Particle Size and PdO–Support Interactions in PdO/CeO2-γ Al2O3 Catalysts and Effect on Methane Combustion
Abstract
1. Introduction
2. Results
2.1. Catalysis Synthesis
2.2. Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)
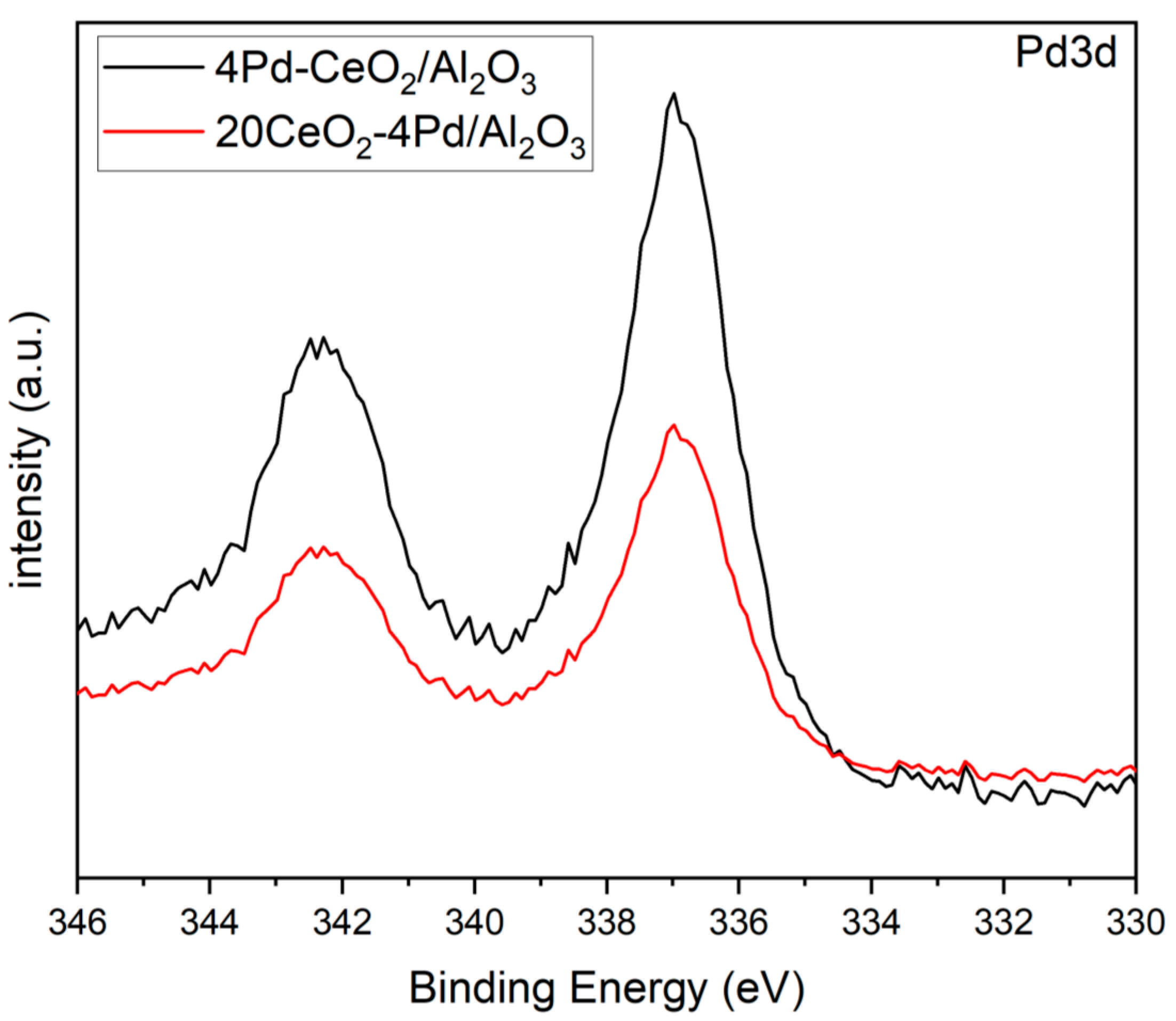
