2.1. Catalyst Characterization
The commercial porous silica support of Ni catalysts for comparing the catalytic efficiency with silica fiber was CARiACT Q10 in the bead form (particle size = 1.18–2.36 mm; bulk density of 0.41 g cm
−3), obtained from Fuji Silysia Chemical Co., Ltd. (Kasugai Aichi, Japan). CARiACT Q10 in the powder form was used as the support of palladium (Pd) for the partial hydrogenation of biodiesel derived from rapeseed oil reported by Numwong et al. [
9]. They found that the Pd/Q10 catalyst had the appropriate pore size for the pore diffusion of C18:2 and facilitates hydrogenation to get the monounsaturated C18:1 easily. This was suitable for palm oil-derived FAME containing a high fraction of C18:2 with a very small amount of C18:3 composition.
In the case of Ni/SF catalysts, the silica fiber was synthesized via a sol–gel electrospinning technique using tetraethylorthosilicate (TEOS) as a silica precursor. The obtained silica fiber had a bulk density of ca. 0.08 g cm
−3 with a diameter of 320 ± 80 nm. The textural properties, in terms of the surface area, pore volume and pore diameter, of both the porous silica ball and silica fiber supports were measured by the N
2 adsorption–desorption technique, as shown in
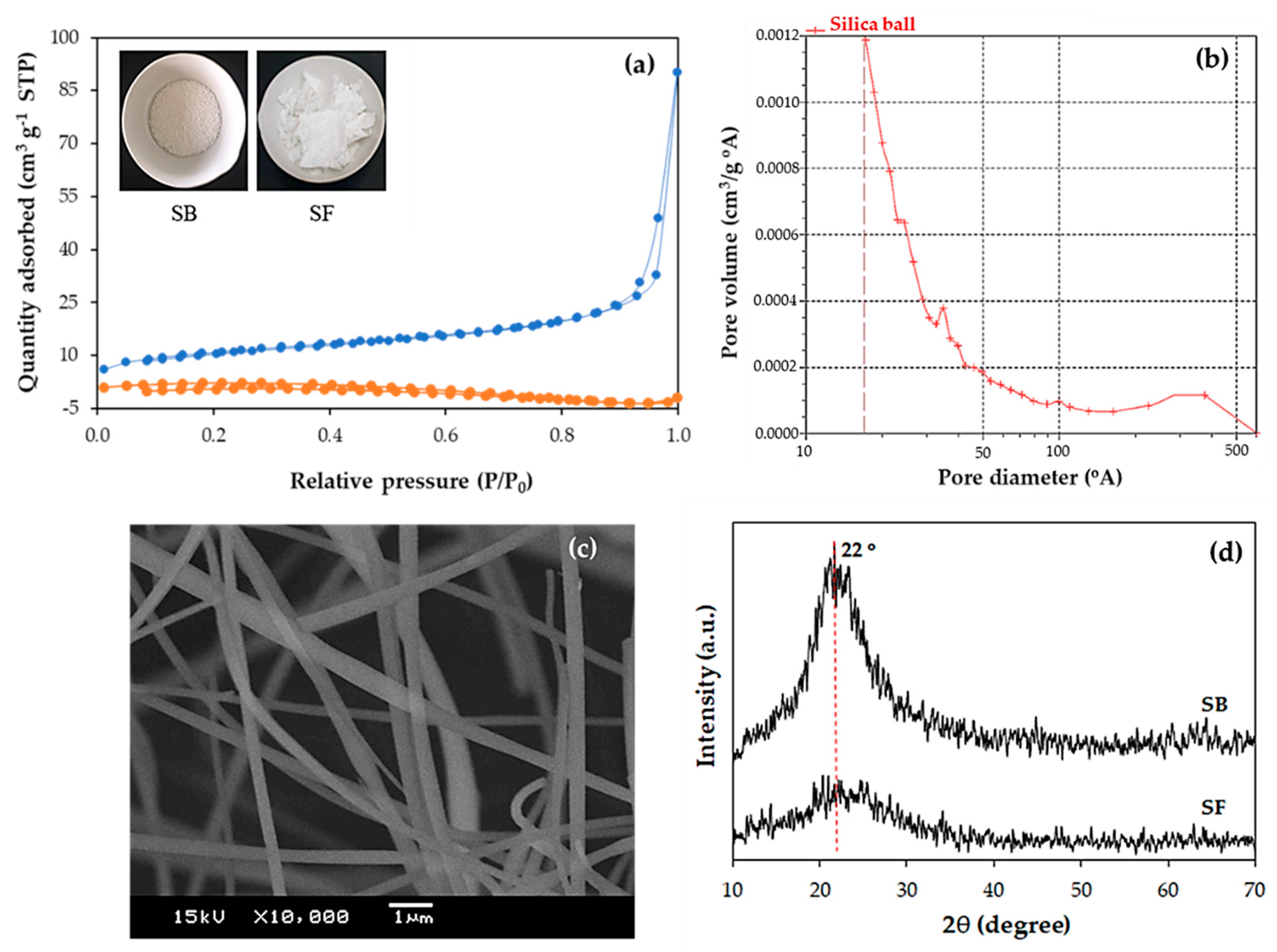
Figure 1a and summarized in
Table 1. It was found that the BET surface area of the silica ball was 38.1 m
2 g
−1, with a 0.14 cm
3 g
−1 pore volume and 14.6 nm average pore diameter. Since the commercial silica used in this research was in the bead form, the pore size distribution shown in
Figure 1b indicated the large pore size in the range of 20–40 nm due to the effect of interparticle voids between the silica beads. The obtained average pore size at 14.6 nm of the commercial silica ball was sufficient for the diffusion of FAME into the pores (the size of the FAME molecules was estimated from the size of methyl oleate as having ~2.5 nm for C–C segments and ~0.5 nm for the cross-section [
