Abstract
The application and advantages of variable frequency microwaves (VFM; range, 5.85–6.65 GHz) are reported for the first time in microwave chemistry, particularly when carrying out reactions catalyzed by metallic conductive catalysts so as to avoid the formation of arc discharges, and especially when using a strong microwave absorber such as activated carbon (AC) particulates as supports of metal-based catalysts. Two model reactions performed in low boiling point nonpolar solvents are described wherein arc discharges easily occur under the more conventional fixed frequency microwave (FFM) approach: (i) the synthesis of 4-methylbiphenyl (4MBP) by the Suzuki-Miyaura cross-coupling process catalyzed by Pd particles supported on AC particulates (Pd/AC), and (ii) the synthesis of toluene via the dehydrogenation of methylcyclohexane (MCH) catalyzed by Pt particles dispersed on AC particulates (Pt/AC). Contrary to the usage of fixed frequency microwaves (5.85 GHz and 6.65 GHz), the use of VFM microwaves increased the chemical yields of 4MBP {49% versus 5–8% after 60 min} and toluene {89% versus 24% after 10 min} by suppressing the formation of discharges that otherwise occur on the catalyst/AC surface with FFM microwaves. Consequently, relative to the latter approach, the VFM technology is significantly advantageous, especially in reactions with solid conductive catalysts, not least of which are the reduction in power consumption, thus energy savings, and the prevention of potential mishaps.
1. Introduction
It is not uncommon to experience that whenever a crumpled aluminum foil is microwave- irradiated in a domestic oven, it causes the formation of arc discharges that may subsequently be followed by ignition (fire) of the foil, because the free electrons affected by the microwave radiation are arc-discharged at the tips of the creased aluminum foil. Similar incidents have been reported with fire occurring in old microwave ovens that had been used for several years as a result of lumps of oil/food carbon residues on the ovens’ walls, at which the microwaves concentrate and cause their ignition. Microwave-assisted chemical reactions actively investigated worldwide since the 1990s may also experience similar mishaps whenever a solid metal-based catalyst is used directly or is supported on activated carbon (AC) particulates. Accordingly, performing such catalytic reactions in a commercial microwave chemical reaction apparatus is now prohibited by the vendors. Nonetheless, solid–liquid reaction systems continue to be very attractive in microwave chemistry because of the use of a non-equilibrium reaction field to heat the catalysts selectively [1,2,3,4]; the potential ensuing risks in carrying out such reactions are now well recognized.
In this regard, microwave selective heating of AC particles dispersed in methylcyclohexane was investigated earlier by Horikoshi and coworkers [5], who performed simulations of microwave heat transfer, the results of which show a temperature difference of 217 °C between cyclohexane (model solvent, 340 °C; AC particles, 557 °C) and the Pd/AC catalytic assembly. This means that if the reaction field was kept high enough without raising the temperature of the bulk solution, then new chemical processes could be attained in an industrial context so as to take full advantage of the electromagnetic waves of microwave radiation [6]. Such selective heating of a catalyst assembly through which the reaction proceeds can lead to a remarkable decrease in power consumption. On the other hand, this condition can—in principle—also lead to some undesirable mishaps. That is, if a strong microwave absorber catalyst or catalyst support were used in nonpolar solvents, discharges could occur at or near the conductive catalyst. To avoid such risk, it would be wise to use polar solvents and low-absorbing microwave catalysts or catalyst supports in chemical syntheses. Even so, however, under such conditions it is still possible to take full advantage of microwaves [7], although microwave selective heating may be lost, and so a trade-off must be considered.
In earlier studies, Horikoshi and coworkers [8] and Petricci et al. [9] proposed experiment-based mechanisms to describe discharges occurring on microwave absorbing catalysts in nonpolar solvents. On their part, Wang and coworkers [10] described a theoretical mechanism based on a numerical simulation of the effects of hot spots when microwave heating a strong microwave- absorbing medium. Additional attempts have been reported in elucidating the suppression of arc discharges in various fields, regardless of the nature of the microwave chemistry, such as, for example, suppressing the arc discharge phenomenon occurring in grapes in a microwave oven [11]. These earlier studies demonstrated that the electric field (E-field) of the microwaves causes a resonance between two particles, thereby producing a standing wave that leads to the occurrence of discharges. Accordingly, this calls attention to suppressing the standing wave locally and thus suppressing the discharges.
Following the variable frequency microwave (VFM) technology developed in 1991 at the Oak Ridge National Laboratories [12,13], the present study used a bandwidth of frequencies from 5.85 to 6.65 GHz that were rapidly swept in a continuous saw tooth manner to be supplied to the reaction chamber. The bandwidth was divided into 4096 discrete frequencies that were provided every 0.1 s, such that the residence time for any of the 4096 frequency standing wave patterns was 25 μs, which rapidly changed with the sweeping frequency. This rapid sweep approach provided not only a uniform microwave field over the entire process volume, but also eliminated charge build-up on a metallic surface, thereby suppressing arcing and discharge phenomena. In this regard, Cauchois and coworkers [14] described the usage of VFM microwaves in the thermal sintering of the microstructure of inkjet-printed silver nanoparticles.
We herein demonstrate for the first time that VFM microwaves can also be used in microwave- assisted chemistry with some advantages in carrying out chemical reactions in the presence of conductive catalysts (e.g., solid metals), heretofore difficult to perform. A single-mode applicator with high microwave irradiation efficiency that could use VFM technology was prototyped and used in carrying out some catalyzed reactions. First, however, metallic powders of various sizes (aluminum, stainless steel, lead, iron), ceramics (aluminum oxide and silicon carbide) and activated carbon (AC) dispersed in various polar to nonpolar solvents were tested to confirm the occurrence or non-occurrence of discharges when using fixed frequency microwaves. The results showed that a combination of activated carbon and low-boiling point nonpolar solvents could easily generate discharges (hot spots). One reason for this is that activated carbon has a significantly high microwave absorption capacity, in addition to which the dielectric loss of activated carbon increases with increasing temperature [15]. Moreover, Joule heating of activated carbon particulates by the microwaves’ electric field leads to arcing, owing to the migration of free electrons between AC particles [16]. Activated carbon was the support of choice to substantiate the above inferences as, to the best of our knowledge, there are no other solid catalyst supports with such microwave properties.
