Abstract
Ivabradine (Corlanor®), is a chiral benzocycloalkane currently employed and commercialized for the treatment of chronic stable angina pectoris and for the reduction in sinus tachycardia. The eutomer (S)-ivabradine is usually produced via chiral resolution of intermediates, by employing enantiopure auxiliary molecules or through preparative chiral HPLC separations. Recently, more sustainable biocatalytic approaches have been reported in literature for the preparation of the chiral amine precursor. In this work, we report on a novel biocatalyzed pathway, via a resolution study of a key alcohol intermediate used as a precursor of the chiral amine. After screening several enzymatic reaction conditions, employing different lipases and esterases both for the esterification and hydrolysis reactions, the best result was achieved with Pseudomonas cepacia Lipase and the final product was obtained in up to 96:4 enantiomeric ratio (e.r.) of an ivabradine alcohol precursor. This enantiomer was then efficiently converted into the desired amine in a facile three step synthetic sequence.
1. Introduction
Ischemic heart disease is conventionally treated with β-blockers, calcium antagonists, and ACE inhibitors []. Despite the use of different therapies, some patients still exhibit refractory angina or vascular side effects. In particular, high values of heart rate (HR) have been proved to be critical in patients suffering from chronic heart failure (CHF) []. The actual insufficient prevention of mortality due to major coronary events is the driving force for the research of new anti-ischemic drugs. In this context, new therapeutics are necessary to modulate HR in those conditions [,,]. HR is determined by sinoatrial (pacemaker) nodal cells among the cardiac myocytes, which generate spontaneous depolarization current, controlled by If (funny) channels [,]. In recent literature, it was reported that the If current can be inhibited by some benzocycloalkane derivatives; particularly, the (S)-ivabradine 1 (Scheme 1) [,,,] was selected as the best compound for activity and hemodynamic values [], and approved by the European Medicines Agency in 2005 for the treatment of stable angina pectoris [,]. Like for many other medicines, stereochemistry is crucial in ivabradine’s structure; only its (S)-enantiomer is active as a drug, therefore, either a chiral synthesis or a racemic resolution of intermediates is necessary in its production. According to literature, including patents, most synthetic approaches aim at obtaining the secondary amine 2 as the chiral building block (Scheme 1) needed for ivabradine’s final structure [,,]. Thus, the target (S)-2 can be obtained via resolution with chiral acids or by preparative chiral HPLC [,]. The described preparations run into problems like low-resolution yields of enantiomerically pure amine, due to several crystallization cycles needed, or not practical scale-up processes. Moreover, chemical reactions involving metal catalysts (e.g., reductions with LiAlH4) [] produce large amount of waste in industrial productions; in a time where manufacturing is focusing more and more on greener chemical processes [,,,], a biocatalytic approach can be also exploited as a valid alternative for the industrial ivabradine’s synthesis. Indeed, enzyme-based processes are already in use in the pharmaceutical productions, such as in the syntheses of Pregabalin-Lyrica® [], an antiepileptic drug, Sitagliptin-Januvia® [,,], a DPP-4 inhibitor for treating type 2 diabetes, or Zanamivir-Relenza® [], a neuraminidase inhibitor against influenza.
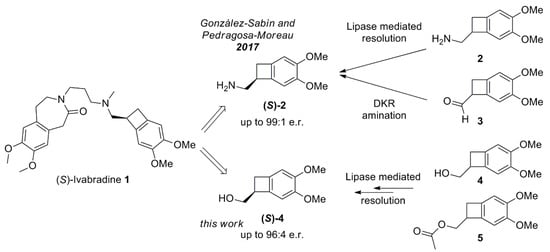
Scheme 1.
Overview on ivabradine 1 and the possible biocatalytic routes [] to the synthesis of its precursors (S)-2 and (S)-4.
An important goal in industrial field today is to perform reactions at ambient temperature and pressure, reducing the use of organic solvents or metal catalysts []. Enzymes often constitute an environmentally friendly alternative to conventional chemical catalysts; they can operate under mild conditions and at low temperature and are nontoxic and biodegradable. The high chemo-, regio-, and stereoselectivity often exhibited enable purer and more selective reactions, and may even diminish the need for functional group protections, which will increase the atom economy and afford shorter synthetic routes [,,]. This is attractive from both an economical and an environmental point of view, so that in recent years, there has been an increase in the use of biocatalysis in industry [,], with hydrolases being the most common enzymes employed [,,,], the class to which esterases and lipases belong to. In their natural environment, their role deals with the hydrolysis of ester bonds [,], such as in the case of free fatty acids, and water is the solvent where they operate. Nevertheless, if lipases are used in organic solvents, condensations, and transacylations can be easily performed. These are reactions, which are unfavored in aqueous solution [,,]. Other common examples of nonhydrolytic processes that can be performed if lipases are used in organic solvents are esterifications, transesterifications, aminolysis, and thiolysis. Lipases are among the most commonly employed enzymes, especially for molecules that resemble their natural lipophilic substrates [,].
In this scenario, the excellent properties displayed by enzymes in terms of selectivity and reactivity under mild reaction conditions make therefore biocatalysis an attractive alternative for the production of enantiopure building blocks and pharmaceuticals [,,].