13]). Regarding the silica fiber (
Figure 1c), the BET surface area of the silica fiber was only 7.10 m
2 g
−1. Moreover, the hysteresis loop of the N
2 adsorption–desorption isotherm of the silica fiber was not observed (
Figure 1a), which reflects the characteristics of non-porous materials [
8]. To evaluate the phase of silica,
Figure 1d shows the broad XRD peak at 2θ of 22° corresponded to the (101) plane of the amorphous silica for both the silica ball and silica fiber supports [
8,
14]. However, the peak intensity of the silica fiber was significantly lower than that of the silica ball, implying that the silica fiber had lower crystallinity. This was possibly due to the low calcination temperature (500 °C) for preparing the silica fiber. This phenomenon was also observed by Mitra et al. [
14], and they explained that the calcination temperature affected the preferential growth of the silica crystallites. For the acidity of the silica supports,
Table 1 shows that the silica fiber had higher acidity (0.587 mmol NH
3/g) than the silica ball (0.162 mmol NH
3/g). This was also a function of the preparation conditions [
9].
For the Ni-based catalysts, the amount of Ni loading was controlled at 10–50 wt%, and the actual Ni content for each catalyst, as confirmed using ICP-OES analysis, was in good agreement (
Table 2). It was observed that the surface area of the Ni/SB catalyst significantly decreased when the Ni loading was increased due to the partial blockage of the mesopores in the silica ball. To confirm the results from the N
2 adsorption–desorption, the XRD analyses and SEM images of the calcined Ni-based catalysts are shown in
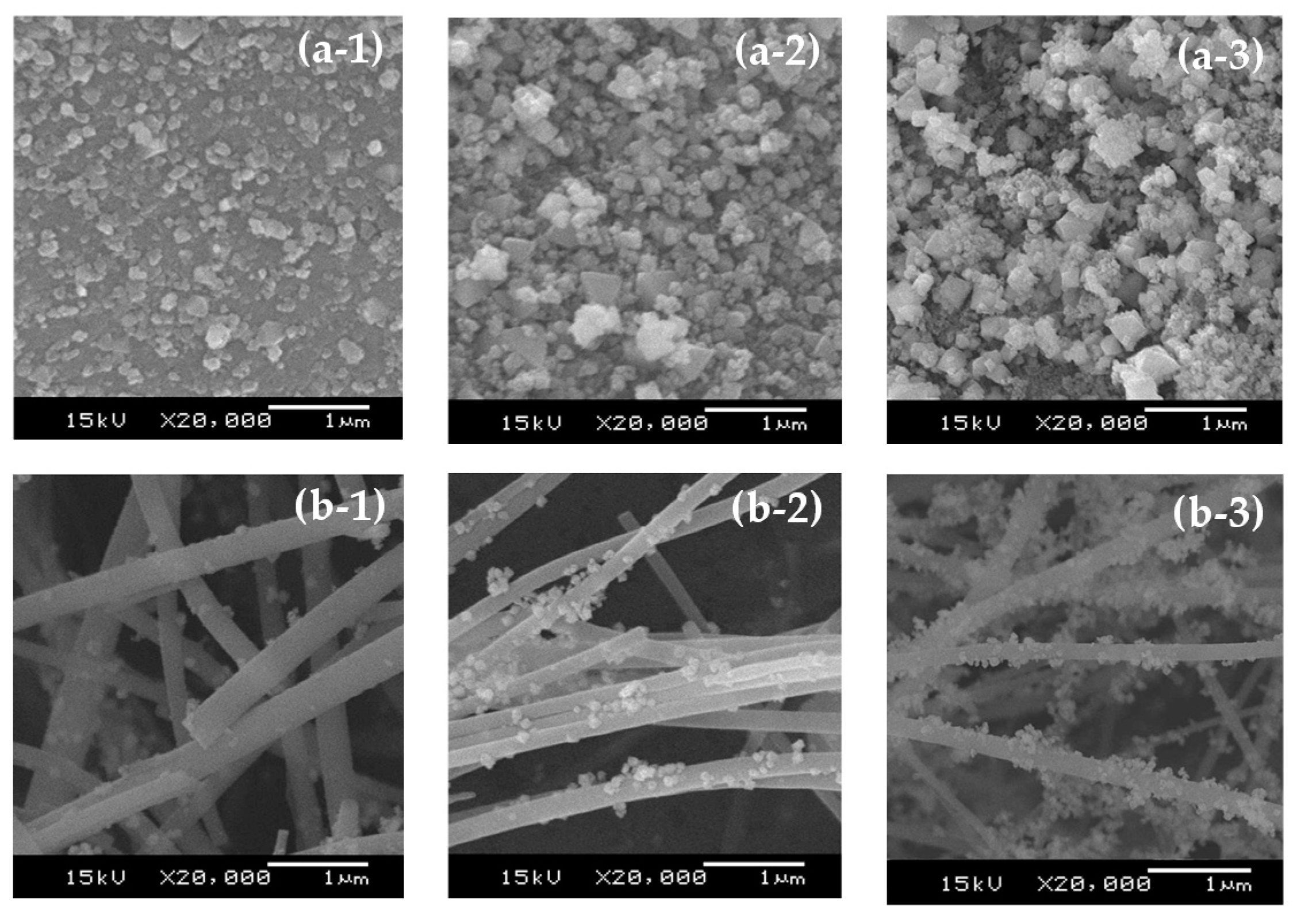
Figure 2 and
