Ordered Mesoporous Carbon as a Support for Palladium-Based Hydrodechlorination Catalysts
Abstract
1. Introduction
2. Results and Discussion
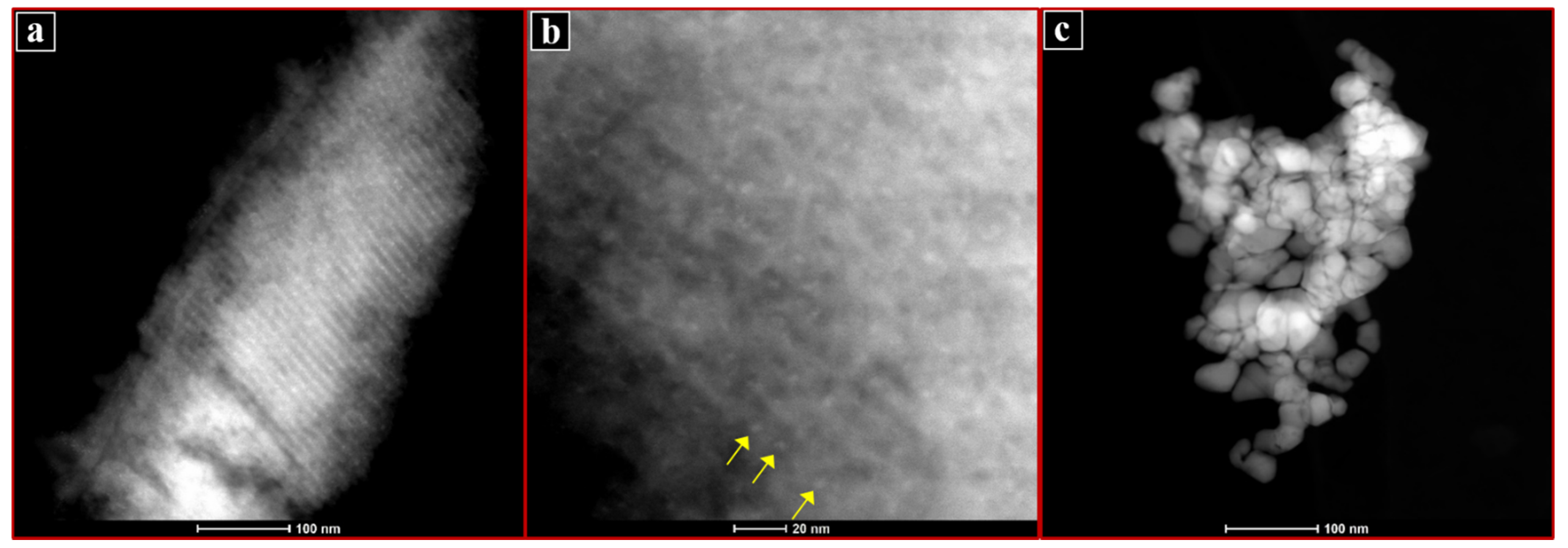
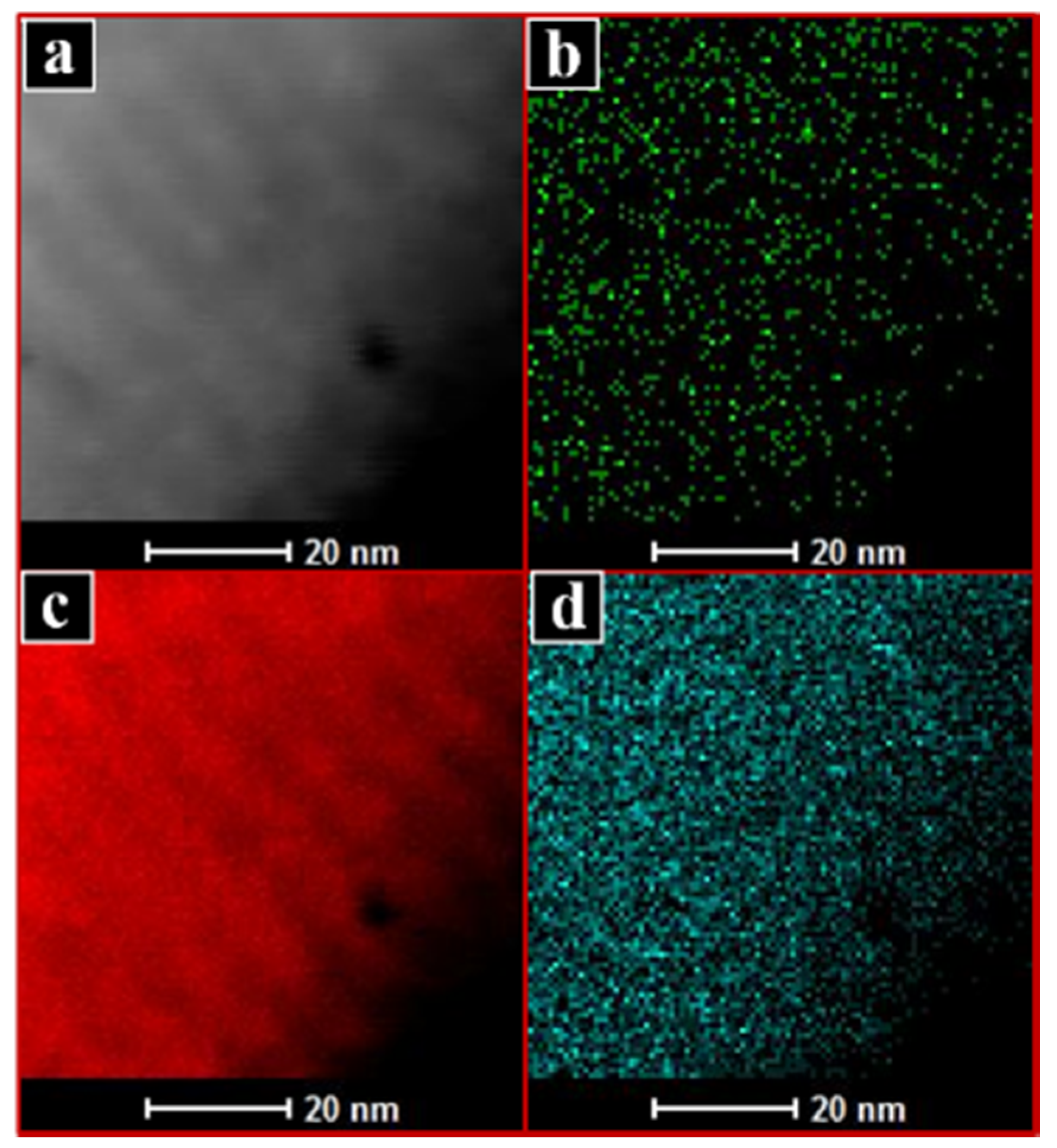
2.1. Characterisation of Catalysts
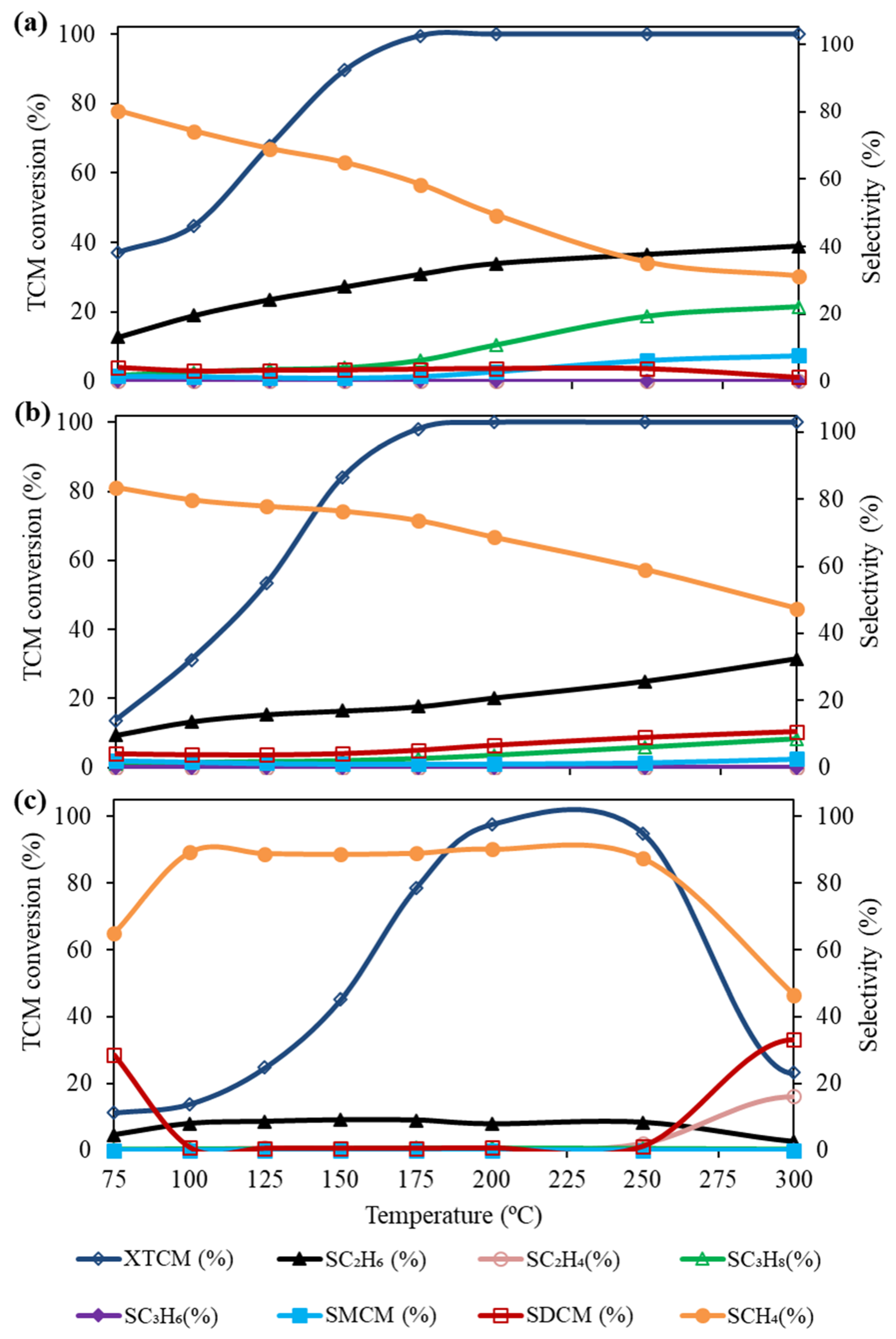
2.2. Catalytic Activity
3. Experimental Section
3.1. Catalysts Preparation
3.2. Catalyst Characterisation
3.3. Catalytic Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bedia, J.; Gómez-Sainero, L.M.; Grau, J.M.; Busto, M.; Martin-Martinez, M.; Rodriguez, J.J. Hydrodechlorination of dichloromethane with mono- and bimetallic Pd–Pt on sulfated and tungstated zirconia catalysts. J. Catal. 2012, 294, 207–215. [Google Scholar] [CrossRef]
- Lee, S.R.; Cho, J.M.; Son, M.; Park, M.J.; Kim, W.Y.; Kim, S.Y.; Bae, J.W. Selective hydrodechlorination of trichloromethane to dichloromethane over bimetallic Pt-Pd/KIT-6: Catalytic activity and reaction kinetics. Chem. Eng. J. 2018, 331, 556–569. [Google Scholar] [CrossRef]