The present study is part of our systematic studies in technology development for which the relevance finds its raison-d′être on how to prevent discharges from occurring on the surfaces of solid catalysts. Accordingly, we examined the possibility of suppressing such arc discharges using the VFM methodology. After several experiments, conditions were selected that could easily generate arc discharges (hot spots) at a low fixed microwave power in combination with nonpolar solvents and activated carbon in model reactions. Explicitly, we examined the synthesis of 4-methylbiphenyl (and biphenyl as a by-product) via the Suzuki–Miyaura cross-coupling and homocoupling reactions in the nonpolar solvent toluene in the presence of Pd/AC catalyst particulates (one of two model reactions examined). Our clear objectives were to determine the yields of products and/or by-products formed by the VFM technology vis-à-vis the fixed frequency microwave (FFM) approach and the conventional oil bath methodology. It should be noted that the Suzuki–Miyaura cross-coupling reaction has been used extensively in the past to explore the benefits of microwave chemistry [17]. The other model reaction was the synthesis of toluene through the dehydrogenation of methylcyclohexane (MCH) using a commercially available Pt/AC solid catalyst. A single-mode applicator was used inasmuch as the catalytic reaction can be performed at low microwave power levels, and therefore the added advantage of energy savings.
2. Results and Discussion
2.1. Synthesis Yields in Product/by-Product Formation
The characteristics of variable frequency microwaves (VFM: 5.85 GHz to 6.65 GHz; fixed power, 6 Watts) and fixed frequency microwaves (FFM: 5.85 GHz and 6.65 GHz; fixed power, 6 Watts) were examined from the viewpoint of a chemical reaction that employed Pd particles supported on activated carbon particulates (Pd/AC) as the solid catalyst. The chemical yield of 4-methylbiphenyl produced with the VFM microwaves was 49% for a reaction time of 60 min (Figure 1a), in contrast to a chemical yield of only 8% and 5%, respectively, for 4MBP when using FFM microwaves at a frequency of 5.85 and 6.65 GHz, also for a reaction time of 60 min. The heating time to reach an internal temperature of 100 °C was 15 s with the VFM microwaves, while with the FFM (5.85 GHz) microwaves, the heating time to reach 100 °C was somewhat longer (135 s). Whether VFM or FFM microwaves were used, the temperatures of the reactions oscillated within the range 98 ± 7 °C for a reaction time of 60 min. Differences in VFM and FFM rates were negligible and thus had little effect on the chemical yields. The synthesis yield of 4-methylbiphenyl from the oil bath heating method using the same reaction vessel was 17% (see Table 1).
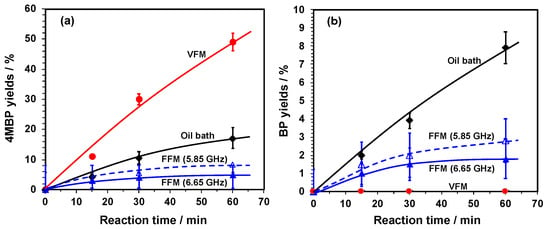
Figure 1.
Product yields of (a) 4-methylbiphenyl (4MBP) and (b) biphenyl (BP) as a by-product in toluene/1-hexanol solvent under irradiation using fixed frequency microwaves (FFM) at 5.85 or 6.65 GHz, and with variable frequency microwaves (VFM) at 5.85–6.65 GHz, and oil bath heating.

Table 1.
Chemical yields in the formation of 4-methylbiphenyl (4MBP) and biphenyl (BP) via the Suzuki–Miyamura cross-coupling and homo-coupling process, respectively.
Similar experiments were carried out in an earlier study [8] using a single-mode applicator with 2.45 GHz FFM microwaves with irradiation either by the electric field (E-field) or by the magnetic field (H-field) of the 70-Watt microwaves. The chemical yields of 4-methylbiphenyl were, respectively, 6% (E-field) and 38% (H-field) for a reaction time of 60 min. Nonetheless, irradiation with the microwaves’ electric field resulted in the formation of arc discharges, so that it was necessary to place the sample at the maximal position of the microwave magnetic field to avoid their occurrence. To meet this requirement, however, necessitated the sample size to be at most a few centimeters, or at best a few millimeters; this made any attempt at scaling up the process to an industrial level using continuous flow reactors rather difficult. On the contrary, application of the microwave VFM technology can result not only in higher chemical yields of products but compared to the 2.45 GHz microwave device used in the previous study a power consumption of less than 8% (= 6 W / 70 W) was also achieved, and thus advantageous for industrial scale-up.
Possible side reactions when using the Suzuki–Miyaura methodology—e.g., formation of biphenyl through the Suzuki–Miyaura homocoupling and 4,4′-dimethylbiphenyl through the Ullmann-type coupling process (Figure 2)—have also been examined by Cravotto and coworkers [18] under various microwave conditions; no 4,4′-dimethylbiphenyl by-product was detected under all conditions. The chemical yields of biphenyl generated through the homocoupling process are displayed in Figure 1b, which shows that under VFM irradiation the yield were 0% for a 60 min period, while under FFM microwave irradiation at 5.85 and at 6.65 GHz, also for 60 min, the yields of biphenyl were, respectively, 3% and 2%; conventional heating with an oil bath yielded 8% of biphenyl (see also Table 1). Clearly, compared with oil bath heating, the efficiency of the Suzuki–Miyaura cross-coupling reaction was greater under VFM irradiation, while that of the homocoupling process witnessed a significant decrease in efficiency. Hence, when a discharge occurs, the synthesis yields tend to decrease and the number of by-products tends to increase, which confirms observations of a previous study [8].