Figure 3, respectively. Regarding the XRD patterns, the cubic structures of the NiO phase in both calcined Ni/SB and Ni/SF catalysts are observed at 2θ of 37.3°, 43.3°, 62.9°, 75.4° and 79.4°, attributed to the (111), (200), (220), (311) and (222) planes, respectively [
8]. Although the XRD pattern of the NiO phase was not affected by the shape of the silica support, the NiO crystallite size was dependent on the form of the silica.
Table 2 indicated that the increase in the amount of Ni loading from 10–50 wt% for Ni/SB catalysts significantly enlarged the NiO crystallite size from 21.8 to 36.6 nm. It is possible that the porous characteristics of the silica ball could retard the diffusion of the Ni precursor solution, resulting in the agglomeration and formation of larger NiO particles on the outer surface of the silica ball, as seen in the SEM images (
Figure 3(a-1–a-3)). This phenomenon, in general, has been previously observed for porous Ni/SiO
2 catalysts prepared via impregnation using a high concentration of Ni aqueous solutions [
15].
To consider the Ni/SF catalysts, the NiO particles deposited on the surface of the silica fiber induced the higher BET surface area from 7.10 to 10–12 m
2 g
−1. Although the pore volume and average pore diameter of Ni/SF catalysts could be calculated from the Barrett–Joyner–Halenda (BJH) desorption data, the fluctuate pore diameters with very small pore volume in the range of 0.01–0.03 cm
3 g
−1 were observed. These values were generated from the overlap of the fibers (
Figure 3(b-1,b-2)) [
8]. To detect the NiO crystallite size, this value slightly increased with increasing Ni loading levels, staying in the range of 21–24 nm. The clusters of agglomerated NiO particles were rarely observed, as shown in
Figure 3(b-1–b-3). These results suggested that the silica fiber prevented the agglomeration of Ni and could thus be applied as a support in the preparation of catalysts requiring a high loading level of the active metals.
