Recent Advances in Microwave-Assisted Copper-Catalyzed Cross-Coupling Reactions
Abstract
1. Introduction
2. Copper-Catalyzed Cross-Coupling Reactions under Microwave Irradiation
2.1. Ullmann-Type Cross-Coupling Reactions
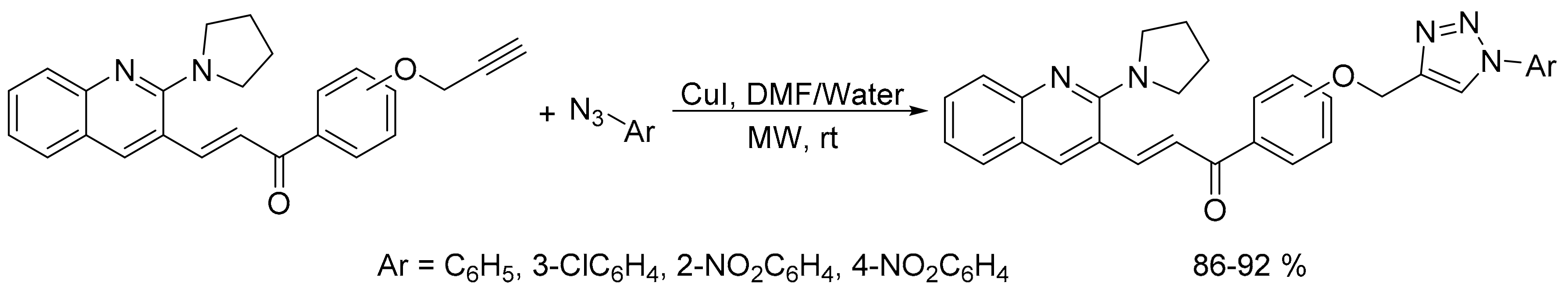
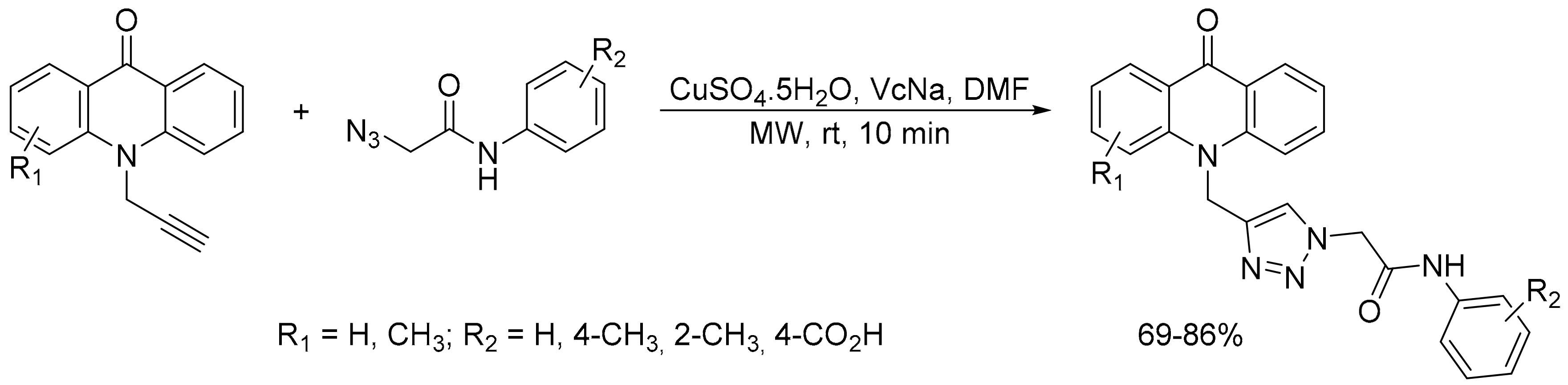
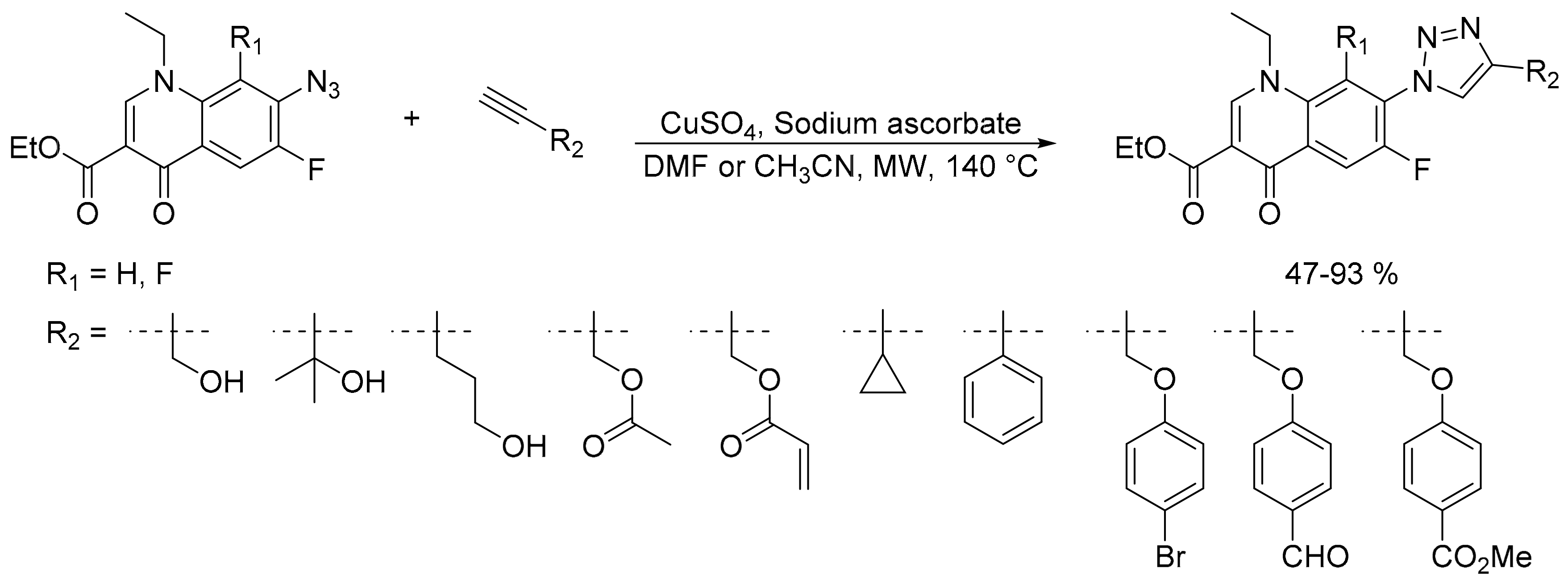
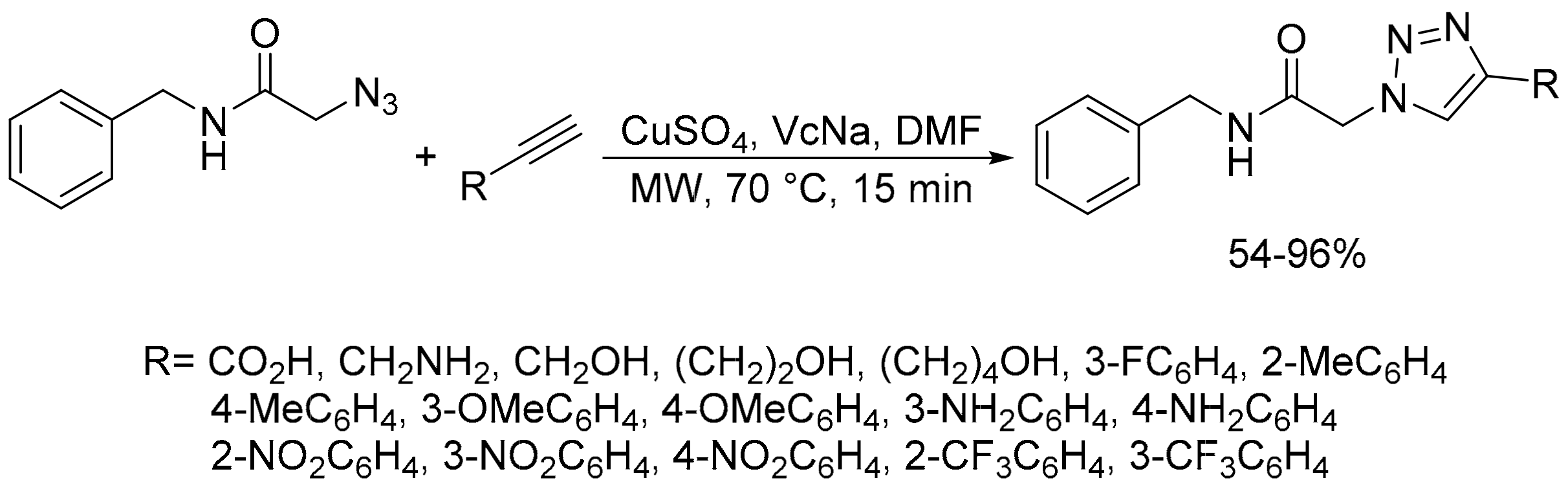
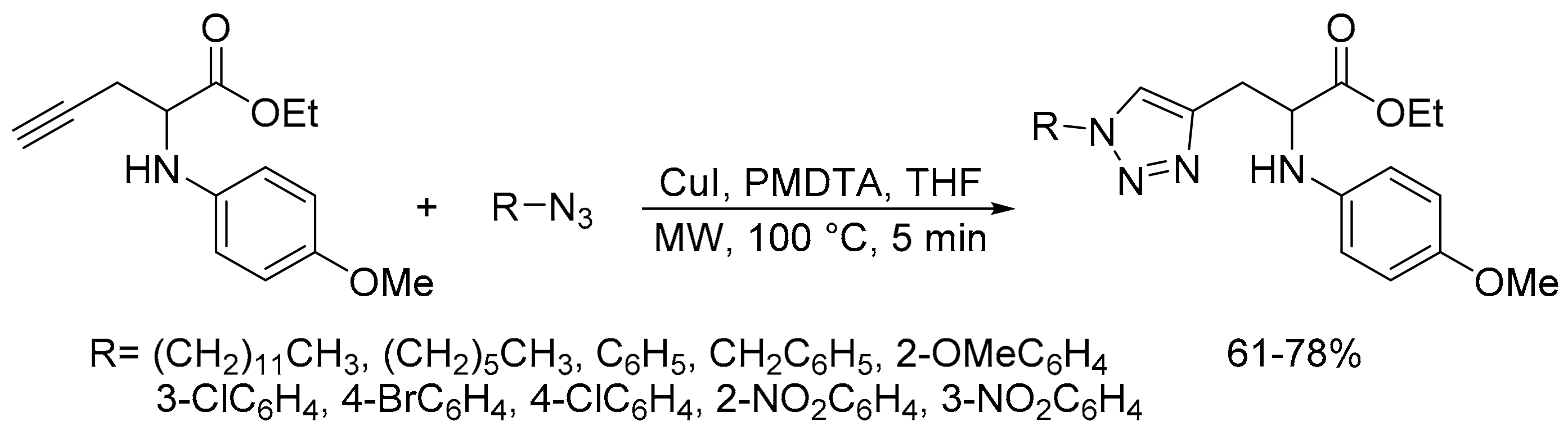
2.2. Azide-Alkyne Huisgen [2 + 3] Cycloaddition Reaction
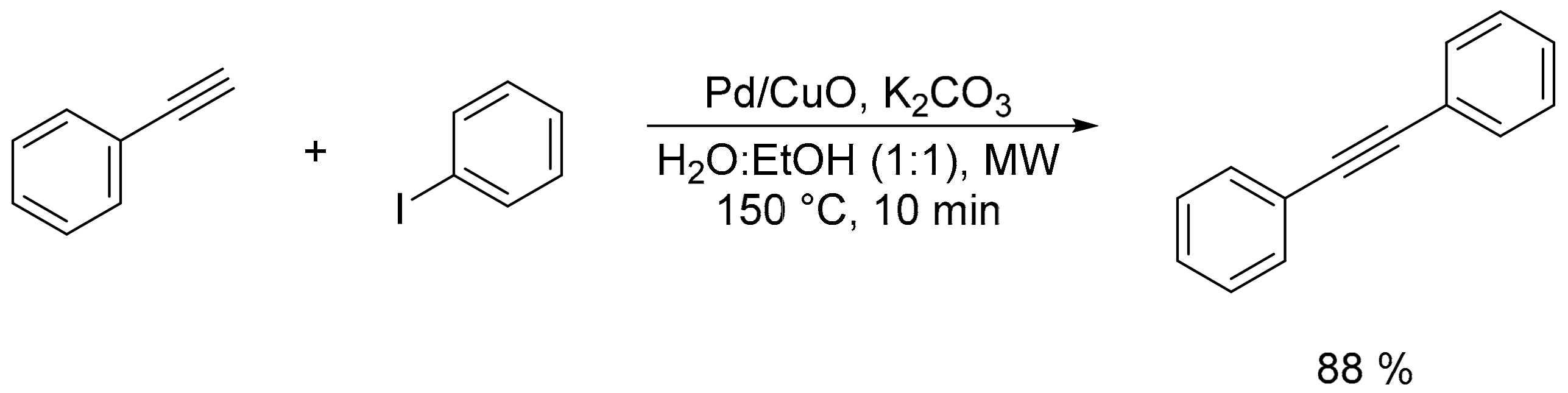
2.3. Carbon–Carbon (C–C) Bond Formation
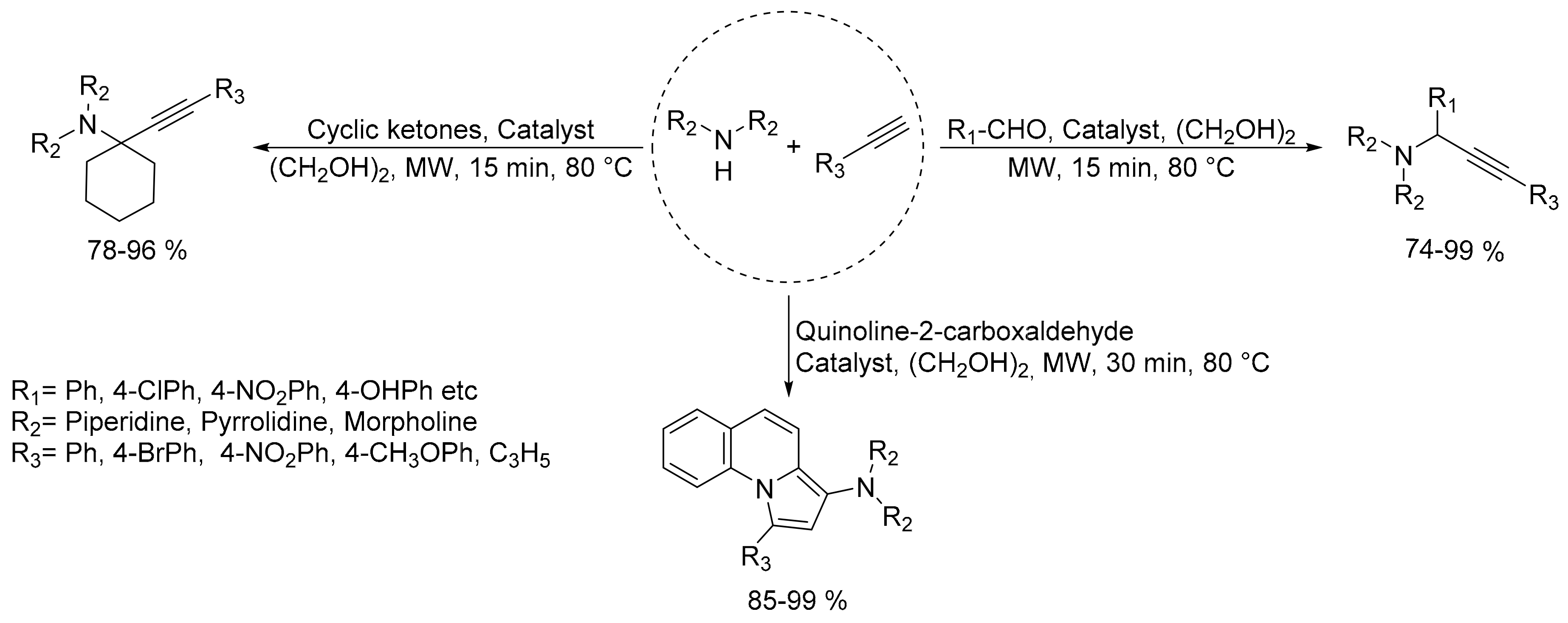
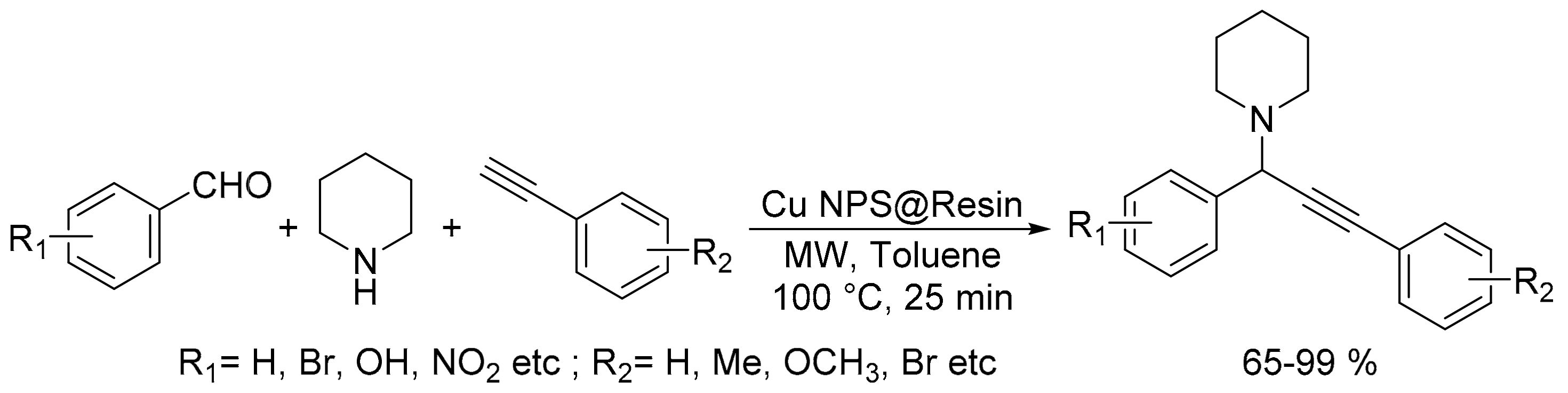
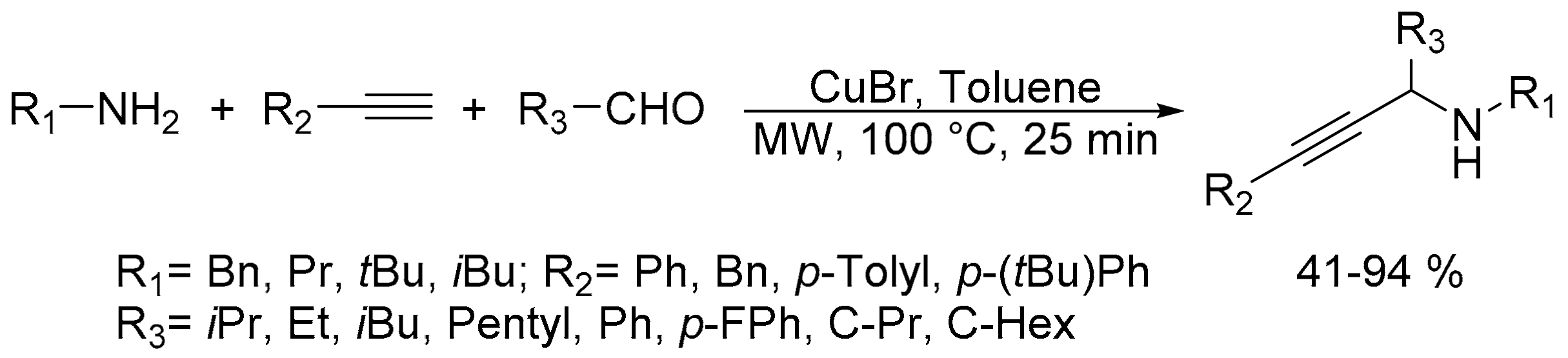
2.4. Synthesis of Propargylamines
2.5. Synthesis of Heterocyclic Systems
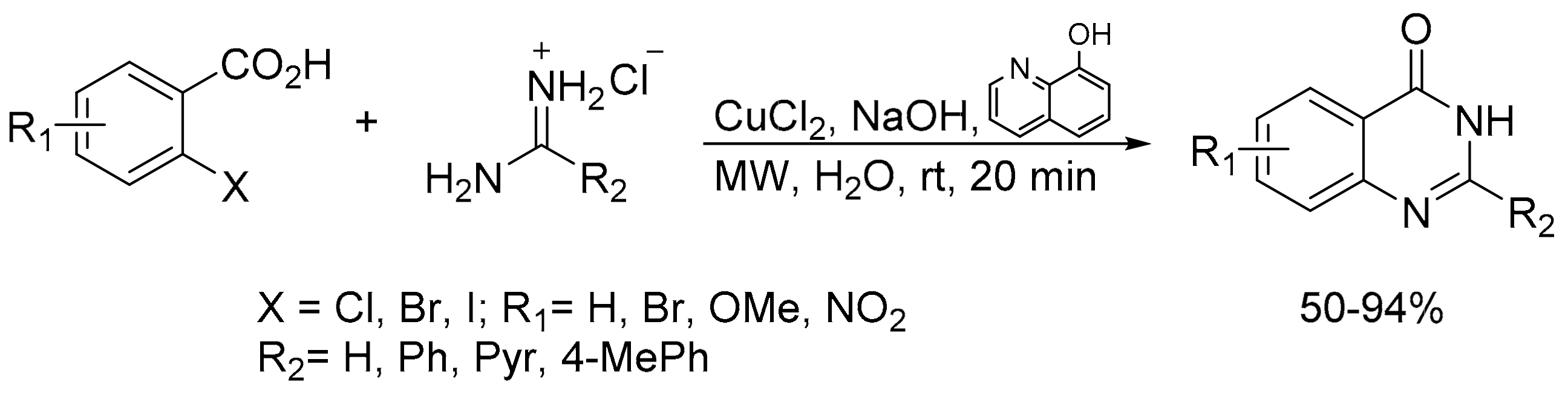
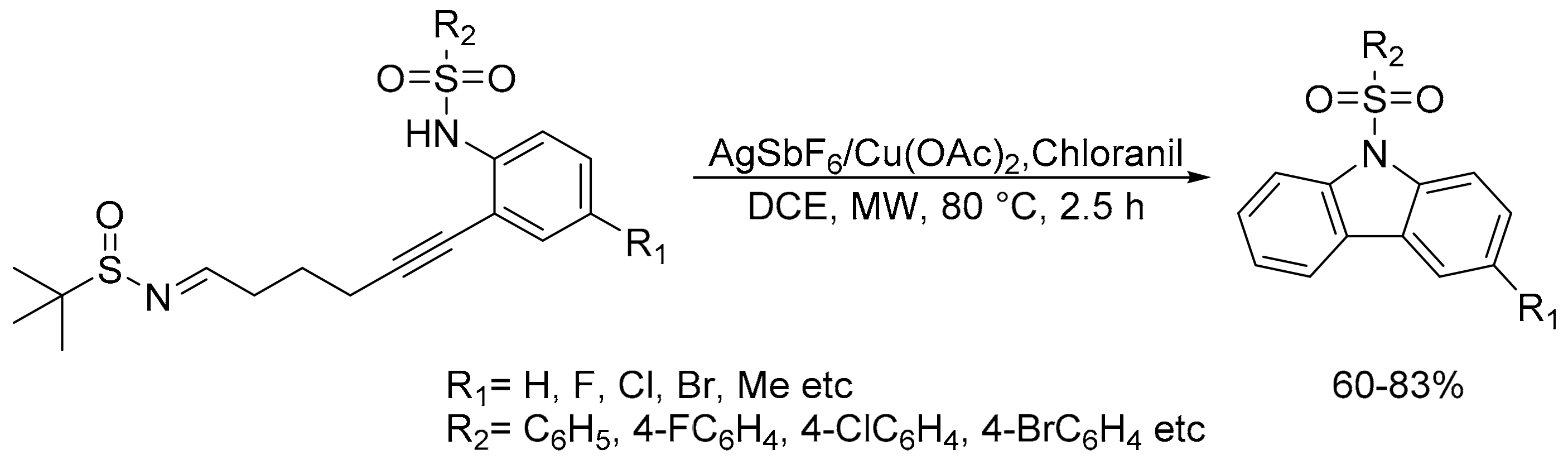
2.6. Carbon–Hydrogen (C–H) Bond Functionalization
2.7. N-(Hetero)Arylation and N-Alkylation Reaction
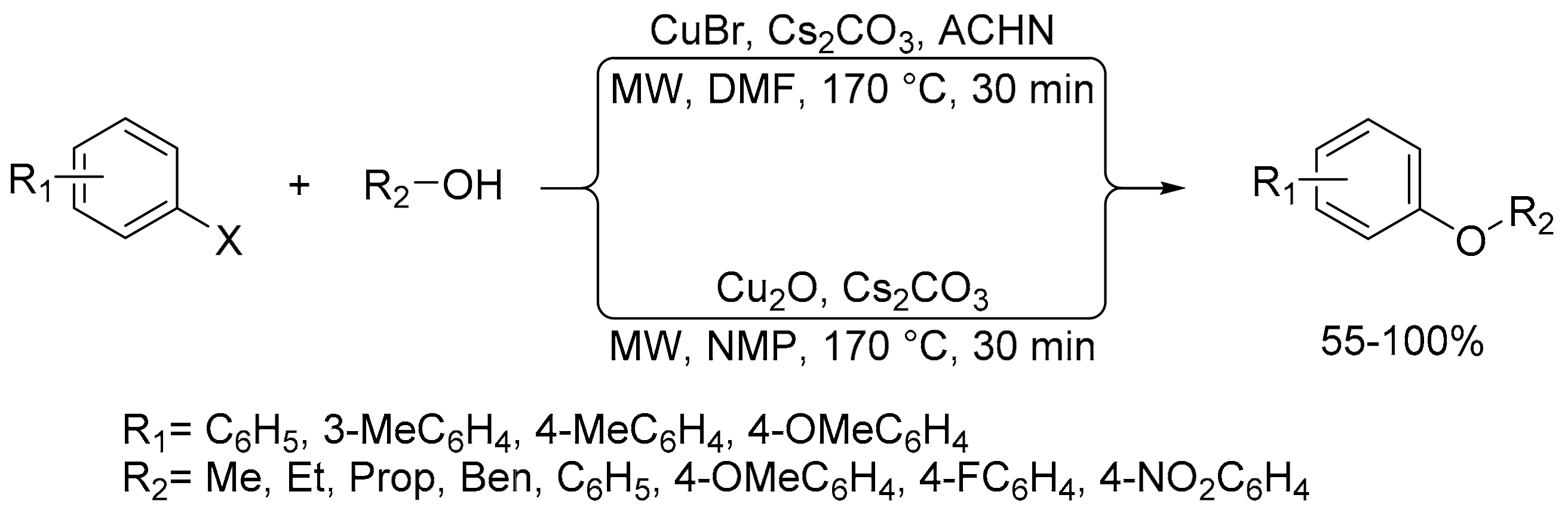
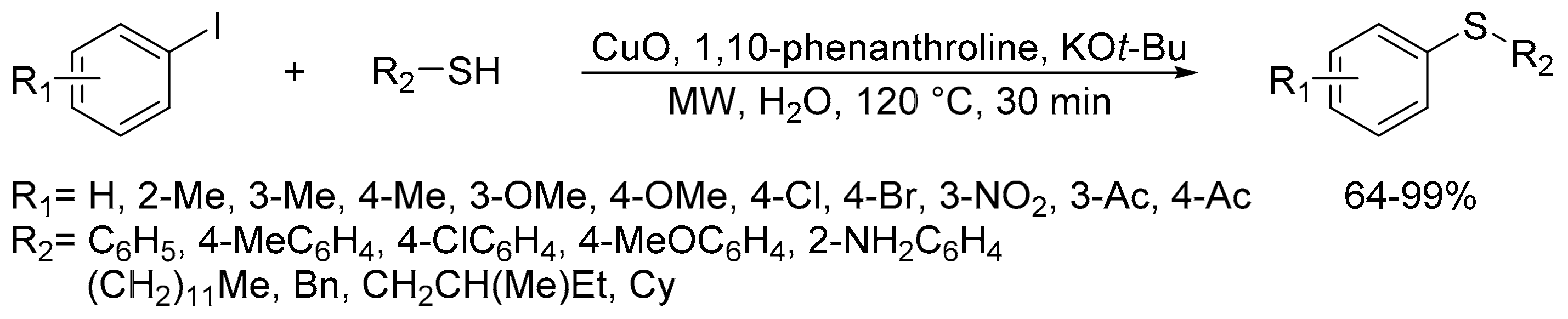
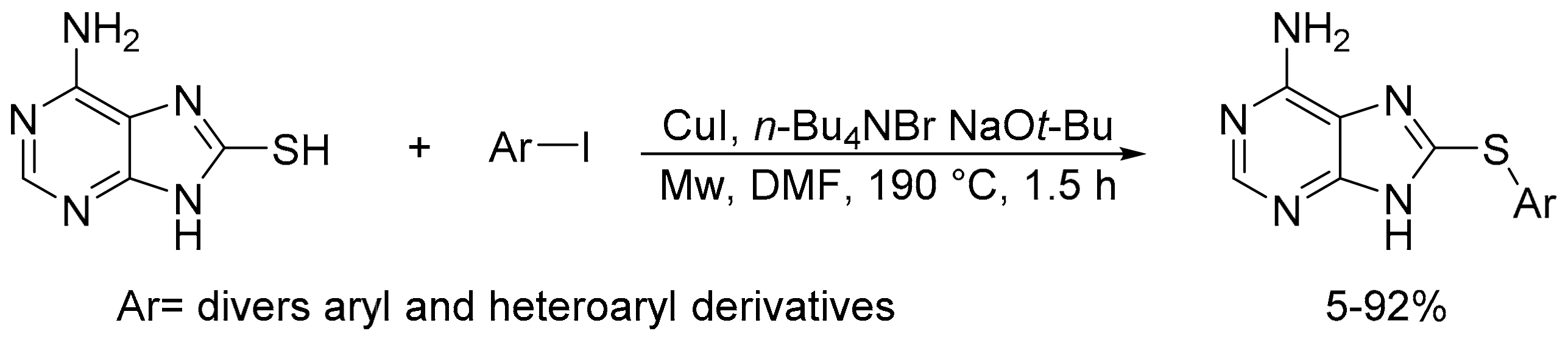
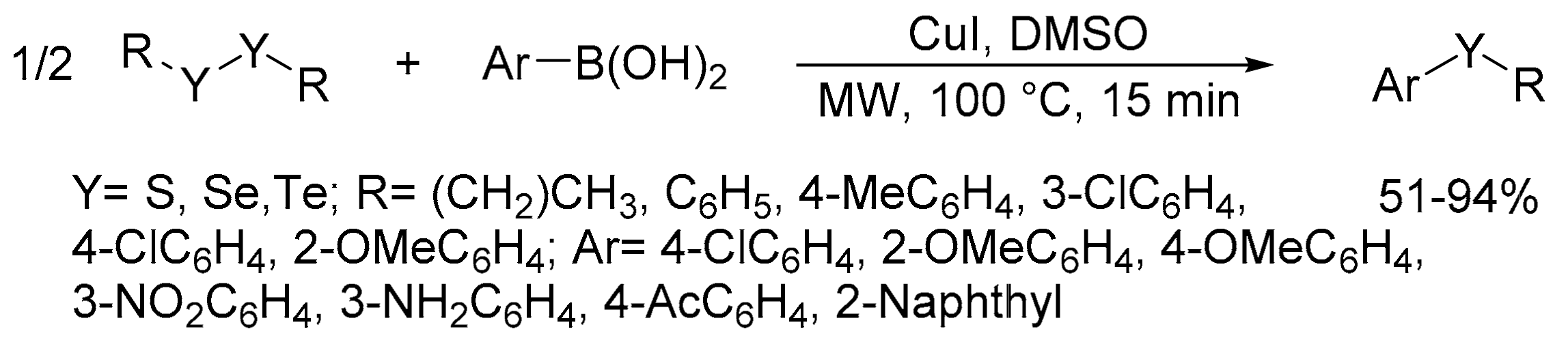
2.8. Synthesis of Carbon-Chalcogen Bonds (C–O, C–S, C–Se, and C–Te)
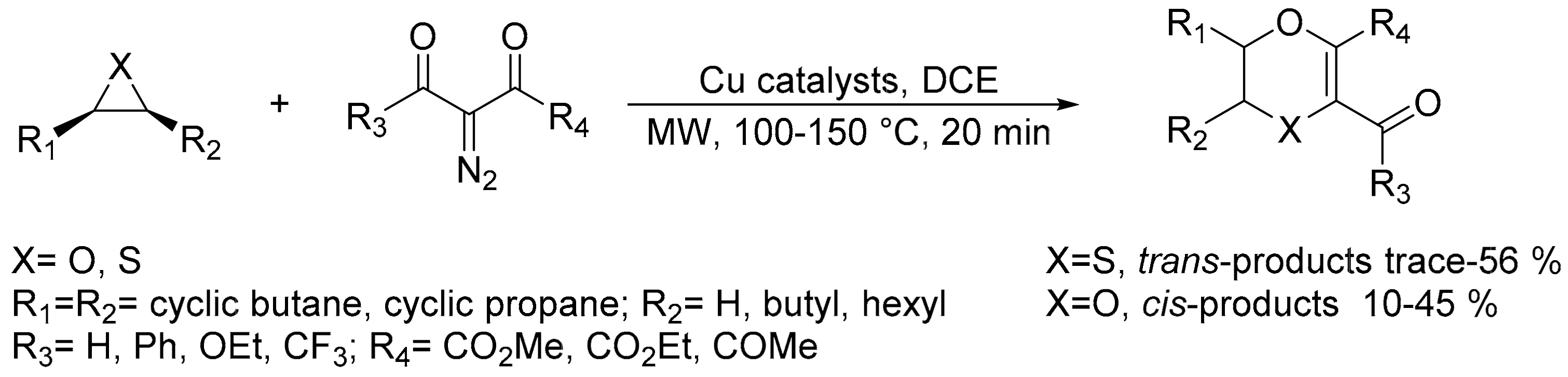
2.9. Miscellaneous Synthesis
3. Conclusions
Funding
Conflicts of Interest
References
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of palladium-catalyzed C–N cross-coupling reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef] [PubMed]
- Giri, R.; Brusoe, A.; Troshin, K.; Wang, J.Y.; Font, M.; Hartwig, J.F. Mechanism of the Ullmann biaryl ether synthesis catalyzed by complexes of anionic ligands: Evidence for the reaction of iodoarenes with ligated anionic CuI intermediates. J. Am. Chem. Soc. 2018, 140, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Salih, K.S.M.; Baqi, Y. Microwave-assisted palladium-catalyzed cross-coupling reactions: Generation of carbon-carbon bond. Catalysts 2020, 10, 4. [Google Scholar] [CrossRef]
- Altman, R.A.; Buchwald, S.L. Cu-catalyzed Goldberg and Ullmann reactions of aryl halides using chelating N- and O-based ligands. Nat. Protoc. 2007, 2, 2474–2479. [Google Scholar] [CrossRef]
- De la Hoz, A.; Loupy, A. Microwaves in Organic Synthesis, 3rd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013. [Google Scholar] [CrossRef]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-assisted chemistry: Synthetic applications for rapid assembly of nanomaterials and organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef]
- Ullmann, F.; Bielecki, J. Ueber Synthesen in der Biphenylreihe. Berichte der Deutschen Chemischen Gesellschaft 1901, 34, 2174–2185. [Google Scholar] [CrossRef]
- Ullmann, F. Ueber eine neue Bildungsweise von Diphenylaminderivaten. Berichte der Deutschen Chemischen Gesellschaft 1903, 36, 2382–2384. [Google Scholar] [CrossRef]
- Ullmann, F. Ueber eine neue Darstellungsweise von Phenyläthersalicylsäure. Berichte der Deutschen Chemischen Gesellschaft 1904, 37, 853–854. [Google Scholar] [CrossRef]
- Ullmann, F.; Sponagel, P. Ueber die Phenylirung von Phenolen. Berichte der Deutschen Chemischen Gesellschaft 1905, 38, 2211–2212. [Google Scholar] [CrossRef]
- Goldberg, I. Ueber Phenylirungen bei Gegenwart von Kupfer als Katalysator. Berichte der Deutschen Chemischen Gesellschaft 1906, 39, 1691–1692. [Google Scholar] [CrossRef]
- Baqi, Y.; Müller, C.E. Antithrombotic P2Y12 receptor antagonists: Recent developments in drug discovery. Drug Discov. Today 2019, 24, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Baqi, Y. Anthraquinones as a privileged scaffold in drug discovery targeting nucleotide-binding proteins. Drug Discov. Today 2016, 21, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Baqi, Y. Ecto-nucleotidase inhibitors: Recent developments in drug discovery. Mini Rev. Med. Chem. 2015, 15, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Baqi, Y.; Atzler, K.; Köse, M.; Glänzel, M.; Müller, C.E. High-affinity, non-nucleotide-derived competitive antagonists of platelet P2Y12 receptors. J. Med. Chem. 2009, 52, 3784–3793. [Google Scholar] [CrossRef]
- Baqi, Y.; Weyler, S.; Iqbal, J.; Zimmermann, H.; Müller, C.E. Structure-activity relationships of anthraquinone derivatives derived from bromaminic acid as inhibitors of ectonucleoside triphosphate diphosphohydrolases (E-NTPDases). Purinerg. Signal. 2009, 5, 91–106. [Google Scholar] [CrossRef]
- Baqi, Y.; Lee, S.-Y.; Iqbal, J.; Ripphausen, P.; Lehr, A.; Scheiff, A.B.; Zimmermann, H.; Bajorath, J.; Müller, C.E. Development of potent and selective inhibitors of ecto-5′-nucleotidase based on an anthraquinone scaffold. J. Med. Chem. 2010, 53, 2076–2086. [Google Scholar] [CrossRef]