Recently, the biocatalytic tools have been applied to the preparation of the ivabradine precursor chiral amine 2. Excellent results have been obtained [] with the use of a lipase from Pseudomonas cepacia (PSC-II) mediated resolution of 2 (99% ee) with diethyl carbonate and 2-Me-THF as a solvent, whereas with the biocatalytic amination of the aldehyde 3, the desired amine could be obtained in 90% ee under dynamic kinetic resolution (DKR) conditions. Despite the very good results hitherto obtained, the use of organic solvent as 2-Me-THF or of very expensive enzymes as transaminase ω-TA, still can represent a limit to a broad application of this transformation. Following these observations, we decided to investigate the possibility to obtain (S)-2 from chiral alcohol (S)-4, which could be, in turn, obtained by an enzymatic resolution of its racemic form. We decided to explore both the acylation of 4 and the hydrolysis of 5. This last reaction can be specially attracting for the use of water as the solvent and very mild reaction conditions. In this work, we tested several easily commercially available Lipase and Esterase in different reaction conditions. Once the enantioenriched intermediate (S)-4 has been obtained, it could be easily converted into the desired amine precursor (S)-2 in a few chemical steps.
2. Results and Discussion
The general scheme of the synthetic pathway to 4 and 5 is reported in Scheme 2. The aim was to obtain both the racemic alcohol 4 and the acylated compound 5 as analytical references and enzymes substrates. The staring material was the commercially available cyano-compound 6. This last was easily hydrolyzed in basic environment to the corresponding carboxylic acid, which was further submitted to a Fischer esterification in methanol to form the methyl ester 7 in 72% overall yield. The racemic alcohol 4 was then achieved by reduction in 7 with LiAlH4. Compound 4 was acetylated using acetic anhydride and pyridine, to get the derivative 5. Biocatalytic resolutions studies were performed on both substrates 4 and 5, employing several types of Lipases and Esterases with different reaction conditions, as discussed in the following paragraphs.
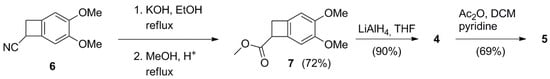
Scheme 2.
Synthesis of racemic substrates 4 and 5.
2.1. Studies on the Enzymes-Catalyzed Acetylation of Racemic 4
Among Lipases and Esterases, different types of enzymes were exploited to achieve enantiomerically enriched compound (S)-5 (Scheme 3). Namely, three esterases (CLEA-Esterase BS2, CLEA-Esterase BS3, and Amano Acylase) and three lipases (Candida antarctica Lipase B-CAL-B, Amano Pseudomonas cepacia Lipase, and Porcine Pancreas Lipase PPL-Type II) were employed. The enzymes were tested varying the reaction medium, using MTBE, tetrahydrofuran, diethyl ether, and mixtures of these organic solvents with drops of buffer. Attempts were also made to substitute the vinyl acetate with butyric anhydride or acetic anhydride, but with a complete loss of selectivity. Temperature was also controlled, and tested between 20 and 40 °C. After exploiting the different conditions, reactions were performed in tert-butyl methyl ether (MTBE) as a solvent and with the use of vinyl acetate as the acyl donor at room temperature.
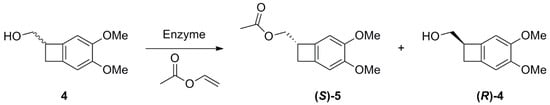
Scheme 3.
Resolution by enzymes-catalyzed acetylation of 4.
The screening was performed on 50 mg of substrate; reactions were monitored up to 48 h with GC-MS for the conversions’ evaluation, whereas the enantiomeric ratio (e.r.) was measured through a chiral HPLC. Results are reported in Table 1. Since the enantiomeric ratio is the most important parameter in this study, the reaction times reported in Table 1 refer to the time at which the best balance between high e.r. and conversions was measured.

Table 1.
Results from screening of enzymes on the acetylation of racemic 4.
As shown, the Esterases exhibited a poor reactivity in these conditions, and generally very low conversions and e.r. values were obtained. On the contrary, Lipases showed a better activity: the most interesting result was achieved with Lipase PS (Amano), which produced a 41% conversion and a 90:10 e.r. of the acylated product (S)-5 after only 1 h (entry 5, Table 1). These enzymes showed a preference for the (S)-enantiomer of the substrate (for the determination of the configuration vide infra), forming enantioenriched acylated compound (S)-5. Anyway, due to a scarce selectivity, each reaction revealed a progressive decrease in enantiomeric excess value during the increase in conversion in product. Indeed, using as an example the results with Lipase PS, after 2 h, the conversion increased up to 57% and e.r. dropped to 86:14. The calculated selectivity factor for this biotransformation is E = 17; with this value, it would be possible to obtain the enantiopure substrate (R)-4 in 30% yield (70% conversion). This was, however, the undesired enantiomer for the synthesis of the target compound would be obtained.
2.2. Studies on the Enzymes-Catalyzed Hydrolysis of Racemic 5
We then moved toward the investigation of the enzymes-catalyzed selective hydrolysis of the acetylated racemic compound 5 (Scheme 4).
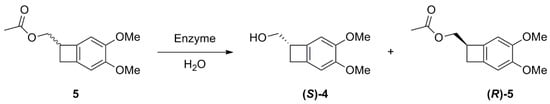
Scheme 4.
Resolution by enzymes-catalyzed hydrolysis of 5.
The same set of the aforementioned Lipases and Esterases were used. The optimized setup for the reaction was a mixture of Na2PO4 buffer at pH 7.4 and EtOH (5:1), operating at room temperature. Moreover, in this case, reactions were monitored up to 48 h with GC-MS for the conversions’ evaluation, whereas the enantiomeric ratio (e.r.) was measured through a chiral HPLC. The results are reported in Table 2. The reaction times reported refer to the time at which the best balance between high e.r. and conversions was measured.

