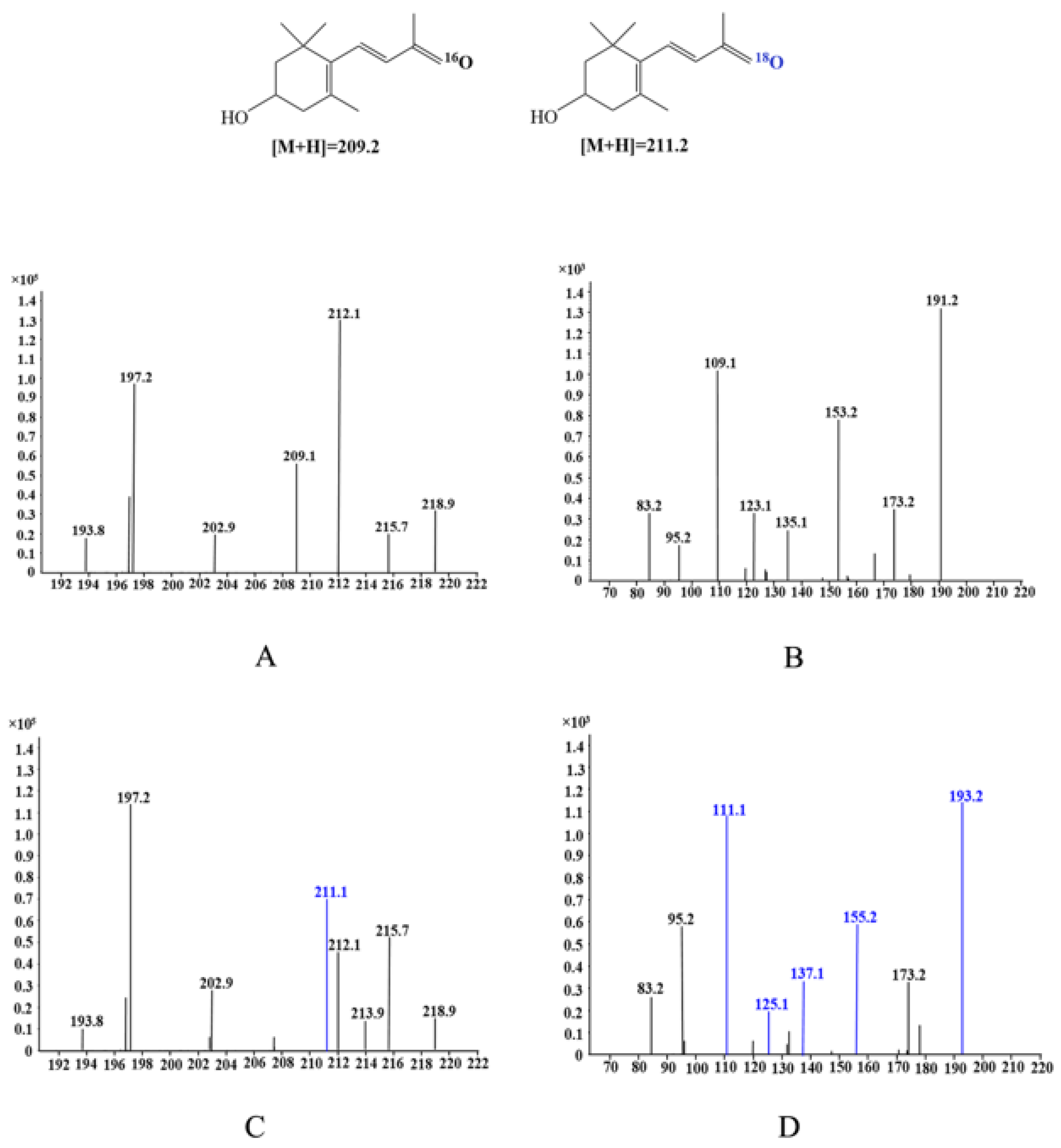
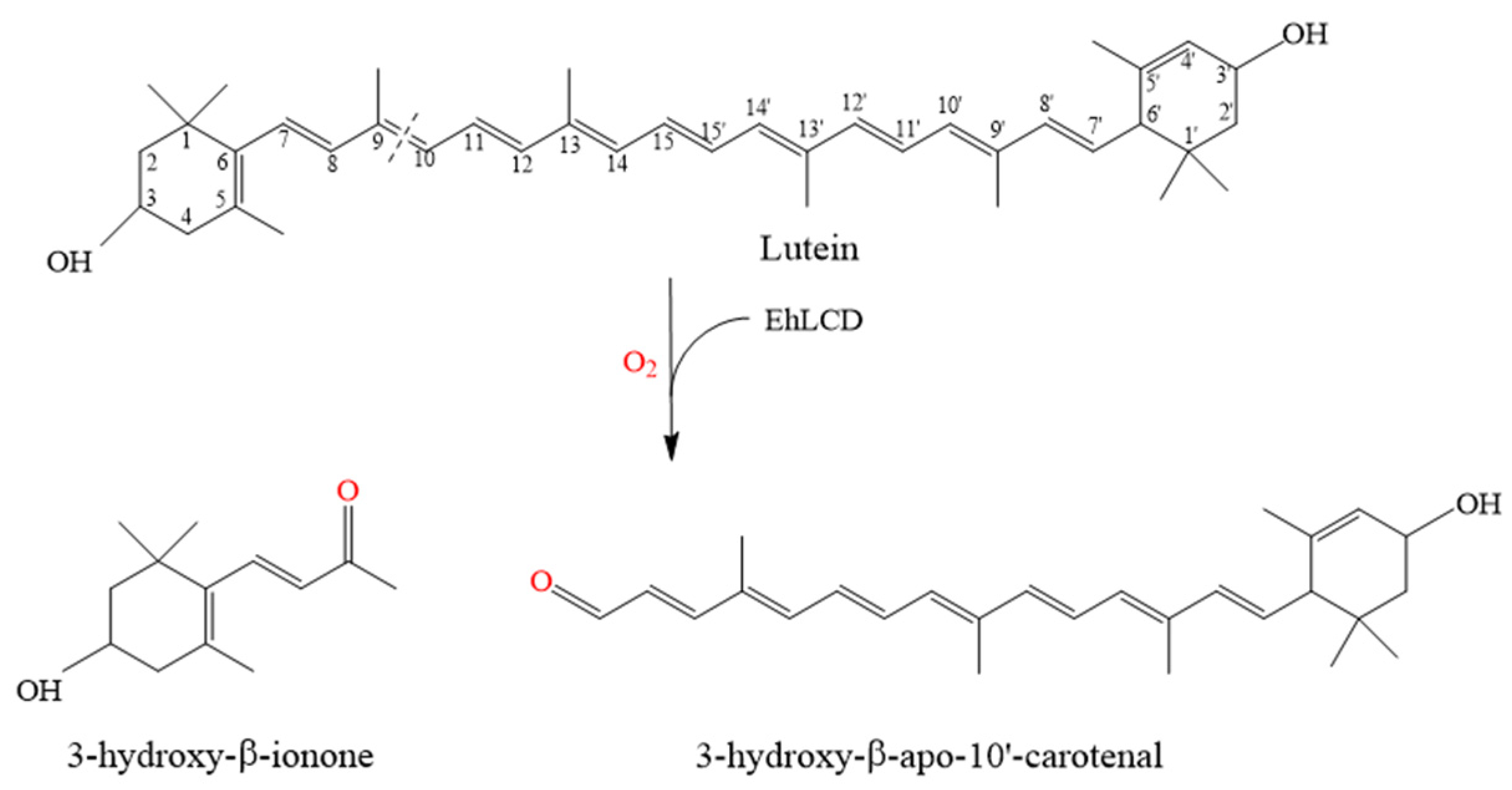
2.3. Reaction Mechanism of Lutein Cleavage by EhLCD
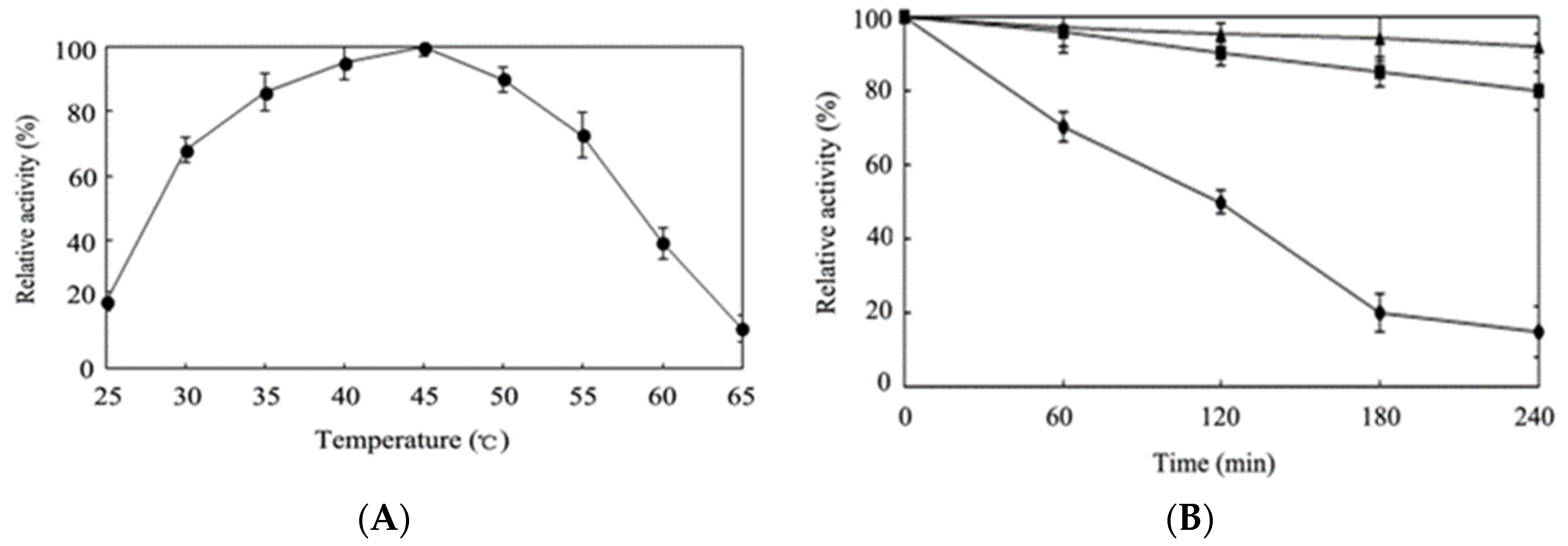
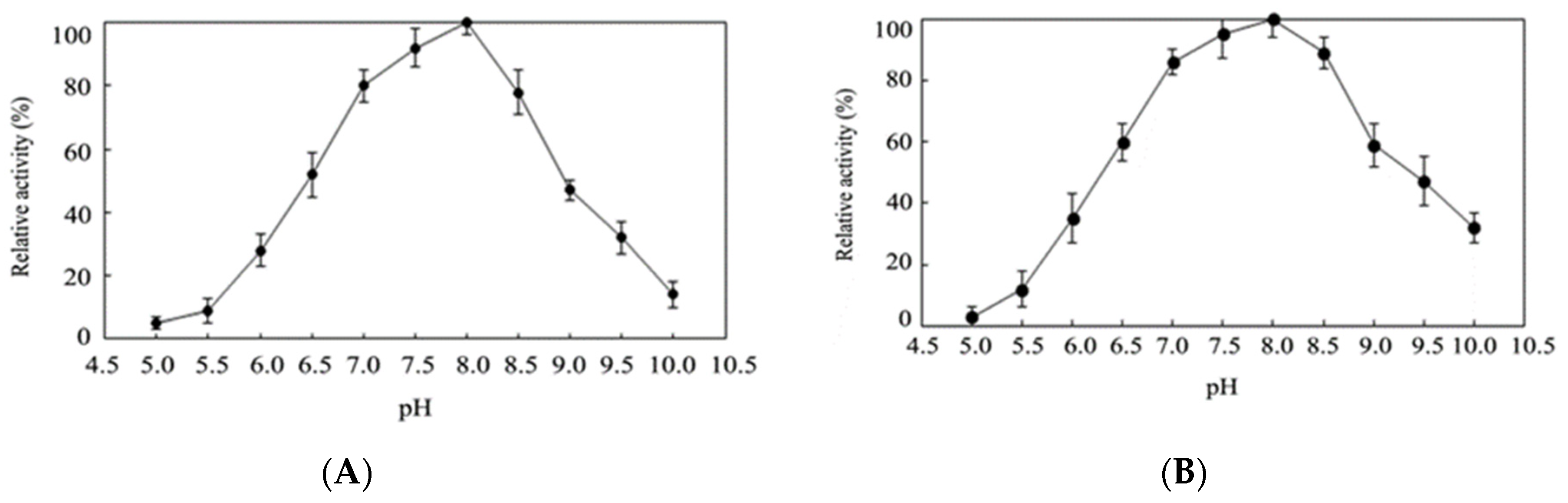
2.4. Effects of Temperature and pH on Activity of Purified EhLCD
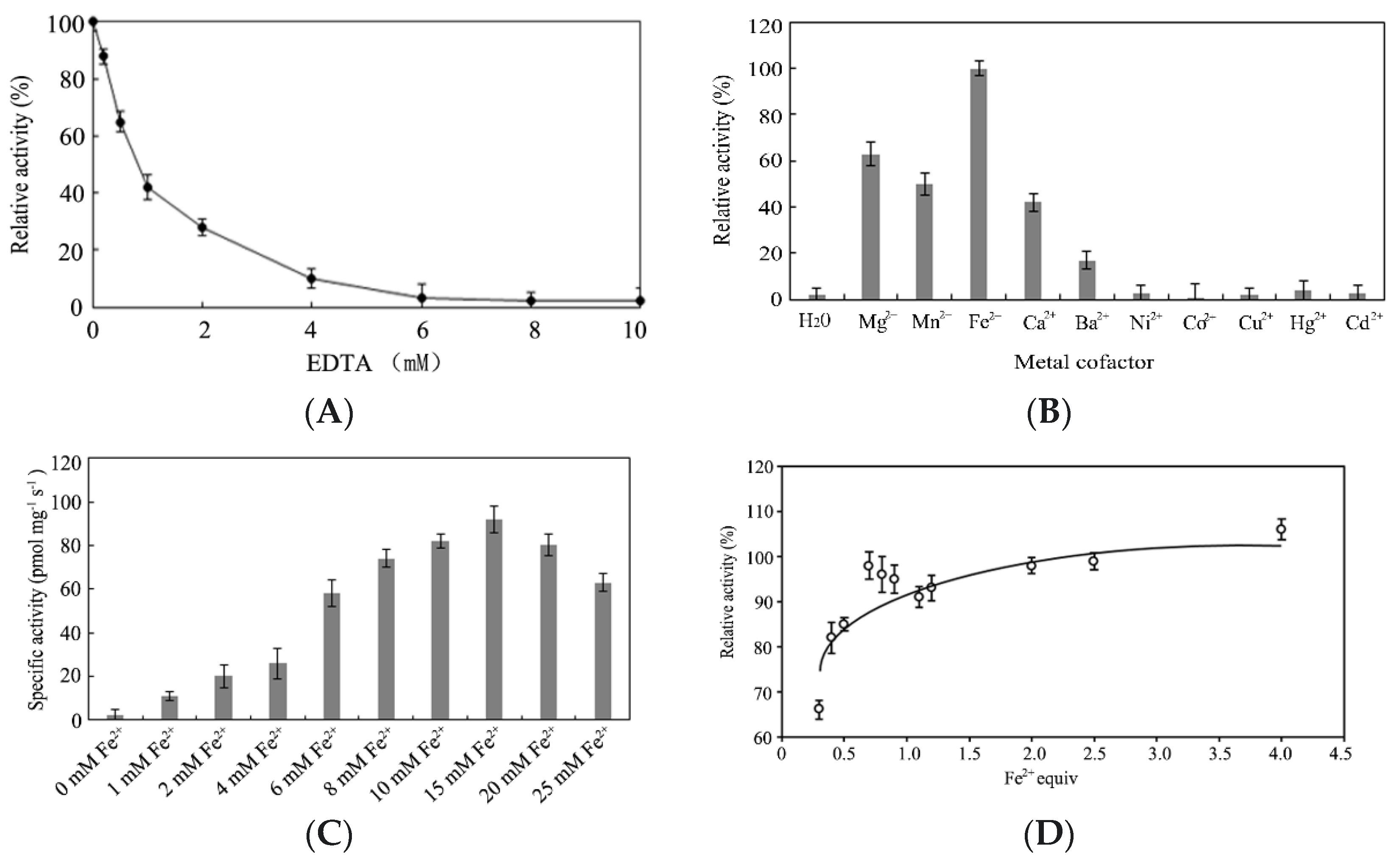
2.5. Effect of Metal Ions on Enzymatic Activity
2.6. Effects of Organic Solvent and Detergent on Enzymatic Activity
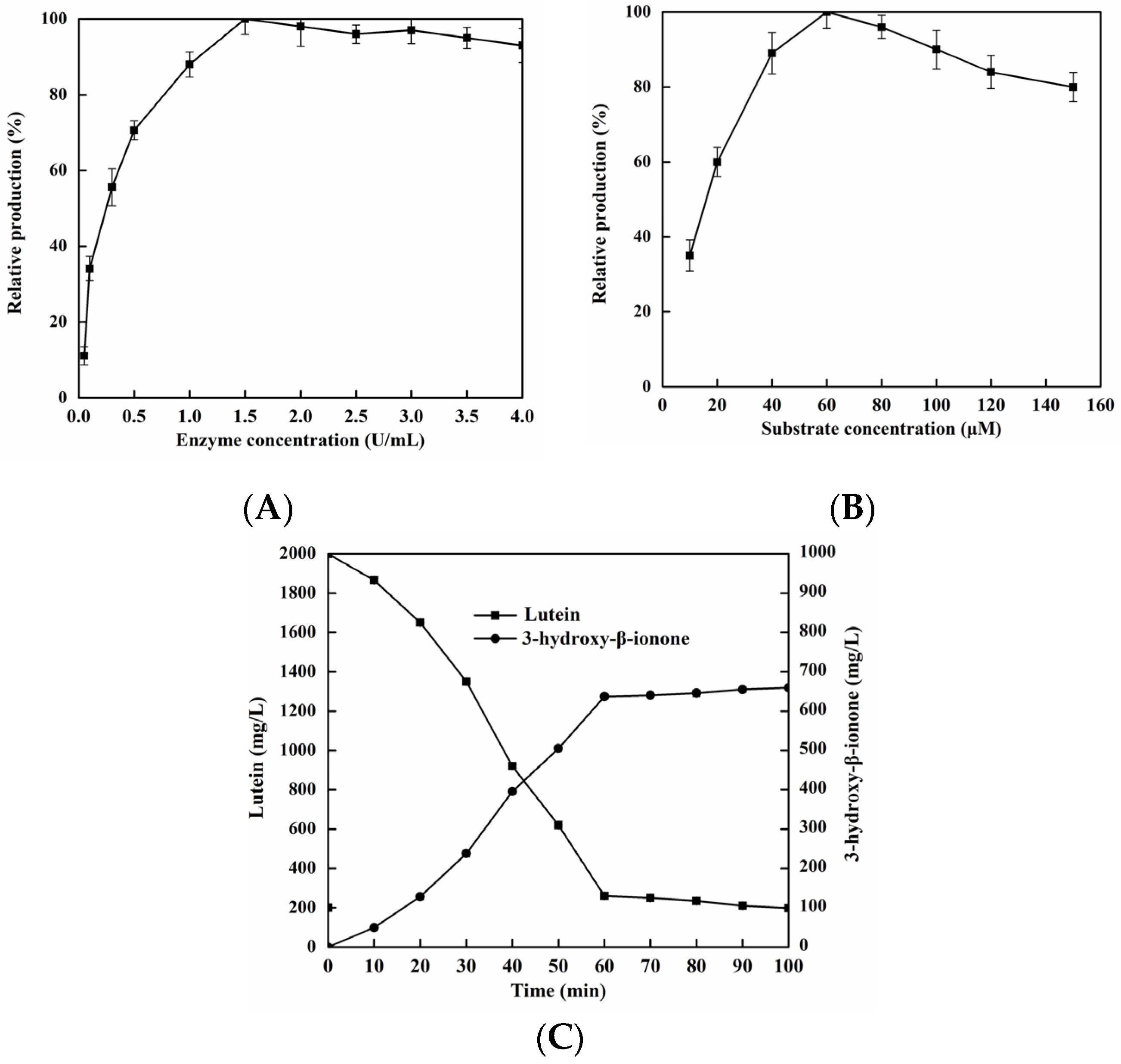
2.7. Production of 3-hydroxy-β-ionone from Lutein by EhLCD
3. Materials and Methods
3.1. Chemicals, Stains, and Plasmids
3.2. Purification of EhLCD
3.3. Enzyme Assay
3.4. Analysis of Amino Acid Sequence of EhLCD
3.5. Catalytic Reaction Mechanism of EhLCD
3.6. Catalytic Properties of EhLCD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, J.H.; Lee, D.J.; Chang, J.S. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef]
- Barbara, D.A.; William, W.A. Antioxidants in photosynthesis and human nutrition. Science 2002, 298, 2149–2153. [Google Scholar]
- Li, X.M.; Li, X.H.; Wu, Z.Z.; Wang, Y.; Cheng, J.S.; Wang, T.; Zhang, B. Chitosan hydrochloride/carboxymethyl starch complex nanogels stabilized Pickering emulsions for oral delivery of β-carotene: Protection effect and in vitro digestion study. Food Chem. 2020, 315, 126288. [Google Scholar] [CrossRef]
- Ceron, M.C.; Campos, I.; Sánchez, J.F.; Acien, F.G.; Molina, E.; Fernandez Sevilla, J.M. Recovery of lutein from microalgae biomass: Development of a process for Scenedesmus almeriensis. J. Agric. Food Chem. 2008, 56, 11761–11766. [Google Scholar] [CrossRef]