- Baqi, Y.; Hausmann, R.; Rosefort, C.; Rettinger, J.; Schmalzing, G.; Müller, C.E. Discovery of potent competitive antagonists and positive modulators of the P2 × 2 receptor. J. Med. Chem. 2011, 54, 817–830. [Google Scholar] [CrossRef]
- Fiene, A.; Baqi, Y.; Malik, E.M.; Newton, P.; Li, W.; Lee, S.Y.; Hartland, E.L.; Müller, C.E. Inhibitors for the bacterial ectonucleotidase Lp1NTPDase from Legionella pneumophila. Bioorg. Med. Chem. 2016, 24, 4363–4371. [Google Scholar] [CrossRef]
- Weyler, S.; Baqi, Y.; Hillmann, P.; Kaulich, M.; Hunder, A.M.; Müller, I.A.; Müller, C.E. Combinatorial synthesis of anilinoanthraquinone derivatives and evaluation as non-nucleotide-derived P2Y2 receptor antagonists. Bioorg. Med. Chem. Lett. 2008, 18, 223–227. [Google Scholar] [CrossRef]
- Baqi, Y.; Müller, C.E. Rapid and efficient microwave-assisted copper(0)-catalyzed Ullmann coupling reaction: General access to anilinoanthraquinone derivatives. Org. Lett. 2007, 9, 1271–1274. [Google Scholar] [CrossRef]
- Baqi, Y.; Müller, C.E. Synthesis of alkyl- and aryl-amino-substituted anthraquinone derivatives by microwave-assisted copper(0)-catalyzed Ullmann coupling reactions. Nat. Protoc. 2010, 5, 945–953. [Google Scholar] [CrossRef] [PubMed]
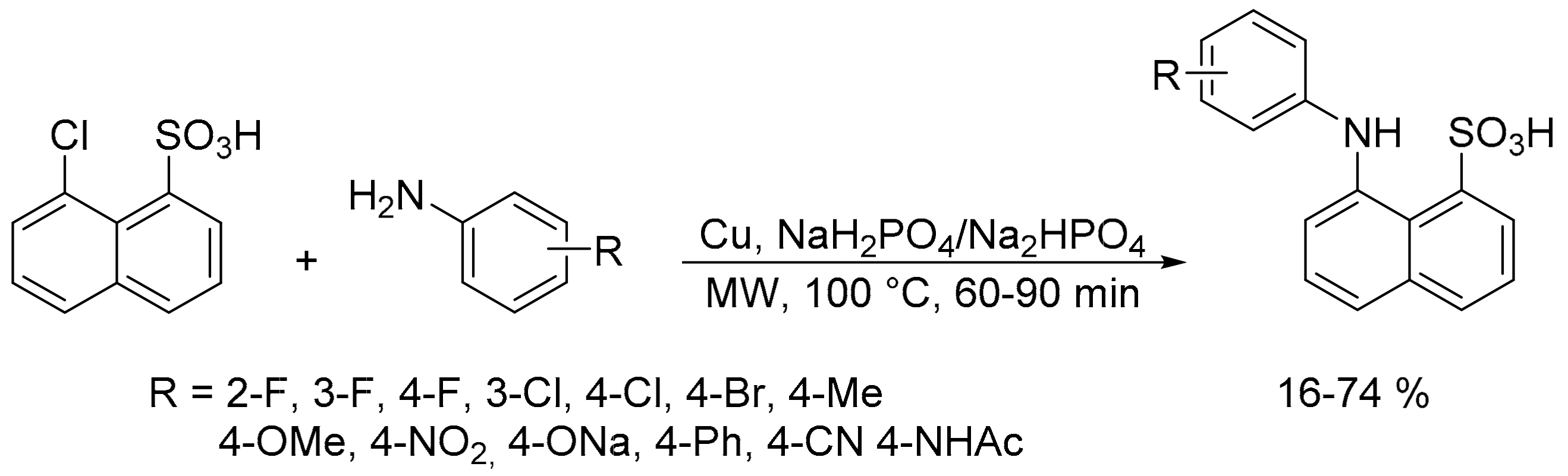
- Wang, N.; Faber, E.B.; Georg, G.I. Synthesis and spectral properties of 8-anilinonaphthalene-1-sulfonic acid (ANS) derivatives prepared by microwave-assisted copper(0)-catalyzed Ullmann reaction. ACS Omega 2019, 4, 18472–18477. [Google Scholar] [CrossRef] [PubMed]
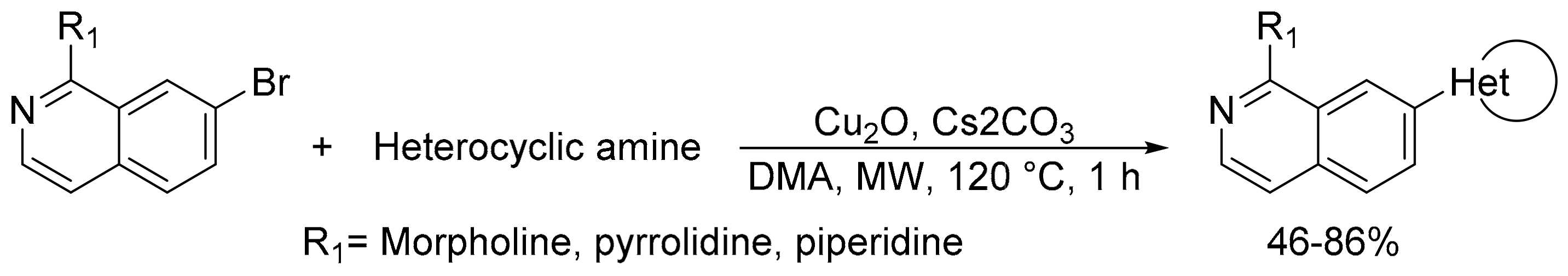
- Park, A.R.; Yum, E.K. Introduction of diverse functional groups to isoquinolines by microwave-assisted transition metal-catalyzed coupling reactions. Bull. Korean Chem. Soc. 2018, 39, 1259–1265. [Google Scholar] [CrossRef]
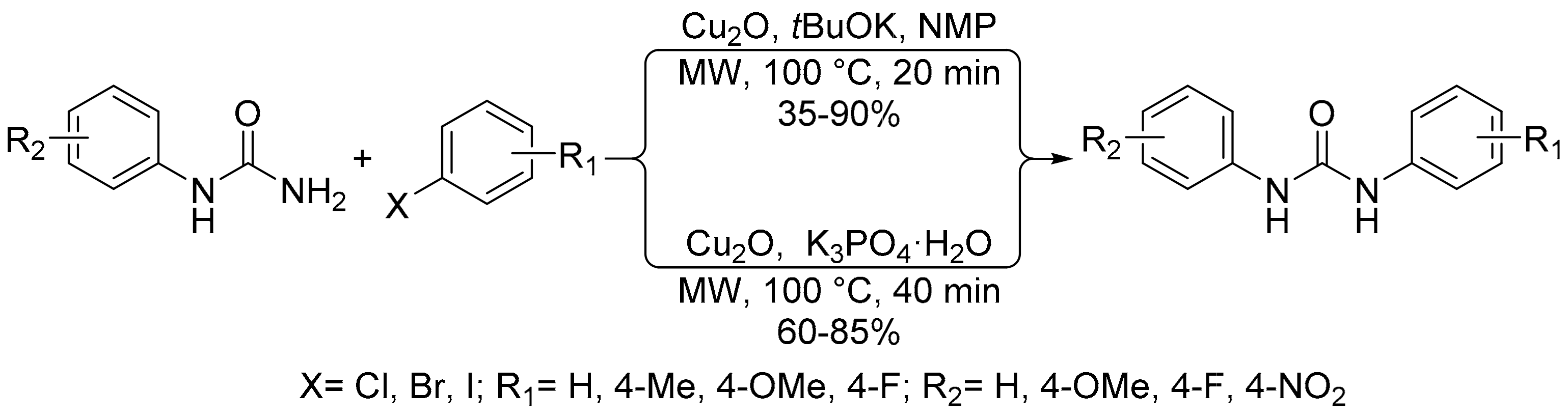
- Gavade, S.; Shingare, M.; Mane, D. Microwave assisted, solvent- and ligand-free copper catalyzed n-arylation of phenylurea with aryl halides. Bull. Korean Chem. Soc. 2011, 32, 4167–4170. [Google Scholar] [CrossRef]
- Gavade, S.N.; Balaskar, R.S.; Shingare, M.; Mane, M.S.; Pabrekar, P.N.; Shingare, M.S.; Mane, D.V. Microwave assisted, ligand free, copper catalyzed reaction of aryl halides with phenyl urea. Chin. Chem. Lett. 2011, 22, 292–295. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Vors, J.-P.; Gesing, E.R.F.; Bolm, C. Microwave-assisted solvent- and ligand-free copper-catalysed cross-coupling between halopyridines and nitrogen nucleophiles. Green Chem. 2011, 13, 42–45. [Google Scholar] [CrossRef]
- Oh, Y.J.; Yum, E.K. Microwave-assisted transition metal-catalyzed coupling approach to indazole diversity. Bull. Korean Chem. Soc. 2019, 40, 404–411. [Google Scholar] [CrossRef]
- Kumari, S.; Shakoor, S.M.A.; Bajaj, K.; Nanjegowda, S.H.; Mallu, P.; Sakhuja, R. Copper-catalyzed C-N/C-O coupling in water: A facile access to N-coumaryl amino acids and fluorescent tyrosine & lysine labels. Tetrahedron Lett. 2016, 57, 2732–2736. [Google Scholar] [CrossRef]
- Kwon, J.-K.; Lee, J.-H.; Kim, T.-S.; Yum, E.-K.; Park, H.-J. Diversification of indoles via microwave-assisted ligand-free copper-catalyzed N-arylation. Bull. Korean Chem. Soc. 2016, 37, 1927–1933. [Google Scholar] [CrossRef]
- Breugst, M.; Reissig, H.U. The Huisgen reaction: Milestones of the 1,3-dipolar cycloaddition. Angew. Chem. Int. Ed. Engl. 2020, 59, 12293–12307. [Google Scholar] [CrossRef]
- Pradeep, M.; Vishnuvardhan, M.; Krishna, V.B.; Raju, R.M. An efficient microwave assisted synthesis and antimicrobial activty of 1,2,3-triazolyl-pyrrolidinyl-quinolinolines. Russ. J. Gen. Chem. 2019, 89, 313–318. [Google Scholar] [CrossRef]
- Arrjane, M.; Slassi, S.; Tazi, B.; Maouloua, M.; Amine, A. Novel series of acridone-1,2,3-triazole derivatives: Microwave-assisted synthesis, DFT study and antibacterial activities. J. Chem. Sci. 2019, 131, 85. [Google Scholar] [CrossRef]
- Hernández-López, H.; Leyva-Ramos, S.; Moncada-Martínez, R.D.; López, J.A.; Cardoso-Ortiz, J. Copper(I)-catalyzed azide-alkyne cycloaddition microwave-assisted: Preparation of 7-(4-substituted-1H-1,2,3-triazol-1-yl)-fluoroquinolones. ChemistrySelect 2019, 4, 11899–11902. [Google Scholar] [CrossRef]
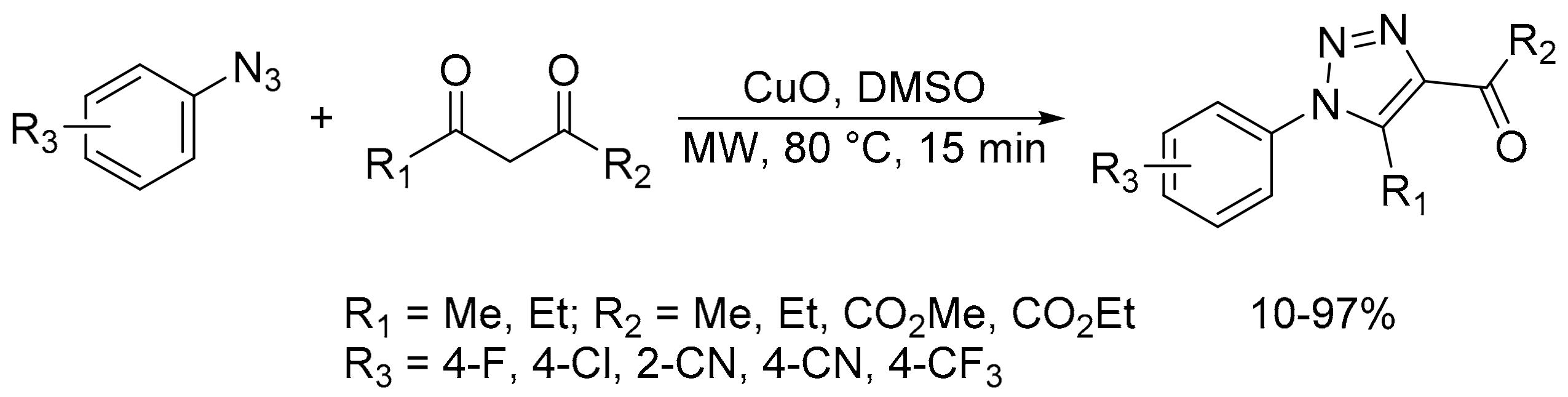
- Da Silva Dias, C.; de Melo Lima, T.; Lima, C.G.S.; Zuekrman-Schpector, J.; Schwab, R.S. CuO nanoparticles as an efficient heterogeneous catalyst for the 1,3-dipolar cycloaddition of dicarbonyl compounds to azides. ChemistrySelect 2018, 3, 6195–6202. [Google Scholar] [CrossRef]
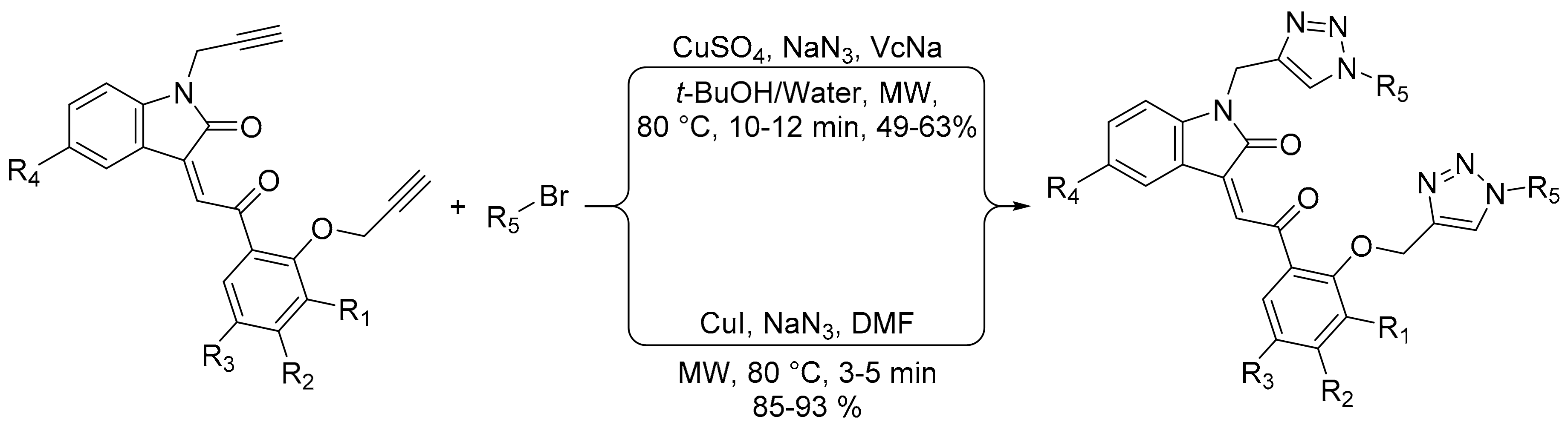