2.3. X-Ray Photoelectron Spectroscopy (XPS)
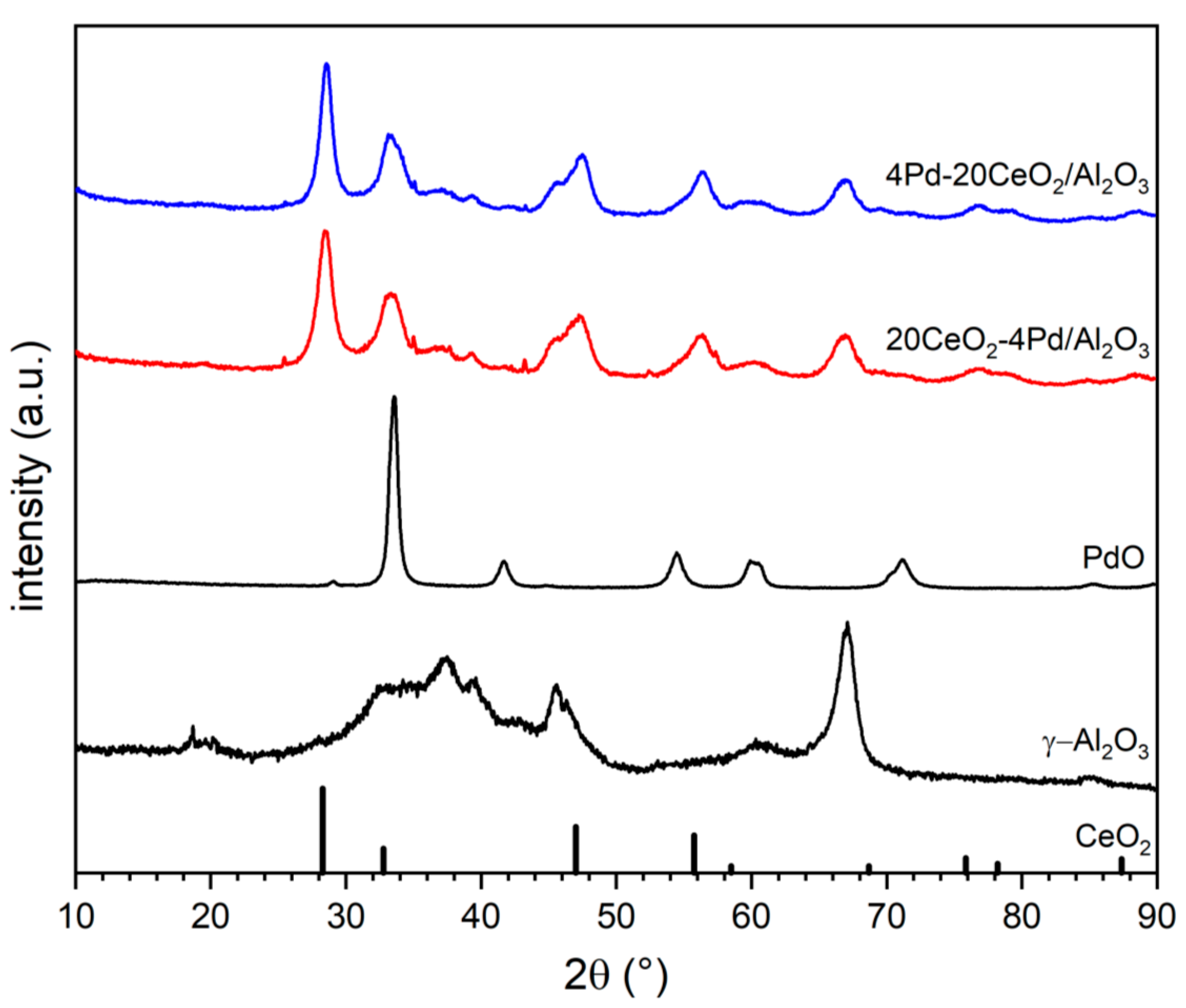
2.4. X-Ray Diffraction (XRD)
2.5. Properties of the Catalysts
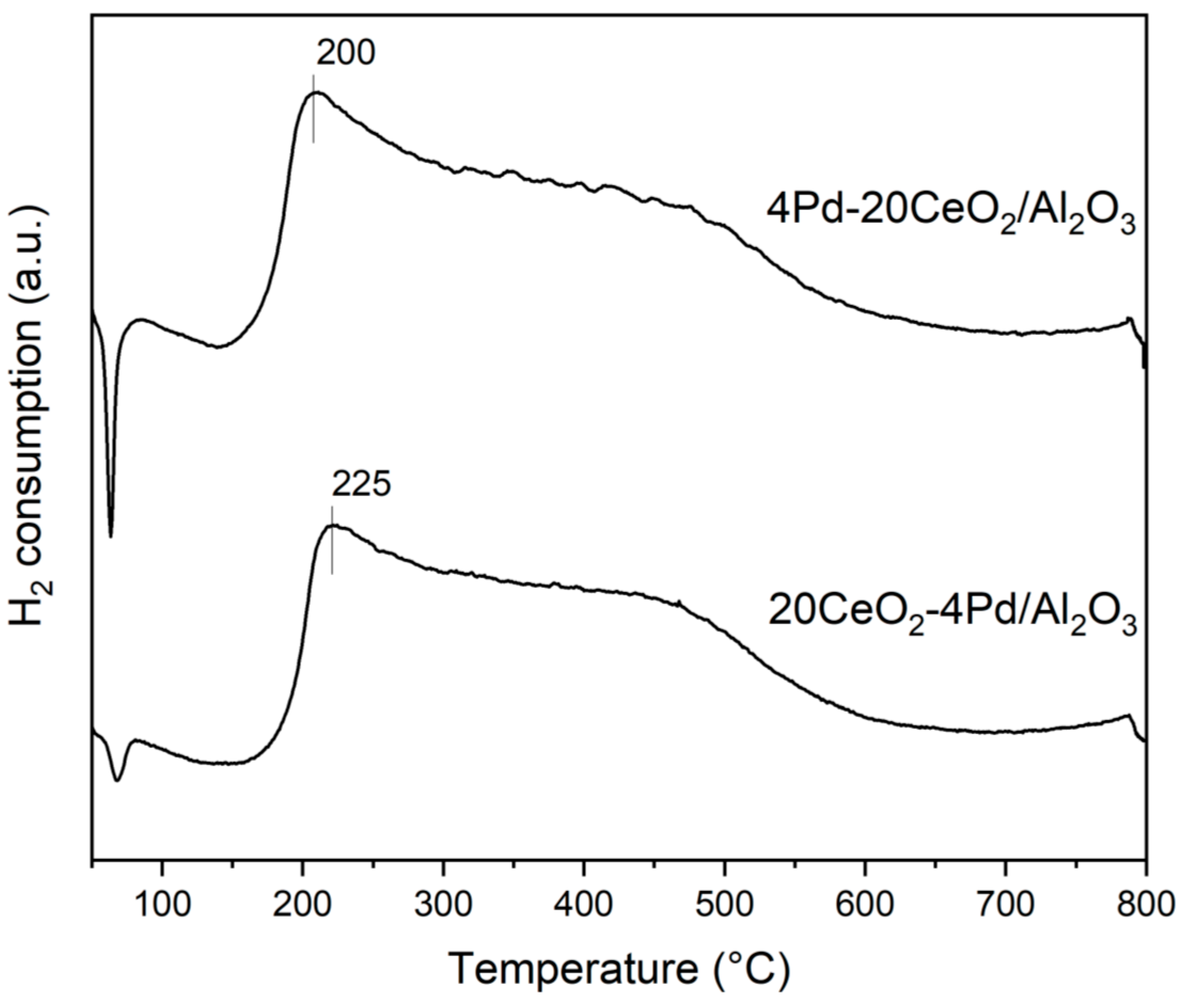
2.6. Hydrogen–Temperature Programmed Reduction (H2-TPR)
2.7. High-Angle Annular Dark-Field Scanning Transmission Electron