- Carpentier, S.; Knaus, M.; Suh, M. Associations between lutein, zeaxanthin, and age-related macular degeneration: An overview. Crit Rev. Food Sci. Nutr. 2009, 49, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.G. Biotechnology of flavours—The next generation. Biotechnol. Lett. 2009, 31, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Lalko, J.; Lapczynski, A.; McGinty, D.; Bhatia, S.; Letizia, C.S.; Api, A.M. Fragrance material review on β-ionone. Food Chem. Toxicol. 2007, 45, S241–S247. [Google Scholar] [CrossRef]
- Lalko, J.; Lapczynski, A.; McGinty, D.; Bhatia, S.; Letizia, C.S.; Api, A.M. Fragrance material review on dihydro-β-ionone. Food Chem. Toxicol. 2007, 45, S225–S228. [Google Scholar] [CrossRef]
- Nacke, C.; Hüttmann, S.; Etschmann, M.M.W.; Schrader, J. Enzymatic production and in situ separation of natural β-ionone from β-carotene. J. Ind. Microbiol. Biotechnol. 2012, 39, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Sefton, M.A.; Skouroumounis, G.K.; Elsey, G.M.; Taylor, D.K. Occurrence, sensory impact, formation, and fate of damascenone. in grapes, wines, and other foods and beverages. J. Agric. Food Chem. 2011, 59, 9717–9746. [Google Scholar] [CrossRef]
- Serra, S. Recent advances in the synthesis of carotenoid-derived flavours and fragrances. Molecules 2015, 20, 12817–12840. [Google Scholar] [CrossRef] [Green Version]
- Gambacorta, G.; Sharley, J.S.; Baxendale, I.R. A comprehensive review of flow chemistry techniques tailored to the flavours and fragrances industries. Beilstein J. Org. Chem. 2021, 17, 1181–1312. [Google Scholar] [CrossRef]
- Cataldo, V.F.; López, J.; Cárcamo, M.; Agosin, E. Chemical vs. biotechnological synthesis of C13-apocarotenoids: Current methods, applications and perspectives. Appl. Microbiol. Biotechnol. 2016, 100, 5703–5718. [Google Scholar] [CrossRef] [PubMed]
- Zelena, K.; Hardebusch, B.; Hülsdau, B.; Berger, R.G.; Zorn, H. Generation of norisoprenoid flavors from carotenoids by fungal peroxidases. J. Agric. Food Chem. 2009, 57, 9951–9955. [Google Scholar] [CrossRef]