- Ashok, D.; Gundu, S.; Aamate, V.K.; Devulapally, M.G. Microwave-assisted synthesis, antioxidant and antimicrobial evaluation of 2-indolinone-based bis-1,2,3-triazole derivatives. Mol. Divers. 2018, 22, 57–70. [Google Scholar] [CrossRef]
- Mahmoud, A.G.; da Silva, M.F.C.G.; Sokolnicki, J.; Smoleński, P.; Pombeiro, A.J.L. Hydrosoluble Cu(I)-DAPTA complexes: Synthesis, characterization, luminescence thermochromism and catalytic activity for microwave-assisted three-component azide-alkyne cycloaddition click reaction. Dalton Trans. 2018, 47, 7290–7299. [Google Scholar] [CrossRef]
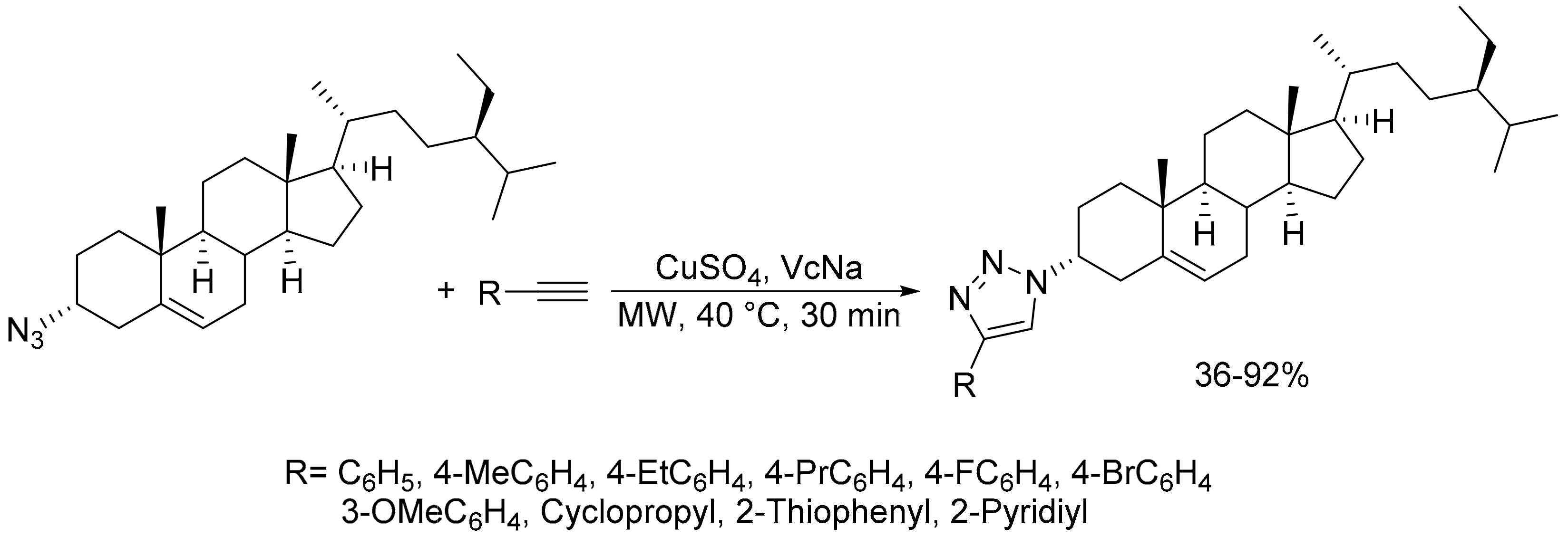
- Yuan, J.-W.; Qu, L.-B. Efficient synthesis of novel β-sitosterol scaffolds containing 1,2,3-triazole via copper (I)-catalyzed click reaction under microwave irradiation. Zeitschrift für Naturforschung B 2017, 72, 717–724. [Google Scholar] [CrossRef]
- Xavier, M.C.D.F.; Xavier, D.M.; Seus, N.; Lenardão, E.J.; Perin, G.; Alves, D. Microwave assisted rapid synthesis of (arylselanyl)phenyl-1h-1,2,3-triazoles by copper catalyzed 1,3-dipolar cycloaddition. Curr. Microwave Chem. 2016, 3, 14–23. [Google Scholar] [CrossRef]
- Steenackers, H.; Ermolat’ev, D.; Trang, T.T.T.; Savalia, B.; Sharma, U.K.; De Weerdt, A.; Shah, A.; Vanderleyden, J.; Van der Eycken, E.V. Microwave-assisted one-pot synthesis and anti-biofilm activity of 2-amino-1H-imidazole/triazole conjugates. Org. Biomol. Chem. 2014, 12, 3671–3678. [Google Scholar] [CrossRef]
- De Andrade, P.; Galo, O.A.; Carvalho, M.R.; Lopes, C.D.; Carneiro, Z.A.; Sesti-Costa, R.; de Melo, D.B.; Silva, J.S.; Carvalho, I. 1,2,3-Triazole-based analogue of benznidazole displays remarkable activity against Trypanosoma cruzi. Bioorg. Med. Chem. 2015, 23, 6815–6826. [Google Scholar] [CrossRef]
- Khan, A.K.; Vasconcelos, S.N.S.; Carrau, G.; Stefani, H.A. Microwave-assisted synthesis of β-1,2,3-triazolyl-α-amino esters. J. Braz. Chem. Soc. 2015, 26, 1457–1465. [Google Scholar] [CrossRef]
- Ding, F.; Ji, L.; William, R.; Chai, H.; Liu, X.-W. Design and synthesis of multivalent neoglycoconjugates by click conjugations. Beilstein J. Org. Chem. 2014, 10, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Potewar, T.M.; Petrova, K.T.; Barros, M.T. Efficient microwave assisted synthesis of novel 1,2,3-triazole-sucrose derivatives by cycloaddition reaction of sucrose azides and terminal alkynes. Carbohydr. Res. 2013, 379, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Worrell, B.T.; Malik, J.A.; Fokin, V.V. Direct evidence of a dinuclear copper intermediate in Cu(I)-catalyzed azide-alkyne cycloadditions. Science 2013, 340, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Elazab, H.A.; Radwan, M.A.; El-Idreesy, T.T. Facile microwave-assisted synthetic approach to palladium nanoparticles supported on copper oxide as an efficient catalyst for Heck and Sonogashira cross-coupling reactions. Int. J. Nanosci. 2019, 18, 1850032. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Lin, Y.-Y.; Wang, Y.-J.; Lee, C.-F. Special topic microwave-assisted copper-catalyzed cross-coupling reaction of alkynes with aryl iodides and vinyl halides. Synthesis 2012, 44, 1507–1510. [Google Scholar] [CrossRef]
- Lei, Y.; Hu, T.; Wu, X.; Wu, Y.; Xiang, H.; Sun, H.; You, Q.; Zhang, X. Microwave-assisted copper- and palladium-catalyzed sonogashira-type coupling of aryl bromides and iodides with trimethylsilylacetylene. Tetrahedron Lett. 2016, 57, 1100–1103. [Google Scholar] [CrossRef]
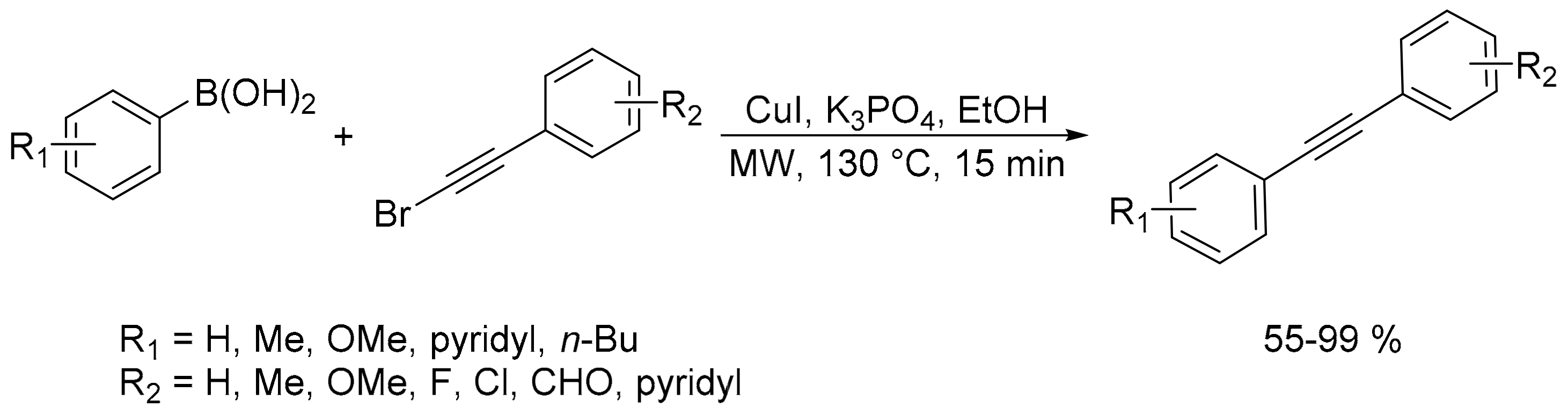
- Babu, S.A.; Saranya, S.; Rohit, K.R.; Anilkumar, G. Ligand-free Cu-catalyzed Suzuki coupling of alkynyl bromides with boronic acids in ethanol under microwave irradiation. ChemistrySelect 2019, 4, 1019–1022. [Google Scholar] [CrossRef]
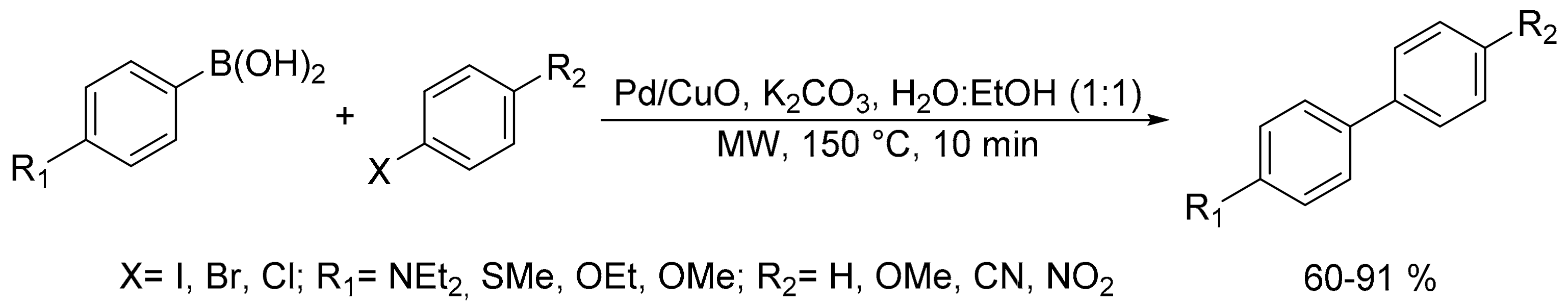
- Elazab, H.A.; Sadek, M.A. Microwave-assisted synthesis of palladium nanoparticles supported on copper oxide in aqueous medium as an efficient catalyst for Suzuki cross-coupling reaction. Adsorpt. Sci. Technol. 2018, 36, 1352–1365. [Google Scholar] [CrossRef]
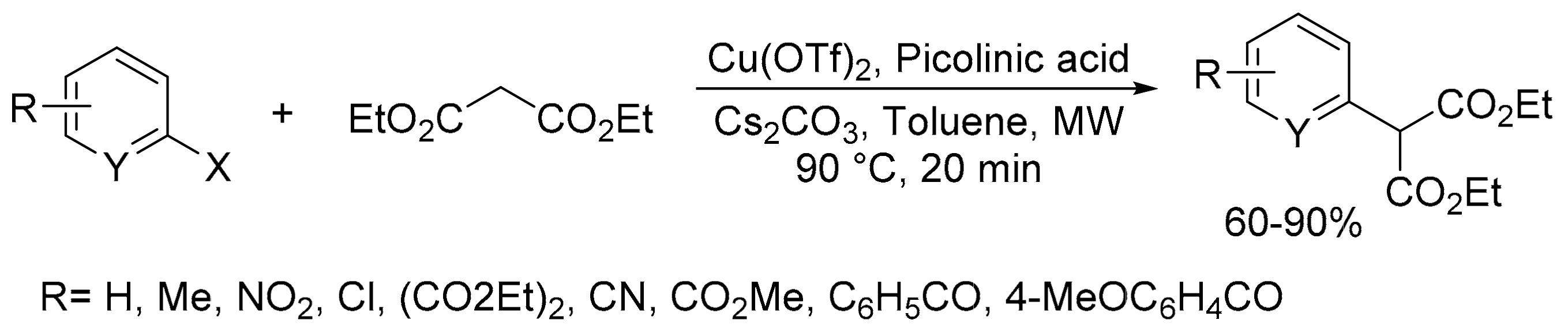
- Ibrahim, M.A. Microwave-assisted synthesis of a -aryl malonates: Key intermediates for the preparation of azaheterocycles. Arabian J. Chem. 2016, 9, S1973–S1983. [Google Scholar] [CrossRef]
- Jha, A.K.; Jain, N. Microwave-assisted ortho-alkylation of azine N-oxides with N-tosylhydrazones catalyzed by copper(I) iodide. Chem. Commun. 2016, 52, 1831–1834. [Google Scholar] [CrossRef] [PubMed]
- Lauder, K.; Toscani, A.; Scalacci, N.; Castagnolo, D. Synthesis and reactivity of propargylamines in organic chemistry. Chem. Rev. 2017, 117, 14091–14200. [Google Scholar] [CrossRef] [PubMed]