Microscopy (HAADF-STEM), Scanning Electron Microscopy (SEM), and Energy Dispersive Spectroscopy (EDS)
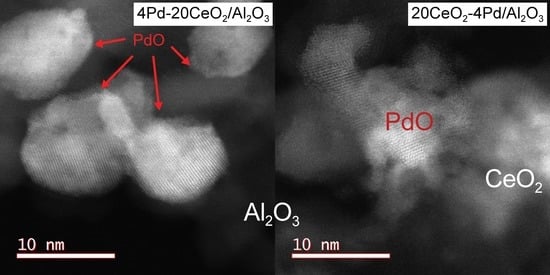
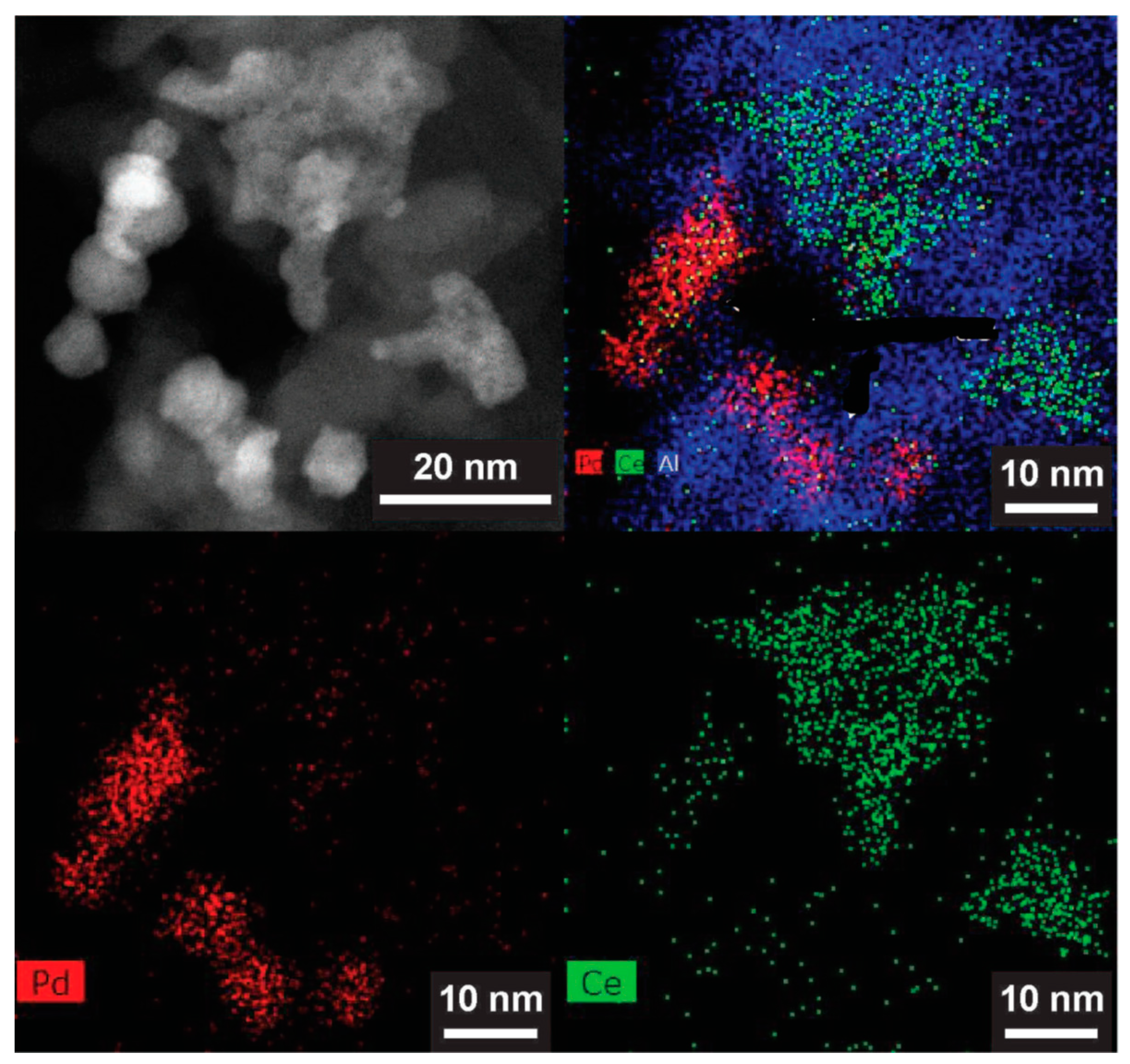
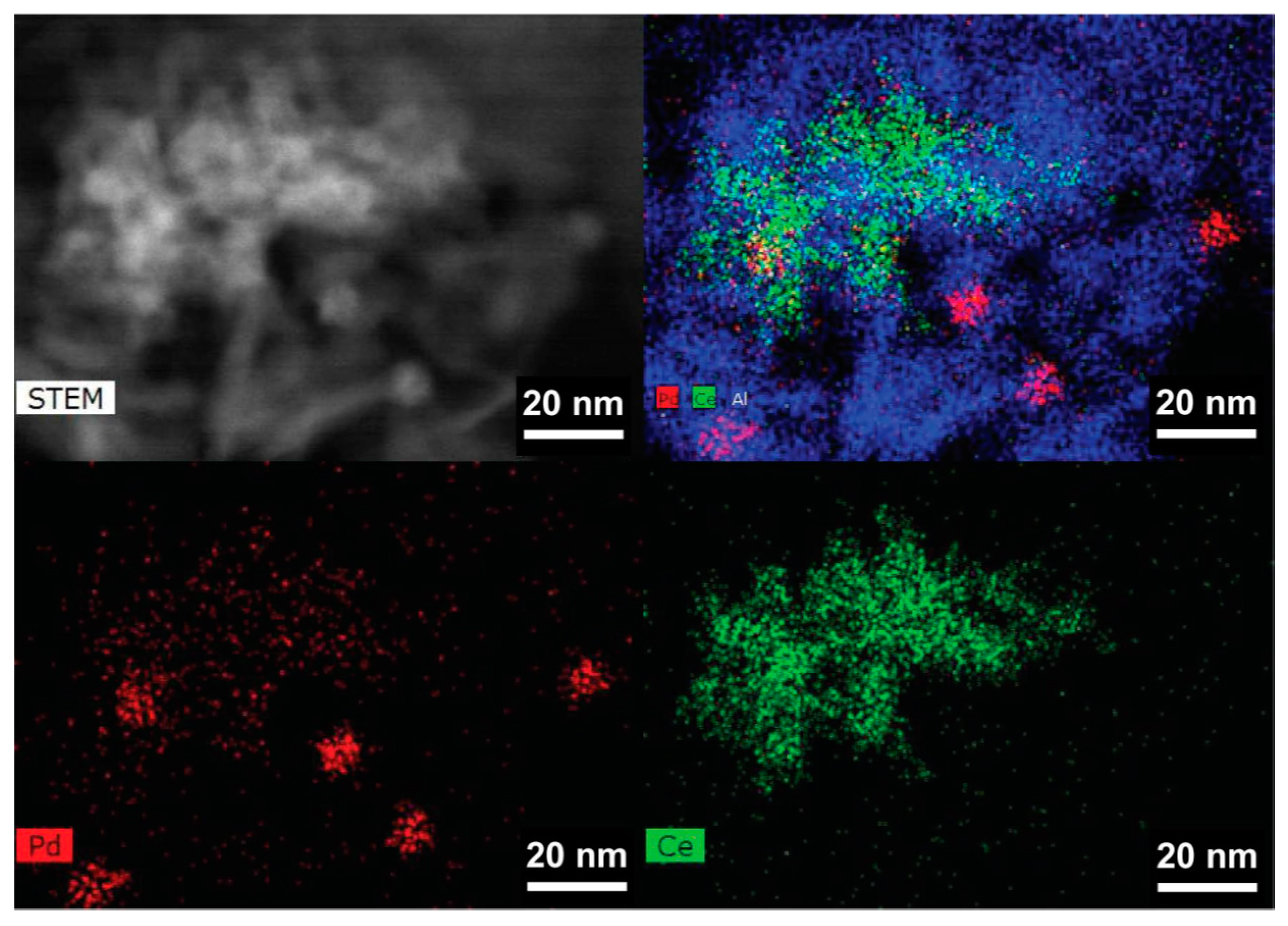
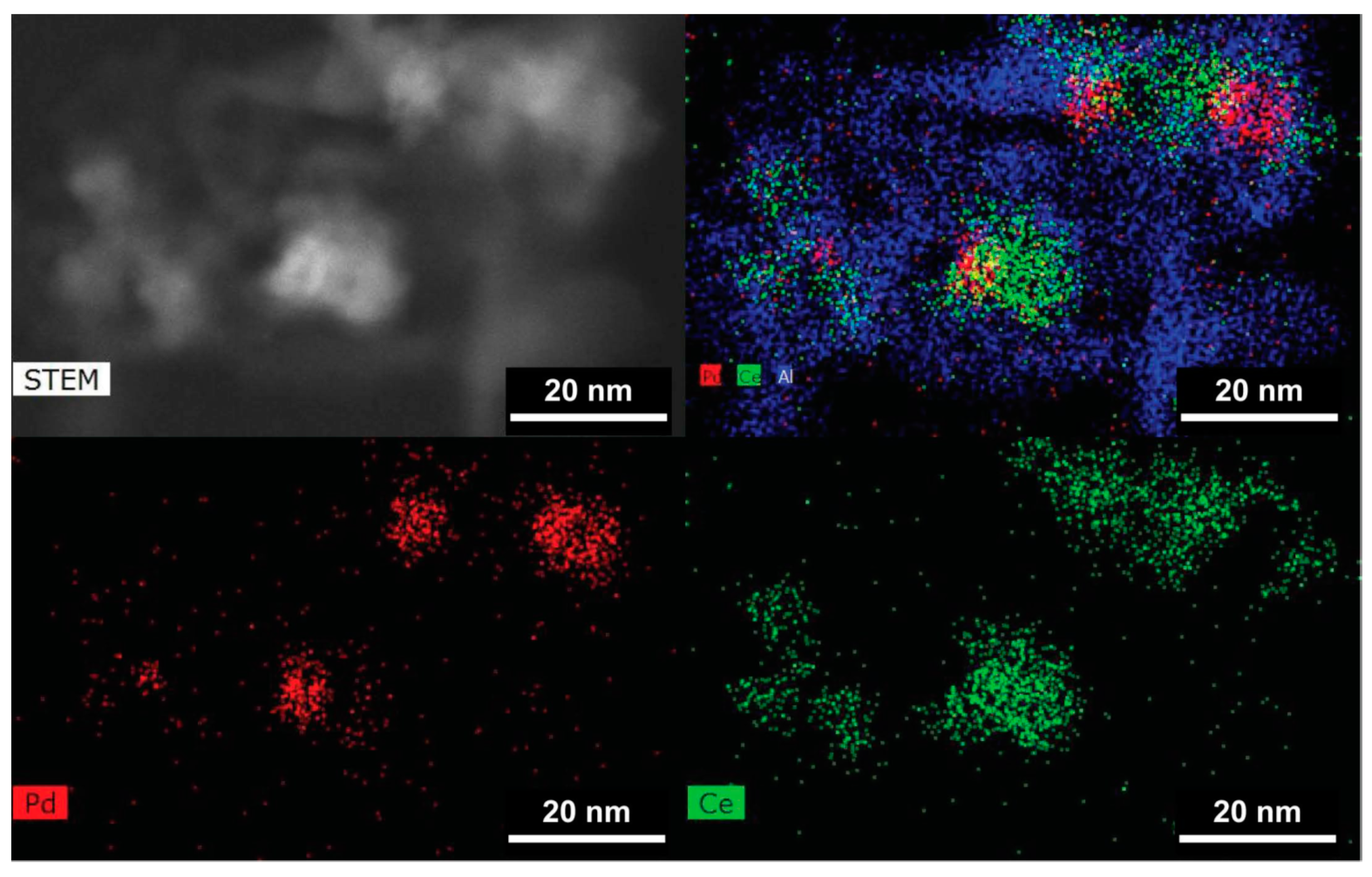
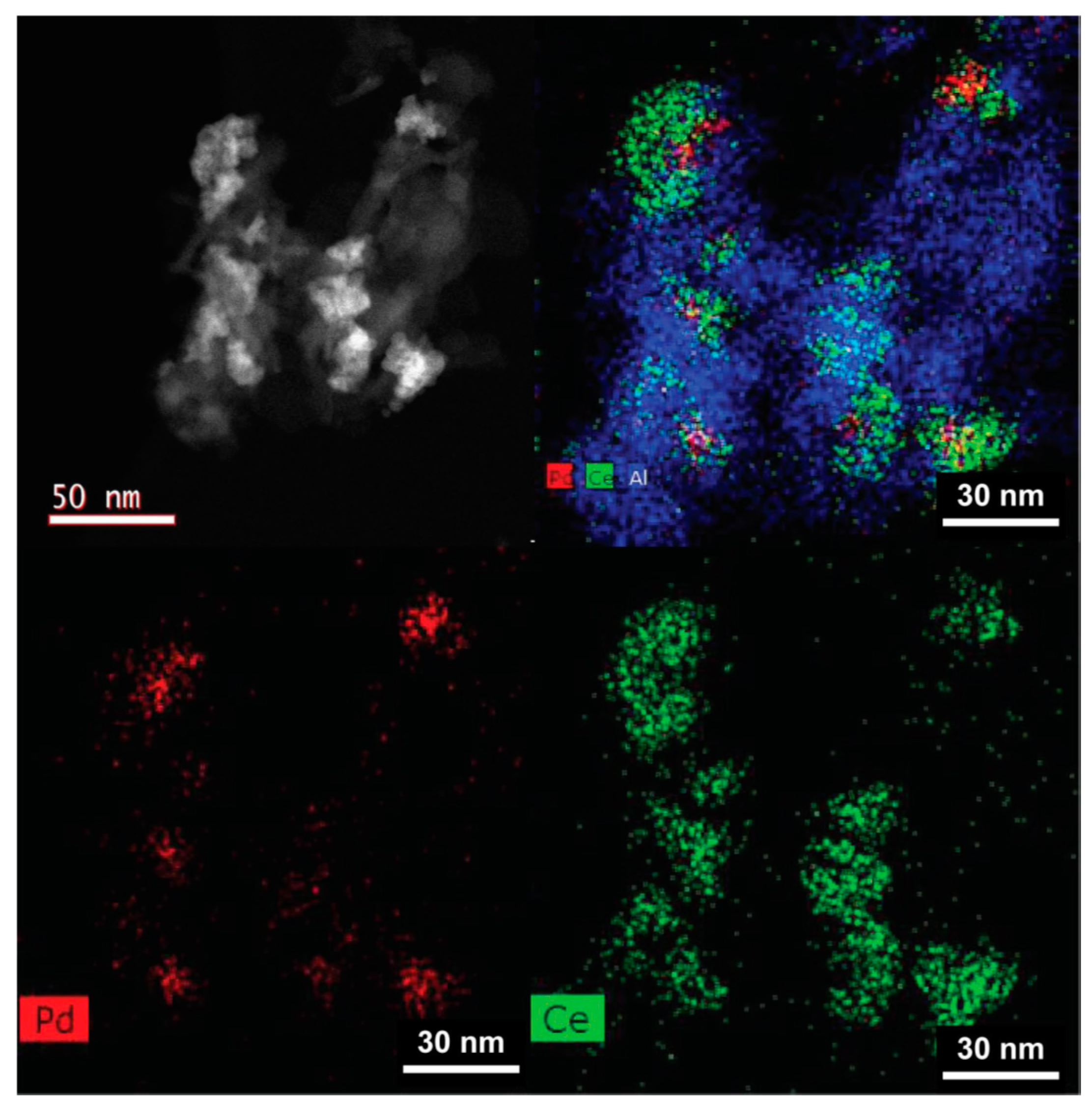