- Sanchez-Contreras, A.; Jimenez, M.; Sanchez, S. Bioconversion of lutein to products with aroma. Appl. Microbiol. Biotechnol. 2000, 54, 528–534. [Google Scholar] [CrossRef]
- Maldonado-Robledo, G.; Rodrıguez-Bustamante, E.; Sanchez-Contreras, A.; Rodrıguez-Sanoja, R.; Sanchez, S. Production of tobacco aroma from lutein. Specific role of the microorganisms involved in the process. Appl. Microbiol. Biotechnol. 2003, 62, 484–488. [Google Scholar] [CrossRef]
- Rodríguez-Bustamante, E.; Maldonado-Robledo, G.; Ortiz, M.A.; Díaz-Avalos, C.; Sanchez, S. Bioconversion of lutein using a microbial mixture-maximizing the production of tobacco aroma compounds by manipulation of culture medium. Appl. Microbiol. Biotechnol. 2005, 68, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhong, G.F.; Yang, X.P.; Hu, X.M.; Mao, D.B.; Ma, Y.P. Bioconversion of lutein to form aroma compounds by Pantoea dispersa. Biotechnol. Lett. 2015, 37, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.F.; Wang, F.F.; Sun, J.H.; Ye, J.B.; Mao, D.B.; Ma, K.; Yang, X.P. Bioconversion of lutein by Enterobacter hormaechei to form a new compound, 8-methyl-alpha-ionone. Biotechnol. Lett. 2017, 39, 1019–1024. [Google Scholar] [CrossRef]
- Mein, J.R.; Dolnikowski, G.G.; Ernst, H.; Russell, R.M.; Wang, X.D. Enzymatic formation of apo-carotenoids from the xanthophyll carotenoids lutein, zeaxanthin and β-cryptoxanthin by ferret carotene-9′,10′-monooxygenase. Arch. Biochem. Biophys. 2011, 506, 109–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amengual, J.; Lobo, G.P.; Golczak, M.; Li, H.N.M.; Klimova, T.; Hoppel, C.L.; Wyss, A.; Palczewski, K.; von Lintig, J. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 2011, 25, 948–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dela Seña, C.; Sun, J.; Narayanasamy, S.; Riedl, K.M.; Yuan, Y.; Curley, R.W.; Schwartz, S.J.; Harrison, E.H. Substrate specificity of purified recombinant chicken β-carotene 9′,10′-oxygenase (BCO2). J. Biol. Chem. 2016, 291, 14609–14619. [Google Scholar] [CrossRef] [Green Version]
- Bandara, S.; Thomas, L.D.; Ramkumar, S.; Khadka, N.; Kiser, P.D.; Golczak, M.; von Lintig, J. The structural and biochemical basis of apocarotenoid processing by β-carotene oxygenase-2. ACS Chem. Biol. 2021, 16, 480–490. [Google Scholar] [CrossRef]
- Thomas, L.D.; Bandara, S.; Parmar, V.M.; Srinivasagan, R.; Khadka, N.; Golczak, M.; Kiser, P.D.; von Lintig, J. The human mitochondrial enzyme BCO2 exhibits catalytic activity toward carotenoids and apocarotenoids. J. Biol. Chem. 2020, 295, 15553–15565. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.H.; Kopec, R.E. Enzymology of vertebrate carotenoid oxygenases. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158653. [Google Scholar] [CrossRef]
- Giordano, E.; Quadro, L. Lutein, zeaxanthin and mammalian development: Metabolism, function and implications for health. Arch. Biochem. Biophys. 2018, 647, 33–40. [Google Scholar] [CrossRef]
- dela Seña, C.; Riedl, K.M.; Narayanasamy, S.; Curley, R.W., Jr.; Schwartz, S.J.; Harrison, E.H. The human enzyme that converts dietary provitamin A carotenoids to vitamin A is a dioxygenase. J. Biol. Chem. 2014, 289, 13661–13666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, M.E.; Ramkumar, S.; Sun, W.Z.; Ortiz, C.C.; Kiser, P.D.; Golczak, M.; von Lintig, J. The biochemical basis of vitamin A production from the asymmetric carotenoid β-cryptoxanthin. ACS Chem. Biol. 2018, 13, 2121–2129. [Google Scholar] [CrossRef]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef]