- Udaykumar, B.; Periasamy, M. Synthesis of propargylamines via Michael dddition using methyl vinyl ketone derivatives, 1-alkynes, and secondary amines catalyzed by copper (I) halides. ACS Omega 2019, 4, 21587–21595. [Google Scholar] [CrossRef] [PubMed]
- Koradin, C.; Polborn, K.; Knochel, P. Enantioselective synthesis of propargylamines by copper-catalyzed addition of alkynes to enamines. Angew. Chem., Int. Ed. 2002, 41, 2535–2538. [Google Scholar] [CrossRef]
- Pereshivko, O.P.; Peshkov, V.A.; Van der Eycken, E.V. Unprecedented Cu(I)-catalyzed microwave-assisted three-component coupling of a ketone, an alkyne, and a primary amine. Org. Lett. 2010, 12, 2638–2641. [Google Scholar] [CrossRef]
- Pierce, C.J.; Nguyen, M.; Larsen, C.H. Copper/Titanium catalyst forms fully substituted carbon centers from the direct coupling of acyclic ketones, amines, and alkynes. Angew. Chem. 2012, 124, 12455–12458. [Google Scholar] [CrossRef]
- Shah, A.P.; Sharma, A.S.; Jain, S.; Shimpi, N.G. Microwave assisted one pot three component synthesis of propargylamine, tetra substituted propargylamine and pyrrolo[1,2-a]quinolines using CuNPs@ZnO-PTh as a heterogeneous catalyst. N. J. Chem. 2018, 42, 8724–8737. [Google Scholar] [CrossRef]
- Kashid, V.S.; Balakrishna, M.S. Microwave-assisted copper(I) catalyzed A3-coupling reaction: Reactivity, substrate scope and the structural characterization of two coupling products. Catal. Commun. 2018, 103, 78–82. [Google Scholar] [CrossRef]
- Sharma, A.S.; Kaur, H.; Barot, N. Microwave-assisted facile synthesis of propargylamine library by robust nitro functionalized cross-linked polystyrene resin supported Cu NPs. J. Phys. Org. Chem. 2017, 31, e3749. [Google Scholar] [CrossRef]
- Li, X.; Chen, N.; Xu, J. Microwave-assisted CuCl-catalyzed three-component reactions of alkynes, aldehydes, and amino alcohols. Synthesis 2019, 51, 3336–3344. [Google Scholar] [CrossRef]
- Bariwal, J.B.; Ermolat’ev, D.S.; Van der Eycken, E.V. Efficient microwave-assisted synthesis of secondary alkylpropargylamines by using A3-coupling with primary aliphatic amines. Chem. Eur. J. 2010, 16, 3281–3284. [Google Scholar] [CrossRef] [PubMed]
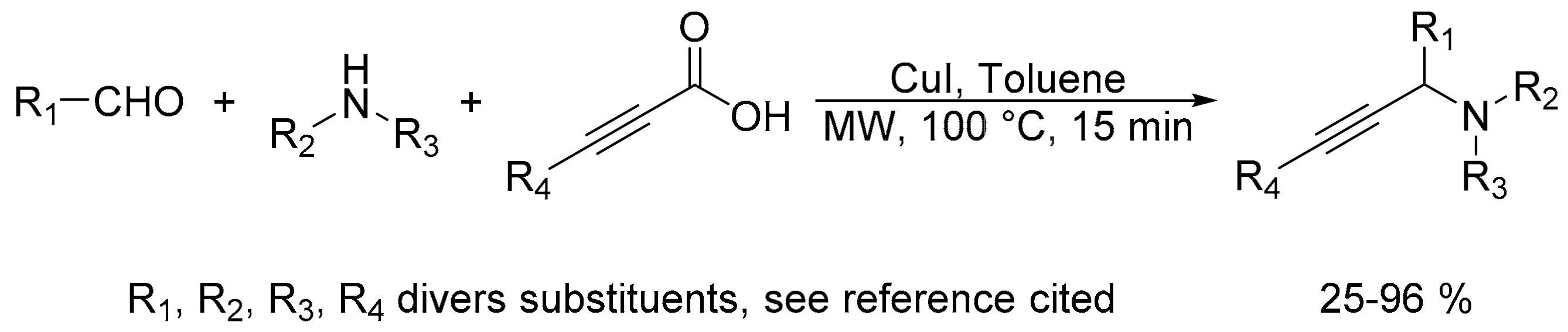
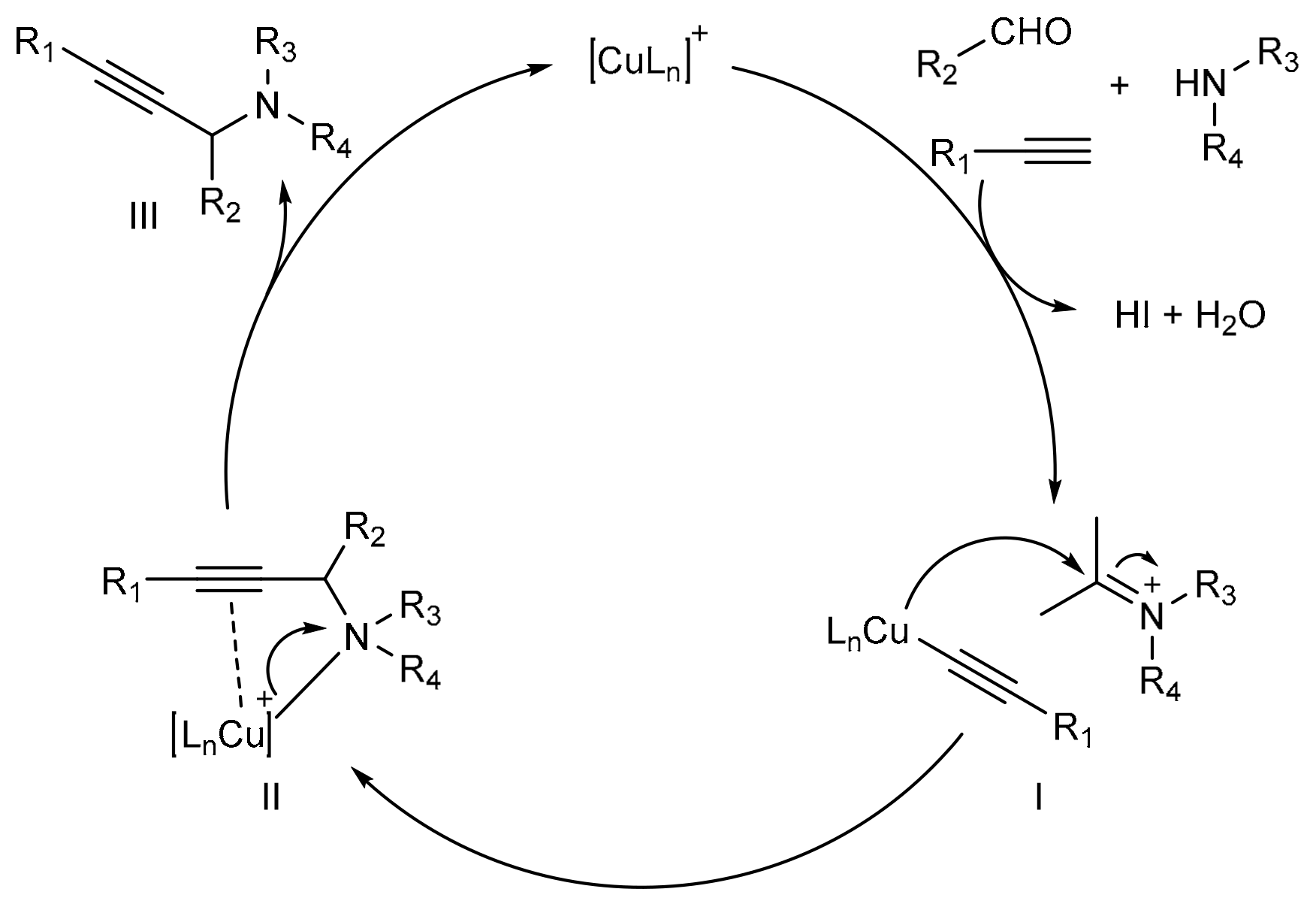
- Ermolat’ev, D.S.; Feng, H.; Song, G.; Van der Eycken, E.V. Copper(I)-catalyzed decarboxylative coupling of propiolic acids with secondary amines and aldehydes. Eur. J. Org. Chem. 2014, 5346–5350. [Google Scholar] [CrossRef]
- Feng, H.; Ermolat’ev, D.S.; Song, G.; Van der Eycken, E.V. Synthesis of symmetric 1,4-diamino-2-butynes via a Cu(I)-catalyzed one-pot A3-coupling/decarboxylative coupling of a propiolic acid, an aldehyde, and an amine. J. Org. Chem. 2012, 77, 5149–5154. [Google Scholar] [CrossRef]
- Feng, H.; Ermolat’ev, D.S.; Song, G.; Van der Eycken, E.V. Microwave-assisted decarboxylative three-component coupling of a 2-oxoacetic acid, an amine, and an alkyne. J. Org. Chem. 2011, 76, 7608–7613. [Google Scholar] [CrossRef]
- Feng, H.; Zhao, P.; Huang, L.; Sun, Z.; Tong, M. Highly selective synergistic copper(I/II)-catalyzed A3 cross coupling/decarboxylative A3 domino reactions in water. Asian J. Org. Chem. 2017, 6, 161–164. [Google Scholar] [CrossRef]
- Shore, G.; Yoo, W.-J.; Li, C.-J.; Organ, M.G. Propargyl amine synthesis catalysed by gold and copper thin films by using microwave-assisted continuous-flow organic synthesis (MACOS). Chem. Eur. J. 2010, 16, 126–133. [Google Scholar] [CrossRef] [PubMed]
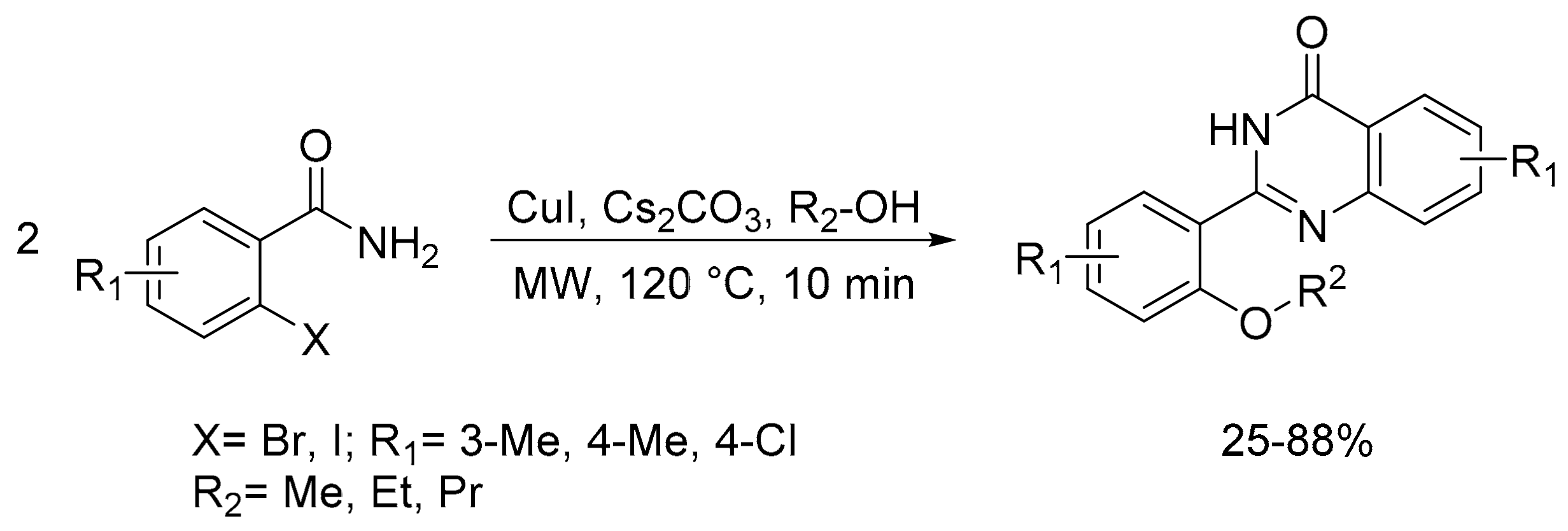
- Sayyad, N.; Cele, Z.; Aleti, R.R.; Bera, M.; Cherukupalli, S.; Chandrasekaran, B.; Kushwaha, N.D.; Karpoormath, R. Copper-catalyzed self-condensation of benzamide: Domino reactions towards quinazolinones. Eur. J. Org. Chem. 2018, 2018, 5382–5388. [Google Scholar] [CrossRef]
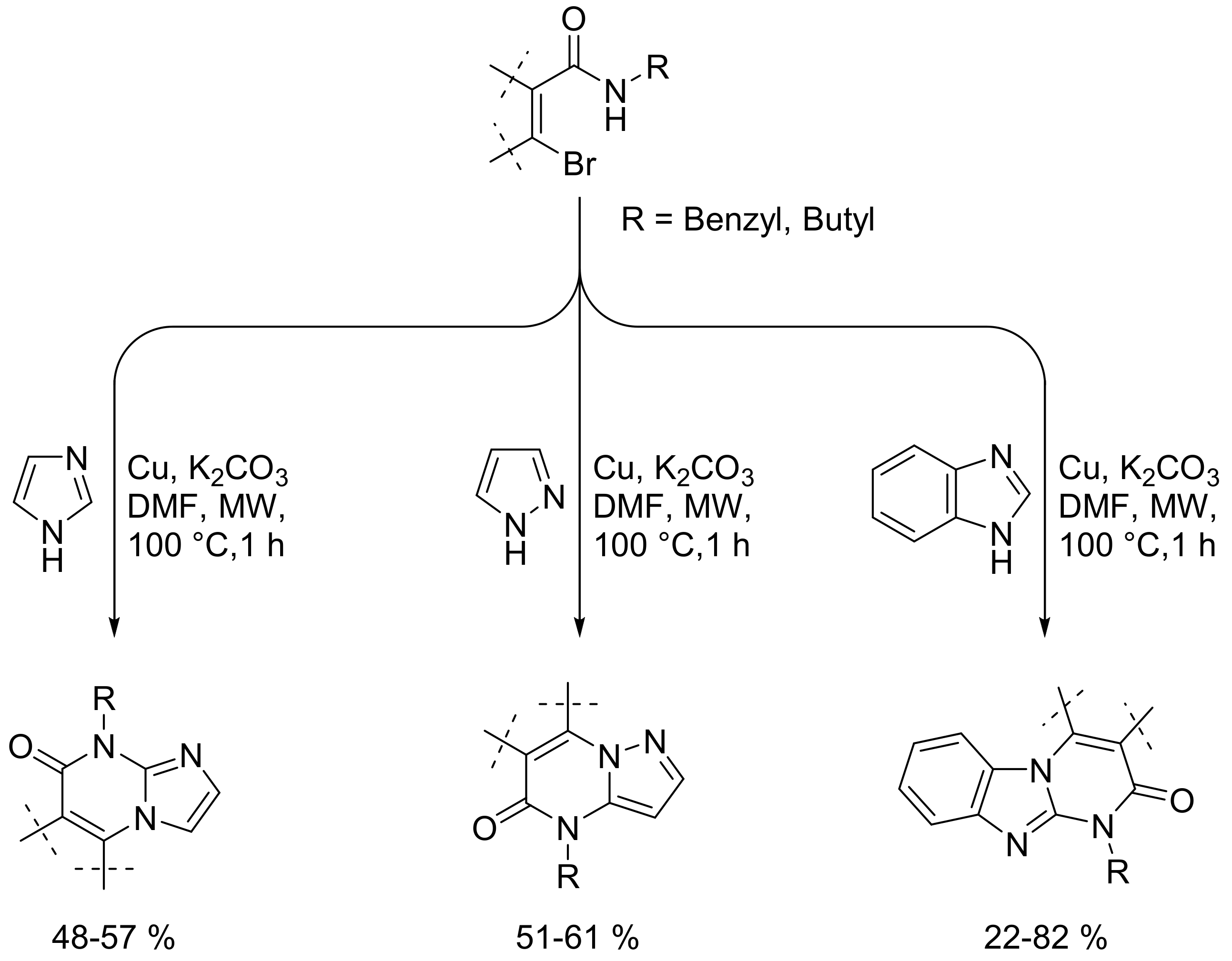
- Dao, P.D.Q.; Cho, C.S.; Ho, S.L.; Sohn, H.-S. Microwave-assisted copper powder-catalyzed synthesis of azole-fused pyrimidinones. Curr. Org. Chem. 2018, 22, 85–93. [Google Scholar] [CrossRef]
- Do Nascimento, J.E.R.; Gonçalves, L.C.C.; Hooyberghs, G.; Van der Eycken, E.V.; Alves, A.; Lenardão, E.J.; Perin, G.; Jacob, R.G. Synthesis of fused 1,2,3-triazolo-1,3,6-triazonines through copper-catalyzed intramolecular Ullmann cross-coupling reaction. Tetrahedron Lett. 2016, 57, 4885–4889. [Google Scholar] [CrossRef]
- Li, Z.; Legras, L.; Kumar, A.; Vachhani, D.D.; Sharma, S.K.; Parmar, V.S.; Van der Eycken, E.V. Microwave-assisted synthesis of 4H-benzo[f]imidazo[1,4]diazepin-6-ones via a post-Ugi copper-catalyzed intramolecular Ullmann coupling. Tetrahedron Lett. 2014, 55, 2070–2074. [Google Scholar] [CrossRef]