Table 2.
Results from screening of enzymes for the hydrolysis of racemic 5.
Esterases showed in all cases good conversion values, but the enantioselection was always very poor. The best result was achieved again using Lipase PS (Amano). With this catalyst, after only 30 min at room temperature, a moderate 30% conversion with a good 96:4 e.r. could be achieved (entry 5, Table 2). The other Lipases also gave good conversions, but lower e.r.s were measured. According to what was previously observed, the enzyme resulted more active on the (S)-enantiomer of substrate 5, thus under hydrolysis conditions, the desired alcohol (S)-4 was obtained. Under these conditions, we were able to prepare 0.5 g of enantioenriched alcohol (S)-4 to be further transformed into the desired amine (S)-2.
2.3. Conversion of Alcohol (S)-4 into Amine (S)-2
To obtain the desired amine (S)-2, a straightforward three steps reaction sequence was performed (Scheme 5). First, the alcohol (S)-4 was converted into the corresponding tosylate using tosyl chloride and pyridine as a base, in dichloromethane. Next, substitution of the activate tosylate moiety with sodium azide at 60 °C in DMF, allowed to isolate, after a chromatographic purification, the azido-derivative (S)-9. Finally, the reduction in the azido group with triphenylphosphine in THF afforded the target compound in 70% overall yield after three steps.
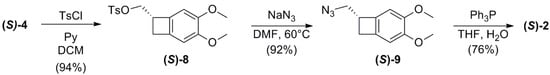
Scheme 5.
Conversion of (S)-4 into the target compound (S)-2.
It is worth to notice that this synthetic sequence is stereoconservative, since no transformation occurred on the stereogenic carbon of the starting alcohol. Thus, by the comparison of the sign of the optical rotatory power of the final product with literature, we could establish the correct (S) configuration of the starting alcohol (S)-4 as resulting from the lipase-mediated hydrolysis of racemic ester 5 ([α]D20 = −7.3 in CHCl3 for (S)-2 obtained from our sequence and [α]D20 = + 7.9 for (R)-2 compound from the literature []).
3. Materials and Methods
3.1. General Remarks
Chemicals and solvents were purchased from Merck KGaA (Darmstadt, Germany) and used without further purifications. Reactions were monitored mostly by thin-layer chromatography (TLC), performed on Merck Kieselgel 60 F254 plates. Visualization was accomplished by UV irradiation at 254 nm and subsequently by treatment with the alkaline KMnO4 reactant or with a phosphomolybdic reagent. 1H and 13C-NMR spectra were recorded on a Bruker 400 spectrometer (1H-NMR, 400 MHz; 13C-NMR, 100 MHz) in CDCl3 solution using TMS as an internal standard; chemical shifts (δ) in the spectra are reported in ppm, and the coupling constants J are reported in Hz. GC-MS analyses were performed on an Agilent HP 6890 gas chromatograph equipped with a HP-5MS column (30 m × 0.25 mm × 0.25 µm), with injection temperature = 250 °C, injecting 1 µL of solution, and using He as a carrier gas (1.0 mL·min−1). The method used for the analyses was: 60 °C (1 min)/6 °C/min/150 °C (1 min)/12 °C/min/280 °C (5 min). Determination of enantiomeric excesses was determined by HPLC Agilent 1260 Infinity equipped with a chiral column Lux 5 µm Cellulose-3 (250 × 4.60 mm), λ = 288 nm. Product (S)-2 isolation was achieved through preparative HPLC Agilent 1260 Infinity equipped with a Luna 10u PREP Silica(3) 100A Column (250 × 21.2 mm), λ:288nm. Optical rotations were performed on JASCO DIP 181 digital polarimeter. The enzymes employed in this work were: Acylase (30,000 U/g from Amano Enzyme Inc., Nagoya, Japan), Esterase BS3 (CLEA) 2500 U/g, and BS2 obtained from Bacillus subtilis 10,000 U/g, immobilized Candida Antarctica Lipase B (CAL-B, Novozym® 435 10,000 U/g from Boehringer Manheim, Germany), Porcine Pancreas Lipase (PPL) (Type II) (30,000 U/g from Merck KGaA, Darmstadt, Germany), and Pseudomonas cepacia Lipase (Lipase PS, 30,000 U/g from Amano Enzyme Inc., Nagoya, Japan).
3.2. Synthetic Procedures
Methyl 3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-triene-7-carboxylate 7
A mixture of 3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-triene-7-carbonitrile 6 (2 g, 10.6 mmol) and KOH (17 eq) in ethanol (20 mL) was refluxed for 8 h. The reaction mixture was cooled down to room temperature and the ethanol was evaporated under reduced pressure. The residue was acidified with 1 N HCl (aq.) and extracted three times with ethyl acetate (3 × 50 mL). The combined extracts were dried over anhydrous Na2SO4, filtered, and then concentrated in vacuum to yield the brown solid carboxylic acid intermediate (1.84 g, 84%). 1H-NMR (400 MHz, CDCl3) δ 6.78 (s, 1H, Ar H), 6.72 (s, 1H, Ar H), 4.25 (dd, J = 4.3, 3.3 Hz, 1H, -CH-), 3.87 (d, J = 1.0 Hz, 6H, -OCH3), 3.44–3.41 (m, 2H, -CH2-). 13C-NMR (101 MHz, CDCl3) δ 178.4 (C=O), 150.8 (Ar), 150.1 (Ar), 135.3 (Ar), 133.1 (Ar), 107.2 (Ar), 106.8 (Ar), 56.3 (-OCH3), 44.7 (-CH-), 33.4 (-CH2-).