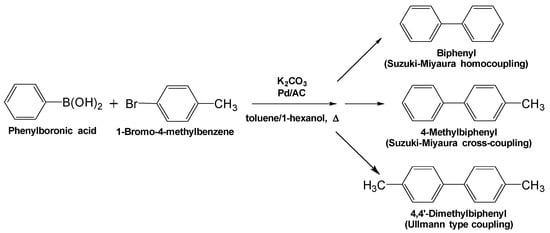
Figure 2.
The synthesis mechanism of 4-dimethylbiphenyl (target), biphenyl (by-product) and 4,4′-dimetyl-biphenyl (by-product).
In accordance with our earlier study [8], one major factor that impacts the chemical yields is the generation of arc discharges (i.e., formation of hot spots) occurring on the surface of the heterogeneous Pd/AC catalyst particles inside the reactor, as observed with a 4K (resolution, 4000) video camera through the hole on the microwave cavity side (see Materials and Methods section). Bubbles could be seen immediately on the Pd/AC surface at the start of microwave irradiation at the fixed frequencies of 5.85 and 6.65 GHz. After approximately 4 s, these discharges appeared as an orange-colored light from the dispersed Pd/AC particles, strong enough to be observed visually. The photographs reported in Figure 3 show the average diameter of the light spot from the discharge to be ca. 0.25 mm; the actual discharge size at the origin was likely smaller. Thereafter, the occurrence of this discharge continued for more than 5 min. Formation of discharges following microwave irradiation impacts negatively on chemical processes, and not least on safety aspects. For example, the spontaneous ignition temperatures of toluene and 1-hexanol occur at 480 and 304 °C [19], respectively; yet the color of the generated discharges indicates the temperature of the orange light to be more like 930 °C [20].
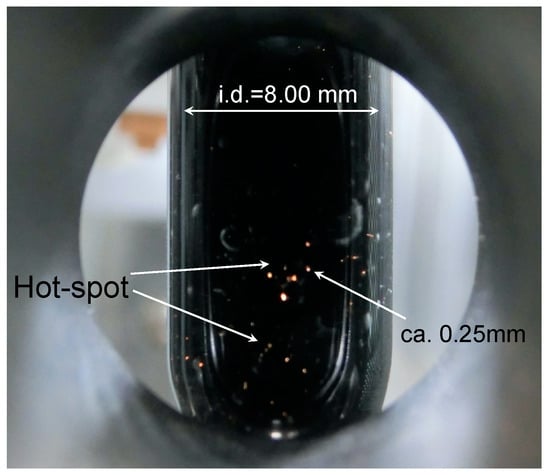
Figure 3.
Photograph of the arc discharges (hot-spots) occurring on the Pd particles deposited on activated carbon (Pd/AC) particle particulates in toluene/1-hexanol solvent during the reaction in a quartz reactor (internal diameter, 8.00 mm; external diameter, 10.0 mm) under 6-W microwave irradiation at the fixed microwave frequency of 5.85 GHz.
Although the occurrence of discharges in the suspension under VFM microwave irradiation did not materialize in the current study, there was nonetheless clear turbulence of the Pd/AC particles in the dispersion medium. Regardless, even an increase in VFM microwave power from 6 to 24 Watts caused no generation of discharges.
Another possible cause for the chemical yields of 4MBP under FFM conditions to be lower than the yield under conventional oil bath heating (Table 1) may be related to the actual activity of the catalyst particulates under those conditions. To confirm such inference, the surface of the Pd/AC catalyst was examined by transmission electron microscopy (TEM; Hitachi Transmission Electron Microscope, Model U-3310; Omuta City, Fukuoka Prefecture, Japan) after irradiating the sample with 6.65-GHz FFM microwaves for 5 min. The photograph in Figure 4a reveals that the Pd catalyst particles (size, 3–5 nm) were adsorbed on the activated carbon surface; nevertheless, aggregates consisting of several tens to hundreds of Pd particles also formed.
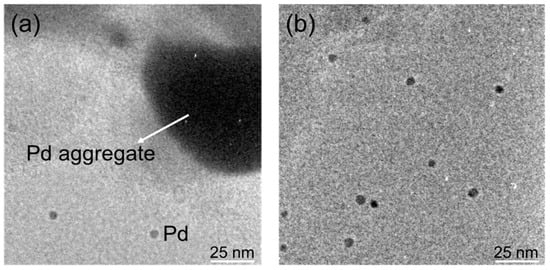
Figure 4.
TEM images of Pd/AC catalyst surface: (a) after 5 min of microwave irradiation at the fixed microwave frequency (FFM) of 6.65 GHz; (b) after 60 min of microwave irradiation with variable frequency microwaves (VFM) in the frequency range 5.85–6.65 GHz.
By contrast, no aggregation of the Pd catalyst particles occurred under the VFM microwave mode as evidenced from the TEM image of the catalyst surface after 60 min (Figure 4b). It is worth pointing out that the melting point of bulk-size palladium is 1554.9 °C, which decreases substantively when it becomes nano-sized. For example, the melting point of the icosahedral-shaped 5-nm Pd particles is significantly lower at ca. 223 °C [21]. It is not unlikely, therefore, that the Pd particles in the vicinity where the discharge occurred may have dissolved and subsequently formed aggregates. Such Pd aggregation was also observed in experiments carried out at the fixed frequency of 2.45 GHz [8], which was also attributed to the occurrence of arc discharges.