- Rout, L.; Kumar, A.; Chand, P.K.; Achary, L.S.K.; Dash, P. Microwave-assisted efficient one-pot multi-component synthesis of octahydroquinazolinone derivatives catalyzed by Cu@Ag core-shell nanoparticle. ChemistrySelect 2019, 4, 5696–5706. [Google Scholar] [CrossRef]
- Ke, F.; Liu, C.; Zhang, P.; Xu, J.; Chen, X. Efficient and selective microwave-assisted copper-catalyzed synthesis of quinazolinone derivatives in aqueous. Synth. Commun. 2018, 8, 3089–3098. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Das, S.; Kundu, L.M. Synthesis of size-expanded nucleobase analogues for artificial base-pairing using a ligand-free, microwave-assisted copper(I)-catalyzed reaction. ChemistrySelect 2018, 3, 13098–13102. [Google Scholar] [CrossRef]
- Raut, A.B.; Tiwari, A.R.; Bhanage, B.M. Ultrasonic irradiation assisted preparation of Cu2O-nanocubes and their high catalytic activity in synthesis of quinazolines. ChemCatChem 2017, 9, 1292–1297. [Google Scholar] [CrossRef]
- Li, S.; Chen, X.; Xu, J. Microwave-assisted copper-catalyzed stereoselective ring expansion of three-membered heterocycles with α-diazo-β-dicarbonyl compounds. Tetrahedron 2018, 74, 1613–1620. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J. Synthesis of 3-acyl-5,6-dihydro-1,4-oxathiines through ring expansion of thiiranes. Tetrahedron Lett. 2017, 58, 1651–1654. [Google Scholar] [CrossRef]
- Baba, N.H.K.; Ashok, D.; Rao, B.A.; Sarasija, M.; Murthy, N.Y.S. Microwave-assisted synthesis of novel benzodifuran-based bis(N-(het)arylthiazol-2-amine) derivatives and their antibacterial and antimycobacterial activities. Chem. Heterocycl. Compd. 2018, 54, 658–663. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, Z.; Song, H.; Liu, Y.; Wang, Q. Silver-copper co-catalyzed cascade intramolecular cyclization/desulfinamide/dehydrogenation: One-pot synthesis of substituted carbazoles. Chem. Commun. 2018, 54, 7143–7146. [Google Scholar] [CrossRef]
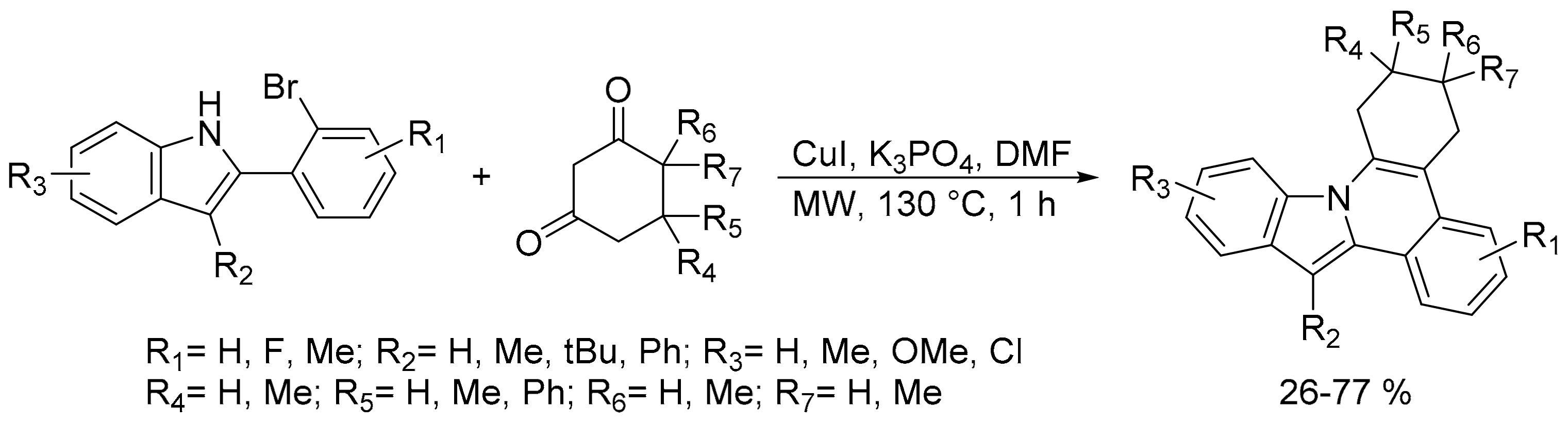
- Lee, H.K.; Dao, P.D.Q.; Kim, Y.-S.; Cho, C.S. Synthesis of indolo[2,1-a]isoquinolines via copper-catalyzed c–c coupling and cyclization of 2-(2-bromoaryl)-1H-indoles with 1,3-diketones. Synthesis 2018, 50, 3243–3249. [Google Scholar] [CrossRef]
- Padmaja, R.D.; Meena, D.R.; Maiti, B.; Chanda, K. [Cu(phen)(PPh3)2]NO3-catalyzed microwave-assisted green synthesis of 5-substituted 1H-tetrazoles. Res. Chem. Intermed. 2017, 43, 7365–7374. [Google Scholar] [CrossRef]
- Chouaïb, K.; Romdhane, A.; Delemasure, S.; Dutartre, P.; Elie, N.; Touboul, D.; Ben jannet, H.; Hamza, M.A. Regiospecific synthesis, anti-inflammatory and anticancer evaluation of novel 3,5-disubstituted isoxazoles from the natural maslinic and oleanolic acids. Ind. Crops Prod. 2016, 85, 287–299. [Google Scholar] [CrossRef]
- Bachon, A.-K.; Opatz, T. Synthesis of 1,2-disubstituted indoles from α-aminonitriles and 2-halobenzyl halides. J. Org. Chem. 2016, 81, 1858–1869. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sharma, N.; Sharma, U.K.; Li, Z.; Song, G.; Van der Eycken, E.V. Microwave-assisted copper-catalyzed oxidative cyclization of acrylamides with non-activated ketones. Chem. Eur. J. 2016, 22, 5878–5882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Yang, Z.-W.; Chen, Z.; Wang, J.; Yang, D.-L.; Shen, Z.; Hu, L.-L.; Xie, J.-W.; Zhang, J.; Cui, H.-L. Tandem copper-catalyzed propargylation/alkyne azacyclization/isomerization reaction under microwave irradiation: Synthesis of fully substituted pyrroles. J. Org. Chem. 2016, 81, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Abda, H.; Aouadi, K.; Brahmi, J.; Msaddek, M.; Vidal, S. Unexpected synthesis of aziridines under Cu(I) catalyzed Kinugasa conditions assisted by microwave irradiation. C. R. Chim. 2016, 19, 275–278. [Google Scholar] [CrossRef]
- Kumar, G.S.; Ragini, S.P.; Kumar, A.S.; Meshram, H.M. Copper-catalyzed multi-component reaction accessing fused imidazo-heterocycles via C−H functionalization. RSC Adv. 2015, 5, 51576–51580. [Google Scholar] [CrossRef]
- Orrego-Hernández, J.; Cobo, J.; Portilla, J. Chemoselective synthesis of 5-alkylamino-1H-pyrazole-4-carbaldehydes by cesium- and copper-mediated amination. Eur. J. Org. Chem. 2015, 5064–5069. [Google Scholar] [CrossRef]
- Hu, Z.; Ou, L.-L.; Li, S.-D.; Yang, L. Efficient copper(I)-catalyzed, microwave-assisted, one-pot synthesis of 3,4-diaryl isoquinolines. Res. Chem. Intermed. 2015, 41, 3461–3469. [Google Scholar] [CrossRef]
- Jiao, Y.; Cho, C.S. Microwave-assisted copper powder-catalyzed coupling and cyclization of β-bromo-α,β-unsaturated amides with amidine hydrochlorides leading to pyrimidinones. Appl. Organometal. Chem. 2015, 29, 372–375. [Google Scholar] [CrossRef]