A mixture of the 3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-triene-7-carboxylic acid obtained (1.84 g, 8.9 mmol) and methanol (20 mL) was stirred for 15 min. Then, a few drops of concentrated H2SO4 were added and the mixture was refluxed for 8 h. The resulting mixture was cooled down at room temperature and the solvent was evaporated under reduced pressure; the residue was washed with NaHCO3 (aq.) and extracted three times with ethyl acetate (3 × 50 mL). The combined extracts were dried over anhydrous Na2SO4, filtered, and then concentrated in vacuum to yield the brown oil. Purification of the crude oil on a column chromatography silica gel (eluent hexane/ethyl acetate from 9:1 to 7:3) afforded 7 (1.65 g, 85%). 1H-NMR (400 MHz, CDCl3) δ 6.77 (s, 1H, Ar H), 6.71 (s, 1H, Ar H), 4.22 (dd, J = 3.9 Hz, 1H, -CH-), 3.85 (bs, 6H, -OCH3), 3.74 (s, 3H, -COOCH3), 3.39 (d, J = 3.9 Hz, 2H, -CH2-). 13C-NMR (101 MHz, CDCl3) δ 173.1 (C=O), 150.6 (Ar), 149.9 (Ar), 135.7 (Ar), 133.7 (Ar), 107.6 (Ar), 106.9 (Ar), 56.4 (-OCH3), 56.3 (-OCH3), 52.0 (-COOCH3), 44.8 (-CH-), 33.4 (-CH2-). (See Figure S1 in the Supplementary Materials for NMR spectra.)
(3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)methanol 4
A solution of compound 7 (1.65 g, 7.4 mmol) in THF (15 mL) was stirred at room temperature for 15 min. Then, a solution of LiAlH4 (1.15 eq) in dry THF (2 mL) was added dropwise and the mixture was refluxed for 8 h. The reaction mixture was quenched with distilled H2O and extracted three times with ethyl acetate (3 × 50 mL) after removing the organic solvent with rotavapor. The combined extracts were dried over anhydrous Na2SO4, filtered, and then concentrated in vacuum to yield the brown oil. Purification of the crude oil on a column chromatography silica gel (eluent hexane/ethyl acetate from 9:1 to 6:4) afforded 4 (1.30 g, 90%). 1H-NMR (400 MHz, CDCl3) δ 6.75 (s, 1H, Ar H), 6.72 (s, 1H, Ar H), 3.85 (bs, 6H, -OCH3), 3.91 (m, 2H, -CH2-OH), 3.64 (dq, J = 6.7 Hz, 1H, -CH-), 3.23 (dd, J = 13.6, 5.0 Hz, 1H, -CH2-), 2.83 (dd, J = 13.6, 2.0 Hz, 1H, -CH2-). 13C-NMR (101 MHz, CDCl3) δ 150.1 (Ar), 149.5 (Ar), 137.0 (Ar), 135.4 (Ar), 107.7 (Ar), 106.9 (Ar), 65.5 (-CH2OH), 56.4 (-OCH3), 56.3 (-OCH3), 44.4 (-CH-), 32.3 (-CH2-). (See Figure S2 in the Supplementary Materials for NMR spectra and S3 for GC-MS chromatograms.)
(3,4-dimethoxybicyclo[4.2.0]octa-1(6),2,4-trien-7-yl)methyl acetate 5
A solution of compound 4 (0.15 g, 0.77 mmol) in dichloromethane (10 mL) was stirred with acetic anhydride (5 eq) and pyridine (2 mL) for 6 h at room temperature. The reaction mixture was then acidified with 1 N HCl (aq.) and extracted three times with dichloromethane (3 × 20 mL). The combined extracts were dried over anhydrous Na2SO4, filtered, and then concentrated in vacuum to yield the brown oil. Purification of the crude oil on a column chromatography silica gel (eluent hexane/ethyl acetate from 9:1 to 7:3) afforded 5 (0.125 g, 69%). 1H-NMR (400 MHz, CDCl3) δ 6.70 (bs, 2H, Ar H), 4.32 (d, 2H, J = 7.0 Hz, -CH2-O), 3.85 (bs, 6H, -OCH3), 3.67 (dq, J = 7.0, 5.0 Hz, 1H, -CH-), 3.26 (dd, J = 13.7, 5.0 Hz, 1H, -CH2-), 2.81 (dd, J = 13.7, 2.2 Hz, 1H, -CH2-), 2.08 (s, 3H, -COCH3). 13C-NMR (101 MHz, CDCl3) δ 171.1 (C=O), 150.3 (Ar), 149.6 (Ar), 136.6 (Ar), 134.9 (Ar), 107.5 (Ar), 106.9 (Ar), 67.1 (-CH2O-), 56.3 (-OCH3), 56.3 (-OCH3), 40.9 (-CH-), 33.0 (-CH2-), 21.0 (-CH3). (See Figure S4 in the Supplementary Materials for NMR spectra and Figure S5 for GC-MS chromatograms.)