The reasons that the usage of FFM 6.65-GHz microwave radiation displayed a lower chemical yield of 4-methylbiphenyl (4-MBP) and biphenyl (BP) compared to the FFM 5.85-GHz microwaves (Table 1) are no doubt related to the number and intensity of the discharges generated. To be sure, observations with a 4K video camera clearly showed that there were a greater number of discharges with the 6.65-GHz microwaves than there were by the 5.85-GHz microwaves, attributable to the greater efficiency of discharge generation at the higher frequency. Our previous study [8] suggested that the places where discharges were triggered optimally were the voids/gaps between the activated carbon particles. To further understand this phenomenon, the distribution and the relationship(s) of the microwaves’ E-field intensity (5.85 versus 6.65 GHz) within the spatial volume between the activated carbon particles were examined by a simulation technique using the RF module and the COMSOL Multiphysics software Version 4.3a (Keisoku Engineering Systems Co. Ltd., Chiyoda-ku, Tokyo, Japan). For the simulation, the relative permittivity (εr′) of toluene and of the activated carbon was taken as 2.35 and 36.26, respectively, while the relative permeability and electrical conductivity of activated carbon were set a 1 and 44.24 S m−1, respectively. The distance between the activated carbon particles (size: 0.65 mm in diameter) in toluene was set up at intervals of 0.001 mm.
The results of the simulation confirm that the discharge between the activated carbon particles was concentrated entirely within the gap that separated the activated carbons. The maximum E-field intensity was 933,584 V m−1 for the 6.65-GHz microwaves (Figure 5) compared to a field intensity of 220,661 V m−1 for the 5.85-GHz microwaves. Nonetheless, even at 5.85 GHz, the microwaves’ electric field was sufficiently concentrated between the particles to generate arc discharges; the discharge efficiency was further increased when using 6.65-GHz microwaves. Accordingly, the lower chemical yields at the higher frequency of 6.65 GHz reported in Table 1 are consistent with such an analysis.
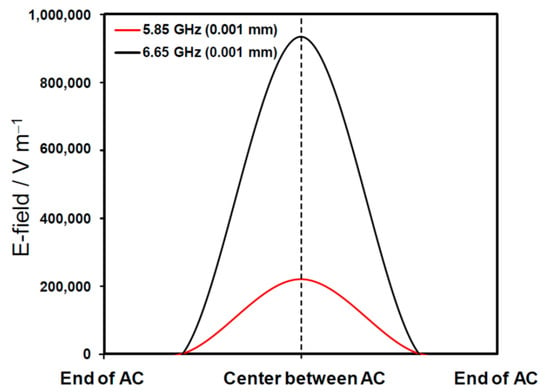
Figure 5.
Distribution of the microwaves’ E-fields (V m−1) around two activated carbon particles in toluene solvent for FFM at 5.85 and at 6.65 GHz simulated with the COMSOL Multiphysics software version 4.3a. The gap between AC particles (end to end of ACs) was set at 0.001 mm.
A word of caution! Whenever a solvent with a low flash point is used and oxygen is present, the arc discharge can easily trigger a fire, as further discussed below. In this regard, on examining various graphite specimens placed on an alumina (Al2O3) board and irradiated with microwaves, Menéndez et al. [22] observed a microwave-induced discharge plasma as a ball lightning plasma, and as a plasma arc as might occur in a domestic microwave oven. They estimated the temperature for the generation of the ball lightning plasma to be less than 400 °C, while estimating the temperature of the plasma arc to be in the 400–700 °C range.
2.2. Synthesis of Toluene from the Dehydrogenation of Methylcyclohexane
The suppression of discharges from the catalyst surface by usage of VFM microwaves was further confirmed by performing the model reaction involving the dehydrogenation of methylcyclo- hexane (MCH) using a commercial Pt/AC catalyst (Figure 6), which yielded toluene (and benzene as a by-product); for comparison, both the VFM and FFM (5.85 GHz) approaches were used. The extent of toluene produced by VFM heating (power, 9 Watts) was 49% (after 5 min) and 89% (10 min), whereas with FFM heating (power, 12 Watts), the quantity of toluene formed was 17% (after 5 min) and 24% (10 min). Formation of the by-product benzene was below 2% by VFM, but was about 7% by FFM heating.
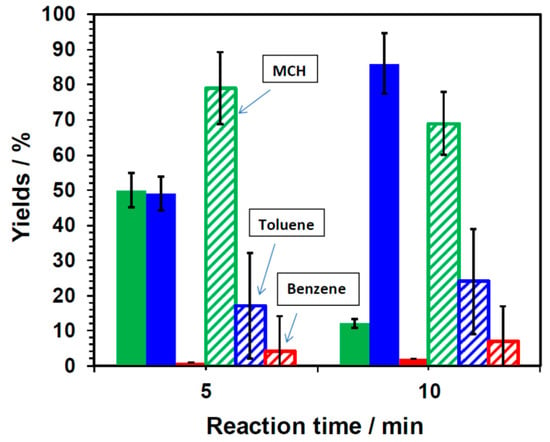
Figure 6.
Percent yields of toluene as a function of reaction times from the dehydrogenation of methylcyclohexane (MCH), and yields of the by-product benzene in Pt/AC with methylcyclohexane as the solvent under irradiation with variable frequency microwaves (VFM; solid colors) at 5.85–6.65 GHz and with fixed frequency microwaves (FFM; striped colors) at 5.85 GHz. (Green: MCH; Blue: toluene; Red: benzene).
The bulk temperature of the MCH solution with catalyst assembly Pt/AC was ca. 180 ± 8 °C under VFM irradiation after 20 s, and 183 ± 14 °C under FFM irradiation after 20 s. Noteworthy is the instantaneous temperature rise observed under FFM irradiation conditions, which sometimes caused bumping in the dispersion owing to an unequal temperature distribution in the reactor. Overheating of the catalyst by arcing was likely responsible for the formation of the benzene by-product. No discharges on the catalyst were observed with the 4K camera (video) when using the VFM approach. On the other hand, several discharges were witnessed with the FFM heating method (same situation as Figure 3). Our observations clearly confirm the discharge phenomena taking place on the surface of the catalyst assembly. Although discharges could not be entirely (100%) precluded under VFM irradiation, there is no doubt that the arc discharges were significantly reduced under the prevalent conditions of the VFM radiation.