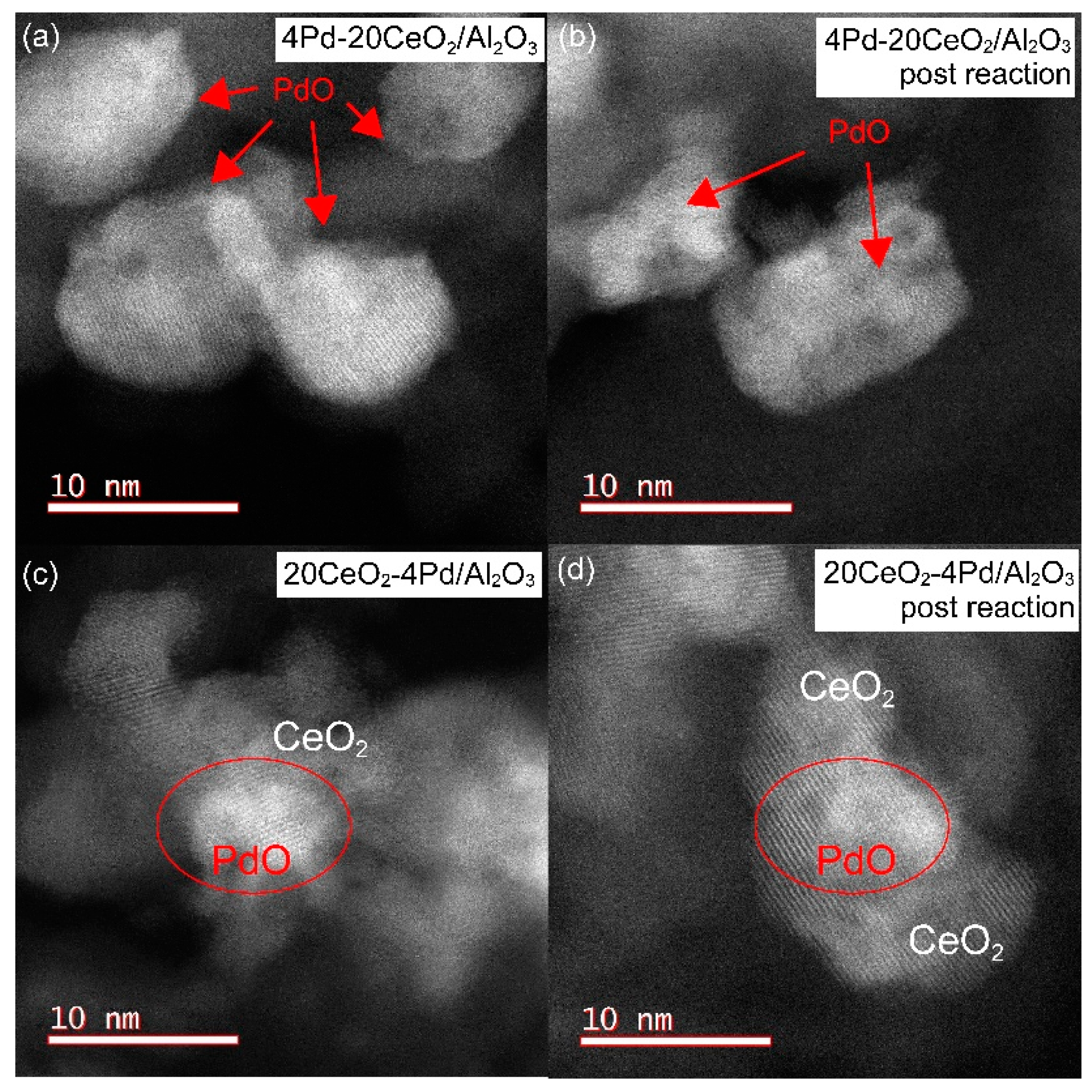
2.7.1. Location of PdO Particles on The Supports
2.7.2. Morphology and PdO–Support Interactions
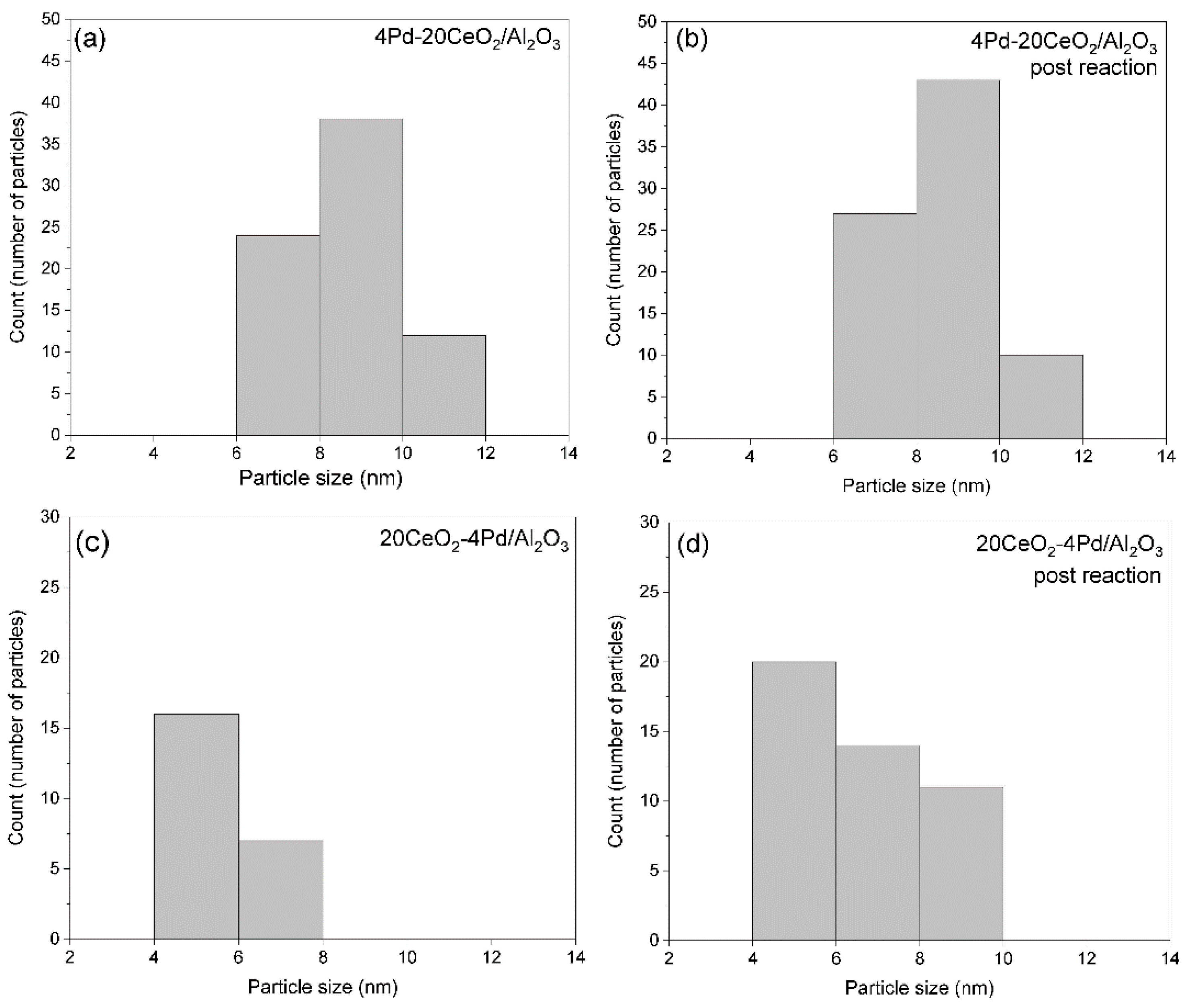
2.7.3. Particle Sizes and Distribution
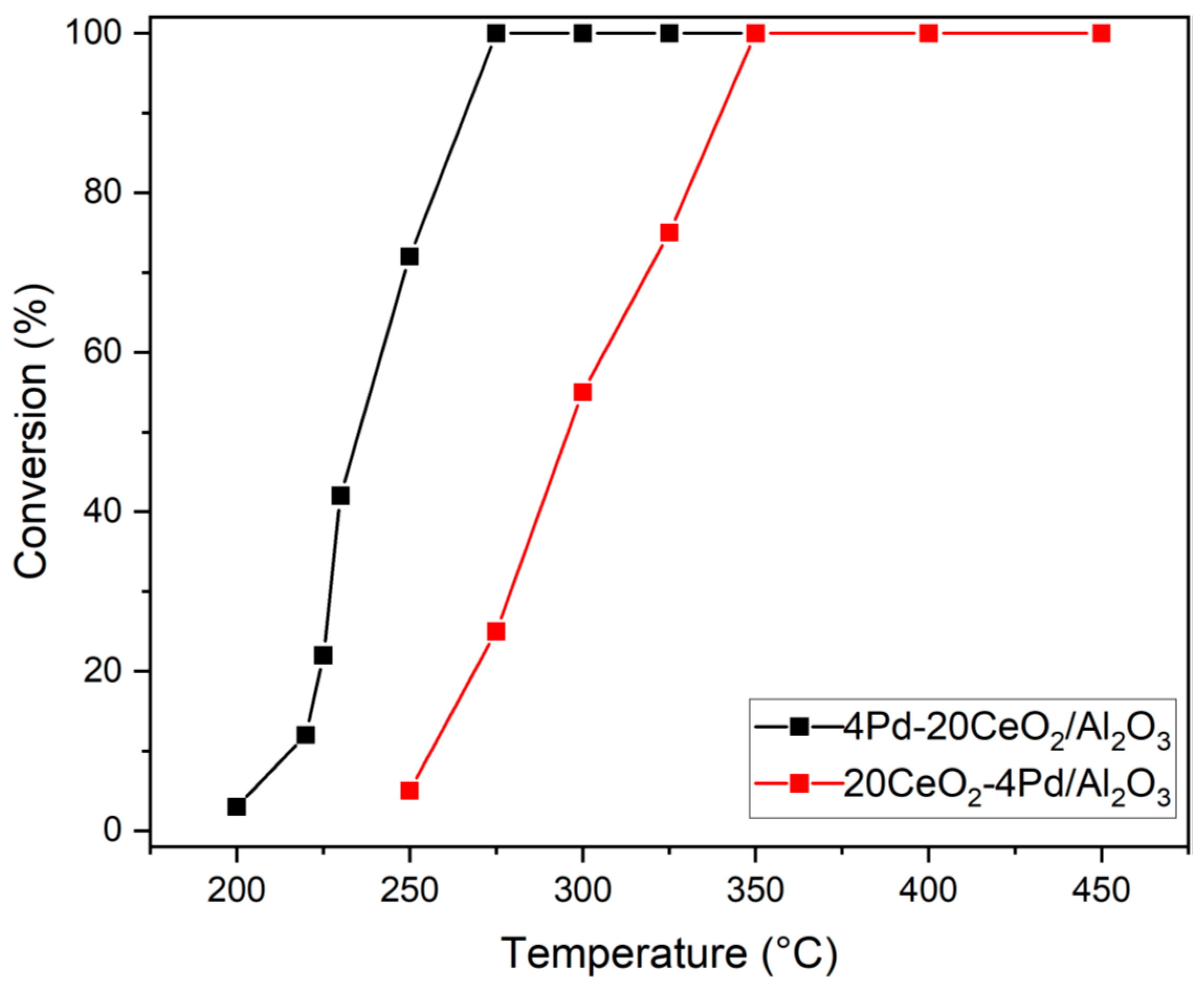
2.8. Activity of the Catalysts
2.9. Activation Energy
3. Discussion
4. Materials and Methods
4.1. Catalyst Synthesis
- Synthesis of 4Pd-20CeO2/Al2O₃ catalyst: This catalyst was prepared by impregnating an aqueous solution of palladium nitrate hydrate to an aqueous slurry of the solid dual support of 20CeO2/Al2O₃ while vortexing for uniform mixing. The slurry was dried and calcined. The ICP-OES analysis gave Pd wt.% as 3.94. The final composition of the catalyst was rounded to 4.0 wt.% Pd (as PdO), 20 wt.% CeO2 and 80 wt.% γ-Al2O₃ [designated in this article as 4Pd-20CeO2/Al2O₃].