General Procedure for enzyme-mediated acetylation of (3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)methanol 4
A mixture of (3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)methanol 4 (50 mg, 0.26 mmol) in tert-butyl methyl ether (TBME, 5 mL) and vinyl acetate (10 eq) was stirred for 10 min at room temperature. Then, enzyme (300 U) was added and the resulting mixture was stirred from 1 to 48 h. To check the flow of the acetylation reaction, 200 µL withdrawals were performed every 30 min or 1 h, according to the type of enzyme. Once withdrawn, the reaction medium was filtrated, diluted with 800 µL of TBME, and analyzed by GC-MS to determine the conversion. The same samples were injected into chiral HPLC to evaluate the enantiomeric excesses of the products. (See Figures S9–S12 in the Supplementary Materials for HPLC chromatograms and Figures S13 and S14 for the selectivity profiles of the kinetic resolution of the racemate.)
General Procedure for enzyme-mediated hydrolysis of (3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)methyl acetate 5
A mixture of (3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)methyl acetate 5 (50 mg, 0.21 mmol), 0.3 M Na2HPO4 buffer solution at pH 7.4 (5 mL), and EtOH as a cosolvent (1 mL) was stirred at room temperature for 10 min. Then the enzyme (300 U) was added and the resulting mixture was stirred from 1 to 48 h. To check the flow of the hydrolysis reaction, 200 µL withdrawals were performed every 30 min or 1 h, according to the type of enzyme. Once withdrawn, the reaction medium was filtered, extracted with TBME and analyzed by GC-MS to determine the conversion. The same samples were injected into chiral HPLC to evaluate the enantiomeric excesses of the products. (See Figures S9–S12 in the Supplementary Materials for HPLC chromatograms and Figures S13 and S14 for the selectivity profiles of the kinetic resolution of the racemate.)
Preparative Synthesis of (S)-4
A mixture of (3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)methyl acetate 5 (2.0 g, 8.5 mmol), 0.3 M Na2HPO4 buffer solution at pH 7.4 (200 mL), and EtOH as a cosolvent (40 mL) was stirred at room temperature until a clear solution has formed. Then 0.5 g of Lipase PS (Amano) was added, and the resulting mixture was stirred for 30 min. Then, the reaction was filtered, extracted three times with TBME (3 × 100 mL), and the solvent was then evaporated under reduced pressure. The enantioenriched alcohol (S)-4 was isolated from the crude mixture after a silica gel column chromatography with eluents in gradient: hexane-ethyl acetate from 9:1 to 7:3 (0.52 g, 31% yield). [α]20D = −4.8 (c 1, CHCl3).
(S)-(3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)methyl 4-methylbenzenesulfonate (S)-8
A solution of compound (S)-4 (0.545 g, 2.8 mmol) in dichloromethane (20 mL) was cooled in ice bath and stirred, while pyridine (3 eq) was added dropwise. Then a solution of tosyl chloride (TsCl, 1.2 eq) in dichloromethane (8 mL) was dropped into the mixture. The ice bath was removed, and the reaction mixture was stirred at room temperature for 48 h. The resulting mixture was washed with 1 N HCl (aq.), the organic solvent was removed under pressure, and the reaction was extracted three times with ethyl acetate (3 × 50 mL). The organic layer was dried over anhydrous Na2SO4, filtered, and concentrated to yield a crude product. The product was purified through a silica gel column chromatography (eluent in gradient: hexane-ethyl acetate) to obtain the desired pure compound (S)-8, as a brownish oil (0.91 g, 94%). [α]20D = +12.4 (c 1, CHCl3). 1H-NMR (400 MHz, CDCl3) δ 7.78–7.76 (m, 2H, Ar H), 7.35–7.33 (m, 2H, Ar H), 6.66 (s, 1H, Ar H), 6.63 (s, 1H, Ar H), 4.28–4.17 (m, 2H, -CH2-O), 3.81 (d, J = 6.6 Hz, 6H, -OCH3), 3.67 (tdd, J = 7.2, 4.9, 2.2 Hz, 1H, (-CH-), 3.21 (dd, J = 13.8, 4.9 Hz, 1H, -CH2-), 2.73 (dd, J = 13.8, 2.1 Hz, 1H, -CH2-), 2.44 (s, 3H, -CH3). 13C-NMR (101 MHz, CDCl3) δ 150.4 (Ar), 149.6 (Ar), 144.7 (Ar), 135.3 (Ar), 134.6 (Ar), 133.2 (Ar), 129.8 (Ar), 127.8 (Ar), 107.5 (Ar), 106.8 (Ar), 72.9 (-CH2O-), 56.3 (-OCH3), 56.19 (-OCH3), 40.7 (-CH-), 32.7 (-CH2-), 20.6 (-CH3). (See Figure S6 in the Supplementary Materials for NMR spectra.)
(S)-7-(azidomethyl)-bicyclo-[4.2.0]-octa-1,3,5-triene (S)-9
A solution of compound (S)-8 (0.5 g, 1.4 mmol) in DMF (5 mL) was stirred for 10 min. Then NaN3 (2 eq) was added into the flask. The mixture was refluxed for 12 h and then washed with brine and extracted five times with dichloromethane (5 × 30 mL). The organic layers were dried over anhydrous Na2SO4, filtered, and concentrated to yield a yellow oil of (S)-9 without further purification (0.270 g, 92%). [α]20D = +6.37 (c 0.85, CHCl3). 1H-NMR (400 MHz, CDCl3) δ 6.75 (s, 1H, Ar H), 6.71 (s, 1H, Ar H), 3.85 (s, 6H, -OCH3), 3.68–3.61 (m, 1H, -CH-), 3.60–3.48 (m, 2H, -CH2-N3), 3.29 (dd, J = 13.7, 4.9 Hz, 1H, -CH2-), 2.80 (dd, J = 13.7, 2.2 Hz, 1H, -CH2-). 13C-NMR (101 MHz, CDCl3) δ 150.4 (Ar), 149.7 (Ar), 136.9 (Ar), 134.5 (Ar), 107.5 (Ar), 106.9 (Ar), 56.4 (-OCH3), 56.3 (-OCH3), 55.1 (-CH2N3), 41.7 (-CH-), 33.5 (-CH2-). (See Figure S7 in the Supplementary Materials for NMR spectra.)