The reduction temperature of the calcined Ni catalysts was investigated using the H
2-TPR technique as depicted in
Figure 4. The reducibility of the Ni(II)O phase in all catalysts was also calculated by comparison of the total H
2 consumption detected by the H
2-TPR analysis and the theoretic value obtained from Ni(II)O + H
2 → Ni(0) + H
2O, as summarized in
Table 2. From
Figure 4, the H
2-TPR profiles indicated that the maximum peak of H
2 consumption for the reduction in NiO in the Ni/SB catalysts increased from 435 to 466 °C as the amount of NiO loading increased from 10 wt% to 40 wt% (
Figure 4a–d). The reduction temperature lower than 578 °C indicated the weak interaction between NiO particles and the support [
16]. The traditional impregnation on the porous support could also easily induce the uncontrolled size and dispersion of the metal on the surface of the supports [
17]. The larger size of NiO particles might block the pores of catalysts resulting in the difficulty of H
2 diffusion into the pores of catalyst. The higher reduction temperature was thus required to activate the NiO phase as the Ni active form. Although the H
2 consumption of Ni/SB catalysts increased from 1.10 to 2.07 mmol g
cat−1 with increasing the NiO content, it was not proportional to the level of Ni loading, resulting in the lower degree of reducibility from 63.3% to 30.9%. In the case of the 50 wt% Ni/SB catalyst, the results in
Table 2 showed that the NiO crystallite size of the 50 wt% Ni/SB catalyst was 36.6 nm reflecting the weak interaction between the bulk NiO particles and silica ball [
15]. It is possible that the precursor solution with a high Ni concentration hardly diffused into the pores of the support, resulting in the agglomeration of some portion of the NiO particles on the outer surface of the silica ball, which made their reduction easier at a lower temperature of 446 °C (
Figure 4e). This phenomenon also provided the lowest H
2 consumption (1.66 mmol g
cat−1) and reducibility (22.6%). This explanation was also confirmed by the CO pulse chemisorption analysis. The results in
Table 2 indicated that the Ni dispersion of Ni/SB catalysts was in the range of 1.3–3.4%, which depended on the amount of Ni loading and was slightly lower than that of Ni/SF catalysts (Ni dispersion = 1.9–3.4%). This implied that some of the Ni particles were agglomerated and mostly deposited on the outer surface of the silica ball at high Ni content.
Providing a comparison of the Ni/SF catalysts at similar Ni loading levels,
Figure 4f–j shows that the maximum peak in the H
2-TPR profiles for the calcined Ni/SF catalyst appeared in the range of 373–438 °C for 10–50 wt% Ni content, with a higher level of H
2 consumption indicating a higher reduction in NiO particles [
18]. It was also observed that the degree of reducibility was in the range of 66.7–76.5% (
Table 1). The lower reduction temperature of the NiO phase in the Ni/SF catalysts compared to that of the porous catalysts was attributed from the open morphology of the NiO particles deposited on the surface of the silica fiber, which could provide a higher opportunity for a direct reaction with the reducing H
2. Moreover, the weaker interaction between NiO particles and the silica fiber surface would facilitate the reduction of NiO to a Ni active phase [
19].
2.2. Comparative Performance of the Ni/SF and Ni/SB Catalysts for the Partial Hydrogenation of FAME
To compare the catalytic performance of the Ni/SB and Ni/SF catalysts, the partial hydrogenation of palm oil-derived FAME was performed in a fixed-bed reactor after the in situ reduction of catalysts in the presence of H2 at 450 °C for 2 h. The central condition for the partial hydrogenation of FAME was conducted under 1 bar H2 pressure at 140 °C with the feed flow rate of H2 and FAME streams at 100 mL min−1 and 0.44 g min−1, respectively. The volume of the catalyst bed was controlled at ca. 5 cm3. According to the different bulk densities of the silica supports, the amount of applied Ni-based catalysts was kept constant at 1.97 and 0.40 g for the Ni/SB and Ni/SF catalysts, respectively.
The obtained liquid product was collected for 4 h and then sampled to analyze the C18 compositions, i.e., methyl stearate (C18:0),
cis-methyl oleate (
cis-C18:1),
trans-methyl oleate (
trans-C18:1), methyl linoleate (C18:2) and methyl linolenate (C18:3), using gas chromatography equipped with a flame ionization detector (GC-FID).