- Hoffmann, H.; Stindl, S.; Ludwig, W.; Stumpf, A.; Mehlen, A.; Monget, D.; Pierard, D.; Ziesing, S.; Heesemann, J.; Roggenkamp, A.; et al. Enterobacter hormaechei subsp. oharae subsp. nov., E. hormaechei subsp. hormaechei comb. nov., and E. hormaechei subsp. steigerwaltii subsp. nov., three new subspecies of clinical importance. J. Clin. Microbiol. 2005, 43, 3297–3303. [Google Scholar] [CrossRef] [Green Version]
- Sutton, G.G.; Brinkac, L.M.; Clarke, T.H.; Fouts, D.E. Enterobacter hormaechei subsp. hoffmannii subsp. nov., Enterobacter hormaechei subsp. xiangfangensis comb. nov., Enterobacter roggenkampii sp. nov., and Enterobacter muelleri is a later heterotypic synonym of Enterobacter asburiae based on computational analysis of sequenced Enterobacter genomes. F1000Research 2018, 7, 521. [Google Scholar]
- Sui, X.; Farquhar, E.R.; Hill, H.E.; von Lintig, J.; Shi, W.; Kiser, P.D. Preparation and characterization of metal-substituted carotenoid cleavage oxygenases. J. Biol. Inorg. Chem. 2018, 23, 887–901. [Google Scholar] [CrossRef] [Green Version]
- Harrison, P.J.; Bugg, T.D. Enzymology of the carotenoid cleavage dioxygenases: Reaction mechanisms, inhibition and biochemical roles. Arch. Biochem. Biophys. 2014, 544, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Daruwalla, A.; Kiser, P.D. Structural and mechanistic aspects of carotenoid cleavage dioxygenases (CCDs). Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2019, 1865, 158590. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Zhang, G.; Lü, X.; Hwang, H.; Aker, W.G.; Guan, H.; Wang, P. Purification and characterization of polysaccharides degradases produced by Alteromonas sp. A321. Int. J. Biol. Macromol. 2016, 86, 86–96. [Google Scholar] [CrossRef] [PubMed]