(S)-(3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)-methanamine (S)-2
A solution of compound (S)-9 (0.150 g, 0.7 mmol) in THF (5 mL) was stirred at room temperature for 10 min. Then, triphenylphosphine (2 eq) was added and stirred for 1 h at room temperature. After that, distilled H2O (1 mL) was added and stirred at room temperature for 12 h. The resulting solution was acidified with 1 N HCl (aq.) and extracted once with diethyl ether (1 × 20 mL) after removing the organic solvent under vacuum. The aqueous phase was made basic with NaOH (pH 12) and extracted twice with diethyl ether (2 × 20 mL). The organic layers were dried over anhydrous Na2SO4, filtered, and concentrated under vacuum to yield a yellow oil. After preparative HPLC purification on a silica column (eluents in gradient: hexane-ethyl acetate) (S)-2 was obtained (0.1 g, 76%). [α]20D = −7.3 (c 1, CHCl3). In accordance with literature values []. 1H-NMR (400 MHz, CDCl3) δ 6.73 (s, 1H, Ar H), 6.71 (s, 1H, Ar H), 3.85 (d, J = 1.8 Hz, 6H, -OCH3), 3.84 (m, 2H, -CH2-NH2), 3.46 (tdt, J = 8.0, 5.2, 2.5 Hz, 1H, -CH-), 3.23 (dd, J = 13.5, 5.0 Hz, 1H, -CH2-), 2.98 (d, J = 6.9 Hz, 2H, -NH2), 2.73 (dd, J = 13.5, 2.2 Hz, 1H, -CH2-). 13C-NMR (101 MHz, CDCl3) δ 150.0 (Ar), 149.6 (Ar), 138.4 (Ar), 135.2 (Ar), 107.7 (Ar), 106.7 (Ar), 56.4 (-OCH3), 56.3 (-OCH3), 46.1 (-CH2NH2), 45.2(-CH-), 33.2 (-CH2-). (See Figure S8 in the Supplementary Materials for NMR spectra.)
4. Conclusions
In the case of (S)-ivabradine, many enzymes have been already tested for the resolution of racemic amine 2, being its main synthetic precursor. An example reported in literature [] is its kinetic resolution via alkoxycarbonylation using lipase from Pseudomonas cepacia (PSC-II), diethyl carbonate, and 2-Me-THF as a solvent. The (S)-carbamate intermediate is then converted into the secondary (S)-2, used to provide (S)-ivabradine in one-step reaction. However, the most common synthetic routes attempted to (S)-2, do not concern biocatalysis; examples are reported by Liu et al., [,] where a synthesis involves the reduction in a cyano group to an amine, its acylation to form an amide, followed by a reduction with LiAlH4. The final step is a resolution of the racemic mixture with the use of the chiral d-camphorsulfonic acid. Alternatively, [,] a primary amine is prepared by reduction in the cyano group and immediately resolved with N-acetyl-L-glutamic acid to produce the desired optical isomer, whose acylation and reduction afford (S)-2. As a last example, the cyano intermediate can be easily converted into a carboxylic derivative, resolved using a chiral amine to get the pure enantiomer that can be reacted to give an amide, finally reduce to (S)-a. []
In this work, we performed the synthesis of the chiral amine (S)-2 by means of a Lipase PS-mediated resolution of the ester precursor 5. One of the advantages of this protocol is that this transformation could be realized under mild conditions, in water and at room temperature on the gram scale, by employing only the 10% m/m of easily available, reusable and cheap catalytic enzyme. This allowed to obtain the alcohol (S)-4 in satisfying yield and in good 96:4 e.r. A three steps sequence allowed then to transform the enantiopure alcohol into the target compound, the essential precursor of (S)-ivabradine.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/11/1/53/s1, Figure S1 to Figure S8: NMR spectra for all the compounds and GC-MS chromatograms for compounds 4 and 5. Figure S9 to Figure S12: HPLC chromatograms for the resolution of compounds 4 and 5. Figure S13 and Figure S14: Selectivity profiles for the resolution of 4 and 5.