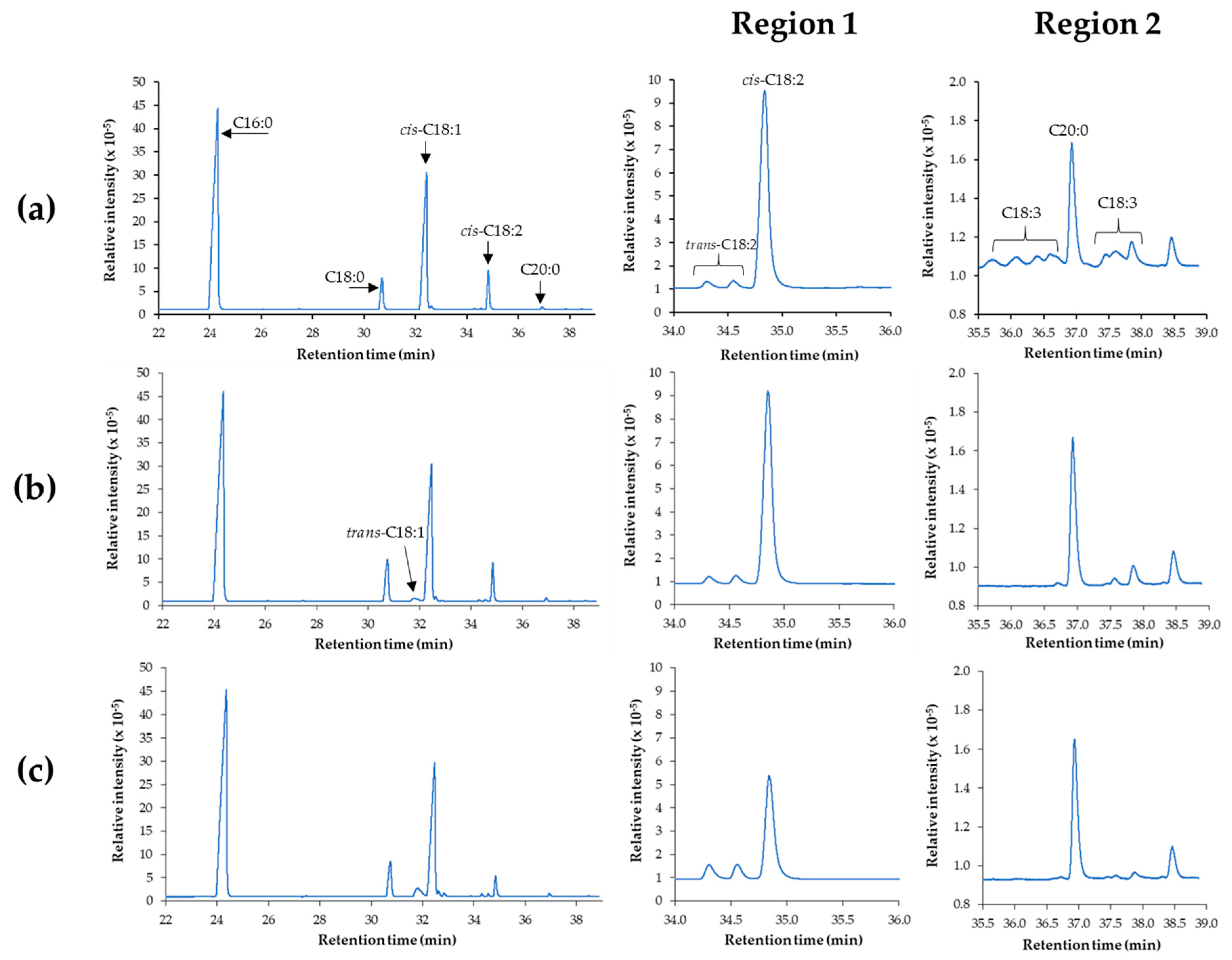
Figure 5a shows a representative GC chromatogram of the FAME, which indicated that it contained a high fraction of methyl palmitate (C16:0; 43.9 mol%) and
cis-C18:1 (40.8 mol%) with smaller amounts of C18:0 (4.4 mol%), C18:2 (9 mol%) and C18:3 (0.4 mol%). Moreover, it was observed that the peak of C20:0 was eluted before some peaks of C18:3 due to the effect of the high polarity of the HP-88 column. This result was consistent with the previous literature that used CP-Sil 88 or SILAR columns [
20,
21]. Chen et al. [
17] reported that the peaks of C18:3 appeared before the peak of C20:0 was in the form of a
trans-structure, while C18:3 eluted after C20:0 contained both
trans- and
cis-configurations. Although the amounts of C18:2 and C18:3 were not large in this palm oil-derived FAME, they had to be minimized due to their fast oxidation rate [
22]. The oxidation rate of linolenic acid containing three C=C bonds was reported to be 2500- and 25-fold greater than those for stearic and oleic acids, respectively [
23,
24]. After partial hydrogenation, the intensity of the
cis-C18:2 and C18:3 peaks decreased with the formation of a small amount of the
trans-C18:1 configuration, especially for the Ni/SF catalyst system (
Figure 5b,c).