- Dobrzyńska, E.; Pośniak, M.; Szewczyńska, M.; Buszewski, B. Chlorinated Volatile Organic Compounds—Old, However, Actual Analytical and Toxicological Problem. Crit. Rev. Anal. Chem. 2010, 40, 41–57. [Google Scholar] [CrossRef]
- Álvarez-Montero, M.A.; Gómez-Sainero, L.M.; Martín-Martínez, M.; Heras, F.; Rodriguez, J.J. Hydrodechlorination of chloromethanes with Pd on activated carbon catalysts for the treatment of residual gas streams. Appl. Catal. B Environ. 2010, 96, 148–156. [Google Scholar] [CrossRef]
- De Pedro, Z.M.; Casas, J.A.; Gomez-Sainero, L.M.; Rodriguez, J.J. Hydrodechlorination of dichloromethane with a Pd/AC catalyst: Reaction pathway and kinetics. Appl. Catal. B Environ. 2010, 98, 79–85. [Google Scholar] [CrossRef]
- Martin-Martinez, M.; Gómez-Sainero, L.M.; Alvarez-Montero, M.A.; Bedia, J.; Rodriguez, J.J. Comparison of different precious metals in activated carbon-supported catalysts for the gas-phase hydrodechlorination of chloromethanes. Appl. Catal. B Environ. 2013, 132–133, 256–265. [Google Scholar] [CrossRef]
- Álvarez-Montero, M.A.; Gómez-Sainero, L.M.; Mayoral, A.; Diaz, I.; Baker, R.T.; Rodriguez, J.J. Hydrodechlorination of chloromethanes with a highly stable Pt on activated carbon catalyst. J. Catal. 2011, 279, 389–396. [Google Scholar] [CrossRef]