Author Contributions
Conceptualization, A.S.; investigation, A.M. and A.R.; supervision, A.S.; writing—original draft preparation, A.S. and A.R.; writing—review and editing, A.S. and A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project VIPCAT, Regione Lombardia, POR FESR 2014-2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parker, J.D.; Parker, J.O. Stable Angina Pectoris: The Medical Management of Symptomatic Myocardial Ischemia. Can. J. Cardiol. 2012, 28, S70–S80. [Google Scholar] [CrossRef]
- Hori, M.; Okamoto, H. Heart rate as a target of treatment of chronic heart failure. J. Cardiol. 2012, 60, 86–90. [Google Scholar] [CrossRef]
- Pedragosa-Moreau, S.; Le Flohic, A.; Thienpondt, V.; Lefoulon, F.; Petit, A.M.; Ríos-Lombardía, N.; Morís, F.; González-Sabín, J. Exploiting the Biocatalytic Toolbox for the Asymmetric Synthesis of the Heart-Rate Reducing Agent Ivabradine. Adv. Synth. Catal. 2017, 359, 485–493. [Google Scholar] [CrossRef]
- Romanelli, M.N.; Sartiani, L.; Masi, A.; Mannaioni, G.; Manetti, D.; Mugelli, A.; Cerbai, E. HCN Channels Modulators: The Need for Selectivity. Curr. Top. Med. Chem. 2016, 16, 1764–1791. [Google Scholar] [CrossRef]
- Sartiani, L.; Mannaioni, G.; Masi, A.; Romanelli, M.N.; Cerbai, E. The hyperpolarization-activated cyclic nucleotide-gated channels: From biophysics to pharmacology of a unique family of ion channels. Pharmacol. Rev. 2017, 69, 354–395. [Google Scholar] [CrossRef]
- Badu-Boateng, C.; Jennings, R.; Hammersley, D. The therapeutic role of ivabradine in heart failure. Ther. Adv. Chronic Dis. 2018, 9, 199–207. [Google Scholar] [CrossRef]
- Pascual Izco, M.; Alonso Salinas, G.L.; Sanmartín Fernández, M.; Del Castillo Carnevalli, H.; Jiménez Mena, M.; Camino López, A.; Zamorano Gómez, J.L. Clinical Experience with Ivabradine in Acute Heart Failure. Cardiology 2016, 134, 372–374. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Anguita, M.; Castillo, J.C.; Rodríguez, S.; Pardo, L.; Durán, E.; Sánchez, J.J.; Ferreiro, C.; Pan, M.; Mesa, D.; et al. Effect of early treatment with ivabradine combined with beta-blockers versus beta-blockers alone in patients hospitalised with heart failure and reduced left ventricular ejection fraction (ETHIC-AHF): A randomised study. Int. J. Cardiol. 2016, 217, 7–11. [Google Scholar] [CrossRef]
- Camici, P.G.; Gloekler, S.; Levy, B.I.; Skalidis, E.; Tagliamonte, E.; Vardas, P.; Heusch, G. Ivabradine in chronic stable angina: Effects by and beyond heart rate reduction. Int. J. Cardiol. 2016, 215, 1–6. [Google Scholar] [CrossRef]
- Weeda, E.R.; Nguyen, E.; White, C.M. Role of Ivabradine in the Treatment of Patients With Cardiovascular Disease. Ann. Pharmacother. 2016, 50, 475–485. [Google Scholar] [CrossRef]
- Malinowski, J.T.; St. Jean, D.J. Next-generation small molecule therapies for heart failure: 2015 and beyond. Bioorganic Med. Chem. Lett. 2018, 28, 1429–1435. [Google Scholar] [CrossRef]
- Sharma, V.; Dewangan, H.K.; Maurya, L.; Vats, K.; Verma, H.; Singh, S. Rational design and in-vivo estimation of Ivabradine Hydrochloride loaded nanoparticles for management of stable angina. J. Drug Deliv. Sci. Technol. 2019, 54, 101337. [Google Scholar] [CrossRef]
- Peglion, J.L.; Vian, J.; Vilaine, J.P.; Villeneuve, N.; Janiak, P.; Bidouard, J.P. Benzocyclobutyl- or indanyl-alkyl-amino-alkyl substituted 3-benzazepin-2-ones Useful in the Treatment of Cardiovascular Diseases. EP Patent 0534859A1, 25 September 1992. [Google Scholar]
- Lerestif, M.J.; Gonzales, I.; Lecouve, J.P.; Brigot, D. Method of Synthesising (1s)-4,5-dimethoxy-1-(methylaminomethyl)-benzocyclobutane and the Addition Salts Thereof, and Use of Same for the Synthesis of Ivabradine and the Pharmaceutically-Acceptable Addition Salts Thereof. WO Patent 2005123659A1, 29 December 2005. [Google Scholar]
- Ujvári, V.; Bódi, J.; Faragó, J.; Szöké, K.; Faigl, F.; Német, Z.; Temesvári, K.; Kiss, R.; Mátravoelgyi, B.; Kassai, F.; et al. Industrial Process for the Synthesis of Ivabradine Salts. WO Patent 2011138625A1, 10 November 2011. [Google Scholar]
- Liu, X.; Liu, Y.; He, H.; Cai, Z.; Yang, Y. New synthetic route to (1s)-4,5-dimethoxy-1-[(methylamino)methyl] benzocyclobutane, a key intermediate of ivabradine. Synth. Commun. 2014, 44, 451–456. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Arends, I.W.C.E.; Hanefeld, U. Introduction: Green Chemistry and Catalysis. In Green Chemistry and Catalysis; Wiley Online Books; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 1–47. ISBN 9783527611003. [Google Scholar]