Based on the difference in the shape and bulk density of the catalysts affecting the total amount of the Ni active phase, the effects of the studied parameters on the activity of the Ni/SB and Ni/SF catalysts in converting the C18:2 structure in the partial hydrogenation of FAME was evaluated in terms of turnover frequency (TOF) calculated with Equation (1), which was adapted from the previous literature [
3]:
where MW
C18:2 and MW
Ni are 294 and 58.69 g/mol, respectively. The actual Ni content in each catalyst and its dispersion degree were obtained from ICP-OES and CO pulse chemisorption techniques, respectively (see
Table 2). The effect of reaction parameters such as Ni loading, H
2 pressure and reaction temperature on TOF and C18 compositions are shown in
Figure 6,
Figure 7 and
Figure 8.
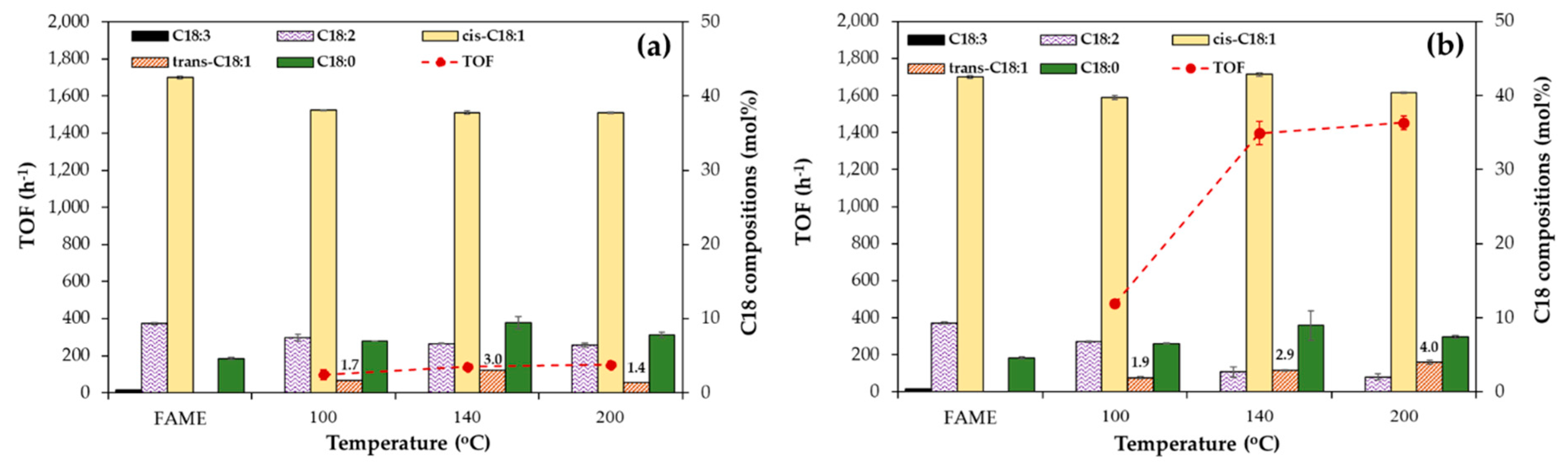
Regarding the effect of the Ni loading, the Ni/SB catalysts (
Figure 6a) exhibited a maximum TOF of 141 h
−1 at 30 wt% Ni content. In addition, the degree of C18:0 content increased from 6.2 to 9.4%, while the amount of the
cis-C18:1 configuration was reduced from 40.6 to 37.8% when the Ni content increased from 10 to 30 wt%. This indicated that some portion of the
cis-C18:1 was converted to a C18:0 configuration. Although a high saturation level is beneficial for oxidation stability, over-saturation can increase the cloud point and pour point of the obtained H-FAME, resulting in the limitation for use in cold weather areas [
3,
5]. Thus, a monounsaturated FAME is preferred since it can balance the properties between oxidation stability and cold flow properties. When the amount of Ni in the Ni/SB catalyst increased to 40–50 wt%, the TOF value decreased to 72–79 h
−1. This indicated that the high Ni loading level induced an oversize of the Ni crystallite structure and blocked the catalyst pores, resulting in the lower catalytic efficiency for hydrogenation or
cis–trans isomerization.