- Ordoñez, S.; Díez, F.V.; Sastre, H. Minimization of the deactivation of palladium catalysts in the hydrodechlorination of trichloroethylene in wastewaters. Appl. Catal. B Environ. 2001, 31, 113–122. [Google Scholar] [CrossRef]
- Coq, B.; Cognion, J.M.; Figueras, F.; Tournigant, D. Conversion under hydrogen of dichlorodifluoromethane over supported palladium catalysts. J. Catal. 1993, 141, 21–33. [Google Scholar] [CrossRef]
- Moon, D.J.; Chung, M.J.; Park, K.Y.; Hong, S.I. Deactivation of Pd Catalyst in the Hydrodechlorination of Chloropentafluoroethane. Appl. Catal. A 1988, 168, 159–170. [Google Scholar] [CrossRef]
- Kim, S.Y.; Choi, H.C.; Yang, O.B.; Lee, K.H.; Lee, J.S.; Kim, Y.G. Hydrodechlorination of tetrachloromethane over supported Pt catalysts. Chem. Commun. 1995, 21, 2169–2170. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, Z.; Wan, H.; Wan, Y.; Chen, H.; Zheng, S.; Zhu, D. Enhanced liquid phase catalytic hydrodechlorination of 2,4-dichlorophenol over mesoporous carbon supported Pd catalysts. Catal. Commun. 2011, 12, 1405–1409. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Jun, S. Synthesis of Highly Ordered Carbon Molecular Sieves via Template-Mediated Structural Transformation. J. Phys. Chem. B 1999, 103, 7743–7746. [Google Scholar] [CrossRef]
- Wang, X.; Liang, C.; Dai, S. Facile Synthesis of Ordered Mesoporous Carbons with High Thermal Stability by Self-Assembly of Resorcinol−Formaldehyde and Block Copolymers under Highly Acidic Conditions. Langmuir 2008, 24, 7500–7505. [Google Scholar] [CrossRef]
- Meng, Y.; Gu, D.; Zhang, F.; Shi, Y.; Cheng, L.; Feng, D.; Wu, Z.; Chen, Z.; Wan, Y.; Stein, A. A Family of Highly Ordered Mesoporous Polymer Resin and Carbon Structures from Organic−Organic Self-Assembly. Chem. Mater. 2006, 18, 4447–4464. [Google Scholar] [CrossRef]
- Zhang, F.; Meng, Y.; Gu, D.; Chen, Z.; Tu, B.; Zhao, D. An Aqueous Cooperative Assembly Route to Synthesize Ordered Mesoporous Carbons with Controlled Structures and Morphology. Chem. Mater. 2006, 18, 5279–5288. [Google Scholar] [CrossRef]
- Xu, J.; Wang, A.; Zhang, T. A two-step synthesis of ordered mesoporous resorcinol–formaldehyde polymer and carbon. Carbon 2012, 50, 1807–1816. [Google Scholar] [CrossRef]