- Synthesis of 20CeO2-4Pd/Al2O₃ catalyst: This catalyst was prepared by impregnating an aqueous solution of cerium nitrate hexahydrate to an aqueous slurry of 5Pd/Al2O₃ while vortexing for uniform mixing. The slurry was dried and calcined. The ICP-OES analysis gave Pd wt.% as 3.56. The final composition of the catalyst was rounded to 20 wt.% CeO2 and 4.0 wt.% Pd (as PdO) on γ-Al2O₃ [designated in this article as 20CeO2-4Pd/Al2O₃].
4.2. Activity Measurements
- [CH4]in = Volume% of methane at 1 atm. and 25 °C in the gas feed entering the reactor.
- [CH4]out = Volume% methane at 1 atm. and 25 °C in the gas mixture exiting the reactor.
4.3. Activation Energy
4.4. Catalyst Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Monai, M.; Montini, T.; Gorte, R.J.; Fornasiero, P. Catalytic Oxidation of Methane: Pd and Beyond. Eur. J. Inorg. Chem. 2018, 2018, 2884–2893. [Google Scholar] [CrossRef]
- Chen, J.; Arandiyan, H.; Gao, X.; Li, J. Recent Advances in Catalysts for Methane Combustion. Catal. Surv. Asia 2015, 19, 140–171. [Google Scholar] [CrossRef]
- Simplício, L.M.T.; Brandão, S.T.; Sales, E.A.; Lietti, L.; Bozon-Verduraz, F. Methane combustion over PdO-alumina catalysts: The effect of palladium precursors. Appl. Catal. B Environ. 2006, 63, 9–14. [Google Scholar] [CrossRef]
- Goodman, E.D.; Dai, S.; Yang, A.C.; Wrasman, C.J.; Gallo, A.; Bare, S.R.; Hoffman, A.S.; Jaramillo, T.F.; Graham, G.W.; Pan, X.; et al. Uniform Pt/Pd Bimetallic Nanocrystals Demonstrate Platinum Effect on Palladium Methane Combustion Activity and Stability. ACS Catal. 2017, 7, 4372–4380. [Google Scholar] [CrossRef]
- Banerjee, A.C.; McGuire, J.M.; Lawnick, O.; Bozack, M.J. Low-temperature activity and PdO-PdOx transition in methane combustion by a PdO-PdOx/γ-AL2O3 catalyst. Catalysts 2018, 8, 266. [Google Scholar] [CrossRef]
- Colussi, S.; Trovarelli, A.; Cristiani, C.; Lietti, L.; Groppi, G. The influence of ceria and other rare earth promoters on palladium-based methane combustion catalysts. Catal. Today 2012, 180, 124–130. [Google Scholar] [CrossRef]
- Ma, J.; Lou, Y.; Cai, Y.; Zhao, Z.; Wang, L.; Zhan, W.; Guo, Y.; Guo, Y. The relationship between the chemical state of Pd species and the catalytic activity for methane combustion on Pd/CeO2. Catal. Sci. Technol. 2018, 8, 2567–2577. [Google Scholar] [CrossRef]
- Cargnello, M.; Jaén, J.J.D.; Garrido, J.C.H.; Bakhmutsky, K.; Montini, T.; Gámez, J.J.C.; Gorte, R.J.; Fornasiero, P. Exceptional Activity for Methane Combustion over Modular Pd@CeO2 Subunits on Functionalized Al2O3. Science 2012, 337, 713–718. [Google Scholar] [CrossRef]
- Li, L.; Zhang, N.; Wu, R.; Song, L.; Zhang, G.; He, H. Comparative Study of Moisture-Treated Pd@CeO2/Al2O3 and Pd/CeO2/Al2O3 Catalysts for Automobile Exhaust Emission Reactions: Effect of Core-Shell Interface. ACS Appl. Mater. Interfaces 2020, 12, 10350–10358. [Google Scholar] [CrossRef]
- Rodrigues, L.M.T.S.; Silva, R.B.; Rocha, M.G.C.; Bargiela, P.; Noronha, F.B.; Brandão, S.T. Partial oxidation of methane on Ni and Pd catalysts: Influence of active phase and CeO2 modification. Catal. Today 2012, 197, 137–143. [Google Scholar] [CrossRef]
- Ramírez-López, R.; Elizalde-Martinez, I.; Balderas-Tapia, L. Complete catalytic oxidation of methane over Pd/CeO2-Al2O3: The influence of different ceria loading. Catal. Today 2010, 150, 358–362. [Google Scholar] [CrossRef]
- Sun, M.; Hu, W.; Yuan, S.; Zhang, H.; Cheng, T.; Wang, J.; Chen, Y. Effect of the loading sequence of CeO2 and Pd over Al2O3 on the catalytic performance of Pd-only close-coupled catalysts. Mol. Catal. 2020, 482, 100332. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Geng, F.; Li, Z.; Li, P.; Gong, W. Synthesis and properties of PdO/CeO2-Al2O3 catalysts for methane combustion. Front. Chem. Sci. Eng. 2012, 6, 34–37. [Google Scholar] [CrossRef]
- Yang, X.; Du, C.; Guo, Y.; Guo, Y.; Wang, L.; Wang, Y.; Zhan, W. Al2O3 supported hybrid Pd–CeO2 colloidal spheres and its enhanced catalytic performances for methane combustion. J. Rare Earths 2019, 37, 714–719. [Google Scholar] [CrossRef]
- Colussi, S.; Trovarelli, A.; Vesselli, E.; Baraldi, A.; Comelli, G.; Groppi, G.; Llorca, J. Structure and morphology of Pd/Al2O3 and Pd/CeO2/Al2O3 combustion catalysts in Pd-PdO transformation hysteresis. Appl. Catal. A Gen. 2010, 390, 1–10. [Google Scholar] [CrossRef]