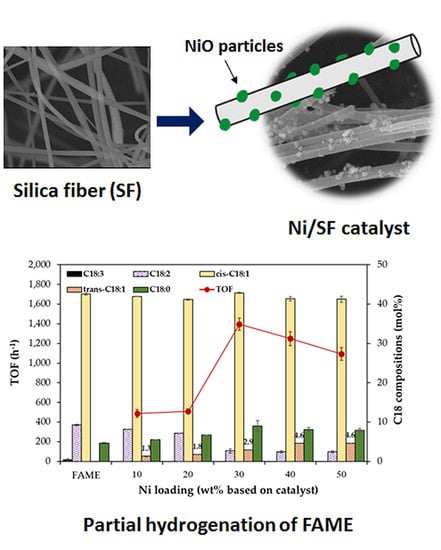
In the comparison of the Ni/SF catalysts at similar Ni loadings, the results indicated in
Figure 6b were in contrast to those for the Ni/SB catalysts (
Figure 6a). The TOF values of the system using Ni/SF catalysts containing 10–30 wt% Ni loading increased from 488 to 1396 h
−1, which was 6–9 times greater than those of Ni/SB catalysts at the same Ni content. Numwong et al. [
9] explained that silica with higher acidity could promote the adsorption of unsaturated bonds in the FAME molecules at the Brønsted acid sites of the silica support. Therefore, not only the open morphology of Ni/SF catalysts could provide a higher direct interaction between FAME molecules and Ni active sites to decrease the reduction temperature, but the higher acidity of the silica fiber could also promote the strong adsorption of C18:2 in FAME, resulting in the higher activity for hydrogenation to achieve the greater TOF values. Moreover, the amount of the C18:0 structure also increased from 5.5 to 8.0–9.0 wt% with a constant level of the
cis-C18:1 configuration (41–43 wt%). This implied that the Ni/SF catalyst having a low bulk density resulting in the small amount of catalyst weight at a given volume of the catalyst bed provided a shorter contact time and consequently inhibited the over-saturation of the monounsaturated FAME. Although the increased Ni loading enhanced the hydrogenation activity, since the bulk Ni particles could decrease the activation energy of H
2 dissociation [
25], the use of the Ni/SF catalyst with an overdose of Ni loading could not increase the reduction in the C18:2 fraction, resulting in the decrease in the TOF to 1248 and 1092 h
−1 for 40 wt% Ni/SF and 50 wt% Ni/SF catalysts, respectively.
To consider the formation of the
trans-C18:1 structure, the amount of the
trans-configuration should be minimized during hydrogenation due to its higher melting point than the
cis-structure at the same carbon number. This structure can remain in a solid form at room temperature and plug the fuel line in vehicles [
26]. The results in
Figure 6b indicated that Ni/SF catalysts containing 10–30 wt% Ni loading produced the lowest amount of the
trans-C18:1 structure (1.3–2.9 mol%) in the obtained H-FAME. This was due to the effect of the non-porous support, which could suppress the
trans-selectivity, as suggested in the previous work [
9]. However, the increase in the Ni loading to 40–50 wt% only slightly increased the amount of
trans-C18:1 to 4.6 mol%. This was possible because the larger metal crystallite size could induce the higher adsorption of unsaturated FAME on the metal surface and facilitate
cis–trans isomerization during partial hydrogenation [
3]. Thus, it can be concluded that the Ni/SF catalyst had high activity for partial hydrogenation to obtain H-FAME with high levels of monounsaturated structures, while also exhibiting low selectivity for
cis–trans isomerization.
The effect of the H
2 pressure on the hydrogenation of FAME at 140 °C was evaluated from 1–4 bar.
Figure 7a shows that the increasing H
2 pressure from 1 to 2 or 4 bar significantly enhanced the TOF value for the system using the 30 wt% Ni/SB catalyst from 141 to 405 or 440 h
−1, respectively. This implied that a higher H
2 pressure was required for the porous catalyst to overcome the mass transfer resistance. In addition, the higher H
2 pressure provided a larger amount of H
2 molecules, which accelerated hydrogenation [
27]. In the case of the 30 wt% Ni/SF catalyst, the degree of TOF for this system increased from 1396 to 1485 or 1659 h
−1 (only ca. 6–19%) when the H
2 pressure increased from 1 to 2 or 4 bar (
Figure 7b), respectively. This result also confirmed that the fibrous catalysts provided a greater accessibility to the Ni active sites for the reaction between H
2 and FAME molecules to occur and achieve the high TOF values at atmospheric pressure. Although the increased H
2 pressure enhanced the TOF for both catalytic systems, the obtained H-FAME contained a large amount of C18:0 (up to 18–20 wt%) with a significant reduction in the
cis-C18:1 content, which would induce poor cold flow properties.