2.3. Safety When Using Microwave Radiation in Chemical Reactions
Safety is always one of the concerns when using either metal or metallic particles on supports as catalysts in chemical reactions carried out under microwave irradiation, particularly when they are run in domestic microwave ovens. To clarify some of the safety issues that may be encountered when using FFM versus VFM microwaves, we conducted some relevant experiments involving volatile combustible organic solvents. For instance, a deep petri dish containing a toluene suspension of activated carbon particles (diameter, 0.65 to 1.10 mm) (Figure 7a) was heated with 5.85-GHz microwaves in a multi-mode VFM microwave irradiation apparatus. Irradiation with 350-Watt FFM microwaves caused the generation of vigorous arc discharges (hot spots) about 10 s after in the vicinity where the activated carbon particulates were mostly concentrated (Figure 7b). Continued microwave irradiation for ca. 32 s caused the toluene solvent to ignite (i.e., fire; see Figure 7c). In this regard, it is worth noting that the boiling point of toluene is 110.6 °C, so that it was sufficiently vaporized by the microwave heating of the activated carbon; also, the flash point of toluene is ~5 °C and the ignition point ca. 552 °C. Accordingly, there is no doubt that the activated carbon surface was ignited by the arc discharge (hot spot), with the toluene solvent being vaporized at the high temperature. By contrast, when the same experiment was performed under conditions that involved VFM microwaves (5.85 to 6.65 GHz), continuous microwave irradiation for 5 min caused no discharges to be formed nor ignition/burning of toluene to occur.

Figure 7.
Photographs of the ignition of toluene associated with microwave irradiation in a dispersion of activated carbon particles (diameter, 0.65 to 1.10 mm): (a) No microwave irradiation; (b) appearance of a discharge (hot spot) with the multimode applicator type VFM chemical equipment after irradiation with FFM microwaves (5.85 GHz; 350 W) for 30 s; (c) fire that occurred 49 s later.
During the several years into our development of microwave chemistry studies [4,5,6,8,23], we experienced two incidents that occurred in one of our laboratories during the performance of microwave-assisted chemical reactions that involved the use of a Pt/AC solid catalyst and a commercial multi-mode applicator microwave chemical reactor system using microwaves at a fixed frequency (FFM) of 2.45 GHz. The setup used initially to carry out the continuous dehydrogenation of methylcyclohexane in a flow-type reactor is displayed in Figure 8a. We deduced that the root cause of the accident was the formation of hot spots on the catalyst surface heated to such a high temperature that exceeded the flash point of methylcyclohexane, causing it to be ignited and combusted, and not least causing considerable damage to the multi-mode microwave chemical equipment. In addition, the quartz reactor was totally destroyed by the explosion because hydrogen gas was generated in the reaction.
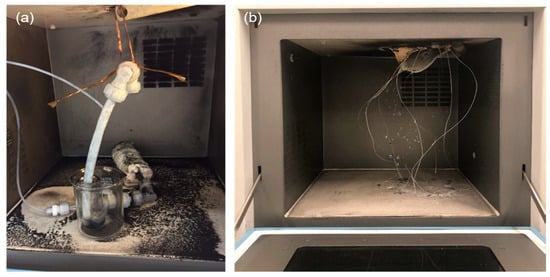
Figure 8.
Photographs showing the results of the accidents that occurred in a 2.45-GHz multi-mode applicator domestic microwave device: (a) fire-caused accident during the dehydrogenation of methylcyclohexane using Pt/AC solid catalyst particles; (b) soot formed after the fire that burned the resin that adhered to the applicator.
As a further demonstration of potential mishaps, Figure 8b illustrates the outcome of a fire that occurred during the sintering of ceramics. We deduced that the fire was not caused by the microwave sintering of the ceramics, however, but rather by the presence of some carbonized material (or dirt) deposits on the upper wall of the microwave device that had been in use for some time.
Accordingly, it is important to recognize that when performing chemical reactions with microwave radiation, particularly when carbon particulates are used to support metal-based catalysts or carbon residues are present, the potential risks just described can be minimized and considerably lessened by the VFM methodology vis-à-vis FFM microwaves in a VFM-dedicated microwave apparatus.
3. Materials and Methods
3.1. Microwave Single-Mode Setup
The experimental setup used in the synthesis of 4-methylbiphenyl (4MBP) in toluene solvent in the presence of Pd catalyst deposits on activated carbon particulates (Pd/AC) that were introduced in the quartz reactor (internal dia., 8.00 mm; external dia., 10.0 mm; height, 150 mm), and subsequently placed in the single-mode applicator is illustrated in Figure 9. The tip of the reactor was fixed with a guide so that it was always at the same position. The Teflon pipe (diameter, 0.75 mm) was connected to the upper part of the quartz reaction vessel as the condenser; cooling of the Teflon pipe was achieved by air flow. The GaN microwave semiconductor generator used (maximal power output, 180 Watts) was connected to the single-mode applicator equipped with a WR-137 waveguide (Dimension: 34.8488 × 15.7988 mm). This device can irradiate the reactor components either in a set frequency range in the variable frequency microwave (VFM) mode or in a fixed microwave frequency (FFM) mode. The temperature of the reactor was measured with a radiation thermometer through a hole opened in the reverse side of the single mode applicator. Although the temperature observed was the temperature of the quartz reactor vessel, the difference between the internal temperature and the reaction vessel temperature measured in advance displayed a difference of less than 1 °C. Microwave irradiation was performed for 15, 30, and 60 min. The liquid level of the solution in the reaction vessel did not change under these conditions.