- Ho, S.L.; Cho, C.S. Microwave-assisted copper-powder-catalyzed synthesis of pyrimidinones from β-bromo-α,β-unsaturated carboxylic acids and amidines. Synlett 2013, 24, 2705–2708. [Google Scholar] [CrossRef]
- Pasunooti, K.K.; Chai, H.; Jensen, C.N.; Gorityala, B.K.; Wang, S.; Liu, X.-W. A microwave-assisted, copper-catalyzed three-component synthesis of dihydropyrimidinones under mild conditions. Tetrahedron Lett. 2011, 52, 80–84. [Google Scholar] [CrossRef]
- Murugavel, G.; Punniyamurthy, T. Microwave-assisted copper-catalyzed four-component tandem synthesis of 3-N-sulfonylamidine coumarins. J. Org. Chem. 2015, 80, 6291–6299. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.L.; Cho, C.S.; Sohn, H.-S. Microwave-assisted copper-powder-catalyzed coupling and cyclization of β-bromo-α,β-unsaturated carboxylic acids with 1,3-diketones leading to 2H-pyran-2-ones. Synthesis 2015, 47, 216–220. [Google Scholar] [CrossRef]
- Naresh, G.; Kant, R.; Narender, T. Copper(II) catalyzed expeditious synthesis of furoquinoxalines through a one-pot three-component coupling strategy. Org. Lett. 2014, 16, 4528–4531. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, L.; Liu, H.; Zhou, S.; Liu, H. Microwave-assisted synthesis of 5,6-dihydroindolo[1,2-a]quinoxaline derivatives through copper-catalyzed intramolecular N-arylation. Beilstein J. Org. Chem. 2013, 9, 2463–2469. [Google Scholar] [CrossRef]
- Shekarrao, K.; Kaishap, P.P.; Gogoi, S.; Boruah, R.C. Efficient synthesis of isoquinolines and pyridines via copper(I)-catalyzed multi-component reaction. RSC Adv. 2014, 4, 14013–14023. [Google Scholar] [CrossRef]
- Dell’Acqua, M.; Abbiati, G.; Rossi, E. Palladium-catalyzed, microwave-enhanced three-component synthesis of isoquinolines with aqueous ammonia. Synlett 2010, 17, 2672–2676. [Google Scholar] [CrossRef]
- Kosurkar, U.B.; Dadmal, T.L.; Appalanaidu, K.; Rao, Y.K.; Nanubolu, J.B.; Kumbhare, R.M. Microwave assisted synthesis of 2-aminooxazolo [4,5-b] pyridine derivatives via intramolecular C–O bond formation in aqueous medium. Tetrahedron Lett. 2014, 55, 1296–1298. [Google Scholar] [CrossRef]
- Vasilyev, E.S.; Agafontsev, A.M.; Tkachev, A.V.; Vasilyev, E.S.; Agafontsev, A.M.; Tkachev, A.V. Microwave-assisted synthesis of chiral nopinane-annelated pyridines by condensation of pinocarvone oxime with enamines promoted by FeCl3 and CuCl2. Synth. Commun. 2014, 44, 1817–1824. [Google Scholar] [CrossRef]
- Pasunooti, K.K.; Jensen, C.N.; Chai, H.; Leow, M.L.; Zhang, D.-W.; Liu, X.-W. Microwave-assisted copper (II)-catalyzed one-pot four-component synthesis of multifunctionalized dihydropyridines. J. Comb. Chem. 2010, 12, 577–581. [Google Scholar] [CrossRef]
- Gyuris, M.; Puskás, L.G.; Tóth, G.K.; Kanizsai, I. Synthesis of novel pyrazole-based heterocycles via a copper (II)-catalysed domino annulation. Org. Biomol. Chem. 2013, 11, 6320–6327. [Google Scholar] [CrossRef]
- Mastalir, M.; Rosenberg, E.E.; Kirchner, K. A practical synthesis of substituted 2,6-diaminopyridines via microwave-assisted copper-catalyzed amination of halopyridines. Tetrahedron 2015, 71, 8104–8110. [Google Scholar] [CrossRef]
- Suh, J.; Kang, H.S.; Kim, J.-E.; Yum, E.K. Diversification of pyrazoles by microwave-assisted ligand free copper catalyzed N-arylation. Bull. Korean Chem. Soc. 2012, 33, 2067–2070. [Google Scholar] [CrossRef]
- Olson, J.P.; Carroll, F.I. An improved synthesis of phenylethynyl[1,2,4]methyltriazines. Synthesis 2011, 3, 0409–0412. [Google Scholar] [CrossRef]
- Kumar, A.S.; Rao, P.V.A.; Nagarajan, R. Microwave-assisted one-pot synthesis of pyrazolo[3,4-b]indoles and new isoxazolo[5,4-b]indoles via copper-catalyzed intramolecular C–N/C–O bond formation. Synthesis 2011, 23, 3878–3886. [Google Scholar] [CrossRef]
- Gu, L.; Li, X. Microwave-assisted synthesis of indole-2-carboxylic acid esters in ionic liquid. J. Braz. Chem. Soc. 2011, 22, 2036–2039. [Google Scholar] [CrossRef]
- Zhong, Q.-F.; Sun, L.-P. An efficient synthesis of 6,9-disubstituted purin-8-ones via copper-catalyzed coupling/cyclization. Tetrahedron 2010, 66, 5107–5111. [Google Scholar] [CrossRef]
- Peshkov, V.A.; Pereshivko, O.P.; Donets, P.A.; Mehta, V.P.; Van der Eycken, E.V. Diversity-oriented microwave-assisted synthesis of the 3-benzazepine framework. Eur. J. Org. Chem. 2010, 4861–4867. [Google Scholar] [CrossRef]
- Bariwal, J.B.; Ermolat’ev, D.S.; Glasnov, T.N.; Van Hecke, K.; Mehta, V.P.; Van Meervelt, L.; Kappe, C.O.; Van der Eycken, E.V. Diversity-oriented synthesis of dibenzoazocines and dibenzoazepines via a microwave-assisted intramolecular A3-coupling reaction. Org. Lett. 2012, 12, 2774–2777. [Google Scholar] [CrossRef]