In the case of reaction temperature, the partial hydrogenation of FAME was generally operated in the range of 80–200 °C depending on the type of catalyst [
3,
5,
10]. In this work, the reaction was performed using 30 wt% Ni/SB or 30 wt% Ni/SF under 1 bar H
2 pressure. In the case of the Ni/SB catalyst system,
Figure 8a showed that the increase in the reaction temperature from 100 to 200 °C slightly increased the TOF from 98 to 152 h
−1. Although the increase in the reaction temperature could improve the mass transfer coefficient for H
2 molecules from FAME to the catalyst surface [
28], the conventional Ni-based porous catalysts still had low activity for partial hydrogenation under mild conditions [
3]. This disadvantage of the porous Ni catalysts would be solved by using fibrous supports.
Figure 8b indicates that the use of Ni/SF catalyst provided the enhancement of the TOF value from 478 to 1454 h
−1 at the same reaction temperature range. This was due to the effect of the open geometry of the Ni active phase deposited on the SF surface, which could provide a higher accessibility of FAME and H
2 molecules to be adsorbed and reacted on the surface of the catalyst. However, the use of higher reaction temperatures promoted the
cis–trans isomerization to achieve the higher amount of the
trans-C18:1 structure from 1.9 to 4.0 mol% [
29].
2.3. Catalyst Stability of Ni/SF and Ni/SB Catalysts
The stability of the 30 wt% Ni/SB and 30 wt% Ni/SF catalysts was comparatively evaluated using a FAME feed rate of 0.44 g min
−1 under 1 bar H
2 at 140 °C. The samples of the H-FAME obtained from each catalyst system were collected every 1–2 h during the time on stream for 16 h, and the C18:2 conversion level was then analyzed. The FAME hydrogenation using the 30 wt% Ni/SB catalyst maintained a C18:2 conversion level of 26–28% throughout the course of the reaction (
Figure 9). The 30 wt% Ni/SF catalyst exhibited a high C18:2 conversion level (70–71%) at the beginning of the reaction, but then the C18:2 conversion level declined, with longer reaction times down to ca. 61% after 16 h. To consider the cumulative C18:2 conversion,
Figure 9 shows that the partial hydrogenation using the 30 wt% Ni/SB catalyst still maintained the cumulative C18:2 conversion at ca. 27% for the long period of operation. On the other hand, the cumulative C18:2 conversion of the system using the 30 wt% Ni/SF catalyst would be decreased to 27% when the reaction was allowed for 5.79 days. Above this point, the cumulative C18:2 conversion of the 30 wt% Ni/SF catalyst trended to be lower than that of the system using the 30 wt% Ni/SB catalyst.
The results from the analysis of the energy-dispersive X-ray fluorescence spectrometer (EDX) as shown in
Table 3 indicated that FAME, used as the raw material of this research, had only Zn species (36 ppm), possibly due to the contaminant or nutrient in the palm oil feedstock used for FAME production [
30,
31]. After the partial hydrogenation, H-FAME obtained from the 30 wt% Ni/SF catalyst contained Ni particles of 93.9 ppm, whereas H-FAME obtained from the 30 wt% Ni/SB catalysts had no Ni species. Presumably, the open geometry with weaker metal–support interaction, as seen in the H
2-TPR analysis (
Figure 4) of Ni/SF catalysts, led the quick deactivation via agglomeration [
19]. This could facilitate the loss of Ni particles from the surface of the support compared to that in the porous catalysts. Although the amount of silica could not be detected in all H-FAMEs, Chen et al. [
5] reported that the amorphous nature of silica or alumina was slightly leached out under the mild hydrogenation condition. Thus, it was possible that the silica fiber having the lower degree of crystallinity than the commercial porous silica ball might have lower stability. According to the results obtained from the EDX analysis and the previous literature [
5], the degree of crystallinity and the interaction between active metals and the surface of the silica fiber should be improved in order to increase the lifetime of the catalyst for applying in the commercialized scale of H-FAME production.