- During, A.; Harrison, E.H. Intestinal absorption and metabolism of carotenoids: Insights from cell culture. Arch. Biochem. Biophys. 2004, 430, 77–88. [Google Scholar] [CrossRef]
- Wyss, A.; Wirtz, G.M.; Woggon, W.D.; Brugger, R.; Wyss, M.; Friedlein, A.; Riss, G.; Bachmann, H.; Hunziker, W. Expression pattern and localization of β,β-carotene 15,15′-dioxygenase in different tissues. Biochem. J. 2001, 354, 521–529. [Google Scholar] [CrossRef]
- Kim, Y.S.; Yeom, S.J.; Oh, D.K. Production of β-apo-10′-carotenal from β-carotene by human β-carotene-9′, 10′-oxygenase expressed in E. coli. Biotechnol. Lett. 2011, 33, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- López., J.; Bustos, D.; Camilo, C.; Arenas, N.; Saa, P.A.; Agosin, E. Engineering Saccharomyces cerevisiae for the Overproduction of β-Ionone and Its Precursor β-Carotene. Front. Bioeng. Biotechnol. 2020, 8, 578793. [Google Scholar] [CrossRef]
- Schempp, F.M.; Drummond, L.; Buchhaupt, M.; Schrader, J. Microbial cell factories for the production of terpenoid flavor and fragrance compounds. J. Agric. Food Chem. 2018, 66, 2247–2258. [Google Scholar] [CrossRef] [PubMed]
- Czajka, J.J.; Nathenson, J.A.; Benites, V.T.; Baidoo, E.E.K.; Cheng, Q.; Wang, Y.; Tang, Y.J. Engineering the oleaginous yeast Yarrowia lipolytica to produce the aroma compound β-ionone. Microb. Cell Fact. 2018, 17, 136. [Google Scholar] [CrossRef]
- Shikama, K. Nature of the FeO2 bonding in myoglobin and hemoglobin: A new molecular paradigm. Prog. Biophys. Mol. Biol. 2006, 91, 83–162. [Google Scholar] [CrossRef]