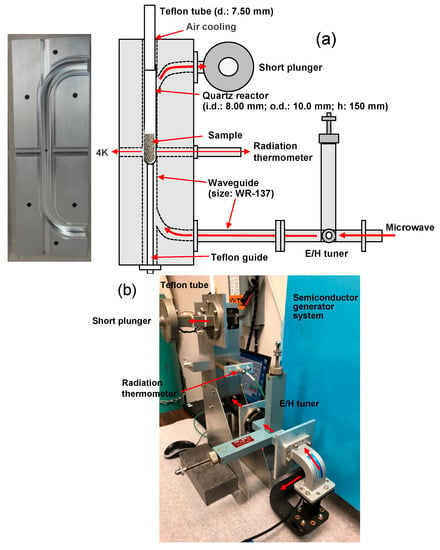
Figure 9.
(a) Schematic illustrating the details of the experimental setup in the variable frequency microwave (VFM) chemical reaction equipment and the quartz reactor within the cavity (inset photo on the left shows the cross-section image of the single-mode applicator); (b) actual photograph of the VFM chemical reaction equipment.
The variable frequency range for the VFM mode spanned 5.85 to 6.65 GHz, whereas in the fixed frequency microwave (FFM) mode, the microwave frequencies were 5.85 or 6.65 GHz. Placing the sample material into this VFM field affects both the uniformity of the field and the power dissipation as per the expression in Equation (1).
Pav = ω εo ε″eff E2rms V
Note that the power dissipation will be correspondingly lower whenever a very small volume of material is used. For instance, the attempt to cure a thin layer of resin on a small metallic object (e.g., a syringe needle) will be impractical because the heat from the energy absorbed on the film will be transferred inductively to the metal and immediately transferred convectively to the air environment. An encountered, albeit unexpected, result was that the larger the volume of the absorbing material was, the higher was the efficiency of heat transfer (Equation (1)). In general, the holding fixtures for samples to introduce into a VFM field should be chosen so that they have low thermal conductivities (poor heat sinks) and possess a high loss modulus (ε″).
The use of a rapidly swept frequency system providing uniform field and temperature also allows the power level to be applied in a closed loop feedback with the measured temperature of the target material. In this way, the power can be adjusted automatically in real time to match the programmed sample temperature. Not only is this a direct method of applying only the needed power to heat the samples to the required temperature, but it also makes the total applied power subject to the total number of samples placed in the field (volume V in Equation (1)). The number of samples in any run does not require manual adjustment of the power.
The applicator is technically of a single mode design, especially when a single fixed frequency is used. However, when we introduce a variable frequency microwave into this (single mode type) applicator, we are sweeping through 4096 individual frequencies in 100 milliseconds, which introduces different modes in different locations throughout the length of the waveguide or applicator every 25 microseconds. The TE10 mode is excited in the waveguide at all frequencies.
3.2. Synthesis of 4-Methylbiphenyl Catalyzed by Pd/AC Particulates
The Pd/AC catalyst was prepared using the following procedure: the activated carbon particles (ACs; 5 g) were washed with ultrapure water and quantities of NaOH (2 M, 50 mL) for 24 h under ambient temperature conditions, after which the ACs were washed once again with ultrapure water and dried for a few hours at 100 °C. The so-washed ACs (1 g) were then introduced into an aqueous PdCl2 (0.034 g) and HCl (1 M) solution (50 mL), following which the solution was brought to pH 14 by the addition of NaOH. Subsequently, NaBH4 (0.016 M) was added to the solution and stirred for 3 h; stirring was continued for an additional 2 h at 60 °C. Finally, the colloidal Pd/AC particles in the solution were filtered, washed with ultrapure water, and then dried at 100 °C overnight. The quantity of Pd on the activated carbon particulates support (diameter, ca. 0.65 mm) was ca. 1.6 wt.% in Pd ascertained by atomic emission spectroscopy using a Shimadzu ICPE-9000 apparatus.
The synthesis of 4-methylbiphenyl by the Suzuki–Miyaura cross-coupling reaction (Figure 2) was carried out so as to compare the results with our earlier study [23]. Note that the chemical yield of 4-methylbiphenyl can be compared with our previous study using FFM at 2.45 GHz. The palladium deposits on activated carbon (Pd/AC) catalyst particulates (0.18 g of which the amount of Pd was 1.6 wt.% or 2.88 mg or 27.1 μmol; mesh size of AC support, 0.95 mm), phenylboronic acid (0.96 mmol; 0.12 g), 1-bromo-4-methylbenzene (0.72 mmol; 0.12 g), K2CO3 as the base (1.4 mmol; 0.20 g), and the toluene/1-hexanol mixed solvent (12 mL; volume ratio, 1:1) were mixed and subsequently added to the cylindrical quartz reactor under an Ar atmosphere. The relative dielectric loss factor (microwave frequency, 5.80 GHz) of a sample solution without the Pd/AC catalyst particles was εr″ = 0.25 at 25 °C (analyzed with a Keysight Technologies HP-85070B Network Analyzer and a Keysight Technologies high temperature probe; Keysight Technologies, Santa Rosa, CA, USA), which by comparison is significantly smaller than that of pure distilled water (εr″ = 20.57 for 5.80 GHz at 25 °C). Hence, the toluene solvent was slightly heated by the microwave radiation. Note that as the frequency increases, the dielectric loss factor of the sample solution will decrease. The main source of heating by the microwaves originated from the dispersed Pd/AC catalyst particles used to carry out the reactions [4].