- Sakina, F.; Baker, R.T. Metal- and halogen-free synthesis of ordered mesoporous carbon materials. Microporous Mesoporous Mater. 2019, 289, 1096222. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D.; Chastain, J.; King, R.C., Jr. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics Inc.: Eden Prairie, MN, USA, 1995. [Google Scholar]
- Fernandez-Ruiz, C.; Bedia, J.; Bonal, P.; Rodriguez, J.J.; Gómez-Sainero, L.M. Chloroform conversion into ethane and propane by catalytic hydrodechlorination with Pd supported on activated carbons from lignin. Catal. Sci. Technol. 2018, 8, 3926–3935. [Google Scholar] [CrossRef]
- Gómez-Sainero, L.M.; Palomar, J.; Omar, S.; Fernández, C.; Bedia, J.; Álvarez-Montero, A.; Rodriguez, J.J. Valorization of chloromethanes by hydrodechlorination with metallic catalysts. Catal. Today 2018, 310, 75–85. [Google Scholar] [CrossRef]
- Martin-Martinez, M.; Gómez-Sainero, L.M.; Bedia, J.; Arevalo-Bastante, A.; Rodriguez, J.J. Enhanced activity of carbon-supported Pd–Pt catalysts in the hydrodechlorination of dichloromethane. Appl. Catal. B Environ. 2016, 184, 55–63. [Google Scholar] [CrossRef]