- Yadav, A.; Biswas, S.; Mobin, S.M.; Samanta, S. Efficient Cu (OTf)2-catalyzed and microwave-assisted rapid synthesis of 3,4-fused chromenopyridinones under neat conditions. Tetrahedron Lett. 2017, 58, 3634–3639. [Google Scholar] [CrossRef]
- Yang, H.J.; Mathew, B.P.; Oh, D.G.; Myung, K.; Kwak, J.H.; Hong, S.Y. Efficient copper catalysts for CH bond arylation under microwave heating: Direct access to multi-substituted pivanilides. Catal. Commun. 2017, 90, 83–86. [Google Scholar] [CrossRef]
- Harari, M.; Couly, F.; Fruit, C.; Besson, T. Pd-catalyzed and copper assisted regioselective sequential C2 and C7 arylation of thiazolo[5,4-f]quinazolin-9(8H)-one with aryl halides. Org. Lett. 2016, 18, 3282–3285. [Google Scholar] [CrossRef] [PubMed]
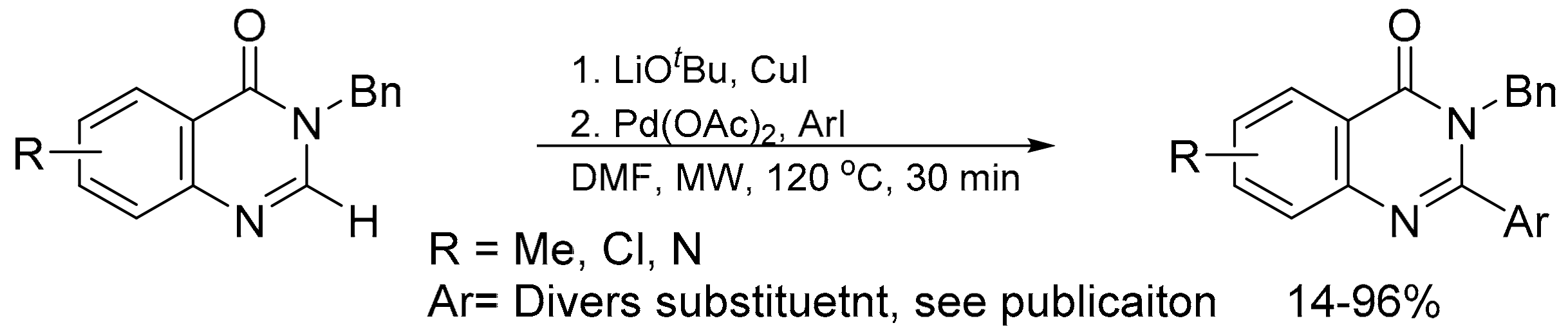
- Laclef, S.; Harari, M.; Godeau, J.; Schmitz-Afonso, I.; Bischoff, L.; Hoarau, C.; Levacher, V.; Fruit, C.; Besson, T. Ligand-free Pd-catalyzed and copper-assisted C–H arylation of quinazolin-4-ones with aryl iodides under microwave heating. Org. Lett. 2015, 17, 1700–1703. [Google Scholar] [CrossRef] [PubMed]
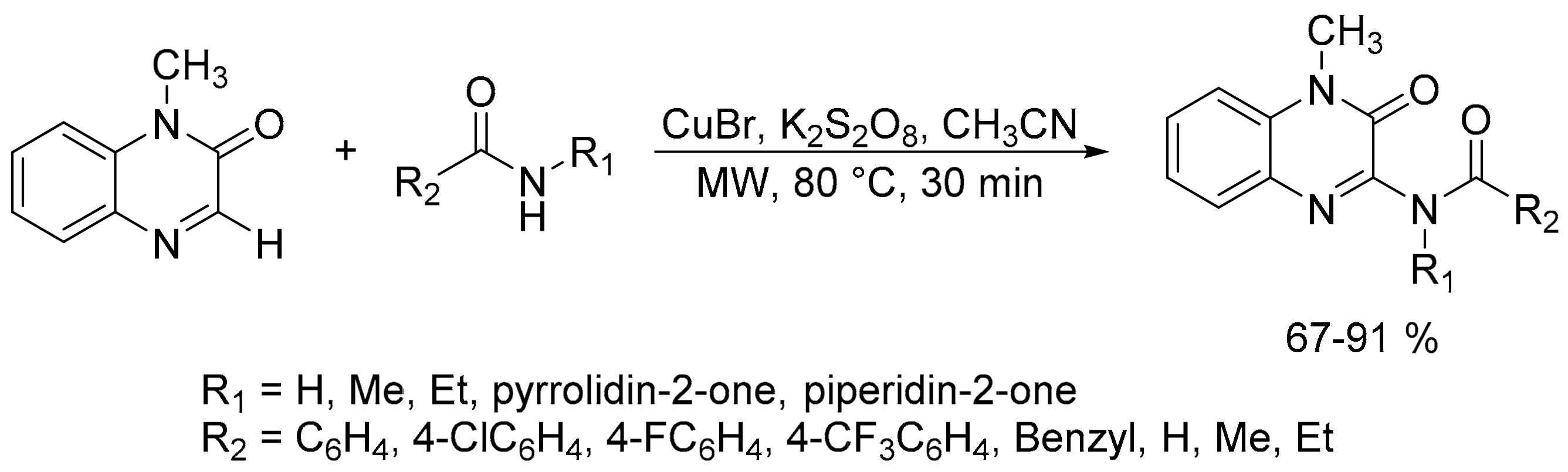
- Yuan, J.; Zhu, J.; Fu, J.; Yang, L.; Xiao, Y.; Mao, P.; Du, X.; Qu, L. Highly efficient copper-catalyzed direct C–H amidation of quinoxalin-2(1H)-ones with amidates under microwave irradiation. Org. Chem. Front. 2019, 6, 925–935. [Google Scholar] [CrossRef]
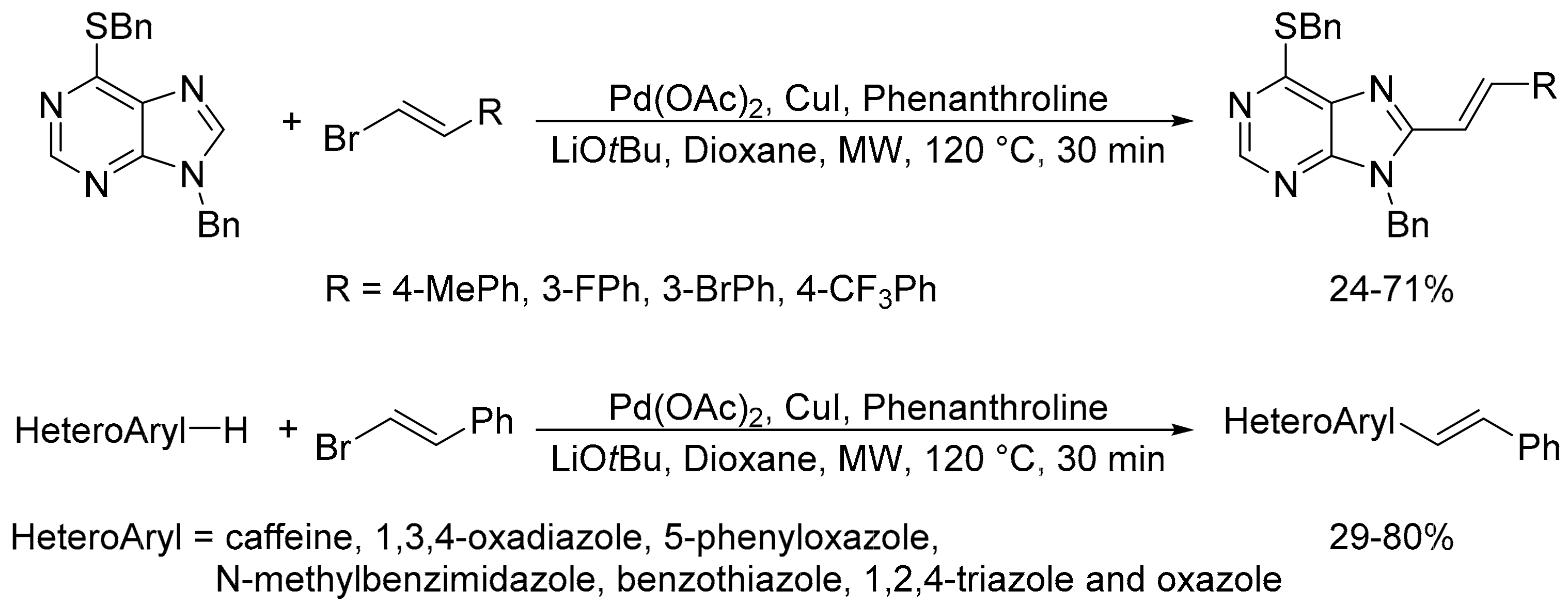
- Vabre, R.; Chevot, F.; Legraverend, M.; Piguel, S. Microwave-assisted Pd/Cu-catalyzed C-8 direct alkenylation of purines and related azoles: An alternative access to 6,8,9-trisubstituted purines. J. Org. Chem. 2011, 76, 9542–9547. [Google Scholar] [CrossRef]
- Abe, T.; Takeda, H.; Takahashi, Y.; Miwa, Y.; Yamada, K.; Ishikura, M. Metal-catalyzed reactions between 2-azabicyclo[2.2.1]hept-5-en-3-ones and arylboronic acids. Eur. J. Org. Chem 2010, 3281–3294. [Google Scholar] [CrossRef]
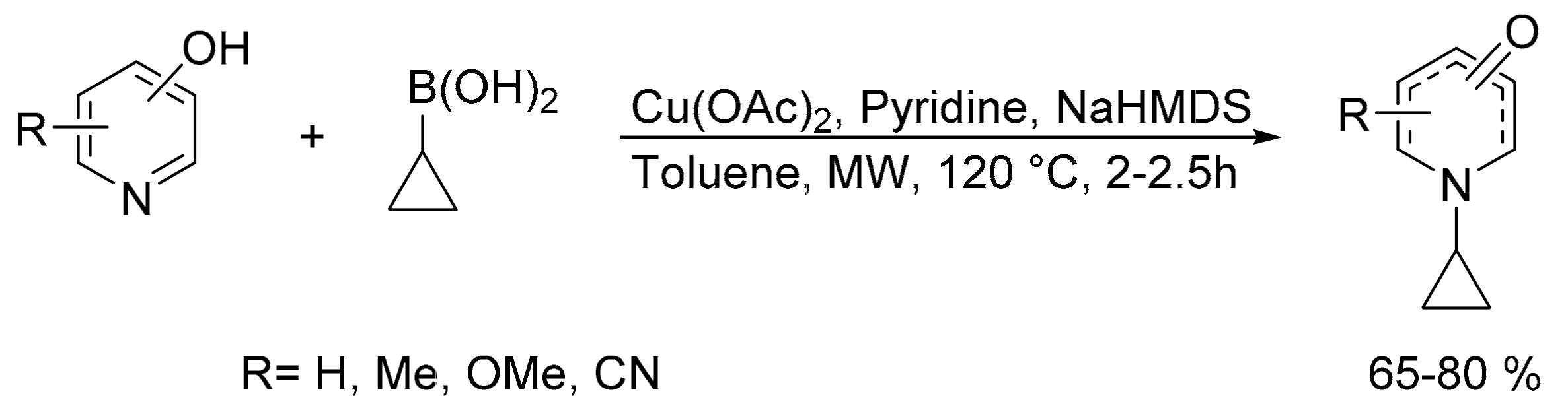
- Tambe, Y.B.; Sharma, S.; Pathak, A.; Reddy, L.K. Microwave-assisted N-cyclopropylation of pyridinols with cyclopropyl boronic acid. Synth. Commun. 2012, 42, 1341–1348. [Google Scholar] [CrossRef]
- Al-Masum, M.; Islam, M.S.; Shaban, W. Cu-Pd dual catalyst system for amide styrylation reaction from potassium styryltrifluoroborates and amides. Int. J. Org. Chem. 2017, 7, 254–262. [Google Scholar] [CrossRef]
- Chang, R.K.; Clairmont, B.P.; Lin, S.; MacArthur, A.H.R. Amidation of aryl chlorides using a microwave-assisted, copper-catalyzed concurrent tandem catalytic methodology. Organometallic 2019, 38, 4448–4454. [Google Scholar] [CrossRef]
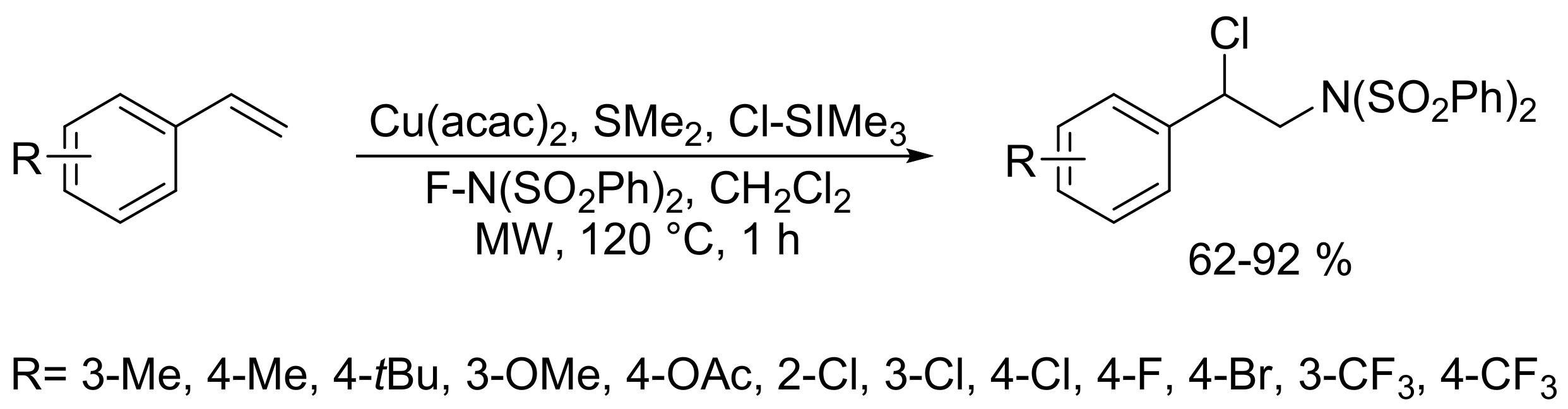
- Iwasaki, M.; Xu, J.; Tani, Y.; Fu, L.; Ikemoto, Y.; Ura, Y.; Nishihara, Y. Copper-catalyzed regioselective chloroamination of alkenes with chlorotrimethylsilane and N-fluorobenzenesulfonimide under microwave-assisted conditions. Chem. Lett. 2019, 48, 281–284. [Google Scholar] [CrossRef]
- Taj, M.B.; Raheel, A.; Alelwani, W.; Babteen, N.; Kattan, S.; Alnajeebi, A.; Sharif, M.; Ahmad, R.H.; Abbas; Hazeeq, A.; et al. One-pot CuO-catalyzed green synthesis of N(N)-arylbenzamidines as potential enzyme inhibitors. Russ. J. Org. Chem. 2019, 55, 1047–1052. [Google Scholar] [CrossRef]
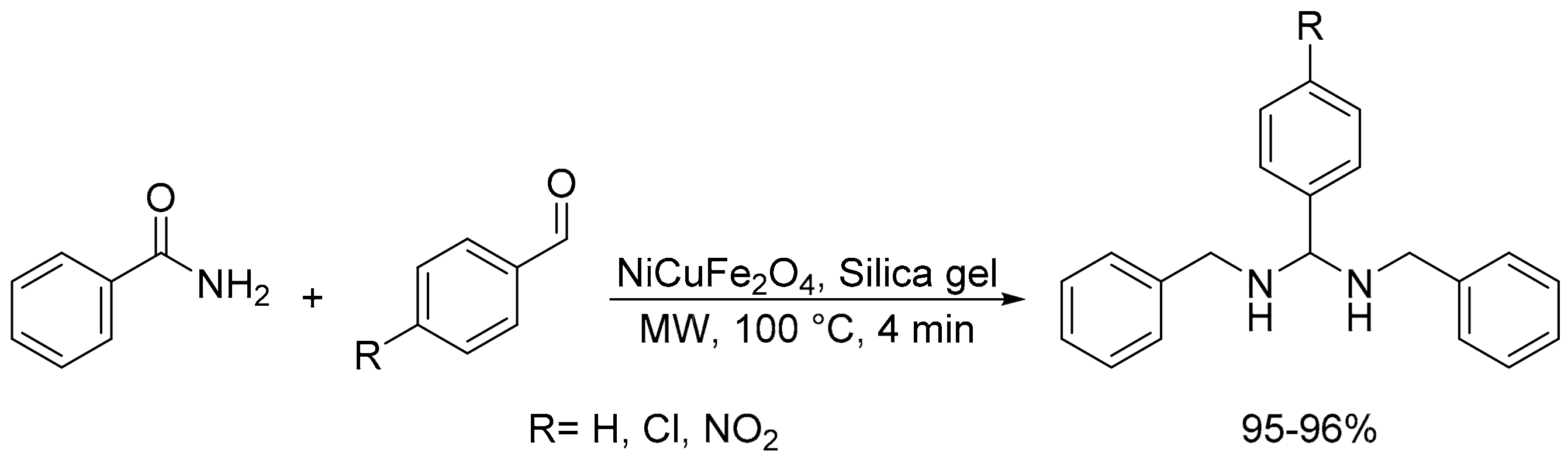
- Sarada, R.; Jagannadharao, V.; Govindh, B.; Padma, M. A facile microwave assisted neat synthesis of bis-amides using nano nickel copper ferrite as amicable catalyst and study of their fluorescence studies. Pharma Chem. 2017, 9, 115–119. [Google Scholar]
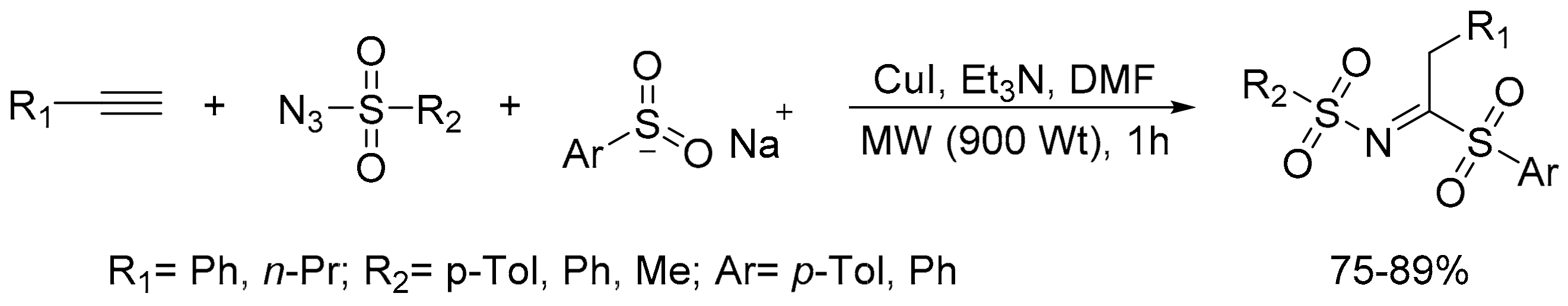
- Nematpour, M.; Abedi, E. A microwave-assisted new synthesis of sulfonylidene-sulfonamide via reactions of N-sulfonylketenimine and sodium arylsulfinates. J. Sulfur Chem. 2017, 38, 76–82. [Google Scholar] [CrossRef]
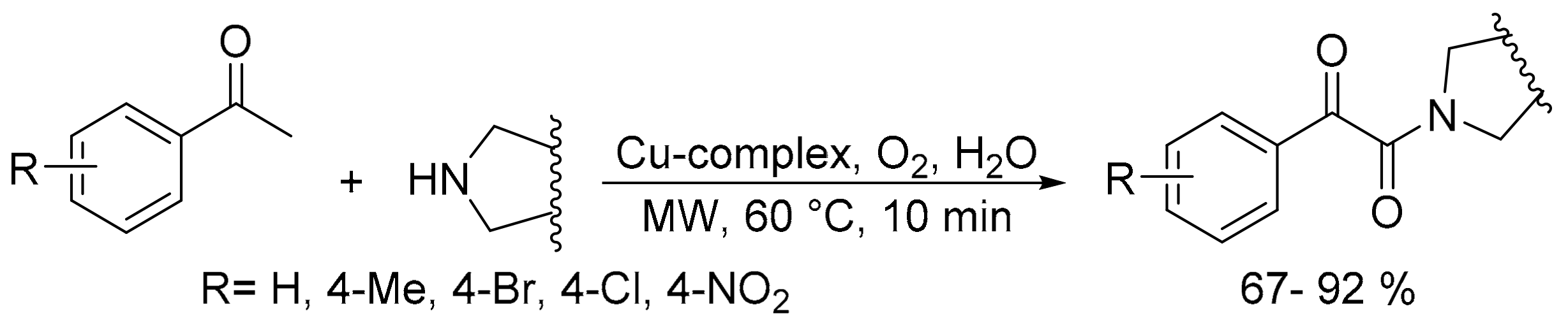
- Nekkanti, S.; Veeramani, K.; Kumar, N.P.; Shankaraiah, N. Microwave-assisted direct oxidative synthesis of α-ketoamides from aryl methyl ketones and amines by a water soluble Cu (I)-complex. Green Chem. 2016, 18, 3439–3447. [Google Scholar] [CrossRef]
- Navarro, L.; Pujol, M.D. Microwave assisted synthesis of selected diaryl ethers under Cu (I)-catalysis. Tetrahedron Lett. 2015, 56, 1812–1815. [Google Scholar] [CrossRef]
- Mehmood, A.; Devine, W.G.; Leadbeater, N.E. Development of methodologies for copper-catalyzed C–O bond formation and direct cyanation of aryl iodides. Top. Catal. 2010, 53, 1073–1080. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Badsara, S.S.; Tsai, W.-T.; Lee, C.-F. Microwave-assisted copper-catalyzed cross-coupling reaction of thiols with aryl iodides in water. Synthesis 2015, 47, 181–186. [Google Scholar] [CrossRef]