- Walter, M.H.; Strack, D. Carotenoids and their cleavage products: Biosynthesis and functions. Nat. Prod. Rep. 2011, 28, 663–692. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhao, C.M.; Jia, W.W.; Huang, S.; Mao, D.B. Expression and purification of fructosyl transferase AoFT in Pichia pastoris and study on its enzymatic properties. J. Light Ind. 2020, 35, 1–10. [Google Scholar]
- Chai, S.C.; Lu, J.P.; Ye, Q.Z. Determination of binding affinity of metal cofactor to the active site of methionine aminopeptidase based on quantitation of functional enzyme. Anal. Biochem. 2009, 395, 263–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Step | Total Protein (mg) | Total Activity (U) | Specific Activity (U/mg) | Fold | Yield (%) |
|---|---|---|---|---|---|
| Crude cell extract | 536.3 | 125.6 | 0.23 | 1 | 100 |
| (NH4)2SO4 precipitation | 300.5 | 113.9 | 0.37 | 1.61 | 56.0 |
| Q-Sepharose column | 129.5 | 98.6 | 0.76 | 3.30 | 24.1 |
| Phenyl Sepharose | 80.6 | 87.7 | 1.09 | 4.73 | 15.0 |
| Superdex 200 | 29.5 | 66.9 | 2.27 | 9.90 | 5.5 |
| Substrate | Km (μM) | kcat (s−1) | Vmax (pmol mg−1·s−1) | kcat/Km (s−1 mM−1) |
|---|---|---|---|---|
| Lutein | 14 ± 5 | 5.5 ± 1.4 | 89.5 ± 2.6 | 0.393 × 103 |
| Zeaxanthin | 20 ± 2 | 3.8 ± 0.6 | 61.8 ± 1.7 | 0.190 × 103 |
| β-cryptoxanthin | 52 ± 6 | 1.5 ± 0.9 | 24.4 ± 2.1 | 0.028 × 103 |
| β-carotene | 128 ± 3.5 | 0.6 ± 0.1 | 9.76 ± 1.2 | 0.005 × 103 |
| Enzymatic Reaction | 18O-3-hydroxy-β-ionone (%) | 16O-3-hydroxy-β-ionone (%) |
|---|---|---|
| EhLCD + 16O-3-hydroxy-β-ionone + H218O | 5 ± 2 | 95 ± 5 |
| EhLCD + 18O-3-hydroxy-β-ionone + H216O | 97 ± 4 | 3 ± 1 |
| EhLCD + lutein (16O2–H218O) | 4 ± 2 | 92 ± 3 |
| EhLCD + lutein e (18O2–H216O) | 90 ± 5 | 6 ± 2 |
| Organic Solvents/Detergents | Concentration (%) | Relative Activity (%) |
|---|---|---|
| None | - | 100 |
| Methanol | 50 (v/v) | 89 ± 5 |
| Ethanol | 50 (v/v) | 72 ± 6 |
| Acetone | 50 (v/v) | 30 ± 2 |