- Xu, L.; Bhandari, S.; Chen, J.; Glasgow, J.; Mavrikakis, M.T. Chloroform Hydrodechlorination on Palladium Surfaces: A Comparative DFT Study on Pd(111), Pd(100), and Pd(211). Top. Catal. 2020, 63, 762–776. [Google Scholar] [CrossRef]
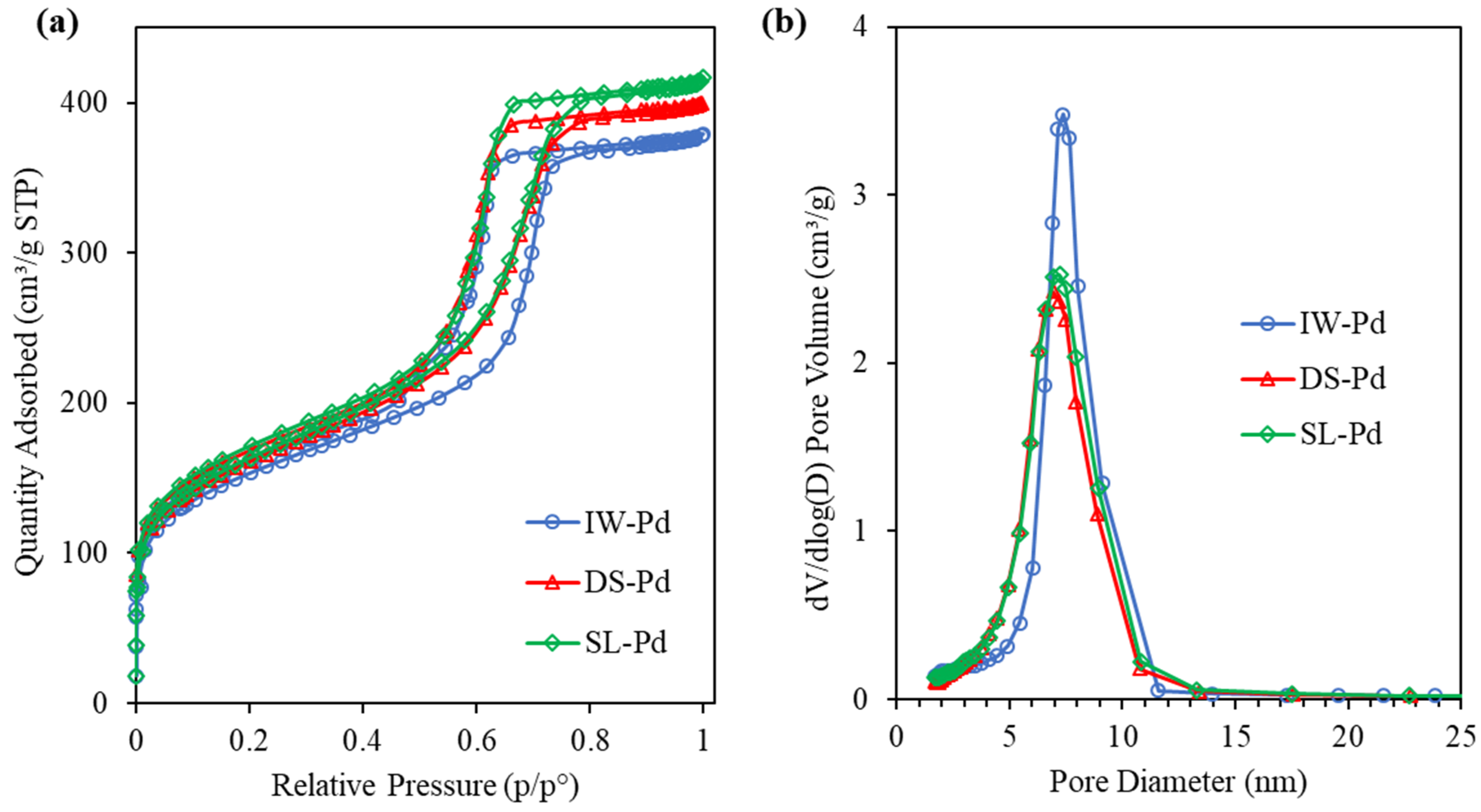
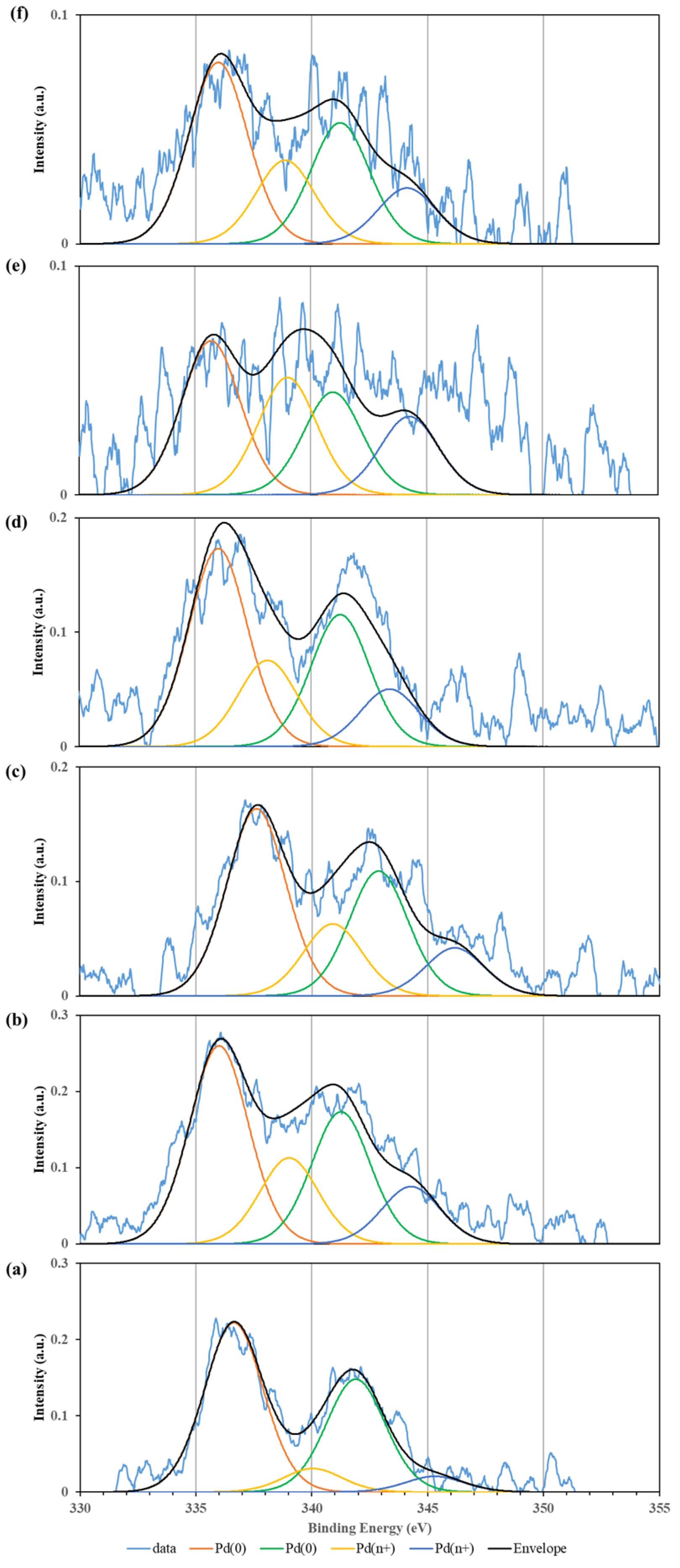
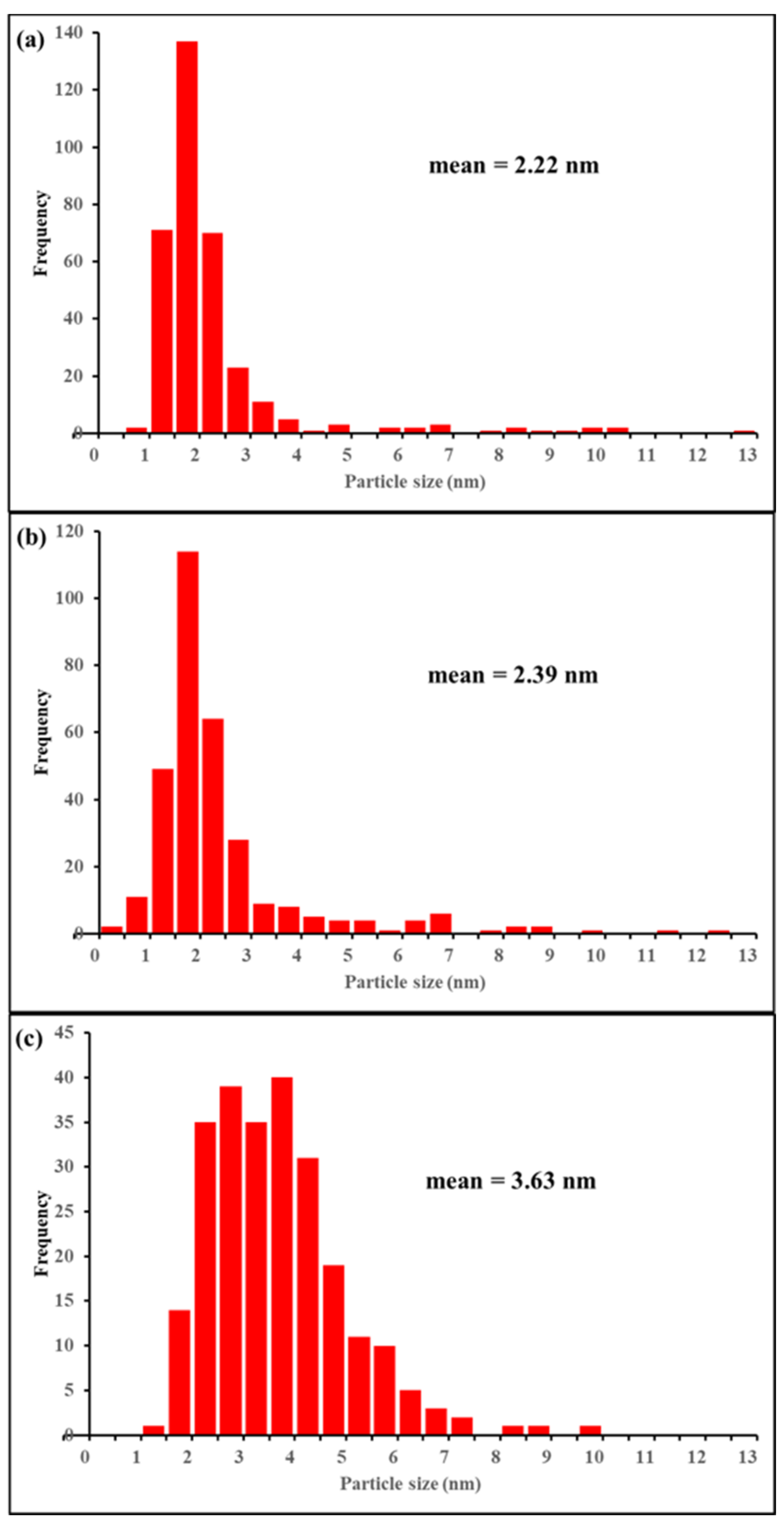


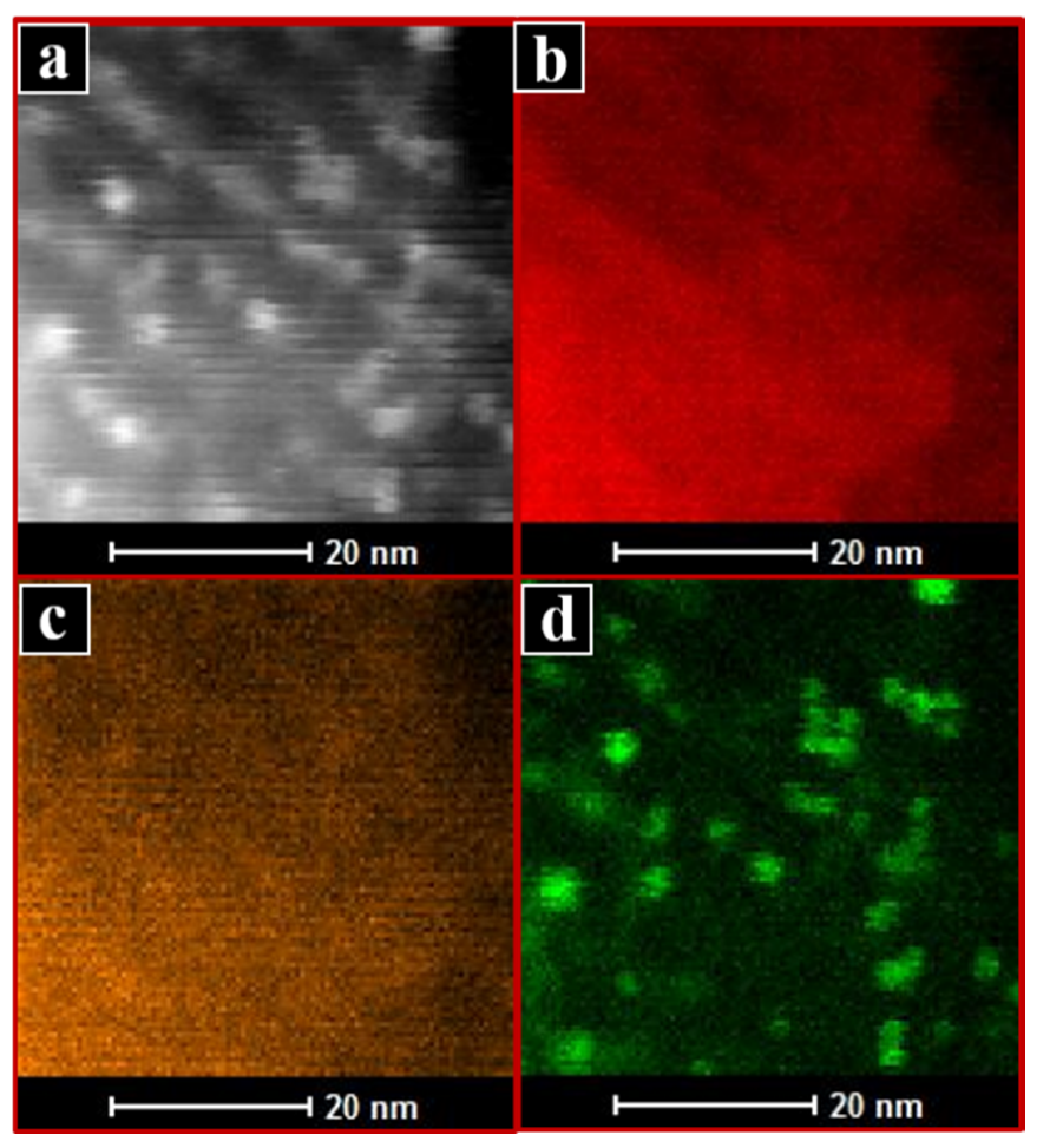
| Sample ID | SSA (m2/g) | VP (cm3/g) | Vmicro (cm3/g) | Dp(nm) |
|---|---|---|---|---|
| IW-Pd | 523 | 0.54 | 0.037 | 7.4 |
| DS-Pd | 551 | 0.58 | 0.040 | 7.0 |
| SL-Pd | 560 | 0.61 | 0.032 | 7.1 |
| % | IW-Pd Fresh | IW-Pd TCM | DS-Pd Fresh | DS-Pd TCM | SL-Pd Fresh | SL-Pd TCM |
|---|---|---|---|---|---|---|
| C (1s) | 77.54 | 77.64 | 78.2 | 78.28 | 79.25 | 78.73 |
| O (1s) | 20.03 | 19.8 | 19.35 | 19.41 | 19.19 | 20.23 |
| Pd (3d) | 2.43 | 2.56 | 2.44 | 2.31 | 1.56 | 1.04 |
| Pd0 | 87.8 | 69.71 | 72.22 | 69.65 | 56.74 | 68.45 |
| Pdn+ | 12.2 | 30.29 | 27.79 | 30.35 | 43.26 | 31.54 |
| Reaction Temperature (°C) | SCH₄ (%) | SC2s | SC2+ (%) | SC1+ (%) | SCMs (%) |