- Sheldon, R.A.; Woodley, J.M. Role of Biocatalysis in Sustainable Chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Woodley, J.M. New opportunities for biocatalysis: Making pharmaceutical processes greener. Trends Biotechnol. 2008, 26, 321–327. [Google Scholar] [CrossRef]
- Pollard, D.J.; Woodley, J.M. Biocatalysis for pharmaceutical intermediates: The future is now. Trends Biotechnol. 2007, 25, 66–73. [Google Scholar] [CrossRef]
- Patel, R.N. Biocatalysis for synthesis of pharmaceuticals. Bioorg. Med. Chem. 2018, 26, 1252–1274. [Google Scholar] [CrossRef]
- Hansen, K.B.; Hsiao, Y.; Xu, F.; Rivera, N.; Clausen, A.; Kubryk, M.; Krska, S.; Rosner, T.; Simmons, B.; Balsells, J.; et al. Highly Efficient Asymmetric Synthesis of Sitagliptin. J. Am. Chem. Soc. 2009, 131, 8798–8804. [Google Scholar] [CrossRef]
- Desai, A.A. Sitagliptin Manufacture: A Compelling Tale of Green Chemistry, Process Intensification, and Industrial Asymmetric Catalysis. Angew. Chemie Int. Ed. 2011, 50, 1974–1976. [Google Scholar] [CrossRef]
- Savile, C.K.; Janey, J.M.; Mundorff, E.C.; Moore, J.C.; Tam, S.; Jarvis, W.R.; Colbeck, J.C.; Krebber, A.; Fleitz, F.J.; Brands, J.; et al. Biocatalytic Asymmetric Synthesis of Chiral Amines from Ketones Applied to Sitagliptin Manufacture. Science 2010, 329, 305–309. [Google Scholar] [CrossRef]
- Ran, N.; Zhao, L.; Chen, Z.; Tao, J. Recent applications of biocatalysis in developing green chemistry for chemical synthesis at the industrial scale. Green Chem. 2008, 10, 361–372. [Google Scholar] [CrossRef]
- Kazlauskas, R.J.; Kim, B.-G. Biotechnology Tools for Green Synthesis: Enzymes, Metabolic Pathways, and their Improvement by Engineering. Biocatal. Green Chem. Chem. Process Dev. 2011, 1–22. [Google Scholar] [CrossRef]
- Sheldon, R.A. Enzyme Immobilization: The Quest for Optimum Performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Fiorati, A.; Berglund, P.; Humble, M.S.; Tessaro, D. Application of Transaminases in a Disperse System for the Bioamination of Hydrophobic Substrates. Adv. Synth. Catal. 2020, 362, 1156–1166. [Google Scholar] [CrossRef]
- Turner, N.J.; Truppo, M.D. Biocatalysis enters a new era. Curr. Opin. Chem. Biol. 2013, 17, 212–214. [Google Scholar] [CrossRef]
- Adrio, J.L.; Demain, A.L. Microbial enzymes: Tools for biotechnological processes. Biomolecules 2014, 4, 117–139. [Google Scholar] [CrossRef]
- García-Urdiales, E.; Alfonso, I.; Gotor, V. Enantioselective Enzymatic Desymmetrizations in Organic Synthesis. Chem. Rev. 2005, 105, 313–354. [Google Scholar] [CrossRef]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef]
- Jaeger, K.-E.; Eggert, T. Lipases for biotechnology. Curr. Opin. Biotechnol. 2002, 13, 390–397. [Google Scholar] [CrossRef]
- Hasan, F.; Shah, A.A.; Hameed, A. Industrial applications of microbial lipases. Enzyme Microb. Technol. 2006, 39, 235–251. [Google Scholar] [CrossRef]
- Ollis, D.L.; Cheah, E.; Cygler, M.; Dijkstra, B.; Frolow, F.; Franken, S.M.; Harel, M.; Remington, S.J.; Silman, I.; Schrag, J. The alpha/beta hydrolase fold. Protein Eng. 1992, 5, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Holmquist, M. Alpha/Beta-hydrolase fold enzymes: Structures, functions and mechanisms. Curr. Protein Pept. Sci. 2000, 1, 209–235. [Google Scholar] [CrossRef] [PubMed]
- Klibanov, A.M. Improving enzymes by using them in organic solvents. Nature 2001, 409, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, P.; Muderhwa, J.M.; Graille, J.; Haas, M.J. Customizing lipases for biocatalysis: A survey of chemical, physical and molecular biological approaches. J. Mol. Catal. B Enzym. 2000, 9, 113–148. [Google Scholar] [CrossRef]
- Reetz, M.T. Lipases as practical biocatalysts. Curr. Opin. Chem. Biol. 2002, 6, 145–150. [Google Scholar] [CrossRef]
- Schrittwieser, J.H.; Resch, V. The role of biocatalysis in the asymmetric synthesis of alkaloids. RSC Adv. 2013, 3, 17602–17632. [Google Scholar] [CrossRef]
- Simon, R.C.; Mutti, F.G.; Kroutil, W. Biocatalytic synthesis of enantiopure building blocks for pharmaceuticals. Drug Discov. Today Technol. 2013, 10, e37–e44. [Google Scholar] [CrossRef]
- Chen, C.S.; Fujimoto, Y.; Girdaukas, G.; Sih, C.J. Quantitative analyses of biochemical kinetic resolutions of enantiomers. J. Am. Chem. Soc. 1982, 104, 7294–7299. [Google Scholar] [CrossRef]
- Bose, P.; Kandadai, A.S.; Siripalli, U.B.R. Process for Preparation of Ivabradine. WO Patent 2010072409A1, 1 July 2010. [Google Scholar]
- Sun, P.Y.; Chen, Y.J.; Yu, G.L. Resolution of 4,5-dimethoxy-1-(methylaminomethyl)-benzocyclobutane. WO Patent 2009062377A1, 22 May 2009. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).