Reaction yields of 4-methylbiphenyl were determined by gas chromatographic analyses using a Shimadzu model 2014 chromatograph (Shimadzu Corporation, Kyoto, Japan) equipped with a Shimadzu GLC Ultra alloy-1 capillary column (Shimadzu Corporation, Kyoto, Japan; He was the carrier gas; column temperature was changed from 100 to 260 °C at 20 °C min−1). The samples were appropriately prepared from the various dispersions using a 4-methylbiphenyl calibration standard (100% GC standard; Fujifilm Wako Pure Chemical Co., Osaka, Japan). The yields of the by-product biphenyl from the Suzuki–Miyaura homo-coupling process and 4,4′-dimethylbiphenyl from the Ullmann-type coupling were ascertained by gas chromatography.
3.3. Synthesis of Toluene Catalyzed by Pt/AC Particulates
The synthesis of toluene from the dehydrogenation of methylcyclohexane (MCH) was carried out to compare the results with our earlier study [5]. The Pt catalyst supported on an activated carbon support (Fujifilm Wako Chemicals Co. Pt/AC particulates; Pt: 5%; 1.00 g) and methylcyclo- hexane (Fujifilm Wako Chemicals Co. 14.0 mL) were loaded onto a cylindrical quartz reactor under an Ar atmosphere. As a non-polar material, MCH is a poor absorber of microwaves (dielectric loss εr″ = 0.01 at 25 °C), and thus was not directly heated by the microwave radiation. At the completion of the reaction, the Teflon pipe and the container were washed with 15 mL of ethyl acetate to collect any of the sample that adhered to the reaction vessel wall. Reaction yields of the dehydrogenation of MCH (sample in ethyl acetate solution) were determined by gas chromatographic analyses (FID-GC; Shimadzu model 2014; Restek Rtx-5 capillary column; Shimadzu Corporation, Kyoto, Japan; He was the carrier gas; column temperature was varied from 70 to 120 °C at 20 °C min−1) using standard samples of MCH and toluene. The by-products benzene and cyclohexane were also analyzed by the GC system. Yields of the by-products (1,1′-bicyclohexyl, ethylcyclopentane and 1,1-dimethylcyclo- pentane) were negligible as determined from the decrease in the quantity of the organic hydride MCH.
4. Concluding Remarks
This study reports for the first time the application of the variable frequency microwave (VFM) technology in carrying out microwave-assisted catalytic chemistry that precludes the deactivation of the solid catalyst, often a significant problem encountered in microwave chemistry. By suppressing the standing waves, the VFM approach can prevent the formation of arc discharges caused by the interaction between the microwaves and the conductive materials. Using two model reactions, the study has demonstrated significant increases in the chemical yields of products with the VFM approach relative to the yields obtained when using the FFM method. In addition, the usage of VFM microwaves can lead to significant energy savings, and not least eradicate mishaps that can cause undue fire damage to the equipment. Examples of incidents have been described in the usage of fixed frequency microwaves (FFM) in microwave chemistry and how the use of novel microwave irradiation methods can avoid them. Commercial vendors now prohibit the execution of chemical reactions that employ heterogeneous metal-based catalysts in their microwave chemical equipment. Regardless, chemical reactions continue to be carried out in commercial microwave ovens, and inevitably some incidents (such as fire) may at times occur when using conventional fixed frequency microwaves, although the number of incidents is rather limited. Accordingly, the VFM technology should be of particular importance not only in microwave-assisted chemistry but also in an industrial setting, as its usage should prove advantageous in exploiting and advancing industrial- level microwave chemistry.
In this regard, the use of the VFM technology within an industrial context has thus far been intended primarily at processing advanced materials, as it displays some specific advantages that include, among others, [24] (i) uniform heating, (ii) arc-free processing, (iii) significant controllability, (iv) higher coupling efficiency, and not least (v) the ability to choose “specific” frequency or frequency bands suitable for particular applications. The recent (2016) review article by Antonio and coworkers [24] on the usage of the VFM Technology in material processing presented some interesting examples, and it further emphasized that sweeping through a bandwidth of frequencies can lead to a time-averaged heating uniformity, can eliminate heating non-uniformities, and can avoid catastrophic thermal excursions sometimes experienced in the use of conventional FFM microwave technology.
In the food industry, microwave food processing has generally been based on a single frequency microwave source (e.g., 2.45 GHz), but as Bows [25] reported long ago (1999), the usage of variable frequency microwave ovens allows for exploiting the frequency dependence of the foods’ permittivity and/or the choice of heating frequency as a novel route to achieve targeted heating.
Clearly then, the active use of metal-based catalytic particulates in microwave-assisted chemistry requires awareness and acquaintance of innovative technologies to avoid the deleterious arc discharges, to enhance chemical yields of products, and to minimize or altogether prevent possible mishaps. The VFM technology is one such novel approach that should prove advantageous in many respects in microwave chemistry.
Author Contributions
S.H. conceived the study, designed the experiments and wrote the first draft; I.A. designed the single mode VFM; Y.A., I.A., C.D., K.H. and B.S. performed the experiments; N.S. discussed and examined the data’s interpretation with S.H. and contributed to the writing of subsequent drafts and final draft of the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the Japan Society for the Promotion of Science (JSPS) through a Grant-in-aid for Scientific Research (No. 19K22316) to S.H.