|---|---|---|---|---|---|
| 75 | 80.3 | 12.8 | 1.6 | 14.4 | 5.4 |
| 100 | 74.2 | 19.0 | 2.6 | 21.6 | 4.2 |
| 125 | 69.0 | 23.6 | 3.2 | 26.8 | 4.2 |
| 150 | 64.9 | 27.2 | 3.8 | 31.0 | 4.2 |
| 175 | 58.3 | 30.9 | 5.9 | 36.8 | 4.9 |
| 200 | 49.3 | 33.9 | 10.4 | 44.3 | 6.4 |
| 250 | 35.2 | 36.6 | 18.6 | 55.2 | 9.6 |
| 300 | 31.2 | 38.9 | 21.4 | 60.3 | 8.5 |
| Reaction Temperature (°C) | SCH₄ (%) | SC2s | SC2+ (%) | SC1+ (%) | SCMs (%) |
|---|---|---|---|---|---|
| 75 | 83.6 | 9.4 | 1.0 | 10.4 | 6.1 |
| 100 | 79.8 | 13.3 | 1.5 | 14.8 | 5.4 |
| 125 | 77.9 | 15.4 | 1.8 | 17.2 | 5.0 |
| 150 | 76.4 | 16.4 | 2.0 | 18.4 | 5.2 |
| 175 | 73.6 | 17.6 | 2.6 | 20.2 | 6.1 |
| 200 | 68.6 | 20.1 | 3.6 | 23.7 | 7.6 |
| 250 | 59.0 | 25.0 | 5.9 | 30.8 | 10.2 |
| 300 | 47.4 | 31.4 | 8.3 | 39.8 | 12.8 |
| Reaction Temperature (°C) | SCH₄ (%) | SC2s | SC2+ (%) | SC1+ (%) | SCMs (%) |
|---|---|---|---|---|---|
| 75 | 64.9 | 4.6 | 1.9 | 6.5 | 28.6 |
| 100 | 89.2 | 8.2 | 1.7 | 10.0 | 0.9 |
| 125 | 88.8 | 9.3 | 1.3 | 10.6 | 0.6 |
| 150 | 88.6 | 9.6 | 1.2 | 10.8 | 0.6 |
| 175 | 88.9 | 9.1 | 1.3 | 10.4 | 0.6 |