- Sun, W.; Patel, P.D.; Stephani, R.A.; Chiosis, G. An efficient copper-catalyzed microwave-assisted S-arylation towards the synthesis of 8-arylsulfanyl adenines. Synlett 2011, 20, 3008–3012. [Google Scholar] [CrossRef]
- Botteselle, G.V.; Godoi, M.; Galetto, F.Z.; Bettanin, L.; Singh, D.; Rodrigues, O.E.D.; Braga, A.L. Microwave-assisted one-pot synthesis of symmetrical diselenides, ditellurides and disulfides from organoyl iodides and elemental chalcogen catalyzed by CuO nanoparticles. J. Mol. Catal. A Chem. 2012, 365, 186–193. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Sigeev, A.S.; Peregudov, A.S.; Petrovskii, P.V.; Khrustalev, V.N. Microwave-assisted synthesis of diaryl selenides. Elucidation of Cu(I)-catalyzed reaction mechanism. Chem. Lett. 2010, 39, 720–722. [Google Scholar] [CrossRef]
- Saba, S.; Botteselle, G.V.; Godoi, M.; Frizon, T.E.A.; Galetto, F.Z.; Rafique, J.; Braga, A.L. Copper-catalyzed synthesis of unsymmetrical diorganyl chalcogenides (Te/Se/S) from boronic acids under solvent-free conditions. Molecules 2017, 22, 1367. [Google Scholar] [CrossRef] [PubMed]
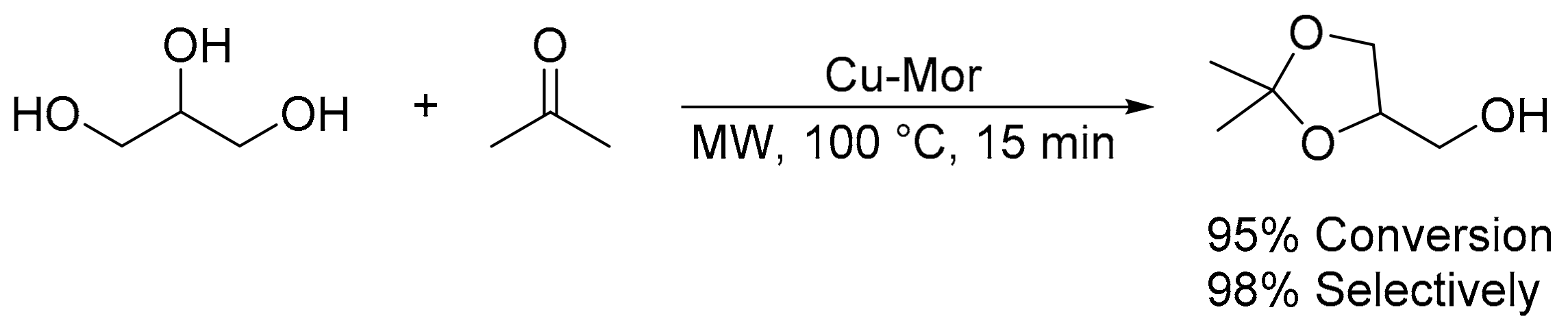
- Priya, S.S.; Selvakannan, P.R.; Chary, K.V.R.; Kantam, M.L.; Bhargava, S.K. Solvent-free microwave-assisted synthesis of solketal from glycerol using transition metal ions promoted mordenite solid acid catalysts. Mol. Catal. 2017, 434, 184–193. [Google Scholar] [CrossRef]
- Nandi, G.C. An efficient Cu-catalyzed microwave-assisted synthesis of diaryl sulfones. Synth. Commun. 2017, 47, 319–323. [Google Scholar] [CrossRef]
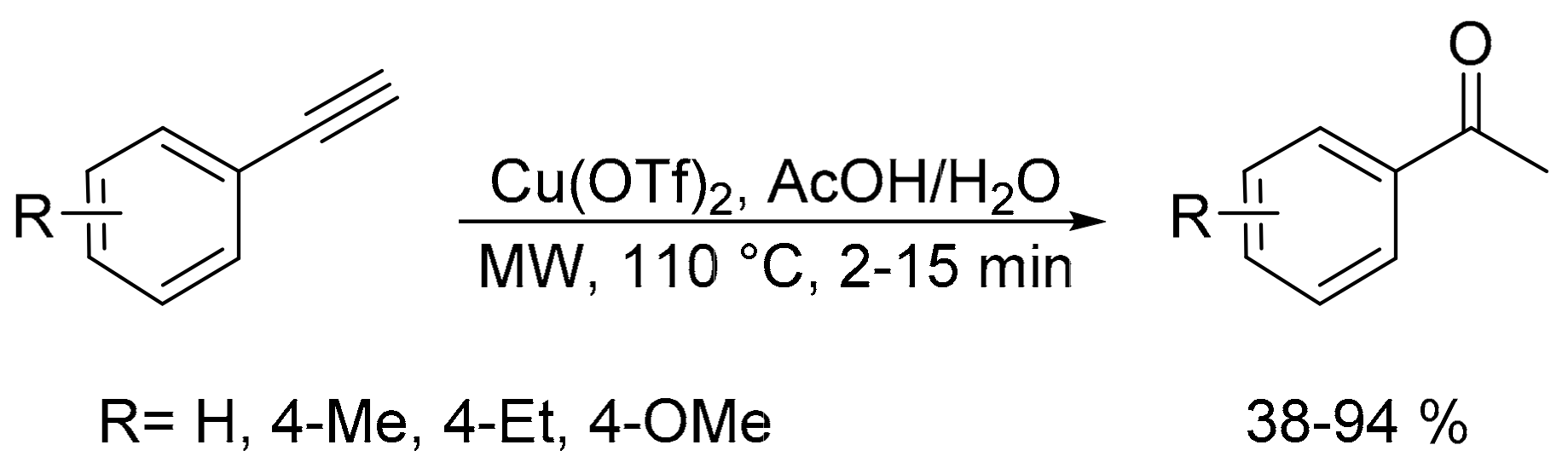
- Jha, M.; Shelke, G.M.; Pericherla, K.; Kumar, A. Microwave assisted copper triflate-catalyzed rapid hydration of aryl acetylenes. Tetrahedron Lett. 2014, 55, 4814–4816. [Google Scholar] [CrossRef]
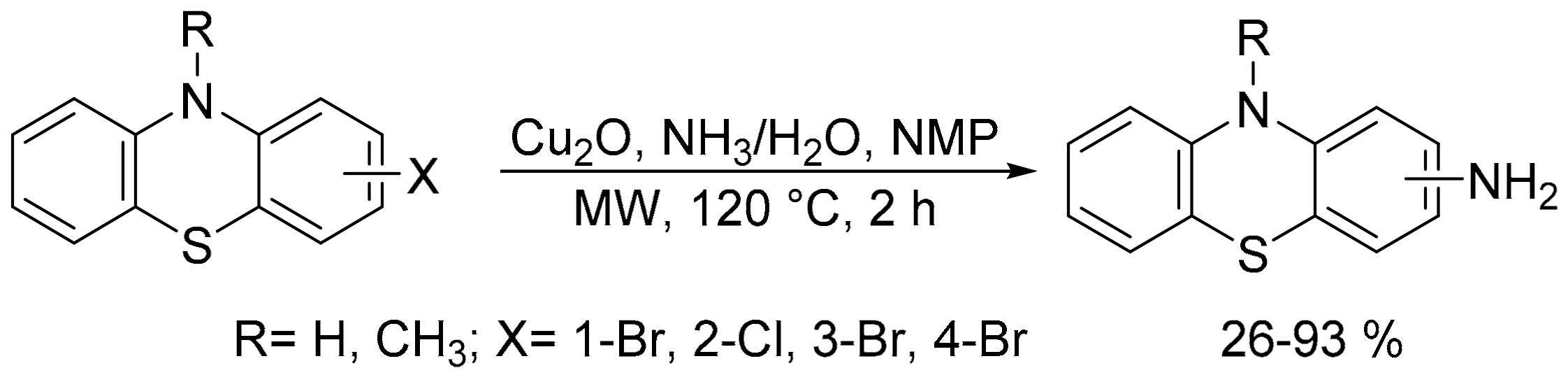
- Gáiná, L.I.; Mátárângá-Popa, L.N.; Gal, E.; Boar, P.; Lönnecke, P.; Hey-Hawkins, E.; Bischin, C.; Silaghi-Dumitrescu, R.; Lupan, I.; Cristea, C.; et al. Microwave-assisted catalytic amination of phenothiazine; Reliable access to phenothiazine analogues of Tröger’s base. Eur. J. Org. Chem. 2013, 5500–5508. [Google Scholar] [CrossRef]
- Ke, F.; Chen, X.; Li, Z.; Xiang, H.; Zhou, X. Microwave-assisted copper-catalyzed hydroxylation of aryl halides in water. RSC Adv. 2013, 3, 22837–22840. [Google Scholar] [CrossRef]
- Paik, S.; Jung, M.G. Rapid microwave-assisted copper-catalyzed nitration of aromatic halides with nitrite salts. Bull. Korean Chem. Soc. 2012, 33, 689–691. [Google Scholar] [CrossRef][Green Version]
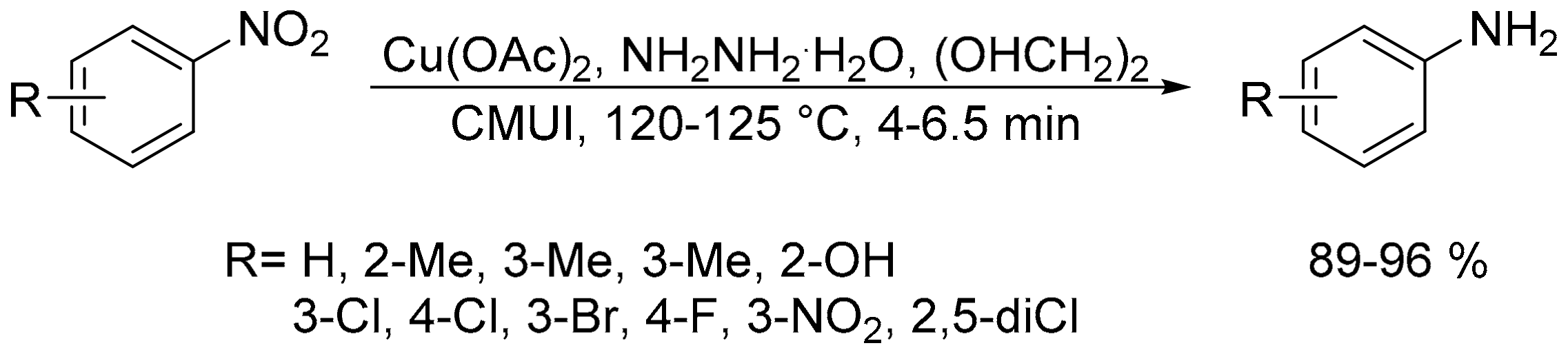
- Feng, H.; Li, Y.; Lin, S.; Van der Eycken, E.V.; Song, G. Nano Cu-catalyzed efficient and selective reduction of nitroarenes under combined microwave and ultrasound irradiation. Sustain. Chem. Proces. 2014, 2, 14. [Google Scholar] [CrossRef]
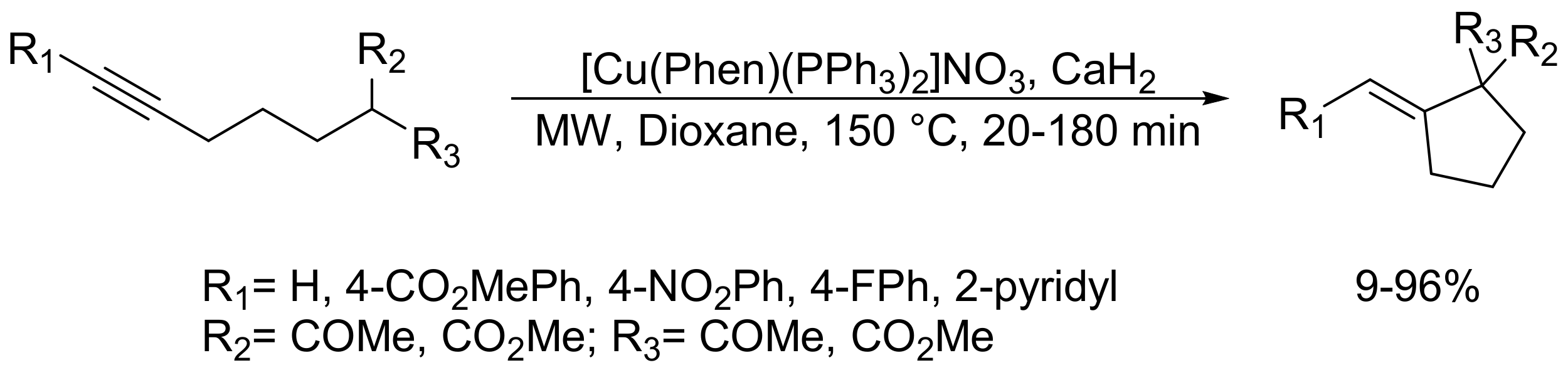
- Montel, S.; Bouyssi, D.; Balme, G. An efficient and general microwave-assisted copper-catalyzed conia-ene reaction of terminal and internal alkynes tethered to a wide variety of carbonucleophiles. Adv. Synth. Catal. 2010, 352, 2315–2320. [Google Scholar] [CrossRef]
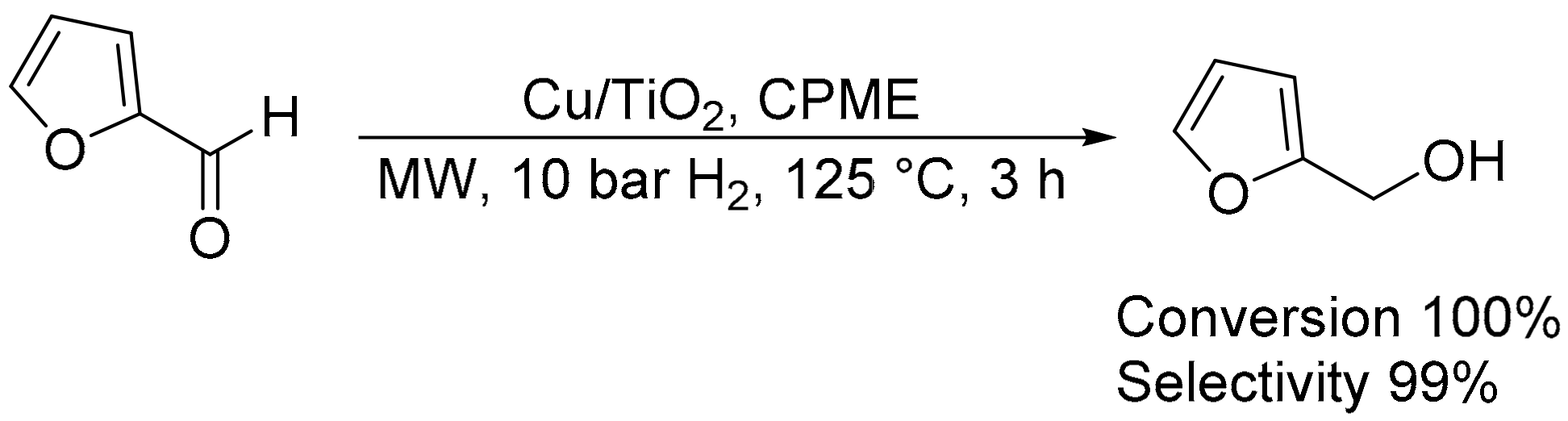
- Romano, P.N.; de Almeida, J.M.A.R.; Carvalho, Y.; Priecel, P.; Sousa-Aguiar, E.F.; Lopez-Sanchez, J.A. Microwave-assisted selective hydrogenation of furfural to furfuryl alcohol employing a green and noble metal-free copper catalyst. ChemSusChem 2016, 9, 3387–3392. [Google Scholar] [CrossRef]
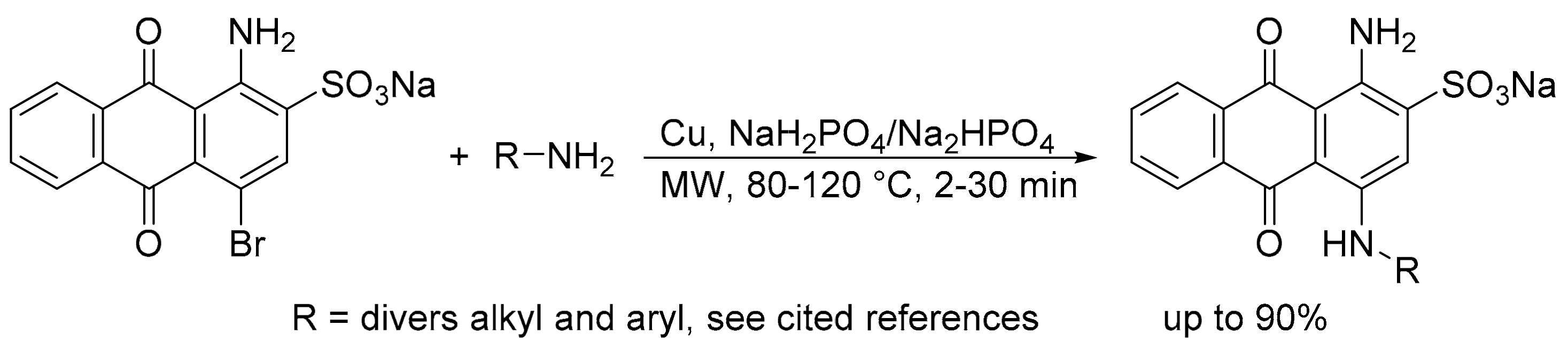
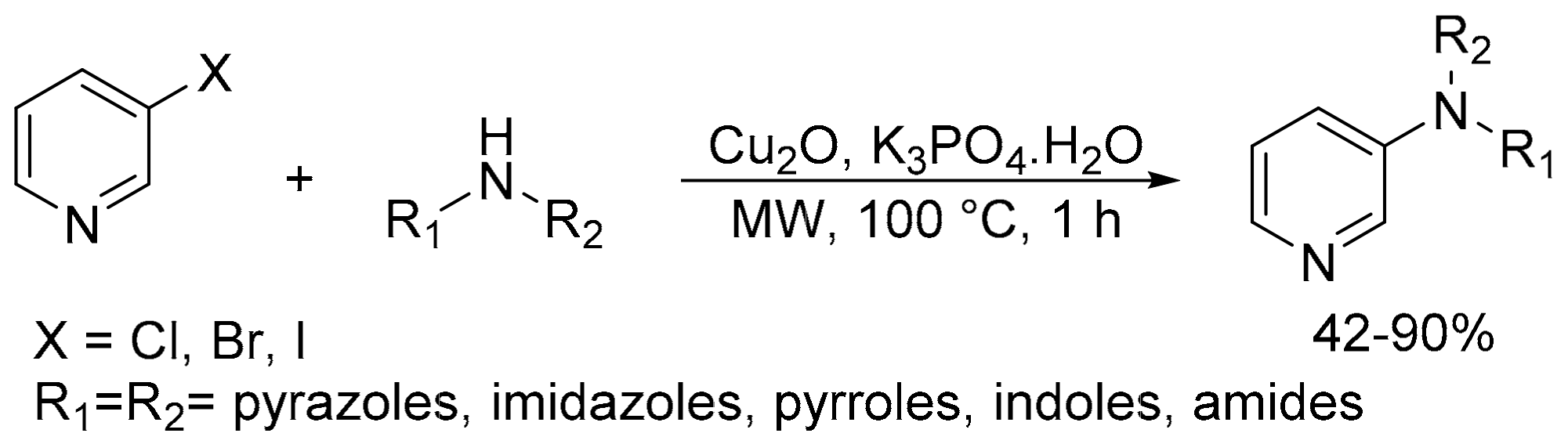






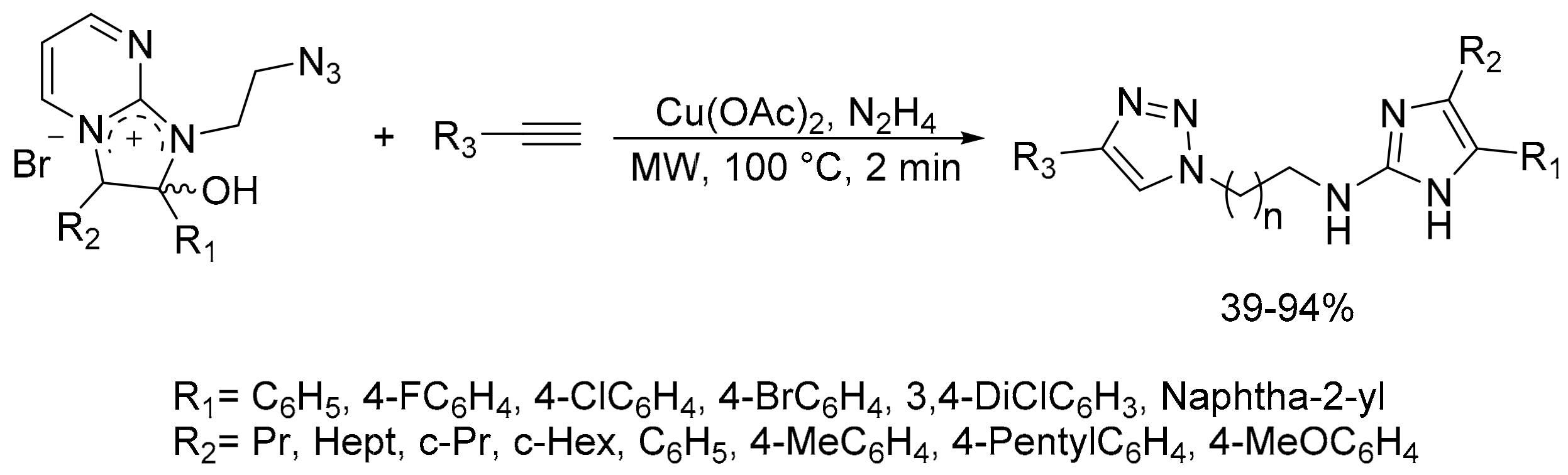





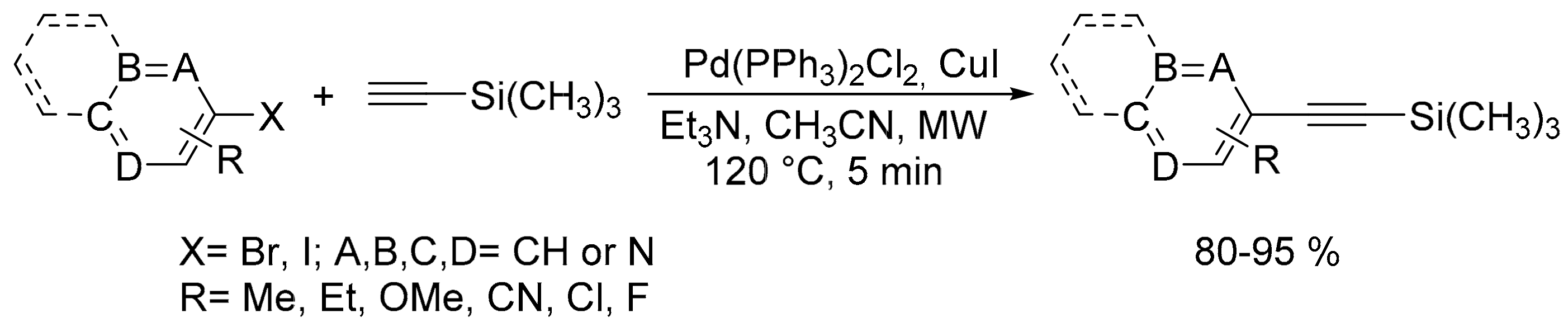
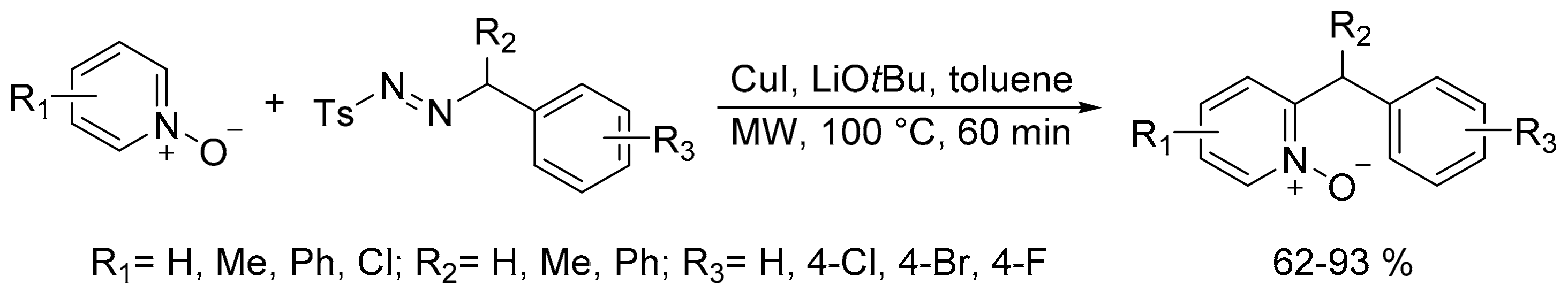

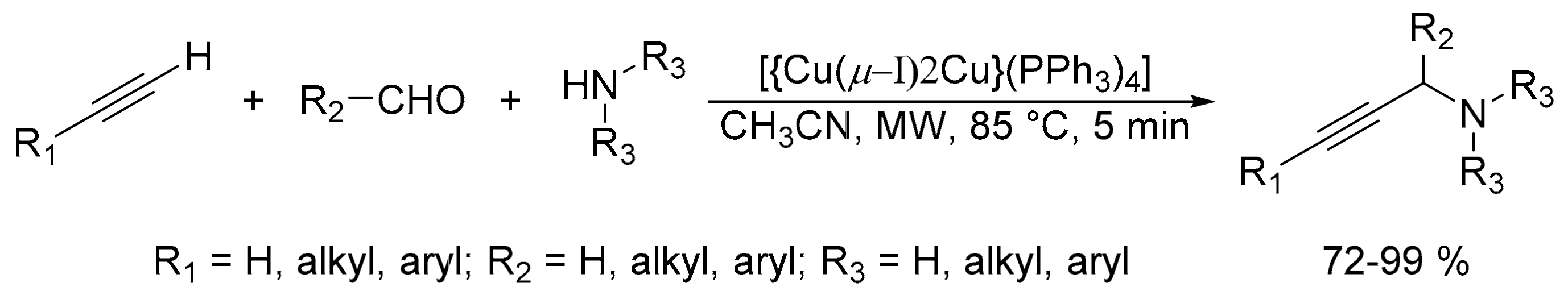




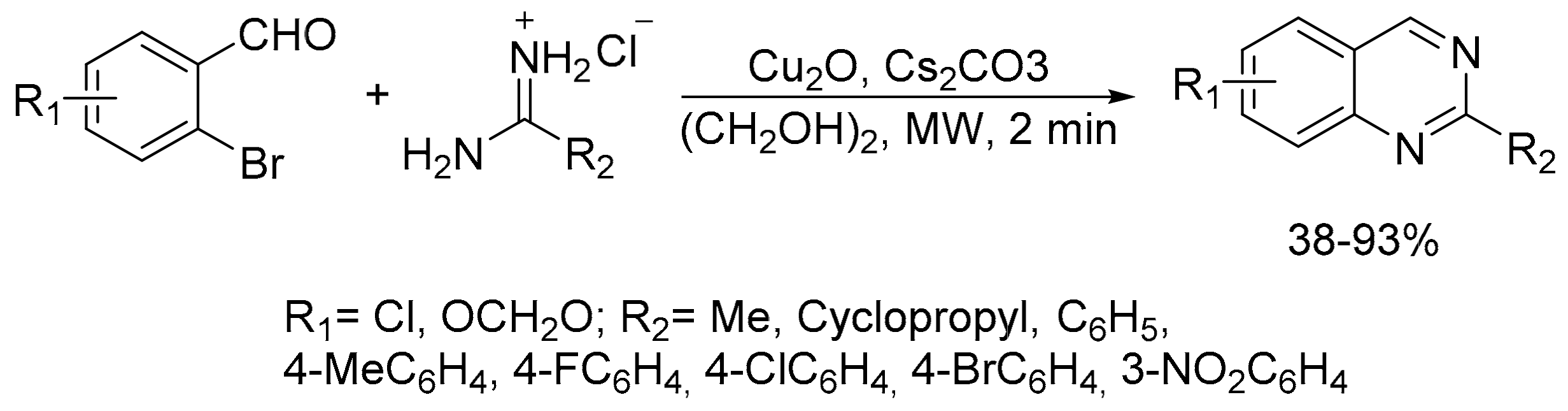




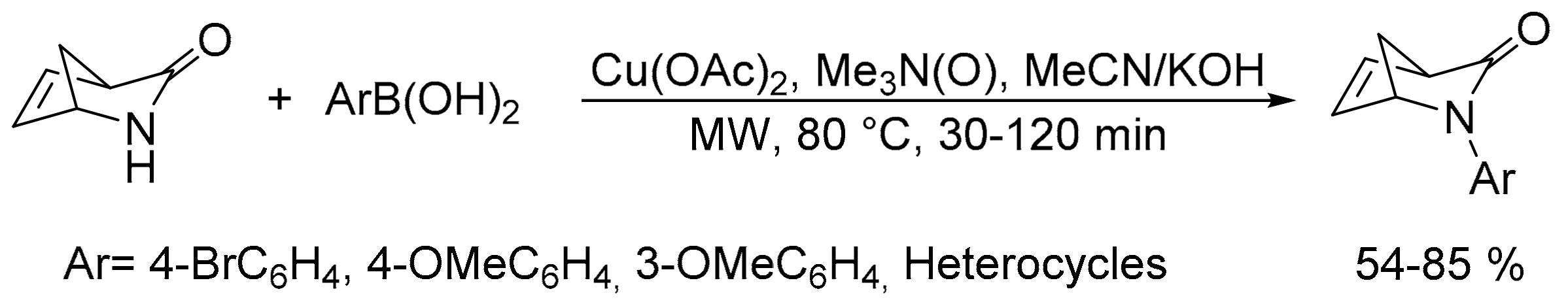
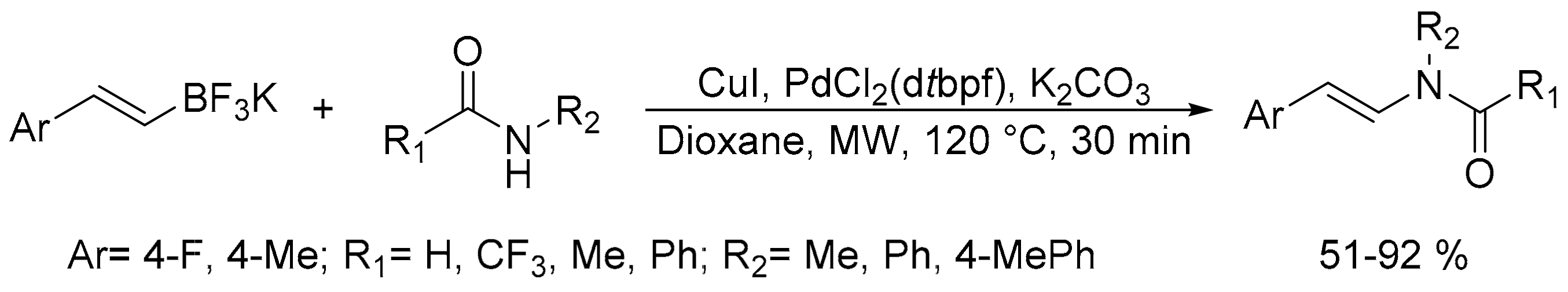
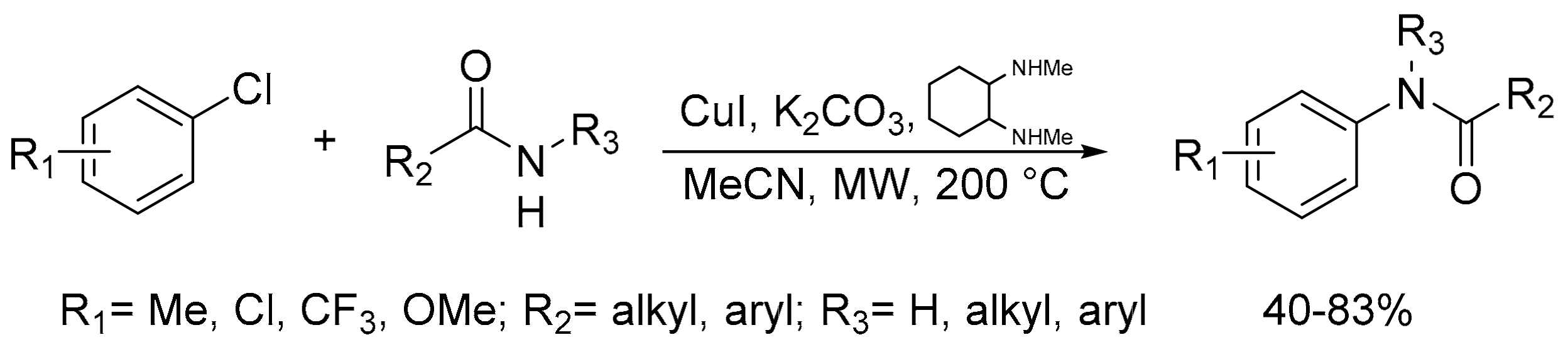




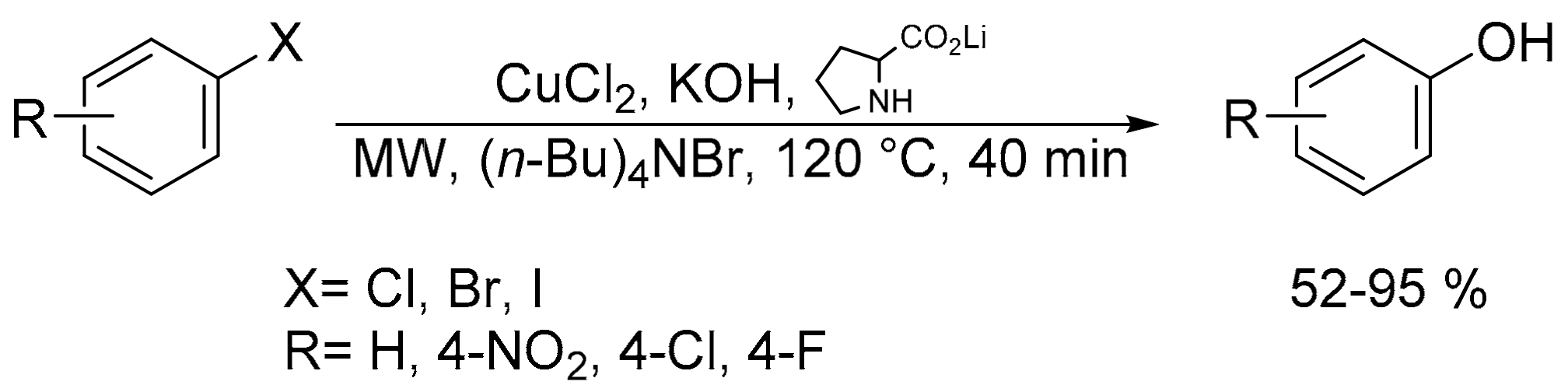

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baqi, Y. Recent Advances in Microwave-Assisted Copper-Catalyzed Cross-Coupling Reactions. Catalysts 2021, 11, 46. https://doi.org/10.3390/catal11010046
Baqi Y. Recent Advances in Microwave-Assisted Copper-Catalyzed Cross-Coupling Reactions. Catalysts. 2021; 11(1):46. https://doi.org/10.3390/catal11010046
Chicago/Turabian StyleBaqi, Younis. 2021. "Recent Advances in Microwave-Assisted Copper-Catalyzed Cross-Coupling Reactions" Catalysts 11, no. 1: 46. https://doi.org/10.3390/catal11010046
APA StyleBaqi, Y. (2021). Recent Advances in Microwave-Assisted Copper-Catalyzed Cross-Coupling Reactions. Catalysts, 11(1), 46. https://doi.org/10.3390/catal11010046