Acknowledgments
N.S. is grateful to the staff of the PhotoGreen Laboratory at the University of Pavia (Italy) for their continued hospitality.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Zhang, X.; Hayward, D.O.; Mingos, D.M.P. Apparent equilibrium shifts and hot-spot formation for catalytic reactions induced by microwave dielectric heating. Chem. Commun. 1999. [Google Scholar] [CrossRef]
- Tsukahara, Y.; Higashi, A.; Yamauchi, T.; Nakamura, T.; Yasuda, M.; Baba, A.; Wada, Y. In situ observation of nonequilibrium local heating as an origin of special effect of microwave on chemistry. J. Phys. Chem. C 2010, 114, 8965–8970. [Google Scholar] [CrossRef]
- Gutmann, B.; Schwan, A.M.; Reichart, B.; Gspan, C.; Hofer, F.; Kappe, C.O. Activation and deactivation of a chemical transformation by an electromagnetic field: Evidence for specific microwave effects in the formation of Grignard reagents. Angew. Chem. Int. Ed. 2011, 50, 4636–4640. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, S.; Serpone, N. Role of microwaves in heterogeneous catalytic systems. Catal. Sci. Technol. 2014, 4, 1197–1210. [Google Scholar] [CrossRef]
- Horikoshi, S.; Kamata, M.; Sumi, T.; Serpone, N. Selective heating of Pd/AC catalyst in heterogeneous systems for the microwave assisted continuous hydrogen evolution from organic hydrides: Temperature distribution in the fixed-bed reactor. Int. J. Hydrogen Energy 2016, 41, 12029–12037. [Google Scholar] [CrossRef]
- Horikoshi, S.; Watanabe, T.; Narita, A.; Suzuki, Y.; Serpone, N. The electromagnetic wave energy effect(s) in microwave-assisted organic syntheses (MAOS). Sci. Rep. 2018, 8, 5151. [Google Scholar] [CrossRef] [PubMed]
- Cini, E.; Petricci, E.; Taddei, M. Pd/C Catalysis under Microwave Dielectric Heating. Catalysts 2017, 7, 89. [Google Scholar] [CrossRef]
- Horikoshi, S.; Osawa, A.; Sakamoto, S.; Serpone, N. Control of microwave-generated hot spots. Part V. Mechanisms of hot-spot generation and aggregation of catalyst in a microwave-assisted reaction in toluene catalyzed by Pd-loaded AC particulates. App. Catal. A Gen. 2013, 460–461, 52–60. [Google Scholar] [CrossRef]
- Petricci, E.; Risi, C.; Ferlin, F.; Lanari, D.; Vaccaro, L. Avoiding hot-spots in microwave assisted Pd/C catalysed reactions by using the biomass derived solvent γ-Valerolactone. Sci. Rep. 2018, 8, 10571. [Google Scholar] [CrossRef]
- Wang, W.; Wang, B.; Sun, J.; Mao, Y.; Zhao, Z.; Song, Z. Numerical simulation of hot-spot effects in microwave heating due to the existence of strong microwave-absorbing media. RSC Adv. 2016, 6, 52974–52981. [Google Scholar] [CrossRef]
- Khattaka, H.K.; Bianuccib, P.; Slepkova, A.D. Linking plasma formation in grapes to microwave resonances of aqueous dimers. Proc. Natl. Acad. Sci. USA 2019, 116, 4000–4005. [Google Scholar] [CrossRef] [PubMed]
- Bible, D.W.; Lauf, R.J. Variable Frequency Microwave Furnace System. US Patent applied for on 14 November 1991. US Patent No. US5321222A, 14 June 1994. [Google Scholar]
- Lauf, R.; Bible, D.W.; Johnson, A.C.; Everleigh, C. 2 to 8 GHz broadband microwave heating systems. Microw. J. 1993, 36, 34. [Google Scholar]
- Cauchois, R.; Saadaoui, M.; Yakoub, A.; Inal, K.; Dubois-Bonvalot, B.; Fidalgo, J.-C. Impact of variable frequency microwave and rapid thermal sintering on microstructure of inkjet-printed silver nanoparticles. J. Mater. Sci. 2012, 47, 7110–7116. [Google Scholar] [CrossRef]
- Atwater, J.E.; Wheeler, R.R. Microwave permittivity and dielectric relaxation of a high surface area activated carbon. Appl. Phys. 2004, 79, 125–129. [Google Scholar] [CrossRef]
- Kim, T.; Jaegaeun, L.; Lee, K.-H. Microwave heating of carbon-based solid materials. Carbon Lett. 2014, 15, 15–24. [Google Scholar] [CrossRef]
- Salih, K.S.M.; Baqi, Y. Microwave-assisted palladium-catalyzed cross-coupling reactions: Generation of carbon-carbon bond. Catalysts 2020, 10, 4. [Google Scholar] [CrossRef]
- Cravotto, G.; Beggiato, M.; Penoni, A.; Palmisano, G.; Tollari, S.; Lévêques, J.-M.; Bonrath, W. High- intensity ultrasound and microwave, alone or combined, promote Pd/C-catalyzed aryl–aryl couplings. Tetrahedron Lett. 2005, 6, 2267–2271. [Google Scholar] [CrossRef]
- International Chemical Safety Cards. Available online: http://www.nihs.go.jp/ICSC/ (accessed on 10 July 2020).
- Thermal Radiation. Available online: http://en.wikipedia.org/wiki/Thermal_radiation (accessed on 10 July 2020).
- Guisbiers, G.; Abudukelimu, G.; Hourlier, D. Size-dependent catalytic and melting properties of platinum-palladium nanoparticles. Nanoscale Res. Lett. 2011, 6, 396. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, J.A.; Juárez-Pérez, E.J.; Ruisánchez, E.; Bermúdez, J.M.; Arenillas, A. Ball lightning plasma and plasma arc formation during the microwave heating of carbons. Carbon 2011, 49, 346–349. [Google Scholar]
- Horikoshi, S.; Kamata, M.; Mitani, T.; Serpone, N. Control of microwave-generated hot spots. 6. Generation of hot spots in dispersed catalyst particulates and factors that affect catalyzed organic syntheses in heterogeneous media. Ind. Eng. Chem. Res. 2014, 53, 14941–14947. [Google Scholar] [CrossRef]
- Antonio, C.; Deam, R.; Taube, A. A Review of the variable frequency microwave technology in material processing. J. Microw. Power Electromagn. Energy 2003, 38, 75–87. [Google Scholar] [CrossRef]
- Bows, J.R. Variable frequency microwave heating of food. J. Microw. Power Electromagn. Energy 1999, 34, 227–238. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).