| 200 | 90.1 | 7.8 | 1.3 | 9.1 | 0.7 |
| 250 | 87.4 | 10.4 | 1.0 | 11.4 | 1.2 |
| 300 | 46.3 | 18.7 | 1.9 | 20.5 | 33.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakina, F.; Fernandez-Ruiz, C.; Bedia, J.; Gomez-Sainero, L.; Baker, R.T. Ordered Mesoporous Carbon as a Support for Palladium-Based Hydrodechlorination Catalysts. Catalysts 2021, 11, 23. https://doi.org/10.3390/catal11010023
Sakina F, Fernandez-Ruiz C, Bedia J, Gomez-Sainero L, Baker RT. Ordered Mesoporous Carbon as a Support for Palladium-Based Hydrodechlorination Catalysts. Catalysts. 2021; 11(1):23. https://doi.org/10.3390/catal11010023
Chicago/Turabian StyleSakina, Farzeen, Carlos Fernandez-Ruiz, Jorge Bedia, Luisa Gomez-Sainero, and Richard T. Baker. 2021. "Ordered Mesoporous Carbon as a Support for Palladium-Based Hydrodechlorination Catalysts" Catalysts 11, no. 1: 23. https://doi.org/10.3390/catal11010023
APA StyleSakina, F., Fernandez-Ruiz, C., Bedia, J., Gomez-Sainero, L., & Baker, R. T. (2021). Ordered Mesoporous Carbon as a Support for Palladium-Based Hydrodechlorination Catalysts. Catalysts, 11(1), 23. https://doi.org/10.3390/catal11010023