| Toluene | 50 (v/v) | 90 ± 4 |
| Benzene | 50 (v/v) | 75 ± 3 |
| Chloroform | 50 (v/v) | 16 ± 2 |
| DMSO | 50 (v/v) | 20 ± 5 |
| DMF | 50 (v/v) | 18 ± 4 |
| n-hexane | 50 (v/v) | 48 ± 3 |
| Span 20 | 1% (w/v) | 18 ± 2 |
| Span 80 | 1% (w/v) | 18 ± 1 |
| Triton X-100 | 1% (w/v) | 18 ± 5 |
| Tween 20 | 1% (w/v) | 68 ± 3 |
| 2% (w/v) | 79 ± 6 | |
| Tween 40 | 1% (w/v) | 94 ± 2 |
| 2% (w/v) | 108 ± 3 | |
| Tween 80 | 1% (w/v) | 78 ± 2 |
| 2% (w/v) | 86 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, Z.; Duan, N.; Xue, Y.; Wang, M.; Li, J.; Su, Z.; Liu, Q.; Mao, D.; Wei, T. Characterization of a Novel Lutein Cleavage Dioxygenase, EhLCD, from Enterobacter hormaechei YT-3 for the Enzymatic Synthesis of 3-Hydroxy-β-ionone from Lutein. Catalysts 2021, 11, 1257. https://doi.org/10.3390/catal11111257
Long Z, Duan N, Xue Y, Wang M, Li J, Su Z, Liu Q, Mao D, Wei T. Characterization of a Novel Lutein Cleavage Dioxygenase, EhLCD, from Enterobacter hormaechei YT-3 for the Enzymatic Synthesis of 3-Hydroxy-β-ionone from Lutein. Catalysts. 2021; 11(11):1257. https://doi.org/10.3390/catal11111257
Chicago/Turabian StyleLong, Zhangde, Naixin Duan, Yun Xue, Min Wang, Jigang Li, Zan Su, Qibin Liu, Duobin Mao, and Tao Wei. 2021. "Characterization of a Novel Lutein Cleavage Dioxygenase, EhLCD, from Enterobacter hormaechei YT-3 for the Enzymatic Synthesis of 3-Hydroxy-β-ionone from Lutein" Catalysts 11, no. 11: 1257. https://doi.org/10.3390/catal11111257
APA StyleLong, Z., Duan, N., Xue, Y., Wang, M., Li, J., Su, Z., Liu, Q., Mao, D., & Wei, T. (2021). Characterization of a Novel Lutein Cleavage Dioxygenase, EhLCD, from Enterobacter hormaechei YT-3 for the Enzymatic Synthesis of 3-Hydroxy-β-ionone from Lutein. Catalysts, 11(11), 1257. https://doi.org/10.3390/catal11111257