- Onn, T.M.; Zhang, S.; Arroyo-Ramirez, L.; Xia, Y.; Wang, C.; Pan, X.; Graham, G.W.; Gorte, R.J. High-surface-area ceria prepared by ALD on Al2O3 support. Appl. Catal. B Environ. 2017, 201, 430–437. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Ahmadi, M.; Mistry, H.; Cuenya, B.R. Tailoring the Catalytic Properties of Metal Nanoparticles via Support Interactions. J. Phys. Chem. Lett. 2016, 7, 3519–3533. [Google Scholar] [CrossRef]
- Banerjee, A.C.; Golub, K.W.; Hakim, M.A.; Billor, M.Z. Comparative study of the characteristics and activities of Pd/γ-Al2O3 catalysts prepared by vortex and incipient wetness methods. Catalysts 2019, 9, 336. [Google Scholar] [CrossRef]
- Huang, F.; Chen, J.; Hu, W.; Li, G.; Wu, Y.; Yuan, S.; Zhong, L.; Chen, Y. Pd or PdO: Catalytic active site of methane oxidation operated close to stoichiometric air-to-fuel for natural gas vehicles. Appl. Catal. B Environ. 2017, 219, 73–81. [Google Scholar] [CrossRef]
- Lan, L.; Chen, S.; Zhao, M.; Gong, M.; Chen, Y. The effect of synthesis method on the properties and catalytic performance of Pd/Ce0.5Zr0.5O2-Al2O3 three-way catalyst. J. Mol. Catal. A Chem. 2014, 394, 10–21. [Google Scholar] [CrossRef]
- Murata, K.; Kosuge, D.; Ohyama, J.; Mahara, Y.; Yamamoto, Y.; Arai, S.; Satsuma, A. Exploiting Metal-Support Interactions to Tune the Redox Properties of Supported Pd Catalysts for Methane Combustion. ACS Catal. 2020, 10, 1381–1387. [Google Scholar] [CrossRef]
- Murata, K.; Ohyama, J.; Yamamoto, Y.; Arai, S.; Satsuma, A. Methane Combustion over Pd/Al2O3 Catalysts in the Presence of Water: Effects of Pd Particle Size and Alumina Crystalline Phase. ACS Catal. 2020, 10, 8149–8156. [Google Scholar] [CrossRef]
- Miller, J.B.; Malatpure, M. Pd catalysts for total oxidation of methane: Support effects. Appl. Catal. A Gen. 2015, 495, 54–62. [Google Scholar] [CrossRef]
- Schwartz, W.R.; Pfefferle, L.D. Combustion of methane over palladium-based catalysts: Support interactions. J. Phys. Chem. C 2012, 116, 8571–8578. [Google Scholar] [CrossRef]
- Feng, X.; Li, W.; Liu, D.; Zhang, Z.; Duan, Y.; Zhang, Y. Self-Assembled Pd@CeO2/γ-Al2O3 Catalysts with Enhanced Activity for Catalytic Methane Combustion. Small 2017, 13, 1700941. [Google Scholar] [CrossRef]
- Nilsson, J.; Carlsson, P.A.; Fouladvand, S.; Martin, N.M.; Gustafson, J.; Newton, M.A.; Lundgren, E.; Grönbeck, H.; Skoglundh, M. Chemistry of Supported Palladium Nanoparticles during Methane Oxidation. ACS Catal. 2015, 5, 2481–2489. [Google Scholar] [CrossRef]
- Puigdollers, A.R.; Schlexer, P.; Tosoni, S.; Pacchioni, G. Increasing oxide reducibility: The role of metal/oxide interfaces in the formation of oxygen vacancies. ACS Catal. 2017, 7, 6493–6513. [Google Scholar] [CrossRef]
- Sulmonetti, T.P.; Pang, S.H.; Claure, M.T.; Lee, S.; Cullen, D.A.; Agrawal, P.K.; Jones, C.W. Vapor phase hydrogenation of furfural over nickel mixed metal oxide catalysts derived from layered double hydroxides. Appl. Catal. A Gen. 2016, 517, 187–195. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, Y.; Pradel, K.C.; Zhang, W.; Liu, M.; Wang, Z.L. Domain structures and Pr co antisite point defects in double-perovskite. Ultramicroscopy 2018, 193, 64–70. [Google Scholar] [CrossRef]

| Properties | Catalysts | |
|---|---|---|
| 4Pd-20CeO2/Al2O3 | 20CeO2-4Pd/Al2O3 | |
| Pd wt.% (ICP-OES) | 3.93 | 3.54 |
| Surface Area (m2/g) | 117 | 116 |
| CO uptake in chemisorption | 38.3 µmol/g | 87.4 µmol/g |
| Dispersion | 10.2% | 26.4% |
| Metal Surface Area | 1.8 m2/g | 4.1 m2/g |
| Nanoparticle diameter (hemisphere) | 10.9 nm | 4.2 nm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fertal, D.R.; Bukhovko, M.P.; Ding, Y.; Billor, M.Z.; Banerjee, A.C. Particle Size and PdO–Support Interactions in PdO/CeO2-γ Al2O3 Catalysts and Effect on Methane Combustion. Catalysts 2020, 10, 976. https://doi.org/10.3390/catal10090976
Fertal DR, Bukhovko MP, Ding Y, Billor MZ, Banerjee AC. Particle Size and PdO–Support Interactions in PdO/CeO2-γ Al2O3 Catalysts and Effect on Methane Combustion. Catalysts. 2020; 10(9):976. https://doi.org/10.3390/catal10090976
Chicago/Turabian StyleFertal, Domenica R., Maxim P. Bukhovko, Yong Ding, Mehmet Z. Billor, and Anil C. Banerjee. 2020. "Particle Size and PdO–Support Interactions in PdO/CeO2-γ Al2O3 Catalysts and Effect on Methane Combustion" Catalysts 10, no. 9: 976. https://doi.org/10.3390/catal10090976
APA StyleFertal, D. R., Bukhovko, M. P., Ding, Y., Billor, M. Z., & Banerjee, A. C. (2020). Particle Size and PdO–Support Interactions in PdO/CeO2-γ Al2O3 Catalysts and Effect on Methane Combustion. Catalysts, 10(9), 976. https://doi.org/10.3390/catal10090976